Abstract
Background
Sudden sensorineural hearing loss (SSNHL) is a common disease in otology, and steroids play an important role in its treatment. Steroids can be administered systemically or locally, and the efficacies of different administration routes remain controversial.
Methods
We searched the Cochrane, EMBASE, PubMed, Web of Science, CNKI, Wanfang and Weipu databases for randomized controlled trials (RCTs) on glucocorticoid treatments for SSNHL to compare the efficacy of topical and systemic steroid administration. The Review Manager 5.4 software was used for synthesis of data: the rate of reported hearing improvement and change in pure-tone audiometry (PTA).
Results
In all the included studies, when intratympanic administration was compared to systemic therapies, the risk difference (RD) using reported hearing improvement as an outcome measure was 0.08 (95% CI: 0.01–0.14, I2 = 45%). Using PTA changes as an outcome measure in 4 studies, the mean difference (MD) was 10.43 dB (95% CI: 3.68–17.18, I2 = 81%). Hearing improvement RD was also compared among different types of steroid, recovery criteria, follow-up times and diagnostic criteria, and showed no significant differences exception for recovery criteria (>10 dB) (RD -0.06, 95% CI: 0.14-0.2, I2 = 0%).
Conclusion
As the initial treatment for SSNHL, topical steroids seem to be superior to systemic steroid administration, especially in patients with contraindications to systemic steroids usage. However, further verification based on high-quality research is needed.
Keywords: Intratympanic treatment, Systemic treatment, meta-Analysis, Sudden sensorineural hearing loss
1. Introduction
Disabling hearing loss affects greater than 6.8% of the world’s population and people of all ages (Neumann et al., 2019). Patients with hearing loss experience impaired quality of life, with emotional and financial burdens seriously affecting their livelihood and families. In 1944, Kleyn first described and defined sudden sensorineural hearing loss (SSNHL) as hearing loss of at least 30 dB at three or more consecutive frequencies on pure-tone audiogram in 3 days or less (Kleyn, 1994). SSNHL affects 5–27 (US) and 160–400 (Germany) per 100,000 people each year (Steth et al., 2019). However, the exact cause of this disease remains unclear. Infections, congenital diseases, autoimmune mechanisms, ototoxic drugs and other conditions have been reported as risk factors for the occurrence of SSNHL (Dan et al., 2016). If is not detected and treated in due time, SSNHL can lead to persistent hearing loss and significantly compromised quality of life (QOL) in patients. Therefore, early management and intervention are essential.
However, treatments and interventions for this disease remain a subject of ongoing debate. To date, many treatment methods have been reported, including various kinds of corticosteroids, vasoactive agents, hyperbaric oxygen (Dan et al., 2016) and antiviral therapies. Since the 1980s, two double-blind trials have shown the efficacy of corticosteroids, including the Wilson study. Corticosteroids have become one of the most commonly used and effective clinical treatments for SSNHL, probably due to their anti-inflammatory actions and effects on blood rheology.
Currently, there are two methods for inner ear steroids administration, namely, systemic steroid treatment (SST) and local therapy, with systemic use being the most widely accepted.
Intratympanic (IT) steroids treatment was first used on the basis of injection of streptomycin in 1956 to relieve symptoms of Meniere’s disease (Schuknecht, 1956). Bird et al. (2007) concluded that intratympanic use of dexamethasone (DEX) resulted in a 1.270-fold increase in its perilymphatic concentration along with a decrease in plasma concentrations. Since then, IT route has been used for inner ear steroids administration and become increasingly common in practice (Fig. 1), especially in patients with contraindications or resistance to systematic use of steroids. IT steroids have also often been used in rescue treatment for those who have failed regular treatments.
Fig. 1.
A schematic representation of inner ear anatomy and IT drug delivery.
Given the widespread application of IT steroids therapy, we wonder if it may be used as a first-line treatment for SSNHL. The purpose of this meta-analysis was to evaluate the efficacy and safety of IT steroids therapy as the initial treatment for SSNHL using published studies in the Chinese and English literature, as it may represent a promising technology to administer small amounts of medications and to reduce systemic adverse events.
2. Methods and materials
2.1. Literature retrieval
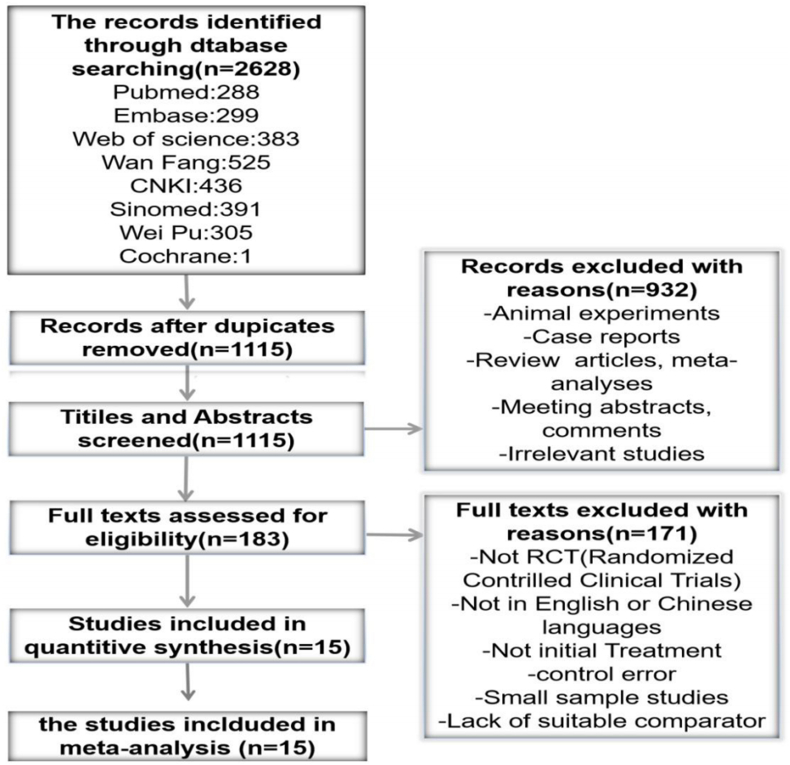
This study was designed based on the Preferred Reporting Items for Systematic Review Protocols (PRISMA-P) Statement 7 (Shamseer et al., 2015). An electronic database search was conducted to identify all available randomized controlled trials (RCTs) examining the use of corticosteroids in SSNHL. The Cochrane, EMBASE, PubMed, Web of Science, CNKI, Wanfang and Weipu electronic databases were searched for articles published before May 25, 2020. The following search terms were used: “sudden hearing loss”, “sudden deafness”, “sudden sensorineural hearing loss”, “idiopathic sensorineural hearing loss”, “idiopathic deafness”, “corticosteroids”, “dexamethasone”, “betamethasone”, “methylprednisolone”, “steroids”, “glucocorticoid”, “intratympanic”, “tympanic”, “auripuncture” and “eustachian tube”. The search was limited to the English and Chinese languages. A flow chart of this process is shown in Fig. 2.
Fig. 2.
Flow chart of articles review and inclusion.
2.2. Inclusion criteria
The research sought to compare pure-tone audiometry (PTA) improvement, recovery rate and complications between IT steroids and SST. The following inclusion criteria were used: (a) studies investigating patients with valid SSNHL diagnosis; (b) initial treatment studies; (c) while nonsteroid treatments could be combined into in therapies, steroids were the main therapy; (d) studies reporting a well-defined hearing outcomes efficacy parameter; (e) studies comparing IT (via auripuncture or eustachian catheter or tympanotomy tube) and SST (oral or intravenous) administration; (f) not restricted by region, age, race or underlying diseases (such as hypertension and diabetes); (g) RCTs.
2.3. Exclusion criteria
Studies with following characteristics were excluded: (a) underreporting results or treatment methodologies; (b) not in English or Chinese; (c) involving patients with identifiable causes of sensorineural hearing loss, such as Meniére disease; (d) involving small patient samples; (e) review articles, conference abstracts or case reports; (f) duplicate articles; and (g) a sham treatment was used as main therapy in the control group.
2.4. Data extraction and collection
All qualified studies were independently read by two reviewers (Yang and Li), including titles and abstracts, to exclude irrelevant publications. Data were summarized and analyzed according to treatment modalities: i.e. intratympanic (treatment group) and SST (control group) administration. From full text review of all eligible articles, information regarding country of origin, authorship, sample size, mean age, time to onset, side affected, sex, initial pure-tone audiometry and accompanying symptoms (such as tinnitus and vertigo) was extracted (summarized in Table 1), as well as details of treatments (e.g. type of drugs used, mode of administration and treatment time) (Table 2) and treatment efficacy (including PTA changes and adverse reactions) (Table 3). Data extraction was performed by one reviewer (Chen), and verified by another reviewer (Liu).
Table 1.
Characteristics of eligible studies.
| Study (Publication Year) | Country | Sample Size |
Mean Age |
Time to Onset(d) |
Affected Ear(R) |
Sex(F) |
With Tinnitus(n) |
With Vertigo(n) |
Initial PTA(dB) |
Diabetic population | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | T | C | T | C | T | C | T | C | T | C | T | C | |||
| [1]Chen, 2018 | China | 40 | 40 | 39.8 ± 2.4 | 39.9 ± 2.5 | NA | 19 | 19 | 19 | 20 | NA | NA | 69.4 ± 14.6 | 69.5 ± 14.7 | NA | |||
| [2]Gülce et al., 2017 | Turkey | 19 | 16 | 49.68 | 41.06 | 3.74 | 2.69 | NA | 7 | 5 | 18 | 13 | NA | NA | NA | |||
| [3]Hong et al., 2009 | Korea | 32 | 31 | 56.9 | 56.2 | NA | 14 | 13 | 19 | 20 | NA | 0 | 77.5 ± 27.6 | 79.9 ± 23.5 | NO | |||
| [4]Liang et al., 2012 | China | 60 | 50 | 21–79 | 17–78 | 8.5 | 7.5 | NA | 23 | 22 | NA | NA | 66.55 ± 16.52 | 66.2 ± 16.43 | NO | |||
| [5]Li Hui et al., 2013 | China | 38 | 38 | 45.3 ± 13.5 | 47.6 ± 15.2 | 6.34 ± 2.3 | 5.8 ± 3.5 | NA | 22 | 24 | 29 | 30 | 8 | 7 | 68.3 ± 23.5 | 63.2 ± 21.7 | NO | |
| [6]Lim, 2013 | Korea | 20 | 20 | 53.3 ± 15.3 | 51.3 ± 14.5 | 10.1 ± 8.1 | 5.4 ± 3.1 | 10 | 8 | 9 | 10 | NA | NA | 58.9 ± 31.2 | 57.8 ± 28.5 | did’t place limitation | ||
| [7]LI Zhi et al., 2015 | China | 32 | 32 | 45.2 ± 11.3 | 44.1 ± 10.5 | 4.9 ± 2.8 | 5.1 ± 1.9 | NA | 12 | 13 | NA | NA | 64.5 ± 20.1 | 65.8 ± 18.7 | NA | |||
| [8]Michael, 2018 | Greece | 34 | 35 | 53.2 ± 12.0 | 50.1 ± 17.3 | 4.6 ± 3.0 | 3.1 ± 3.0 | 20 | 18 | 16 | 15 | 31 | 32 | 13 | 16 | 81.4 ± 23.3 | 81.1 ± 28.8 | NO |
| [9]Peng et al., 2008 | China | 21 | 21 | 43.8 ± 13.4 | 42.1 ± 10.2 | 6.2 ± 2.4 | 5.2 ± 2.8 | NA | NA | NA | NA | 72.0 ± 18.6 | 69.0 ± 16.5 | NA | ||||
| 21 | 21 | 42.5 ± 11.6 | 45.2 ± 11.5 | 5.8 ± 3.5 | 5.7 ± 3.1 | NA | NA | NA | 71.0 ± 18.67 | 70.0 ± 17.6 | ||||||||
| [10]Qu et al., 2015 | China | 57 | 69 | 18–65 | 18–65 | ≤14 | ≤14 | NA | 25 | 33 | 31 | 33 | 12 | 13 | NA | did’t place limitation | ||
| [11]Tang et al., 2015 | China | 25 | 25 | 35.8 ± 9.0 | 34.2 ± 8.6 | 2.6 ± 1.3 | 2.5 ± 1.3 | 10 | 13 | 13 | 11 | 15 | 17 | 8 | 7 | 85.4 ± 5.6 | 84.8 ± 5.6 | YES |
| [12]Zhang et al., 2016 | China | 42 | 49 | 45.14 ± 13.56 | 45.22 ± 13.81 | 4.64 ± 3.63 | 4.98 ± 3.38 | 19 | 22 | 20 | 24 | 33 | 33 | 32 | 35 | 76.95 ± 15.49 | 78.08 ± 15.90 | did’t place limitation |
| [13]Rauch, 2011 | America | 129 | 121 | 51(19–85) | 52(18–80) | 7.0(6.4–7.6) | 6.7(6.1–7.4) | NA | 52 | 46 | 106 | 103 | 53 | 58 | 86.4(82.8–90.0) | 86.7(82.9–90.6) | NA | |
| [14]Kosyakov et al., 2011 | Tanzania | 24 | 25 | 49(35–52) | 40(32–53) | ≤30 | NA | 10 | 12 | NA | 4 | 6 | 41.0 ± 12.87 | 39.1 ± 16.97 | NO | |||
| [15]Dispenza et al., 2011 | Italy | 25 | 21 | 39–59 | 3–10 | 20 | 18 | 36 | 20 | 65 | 51 | NO | ||||||
T, Treatment group C, control group PTA, pure tone average NA, not available.
Table 2.
Details of treatment condition.
| Study (Publication Year) | SSNHL Definition | Drug |
Mode of administration |
Duration fo Treatment |
Dose and Frequency |
adjuvant therapy |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | T | C | T | C | T | C | ||
| [1]Chen, 2018 | At least 20 dB hearing lossin 2 CFs occurring within 3 days | MP | MP | auripuncture injection | IV | 10d | NA | 0.5~0.7 mL/d every other day for 5 times | 40 g/L:8mg/(kg·d);reduced and stopped gradually after 6 days | all patients received vasodilators and neurotrophic agents | |
| [2]Gülce et al., 2017 | At least 20 dB hearing lossin 3 CFs occurring within 3 days | DEX | P | auripuncture injection | oral | 6d | NA | 8mg/2 mL, 0.5–0.7 cc, 3 times every other day | 1 mg/kg (maximum 80 mg) and tapering 10 mg every 3 days | All patients received the standard treatment protocol of our institu_×005f tion for SSHL; intravenous low molecular weight dextran (5 cc/kg) for 5–10 days, an oral diuretic agent (acetazola mide) for a month, an oral antiviral agent (acyclovir) for 5 days, an oral vasodilator agent (betahistine), and an oral cytoprotective agent (trimetazidine) for 3 months. | |
| [3]Hong et al., 2009 | At least 20 dB hearing lossin 3 CFs occurring within 3 days | DEX | P | auripuncture injection | oral | 8d | 8d | 5 mg/mL, 0.3–0.4 cc once a day | 60 mg for 4 days followed by 40 mg for 2 days and 20 mg for 2 days | treating with other medications, such as a peripheral vasodilator and ginkgo biloba extract | |
| [4]Liang et al., 2012 | At least 20 dB hearing lossin 2 CFs occurring within 3 days | DEX | P | Eustachian tube injection | IV/oral | 10d | 37d | 5 mg/mL, every other day for 5 times | IV:10 mg/d DX for 7 days and followed by oral P 30 mg/d, reduce 5 mg every 7 days | intravenous Ginato 87.5 mg/d for 14 days and | |
| [5]LI Hui et al., 2013 | At least 20 dB hearing lossin 2 CFs occurring within 3 days | DEX | DEX | auripuncture injection | IV | 10d | 14d | 5 mg/mL, 1 mL/d once a day | 10 mg/d for 7 days, halve the dosage for another 7 days | IV:shuxuening injection 20 mL/d and Vinpocetine 30 mg/d; intramuscular injection: Vitamin1 0.1 g/d and Vitamin12 0.5 mg/d for 2 weeks | |
| [6]Lim, 2013 | At least 20 dB hearing lossin 3 CFs occurring within 3 days | DEX | P | auripuncture injection | oral | 14d | 10d | 5 mg/mL,0.3–0.4 mL, twice a week for 2 weeks, for a total of 4 times | 60 mg/d for 5 days,40 mg/d for 2 days,20 mg/d for 2 days, and 10 mg/d for 1 day | NA | |
| [7]LI Zhi et al., 2015 | At least 20 dB hearing lossin 2 CFs occurring within 3 days | Steroids | auripuncture injection | oral | 10d | 10d | 5 mg/mL, every other day for 5 times | 1mg/(kg·d) | all patients received vasodilators and neurotrophic agents | ||
| [8]Michael, 2018 | At least 20 dB hearing lossin 3 CFs occurring within 3 days | MP | P | auripuncture injection | IV/oral | 10d | 17d | 62.5 mg/mL, 0.4–0.6 mL every day for 1, 3, 5, 10 days after presentation(total of 4 times) | IV,P 1 mg/(kg·d)for 7 days followed by 0.5mg/(kg·d)for another 3 days;then oral MP:32 mg/d for 4 days followed by oral MP 16 mg/d for another 3 days | NA | |
| [9]Peng et al., 2008 | At least 20 dB hearing lossin 2 CFs occurring within 3 days | DEX | DEX | auripuncture injection | oral | 10d | 10d | 5 mg/d, once a day for 10 times | 0.75mg × 3/d for 7 dyas and 0.75 × 2 for another 2 days | IV:Low molecular dextran 500 mL, buflomedil 0.2g, intramuscular injection: Vitamin1 0.1 g/d and Vitamin12 0.5 mg/d for 10d or 20d | |
| DEX | DEX | Eustachian tube injection | IV | 10d | 10d | 5 mg/d for 10 times | 10 mg for 4 days and halve the dosage for another 6 days | ||||
| [10]Qu et al., 2015 | NA | MP | MP | auripuncture injection | IV | 10d | 7d | 40 mg/d every other day for 10 days | 80 mg/d for 4 days and reduced to 40 mg/d for another 3 days | all patients received vasodilators and neurotrophic agents | |
| [11]Tang et al., 2015 | NA | DEX | DEX | auripuncture injection | IV | 12d | 6d | 5 mg/d every 3 day for 4 times | 10 mg/d for 3 days and halve the dosage for another 3 days | IV:Cinepazide maleate 240 mg/d, ginkgo leaf extract and dipyridamole 30 mL/d, mecobalamin 0.5 mg/d;Oral:flururizine gum capsule,5 mg/d for 10 days | |
| [12]Zhang et al., 2016 | At least 20 dB hearing lossin 2 CFs occurring within 3 days | MP | P | auripuncture injection | oral | 7d | 7/14d | 0.4–1 mL/d, 7 times of treatment | 60 mg/d for 4 days, reduce 10 mg every 2 days to stop drug | IV:Ginaton 105 mg/d for 7 days or 14 days | |
| [13]Rauch, 2011 | hearing loss with onset in less than 72 h | MP | P | auripuncture injection | oral | 2W | 19d | 40 mg/mL every 3–4 days for 1 times | 60 mg/d for 14 days, followed by a 5-day taper(50 mg, 40 mg, 30 mg, 20 mg and to 10 mg) | NA | |
| [14]Kosyakov et al., 2011 | At least 20 dB hearing lossin 3 CFs occurring within 3 days | DEX | DEX | auripuncture injection | IV | 6M | 10d | 4cc for 10 days, 4 mg/d for 20 days and 4 mg 2 times a week over 5 months | 0.1mg/(kg·d) | NA | |
| [15]Dispenza et al., 2011 | At least 20 dB hearing lossin 3 CFs occurring within 3 days | P | DEX | auripuncture injection | oral | 4W | 14d | 4 mg/mL weekly for a total of four injections | 60 mg tapered | NA | |
T, Treatment group C, control group NA, not available MP, Methylprednisolone P, prednisone DEX, dexamethasone M, month d, day CF, continuous frequency PTA, pure tone average.
Table 3.
Summary of treatment outcomes.
| Study(Publication Year) | Criterion for hearing recovery |
Outcome(Recovery Rate[%]) |
PTA Before Treatment(dB) |
PTA Differences(dB) |
Frequency for PTA(kHz) |
Follow-up Period |
Results | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | T | C | T | C | T | C | T | C | ||
| [1]Chen, 2018 | >15 dB improvement in PTA | 67.5 | 37.5 | 69.4 ± 14.6 | 69.5 ± 14.7 | NA | NA | NA | injection > IV | ||||
| [2]Gülce et al., 2017 | >10 dB improvement in PTA | 87.5 | 84.2 | NA | NA | 0.5, 1.0, and 2.0 | 3M | injection = oral | |||||
| [3]Hong et al., 2009 | >15 dB improvement in PTA | 75 | 72 | 77.5 ± 27.6 | 79.9 ± 23.5 | NA | 0.5, 1, 2 and 3 | 3M | injection = oral | ||||
| [4]Liang et al., 2012 | >15 dB improvement in PTA | 91.7 | 80 | 66.55 ± 16.52 | 66.2 ± 16.43 | NA | NA | 30d | Eustachian tube injection > IV/oral | ||||
| [5]LI Hui et al., 2013 | >15 dB improvement in PTA | 84.2 | 60.5 | 68.3 ± 23.5 | 63.2 ± 21.7 | NA | 0.5, 1, 2 and 4 | 1M | injection > IV | ||||
| [6]Lim, 2013 | >10 dB improvement in PTA | 55 | 60 | 58.9 ± 31.2 | 57.8 ± 28.5 | 12.1 ± 14.6 | 12.8 ± 15.4 | 0.5, 1, 2 and 3 | 3W | injection = IV | |||
| [7]LI Zhi et al., 2015 | >15 dB improvement in PTA | 81.3 | 75 | 64.5 ± 20.1 | 65.8 ± 18.7 | 13.9 ± 17.16 | 14.8 ± 16.5 | NA | NA | injection > IV | |||
| [8]Michael, 2018 | >15 dB improvement in PTA | 70.6 | 77.1 | 81.4 ± 23.3 | 81.1 ± 28.8 | 27 | 29 | 0.5, 1, 2 and 4 | 90d | injection = IV/oral | |||
| [9]Peng et al., 2008 | >15 dB improvement in PTA | 80.95 | 61.9 | 72.0 ± 18.6 | 69.0 ± 16.5 | 43.2 ± 21.5 | 21.3 ± 16.6 | 0.5, 1, 2 and 4 | 1W | injection/Eustachian tube injection > IV/oral | |||
| 85.7 | 66.7 | 71.0 ± 18.67 | 70.0 ± 17.6 | 48.1 ± 15.2 | 27.5 ± 14.5 | ||||||||
| [10]Qu et al., 2015 | >15 dB improvement in PTA | 80.7 | 78.26 | NA | NA | NA | NA | injction = IV | |||||
| [11]Tang et al., 2015 | NA | 84 | 68 | 85.4 ± 5.6 | 84.8 ± 5.6 | 53.7 ± 5.17 | 36.6 ± 5.28 | NA | 15d | injection > IV | |||
| [12]Zhang et al., 2016 | >15 dB improvement in PTA | 76.19 | 73.74 | 76.95 ± 15.49 | 78.08 ± 15.90 | 25.88 ± 14.46 | 22.85 ± 12.37 | NA | 8W | injection = IV | |||
| [13]Rauch, 2011 | >10 dB improvement in PTA | 76.7 | 84.3 | 86.4(82.8–90.0) | 86.7(82.9–90.6) | NA | NA | 2M | injection = oral | ||||
| [14]Kosyakov et al., 2011 | >15 dB improvement in PTA | 88 | 56 | 41.0 ± 12.87 | 39.1 ± 16.97 | 24.8 ± 5.83 | 14.0 ± 3.58 | 0.5, 1, 2 and 4 | 6M | 1 month treatment, injection = IV; Long-term treatment, injection > IV | |||
| [15]Dispenza et al., 2011 | >10 dB improvement in PTA | 80 | 81 | 65 | 51 | NA | 0.5, 1, 2, and 4 | 6M | injection = IV | ||||
T, Treatment group C, control group PTA, pure tone average NA, not available.
2.5. Evaluation of studies quality
The Cochrane Collaboration Group tool was used to evaluate methodological quality of RCTs to assess the risk of bias by two reviewers (Zhou and Yang) using the Cochrane Handbook 5.4 software. In particular, the following domains were considered: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. We judged each domain as having either a low, unclear or high risk of bias.
2.6. Statistical methods
The RevMan 5.4 and Stata 15.1 software was used for data analysis. We pooled the data and used the risk difference (RD) with 95% CI to process dichotomous results: i.e. hearing recovery, and the mean difference (MD) with 95% CI for continuous measures: i.e. PTA changes in dB. Tests of heterogeneity were conducted using the chi-square test as an X2 variable.
Heterogeneity between studies was evaluated using the I2 value to represent the chance that variability between different effect estimates exceeded expectations, which was categorized as following using the Nordic Cochrane Centre (2011) reference we considered the following:
| I2 = 0–40%, no important heterogeneity; I2 = 30–60%, moderate heterogeneity; I2 = 50–90%, substantial heterogeneity; I2 = 75–100%, considerable heterogeneity. |
2.7. Subgroup variable analysis
The following potential subgroup variables were also analyzed: type of steroids (methylprednisolone [MP] or dexamethasone [DEX]), recovery criteria (>10 dB or 15 dB), follow-up time (<3 or ≥3 months), mode of drug administration and diagnostic criteria (20 dB hearing loss involving 2 or 3 consecutive frequencies).
3. Results
3.1. Search results and the characteristics of included studies
We obtained 2628 articles, of which 1513 were duplicate results and therefore discarded. Title and abstract review of the remaining 1115 studies yielded 183 full-text candidates. After excluding animal experiments, case reports, review articles, conference abstracts, comments and irrelevant studies, 15 final studies were identified as randomized studies [1–15] focusing on the efficacy of corticosteroids as the initial treatment for SSNHL (Fig. 2), published from 2008 to 2018. These studies were conducted in China [1,3,4,7,9–12], Turkey [2], Korea [6], Greece [8], the US [13], Tanzania [14] and Italy [15], involving a total of 1233 patients, with 619 in the IT group (50.2%), and 614 in the SST group (49.8%). The average patient age in these trials ranged from 34.2 to 56.9 years, and the follow-up time from 1 week to 6 months.
3.2. Risk-of-bias assessment
All included studies maintained random sequence generation. However, only 7 studies described the specific methods used, including random number tables and mechanical randomization. In one article [9], randomization was achieved based on the order of admission, which was considered an incorrect randomization method. Only 2 studies [8,13] clearly described concealment of allocation. Regarding blinding of participants and personnel, only 3 studies [3,6,13] followed the rules and clearly described their blinding procedures. Three studies [8,9,11] did not report on complications and were considered to have incomplete data. All the studies included were free of result evaluation combination bias, selective reporting bias, or other biases (Fig. 3).
Fig. 3.
Risk of bias analysis.
3.3. Meta-analysis
In the 15 included studies, DEX, MP and prednisolone were used in intratympanic therapies through auripuncture or eustachian tubes, and administered orally or intravenously. The data from these 15 studies were pooled for meta-analysis.
3.3.1. Treatment outcome analysis
Based on descriptions in each study, we extracted complete or partial recovery rates to provide a recovery rate for evaluation. The treatment status and prognostic information are listed in Table 2, Table 3
Dosage of orally or intravenously administered corticosteroids (prednisone, DEX and MP) was 10–60 mg/d for 6–14 days, while that administered intratympanically was 0.3–40 mg/injection once a day or once every 2 days for 6 days to 4 weeks. The dosage of intratympanic steroid treatment was significantly lower than that of systemic administration but typically required a longer course of treatment.
Forest plots were created to depict results of individual studies along with summarized results derived from meta-analysis, showing significantly improved hearing in patients receiving IT steroids compared with patients receiving SST.
Fig. 4a compares the overall efficacy represented by the recovery rate between IT steroids and SST. With I2 = 35% < 40% and P = 0.08 < 0.10, indicating minimal heterogeneity and reasonable random-effects model, the summarized hearing recovery RD at 0.08 (95% CI: 0.01–0.14, P = 0.02) indicated better total effective rate with IT steroids than with SST.
Fig. 4.
Meta-analysis forest map comparing the rate of hearing recovery and mean PTA gain (in dB) between IT steroids and SST.
When comparing PTA changes (pre/post treatment differences) between the IT and SST groups in 4 studies after excluding studies with high heterogeneity (Fig. 4b), the random-effects model used due to high heterogeneity and justification (P = 0.0003, I2 = 81%). The pooled PTA MD was 10.43 dB (95% CI: 3.68–17.18 dB), again indicating better PTA improvement with IT steroids than with SST.
3.3.2. Side-effects
Ten of the 15 studies reported adverse reactions (473 in the IT group and 475 in the control group), including 1 study [10] that reported absence of side effects in either group (Fig. 5).
Fig. 5.
Adverse events comparison between IT and systemic steroids groups.
The main adverse effects reported in the SST group were sleep loss (n = 69), and fluctuations in blood glucose and blood pressure (n = 36). Other side effects included mood swings, appetite changes, increased thirst or dry mouth and gastrointestinal distress. After discontinuing steroids, most of these symptoms gradually disappeared, although some patients needed drug intervention. For example, the 34 patients with blood glucose and pressure fluctuations reported by Tang [11], Li [5], and Zhang [12] needed drug intervention, especially for those with diabetes and hypertension. The 5 patients with gastrointestinal symptoms reported by Tang [11] needed antacid therapy. Rauch [13] reported a serious complication in 2011 as a mild deterioration of preexisting renal insufficiency in a patient with type 2 diabetes.
The IT group experienced typical side effects of topical treatments, with transient earache being the most common [13]. Short-term caloric vertigo and ear infections were also reported. However, most of these side effects were surgery related and short term. Eardrum perforation was reported in 6 cases [12,13], and persisted after 6 months in 1 case [12]. However, no other serious or life-threatening side effects were reported.
In short, besides a risk of local side effects, such as earache and transient vertigo, fewer complications were noted in the IT group as compared with the SST group.
3.4. Subgroup variable analysis
Analysis of subgroup variables such as types of steroids used, criteria of recovery, follow-up time, medication use and diagnostic criteria showed mixed effect sizes (Fig. 6).
Fig. 6.

Meta-analysis forest map of subgroup variables.
3.4.1. Types of steroids used
As shown in Fig. 6a, studies using MP exhibited substantial heterogeneity (I2 = 79%, P = 0.03), while those using DEX exhibited no significant heterogeneity (I2 = 0%, P = 0.91). There were no obvious differences in treatment outcomes noted between the two types of steroids in IT vs SST comparison (MP, RD = 0.15, 95% CI = −0.12–0.42; DEX, RD = 0.22, 95% CI = 0.12–0.33), suggesting that IT steroids might be a superior treatment modality for SSNHL, regardless of the type of steroid.
3.4.2. Criteria of recovery
The studies were divided into two groups based on their criteria (definition) for hearing recovery: i.e. >10 dB or >15 dB. IT vs SST hearing recovery RD was −0.06 (95% CI = −0.14-0.02, I2 = 0%) for studies using the >10 dB criterion and 0.10 (95% CI = 0.04–0.17, I2 = 22%) for those using the >15 dB criterion (Fig. 6b), although spontaneous recovery would be more difficult to be excluded for studies using the >10 dB criterion, as some researchers have reported spontaneously recovery independent of medical treatment in some patients (Mattox and Simmons, 1977).
3.4.3. Follow-up time
When comparing studies with follow-up time <3 months to those with follow up time ≥3 months, the IT vs SST hearing recovery RD was 0.09 (95% CI: 0.01-0.18, I2 = 52%) for those with short follow up times vs 0.05 (95% CI: 0.09-0.18, I2 = 41%) for those with longer follow up times (Fig. 6c), showing no significant differences.
3.4.4. Modality of steroids administration
Comparison of steroid administration modalities showed: (a) hearing recovery RD was 0.11 (95% CI: 0.02-0.24, I2 = 74%) when the auripuncture route was compared to intravenous administration; (b) the RD was 0.08 (95% CI: 0.01-0.16, I2 = 0%) when auripuncture injection was compared to oral administration; and (c) the RD was 0.13 (95% CI: 0.02–0.25, I2 = 0%) when injection via eustachian tube was compared to intravenous administration, indicating that different routes of steroid administration are not a source of heterogeneity (Fig. 6d).
3.4.5. Diagnostic criteria
The IT vs SST hearing recovery RD was 0.12 (95% CI = 0.03–0.20, I2 = 33%) when 20 dB hearing loss at 2 continuous frequencies (CFs) within 3 days was used as the diagnostic criteria and 0.06 (95% CI: 0.07-0.19, I2 = 34%) when 20 dB hearing loss at 3 CFs within 3 days was used, showing that this factor did not completely negate the study.
3.5. Sensitivity analysis
When each study was sequentially excluded based on the rule of omission to assess stability of final results, we found that no individual study alone significantly affected the pooled risk estimate (Fig. 7a).
Fig. 7.
Sensitivity analysis and funnel plot of hearing recovery rate for IT with systemic steroids treatments.
3.6. Publication bias
Potential meta-analysis biases were evaluated by funnel plots, as shown in Fig. 7b. The essentially symmetrically distributed dots indicated no obvious publication bias in our results.
4. Discussion
Etiologies and pathophysiological mechanisms of SSNHL have not been completely elucidated. Nonetheless, steroid therapy has been the recommended first-line treatment for this disease (Seth et al., 2019), with long records of safety and efficacy. When SST fails, IT steroids has commonly been used as a rescue treatment, although in recent years, an increasing number of clinicians have started to use it as an initial treatment, with its efficacy still being controversial.
4.1. Condition of existing studies
A number of systematic reviews and meta-analyses studies on steroid treatment of SSNHL exist, of which 7 compared effects of IT steroids with SST as the initial treatment (Mirian and Ovesen, 2020; Garavello et al., 2015; Han et al., 2017; Lai et al., 2017; Qiang et al., 2017; Ding et al., 2013; Chen et al., 2015). Mirian and Ovesen (2020), Garavello et al. (2015) and Han et al. (2017) concluded that there were no statistically significant differences between the two treatment modalities, while 3 other studies (Han et al., 2017; Lai et al., 2017; Chen et al., 2015) showed better outcomes with IT steroids, similar to the current study. Chen et al. found a significant difference in the effective rate between IT and intravenous steroid therapies (RR = 1.17, 95% CI: 1.02–1.34, P < 0.05), but not between IT and oral steroid administration (RR = 1.15, 95% CI: 0.92–1.42, P > 0.05). Ding et al. reported no significantly different rates of recovery between patients receiving systemic and IT steroids (RR = 1.11 95% CI: 0.96–1.28, P = 0.15), although a difference was noticed between intraperitoneal and systemic steroids in diabetic patients (RR = 1.24, 95% CI = 1.02–1.50, P = 0.03).
4.2. Strengths and weaknesses of the study
The current meta-analysis study included RCTs in English and Chinese literatures for the first time and analyzed subgroup variables such as type of drugs, recovery criteria, follow-up time, steroid administration routes and diagnostic criteria. Literature search was comprehensive involving 8 large databases with a systematic review following PRISMA guidelines, including systematic evaluation of quality and treatment outcomes in all studies published to date. Given emergence of new evidence regarding IT steroids for SSNHL, we believe that this route is more effective than SST.
However, the following limitations regarding this study need to be considered. First, the research included in this article exhibits a risk of bias. Most articles did not explain their specific methods of randomization and concealment. Second, we could not adjust for the heterogeneity implied by the use of different assessment schemes nor could we adjust for differences at baseline, including (a) age, (b) dosage and duration of treatment, (c) time to treatment start, and (d) audiometric pattern/severity. We were unable to analyze these factors due to the small sample size and lack of detailed classification in the original literature. Third, the possibility of spontaneous recovery unrelated to treatment cannot be excluded. Fourth, some of the studies combined non-steroid therapies as part of the treatment regimen, including vasodilators and vasoactive substances. The effects of these substances on SSNHL has not been proven, but they may potentially affect the outcomes and accuracy of analysis to some extent. The medications are all listed in Table 2. Given the variations in dosage, types of drug, course of treatment and administration routes across the studies, it was difficult to determine how they could affect outcomes or was a source of heterogeneity. Finally, few high-quality studies on IT steroids as the initial treatment for patients with diabetes or hypertension are available, making analyzing these subgroups variables impossible.
4.3. Future practice and research
From our study, we suggest that future studies need to focus on (a) recording hearing recovery based on classifications of audiometric pattern/severity (b) taking age and underlying conditions (e.g. diabetes and hypertension); into consideration when grouping subjects, with corresponding standard treatment protocols; (c) quantifying adverse side effects using appropriate scales for comprehensive and complete assessment; (d) establishing standardized follow-up criteria in large sample studies to evaluate short- and long-term treatment impact and safety; (e) standardizing and improving recovery criteria to allow comparability between trials; and (f) comparing various techniques of inner ear steroids delivery through the tympanic cavity. The traditional auripuncture injection has been reported to be associated with loss of drug solution through the eustachian tube, shortening inner ear exposure time. Moreover, this approach is invasive and requires surgical skills. New techniques and tools allowing direct steroids delivery to the round window membrane and taking advantage of the eustachian tube route are therefore being studied. As more data become available, new IT steroids delivery techniques will allow clinicians to apply local drug delivery systems designed for specific groups of patients for improved tissue targeting and drug delivery efficacy.
5. Conclusion
In summary, this meta-analysis review evaluated the correlation between different steroids administration routes in initial SSNHL treatment involving 1233 patients and showed better efficacy with IT steroids than with SST, regardless of type of steroids, diagnostic criteria, follow-up time and mode of administration. Nonetheless, further prospective, randomized and multicenter studies are needed to confirm these findings.
In practice, patients receiving oral drug treatment need only one visit for evaluation and prescription, while patients receiving IT steroids require multiple visits and remaining in a lying down position for 30 min after each injection (Rauch, 2011). Due to the cost of surgery and transportation, intratympanic steroid therapy is more expensive than oral steroids. Therefore, clinicians should fully explain the advantages and disadvantages of both methods and carefully choose the appropriate approach according to the patient’s wishes.
Declaration of competing InterestCOI
All authors have no conflicts of interest relevant to this paper.
Funding
None.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Contributor Information
Ting Yang, Email: 18191619266@163.com.
Hui Liu, Email: liuhui1105@163.com.
Fangyao Chen, Email: chenfy2017@hotmail.com.
An Li, Email: li_an0835@163.com.
Zhou Wang, Email: ikrush@11.com.
Shuangyuan Yang, Email: 18701586769@163.com.
Shiyu Yang, Email: yangshiyu0316@163.com.
Wen Zhang, Email: smileww@foxmial.com.
References
- Bird P.A. Intratympan ic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol. Neurotol. 2007;28(8):1124–1130. doi: 10.1097/MAO.0b013e31815aee21. [DOI] [PubMed] [Google Scholar]
- Chen P. Intratympanic versus systemic steroid initial treatment for idiopathic sudden hearing loss: a Meta-analysis. J Clin Otothinolaryngol Head Neck Surg(China) 2015;29(22):1970–1977. [PubMed] [Google Scholar]
- Chen Comparation of clinical effect of intratympanic injection and intravenous infusion of methylprednisolone in the treatment of sudden deafnes. Chin. Community Doct. 2018;34(17):47–49. [Google Scholar]
- Dan Zhao. A comparison of effects of systemic and intratympanic steroid therapies for sudden sensorineural hearing loss:A meta-analysis. J. Otol. 2016;11:18–23. doi: 10.1016/j.joto.2016.02.002. 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X. Intratympanicversussystem icsteroid treatm entfor idiopathicsudden hearing loss: a Meta-analysis. Chin. J. Otorhinolarygol. Head Neck Surg. 2013;48(5):412–416. [PubMed] [Google Scholar]
- Dispenza Francesco. Treatment of sudden sensorineural hearing loss with transtympanic injection of steroids as single therapy: a randomized clinical study. Eur. Arch. Oto-Rhino-Laryngol. 2011;268(9):1273–1278. doi: 10.1007/s00405-011-1523-0. [DOI] [PubMed] [Google Scholar]
- Garavello W. Intratympanic steroid treatment for sudden deafness: a meta-analysis of randomized controlled trials. Otol. Neurotol. 2015;33(5):724–729. doi: 10.1097/MAO.0b013e318254ee04. [DOI] [PubMed] [Google Scholar]
- Gülce E. Sudden hearing loss: an efectivity comparison of intratympanic and systemic steroid treatments. Eur. Arch. Otorhinolaryngol. 2018;274(10):3584–3591. doi: 10.1007/s00405-017-4691-8. [DOI] [PubMed] [Google Scholar]
- Han Xue. Combined intratympanic and systemic use of steroids as a first-line treatment for sudden sensorineural hearing loss: a meta-analysis of randomized, controlled trials. Otol. Neurotol. 2017;38(4):487–495. doi: 10.1097/MAO.0000000000001361. [DOI] [PubMed] [Google Scholar]
- Hong Seok Min. Hearing outcomes of daily intratympanic dexamethasone alone as a primary treatment modality for ISSHL. OTOLARYNG HEAD NECK. 2009;141(5):579–583. doi: 10.1016/j.otohns.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Hui Li. The effect of tympanic dexamethasone injection in the treatment of sudden deafness. J. Audiol. Speech Pathol. 2013;21(5):539–541. [Google Scholar]
- Kleyn A. Sudden complete or partial loss of function of the octavus-system in apparently normal persons. Acta Otolaryngol. 1994;32(5–6):407–429. [Google Scholar]
- Kosyakov Sergey. Intratympanic steroids for sudden sensorineural hearing loss. J. Int. Adv. Otol. 2011;7(3):323–332. [Google Scholar]
- Lai D. Intratympanic glucocorticosteroid therapy for idiopathic sudden hearing loss: meta-analysis of randomized controlled trials. Medicine (Baltim.) 2017;96(50) doi: 10.1097/MD.0000000000008955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Dongyong. Comparison of the effects of two different administration routes with steroids on sudden hearing loss. Youjiang Med. J. 2012;40(4):513–514. [Google Scholar]
- Lim H.J. Efficacy of 3 different steroid treatments for sudden sensorineural hearing loss: a prospective, randomized trial. Otolaryngol. Head Neck Surg. 2013;148(1):121–127. doi: 10.1177/0194599812464475. [DOI] [PubMed] [Google Scholar]
- Mattox D.E., Simmons F.B. Natural history of sudden sensorineural hearing loss. Ann. Otol. Rhinol. Laryngol. 1977;86(4 Pt 1):463–480. doi: 10.1177/000348947708600406. [DOI] [PubMed] [Google Scholar]
- Michael T. Systemic, intratympanic and combined administration of steroids for sudden hearing loss. A prospective randomized multicenter trial. Eur. Arch. Oto-Rhino-Laryngol. 2018;275(1):103–110. doi: 10.1007/s00405-017-4803-5. [DOI] [PubMed] [Google Scholar]
- Mirian C., Ovesen T. Intratympanic vs systemic corticosteroids in first-line treatment of idiopathic sudden sensorineural hearing loss: a systematic review and meta-analysis. JAMA OTOLARYNGOL. 2020;146(5):421–428. doi: 10.1001/jamaoto.2020.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann Katrin. Newborn and infant hearing screening facing globally growing numbers of people suffering from disabling hearing loss. Int J Neonatal Screen. 2019;5(1):7. doi: 10.3390/ijns5010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Yikun. Clinical investigation of different routes of administration of dexamethasone on sudden deafness. J clin Otorhinolaryngol Head Neck Surg(China) 2008;22(10):442–445. [PubMed] [Google Scholar]
- Qiang Qingfen. A comparison between systemic and intratympanic steroid therapies as initial therapy for idiopathic sudden sensorineural hearing loss: a meta-analysis. Acta Otolaryngol. 2017;137(6):598–605. doi: 10.1080/00016489.2016.1260157. [DOI] [PubMed] [Google Scholar]
- Qu Yongtao. Analysis the treatment of sudden sensorineural hearing loss with steroid from different administration routes. J clin Otorhinolaryngol Head Neck Surg(China) 2015;29(4):324–326. [PubMed] [Google Scholar]
- Rauch S.D. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA, J. Am. Med. Assoc. 2011;305(20):2071–2079. doi: 10.1001/jama.2011.679. [DOI] [PubMed] [Google Scholar]
- Schuknecht H.F. Ablation therapy for the relief of Meniere ’s disease. Laryngoscope. 1956;66:859–870. doi: 10.1288/00005537-195607000-00005. [DOI] [PubMed] [Google Scholar]
- Seth R., Schwartz, Zeitler Daniel. Clinical practice guideline: sudden hearing loss (update) for audiology. Hear. J. 2019;72(12):S1–S45. doi: 10.1177/0194599819859885. [DOI] [PubMed] [Google Scholar]
- Shamseer Larissa. Preferred reporting items for systematic review and meta analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349(2):1–25. doi: 10.1136/bmj.g7647. g7647. [DOI] [PubMed] [Google Scholar]
- Tang zhiping. The effectiveness of intratympanic steroid therapy on the treatment of idiopathic sudden sensorineural hearing loss patients with damaged glucose tolerance. J. Audiol. Speech Pathol. 2015;23(2):160–162. [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration . The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2011. Review Manager (RevMan). 5.1. [Google Scholar]
- Zhang yu. The efficacy of continuously treatment with intraympanic glucocorticoid injection for sudden deafness. J. Journal of Audiology and Speech Pathology. 2016;26(2):184–187. [Google Scholar]
- Zhi Li. Siphon method allowed to give glucocorticoid treatment of sudden deafness clinical observation. Shaanxi Med. J. 2015;44(7):868–869. [Google Scholar]