Abstract
The COVID-19 pandemic has contributed to decreases in breast, colorectal, and cervical cancer screenings between 86 and 94% compared to three-year averages. These postponed screenings have created backlogs that systems will need to address as healthcare facilities re-open for preventive care. The American Cancer Society is leading a 17-month intervention with 22 federally qualified health centers (FQHCs) across the United States aimed at reducing cancer incidence and mortality disparities and alleviating additional strain caused by COVID-19. This study describes COVID-related cancer screening service disruptions reported by participating FQHCs. Selected FQHCs experienced service disruptions and/or preventive care cancellations due to COVID-19 that varied in severity and duration. Fifty-nine percent stopped cancer screenings completely. Centers transitioned to telehealth visits or rescheduled for the future, but the impact of these strategies may be limited by continued pandemic-related disruptions and the inability to do most screenings at home; colon cancer screening being the exception. Most centers have resumed in-person screening, but limited in person appointments and high levels of community transmission may reduce FQHC abilities to provide catch-up services. FQHCs provide critical cancer prevention services to vulnerable populations. The delivery of culturally competent, high-quality healthcare can mitigate and potentially reverse racial and ethnic disparities in cancer prevention testing and treatment. Ensuring and expanding access to care as we move out of the pandemic will be critical to preventing excess cancer incidence and mortality in vulnerable populations.
Keywords: COVID-19, Cancer screening, FQHC
1. Introduction
National shutdowns at the beginning of the COVID-19 pandemic contributed to decreases in breast, colorectal, and cervical cancer screenings between 86 and 94% compared to three-year averages (Epic Health Research Network, 2020). Although screening rates recovered somewhat after initial shutdowns, they remain approximately 25% below baseline levels (Miller et al., 2021). These postponed screenings have created backlogs that systems will need to address as healthcare facilities expand access to preventive services. Health centers need to develop and adopt new processes and protocols to tackle this backlog of missed cancer screenings to avoid future increases in cancer incidence and mortality (Maringe et al., 2020).
Raising screening rates to above pre-pandemic levels in the short term is necessary to address the backlog of patients. However, across the country, health centers and their staff have been greatly impacted by the pandemic. A Health Resources and Services Administration (HRSA) survey estimated that during the week of July 3, 2020 more than 1100 health center sites were temporarily closed, and nearly 6.3% of health center staff were unable to work due to effects of COVID-19. Over 35% of patient visits were taking place virtually (compared to <5% prior to the pandemic), and only colon cancer screenings could be supported via telehealth Health Resources and Services Administration, 2021 (May 28,2021). These issues were magnified in low-income communities and communities of color, which were hardest hit both economically and with COVID-19 disease (Karmakar et al., 2021).
Low-income and minority communities had disproportionately high cancer incidence and mortality prior to the pandemic (Du et al., 2011), and now face the possibility of increased disparities due to reduced access to screenings in the wake of COVID-19 (Maringe et al., 2020). For over a decade, the American Cancer Society (ACS) has offered community grants and supported the implemention evidence-based interventions to build health system capacity to promote health equity and access to screening resources within under-resourced communities. Projects focus on increasing access to timely cancer screenings and appropriate follow-up care. These competitive grants have been awarded to federally qualified health centers (FQHCs), Tribal Health Centers, and Community Health Centers that predominantly provide care in uninsured and underinsured populations. Early data indicate these grant projects produce meaningful change in screening rates (Fedewa et al., 2021).
In 2020, the ACS and the National Football League (NFL) joined forces to offer a new funding opportunity called Cancer Screening during COVID-19, aimed at reducing cancer mortality disparities and alleviating additional strain on Health Centers caused by COVID-19. The program supports FHQCs to resume high-quality screenings for breast, cervical, and colorectal cancer, catch up on missed screenings, and provide timely follow-up care. In the long-term, these projects seek to address the known disparities in cancer incidence and mortality and challenges with limited access to specialty care in under-resourced communities. The purpose of this manuscript is to describe disruptions in cancer screening services among FQHCs participating in this ACS quality improvement initiative.
2. Methods
2.1. Setting and FQHC selection
The Cancer Screening during COVID-19 Projects are quality improvement projects within participating FQHCs that aim to facilitate rapid resumption of cancer screenings to mitigate the impact of COVID-19 on cancer morbidity and mortality. Projects span 17 months, which included a 2-month baseline data collection period (August–September 2020), a 3-month capacity building phase (October–December 2020), and 12-month intervention phase (January – December 2021) (Table 1 ). The ACS invited FQHCs and other safety net clinics who noted dramatic reductions in cancer screening due to COVID-19 but retained staff capacity to address return-to-screening efforts to apply for the project. FQHCs decided on which cancer(s) to focus their efforts: breast, cervical, or colorectal. Additionally, FQHCs were required to be within a 100-mile radius of an NFL team and willing to engage ACS cancer control staff to provide quality improvement support for the project. Criteria for FQHC participation also included the capacity to:
-
•
Provide access to screenings for all eligible patients (this may include referral)
-
•
Capture and track screenings completed and cancers diagnosed
-
•
Collaborate with local partners to improve processes that ensure access to specialty care and timely delivery of diagnostic testing and cancer treatment, if needed
-
•
Complete COVID-19 Progress reporting form (after capacity building, at mid-point, and end of project period)
-
•
Utilize quality improvement methods and tools throughout the project period; providing examples and/or documentation of improvement projects as requested
-
•
Participate in up to six virtual ECHO sessions to share best practices nationally
Table 1.
Back on track with screening quality improvement intervention timeline.
| Baseline data collection phase (month 1–2) |
|---|
|
| Intervention capacity building phase (month 3–5) |
|---|
|
| Implementation phase (month 6–17) |
|---|
|
2.2. COVID impact and screening questionnaire
Prior to beginning their interventions, applicants were asked to provide the following information detailing the impact of COVID-19 on cancer screenings: date disruptions to cancer screening began, date disruptions peaked, which cancer screenings were still available during the height of disruptions for patients seen in-person and virtually, what options for cancer screening were discussed or recommended as a result of COVID-19, what was the health center's current policy or plan for rescheduling screenings that were canceled or deferred, what barriers did they anticipate to getting back on track with cancer screening, and any other impact of COVID-19 on cancer screenings not captured by previous questions. Applicants were also asked to identify preexisting and/or new strategies utilized by the health center or to refer patients to cancer screening including: telehealth, patient reminders, proactive communication to patients, expanded clinic hours, expanding the types of staff allowed to recommend screening, deploying mobile units, home-based screening, off-site screening, structural changes within the clinic, new waiting room protocols, and new or expanded referral processes. Descriptive statistics are used to describe pandemic-related changes in screening practices. This project was approved by the Institutional Review Board of Emory University.
3. Results
Twenty-two FQHC systems agreed to participate and completed baseline questionnaire data. Geographically, systems span 15 states across the country. Collectively, participating FQHCs represent nearly 204,000 eligible screenings for the project period including breast cancer eligible screenings (37,617), cervical cancer eligible screenings (66,598), colorectal cancer eligible screenings (99,695). The average FQHC project partner serves a population that is primarily Hispanic/Latino (37.5%), followed by Black/African American (26.5%), and Non-Hispanic white (24.6%) patients. The remainder of the patient population identified as Other (6.6%), Asian (2.5%), multiracial (1.3%), American Indian Alaska Native (<1%), or Native Hawaiian Pacific Islander (<1%). FQHC systems provided baseline screening rates using Center for Medicare and Medicaid Services (CMS) metrics for the cancer type specified in their application: breast, cervical, and/or colorectal. Twelve of the 22 partners focused on just one cancer type for screening, five focused on two cancer types, and the remaining five focused on all three cancer types. Table 2 provides average screening rates by cancer focus as well as the count of systems who focused on which cancer types.
Table 2.
Average screening rate at baseline by cancer focus.
| Cancer focus | Performance measure | Average screening rate (range) |
|---|---|---|
| Breast (n = 13 projects) | Percentage of women 50–74 years of age who had a mammogram to screen for breast cancer (CMS 125) | 44.2% (20.5% - 64.3%) |
| Cervical (n = 10 projects) | Percentage of patients 21–64 years of age who had the appropriate screening for cervical cancer. (CMS 124) | 57.9% (44.5% - 73.1%) |
| Colorectal (n = 14 projects) | Percentage of patients 50–75 years of age who had the appropriate screening for colorectal cancer. (CMS 130) | 36.6% (15.0% - 58.9%) |
Note: CMS 125: Percentage of women 50–74 years of age who had a mammogram to screen for breast cancer in the 27 months prior to the end of the Measurement Period.
CMS 124: Percentage of patients 21–64 years of age who had the appropriate screening for cervical cancer using the following criteria: cervical cytology performed in the last three years for patients who are at least 21 years old at time of test; cervical cytology/human papillomavirus (HPV) co-testing performed in the last five years for patients who are at least 30 years old at the time of test.
CMS 130: Percentage of patients 5–75 years of age who had the appropriate screening for colorectal cancer, including any of the following: fecal occult blood test (gFOBT or FIT) in the last 12 months; colonoscopy in the last 10 years; FIT-DNA during the last three years; CT colonography in the last five years; and flexible sigmoidoscopy in the last five years.
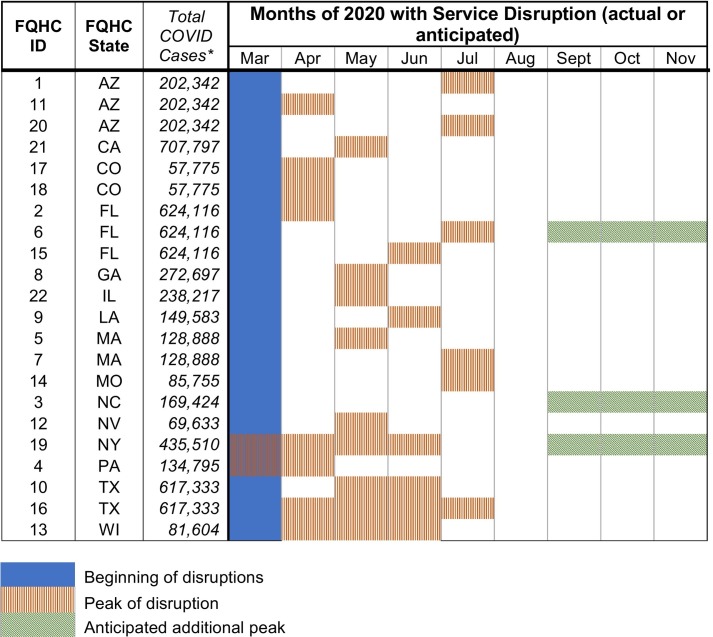
FQHCs provided an overview of disruptions to cancer screening since the start of the COVID-19 pandemic. Data were collected in August–September 2020. Centers were asked to estimate the date disruptions began and when disruptions peaked or were expected to peak. All disruptions started in March 2020 but peak disruptions varied by center (see Fig. 1 ), including some FQHCs who anticipated Fall 2020 peaks.
Fig. 1.
Service disruptions during 2020 at participating FQHC systems.
All systems (N = 22) experienced service disruptions and/or cancellations due to COVID-19 which varied in severity and duration. Of the 22 systems, 11 (50%) reported stopping cancer screening completely for the cancer type specified in their application since the start of COVID-19 disruptions. One center reported never stopping screening entirely for their specified cancer types. See Table 3 for a detailed description of the percentage of the reduction in screenings by cancer type. Colorectal cancer screenings were the most frequently reported cancer screenings to continue. The ability to continue colorectal cancer screening was largely due to existing home-based screening kits (e.g., FIT kits) that could be mailed. Fifty percent of (n = 7) systems focusing on colorectal cancer were already using home-based screening options prior to COVID-19. This percentage increased to 71% (n = 10) post-COVID-19 disruptions.
Table 3.
Reduction in screenings by cancer focus due to COVID-19.
| Cancer focus | Continued screenings | Canceled screenings | Reduction in screenings (%) |
|---|---|---|---|
| Breast (n = 13 projects) | 3 | 10 | 77% |
| Cervical (n = 10 projects) | 1 | 9 | 90% |
| Colorectal (n = 14 projects) | 7 | 7 | 50% |
Over half of all systems reported enforced screening service disruptions/cancellations as a result of state or local COVID-19 restrictions. They also reported a number of patient barriers: hesitancy to seek care, financial difficulties, and transportation challenges. All of these factors led to a reduction in number of cancer screenings (see Table 3) and an increase in virtual visits. FQHCs reported using the following screening options for patients seen virtually in lieu of in-person visits: rescheduling for future visits (86%, n = 19), home-based/mailed stool test (82%, n = 18) (for colorectal cancer), continued referrals to the same location (59%, n = 13), referrals to alternate locations where in-person screening options existed (14%, n = 3), and no screening options discussed (9%, n = 2). The rescheduling for future visits was by far the most frequent strategy, but with ongoing disruptions related to both infection control measures and the financial consequences of the pandemic may compromise the ability to complete the screenings in a timely manner.
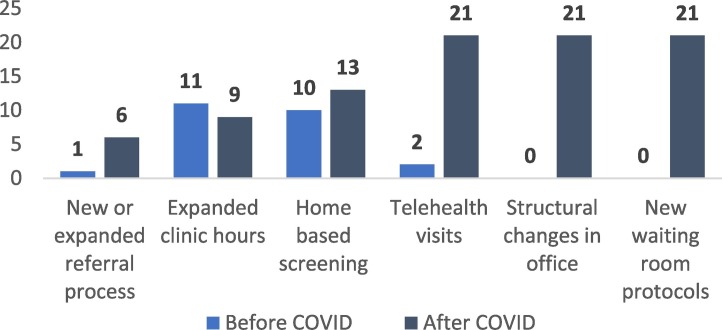
All FQHCs who experienced disruptions and screening cancellations (n = 21) reported the following strategies to maintain some level of care, regardless of the cancer focus specified in their application: switching to telehealth visits (100%), implementing structural changes in the office to increase safety (100%), altering waiting room protocols for any in-person visits (100%), and changing or introducing a new referral process (27%) (see Fig. 2 ). There were no reported differences pre- and post-COVID disruptions for mobile screening units, off-site screening, or patient communication (e.g., proactive communication about screenings, patient reminders).
Fig. 2.
Frequency of strategies used by health centers to provide or refer patients to screenings, pre- & post- COVID.
Health centers also reported anticipated system-level barriers independent of patient-focused concerns, such as challenges with staffing due to COVID-related closures and illness, a lack of dedicated staff to coordinate the high volume of backlogged screenings, and altered workflows and processes that have limited operational capacity.
4. Discussion
COVID-19 disrupted cancer screening service provision. The ability of FQHC systems to address missed screenings in the future is uncertain. During the height of service disruptions, 77% and 90% of clinics stopped offering breast and cervical cancer screenings, respectively, and half discontinued colorectal cancer screening. This is particularly concerning as the majority of eligible patients were not up to date on screening at baseline, and screening participation was well below national averages (Sabatino et al., 2021). Although cancer services have largely resumed, heightened clinic hygiene protocols and physical distancing requirements limit the numbers of in-person visits that can be offered each day (Centers for Disease Control, 2020). Our data also note that some FQHCs anticipated peaks in disruptions after the time point at which data were collected.
COVID-19 rates and preventable cancer rates have been high among low-income and rural communities, as well as Black, indigenous, and people of color (Moore et al., 2020; Muñoz-Price et al., 2020; Singh and Jemal, 2017). Without purposeful intervention, pandemic-related disruptions in preventive services may widen existing cancer disparities (Singh and Jemal, 2017). FQHCs provide critical cancer prevention services to under-resourced rural and urban communities. The delivery of high-quality healthcare can mitigate and potentially reverse racial and ethnic disparities in cancer prevention testing and treatment (Alimena et al., 2020; Lee et al., 2020; Nelson et al., 2020). However, our data indicate that many cancer screenings were delayed or canceled during the COVID-19 pandemic. Although FQHC responses indicated that many patients with deferred screenings were rescheduled or put on recall lists, additional care disruptions due to additional waves of infection, as well as reduced services due to the financial impact of the pandemic may reduce FQHC abilities to provide catch-up services.
The important role of telehealth and self-sampling in maintaining cancer screening services during the pandemic is a key finding from this study. Data indicate that approximately 70% of patients were comfortable receiving visits via telehealth during the pandemic, and telehealth helped keep patients connected to their primary care providers (PCC, 2020). While breast and cervical cancer screenings, which require in-person visits were greatly impacted by COVID-related disruptions in care, colon cancer screenings using at-home stool testing continued in half of FQHCs. HPV self-collection is a promising method of cervical cancer screening that is currently used for reaching underscreened populations in Australia and the United Kingdom (Arbyn et al., 2018). HPV self-collection using PCR-based testing has similar detection of pre-cancer and cancer as clinician-collected samples, both of which are far superior to Pap testing alone (Arbyn et al., 2018; Schiffman et al., 2018). Adoption of HPV self-collection as an acceptable method of cervical cancer screening in the United States has the potential to both increase screening participation and create resilience within cervical cancer screening systems to future disruptions in care (Peeters et al., 2020; Scarinci et al., 2021).
4.1. Limitations
These data represent a small sample of federally qualified health centers during a rapidly evolving pandemic. They may not be generalizable to other settings, and conditions even within the systems surveyed may change rapidly. However, national and international data also support a deficit in cancer screenings with a concern for rising mortality in the future (Maringe et al., 2020), therefore the need to support healthcare systems in resuming care is paramount, particularly within FQHCs who serve largely uninsured and underinsured people, many of whom were underscreened before the pandemic.
5. Conclusions
COVID-19 created significant disruptions in cancer screenings in federally qualified health centers. The Cancer Screening during COVID-19 projects aim to help FQHCs resume cancer prevention services and catch up on missed cancer screenings to mitigate the impact of disruptions in care related to COVID-19 on cancer morbidity and mortality. Ensuring and expanding access to care as we move out of the pandemic will be critical to preventing excess cancer incidence and mortality in underscreened populations. Supplementing existing screening models with self-collected screens may be an important tool to eliminate current screening deficits and prevent future cancers.
Author Contributions
Conceptualization: Marcie Fisher-Borne, Rebecca Perkins; Data curation: Marcie Fisher-Borne, Jennifer Isher-Witt; Formal analysis: Jennifer Isher-Witt; Funding acquisition: Marcie Fisher-Borne; Investigation: Marcie Fisher-Borne, Sara Comstock; Methodology: Sara Comstock; Project Administration: Sara Comstock, Marcie Fisher-Borne; Supervision: Marcie Fisher-Borne; Validation and Visualization: Jennifer Isher-Witt; Writing-Original: Marcie Fisher-Borne, Rebecca Perkins, Sara Comstock, Jennifer Isher-Witt; Writing—review and editing: Marcie Fisher-Borne, Rebecca Perkins, Sara Comstock, Jennifer Isher-Witt.
Funding
Funding was provided by the National Football League (NFL) in partnership with the American Cancer Society. Funding provided by the NFL helps to execute and sustain activities at all project sites. The NFL was not involved in the writing of this research manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alimena S., Manning-Geist B., Pena N., et al. Outcomes by race among women referred to an academic colposcopy clinic with a patient navigation program [published online ahead of print, 2020 Sep 22] J. Women’s Health (Larchmt) 2020 doi: 10.1089/jwh.2020.8381. [DOI] [PubMed] [Google Scholar]
- Arbyn M., Smith S.B., Temin S., et al. Collaboration on self-sampling and HPV testing Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823. doi: 10.1136/bmj.k4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Cdc.gov; 2020. Infection Control Guidance for Healthcare Professionals about Coronavirus (COVID-19)https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html Published September 18. [Google Scholar]
- Du X.L., Lin C.C., Johnson N.J., et al. Effects of individual-level socioeconomic factors on racial disparities in cancer treatment and survival: findings from the National Longitudinal Mortality Study, 1979-2003. Cancer. 2011;117(14):3242–3251. doi: 10.1002/cncr.25854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epic Health Research Network . Delayed Cancer Screenings (ehrn.org); 2020. Preventive Cancer Screenings During COVID-19 Pandemic. Published May. Accessed July 1, 2020. [Google Scholar]
- Fedewa S.A., Cotter M.M., Wehling K., et al. Changes in breast cancer screening rates among 32 community health centers during the COVID-19 pandemic. Cancer. 2021 doi: 10.1002/cncr.33859. Under Review, May 28, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Resources & Services Administration Health Center COVID-19 Survey. 2021. Hrsa.govhttps://bphc.hrsa.gov/emergency-response/coronavirus-health-center-data
- Karmakar M., Lantz P.M., Tipirneni R. Association of social and demographic factors with COVID-19 incidence and death rates in the US. JAMA Netw. Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.36462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.C., Liang H., Chen N., et al. Cancer screening among racial/ethnic groups in health centers. Int. J. Equity Health. 2020;19(1):43. doi: 10.1186/s12939-020-1153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.J., Lanfang X., Jin Q., et al. Impact of COVID-19 on cervical cancer screening rates among women aged 21–65 years in a large integrated health care system — Southern California, January 1–September 30, 2019, and January 1–September 30, 2020. MMWR Morb. Mortal. Wkly Rep. 2021;70(4):109–113. doi: 10.15585/mmwr.mm7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.T., Ricaldi J.N., Rose C.E., et al. Disparities in incidence of COVID-19 among underrepresented racial/ethnic groups in counties identified as hotspots during June 5–18, 2020–22 states, February–June 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(33):1122–1126. doi: 10.15585/mmwr.mm6933e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Price L.S., Nattinger A.B., Rivera F., et al. Racial disparities in incidence and outcomes among patients with COVID-19. JAMA Netw. Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson H.D., Cantor A., Wagner J., et al. Effectiveness of patient navigation to increase cancer screening in populations adversely affected by health disparities: a meta-analysis. J. Gen. Intern. Med. 2020;35(10):3026–3035. doi: 10.1007/s11606-020-06020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters E., Cornet K., Cammu H., et al. Efficacy of strategies to increase participation in cervical cancer screening: GPs offering self-sampling kits for HPV testing versus recommendations to have a pap smear taken - A randomised controlled trial [published correction appears in Papillomavirus Res. 2020 May 8;:100201] Papillomavirus Res. 2020;9:100194. doi: 10.1016/j.pvr.2020.100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primary Care Collaborative . Pcpcc.org; 2020. COVID-19 Patient Primary Care Survey.https://www.pcpcc.org/sites/default/files/news_files/C19%20Patient%20Series%202%20Executive%20Summary.pdf [Google Scholar]
- Sabatino S.A., Thompson T.D., White M.C., et al. Cancer screening test receipt — United States, 2018. MMWR Morb. Mortal. Wkly Rep. 2021;70(2):29–35. doi: 10.15585/mmwr.mm7002a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarinci I.C., Li Y., Tucker L., et al. Given a choice between self-sampling at home for HPV testing and standard of care screening at the clinic, what do African American women choose? Findings from a group randomized controlled trial. Prev. Med. 2021;142:106358. doi: 10.1016/j.ypmed.2020.106358. [DOI] [PubMed] [Google Scholar]
- Schiffman M., Kinney W.K., Cheung L.C., et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J. Natl. Cancer Inst. 2018;110(5):501–508. doi: 10.1093/jnci/djx225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G.K., Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J. Environ. Public Health. 2017;2017:2819372. doi: 10.1155/2017/2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]