Abstract
COVID-19 pandemic has severely affected regular public health interventions including population-based cancer screening. Impacts of such screening delays on the changes in structure and screening process and the resultant long-term outcomes are unknown. It is therefore necessary to develop a systematic framework to assess theses impacts related to these components of quality. Using population-based cancer screening with fecal immunochemical test (FIT) as an illustration, the main analysis was to assess how various scenarios of screening delays were associated with the capacity for primary screening and full time equivalent (FTE) for colonoscopy and impact long-term outcomes based on a Markov decision tree model on population level. The second analysis was to quantify how the extent of COVID-19 epidemic measured by social distancing index affected capacity and FTE that were translated to delays with an exponential relationship. COVID-19 epidemic led to 25%, 29%, 34%, and 39% statistically significantly incremental risks of late cancer for the delays of 0.5-year, 1-year,1.5-year, and 2-year, respectively compared with regular biennial FIT screening. The corresponding statistically findings of four delayed schedules for death from colorectal cancer (CRC) were 26%, 28%, 29%, and 30%, respectively. The higher social distancing index led to a lower capacity of uptake screening and a larger reduction of FTE, resulting in longer screening delay and longer waiting time, which further impacted long-term outcomes as above. In summary, a systematic modelling approach was developed for demonstrating the strong impact of screening delays caused by COVID-19 epidemic on long-term outcomes illustrated with a Taiwan population-based FIT screening of CRC.
Keywords: COVID-19, Colorectal cancer, Screening, Fecal immunochemical test, Social distancing
1. Introduction
While COVID-19 pandemic has claimed over three million of deaths as of April 2021 (WHO, 2021) the colossal number of cases has also engulfed health care resources which in turn affected the allocation and the provision of regular public health intervention programs such as population-based screening for breast cancer and colorectal cancer (CRC). Many of population-based screening programs around the world have been completely halted or electively executed due to the rampant COVID-19 pandemic (Nodora et al., 2020).
Long-term impacts of screening delays and the alterations of relevant structure and process in population-based cancer screening are unknown and worthy of being investigated. However, as population-based screening is a complex process evaluating long-term outcomes requires a systematic framework to assess how and to what degree COVID-19 pandemic have disrupted the provision of population-based cancer screening.
From the quality aspects of population-based screening programs following the Donabedian model (Donabedian, 1988; Haj et al., 2013) for health care system, the impacts of stopping or delaying screening programs are three-fold. These include the first key component “structure” defined as the capacity of uptake screening and subsequent diagnostic workup for confirmatory diagnosis, the second key component “process” defined as the screening flow from uptake screening to confirmatory diagnosis, and the third key component “outcomes” defined as advanced of and mortality from specific-cause of cancer. All these three key components would be compromised from both sides, providers and physicians who are involved in the execution of population-based screening and clients who are invited to attend screen and would be referred to have confirmatory diagnosis if they are positive for the suspected malignancy after the uptake of screen.
Based on the overall framework, an integrated quantitative model is required for assessing the impact of screening delays due to COVID-19 pandemic on these three key components. As the spread of SARS-CoV-2 leading to COVID-19 pandemic is a dynamic process and its influence on population-based screening is also subject to the recovery rate and case-fatality rate of COVID-19 cases, the second goal is to link the extent of the spread of COVID-19, the recovery rate, and the case-fatality to the capacity of structure, the compromised screening process, and the upstaging of cancer and death with the recently proposed measure of social distancing that is defined as the ratio of the cumulative number of COVID-19 cases to the production of the cumulative number of recovery times one minus the case-fatality rate (Chen et al., 2020).
To demonstrate how to apply the methodology developed here to evaluate long-term impacts of population-based screening delays, we selected fecal immunochemical test (FIT) screening as an illustration. In the main analysis, we developed a novel systematic cascade model integrated from three key components of the quality of population-based screening to evaluate how screening delays impact the capacity of manpower for primary screening and full-time equivalent (FTE) to colonoscopy, screening process, and long-term outcomes. In second analysis, we linked the extent of social distancing with the capacity of manpower for primary screening and FTE, translated the capacity and FTE to screening delays, and the resultant long-term outcomes.
2. Materials and methods
2.1. Overall framework of three quality components affected by COVID-19
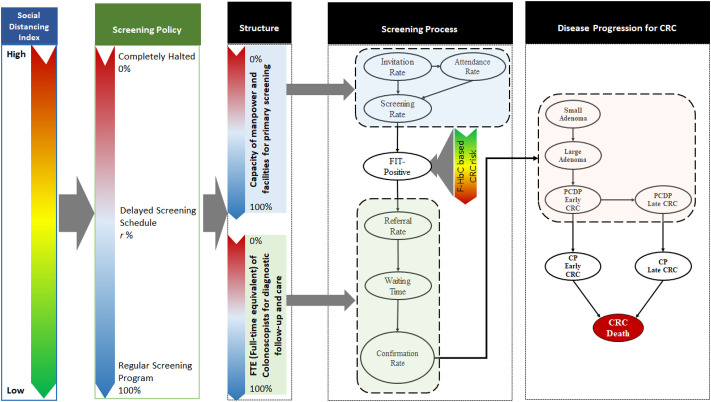
Fig. 1 shows the overall framework of how COVID-19 pandemic affects three key components that are responsible for the quality of population-based screening in the light of the Donabedian model (Donabedian, 1988; Haj et al., 2013). The influences of COVID-19 pandemic in the globe or epidemic in local region is measured with an index of easing social index ranging from 0 to positive integers.
Fig. 1.
The framework for assessing the impact of COVID-19 on FIT-based CRC screening.
The quality components are modelled by three main stochastic processes, including the queue process for accommodating the capacity of uptake screening, the linear queue model for cascade of processes from referral, waiting time, until confirmatory diagnosis for those with positive or suspected malignancy after the uptake of screening, and the tumour-stage-based progressive disease natural model for those ascertained as colorectal neoplasm. Note that the proposed overall framework can be applied to different kinds of screening program with various screening modalities including the endoscopy-based (such as colonoscopy and sigmoidoscopy) and the stool-based modes. Here, CRC screening with FIT is used as an illustration.
2.2. The queue process for modelling the capacity of uptake screening
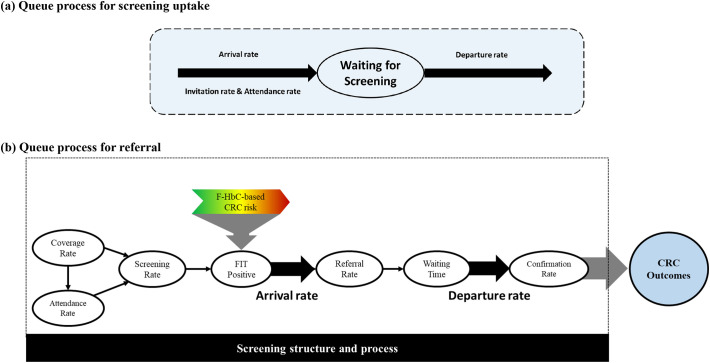
The extent of the delayed schedule can be modelled by a queue process as shown in Fig. 2 (a) with the arrival rate for the uptake of screen and the departure rate for receiving FIT. The arrival rate for screening based on this queue process is determined by the invitation rate and attendance rate during COVID-19 pandemic. As illustrated in Fig. 1, the invitation rate is subject to the capacities of facilities and manpower involved in a screening program, which are both crucial for designing and guiding the delayed screening policy in the era of COVID-19 pandemic. The attendance rate is affected by the health behaviour of clients who may be hesitant to participate in the screening program when invited at regular interval in the face of the epidemic of COVID-19. Note that the lower invitation rate and attendance rate may counterbalance screening delays but those invited but hesitant participants may have the delay in diagnosis that will be captured in the final element of the Markov model following the natural history model. To what extent they impacted long-term outcomes such as advanced CRC and its mortality is highly dependent on how soon (e.g. screening interval) they can come back to screen. Such aggregated net impacts would be quantified by the following Markov cohort approach on population level. The departure rate in this queue process is also possibly influenced by the capacity of providing FIT for participants.
Fig. 2.
The processes for colorectal cancer screening.
(a) Queue process for screening uptake.
(b) Queue process for referral.
2.3. The queue process for cascade of screening process
A quantitative model for modelling cascade of screening processes from referral for positives, waiting time and confirmation for colonoscopy is required. Fig. 2 (b) shows such a queue process. The crucial determinant for this process is subject to the capacity of manpower involved in confirmatory diagnosis of referrals. We were to measure this capacity by using the concept of FTE already developed in literature (Bowman et al., 1983; Burke et al., 2013; Mulhausen and Mcgee, 1989). The FTE devoted to population-based screening affects the confirmation rate through referral rate and waiting time for confirmatory colonoscopy. Information on the collection of FTE is described below.
2.4. Stage-based disease natural history model for the progression of CRC
We applied a multistate assessment model for predicting the influence of COVID-19 on short-term (stage shifting) and long-term (CRC mortality and life-year loss) outcomes pertaining to population-based CRC screening (Wu et al., 2006; Yang et al., 2006). The disease natural history involves a series of states of colorectal neoplasm including small and large adenoma, early and late CRC. Following the evolution of CRC, the disease natural history of CRC is constructed by a seven-state Markov model consisting of normal, small adenoma (adenoma smaller than 1 cm in size), large adenoma (adenoma larger than 1 cm in size), preclinical CRC at early stage (early PCDP, say stage I and II), preclinical CRC at late stage (late PCDP, say stage III and IV), clinical CRC at early CRC (early CP), clinical CRC at late stage (late CP). With the evolution of CRC, the terminal outcome of CRC death may occur according to the force depending on the stage of CRC. The occurrence of other causes of death may also occur along the natural evolution of CRC.
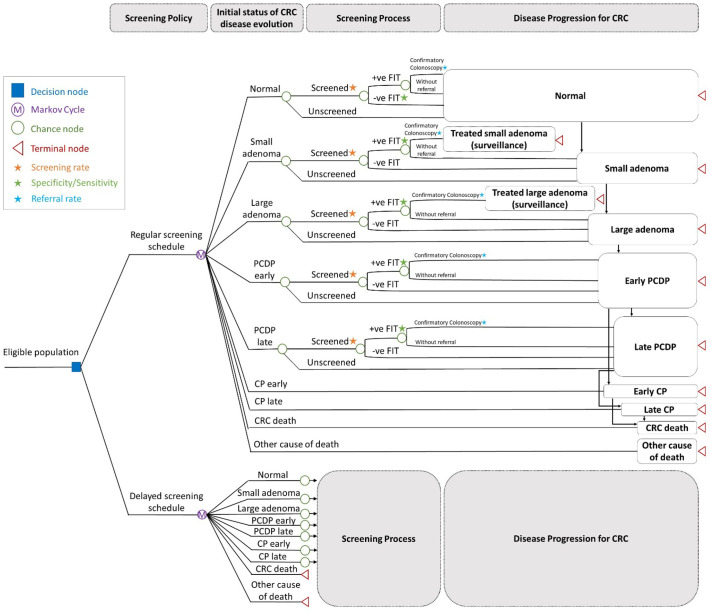
2.5. Markov decision tree model
This network on the implementation of CRC screening with the consideration of structure, process, and outcomes of a screening program with the two queue models and one disease natural history model depicted in Fig. 1, Fig. 2 can be translated into a Markov decision tree to assess the impact of COVID-19 outbreak. Fig. 3 shows the Markov decision trees constructed for regular and the delayed screening schedule (Fig. 3(a)), the decision tree for regular FIT-based screening (Fig. 3(b)), and that for the delayed screening schedule (Fig. 3(c)). The seven-state Markov model for the evolution of colorectal neoplasm depicted in Fig. 2 (c) is the backbone for the decision tree (Fig. 3(a)). Subjects of the eligible population belong to one of the seven transient states on enrolment. For the regular FIT-based screening program, attendees are provided with FIT test on a biennial basis. Subjects with the underlying disease status of adenoma (small and large), PCDP (early and late) are detected with a positive FIT depend on the sensitivities of FIT according to the type of colorectal neoplasm (Fig. 3(b) and Fig. 3(c)). After the provision of FIT test, subjects with positive result are referred to receive confirmatory colonoscopy in the designated hospital (Fig. 3(b) and Fig. 3(c)). Following the identification of poly and polypectomy, the subjects are arranged with surveillance process following current guideline, including a colonoscopy on a triennial basis for small adenoma and once every five years for large adenoma.
Fig. 3.
Markov decision tree for assessing the impact of COVID.
Regarding the delayed screening schedule, how the screening program is delayed in theory can be quantified by linking the actual values of social distancing index with the capacity of uptake screening, namely arrival rate (Fig. 3(c)) using an exponential relationship as described below. Considering the evolution of CRC on the basis of annual cycle, the impact of COVID-19 on the provision of screening test with FIT, the confirmatory colonoscopy, and the corresponding waiting time resulting from the limited medical capacity can be evaluated with the underpinning of natural evolution of CRC.
For the prognosis of CRC, either identified by screening activity during their PCDP states or by the presence of symptoms and signs defined as CP states, the prognosis is determined by the stage (early or late) with the incorporation of differential survival on the basis of annual cycle.
The short-term outcome of stage distribution and the terminal events of death from CRC is incorporated into the assessment to construct a system for evaluating the impact of CRC disease burden associated with the turbulence incurred by COVID-19 outbreak.
2.6. The simulated cohort
A population-based screening using FIT as an illustration with the size of target population of 1000000 was simulated. Average-risk subjects were screened from age 50 years until age 75 or death. A Markov cohort simulation was applied for the derivation of the quantities of outcomes.
Table 1 lists the parameters for the constructed Markov decision trees including the structure parameters (screening rate and referral rate for confirmatory colonoscopy), process parameters (specificity and sensitivities of FIT given the underlying states of colorectal neoplasm), outcome parameters (the rate determining the evolution of colorectal neoplasm and death rates attributable to CRC and other cause) according to Taiwan scenario. The impact of COVID-19 as a result of the provision of screening service in terms of the delayed screening schedule and unmet demand for screening colonoscopy is translated into the screening rate and the compromised referred capacity. This is compared with the optimal scenario with 80% coverage and 80% referral capacity according to the regular screening schedule.
Table 1.
Parameters used for constructing the Markov decision tree including the structure, process, and outcome parameters.
| Variables | Value | Sources |
|---|---|---|
| Structure parameters | ||
| Screening rate of screening program | 80% | |
| Referral rate for confirmatory colonoscopy | 80% | |
| Process parameters | ||
| Specificity of FIT | 96.1% |
Wu et al. (2006); Chiu et al. (2013); Imperiale et al. (2019); Chen et al. (2018) Hsu et al. (2021) |
| Sensitivity of FIT | ||
| Small adenoma | 10.6% | |
| Large adenoma | 36.6% | |
| Early PCDP | 68.7% | |
| Late PCDP | 74.2% | |
| Outcome parameters | ||
| Natural evolution of colorectal neoplasm (annual rate) | ||
| Incidence of colorectal adenoma | 0.01156 | Wu et al., 2006; Estimated results from National Colorectal Cancer Screening Program (Chiu et al., 2015, Chiu et al., 2021) |
| Transition from small to large adenoma | 0.03460 | |
| Transition from large adenoma to early PCDP | 0.02150 | |
| Dwelling in the state of large adenoma | 0.97873 | |
| Transition from large adenoma to early PCDP | 0.01678 | |
| Transition from large adenoma to late PCDP | 0.00202 | |
| Transition from large adenoma to early CP | 0.00208 | |
| Transition from large adenoma to late CP | 0.00039 | |
| Dwelling in the state of early PCDP | 0.61073 | |
| Transition from early PCDP to late PCDP | 0.15843 | |
| Transition from early PCDP to early CP | 0.18023 | |
| Transition from early PCDP to late CP | 0.05061 | |
| Dwelling in the state of late PCDP | 0.59268 | |
| Transition from late PCDP to late CP | 0.40732 | |
| Annual CRC death rate (annual rate) | ||
| CRC at early stage | 0.0206 | Information from Taiwan Nationwide Cancer Registry (Chiang et al., 2019) |
| CRC at late stage | 0.1680 | |
| Other cause of death | Age-specific rate (life table) | Information from Taiwan Death Registry (Lee et al., 2019) |
2.7. Study design for the delayed schedule of population-based screening
Given the postulate that a higher social distancing index would lead to a lower capacity of uptake screening and the longer delayed schedule of the periodical screen. In theory, the degree of screening delay could be quantified in the light of the actual value of index as indicated in Fig. 1 given different scenarios of the epidemic of COVID-19. For ease of presentation, we begin with the demonstration of the suspended screening service in year 2020 followed by four time points as an illustration for resuming the screening service: (1) June 2021 (2) December 2021 (3) June 2022 and (4) December 2022. The impact of COVID-19 for the distribution of CRC stage and CRC death for the four scenarios are evaluated in the year 2023, which correspond to the delays of 0.5-year, 1-year, 1.5-year, and 2-year, respectively. Since we used a Markov cohort simulation approach such screening delays would be handled by randomly selecting 75%, 50%, 25%, and 0% percentage of screened subjects on population level during each Markov cycle.
For each Markov decision, all possible transition states radiated from the decision node with the Markov cycles evaluated on annual basis. Effectiveness was defined as the proportion of CRC at late stage and additional life-year lost as a result of compromised screening service. A discrete-time discrete-state Markov-decision model was applied for predicting the outcomes of stage shifting and life-year lost due to the compromised screening service during COVID-19 outbreak.
For the evaluation of the impact of COVID-19 on CRC, the outcomes of late CRC incidence and mortality rate were used. The relative index of relative risk (RR), and absolute index of risk difference (RD) along with number needed to be screened (NNS) were applied.
2.8. Social distancing index and the capacity of uptake screening in population-based screening with FIT
The impact of COVID-19 outbreak on medical capacity can be assessed by using a social distancing index, which captured the balance between COVID-19 outbreak and medical capacity (Chen et al., 2020). The elaboration and application of this index in depicting the extent of COVID-19 outbreak has been described in full elsewhere (Chen et al., 2020). In brief, an increase in COVID-19 cases due to the outbreak of COVID-19 cases would eat up the regional medical capacity for caring patients, thus resulting in the delay in recovery and the elevated case-fatality rate, which gives a high value of index. On the other hand, the accelerated recovery and low case-fatality rate would lower the value of index for extending the capacity of routine medical care including population-based screening. This index can thus be used to guide the capacity of uptake screening in the era of COVID-19 pandemic (left panel of the index for easing social distancing, Fig. 1).
The higher the value of this index, the lower the invitation rate, resulting in a lower arrival rate, the longer the schedule is required to bring those eligible participants to return the screening program that would have attended the screening regularly had COVID-19 pandemic not occurred. The same logic is applied to a lower attendance rate as a result of hesitancy of invitees to participate in the screening. It can be postulated that the higher the value of this index, the lower the capacity of uptake screening and the longer the screening delay. Based on this conceptual relationship, the delayed or the halted scheduled would be envisaged in the light of the magnitude of index given each scenario of the epidemic of COVID-19. We assume the screening program is halted if the value of this index is larger than 1 whereas the extent of the delayed schedule is proposed below if the value of index is between 0 and 1. The regular scheduled screen is resumed if the value of index is lowered to almost 0.
The index for each country and region can be estimated by using the number of COVID-19 cases, the recovered cases, and case-fatality rate (Chen et al., 2020) with the following formula
To consider a non-linear relationship between social distancing index between 0 and 1 and the capacity of uptake screening and also the time of screening delay, the eq. (1) is proposed for modelling as follows:
| (1) |
λ:capacity of uptake screening modelled by arrival rate.t: time interval.SDI: social distancing index.
Given the fixed time interval t, the higher the value of the index, the lower the capacity of uptake screening captured by arrival rate. In a similar vein, given the fixed arrival rate, the higher the value of index the longer the delayed schedule of the periodical screen. The arrival rate can also be accommodated by attendance rate.
2.9. Data collection
The data used for estimating the index for social distancing were derived from a web-based real-time GitHub repository created by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (Dong et al., 2020). The quantities of the COVID-19 cases, recovered subjects, and deaths results from COVID-19 for each country and area can be retrieved on the web-based interface maintained by CSSE.
We demonstrate how to applying FTE method for measuring the supply of manpower devoted to the CRC screening with a 19-item questionnaire that was designed on the basis of current medical scenario in Taiwan.
This questionnaire includes a string of questions about (1) demographic information, specialty characterization, and hospital setting of the respondents, (2) work content and total endoscopic service amount, (3) colonoscopy loading (including FIT-related), (4) patient waiting time for colonoscopy, and (5) perception of current manpower capacity.
Survey and data collection to all board members from The Digestive Endoscopy Society of Taiwan (DEST) were performed from October to November 2016. Responses were received from 419 of 1402 gastroenterologists, and 98.3% (N = 412) of respondents perform colonoscopies in their daily practice. Based on this survey data, the total number of colonoscopies per endoscopist per month was 70.5. The average service hour for an endoscopist per month can be estimated as 147 h. Average colonoscopy per month for FIT screening was 8. We assume the time for each colonoscopy is 80 min. The FTE was estimated as 2%.
3. Results
3.1. Impact of COVID-19 pandemic on structure
As indicated earlier, the elements related to the structure of population-based screening affected by COVID-19 pandemic involve the capacity of the uptake of screening and confirmatory diagnostic workup for the referral of positive results measured with FTE. In our present illustration with FIT service screening, the capacity of uptake screening is determined by manpower and facilities for primary screening including invitation to screen, the delivery of FIT, the test result of faecal haemoglobin concentrations (f-Hb) conducted in the laboratory, and the dissemination of report. The capacity of referral and confirmatory diagnosis is captured by measuring the FTE of gastroenterologists.
3.2. Capacity and FTE
Given the Taiwan scenario on one round of a biennial screening for CRC with FIT between 2020 and 2021, Table 2 provides three delayed schedules (including 0.5-, 1-, and 1.5-year) and the regular (no delay) schedule on population level as indicated in the method section. Suppose 80% participants invited to screen were affected by COVID-19 pandemic the corresponding arrival rate of attending screening would be considered from 60% for the 0.5-year delay, 40% for the 1-year delay, 20% for the 1.5-year delay, and 80% for the regular schedule.
Table 2.
Influence on waiting time (days) in relation to FTE, invitation rate, and referral rate.
| Time of screening delayed (2021−2022) | Invitation rate (%) | Referral rate (%) | FTE |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 6% |
4.5% |
3% |
1.5% |
||||||
| Positive rate |
Positive rate |
Positive rate |
Positive rate |
||||||
| 7% | 3% | 7% | 3% | 7% | 3% | 7% | |||
| No delay (regular) | 80 | 80 | 90 | 51 | 120 | 77 | 180 | 154 | 360 |
| 40 | 45 | 26 | 60 | 39 | 90 | 77 | 180 | ||
| 20 | 23 | 13 | 30 | 19 | 45 | 39 | 90 | ||
| 0.5-year | 60 | 80 | 68 | 39 | 90 | 58 | 135 | 116 | 270 |
| 40 | 34 | 19 | 45 | 29 | 68 | 58 | 135 | ||
| 20 | 17 | 10 | 23 | 14 | 34 | 29 | 68 | ||
| 1.0-year | 40 | 80 | 45 | 26 | 60 | 39 | 90 | 77 | 180 |
| 40 | 23 | 13 | 30 | 19 | 45 | 39 | 90 | ||
| 20 | 11 | 6 | 15 | 10 | 23 | 19 | 45 | ||
| 1.5-year | 20 | 80 | 23 | 13 | 30 | 19 | 45 | 39 | 90 |
| 40 | 11 | 6 | 15 | 10 | 23 | 19 | 45 | ||
| 20 | 5 | 3 | 8 | 5 | 11 | 10 | 23 | ||
3.3. FTE of confirmatory diagnosis workshop for referral
Since the capacity of manpower involved in confirmatory diagnosis for the referral of those with positive results is pivotal in how much time and resources gastroenterologists are devoted to performing endoscopy resulting from screening defined as FTE to population-based screening as they have to extend a series of clinical practices, administration, and researches.
Referring to data from our Taiwanese CRC screening program with FIT, the results show among 1402 gastroenterologists, based on these estimates, FTE for population-based screening was 2%. The total number of colonoscopies per endoscopist per month was 70.5 (95% CI = 65.0–76.0), resulting in the provision of 47444 numbers of colonoscopies for a regular biennial schedule.
Fig. 4 shows the number of colonoscopy required for the screening program given the capacity and FTE that determines the referral rate of colonoscopy. Given the normal schedule, the number required for colonoscopies were 43218 that can be met by the amount of FTE (47444) as indicated above. The demand for colonoscopies deceased as the delayed schedule became longer. Given the demand of colonoscopies required for the delay with 0.5-year with 80% referral rate as shown in Fig. 4 the supply for colonoscopies should not be lowered to 1.5% FTE (35583). If the FTE is 1% (23722), the referral rate cannot be beyond 40% to meet the demand of 16207. The similar logics are applied to other delayed screening schedules.
Fig. 4.
Number of screening colonoscopy needed given the capacity of screening service in terms of delayed screening schedule with the compromised referral rate.
3.4. Impact of COVID-19 pandemic on the screening process
Under the regular circumstance without COVID-19 epidemic, the number of referrals would be determined by the positive rate of attenders that is further determined by the cut-off of f-Hb.
In parallel with the delayed schedule, the impact of COVID-19 on the component of “process” would be related to waiting time for confirmatory diagnosis after referral given the capacity of uptake screening and the reduced FTE. Table 2 shows a series of results on the waiting time for colonoscopic examination for the regular FTE and three reduced FTE scenarios given 7% positive rate. The less delayed schedule would invoke larger numbers of referral and longer waiting time.
To reduce the burden of referral for confirmatory diagnosis an alternative choice to change the cut-off of f-Hb, Table 2 also shows the waiting time for colonoscopy given 3% positive rate when the cut-off is altered to half of positive rate for the referral of confirmatory diagnosis. It should be noted that whether to change cut-off to meet the demand for colonoscopy or just the application of delayed schedule is subject to the policy made by health policy-maker.
3.5. Impact of COVID-19 pandemic on long-term outcomes
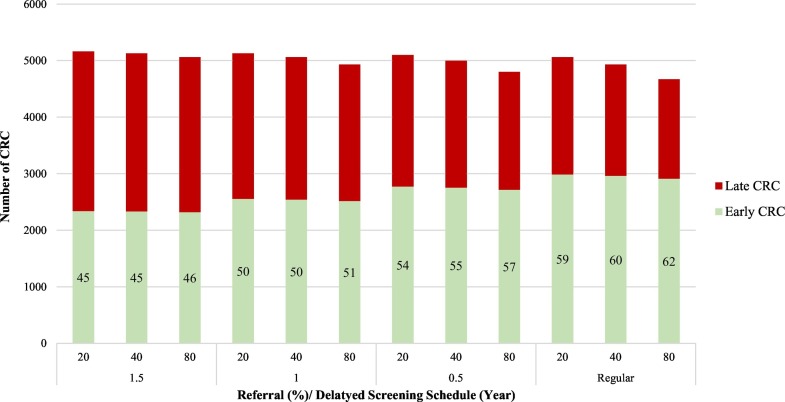
Fig. 5 shows the impact of the delayed screening service on upstaging shifting. The shorter the delayed schedule, the higher the proportion of early CRC. The similar logic is applied to the referral rate given the same delayed schedule. The proportions of early stage CRC were reduced to 46% for the delay of 1.5-year compared with 62% proportion of early stage CRC detected through the regular screening schedule.
Fig. 5.
Stage distribution of CRC by years of delayed screening schedule and referral rate.
Table 3 shows the estimated results of late CRC and risk (per 100000), absolute RD, RR, and NNS regarding the impact of COVID-19 on long-term effectiveness of screening with FIT for the halted and three delayed schedules compared with the regular schedule (no delay), making allowance for 80% attendance rate and 80% referral rate.
Table 3.
Influence of COVID-19 pandemic on outcomes associated with delayed screening schedule.
| Time of screening delay (2021–2022) | Frequency | Risk (pre 105) | Relative risk | Risk difference | NNS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 80% screening rate with 80% referral rate | |||||||||||
| Late CRC | |||||||||||
| No delay (regular) | 1851 | 37.5 | Ref | Ref | Ref | ||||||
| 0.5-year | 2318 | 47.0 | 1.25 | (1.18, | 1.33) | 9.5 | (8.0, | 11.0) | 10549 | (9109, | 12530) |
| 1.0-year | 2378 | 48.2 | 1.29 | (1.21, | 1.37) | 10.7 | (9.2, | 12.2) | 9346 | (8191, | 10882) |
| 1.5-year | 2477 | 50.2 | 1.34 | (1.26, | 1.42) | 12.7 | (11.2, | 14.2) | 7869 | (7025, | 8943) |
| 2.0-year | 2574 | 52.2 | 1.39 | (1.31, | 1.48) | 14.7 | (13.1, | 16.2) | 6813 | (6165, | 7614) |
| CRC death | |||||||||||
| No delay (regular) | 3832 | 26.5 | Ref | Ref | Ref | ||||||
| 0.5-year | 4836 | 33.5 | 1.26 | (1.21, | 1.32) | 7.0 | (5.7, | 8.2) | 14365 | (12159, | 17550) |
| 1.0-year | 4884 | 33.8 | 1.28 | (1.22, | 1.33) | 7.3 | (6.0, | 8.6) | 13709 | (11680, | 16589) |
| 1.5-year | 4937 | 34.2 | 1.29 | (1.24, | 1.34) | 7.7 | (6.4, | 8.9) | 13050 | (11194, | 15644) |
| 2.0-year | 4989 | 34.5 | 1.30 | (1.25, | 1.36) | 8.0 | (6.7, | 9.3) | 12463 | (10755, | 14816) |
3.6. Delayed and halted schedule leading to the excess of late CRC
Table 3 shows a total of 1851 late CRCs with a regular schedule given a million simulated cohort with four years of study period. The corresponding number was increased with the longer delayed screening schedule with the order of 2318, 2378, 2477, and 2574 when the screen schedule is re-opened from June and December 2021, and June and December 2022, respectively.
The RRs of late stage CRC increased from 25% (RR = 1.25, 95% CI: 1.18–1.33) for the delay with 0.5-year, 29% (RR = 1.29, 95% CI: 1.21–1.37) for the delay with 1-year, 34% (RR = 1.34, 95% CI: 1.26–1.42) for the delay with 1.5-year, and 39% for the completely halted schedule (RR = 1.39, 95% CI: 1.31–1.48) resulting from COVID-19 epidemic.
The corresponding RDs (per 100000) also increased from 9.5 for the delay with 0.5-year to 14.7 for the halted schedule. Although the estimated figures of NNS decreased with the delayed schedule and the halted schedule but the differences were not statistically significant due to the overlapping of 95% CI.
3.7. Delayed and halted schedule leading to the excess of death from CRC
Regarding the long-term outcome of death from CRC, there were a total of 3832 deaths from CRCs with a regular schedule given a million simulated cohort with 15 years of follow-up period. Like late CRCs, the corresponding number increased with the longer delayed screening schedule with the order of 4836, 4884, 4937, and 4989 when the screen schedule was re-opened from June and December 2021, and June and December 2022, respectively.
Table 3 also shows the similar corresponding estimated results on the excess death from CRC due to the delayed and the halted schedule of screening ascribed to COVID-19 pandemic. The RRs of dying from CRC increased from 26% (RR = 1.26, 95% CI: 1.21–1.32) for the delay with 0.5-year, 28% (RR = 1.28, 95% CI: 1.22–1.33) for the delay with 1-year, 29% (RR = 1.29, 95% CI: 1.24–1.34) for the delay with 1.5-year, and 30% for the completely halted schedule (RR = 1.30, 95% CI: 1.25–1.36) resulting from COVID-19 epidemic.
3.8. Delayed screening schedule to avert death from COVID-19
A population-based screening with FIT can predispose the attendees to expose the risk of COVID-19 infection in the process of confirmatory colonoscopy in the designated hospital. Table 4 shows the total number of subjects for whom the confirmatory colonoscopy would be expected to be performed, ranging from 10804 to 43218 depending on the delayed schedule of screening program. Based on the previous report on the nosocomial risk of contracting COVID-19 of 1.7% (Rhee et al., 2020) and the current COVID-19 case-fatality of 2.3% (Alamo et al., 2020, Dong et al., 2020), the estimated results on the numbers of COVID-19 death due to confirmatory colonoscopies ranged between 4 for the screening schedule delayed for 1.5-year and 15 for the regular screening schedule.
Table 4.
Estimated number of subjects contacted COVID-19 and death associated with confirmatory colonoscopya
| Time of screening delay (2021–2022) |
Subjects visit hospital for confirmatory colonoscopy | Number of COVID-19 case |
Number of COVID-19 death |
|---|---|---|---|
| No delay (regular) | 43218 | 735 | 15 |
| 0.5-year | 32413 | 551 | 12 |
| 1.0-year | 21609 | 367 | 8 |
| 1.5-year | 10804 | 184 | 4 |
| 2.0-year | – | 0 | 0 |
Risk of nosocomial COVID-19 infection rate: 1.7%; case-fatality rate of COVID-19: 2.1%.
3.9. Precision screening strategies guided with f-Hb concentration
Table 5 demonstrates the results with the provision of FIT for those with f-Hb above the arbitrarily selected cut-off to accommodate various kinds of the delayed schedule based on the dose-response relationship between f-Hb and the risk for CRC (Supplementary Table 1). Compared with the results of late CRCs shown in Table 3, the delivery of FIT based on such a precision screening strategy enables one to alleviate long-term outcomes. We can also use these findings to prioritize various extents of the delayed schedule for different subjects according to risk-guided f-Hb level in the face of dynamic COVID-19 outbreak.
Table 5.
The effect of FIT screening guided by f-Hb concentration (Higher f-Hb with high priority for screening) on late CRC.
| Delay for f-Hb concentration below cut-offs | Relative risk for late CRC |
|
|---|---|---|
| Cut-off at 150 ng/mL | Cut-off at 250 ng/mL | |
| Regular | Ref | Ref |
| 0.5-year | 1.10 (1.04, 1.18) | 1.13 (1.06, 1.21) |
| 1.0-year | 1.11 (1.04, 1.18) | 1.14 (1.08, 1.22) |
| 1.5-year | 1.12 (1.05, 1.19) | 1.16 (1.10, 1.24) |
| 2.0-year | 1.13 (1.06, 1.21) | 1.18 (1.11, 1.25) |
3.10. The social distancing index associated with the impact of COVID-19 pandemic on screening delays
In our second analysis, the social distancing index was used to estimate the impact of COVID-19 on screening delays in a quantitative manner as shown in the eq. (1) for the index between 0 and 1. The screening would be halted when the value of the social distancing index is larger than 1whereas it would return to the regular schedule if the index is almost 0. Supplementary Fig. 1 shows four scenarios of the relationship between the distribution of this index and the halted (2-year delay), the delayed, and the regular screening schedule.
Based on the conceptual relationship between the index of social distancing and the capacity based on the eq. (1), the higher the index, the lower the capacity and the longer delayed schedule. Table 6 shows, following the exponential relationship between the index and the time of screening delay and the capacity of uptake screening captured by the arrival rate of queue process, the relationship between social distancing index and times of screening delay by two different baseline figures associated with the capacity of uptake screening before COVID-19 epidemic (see the first row of Table 6), 1.09% arrival rate (per week) according to Taiwan FIT screening scenarios and 2.3% arrival rate representing one of countries with high human development index (HDI). It is obvious that the higher the index the lower the capacity and the longer the screening delay. It is also very interesting to note that higher baseline arrival rate would accommodate screening delays better than lower baseline arrival rate.
Table 6.
The delayed schedule for screening and parameters related to the queue process on population level in different pandemic level under the scenarios in Taiwan and high HDI countries.
| Social distancing index | Taiwan scenario |
High HDI countries |
||
|---|---|---|---|---|
| Time of screening delayed (2021–2022) | Arrival rate (capacity of uptake screening) | Time of screening delayed (2021–2022) | Arrival rate (capacity of uptake screening) | |
| 0.00 | No delay (regular) | 0.01088 | No delay (regular) | 0.02300 |
| 0.05 | 0.07 | 0.01072 | 0.03 | 0.02265 |
| 0.10 | 0.15 | 0.00824 | 0.07 | 0.01741 |
| 0.15 | 0.23 | 0.00679 | 0.11 | 0.01434 |
| 0.20 | 0.32 | 0.00576 | 0.15 | 0.01217 |
| 0.25 | 0.41 | 0.00496 | 0.19 | 0.01048 |
| 0.30 | 0.50 | 0.00431 | 0.24 | 0.00910 |
| 0.35 | 0.61 | 0.00376 | 0.29 | 0.00794 |
| 0.40 | 0.72 | 0.00328 | 0.34 | 0.00693 |
| 0.45 | 0.84 | 0.00286 | 0.40 | 0.00604 |
| 0.50 | 0.98 | 0.00248 | 0.46 | 0.00524 |
| 0.55 | 1.13 | 0.00214 | 0.53 | 0.00452 |
| 0.60 | 1.30 | 0.00183 | 0.61 | 0.00386 |
| 0.65 | 1.48 | 0.00154 | 0.70 | 0.00326 |
| 0.70 | 1.70 | 0.00128 | 0.81 | 0.00270 |
| 0.75 | 1.96 | 0.00103 | 0.93 | 0.00218 |
| 0.80 | 2.27 | 0.00080 | 1.08 | 0.00169 |
| 0.85 | 2.68 | 0.00058 | 1.27 | 0.00123 |
| 0.90 | 3.25 | 0.00038 | 1.54 | 0.00080 |
| 0.95 | 4.23 | 0.00018 | 2.00 | 0.00039 |
| >1.00 | (halted) | (halted) | ||
The relationship between social distancing index as a reflection of COVID-19 epidemic and the reduced FTE can be derived in a similar way. For example, if we took 2% FTE as the capacity of regular basis and the FTE attributed to COVID-19 pandemic was reduced to 1.5%, 1%, 0.5% analogous to three scenarios of screening delay. The corresponding numbers of colonoscopies in the light of three reduced FTE scenarios were 35583, 23722, and 11861 on biennial basis. The corresponding waiting times can also be derived in a way in the light of Table 3. The higher the value of the index would cause the reduced FTE and longer waiting time. Finally, the development of such a quantitative relationship between the social distancing index and capacity and FTE associated with screening delays may further give the results on the impact of COVID-19 on long-term outcomes in population-based screening as modelled in the result section earlier.
4. Discussion
The current study proposed a systematic framework and an integrated Markov model for the evaluation of screening delays resulting from COVID-19 epidemic on long-term outcomes of population-based cancer screening and the changes in two other components of the quality following the Donabedian model (Donabedian, 1988, Haj et al., 2013). Specifically, the main analysis is that cascade of impacts resulting from COVID-19 were elucidated and quantified with the two queue models for structure and process and with one disease natural history model of colorectal neoplasm for the outcomes on late stage of and death from CRC. In the second analysis, the relationship between social distancing index and capacity and the delayed schedule has been also explored for linking social distancing index with the impact of COVID-19 epidemic on long-term outcomes. We used a Taiwan population-based FIT screening as an illustration.
The results show the epidemic of COVID-19 not only compromises the structure and process of keeping population-based screening in operation but also takes many tolls due to the failure of early detection of CRC with the delayed screening schedule. The impact on the reduced long-term effectiveness are consequential even making allowance for averting deaths from COVID-19 with the delayed schedule.
To minimize such a kind of substantial loss, precision screening strategies may be considered. For example, the recently proposed dose-relationship between f-Hb and the risk for CRC can be exploited to provide a f-Hb-guided personalized risk assessment strategy for prioritizing the eligible target population when the delayed schedule is proposed. This is particularly useful for a temporary replacement for the country or the region that adopts colonoscopy or sigmoidoscopy as the primary screening tool for early detection of CRC. Such a personalized risk assessment can be also applied to two-stage FIT screening if information on baseline f-Hb is available. However, how and whether to use this precision strategy to prioritize screening delays to offset the loss caused by screening delays still require a more delicate analysis.
To evaluate the impacts of COVID-19 on the quality aspect of population-based screening requires a delicate and integrated model as determinants from structure until outcomes are rather complex. Although the proposed methodology is illustrated with a two-stage FIT screening, as a matter of fact, such a methodology can be applied to any kind of population-based organized service screening that may have already the halted or the delayed schedule or is going to happen if information on both supply and demand sides of the cancer in question can be available and relevant parameters can be estimated in a way as shown in the current study. Information provided here plays a crucial role in modelling the impacts of COVID-19 on the quality of population-based screening. It includes the use of an index for social distancing with available data on COVID-19 cases and recovered and death available from open data for the dynamic assessment of the epidemic of SARS-CoV-2 and further provides a guidance for the capacity of uptake screening. Attendance rate and referral rate on how participants are affected by COVID-19 pandemic may have a simple survey with the representative sample. Information on FTE is also crucial for the determination of the capacity of confirmatory diagnosis in our illustration of FIT screening and uptake screening if the colonoscopy is taken as the primary screening tool. The parameters used for building up the screening process from referral rate until confirmatory diagnosis and for the construction of the disease natural history model can refer to the literature or be estimated from the routine data on population-based screening in each country and region. Note that the framework and methodology can also be modified and extended to other kinds of evidence-based population-based screening for CRC, including sigmoidoscopy and colonoscopy (Hardcastle et al., 1996; Kronborg et al., 1996; Mandel et al., 1993; Berry et al., 1997; Rasmussen et al., 1999; Segnan et al., 2005, Segnan et al., 2011; Verne et al., 1998; Kaminski et al., 2015; Chiu et al., 2015, Chiu et al., 2021).
There are some limitations and concerns of the current study. The proposed criteria of designing the delayed screen to accommodate insufficient capacity of uptake screening and reduced FTE is based on the distribution of social distancing index which assume screening is halted if the index is larger than 1 and returns to normal when it drops almost 0 whereas the delayed schedule is adopted when it between 0 and 1. Such criteria may be adequate for some countries but may not be adequate for others with high medical capacity as already indicated in different baseline arrival rates for representing various degrees of capacity. The criteria can be modified in the light of the real medical capacity if data are available. Second, although the delayed schedule design is proposed for the evaluation of the impact of COVID-19 it may not be adequate for the country with very dynamic COVID-19 epidemic with the fluctuated index of social distancing. An even precise delayed schedule with dynamic type of the delayed schedule should be considered. Third, we did not present the result of colorectal adenoma although the Markov decision tree has already incorporated this part. The contents may be too much to be covered in the current manuscript but it can be further studied as the reduction in incidence of CRC due to early detection of adenoma plays an important role in medical expenditure for treatment and therapy of CRC. Finally, as some parameters such as incidence of CRC, disease progression rate and survival were based on Taiwan registry data, the application of the proposed method to other countries may have a more delicate analysis as different countries have dramatically different CRC incidence and mortality rates, and the designs of the programs are very different, including different test sensitivities.
In conclusion, a systematic and integrated Markov model underpinning structure-process-outcomes was developed to evaluate the impact of screening delays due to COVID-19 epidemic on long-term outcome and the associated changes in structure and process of population-based screening in the light of social distancing index. The strong impact of screening delays caused by COVID-19 epidemic on long-term outcomes was illustrated with a Taiwan population-based FIT screening of CRC.
Declaration of competing interest
The authors declare that they have no conflict of interests.
Funding
This study was supported by the Ministry of Science and Technology, Taiwan (MOST 108–2118-M-002-002-MY3; MOST 109–2811-M-002-643; MOST 108–2118-M-038-001-MY3; MOST 108–2118-M-038-002-MY3; MOST 109–2327-B-002-009). The funding source had no role in study design, data collection, analysis, or interpretation, report writing, or the decision to submit this paper for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2021.106597.
Appendix A. Supplementary data
Supplementary material
References
- Alamo T., Reina D.G., Mammarella M., Abella A. Covid-19: Open-Data Resources for Monitoring, Modeling, and Forecasting the Epidemic. Electronics. 2020 [Google Scholar]
- Berry D.P., Clarke P., Hardcastle J.D., Vellacott K.D. Randomized trial of the addition of flexible sigmoidoscopy to faecal occult blood testing for colorectal neoplasia population screening. Br. J. Surg. 1997;84 doi: 10.1002/bjs.1800840922. [DOI] [PubMed] [Google Scholar]
- Bowman M.A., Katzoff J.M., Garrison L.P., Wills J. Estimates of physician requirements for 1990 for the specialties of neurology, anesthesiology, nuclear medicine, pathology, physical medicine and rehabilitation, and radiology: a further application of the GMENAC methodology. JAMA J. Am. Med. Assoc. 1983;250 doi: 10.1001/jama.1983.03340190025025. [DOI] [PubMed] [Google Scholar]
- Burke B.T., Miller B.F., Proser M., Petterson S.M., Bazemore A.W., Goplerud E., Phillips R.L. A needs-based method for estimating the behavioral health staff needs of community health centers. BMC Health Serv. Res. 2013;13(1):1–12. doi: 10.1186/1472-6963-13-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.L.S., Hsu C.Y., Yen A.M.F., Young G.P., Chiu S.Y.H., Fann J.C.Y., et al. Demand for colonoscopy in colorectal cancer screening using a quantitative fecal immunochemical test and age/sex-specific thresholds for test positivity. Cancer Epidemiol. Biomark. Prev. 2018;27 doi: 10.1158/1055-9965.EPI-17-0387. [DOI] [PubMed] [Google Scholar]
- Chen S.L.S., Yen A.M.F., Lai C.C., Hsu C.Y., Chan C.C., Chen T.H.H. An index for lifting social distancing during the COVID-19 pandemic: algorithm recommendation for lifting social distancing. J. Med. Internet Res. 2020;17;22(9) doi: 10.2196/22469. e22469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C.J., Wang Y.W., Lee W.C. Taiwan’s nationwide cancer registry system of 40 years: past, present, and future. J. Formos. Med. Assoc. 2019;118 doi: 10.1016/j.jfma.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Chiu H.M., Lee Y.C., Tu C.H., Chen C.C., Tseng P.H., Liang J.T., et al. Association between early stage colon neoplasms and false-negative results from the fecal immunochemical test. Clin. Gastroenterol. Hepatol. 2013;11 doi: 10.1016/j.cgh.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Chiu H.M., Chen S.L.S., Yen A.M.F., Chiu S.Y.H., Fann J.C.Y., Lee Y.C., et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the one million Taiwanese screening program. Cancer. 2015;121 doi: 10.1002/cncr.29462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H.M., Jen G.H.H., Wang Y.W., Fann J.C.Y., Hsu C.Y., Jeng Y.C., et al. Long-term effectiveness of faecal immunochemical test screening for proximal and distal colorectal cancers. Gut. 2021 doi: 10.1136/gutjnl-2020-322545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donabedian A. The quality of care: how can it be assessed? JAMA J. Am. Med. Assoc. 1988;260 doi: 10.1001/jama.1988.03410120089033. [DOI] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L., et al. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj H.I.El, Lamrini M., Rais N. Quality of care between the DONABEDIAN model and the ISO9001v2008 model. Int. J. Qual. Res. 2013;7 [Google Scholar]
- Hardcastle J.D., Chamberlain J.O., Robinson M.H.E., Moss S.M., Amar S.S., Balfour T.W., et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996:348. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- Hsu W.F., Hsu C.Y., Yen A.M.F., Chen S.L.S., Chiu S.Y.H., Fann J.C.Y., et al. Classifying interval cancers as false negatives or newly occurring in fecal immunochemical testing. J. Med. Screen. 2021;969141320986830 doi: 10.1177/0969141320986830. [DOI] [PubMed] [Google Scholar]
- Imperiale T.F., Gruber R.N., Stump T.E., Emmett T.W., Monahan P.O. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: a systematic review and meta-analysis. Ann. Intern. Med. 2019 doi: 10.7326/M18-2390. [DOI] [PubMed] [Google Scholar]
- Kaminski M.F., Kraszewska E., Rupinski M., Laskowska M., Wieszczy P., Regula J. Design of the Polish Colonoscopy Screening Program: A randomized health services study. Endoscopy. 2015:47. doi: 10.1055/s-0034-1392769. [DOI] [PubMed] [Google Scholar]
- Kronborg O., Fenger C., Olsen J., Jørgensen O.D., Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996:348. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- Lee Y.C., Hsu C.Y., Chen S.L., Yen A.M., Chiu S.Y., Fann J.C., et al. Effects of screening and universal healthcare on long-term colorectal cancer mortality. Int. J. Epidemiol. 2019;48 doi: 10.1093/ije/dyy182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel J.S., Bond J.H., Church T.R., Snover D.C., Bradley G.M., Schuman L.M., et al. Reducing mortality from colorectal Cancer by screening for fecal occult blood. N. Engl. J. Med. 1993;328 doi: 10.1056/nejm199305133281901. [DOI] [PubMed] [Google Scholar]
- Mulhausen R., Mcgee J. Physician need: an alternative projection from a study of large, prepaid group practices. JAMA J. Am. Med. Assoc. 1989;261 doi: 10.1001/jama.261.13.1930. [DOI] [PubMed] [Google Scholar]
- Nodora J.N., Gupta S., Howard N., Motadel K., Propst T., Rodriguez J., Schultz J., Velasquez S., Castañeda S.F., Rabin B., Martínez M.E. The COVID-19 pandemic: identifying adaptive solutions for colorectal cancer screening in underserved communities. J. Natl. Cancer Inst. 2020 doi: 10.1093/jnci/djaa117. djaa117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M., Kronborg O., Fenger C., Jørgensen O.D. Possible advantages and drawbacks of adding flexible sigmoidoscopy to Hemoccult-II in screening for colorectal cancer: a randomized study. Scand. J. Gastroenterol. 1999;34 doi: 10.1080/00365529950172862. [DOI] [PubMed] [Google Scholar]
- Rhee C., Baker M., Vaidya V., Tucker R., Resnick A., Morris C.A., et al. Incidence of nosocomial COVID-19 in patients hospitalized at a large US Academic Medical Center. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segnan N., Senore C., Andreoni B., Arrigoni A., Bisanti L., Cardelli A., et al. Randomized trial of different screening strategies for colorectal cancer: Patient response and detection rates. J. Natl. Cancer Inst. 2005:97. doi: 10.1093/jnci/dji050. [DOI] [PubMed] [Google Scholar]
- Segnan N., Armaroli P., Bonelli L., Risio M., Sciallero S., Zappa M., et al. Once-only sigmoidoscopy in colorectal cancer screening: Follow-up findings of the italian randomized controlled trial - SCORE. J. Natl. Cancer Inst. 2011:103. doi: 10.1093/jnci/djr284. [DOI] [PubMed] [Google Scholar]
- Verne J.E.C.W., Aubrey R., Love S.B., Talbot I.C., Northover J.M.A. Population based randomised study of uptake and yield of screening by flexible sigmoidoscopy compared with screening by faecal occult blood testing. Br. Med. J. 1998;317 doi: 10.1136/bmj.317.7152.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO WHO Coronavirus (COVID-19) Dashboard. 2021. https://covid19.who.int/ (accessed March 23, 2021)
- Wu G.H.M., Wang Y.M., Yen A.M.F., Wong J.M., Lai H.C., Warwick J., et al. Cost-effectiveness analysis of colorectal cancer screening with stool DNA testing in intermediate-incidence countries. BMC Cancer. 2006;6 doi: 10.1186/1471-2407-6-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K.C., Liao C.S., Chiu Y.H., Yen A.M.F., Chen T.H.H. Colorectal cancer screening with faecal occult blood test within a multiple disease screening programme: an experience from Keelung. Taiwan. J. Med. Screen. 2006;13 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material