Abstract
Objectives
The video head impulse test (vHIT) is used as a measure of compensation yet it’s stability in patients with vestibular pathology is unknown.
Methods
144 patients (n = 72 female, mean 54.46 ± 15.8 years) were grouped into one of three primary diagnoses (Peripheral, Central, or Mixed). Subjects were further categorized based on sex (male versus female), ear (left versus right; ipsilesional versus contralesional), age (six groups ranging from 19 to 84 years), and duration between visits (five groups, mean 191.46 ± SE 29.42 days, median 55.5 days). The gain of the VOR during passive head rotation was measured for each semicircular canal (horizontal, anterior, posterior).
Results
There was no difference in the VOR gain within any semicircular canal between the two visits (horizontal: p = 0.179; anterior: p = 0.628; posterior: p = 0.613). However, the VOR gain from the horizontal canals was higher than the vertical canals for each visit (p < 0.001). Patients diagnosed with peripheral vestibular pathology had significantly lower (p ≤ 0.001) horizontal semicircular canal gains at each visit. There was no difference in VOR gain between sex (p = 0.215) or age groupings (p = 0.331). Test-retest reliability of vHIT in patient subjects is good (ICC = 0.801) and the VOR gain values across two separate visits were significant and positively correlated (r = 0.67) regardless of sex, ear, age, or duration between visits.
Conclusion
The vHIT is a stable measure of VOR gain over two different times across a variety of vestibular patients with no influence of age or sex.
Keywords: Video head impulse, Vestibulo-ocular reflex, Gain, Peripheral vestibular hypofunction
1. Introduction
The vestibulo-ocular reflex (VOR) is the briefest human reflex and responsible for gaze stabilization during rapid head rotation. This is achieved by synchronizing eye velocity opposite head velocity for the purpose of keeping images stable on the fovea of the retina. Rehabilitation attempts to improve the effects of a lesioned VOR through the prescription of gaze stability exercises are recommended as standard of care for treating patient with vestibular hypofunction (Hall et al., 2016). Furthermore, advances have been made in vestibular rehabilitation strategies that illustrate the gain of the VOR can be enhanced when lesioned (Migliaccio and Schubert, 2014; Gimmon et al., 2019; Schubert and Migliaccio, 2019). With this progress however, comes an obligation to objectively measure the VOR. Unfortunately, it is atypical for rehabilitation providers to have the necessary equipment in their clinics enabling such measurement of the VOR. Instead of measuring the VOR directly, rehabilitation providers typically rely on behavioral measures of the VOR such as the computerized dynamic visual acuity (DVA) or gaze stabilization tests (GST). Although the computerized versions of each test monitor head velocity at ranges adequate to require a contribution from the VOR, neither test is clinically efficient nor robustly sensitive to confidently identify a vestibular hypofunction (Herdman et al., 1998, 2001; Schubert et al., 2006; Goebel et al., 2007; Honaker and Shepard 2011; Voelker et al., 2015; Riska and Hall 2016). The behavioral nature of both tests increase their vulnerability for false negatives given patients with known vestibular hypofunction can have normal DVA/GST and those with normal VOR function can have abnormal DVA/GST (Millar et al., 2020; Ward et al., 2010; Schubert et al., 2002).
The gain (eye velocity∖head velocity) of the VOR is becoming more common for measuring vestibular function and is typically recorded during the video head impulse test (vHIT). The vHIT incorporates a high-speed (250 Hz) head-mounted camera attached to tight-fitting goggles with an embedded rate sensor for measuring head velocity, all of which enables a rapid determination of the VOR gain across each of the six semicircular canals by applying a small amplitude (∼10°), moderate velocity (∼150°/s) and high acceleration (∼2500°/s2) unpredictable head rotation (Halmagyi and Curthoys, 1988; MacDougall et al., 2009; Alhabib and Saliba, 2017; Halmagyi et al., 2017). We believe that directly measuring the VOR would be useful for knowing the mechanism behind the reported improvement upon completing a gaze stability exercise program, particularly given the high ranges of head velocity and frequency typical of daily life (Grossman et al., 1988) of which the gaze stability exercises attempt to mimic.
Few studies to date have examined the effect of gaze stability training on changing VOR gain. Using the incremental VOR adaptation (IVA) training paradigm, Schubert et al. (2008) revealed that unilateral vestibular hypofunction (UVH, n = 6) can achieve ∼ 18% VOR gain change during active (patient generated) ipsilesional head rotation; the passively measure VOR gain increase was less consistent. Later, Migliaccio and Schubert (2014) reported large changes in ipsilesional only VOR gain for patients with UVH during both active (23%) and passive (79%) impulse testing (Migliaccio and Schubert, 2014). However, each of these earlier studies (2008 and 2014) only measured the VOR gain on the day of training. Using the same IVA method, Gimmon et al. (2019) showed that after 1 year of training daily at home, a patient with bilateral vestibular hypofunction retained an improved VOR gain increase of 380% for rightward head rotation and 179% for leftward head rotation (both passively measured vHIT). In a separate case study measuring retention in a patient with UVH, Rinaudo et al. (2019) used the IVA method to increase the VOR gain by 52% (passively measured). Viziano et al. (2019) examined VOR gain 1 year after traditional gaze stability exercises in participants with UVH and reported the gain was improved by 72%. Recently, Lacour et al. (2020) showed that active head rotation exercises while attempting to identify letters (e.g. DVA task) improved the ipsilesional VOR gain by mean 246% in patients with acute onset vestibular neuritis. In contrast, Millar et al. (2020) found no significant group changes in VOR gain for patients with unilateral vestibular deafferentation that completed gaze stability exercises. Together, these studies reveal the VOR gain is malleable when lesioned, albeit to varying degrees no doubt related in part to any residual VOR function (Gimmon et al., 2019; Lacour et al., 2020, Rinaudo et al., 2019). Although the gain remains pathologically low in most of these instances, the stability of the vHIT in a large sample of patients with variable pathophysiology affecting the vestibular system is unknown. Knowing the error rate associated with repeated vHIT measures of VOR gain is critical if rehabilitation providers are going to rely on the vHIT as an outcome measure of their intervention.
Few studies have focused on repeated measures of VOR gain across days. In healthy controls exposed to the IVA training paradigm for 12 days, Mahfuz et al. (2020) reported that the VOR gain did not deviate by more than 5% for at least 19 days afterwards. Using scleral search coil, Migliaccio and Schubert reported the angular VOR gain was stable when tested across days varying from 3 to 537 days, although this study included healthy controls and those with hearing loss, not patients with known vestibular hypofunction (Schubert and Migliaccio, 2016b, Schubert and Migliaccio, 2016a). Recently, it was reported that the vHIT was reliable, although the study design only examined gain twice over a 48 h period (Singh et al., 2019) and in a small sample size of patients (n = 20) of which 45% were diagnosed with posterior semicircular canal BPPV, a diagnosis we do not expect to see reduced VOR gain. Therefore, variability of the vHIT VOR gain across a greater duration of time in patients with varied vestibular diagnoses is relatively unknown. In this study we sought to understand the test-retest reliability of vHIT by exploring the variability of VOR gain across two separate visits of varying duration while considering diagnosis, sex, measured side and age.
2. Methods
2.1. Subjects
We measured the angular VOR gain from one hundred and forty four patients with symptoms related to various vestibular disorders (n = 72 male, mean 62.97 ± 14.5 years; n = 72 female, mean 54.46 ± 15.8 years; total range = 14.5–92.4 years). Each of the patients underwent clinical oculomotor and vestibular examination that included spontaneous nystagmus with and without fixation removed, head-shaking and skull-vibration induced nystagmus, ocular alignment, positional testing, smooth pursuit and saccadic testing, as well as video head impulse testing (ICS Otometrics, Taastrup, Denmark). Patients with suspected vestibular hypofunction underwent binaural caloric testing to confirm side of lesion, where asymmetry greater than 20% was considered significant. Each patient was initially categorized into one of three main diagnostic groups: Peripheral, Mixed, and Central (Table 1).
Table 1.
Demographic and diagnostic grouping.
| Diagnosis | Sub-Diagnosis | Gender | Age (Mean and SD) | N | |
|---|---|---|---|---|---|
| Peripheral | VH | Female | 56.2 ± 13.70 | 85 (44R/41L) | 105 |
| Male | 63.75 ± 15.47 | ||||
| BPPV | Female | 48.87 ± 22.73 | 9 | ||
| Male | 67.02 ± 9.04 | ||||
| Meniere’s | Female | 61.27 ± 6.73 | 11 | ||
| Male | 71.24 ± 7.80 | ||||
| Mixed | CNS & PVS | Female | 38.61 ± 11.40 | 10 | 20 |
| Male | 55.77 ± 9.49 | ||||
| Migraine & PVS | Female | 50.33 ± 17.33 | 10 | ||
| Male | 62.88 ± 6.82 | ||||
| Central | Pure CNS | Female | 57.02 ± 17.82 | 19 | 19 |
| Male | 54.58 ± 13.96 | ||||
Peripheral: VH – Vestibular Hypofunction (i.e. UVH, BVH, UVD); R – right; L – left; BPPV – Benign Paroxysmal Positional Vertigo. Mixed: CNS - Central Nervous System and PVS - Peripheral Vestibular System diagnoses. Central – CNS only lesions.
No patients within the Peripheral group were seen acutely; their mean duration of time from onset of the peripheral hypofunction was 212.91 ± 50.2 SE days with a median 61 days.
The “Peripheral” group refers to having a peripheral vestibular diagnosis that included vestibular hypofunction (unilateral and bilateral, VH), benign paroxysmal positional vertigo (BPPV), Meniere’s disease, and unilateral vestibular deafferentation (UVD) after vestibular schwannoma resection. The “Mixed” group refers to subjects having both peripheral vestibular system (PVS) and central nervous system (CNS) diagnoses and included migraine with VH; UVD with post-operative complications that involved evidence of cerebellar or brainstem lesion (Koos-4 rating (Erickson et al., 2019), mild traumatic brain injury with VH, migraine with superior canal dehiscence, persistent postural and perceptual dizziness (3PD) with Meniere’s, and 3PD with both migraine with Meniere’s disease. Patients from the “Central” diagnostic group had CNS lesions including ataxia, meningioma, hydrocephaly, mild traumatic brain injury, cerebral ventriculomegaly, migraine, and 3PD (Lopez-Escamez et al., 2015; Staab et al., 2017; Waterston et al., 2012).
Subjects were also categorized based on sex (male versus female), ear (left versus right), age (six groups; 0–40 years, 41–50 years, 51–60 years, 61–70 years, 71–80 years, and >80), and duration between visits (five groups: ≤ 30 days; 31–60 days; 61–180 days, 181–365 days, and >365 days). Only subjects that completed at least two vHIT sessions on two separate days or in two separate sessions in the same day were included. VOR gains of each semicircular canal (SCC) were determined using passive impulses. The horizontal semicircular canal was measured twice in n = 144 patients; the anterior semicircular canal was measured twice in n = 101 patients, and the posterior semicircular canal was measured twice in n = 99 subjects. The study was approved by the Johns Hopkins University Institutional Review Board.
2.2. Passive head impulse testing
Data was collected from the medical records of patients seen in clinic between 2012 and 2020. The head impulse is a passive delivered head rotation of unpredictable timing and direction in the plane of each semicircular canal (Halmagyi and Curthoys, 1988). During the passive head impulse, subjects were instructed to fixate a stationary target located 1m directly in front of them at eye level while the operator turned their head to excite the horizontal, left anterior/right posterior (LARP) and right anterior/left posterior RALP SCC’s.
2.3. Recording system
VOR gains were collected using the vHIT system (Natus Medical Incorporated, Denmark) that included a high speed USB camera to record right eye and head velocity at a frame rate of 250 Hz. The software defines VOR gain as the ratio of the area under the eye velocity curve to the area under the head velocity curve from onset to offset of head velocity. VOR gain values were determined excluding the influence of any compensatory saccade (saccade that occurs in the direction of the slow component eye velocity). Trained personnel examined individual head impulse traces for quality and rejected those impulses for artifacts or when evidence of noise (i.e. loose goggle, wrong calibration, and head-overshoot).
2.4. Statistical analysis
Levene’s test (p > 0.05) for violation of equality of covariance and homogeneity of variance, Pearson-product-moment correlation coefficient (PPMCC), Box’s M test, and calculation of observed power (>80%) were conducted prior to analysis. Although Box’s M test result was significant (p < 0.001), the small alpha level indicated our analysis could proceed given our sample size was greater than 30. Hence, the MANOVA is robust against violations of homogeneity of variance-covariance matrices assumption (Allen and Bennett, 2008). We conducted one-way MANOVA (Multivariate ANOVA) to examine the VOR gain differences across the three semicircular “Canals” (Horizontal, Anterior, Posterior) during two “Visits” (Visit1, Visit2). Post hoc tests were used for pair-wise comparison of VOR gain across three semicircular canals during two visits. Additionally, separate two-way MANOVA examined stability of VOR gain across the two visits within different groups based on categorization (peripheral, mixed, central), sub-diagnosis, gender, age range, and side (right, left). “Visit” along with “Diagnosis”, “Sub-diagnosis”, “Gender”, “Age range”, “Measured Side” were used as independent variables. VOR gain across the two visits were the dependent variables. In addition, we computed the intra-class correlation coefficient (ICC), the Pearson Product Moment Correlation and the coefficient of determination (R2) for VOR gain across the two visits for each factor (semicircular canal, diagnosis, side, sex, age, duration between visits) as estimates of inter-rater reliability, correlational magnitude, and variance. Data is reported as mean VOR gain and 1 standard deviation with 95% confidence intervals at a significance level of p < 0.05. The partial squared eta (ηp2) was used for reporting effect sizes. All analyses employed IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, N.Y., USA).
3. Results
3.1. VOR gain between semicircular canals across visits
We found significant differences in the mean VOR gains across the semicircular canals (p ≤ 0.001) during two visits (First Visit: F (2, 682) = 22.416, p < 001, partial eta squared, ηp2 = 0.062; Second Visit: F (2, 682) = 23.705, p < 001, partial eta squared, ηp2 = 0.065), Table 2. The duration between the two visits varied from 0 to 2247 days.
Table 2.
VOR gain across the semicircular canals at two separate visits for all patients.
| Visit | Semicircular Canal | VOR Gain (1 SD) | 95% CI (Lower – Upper Bound) | Effect Size (ηp2) | Observed Power |
|---|---|---|---|---|---|
| Visit 1 | Horizontal | 0.735 ± 0.260 | 0.704–0.766 | 0.062 | >95% |
| Anterior | 0.572 ± 0.267 | 0.535–0.608 | |||
| Posterior | 0.659 ± 0.269 | 0.622–0.696 | |||
| Visit 2 | Horizontal | 0.724 ± 0.285 | 0.692–0.755 | 0.065 | >95% |
| Anterior | 0.553 ± 0.251 | 0.515–0.590 | |||
| Posterior | 0.644 ± 0.263 | 0.607–0.682 |
SD – Standard Deviation; CI – Confidence Interval; ηp2 – Partial eta-squared indicates the % of variance in the dependent variable, which is small. Observed power has been calculated using alpha = 0.05. The VOR gain from each semicircular canal was significantly different from each other (p < 0.001) within visit 1 and visit 2.
During visit 1, post-hoc testing revealed the mean VOR gain of the horizontal semicircular canal (0.735 ± 0.26) was higher than both the anterior (0.572 ± 0.267, p ≤ 0.001) and posterior (0.659 ± 0.269, p < 0.05) semicircular canals. The mean VOR gain for the anterior semicircular canal was significantly lower than that of posterior semicircular canal, (p ≤ 0.005). Similarly, during visit 2, the mean VOR gain for the horizontal semicircular canal (0.724 ± 0.285) was significantly higher than the anterior (0.553 ± 0.251, p < 0.001) and posterior (0.644 ± 0.263, (p ≤ 0.005) SCC, and the mean VOR gain for the anterior SCC was lower than that of posterior SCC, (p ≤ 0.005), Table 2.
3.2. VOR gain difference within semicircular canal across visits
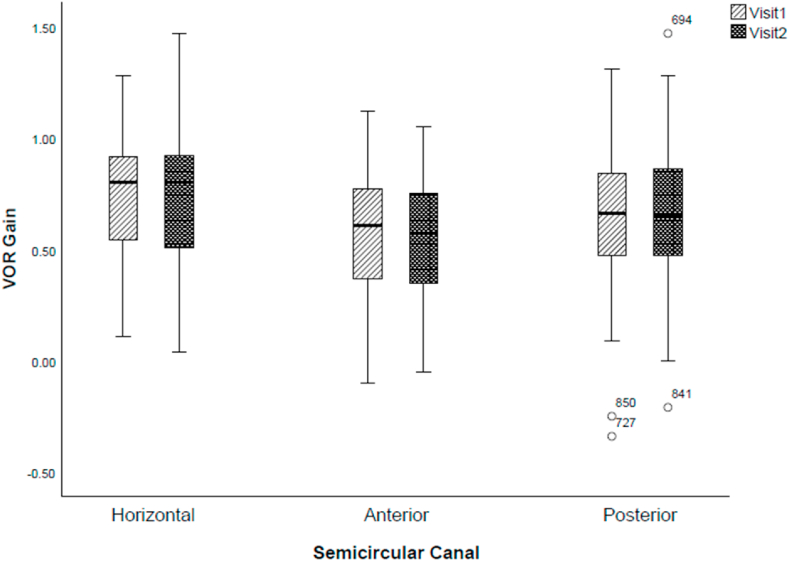
There was no difference in the VOR gain of any semicircular canal between the two visits (horizontal: F(1, 388) = 1.809, p = 0.179, partial eta squared, ηp2 = 0.005; anterior: F(1,388) = 0.239, p = 0.628, partial eta squared, ηp2 = 0.001; posterior: F(1, 388) = 0.256, p = 0.613, partial eta squared, ηp2 = 0.001), Fig. 1.
Fig. 1.
Variability in VOR gain between and within semicircular canals during two separate vHIT sessions. Circles represent outliers.
3.3. VOR gain across broad diagnostic groups
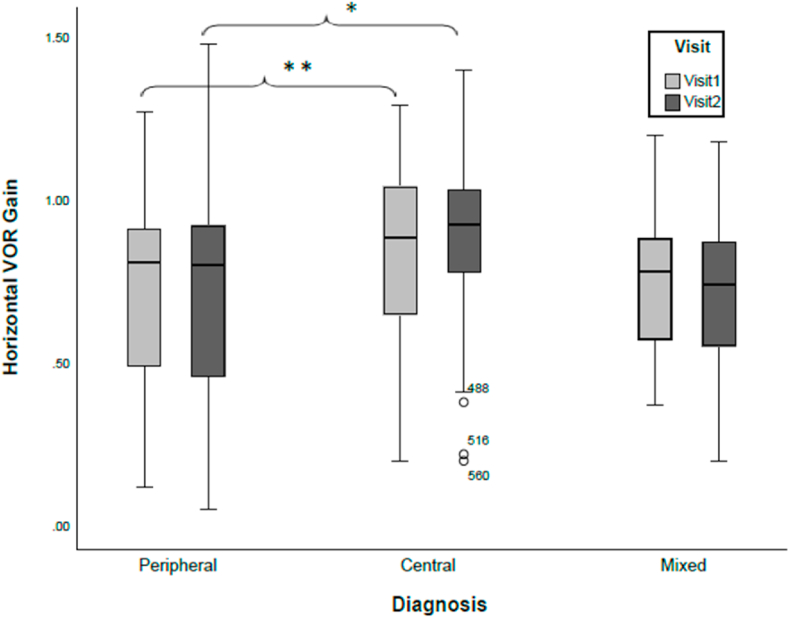
The VOR gain from the horizontal SCC was significantly different across the three broad diagnostic groups (Peripheral, Mixed and Central; F(2,387) = 9.889, p > 0.001). Post-hoc testing revealed differences existed in horizontal SCC VOR gain between the mixed and central groups (p = 0.016), as well as the peripheral and central groups (p > 0.001), but not the mixed and peripheral groups (p = 0.779) Fig. 2. The VOR gain of the horizontal SCC of the Central group was consistently higher than that of the Peripheral group only, for visit 1 (p = 0.022) and visit 2 (p = 0.002). There was no significant differences (p > 0.05) between the mixed and peripheral groups, as well as between the mixed and central groups for visit 1 and visit 2. There was no difference in the anterior and posterior semicircular canal VOR gain among the three main diagnostic groups (anterior: F(2,387) = 0.030, p = 0.971, ηp2 = 0.000; posterior: F(2, 387) = 0.166, p = 0.847, ηp2 = 0.001).
Fig. 2.
Distribution of VOR gain from the horizontal semicircular canal across the three primary diagnostic groups during two visits. Patients with peripheral diagnoses have the largest variability in VOR gain, while the most stability is observed for the patients with central diagnoses (CNS). Significant differences exist between peripheral and central for both visit1 and visit2 (∗ p = 0.002; ∗∗ = p = 0.022). Overall, there is no significant difference in mean VOR gains across the visits for either diagnostic group p < 0.05. Circles represent outliers.
3.4. VOR gain across sub-diagnostic group
The VOR gain from the horizontal and posterior semicircular canals were significantly different across the six sub-diagnostic groups (VH, BPPV, Meniere’s, CNS & PVS, Migraine & PVS and pure CNS), horizontal (F(5,378) = 11.532, p < 0.001, ηp2 = 0.132) and posterior (F(5,378) = 2.198, p = 0.006, ηp2 = 0.043) semicircular canals. However, there was no significant difference for anterior semicircular canal VOR gain among these six sub-diagnostic groups, anterior (F(5,378) = 3.356, p = 0.054, ηp2 = 0.028). Similarly, we found no significant interaction effect within the six sub-diagnostic groups and visit for the horizontal, anterior and posterior SCC VOR gain (Horizontal: F(5,378) = 0.279, p = 0.924, ηp2 0.004; Anterior: F(5,378) = 0.421, p = 0.834, ηp2 = 0.006; Posterior: F(5,378) = 0.632, p = 0.675, ηp2 0.008).
3.5. VOR gain differences between ipsi and contra – lesional rotation in vestibular hypofunction
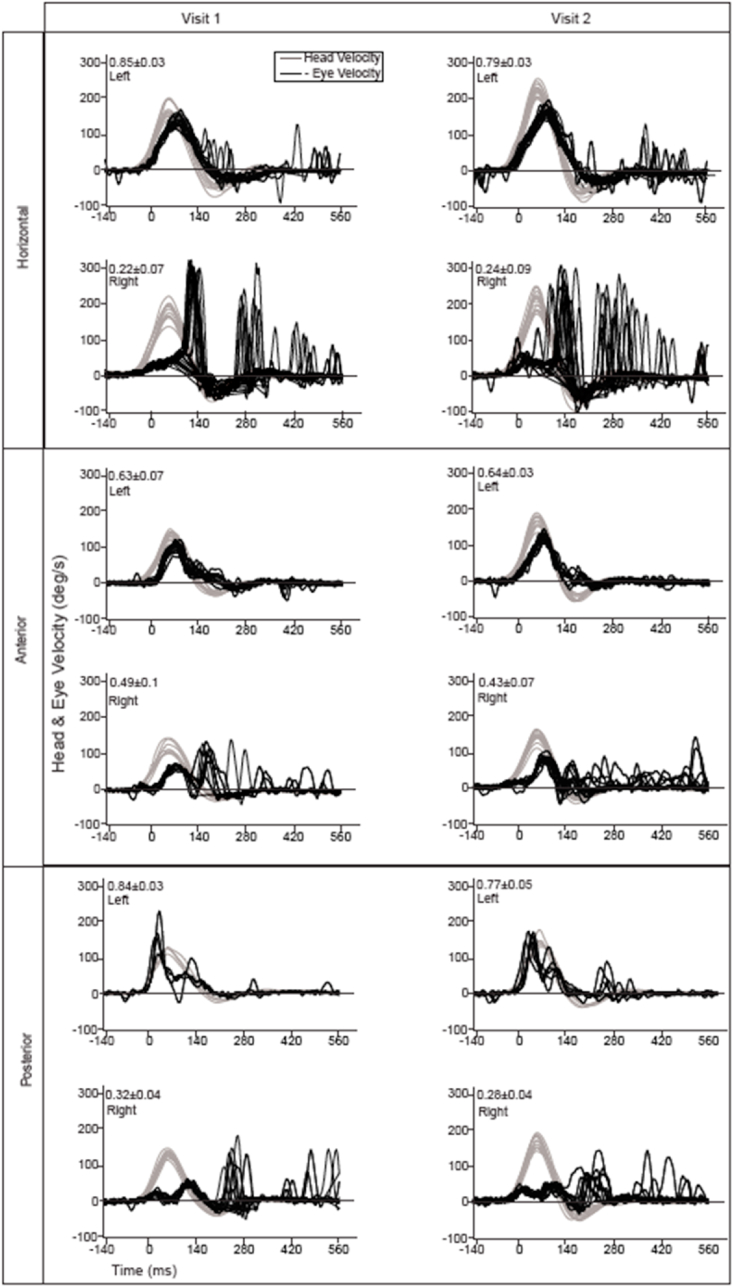
The VOR gain within the VH sub-group for each of the three semicircular canals during ipsilesional rotations were significantly reduced (Horizontal: F(1,234) = 5.355, p ≤ 0.001, ηp2 = 0.292; Anterior: F(1,234) = 3.551, p ≤ 0.001, ηp2 = 0.206; Posterior: F(1,234) = 1.813, p ≤ 0.001, ηp2 = 0.105). However, the ipsilesional and contralesional VOR gains within each semicircular were stable across the two visits (Fig. 3) (Horizontal: F(1,234) = 0.095, p = 0.758, ηp2 = 0.000; Anterior: F(1,234) = 0.019, p = 0.890, ηp2 = 0.000; Posterior: F(5,378) = 0.008, p = 0.930, ηp2 = 0.000, Table 3.
Fig. 3.
VOR gain across two visits for the horizontal, anterior and posterior semicircular canals for a subject with right UVD. There is no significant difference between VOR gains between first and second visit. The duration between two visits was 40 days. A greater variability in compensatory saccades during the 2nd visit is noticeable, particularly during horizontal head rotation.
Table 3.
VOR gain variability for the subjects with vestibular hypofunction.
| Semicircular Canal | Ear/Visit | VOR Gain (Mean ± SD) | 95% CI (Lower–Upper Bound) | Significance (p Value) | Effect Size (ηp2) |
|---|---|---|---|---|---|
| Horizontal | Contra | 0.81 ± 0.21 | 0.77–0.85 | <0.001 | 0.29 |
| Ipsi | 0.51 ± 0.26 | 0.47–0.55 | |||
| Anterior | Contra | 0.67 ± 0.24 | 0.62–0.71 | <0.001 | 0.21 |
| Ipsi | 0.42 ± 0.24 | 0.38–0.46 | |||
| Posterior | Contra | 0.72 ± 0.27 | 0.67–0.76 | <0.001 | 0.11 |
| Ipsi | 0.55 ± 0.24 | 0.5–0.6 | |||
| Horizontal | Visit1 | 0.68 ± 0.27 | 0.64–0.72 | 0.18 | 0.01 |
| Visit2 | 0.64 ± 0.28 | 0.60–0.68 | |||
| Anterior | Visit1 | 0.56 ± 0.28 | 0.52–0.60 | 0.25 | 0.01 |
| Visit2 | 0.53 ± 0.26 | 0.48–0.57 | |||
| Posterior | Visit1 | 0.64 ± 0.27 | 0.59–0.68 | 0.59 | 0.00 |
| Visit2 | 0.62 ± 0.27 | 0.57–0.67 |
VOR – Vestibulo-Ocular Reflex; SD - Standard Deviation; CI - Confidence Interval; ηp2 – Partial Eta squared.
3.6. vHIT test-retest reliability
ICC estimates shows good reliability of the vHIT across two separate test visits for determining VOR gain within the horizontal (ICC = 0.844, p < 0.001; 95% CI at 0.80–0.88) and anterior semicircular canals (ICC = 0.818, p < 0.001; 95% CI (0.76–0.86), but moderate reliability for posterior canal ((ICC = 0.644, p < 0.001; 95% CI (0.53–0.73), Table 4. Good reliability also exists for each of the factors examined (diagnosis, sex and side); however, reliability dropped to moderate for subjects older than 80 years and when duration between visit 1 and 2 was greater than 1 year, Table 4. All other predictors for age and duration group have good test-retest reliability (i.e. ICC >0.75). Overall, the VOR gain across two separate visits as measured by the vHIT in patient subjects was stable as indicated by a good reliability (ICC = 0.801, p < 0.005 with 95% CI (0.77, 0.83) (Koo and Li 2016).
Table-4.
Pearson Correlation Coefficient, R2 and ICC of the vHIT Across Different Factors.
| Factor | ||||||
|---|---|---|---|---|---|---|
| PPMCC (r) | R2 | ICC | ICC 95% CI Lower-Upper Bound | Significance (p value) | ||
| Semicircular Canal | Horizontal | 0.733 | 0.537 | 0.844 | 0.876–0.802 | <0.001 |
| Anterior | 0.693 | 0.480 | 0.818 | 0.862–0.759 | <0.001 | |
| Posterior | 0.475 | 0.226 | 0.644 | 0.731–0.529 | <0.001 | |
| Diagnosis | Peripheral | 0.666 | 0.444 | 0.799 | 0.831–0.761 | <0.001 |
| Central | 0.712 | 0.507 | 0.831 | 0.894–0.732 | <0.001 | |
| Mixed | 0.618 | 0.382 | 0.764 | 0.844–0.643 | <0.001 | |
| Sex | Male | 0.619 | 0.383 | 0.761 | 0.808–0.703 | <0.001 |
| Female | 0.733 | 0.537 | 0.846 | 0.875–0.81 | <0.001 | |
| Age (years) | 0–40 | 0.634 | 0.402 | 0.773 | 0.846–0.665 | <0.001 |
| 41–50 | 0.746 | 0.557 | 0.849 | 0.901–0.771 | <0.001 | |
| 51–60 | 0.71 | 0.504 | 0.829 | 0.875–0.766 | <0.001 | |
| 61–70 | 0.656 | 0.430 | 0.79 | 0.84–0.724 | <0.001 | |
| 71–80 | 0.652 | 0.425 | 0.777 | 0.861–0.642 | <0.001 | |
| >80 | 0.547 | 0.299 | 0.708 | 0.836–0.479 | <0.001 | |
| Duration (days) | ≤30 | 0.655 | 0.429 | 0.79 | 0.843–0.717 | <0.001 |
| 31–60 | 0.707 | 0.500 | 0.828 | 0.869–0.773 | <0.001 | |
| 61–180 | 0.602 | 0.362 | 0.752 | 0.817–0.664 | <0.001 | |
| 181–365 | 0.857 | 0.734 | 0.922 | 0.952–0.873 | <0.001 | |
| >365 | 0.514 | 0.264 | 0.681 | 0.806–0.476 | <0.001 | |
| Side | Right | 0.609 | 0.371 | 0.756 | 0.803–0.698 | <0.001 |
| Left | 0.716 | 0.513 | 0.834 | 0.866–0.794 | <0.001 | |
| Overall | 0.668 | 0.446 | 0.801 | 0.829-0.769 | < 0.001 | |
PPMCC – Pearson’s Product-Moment Correlation Coefficient, ICC – Intraclass Correlation Coefficients, CI – Confidence Interval, R2 = Coefficient of Determination.
3.7. Correlation of VOR gain between visits for the different factors
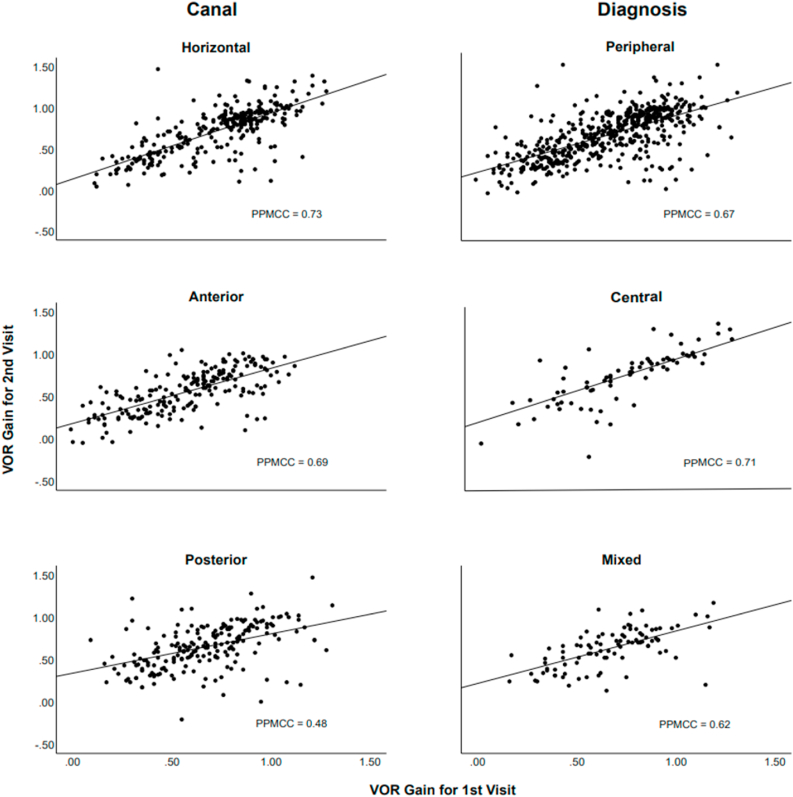
The correlational magnitudes of the VOR gain for each semicircular canal (0.733 horizontal, 0.693 anterior, 0.475 posterior) were significant between visits and the three primary diagnostic groups at a moderate to good strength, Fig. 4. When considering the other factors, the magnitude of the correlations were significant and moderately positive for sex, age, duration, and side (Table 4). Overall, the correlation of VOR gain between two visits (0.668, p < 0.01) is good with a coefficient of determination R2 = 0.446.
Fig. 4.
Scatter plots of the VOR gain and Pearson’s Product-Moment Correlation Coefficient (PPMCC) between the two visits for the semicircular canal and diagnostic group. PPMCC values are significantly positive and of moderate to good strength.
3.8. VOR gain between visits with respect to test duration, sex, age, and side
Although there was a significant main effect (Wilk’s Λ = 0.905, F (12, 382) = 3.221, p < 0.001, ηp2 = 0.33) for VOR gain within the groups of subjects tested at different durations, there was no interaction effect between duration and visit (Wilk’s Λ = 0.982, F (12, 382) = 0.982, p = 0.864, ηp2 = 0.006) which suggests that there is no significant differences in VOR gain across the two visits due to test duration. Similarly, there was no interaction effect between visit and sex (Wilk’s Λ = 0.990, F (3, 384) = 1.325, p = 0.266, ηp2 = 0.010), visit and different age ranges (Wilk’s Λ = 0.989, F (15, 1038) = 0.283, p = 0.997, ηp2 = 0.004), or visit and measured side (Wilk’s Λ = 0.981, F (3, 384) = 2.417, p = 0.66, ηp2 = 0.019). There was no difference in VOR gain between female and male (Wilk’s Λ = 0.988, F (3, 384) = 1.497, p = 0.215, ηp2 = 0.012), nor among the six age ranges (Wilk’s Λ = 0.956, F (15, 1038) = 1.125, p = 0.331, ηp2 = 0.015). The VOR gain for the leftward horizontal semicircular canal was higher (0.723 ± 0.284) than that of the rightward semicircular canal (0.574 ± 0.223), Wilk’s Λ = 0.616, F (3, 384) = 79.946, p ≤ 0.001, ηp2 = 0.384).
4. Discussion
Our study reveals that the VOR gain as determined using ‘off the shelf’ vHIT equipment and the manufacturer recommended test method in patients with various vestibular disorders is higher in the horizontal SCC than that of the anterior and posterior SCC. Prior studies of VOR gain difference across the semicircular canals using the gold-standard scleral search coil recording method have similarly reported the horizontal SCC have VOR gains higher than the vertical SCC (anterior and posterior), in both healthy subjects and those with inner ear disorders. Schubert and Migliaccio (2016) reported VOR gain for the horizontal SCC is ∼1.2 times higher than that of vertical SCCs as applied in canal planes (Schubert and Migliaccio, 2016b, Schubert and Migliaccio, 2016a). In a smaller sample size, Cremer et al. (1998) also used search coil to show the normal horizontal VOR gain (∼0.9) is 1.1–1.3 times higher than the VOR gain (0.7–0.8) for both the RALP and LARP planes (n = 9) (Cremer et al., 1998). When conducting head impulses in SCC planes, the vertical SCC gains are smaller given the contribution from the roll VOR (∼0.6) induces a reduction effect. Aw et al. confirmed this by illustrating the mean VOR gain for impulses applied in the yaw (∼0.98) and pitch (∼1.04) planes are 1.3 and 1.4 times higher than those from the roll plane (0.74) (Aw et al., 1996a). Therefore, a reduction in VOR gain from the roll component occurs for during vertical SCC excitation in pitch (Cremer et al., 1998; Ferman et al., 1987; Leigh et al., 1989). The current vHIT eye tracking method is unable to record torsion, thus torsional VOR gain is unable to contribute any magnitude to the vertical SCC gain value. Manufacturer guidelines recommend testing the vertical SCC using a pitch only head rotation after the head is initially placed into a static position of ∼45 deg yaw, thereby precluding a roll eye rotation. Thus the vHIT method uses a ‘2D’ calculation of VOR gain, which has been validated using search coil (Migliaccio and Cremer, 2011).
Our data did reveal a greater variation (4.9%) in VOR gain of the horizontal semicircular canal across subjects within the three main diagnostic groups (peripheral, mixed and central). This was due to those patients with central causes for their symptoms having larger VOR gain and suggests the patients seen in our clinics with central diagnoses tend to have normal peripheral vestibular function, while the subjects with peripheral lesions (mixed and peripheral only) have lower VOR gain with greater variability. Furthermore, our data reveal the VOR gain during contralesional head impulses was greater by 1.59, 1.58 and 1.32 times than that of ipsilesional rotation for horizontal, anterior and posterior semicircular canals respectively. Cremer et al. (1998) report a similar result in a smaller sample study of 20 patients with UVD; the ipsilesional impulse VOR gain was about 0.2–0.3 regardless of which SCC was tested. In contrast, the contralesional yaw impulse VOR gains were about 0.9–1.0 while the contralesional vertical canals were 0.7–0.8 (Cremer et al., 1998). In patients with UVD, Schubert and Migliaccio, 2016b, Schubert and Migliaccio, 2016a confirm that the magnitude asymmetry in VOR gain between pitch/yaw and roll persists (Aw et al., 1996b). The mean pitch (∼0.67) and yaw (0.56) VOR gains remain nearly 1.4 times higher than that of roll (mean 0.39; 0.23 ipsi, 0.56 contra). Millar et al. (2020) reported ipsilateral horizontal VOR gain of 0.44 against the contralateral horizontal VOR gain of 0.81 in chronic patients with UVD (n = 43). In acutely recovering UVD patients (n = 5), Mantokoudis et al. (2014) reported the ipsilesional yaw VOR gain was 0.23 against the contralateral yaw VOR gain of 0.79 at post-day 2. However, at post op day 4 the contralesional VOR gain recovered to baseline (Mantokoudis et al., 2014).
4.1. VOR gain asymmetric between left and right vertical canals
In our patient population, there exists some asymmetry in VOR gain comparing the vertical SCC. The mean VOR gain for the left posterior SCC was 1.26 times higher (p < 0.001) than that of the right posterior canal yet the mean VOR gain for the rightward anterior SCC was 1.55 times (p < 0.001) higher than that of the leftward anterior canal. There was no difference between left and right (p = 0.76) horizontal SCC gain. We are not the first to report asymmetry within the anterior SCC using vHIT. McGarvie et al. (2015) reported the mean VOR gain of healthy subjects for the right anterior canal was higher than that of left anterior canal as measured using the vHIT regardless of head velocity, however the magnitude differences were not reported (McGarvie et al., 2015). Similarly, in a small sample size of patients with vestibular disorders (n = 12) MacDougall et al. (2013) reported that rightward vertical SCC tests were higher for anterior (∼1.15x) that that of leftward anterior SCC (MacDougall et al., 2013). McGarvie et al. (2015) suggests this result was the vertical equivalent of the horizontal gaze distance dependence due to an increased demand in rotation of the abducting eye rotation (Viirre et al., 1986). One explanation for our patient subjects having vertical VOR SCC gains greater for leftward versus rightward head rotations is the preponderance of right hypofunction in the peripheral group. Another reason for this difference may be related to handedness of the examiner, although the role of dominance has not been examined in vHIT testing.
4.2. VOR gain across age and sex
Recent investigation regarding the effect of aging on the gain of the VOR suggest that for the healthy subjects there is little decrease in VOR gain through the 8th decade of life for the horizontal and anterior SCC. However, McGarvie et al. (2015) did report that the VOR gain of the posterior SCC was significantly reduced with age (p < 0.02) (McGarvie et al., 2015). It is unknown how stable the VOR gain is in patient subjects as they age.
It has been reported that woman have a higher incidence of diagnoses primarily affecting the vestibular system (e.g. BPPV, vestibular neuritis) and related symptoms (e.g. vertigo, nonspecific dizziness, motion sickness) (Kim et al., 2020; Yang et al., 2020; Smith et al., 2019). However, there does not seem to be any significant differences in the SCC or otolith reflexes including the VOR and the vestibular-evoked myogenic potentials (Smith et al., 2019). Our data also suggests that VOR gain is unaffected by sex.
The R2 value we report (0.446) was significant and thereby suggests our predictor variables have a true relationship on VOR gain for patient subjects, however it also suggests that the current vHIT method and equipment have an inherently large amount of unexplained variability (55%) as associated with repeat testing. Adding other variables may refine future regression models, though we suspect that much of the variability is due to differences in lesions affecting the VOR.
4.3. Relevance with vestibular rehabilitation
Our data also reveal that the VOR gain varied little over time (visit 1 and 2) for the horizontal, anterior, and posterior SCC when considering all patient subjects regardless of test duration between visits. The head impulse test is an established clinical test with reliability and variability dependent on lesion severity, with highest validity in those with surgically induced lesions (Cremer et al., 1998; Halmagyi et al., 2017). To date, the vHIT is primarily used to diagnose lesions of the semicircular canal periphery though research is beginning to use metrics from the vHIT to document compensation (Lee and Kim, 2020; McGarvie et al., 2020). Our results suggest that the vHIT is a stable measure of SCC function across different days, thereby establishing vHIT as a valid measure to document change in VOR gain, particularly useful for rehabilitation providers prescribing exercise strategies with the intent of improving such performance.
4.4. Limitations
The VOR gain varies ∼15% depending on whether the recorded eye is abducting or adducting, yet we calculated VOR gain from the right eye only for both directions (Weber et al., 2008). Therefore some of the variability we report may be related to lack of controlling for inter-ocular differences in VOR gain. Although the same experienced provider delivered the head impulses, we did not control for head velocity. Similarly, any directional bias in examiner strength (i.e. rightward vs leftward) albeit un-intended, in delivery of the head rotation from the same experienced provider may have influenced the passive head velocity. Given the VOR is non-linear, it is therefore possible that the variability in VOR gain would have been minimized were head velocity controlled. Finally, although we cover a broad range of diagnoses common to tertiary specialty clinics treating vestibular disorders, our sample size of those patients with central and mixed diagnosis were less than the sample of those patients with purely peripheral diagnoses.
5. Conclusion
The vHIT is a stable measure of the passive VOR gain over two different times across a variety of patients with vestibular disorders with no influence of age or sex.
Funding information
This work was supported in part by the Department of Defense under the Neurosensory and Rehabilitation Research Award Program (Grant Award W81XWH-15-1-0442) and the Psychological Health/Traumatic Brain Injury Research Program Complex Traumatic Brain Injury Rehabilitation Research Clinical Trial Award (Grant Award W8lXWH-l7).
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Contributor Information
M. Muntaseer Mahfuz, Email: mmahfuz1@jhmi.edu.
Jennifer L. Millar, Email: jmillar1@jhmi.edu.
Michael C. Schubert, Email: mschube1@jhmi.edu.
References
- Alhabib S.F., Saliba I. Video head impulse test: a review of the literature. Eur. Arch. Oto-Rhino-Laryngol. 2017;274:1215–1222. doi: 10.1007/s00405-016-4157-4. [DOI] [PubMed] [Google Scholar]
- Allen P., Bennett K. Thomson; 2008. SPSS for the Health & Behavioural Sciences. [Google Scholar]
- Aw S.T., Haslwanter T., Halmagyi G.M., Curthoys I.S., Yavor R.A., Todd M.J. Three-dimensional vector analysis of the human vestibuloocular reflex in response to high-acceleration head rotations I. Responses in normal subjects. J. Neurophysiol. 1996;76:4009–4020. doi: 10.1152/jn.1996.76.6.4009. [DOI] [PubMed] [Google Scholar]
- Aw S.T., Halmagyi G.M., Haslwanter T., Curthoys I.S., Yavor R.A., Todd M.J. Three-dimensional vector analysis of the human vestibuloocular reflex in response to high-acceleration head rotations II. Responses in subjects with unilateral vestibular loss and selective semicircular canal occlusion. J. Neurophysiol. 1996;76:4021–4030. doi: 10.1152/jn.1996.76.6.4021. [DOI] [PubMed] [Google Scholar]
- Cremer P.D., Halmagyi G.M., Aw S.T., Curthoys I.S., McGarvie L.A., Todd M.J., Black R., Hannigan I.P. Semicircular canal plane head impulses detect absent function of individual semicircular canals. Brain. 1998;121:699–716. doi: 10.1093/brain/121.4.699. [DOI] [PubMed] [Google Scholar]
- Erickson N.J., Schmalz P.G.R., Agee B.S., Fort M., Walters B.C., McGrew B.M., Fisher W.S. Koos classification of vestibular schwannomas: a reliability study | neurosurgery. Neurosurgery. 2019;85:409–414. doi: 10.1093/neuros/nyy409. Oxford Academic. [DOI] [PubMed] [Google Scholar]
- Ferman L., Collewijn H., Jansen T.C., Van den Berg A.V. Human gaze stability in the horizontal, vertical and torsional direction during voluntary head movements, evaluated with a three-dimensional scleral induction coil technique. Vis. Res. 1987;27:811–828. doi: 10.1016/0042-6989(87)90078-2. [DOI] [PubMed] [Google Scholar]
- Gimmon Y., Migliaccio A.A., Kim K.J., Schubert M.C. VOR adaptation training and retention in a patient with profound bilateral vestibular hypofunction. Laryngoscope. 2019;129:2568–2573. doi: 10.1002/lary.27838. [DOI] [PubMed] [Google Scholar]
- Goebel J.A., Tungsiripat N., Sinks B., Carmody J. Gaze stabilization test: a new clinical test of unilateral vestibular dysfunction. Otol. Neurotol. 2007;28:68–73. doi: 10.1097/01.mao.0000244351.42201.a7. [DOI] [PubMed] [Google Scholar]
- Grossman G.E., Leigh R.J., Abel L.A., Lanska D.J., Thurston S.E. Frequency and velocity of rotational head perturbations during locomotion. Exp. Brain Res. 1988;70:470–476. doi: 10.1007/BF00247595. [DOI] [PubMed] [Google Scholar]
- Hall C.D., Herdman S.J., Whitney S.L., Cass S.P., Clendaniel R.A., Fife T.D., Furman J.M., Getchius T.S.D., Goebel J.A., Shepard N.T., Woodhouse S.N. Vestibular rehabilitation for peripheral vestibular hypofunction: an evidence-based clinical practice guideline: from the American physical therapy association neurology section. J. Neurol. Phys. Ther. 2016;40:124–155. doi: 10.1097/NPT.0000000000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmagyi G.M., Chen L., MacDougall H.G., Weber K.P., McGarvie L.A., Curthoys I.S. The video head impulse test. Front. Neurol. 2017;8 doi: 10.3389/fneur.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmagyi G.M., Curthoys I.S. A clinical sign of canal paresis. Arch. Neurol. 1988;45:737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- Herdman S.J., Schubert M.C., Tusa R.J. Role of central preprogramming in dynamic visual acuity with vestibular loss. Arch. Otolaryngol. Head Neck Surg. 2001;127:1205–1210. doi: 10.1001/archotol.127.10.1205. [DOI] [PubMed] [Google Scholar]
- Herdman S.J., Tusa R.J., Blatt P., Suzuki A., Venuto P.J., Roberts D. Computerized dynamic visual acuity test in the assessment of vestibular deficits. Am. J. Otol. 1998;19:790–796. [PubMed] [Google Scholar]
- Honaker J.A., Shepard N.T. Use of the Dynamic Visual Acuity Test as a screener for community-dwelling older adults who fall. J. Vestib. Res. Equilib. Orientat. 2011;21:267–276. doi: 10.3233/VES-2011-0427. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Lee J.O., Choi J.Y., Kim J.S. Etiologic distribution of dizziness and vertigo in a referral-based dizziness clinic in South Korea. J. Neurol. 2020 doi: 10.1007/s00415-020-09831-2. [DOI] [PubMed] [Google Scholar]
- Koo T.K., Li M.Y. A guideline of selecting and reporting Intraclass correlation coefficients for reliability research. Chiropr. Med. 2016 Jun;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacour M., Tardivet L., Thiry A. Rehabilitation of dynamic visual acuity in patients with unilateral vestibular hypofunction: earlier is better. Eur. Arch. Otorhinolaryngol. 2020 doi: 10.1007/s00405-019-05690-4. [DOI] [PubMed] [Google Scholar]
- Lee J.-Y., Kim M.-B. Importance of video head impulse test parameters for recovery of symptoms in acute vestibular neuritis. Otol. Neurotol. 2020;41:964–971. doi: 10.1097/MAO.0000000000002669. [DOI] [PubMed] [Google Scholar]
- Leigh R.J., Maas E.F., Grossman G.E., Robinson D.A. Visual cancellation of the torsional vestibulo-ocular reflex in humans. Exp. Brain Res. 1989;75:221–226. doi: 10.1007/BF00247930. [DOI] [PubMed] [Google Scholar]
- Lopez-Escamez J.A., Chung W.-H., Magnusson M., Mandalà M., Carey J., Goebel J.A., Newman-Toker D.E., Strupp M., Suzuki M., Trabalzini F., Bisdorff A. Diagnostic criteria for Menière’s disease. J. Vestib. Res. 2015;25:1–7. doi: 10.3233/VES-150549. [DOI] [PubMed] [Google Scholar]
- MacDougall H.G., McGarvie L.A., Halmagyi G.M., Curthoys I.S., Weber K.P. The video head impulse test (vHIT) detects vertical semicircular canal dysfunction. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall H.G., Weber K.P., McGarvie L.A., Halmagyi G.M., Curthoys I.S. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology. 2009;73:1134–1141. doi: 10.1212/WNL.0b013e3181bacf85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantokoudis G., Schubert M.C., Saber Tehrani A.S., Wong A.L., Agrawal Y. Early adaptation and compensation of clinical vestibular responses after unilateral vestibular deafferentation surgery. Otol. Neurotol. 2014;35:148–154. doi: 10.1097/MAO.0b013e3182956196. [DOI] [PubMed] [Google Scholar]
- McGarvie L.A., MacDougall H.G., Halmagyi G.M., Burgess A.M., Weber K.P., Curthoys I.S. The video head impulse test (vHIT) of semicircular canal function - age-dependent normative values of VOR gain in healthy subjects. Front. Neurol. 2015;6 doi: 10.3389/fneur.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarvie L.A., MacDougall H.G., Curthoys I.S., Halmagyi G.M. Spontaneous recovery of the vestibulo-ocular reflex after vestibular neuritis; long-term monitoring with the video head. Impulse Test Single Patient. 2020 Jul 28;11:732. doi: 10.3389/fneur.2020.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A.A., Cremer P.D. The 2D modified head impulse test: a 2D technique for measuring function in all six semi-circular canals. J. Vestib. Res. Equilib. Orientat. 2011;21:227–234. doi: 10.3233/VES-2011-0421. [DOI] [PubMed] [Google Scholar]
- Migliaccio A.A., Schubert M.C. Pilot study of a new rehabilitation tool: improved unilateral short-term adaptation of the human angular vestibulo-ocular reflex. Otol. Neurotol. 2014;35 doi: 10.1097/MAO.0000000000000539. e310–e316. [DOI] [PubMed] [Google Scholar]
- Millar J.L., Gimmon Y., Roberts D., Schubert M.C. Improvement after vestibular rehabilitation not explained by improved passive VOR gain. Front. Neurol. 2020;11:79. doi: 10.3389/fneur.2020.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfuz M.M., Schubert M.C., Figtree W.V.C., Migliaccio A.A. Retinal image slip must pass the threshold for human vestibulo-ocular reflex adaptation. JARO J. Assoc. Res. Otolaryngol. 2020 doi: 10.1007/s10162-020-00751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaudo C.N., Schubert M.C., Cremer P.D., Figtree W.V.C., Todd C.J., Migliaccio A.A. Improved oculomotor physiology and behavior after unilateral incremental adaptation training in a person with chronic vestibular hypofunction: a case report. PubMed. Phys. Ther. 2019;99:1326–1333. doi: 10.1093/ptj/pzz083. academic.oup.com [DOI] [PubMed] [Google Scholar]
- Riska K.M., Hall C.D. Reliability and normative data for the dynamic visual acuity test for vestibular screening. Otol. Neurotol. 2016;37:545–552. doi: 10.1097/MAO.0000000000001014. [DOI] [PubMed] [Google Scholar]
- Schubert M.C., Della Santina C.C., Shelhamer M. Incremental angular vestibulo-ocular reflex adaptation to active head rotation. Exp. Brain Res. 2008;191:435–446. doi: 10.1007/s00221-008-1537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M.C., Herdman S.J., Tusa R.J. Vertical dynamic visual acuity in normal subjects and patients with vestibular hypofunction. Otol. Neurotol. 2002;23:372–377. doi: 10.1097/00129492-200205000-00025. [DOI] [PubMed] [Google Scholar]
- Schubert M.C., Migliaccio A.A. New advances regarding adaptation of the vestibulo-ocular reflex. J. Neurophysiol. 2019;122:644–658. doi: 10.1152/jn.00729.2018. [DOI] [PubMed] [Google Scholar]
- Schubert M.C., Migliaccio A.A. Stability of the aVOR to repeat head impulse testing. Otol. Neurotol. 2016;37:781–786. doi: 10.1097/MAO.0000000000001055. [DOI] [PubMed] [Google Scholar]
- Schubert M.C., Migliaccio A.A. Stability of the aVOR to repeat head impulse testing. Otol. Neurotol. 2016;37:781–786. doi: 10.1097/MAO.0000000000001055. [DOI] [PubMed] [Google Scholar]
- Schubert M.C., Migliaccio A.A., Della Santina C.C. Dynamic visual acuity during passive head thrusts in canal planes. JARO J. Assoc. Res. Otolaryngol. 2006;7:329–338. doi: 10.1007/s10162-006-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N.K., Govindaswamy R., Jagadish N. Test–retest reliability of video head impulse test in healthy individuals and individuals with dizziness. J. Am. Acad. Audiol. 2019;30:744–752. doi: 10.3766/jaaa.17080. [DOI] [PubMed] [Google Scholar]
- Smith P.F., Agrawa Y., Darlington C.L. Sexual dimorphism in vestibular function and dysfunction. J. Neurophysiol. 2019;121:2379–2391. doi: 10.1152/jn.00074.2019. [DOI] [PubMed] [Google Scholar]
- Staab J.P., Eckhardt-Henn A., Horii A., Jacob R., Strupp M., Brandt T., Bronstein A. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the committee for the classification of vestibular disorders of the barany society. J. Vestib. Res. Equilib. Orientat. 2017;27:191–208. doi: 10.3233/VES-170622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viirre E., Tweed D., Milner K., Vilis T. A reexamination of the gain of the vestibuloocular reflex. J. Neurophysiol. 1986;56:439–450. doi: 10.1152/jn.1986.56.2.439. [DOI] [PubMed] [Google Scholar]
- Viziano A., Micarelli A., Augimeri I., Micarelli D., Alessandrini M. Long-term effects of vestibular rehabilitation and head-mounted gaming task procedure in unilateral vestibular hypofunction: a 12-monthfollow-up of a randomized controlled trial. Clin. Rehabil. 2019;33:24–33. doi: 10.1177/0269215518788598. [DOI] [PubMed] [Google Scholar]
- Voelker C.C.J., Lucisano A., Kallogjeri D., Sinks B.C., Goebel J.A. Comparison of the gaze stabilization test and the dynamic visual acuity test in unilateral vestibular loss patients and controls. Otol. Neurotol. 2015;36:746–753. doi: 10.1097/MAO.0000000000000689. [DOI] [PubMed] [Google Scholar]
- Ward B.K., Mohammad M.T., Whitney S.L., Marchetti G.F., Furman J.M. The reliability, stability, and concurrent validity of a test of gaze stabilization. J. Vestib. Res. Equilib. Orientat. 2010;20:363–372. doi: 10.3233/VES-2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston J.A., Seemungal B.M., Lempert T., Olesen J., Furman J., Waterston J., Seemungal B., Carey J., Bisdorff A., Versino M., Evers S., Newman-Toker D. Vestibular migraine: diagnostic criteria. Artic. J. Vestib. Res. 2012;22:167–172. doi: 10.3233/VES-2012-0453. [DOI] [PubMed] [Google Scholar]
- Weber K.P., Aw S.T., Todd M.J., McGarvie L.A., Pratap S., Curthoys I.S., Halmagyi G.M. Progress in Brain Research. Elsevier; 2008. Inter-ocular differences of the horizontal vestibulo-ocular reflex during impulsive testing; pp. 195–198. [DOI] [PubMed] [Google Scholar]
- Yang T., Xirasagar S., Cheng Y., Wu C., Kuo N., Lin H. Peripheral vestibular disorders: nationwide evidence from taiwan. Laryngoscope lary. 2020 doi: 10.1002/lary.28877. [DOI] [PubMed] [Google Scholar]