Abstract
At different parts of the world, Red Seaweeds are one component of human diets especially at Southeast Asia. Red Seaweeds structurally contain bioactive molecules so; we studied the effect of Chondrus crispus on increasing the male albino rat fertility. Twelve male albino rats are used in this study as two group pre-treated group and post- treated one each with 6 animals. The pretreated group was dissected before the post-treated group injection. Each post treated rat injected intramuscular with 1 mg of Chondrus crispus with dose 0.1 ml/ twice per week for 48 day (Mukhtar et al., 2013).
The results showed that increasing on the total testosterone levels insignificantly, sperm motility significantly, and decreasing in both FSH and DPPH levels insignificantly and significantly for the MDA levels in the post-treated group. The morphological appearance and histological examination for the sperm, testis and liver were normal as the pretreated group. The molecular studies showed absence of any DNA fragmentation for the testis of both group.
The Red Seaweed has an enhanced effect in the testicular function of the animal which might increase their fertility and sexual activities.
Keywords: Red Seaweed, Chondrus crispus, Fertility, Albino rat, Testis
1. Introduction
Many species of marine algae contain significant quantities of complex structural sulphated polysaccharides that have been shown to inhibit the replication of enveloped viruses. Other compounds, both of red algae (e.g., the lectin griffithsin and the phycocolloid carrageenan), and other sulphated polysaccharides extracted from green algae (i.e., ulvans) and brown algae (i.e., fucoidans) could be potential antiviral therapeutic agents against SARS-CoV (Pereira and Critchley, 2020).
Seaweeds as brown, red and green in several parts of the world are important component in human diets especially in Southeast Asia (Lee et al., 2011, Nwosu et al., 2011). From seaweeds, there are different biological activities compounds, such as anti-microbial, anti-inflammatory, anti-coagulant and anti-obesity, anti-oxidant, anti-cancer, activities (Liu et al., 2013, Mohamed et al., 2012). Also, in whole animals' studies, seaweeds made neuro-protective action against β-amyloid toxicity (Suganthy et al., 2010). A cultivated Chondrus crispus (Irish Moss) used in feeding at several parts around the world with significant beneficial bioactivity (Sangha et al., 2013) and contain various polysaccharides as carrageenans, fatty acids and sterols (Tasende, 2000). Chondrus crispus contain bioactive peptides and prebiotics which is evidence for their health benefit (Harnedy and FitzGerald, 2011, Lordan et al., 2011). Methanolic extraction of chondrius crispus (CCME) showed protection against the oxidative stress in worm (Tânia Melo et al., 2015).
Historically, some data found from 1830s about Irish moss or carrageen which is a mixture of naturally co-occurring Chondrus crispus and Mastocarpus stellatus. This mixture has a large number of medical applications which still used in Ireland to make traditional medicinal teas and cough medicines to treat colds, bronchitis and chronic coughs as it has mucus dislodging action and also has antiviral properties (Pereira, 2018).
Here we aim to study the effect of Chondrus crispus on increasing the male albino rat fertility.
2. Materials and methods
2.1. Grouping animals
Rattus norvegicus were used in this study as they characterized by their genetic stability and a very low rate of spontaneous malformation (Tuchmann- Duplessis, 1977). The animals were selected from a pure strain so that the genetic influence was kept at constant and uniform level. The standard guidelines of National Organization for Drug Control and Research (NODCAR) were used in handling animals. Twelve male albino rat selected with weight of (150–200 gm). Animals will have free access to food and water ad libitum. They will be maintained at 21–24 °C and 40–60% relative humidity with 12-h light–dark cycle. All animals’ procedures will be performed in accordance to the institutional Ethics Committee and in accordance with the recommendations for the proper care and use of laboratory animals. Unnecessary disturbance of animals will be avoided. Animals will be treated gently; squeezing, pressure and tough maneuver will be avoided.
2.2. Preparation of Chondrus crispus
Chondrus crispus were collected from the shore of Red Sea, Hurgada, Egypt, and then the algae washed to remove any salts or the sands, dried in a vaccum dryer at temperature of 30 °C until it has completely dried. Algae were milled to obtain a fine powder, mixed with ethanol in a concentration of 1/50 ml ethanol. The mixture macerated for three days with frequent shaking, stirred for 6 h, sonicated for three hours. The mixture then filtered, ethanol evaporated in a rotary evaporator (lab first scientific, China) at temperature of 45 °C at a speed of 50 rpm.
The dry extract then was mixed with buffer pH 7 composed of Na2HPO4, KH2PO4, NaCl, KCl, added and stirred for 2 h, propylene glycol was added in a concentration of 1% as a solvent. The final concentration was 10 mg per ml; each 0.1 ml contains 1 mg of Chondrus crispus.
2.3. Experimental design
Twelve animals are randomly chosen and divided into two groups. The 1st group (called pretreated) was kept as a normal control and received buffer intra-muscularly to examine safety of the these buffer (composed of Na2HPO4, KH2PO4, NaCl, KCl stirred for 2 h, propylene glycol was added in a concentration of 1% as a solvent) twice per week for 48 day (Mukhtar et al., 2013) then we scarifies them. After that, the 2nd group (called post-treated) started injected intra-muscularly with 1 mg of Chondrus crispus with dose 0.1 ml twice per week (Collén et al., 2014) for 48 day (Mukhtar et al., 2013).
2.4. Effect of the Chondrus crispus on male fertility
The effect of the Chondrus crispus on male fertility was studied by epididymal spermatozoa as well as histopathological examination.
2.5. Sperm analysis
A droplet from epididymal content added to a droplet of sodium citrate solution (2.9%) on clear glass slide then the slide was gently warmed. Many fields examined using light microscope with magnification (X100) to estimate the percentage of progressive sperms motility according to Bearden and Fluquary (1980).
Sperm concentration performed by using pipette of counting erythrocytes (haemocytometer) according to Bearden and Fluquary (1980). The epididymal content drawn up to the mark 0.1 then the pipette filled up to the mark 101 by sodium citrate (0.9%) solutions. The content of the pipette mixed and shacked well. One drop of diluted epididymal content spread between the haemocytometer chamber and the cover slide and count as R.B.C counting chambers.
Epididymal sperm abnormalities: for each rat, a droplet from epididymal content took immediately and mixed with one drop of Eosin-Nigrosin stain for detection of dead and malformed sperm. Then films examined at random per slide under × 200 and the type (live and dead). Also, percentage of sperms abnormalities recorded.
2.6. Histopathological examination
The rat's testis and livers were fixed in 10% formaldehyde. Prepared the paraffin sections with thickness (4–5 μm) then stained with hematoxylin and eosin (H&E) stain. The sections examined under light microscopy for histological changes according to method described by Banchroft et al. (1996).
2.7. Total testosterone and follicle stimulating hormone (FSH)
Blood collected from rats to estimate sex hormones serum levels. Serum separated into clean bottles, stored frozen and then used for the estimation of testosterone and folliclestimulating hormone (FSH) (Tatli-Çankaya et al., 2014).
2.8. Malondialdehyde (MDA) measurements
Reagents and chemicals: TBA (Thiobarbituric acid) purchased from BDH (England), the MDA salt (malon dialdehyde tetra-butyl ammonium salt 96%) and methanol (99.8%) both from Sigma-Aldrich (Germany), glacial acetic acid (99–101%) from Daejung (Korea), ultra-pure deionized double distilled water with<5 mΩ was used. Other chemicals and reagents were of an analytical standard with high purity at NODCAR laboratories.
Preparation of TBA Reagent: standard solution of 4.0 mM of TBA was prepared in glacial acetic acid by 57.66 mg of TBA dissolved in 100 ml of glacial acetic acid. Freshly solution of TBA prepared daily.
Analytical Procedure
We take 0.1 ml of homogenate in a 10 ml test tube and mixed with TBA (1 ml). The mixture heated in a boiling bath water at 95 °C for 60 min. The test tubes were cooled at room temperature and then we measure the absorbance at 532 nm against blank using UV–visible spectrophotometer.
Concentration was calculated from standard curve concentration of MDA from 5 to 35 ng.
2.9. 2,2-diphenyl 1- picrylhydrazyl (DPPH) evaluation
0.5 ml of homogenate was added to 1.5 ml of DPPH reagent, incubated at 37 °C in incubator for 30 min. The initial absorbance of blank or the DPPH solution was measured against air (purple colour).
The absorbances, then were read against air to find the decrease in the absorbances, correlated with the electron scavenging activity and the color has changed from purple to yellowish purple.
The electron scavenging activity calculated as follow
2.10. DNA fragmentation methods
2.10.1. Extraction of genomic DNA
The DNA fragmentation assay (DNA extraction and detection of apoptosis) were done by the methods of Salting out extraction which used by Aljanabi and Martinez (1997) with some modifications of Hassab El-Nabi et al. (2004).
2.10.2. DNA fragmentation assay
These made by using agarose gel electrophoresis as the DNA (isolated genomic) for the experimental animals were fractionated on 1.8% agarose gel electrophoresis according to Surzycki, 2000 then about 15 μg of the sample with 5 μl of the loading dye and marker DNA were loaded carefully in to the respective wells without disturbing the gel. Then conduct the electrophoresis at 50 mV, the agarose gel was photographed using gel doc.
2.11. Statistical analysis
All the Statistical analysis values were presented as means ± standard errors of the means (S.E.M.) by using unpaired t-test at level of significance P < 0.05. Graph Pad Software Instate (version 5) was used to carry out the statistical tests (Armitage and Berry, 1987).
3. Results
3.1. Sperm analysis
3.1.1. Morphological examination
Normal sperm is highly motile body. It consists of (1) head which is pyriform shape and mainly formed of nucleus. The anterior of the nucleus is covered by the acrosomal cap, (2) the mid-piece contains tightly packed mitochondria and (3) the tail which is formed of the axial filament. Both the pretreated and post-treated groups have large percentages of normal morphological appearance for the sperms. The head abnormalities are significantly decreased in post-treated group by 51% than the pre-treated group. Also, coiled tails and dead sperms are decreased insignificantly in post-treated group than pretreated one (Table 1).
Table 1.
Each value indicates the mean ± S.E.M. Statistical analysis was carried out by using unpaired t-test at level of significance P < 0.05. Pre-treated group received buffer intramuscularly twice per week for 48 days, Post-treated group injected intramuscularly with 1 mg of Chondrus crispus with dose 0.1 ml/ twice per week for 48 days.
| Groups |
Parameter |
||
|---|---|---|---|
| Head abnormalities (%) | Coiled tail (%) | Dead sperm (%) | |
| Pre-treated | 2.602 ± 0.3397 | 1.382 ± 0.1474 | 1.297 ± 0.1877 |
| Post-treated | 1.273 ± 0.2101* | 1.896 ± 0.2523 | 0.8270 ± 0.2368 |
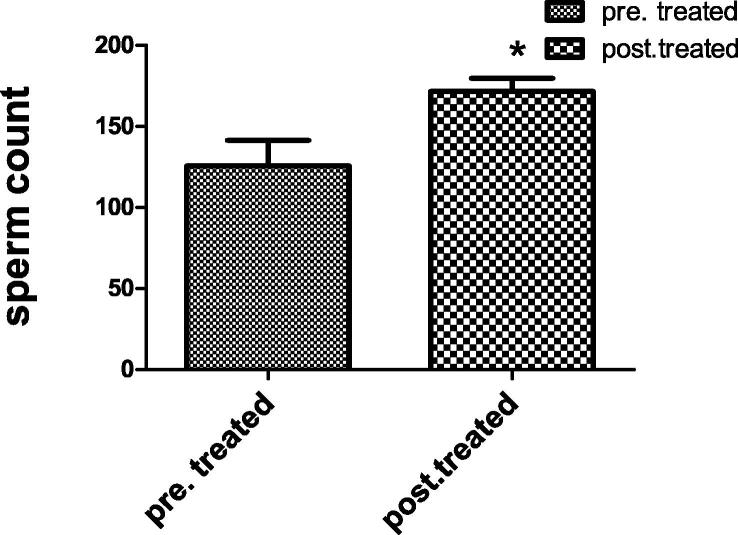
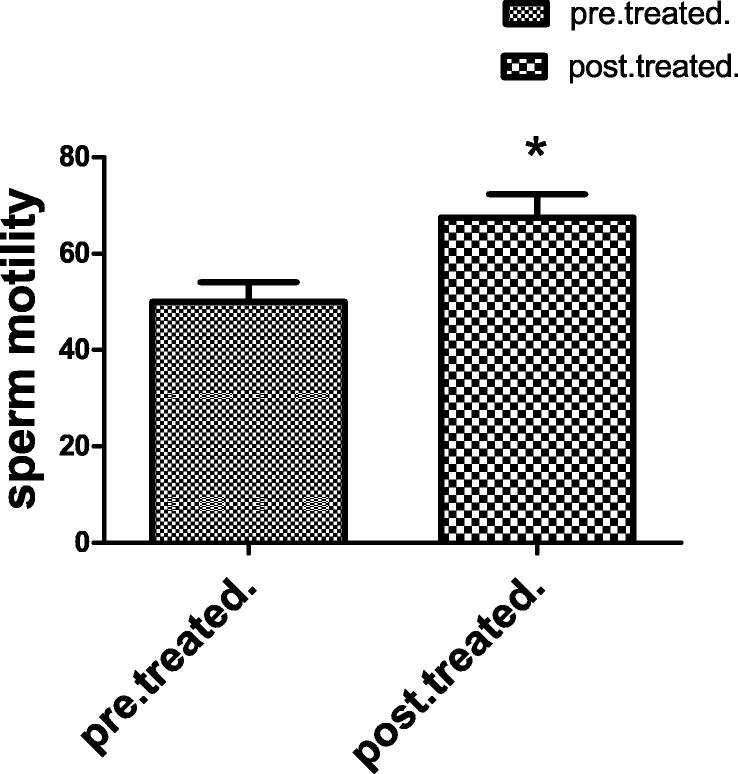
3.1.2. Sperm motility and count
The sperm count and motility for post-treated group increases significantly by 36.6% and 35% than pre-treated group respectively (Fig. 1, Fig. 2).
Fig. 1.
Each value indicates the mean ± S.E.M. Statistical analysis was carried out by using unpaired t-test at level of significance P < 0.05. Pre-treated group received buffer intramuscularly twice per week for 48 day, Post-treated group injected intra muscular with 1 mg of Chondrus crispus with dose 0.1 ml/ twice per week for 48 day.
Fig. 2.
Each value indicates the mean ± S.E.M. Statistical analysis was carried out by using unpaired t-test at level of significance P < 0.05. Pre-treated group received buffer intramuscularly twice per week for 48 day, Post-treated group injected intra muscular with 1 mg of Chondrus crispus with dose 0.1 ml/ twice per week for 48 day.
3.2. Histopathological examination
3.2.1. Testis examination
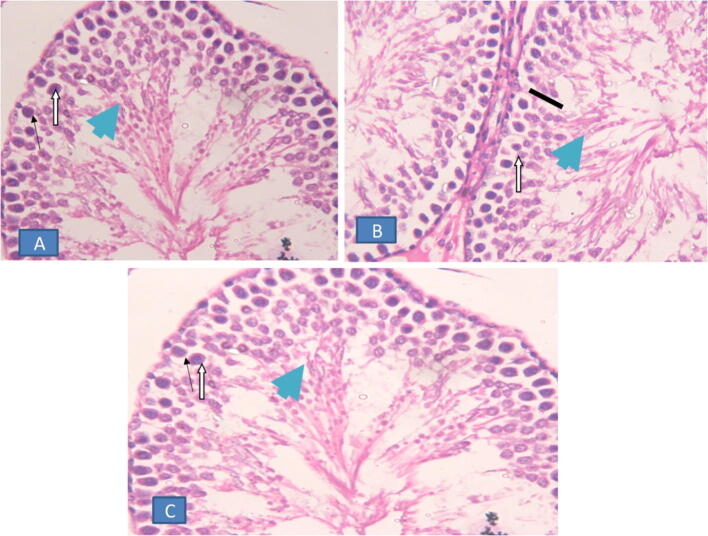
The histological architecture of testes of pre-treated and post-treated groups (Fig. 3) is covered with a connective tissue coat, the tunica alboginea; which is followed by a thin layer of highly vascular loose connective tissue, the tunica vasculosa. The testes are composed of a large number of seminiferous tubules which appear as round or oval structure. Each tubule consists of a germinal epithelium (containing spermatogenic stages) resting on basement membrane and is surrounded by a thin connective tissue layer. The seminiferous tubules are long and extremely convoluted. Each seminiferous tubule is lined with germinal epithelium resting on a thin basement membrane and consisting of several rows of spermatogenic cells with supporting cells of Sertoli cells scattered in between. The spermatogenic cells include the successive stages of spermatogenesis i.e. spermatogonia, primary spermatocytes, secondary spermatocytes, spermatides and spermatozoa.
Fig. 3.
Histological section of testis: (A) normal pre-treated rats. (B &C) post-treated group. Both groups showed normal spermatogenesis where, normal spermatogonia (black thin arrow), normal spermatocytes (white block arrow), and normal spermatides (arrow head) (H&E X40).
Spermatogonia are considered as the mother cells of the whole series of spermatogenic stages. They are numerous rounds or hemispherical cells lying adjacent to the basement membrane of the seminiferous tubules. The primary spermatocytes are produced by the growth of the daughter spermatogenic cells observed in seminiferous tubules.
The secondary spermatocytes are much smaller than the primary spermatocytes and possess proportionally smaller nuclei .which are round or ovoid in shape and contain one or more nucleoli. The spermatides are the smallest spermatogenic cells that constitute the most superficial layers of the germinal epithelium and are lying usually in groups close to the lumina of seminiferous tubules. The spermatozoon is specialized cells that are efficiently packed to carry the paternal DNA condensed in the head to the ovum clusters of spermatozoa are found in abundance with their deeply staining heads directed towards and embedded in the cytoplasm of the Sertoli cells.
3.2.2. Liver examination
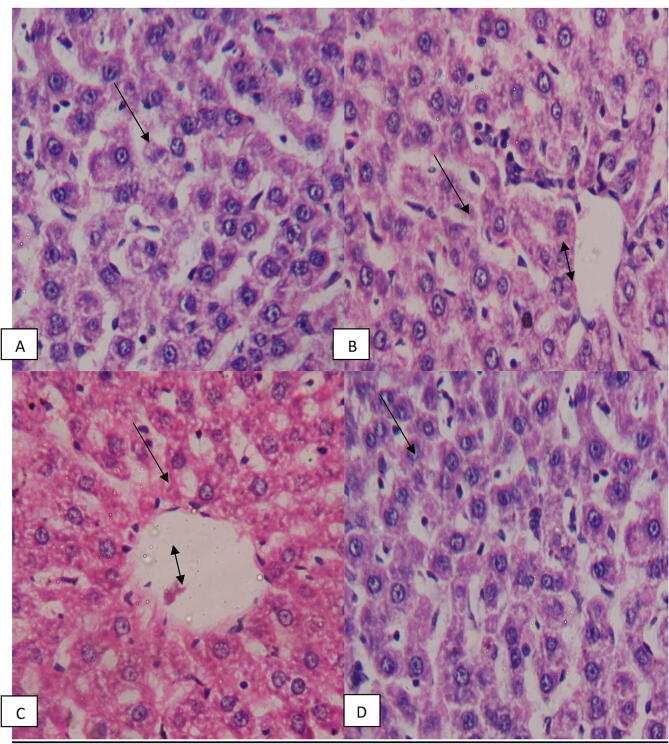
The pre-treated group and post-treated group (Fig. 4) shows hepatic lobules which are separated from each other by hardly distinct connective tissue. It is covered with thin connective tissue. Each hepatic lobule contains a central vein from which radiate branching cords or strands of hepatic cells.
Fig. 4.
Histological section of liver: (A &B) pre-treated group, (C & D) post-treated rats, both showing normal archicture, normal central vien (double head arrow) and normal hepatocytes.
The hepatic lobules are composed of hepatocytes arranged in cords or strands, each being one or two cells thick. Each cell possesses homogeneous finely granulated acidophilic cytoplasm and a large spherical nucleus containing one or more distinct nucleoliand chromatin.
3.3. Biochemical analysis
3.3.1. Total testosterone and follicle stimulating hormone (FSH)
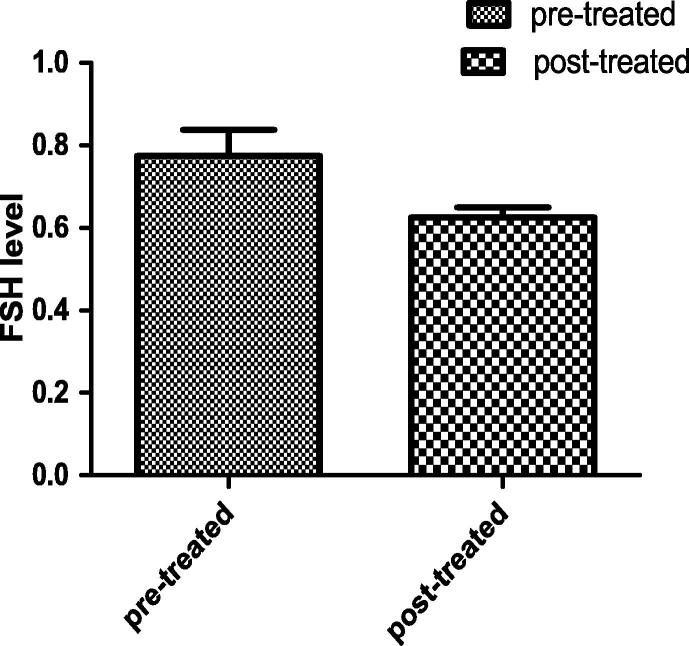
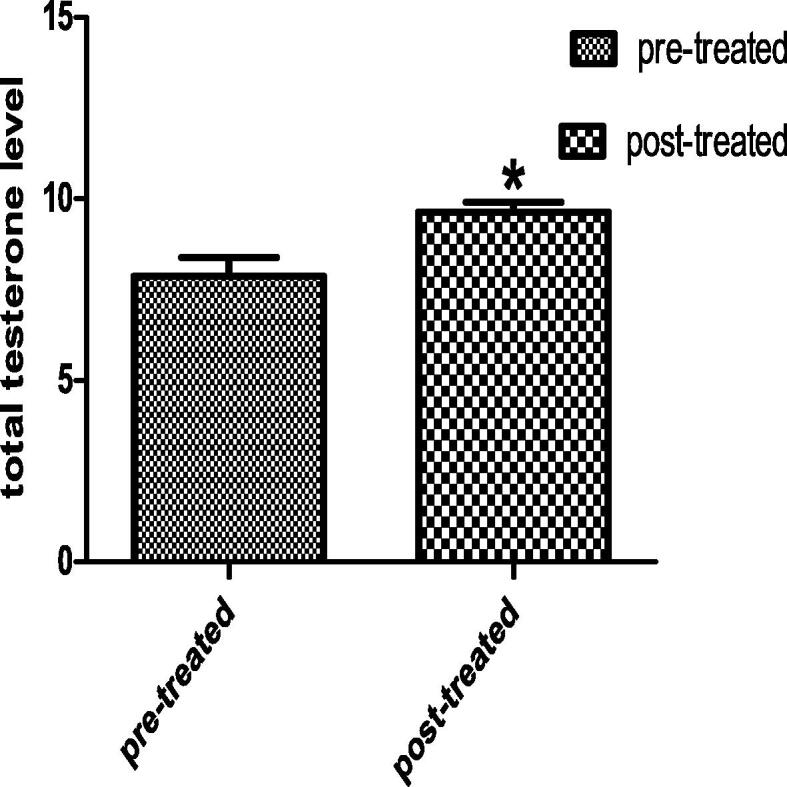
In the post-treated group, the total testosterone hormone was increased significantly than the pre-treated group by 22.6% and also the FSH was decreased insignificantly than the pre-treated group by 19.3%. The results is representing in Figs. 5 and 6.
Figs. 5 and 6.
Each value indicates the mean ± S.E.M. Statistical analysis was carried out by using unpaired t-test at level of significance P < 0.05. Fig. 5, FSH hormone level, Fig. 6, total testosterone hormone. In both figure, Pre-treated group received buffer intra-muscularly twice per week for 48 day, Post-treated group injected intra-muscular with 1 mg of Chondrus crispus with dose 0.1 ml/ twice per week for 48 day.
3.3.2. 3-Melandialdihyde (MDA) and 2,2, diphenyl 1- picrylhydrazyl (DPPH)
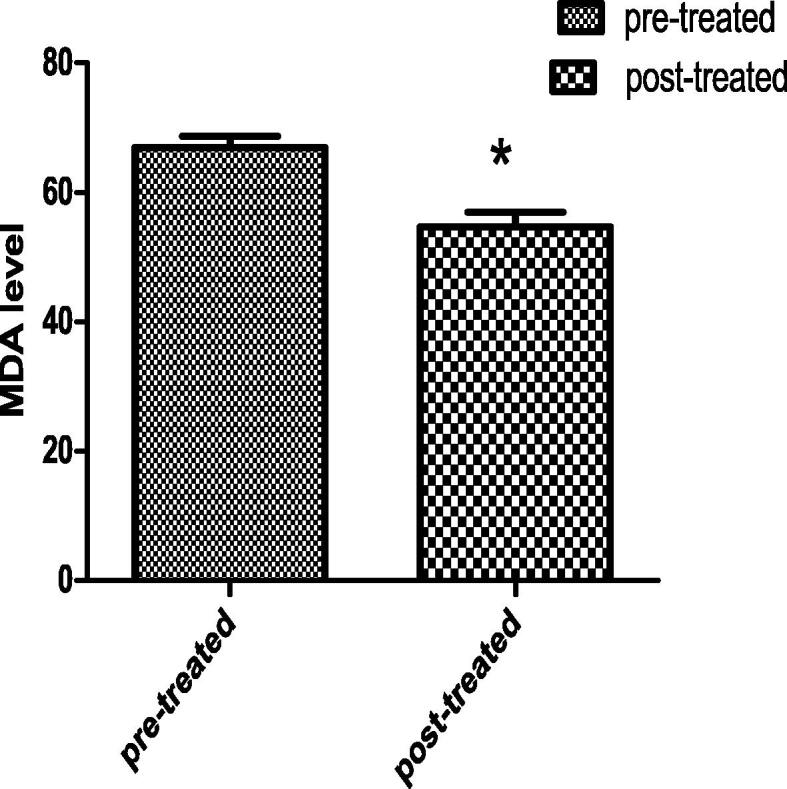
Both the MDA and DPPH are decreased significantly in the post-treated group by18.2% and 11.7% respectively than the pre-treated group (Figs. 7 and 8).
Figs. 7 and 8.
Each value indicates the mean ± S.E.M. Statistical analysis was carried out by using unpaired t-test at level of significance P < 0.05. Fig. 7 for MDA level, Fig. 8 for DPPH level. In both figure, Pre-treated group received buffer intramuscularly twice per week for 48 day, Post-treated group injected intra muscular with 1 mg of Chondruscrispus with dose 0.1 ml/ twice per week for 48 day.
3.4. Molecular studies
Both the pre-treated and post-treated group show normal DNA without any fragmentation (Fig. 9).
Fig. 9.
Agarose Gel Electrophoresis of Genomic DNA from testis of pre-treated (1) and post-treated groups (2) showing:normal DNA bands for both group.
4. Discussion
Algae is one of most widely used in many parts of the world as a source of nutrients for human because it contain many essential compound, also it is used for the cosmetic and pharmaceutical industries (Thomas and Kim, 2011). Due to their protein excellent quality, they are source of proteins as algae contain all the essential amino acids; polyunsaturated fatty acids especially as the omega-3 family and other essential fatty acids (Paul and Pohnert, 2011).
Vo et al. (2012) reported that algae also contain a lot of vitamins; carbohydrates; dietary fibers (alginates, agar and carrageenans) minerals (magnesium and calcium) and bioactive secondary metabolites (phytosterols and polyphenols).
Brewer et al. (1974), reported that chondrius crispus at doses (250 and 500 g wet wt/wk) is not acutely toxic. In the present study, chondrius crispus used with only dose 1 mg of Chondrus crispus and injected twice per week for 48 day (Collén et al., 2014).
Our result of this study revealed that chondrius crispus administration caused decreasing in the sperm head abnormalities, coiled tail and dead sperm. Also, the histopathlogical structures of testis were kept normal within pre-treated and post-treated group which is evidence for the chondrius crispus was not toxic substance. Seaweed is considered as basic diet item in China, Korea and Japan since prehistoric times. In 600 BCE. Every day at Japan, about 21 species of algae is cooked (six of them since the 8th century (Indergaard, 1983).
Chondriuscrispus consists of multiple bioactive compounds. Also, it has neuroprotective effect in C. elegans, due to the effect of the unsaturated fatty acids, glycolipids, floridoside, isothionic acid and other components such as pigments and free amino acids. Interestingly, the major component of C. crispus, κ-carrageenan, was recently shown to have potentials for anti-inflammation (Liu et al., 2013) and preventing the neurodegenerative processes (Barbosa et al., 2014). Liu et al. (2015) found that dietary supplementation of C. crispus enhanced the oxidative stress tolerance in C. elegans due to the up-regulation of the stress response genes sod-3 and significant increase in the skn-1 gene expression. SKN-1 was involved in homeostatic functions that extend more than Nrf2 which is the best known regulator of antioxidant and xenobiotic defense responses to acute stress (Jinghua et al., 2015).
On the other hand, SKN-1 has important role in C. elegans longevity suggesting that mechanisms regulated by SKN-1 may be of conserved importance in aging. These C. elegans studies predict that mammalian Nrf/CNC protein functions and regulation may be similarly complex and that the proteins and processes that they regulate are likely to have a major influence on mammalian life- and health span (Keith et al., 2015).
Sangha et al. (2015) reported that, the extraction of chondrius crispus showing strong anti-inflammatory properties due to the presences of Floridoside and isethionic acid by percentage 7.51% and 9.08%, respectively (Sangha et al., 2015) and both have antioxidant potential (Li et al., 2010). These agree with our histological results as the post-treated testis (injected with chondrius crispus) showed normal structure without any inflammation.
Usually, aging of male is accompanied with decrease in serum testosterone concentration (Sangha et al., 2015). The level of testosterone hormone in serum can be affected by many physiological and biochemical factors related with aging (Abidi et al., 2008). Many studies showed that the level of ROS increase in Leyding cells with aging, particularly lipid peroxidation-mediated damage to cell membrane, is related with intracellular cholesterol transport, which may weaken the Leyding cells function leading to decreased steroidogenesis (Hanukoglu, 2006). Floridoside which present as 7.51% in C. crispus has the ability to suppress pro-inflammatory responses in lipo-polysaccharide (LPS)-activated microglia cells via blocking p38 and MAPK signaling pathways (Li et al., 2010). Activation of both p38 MAPK → COX2 and NF-κB → COX2 signaling pathways are functionally linked to the oxidative stress response and chronic inflammation during aging, and mediate their inhibitory effects on testosterone production ((Pereira, 2018; Kim et al., 2013). So, C. crispus injection in our results showed increasing in the total testosterone hormone.
5. Conclusion
The Red Seaweed induces significant changes in the testicular function of the animal which might increase their fertility and sexual activities.
Authors' contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Nehad Ibrahim, Shimaa Ibrahim, Osama Ashour and Rania Ali. The first draft of the manuscript was written by Tharwat Abdel-Kader and revised by Monaser Hassan. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are grateful to the Deanship of Scientific Research for funding this article by Taif University Research Supporting Project number (TURSP-2020/119). Taif University. Taif, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abidi, P., Leers-Sucheta, S., Cortez, Y., Han, J., Azhar, S., 2008. Evidence that age-related changes in p38 MAP kinase contribute to the decreased steroid production by the adrenocortical cells from old rats. Aging Cell. 2008 Mar;7(2):168-78. doi: 10.1111/j.1474-9726.2007.00364.x. Epub 2008 Jan 28. PMID: 18241324. [DOI] [PubMed]
- Aljanabi S.M., Martinez I. Universal and rapid salt extraction of high genomic DNA for PCR based techniques. Nucleic Acids Res. 1997;25(22):4692–4693. doi: 10.1093/nar/25.22.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage P., Berry G. Statistical methods in Medical Research. Blackwell, Oxford, UK, page. 1987:93–213. [Google Scholar]
- Banchroft J.D., Stevens A., Turner D.R. fourth ed. Churchil Livingstone; New York, London, San Francisco, Tokyo: 1996. Theory and practice of histological technique. [Google Scholar]
- Barbosa, M., Valentão, P., Andrade, P.B., 2014. Bioactive compounds from macroalgae in the new Millennium: Implications for neurodegenerative diseases. Mar. Drugs 2014, 12, 4934–4972. Chondruscrispus. [DOI] [PMC free article] [PubMed]
- Bearden H., Fluquary J. Restor Published Co., Inc.; Reston Virginia: 1980. Applied Animal Reproduction; pp. 158–160. [Google Scholar]
- Brewer, D., Mcl-e.cnreN, J., Nnrsu, A.C., Snecrlocx, P.F., Lvron, T., 1974. Effects of Chondrus crisptts on fertility, pregnancy, post-natal welfare of Shropshire ewes. Can. J. Anim. Sci. 54: 4145.
- Collén J., Cornish M.L., Craigie J., Ficko-Blean E., Hervé C., Krueger-Hadfield S.A., Boyen C. Chondruscrispus – A present and historical model organism for Red Seaweeds. Sea Plants. 2014;53–89 doi: 10.1016/b978-0-12-408062-1.00003-2. [DOI] [Google Scholar]
- Hanukoglu, I., 2006. 200 Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug metabolism reviews. 38, 171–96 (2006). [DOI] [PubMed]
- Harnedy P.A., FitzGerald R.J. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 2011;2011(47):218–232. doi: 10.1111/j.1529-8817.2011.00969.x. [DOI] [PubMed] [Google Scholar]
- Hassab El-Nabi S.E. Molecular and cytogenetic studies on the antimutagenic potential of eugenol in human lymphocytes culture treated with depakine and apetryl drugs. J. Drug Ger. Soc. Zool. 2004;43(2):171–196. [Google Scholar]
- Jinghua Liu, Arjun H., Banskota, Alan T., Critchley, Jeff Hafting E., Balakrishnan Prithiviraj, 2015. Neuroprotective effects of the cultivated chondruscrispusin a C. elegansModel of Parkinson’s disease Mar. Drugs 2015, 13, 2250-2266; doi:10.3390/md13042250 [DOI] [PMC free article] [PubMed]
- Kim M., Li Y.-X., Dewapriya P., Ryu B., Kim S.-K. Floridoside suppresses pro-inflammatory responses by blocking MAPK signaling in activated microglia. BMB Rep. 2013;2013(46):398–403. doi: 10.5483/BMBRep.2013.46.8.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith D.A. Assessing and managing risks to ecosystem biodiversity. Austral Ecol. 2015;40 in press doi:10.1111/aec.12249. [Google Scholar]
- Lee O.H., Yoon K.Y., Kim K.J., You S., Lee B.Y. Seaweed extracts as a potential tool for the attenuation of oxidative damage in obesity-related pathologies. J. Phycol. 2011;2011(47):548–556. doi: 10.1111/j.1529-8817.2011.00974.x. [DOI] [PubMed] [Google Scholar]
- Pereira Leonel, Critchley Alan T. The COVID 19 novel coronavirus pandemic 2020: seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J. Appl. Phycol. 2020;32:1875–1877. doi: 10.1007/s10811-020-02143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.X., Li Y., Lee S.H., Qian Z.J., Kim S.J.I. Inhibitors of oxidation and matrix metalloproteinases, floridoside, and D-isofloridoside from marine red alga Laurenciaundulata. J. Agric. Food. Chem. 2010;2010(58):578–586. doi: 10.1021/jf902811j. [DOI] [PubMed] [Google Scholar]
- Liu J., Hafting J., Critchley A.T., Banskota A.H., Prithiviraj B. Components of the cultivated red seaweed Chondrus crispus enhance the immune response of Caenorhabditis elegansto Pseudomonas aeruginosathrough the pmk-1, daf-2/daf-16, and skn-1 pathways. Appl. Environ. Microbiol. 2013;2013(79):7343–7350. doi: 10.1128/AEM.01927-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lordan S., Ross R.P., Stanton C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs. 2011;2011(9):1056–1100. doi: 10.3390/md9061056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indergaard, M., 1983. The aquatic resource. I. The wild marine plants: a global bioresource. In: Cote, W. A. Biomass utilization. Vol. Plenum Publishing Corporation, 137-168
- Mohamed S., Hashim S.N., Rahman H.A. Seaweeds A sustainable functional food for complementary and alternative. Trends Food Sci. Technol. 2012;2012(23):83–96. [Google Scholar]
- Mukhtar, A., Al-Daghri, N., Alokail, M.S., Hussain, T., 2013. Potential changes in rat spermatogenesis and sperm parameters after inhalation of Boswelliapapyrifera and Boswelliacarteriiincenseint. J. Environ. Res. Public Health 2013, 10, 830-844; doi:10.3390/ijerph10030830 [DOI] [PMC free article] [PubMed]
- Nwosu F., Morris J., Lund V.A., Stewart D., Ross H.A., McDougall G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011;2011(126):1006–1012. [Google Scholar]
- Paul, C., Pohnert, G.,2011. Production and role of volatile halogenated compounds from marine algae. Nat Prod Rep. Feb;28(2):186-95. doi: 10.1039/c0np00043d. Epub 2010 Dec 1. PMID: 21125112 [DOI] [PubMed]
- Pereira L. Biological and therapeutic properties of the seaweed polysaccharides. Int. Biol. Rev. 2018;2018(2):1–50. [Google Scholar]
- Sangha J.S., Di F., Banskota A.H., Stefanova R., Khan W., Hafting J., Craigie J.S., Critchley A.T., Prithiviraj B. Bioactive components of the edible strain of red alga, Chondruscrispus, enhance oxidative stress tolerance in Caenorhabditiselegans. J. Func. Foods. 2013;2013(5):1180–1190. [Google Scholar]
- Sangha J.S., Wally O., Banskota A.H., Stefanova R., Hafting J.T., Critchley A.T., Prithiviraj B. Dalhousie University; Truro, Canada: 2015. A cultivated strain of a red seaweed (Chondrus crispus), suppresses β-amyloid-induced paralysis in Caenorhabditis elegans. Unpublished work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganthy N., Karutha P.S., Pandima D.K. Neuroprotective effect of seaweeds inhabiting South Indian coastal area (Hare Island, Gulf of Mannar Marine Biosphere Reserve): Cholinesterase inhibitory effect of Hypneavalentiaeand Ulvareticulata. Neurosci. Lett. 2010;2010(468):216–219. doi: 10.1016/j.neulet.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Surzycki, S., 2000. Agarose Gel electrophoresis of DNA in Basic techniques in Molecular Biology (ed): 175-178.
- TâniaMelo A.L., ElianaAlves A.L., VítorAzevedoa Ana Sofia, Martins A., Bruno Neves A., Dominguesa Pedro, RicardoCalado B., Maria H., Abreu C. Lipidomics as a new approach for the bioprospecting of marine. Maria Rosário Domingues Algal Research. 2015;8:181–191. [Google Scholar]
- Tasende M.G. Fatty acid and sterol composition of gametophytes and saprophytes of Chondruscrispus. Sci. Marina. 2000;2000(64):421–426. [Google Scholar]
- Tatli-Çankaya I., Alqasoumi S.I., Abdel-Rahman R.F., Yusufoglu H., Anul S.A., Akaydin G. Evaluating the antifertility potential of the ethanolic extracts of Bupleurum sulphureum and Cichorium intybus in male rats. Asian J. Pharm. Clin. Res. 2014;7(7):211–218. [Google Scholar]
- Thomas, N.V., Kim, S.K., 2011. Potential cosmeceutical applications of phlorotannins and fucoidans from marine algae in the treatment of atopic dermatitis. In Marine Cosmeceuticals: Trends and Prospects; Kim, S.K., Ed.; CRC Press: Boca Raton, FL, USA,; pp. 257–264
- Tuchmann-Duplessis H. ADIS Press; New York: 1977. Drug effect on the fetus: General principles of drug induced congenital abnormalities. [Google Scholar]
- Vo T.S., Ngo D.H., Kim S.K. Potential targets for anti-inflammatory and anti-allergic activities of marine algae: An overview. Inflame. Allergy Drug Targets. 2012;2012(11):90–101. doi: 10.2174/187152812800392797. [DOI] [PubMed] [Google Scholar]