Abstract
Xylene is a common pollutant in the environment that enters the body of animals and humans in various ways, but most often through the respiratory tract and adversely affects their overall health. However, xylene effects after oral exposure have not been sufficiently studied. This study aimed to investigate the effects of xylene exposure on the mouse organism and to identify possible beneficial effects of flaxseed on such exposure. Eighty mice were divided into four groups: control group C (basal diet + no xylene exposure), group X (oral exposure by 400 mg/kg/day xylene), group F (10% flaxseed supplementation of basal diet), and group XF (10% dietary flaxseed + oral exposure by xylene). Experimental trial took 14 days. Clinical examination, spectroscopic analysis of tissue aminotransferases, total lactate dehydrogenase (TLDH), and acetylcholinesterase (AchE) activities, electrophoretic analysis of LDH isoenzymes, western blot and immunohistochemical analysis of apoptosis as well as routine histology of the kidneys and jejunum, and transmission electron microscopy of the liver were performed. Marked restlessness in group X and high weight losses in mice of all groups were recorded during the experiment. Xylene promoted apoptosis (caspase-3 expression) without causing marked structural changes in the liver and jejunum, although renal cortex structure was affected adversely. In the brain, liver, and kidney of mice, xylene increased levels of liver transaminases, LDH, and decreased AchE activities, reflecting cell membrane damage. Flaxseed feeding improved animal behaviour, leakage of enzymes and prevented selected tissue toxic damage induced by xylene by protecting cell membrane integrity and fluidity and by suppressing apoptosis. These results point at the protective effect of flaxseed consumption on mice.
Keywords: Flaxseed, Xylene, Aminotransferases, Lactate dehydrogenase, Acetylcholinesterase, Apoptosis
1. Introduction
Fuels, plastics, acrylates, paints, cleaning agents, textile fibers, insecticides, and other auxiliaries are derived from oil and its products. The environmental impacts of oil extraction can lead to air pollution, ozone depletion, acid rains and various diseases in humans and animals (Kandyala et al., 2010). Harmful effects of xylene exposure were well studied, but there is a lack of information that would precisely define this effect on the level of cell membrane integrity. Scientists, laboratory technicians, and other people manipulating with xylenes are most often exposed by inhalation or dermal exposure (ATSDR, 2007). Xylene, or dimethyl benzene (C8H10) is an organic aromatic hydrocarbon. The individual isomers of xylene include o-, m-, and p-xylene. In addition to air pollution, xylene can enter the environment by leaking into soil, surface water, or groundwater, where once it is present can contaminate drinking water for several months (ATSDR, 2007). Therefore, it is necessary to improve control measures reffering to the amount of environmental contaminants, e.g. in the air, water, or soil and focus on the prevention of their harmful effects. Xylene is rapidly absorbed through the lungs, digestive tract and skin, and quickly distributed throughout the body (Langman, 1994). The lipophilic property of xylene, which dissolve lipid membranes, is responsible for irritation of mucous membranes, eyes, and skin (Riihimaki, 1979). Animal studies confirmed that xylene has adverse effects on many organ systems, including the CNS, liver, kidney, haemopoietic tissues and respiratory tract (ATSDR, 2007, Salimi et al., 2017). Lungs evaporate unmetabolized xylene, but approximately 95% of the xylene is metabolised in the liver to methylhippuric acid and excreted in the urine. There are differences between animal species and between animals and humans, in the metabolism of, sensitivity to, as well as conditions of exposure to, xylene (Salimi et al., 2017).
Current research focuses on the use of biologically active additives of natural origin, such as flaxseed, whose targeted application can affect animal health through activation of specific molecular mechanisms (Bomba et al., 2012, Strojný et al., 2014). The health-promoting effects of flaxseed have been described in many studies related to gastrointestinal diseases (Strojný et al., 2014, Laurino et al., 2017), including functions in hepatoprotection (Andrejčáková et al. 2016) and hypocholesterolaemic benefits (Sopková et al., 2017). Other positive effects of flaxseed include assistance in the prevention of diabetes (Mohammadi-Sartang et al., 2018), cancer (Calado et al., 2018), and cardiovascular diseases (Bomba et al., 2012, Prasad and Jadhav, 2016). Flaxseed is a rich plant source of n-3 polyunsaturated fatty acids (PUFA), mainly essential α-linolenic acid (ALA). The PUFAs of the n-6 and n-3 series are an integral part of cell membranes and are responsible for their fluidity and physicochemical properties. Fatty acids of the n-3 PUFA series give rise to eicosanoids, which act as tissue hormones involved in reproduction, immunity, as well as secretory and growth processes (Bomba et al., 2012). Lignans originated from flaxseed are absorbed through the intestinal wall into the blood and tissues (Clavel et al., 2005). These substances have a similar chemical structure to 17β-oestradiol. They bind to oestrogen receptors of target cells and promote cell proliferation or apoptosis (Rietjens et al., 2013, Yanagihara et al., 2014). In vitro (Kádasi et al., 2015) and in vivo (Vlčková et al., 2018) studies revealed that dietary flaxseed promotes apoptosis of somatic cells of the porcine ovaries. Some studies confirmed its pro-apoptotic effect due to high content of antioxidants such as flavonoids (Yanagihara et al., 2014).
In our previous studies performed on piglets (Andrejčáková et al., 2016, Sopková et al., 2017, Vlčková et al., 2018) and mice (Andrejčáková et al., 2020) we identified beneficial and protective effects of flaxseed consumption on cell membranes and overall health of animals with bacterial infections. We conducted preliminary study to test the effects of xylene exposure as well as the effects of dietary flaxseed on the mouse organism (Kuráňová et al., 2020), but this study lacked a view of the whole organism, including effect on various tissues cell survival and membrane integrity.
This study focused on the observation of orally applied xylene induced toxicity in mice. We also tested wheather the flaxseed can prevent or even suppress adverse effects of xylene exposure in mice. Our study highlighted the significance of measuring clinical status, such as animal behaviour and body weight, changes in the activity of liver transaminases, lactate dehydrogenase and cholinesterases, expression of caspase-3 in various tissue types, along with the structure of the liver and kidneys in mice fed flaxseed, xylene or combination of both.
2. Material and methods
2.1. Experimental design
A total 80 female CD-1 ICR mice (VELAZ s.r.o., Czech Republic) aged 35 days were used in the study (at 28 days of age they were transported from the breeding facility to the quarantine of the experimental facility of the Institute of Animal Physiology of the Slovak Academy of Science). Mice were randomly divided into four separate groups: the control group (C; n = 20), whose standard M3 diet (BONAGRO a.s. Blazovice, CZ; Table 1) was administered at dose of 4 g per day (divided into two separate doses); the xylene group (X; n = 20) diet included M3 and administration of xylene (mixed xylene p.a.; dilution 1:10 in water; 10 µl/head/day equal to 400 mg/kg/day) per os by cannula; the flaxseed group (F; n = 20) diet included M3 supplemented with crushed flaxseed (10%; var. Libra; 57% content of ALA); and the combined group (XF; n = 20) who received M3, flaxseed and xylene at the same doses as group X and F. Animals had ad libitum access to water. Mice were kept at temperatures maintained between 20 and 24 °C, with a relative humidity of 45 – 65%, under a 12-h light/dark regimen. Bedding intended for barrier breeding was used (Lignocel 3-4S; VELAZ s.r.o., Prague, Czech Republic). During the experimental period (total 14 days), animals were observed for normal behaviours and were weighed recurrently (Day 1, 7 and 14). Animals were humanely euthanized by cervical dislocation on day 14 (experiment approval number 598/18–221/3).
Table 1.
Composition of standard diet M3 administred to mice.
| Component | Unit | Component | Unit |
|---|---|---|---|
| Crude protein | 22% | Vitamin A | 20 000 IU |
| Crude fiber | 3.14% | Ferrous sulphate monohydrate | 17 000 mg |
| Coarse fat | 3.31% | Potassium iodide | 0.65 mg |
| Ash | 6.16% | Cobalt (bis) carbonate | 0.4 mg |
| Calcium | 1.01% | Copper sulphate pentahydrate | 15 000 mg |
| Phosphorus | 0.53% | Manganese oxide | 45 000 mg |
| Sodium | 0.11% | Zinc oxide | 71 000 mg |
| Vitamin D3 | 2000 IU | Selenium | 0.15 g0 |
2.2. Tissue collection and sample preparation
Livers, hearts, brains, kidneys and jejunums were collected for selected analyses. Tissue extracts of livers were used for analysis of total activity of lactate dehydrogenase (TLDH) and its isoenzymes LDH-1 – LDH-5, concentration of AST, ALT, and a AST to ALT ratio (De Rites index). Tissue extracts of mice kidneys were also used for LDH analysis. The samples of tissues were cut into small pieces (5 mm × 5 mm) and washed in buffered-saline to remove the excess blood and connective tissue. Livers and kidneys were then homogenised in ratio of 1 to 10 volumes of cold buffer (0.05 M Tris-HCl buffer, pH 7.3) in line with Heinová et al. (1999) and expressed in international units per gram of protein (U/g).
Tissue extracts from the brains, livers, and kidneys of mice were used for measuring of cholinesterases activity, prepared according to manufacturer's instructions for Acetylcholinestarase Assay Kit (Abcam), and expressed in international units per gram of protein (U/g).
Tissue extracts from the brain, liver, and half of the jejunum was used for western blot analysis of anti-caspase-3 antibody determination. Tissues were homogenised on ice with a cold buffer (50 mM Tris-HCl with pH 7.5, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 1 mM PMSF, 1 mM EDTA, protease inhibitor according to manufacturer's instructions; Thermo Scientific), sonicated and centrifuged at 10,000g for 30 min at 4 °C. Prior to the western blot analysis, the samples were denaturated at 95 °C for 5 min with the addition of Laemmli buffer (Sigma-Aldrich Chemie GmbH, Germany). The total protein concentration of extracted samples was measured using spectroscopy.
2.3. Spectroscopic analysis of enzymes
In the tissue extracts, the concentration of TLDH, alanine aminotranspherase (ALT), aspartate aminotranspherase (AST, all λ 340 nm) and total protein concentration (λ 540 nm) were determined using commercial diagnostic kits (Randox, United Kingdom) with an ALIZÉ automatic biochemical analyzer (Lisabio, France).
For cholinesterase analysis, supernatants of prepared brain, liver and kidney tissue extracts of individual groups of mice were used. The Acetylcholinesterase (AChE) Assay Kit (colorimetric; Abcam, Shanghai, China) was used to determine total cholineesterases (ChE) in tissue samples according to manufacturer's instructions and adapted for reading on microplates for ELISA device (λ 410 nm; Multiskan®EX Spectrometer, Thermo-Fisher, Abingdon, UK). The same procedure with the addition of Donepezil hydrochloride (1 µM, inhibitor of AChE; Abcam) to the tested samples was used to determine ButyrylChE (BChE) activity. Differences between ChE and BChE were calculated as the AChE activity.
2.4. Electrophoretic analysis of LDH isoenzyme fractions
For the electrophoretic study, the samples of the liver and kidney extracts were used for each separation. A HYDRASYS device (Sebia, Lisses, France) was used to determine LDH isoenzyme activities according to protocols outlined in our previous study (Andrejčáková et al., 2016, Andrejčáková et al., 2020). The samples were separated using commercial Hydragel 15 ISO-LDH electrophoretic kits (Ecomed, Žilina, Slovak Republic). Qualitative evaluations of gels were done directly from the electrophoretograms. Densitometric curves of the separations were created by means of Epson Perfection V 700 Photo densitometer scanning at λ 570 nm and evaluated using the PHORESIS software (Version 9.20, 2015, Sebia, Lisses, France).
2.5. Light microscopy and transmission electron microscopy (TEM)
The tissue excisions of kidneys and livers up to 1 mm3 were fixed by immersion in 3% glutaraldehyde and postfixed in 1% osmium tetraoxide (both in 0.15 M phosphate buffer, pH 7.3). After dehydration in acetone and clearing in propylene oxide, they were embedded in DurcupanTM ACM (Sigma-Aldrich Chemie GmbH, Germany). The semithin sections of kidney (0.5–1 μm) and ultrathin sections (90–120 nm) of liver samples were cut with ultramicrotome (LKB Nova, Sweden). The renal tissue sections were stained with toluidine blue and examined under a light microscope Zeiss Axio Lab A1 (Zeiss, Germany) documented with camera Axio Cam ERc 5 (Zeiss, Germany). The hepatal tissue sections were double contrasted with 1% uranyl acetate and 0.3% lead citrate and examined under an electron microscope Tesla BS 500 (Tesla Brno, Czech Republic). Every 20th section (n = 6 per sample) were analysed.
2.6. Immunohistochemistry (IHC)
Part of the jejunum was fixed in 4% paraformaldehyde for 24 h, dehydrated, embedded in paraffin and cut into 5 µm thick serial sections by use of Leica RM2255 microtome (Leica Microsystems Nussloch GmbH, Nussloch, Germany) for routine histology (haematoxylin-eosin staining, H-E; data not shown) and immunohistochemistry (anti-caspase-3 antibody). For immunohistochemistry the paraffin sections of the jejunum were deparaffinised and rehydrated. For detection, antigen retrieval was performed by boiling the slides in 10-mM citrate buffer (pH 6.0) for 6 min. To block endogenous peroxidase activity, the slides were incubated in methanol with 3% H2O2 addition for 30 min. To block non-specific binding, the sections were incubated for 1 hr with 1% bovine serum albumin (BSA) in TBS (0.05 M Tris–HCl plus 0.15 M NaCl, pH 7.6). In addition, monoclonal mouse anti-caspase-3 (dilution 1:250) antibody (Santa Cruz Biotechnology Inc., Dallas, USA) were applied and incubated overnight at 4 °C. After rinsing with TBST (TBS containing 0.1% Tween20), the sections were incubated with goat anti-mouse secondary antibodies (Dako REAL™ EnVision™/HRP, Rabbit/Mouse (ENV), ready-to-use, Dako, Denmark) for 2 hrs. Following the incubation, the specimens were rinsed in TBST and then in TBS. A colour reaction was conducted with diaminobenzidine as a chromogen (Dako REAL™ DAB + Chromogen, Dako, Denmark). The stained sections were rinsed with distilled water, counterstained with haematoxylin to visualise nuclei, dehydrated and immersed in DPX (Distyrene Plasticiser and Xylene; Buchs, Switzerland). Preparing a negative control for each sample, the primary antibody was omitted. Photographic documentation was obtained using optical microscope (Olympus BX43, Olympus Corporation, Tokyo, Japan) coupled to a camera (Olympus UC30, Olympus Corporation, Tokyo, Japan) and computer.
To evaluate the intensity of the immunohistochemical reaction quantitatively, approximately 6 images from the sections of each examined animal (n = 6 for each group) were analysed by using the public-domain ImageJ software (National Institutes of Health, Bethesda, MD, USA). The outlines of all cells, which demonstrated an immunopositive signal in the jejunum, were marked manually and grey level (GL) of the marked areas was measured. The intensity of the IHC reaction was expressed as the relative optical density (ROD) of the DAB brown reaction products and was calculated using the formula described by Smolen (1990) as follows:
where GL is the grey level of the stained area (specimen) and unstained area (background) and blank is the GL measured after the slide was removed from the light path.
2.7. Western blot analysis
For western blot analysis, the supernatants of prepared liver, brain and jejunum tissues were used. Samples containing 30 µg of protein were separated on 12% SDS-PAGE (25 mM Tris, 192 mM glycine, 0.1% w/v SDS) under reducing conditions. Separated proteins were electroblotted onto a PVDF (polyvinylidene difluoride; Thermo Scientific) membrane using a wet blotter in transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol). Thereafter, membranes were blocked in 5% non-fat milk in Tris-buffered saline (TBS; 20 mM Tris-HCl, pH 7.5; 137 mM NaCl; pH 7.4) containing 0.1% Tween 20 (TBST) for 1 h at room temperature and incubated overnight at 4 °C with primary monoclonal Anti-caspase-3 antibody of dilution 1: 1000 (Santa Cruz Biotechnology Inc., Dallas, USA) or polyclonal rabbit anti-β-actin antibody (Thermo Scientific) of dilution 1:10 000 as a control. The 24 h later, the membranes were washed in TBST buffer and the secondary anti-mouse IgG antibodies of dilution 1:20 000 (Sigma-Aldrich) were applied for one hour at room temperature. After incubation, the membranes were washed four times in TBST (5 min each) and then in TBS (5 min). Immunoreactive proteins were detected by chemiluminescence using Clarity™ Western ECL Blotting Substrate (Bio-Rad Laboratories Inc.) and Fusion Fx7 system (Vilber Lourmat, Eberhardzell, Germany). The molecular weights of target proteins were estimated by reference to standard proteins (BlueEye Prestained Protein Ladder; Sigma Aldrich). The relative intensities of the bands were densitometrically quantified and normalized to their corresponding β-actin bands using ImageJ software (National Institutes of Health, Bethesda, MD, USA). To confirm protective effect of flaxseed on the jejunum of intoxicated mice, caspase-3 activity in groups X and XF was measured.
2.8. Statistical analysis
The dynamics of weight gain/loss of mice were statistically assessed using One-way ANOVA with Tukey's post hoc analysis, while the differences in other parameters between the groups were analysed using unpaired t-test (GraphPad Prism 5.0 for Windows, GraphPad Software, San Diego, CA, USA) at the end of experiment. The values listed in the tables and figures are the average values obtained from 20 animals in each group. All data are means with standard error of mean (SEM). Differences within the groups are marked with superscript letters a,b,c,x,y,z and considered significant at levels of a, x = p < 0.05, b, y = p < 0.01, and c, z = p < 0.001. Correlations between the different variables (TLDH and AChE in the liver and kidneys) of mice at p value ≤ 0.05 were considered statistically significant.
3. Results
3.1. Clinical status and weight changes in mice
During the observation period, all animals were spry, feed intake was normal, and faeces were assessed as solid. The group of xylene mice showed increased hypersensitivity and restlessness. Body weights of mice exposed or not exposed to xylene and fed or not fed the diet supplemented with flaxseed during 14-day period showed weight loss (Table 2). Weight loss was observed in mice of all groups on Day 7 with the lowest weight loss in XF group compared to mice of X group (p < 0.05). At the end of experiment, mice of control (p < 0.001), X and F groups (both p < 0.01) gained some weight compared to Day 7 as well as in XF group, although not significantly.
Table 2.
Body weights of the experimental mice.
| Group |
Weighing days |
||
|---|---|---|---|
| 1 | 7 | 14 | |
| C | 27.02 ± 0.35z | 24.69 ± 0.23z | 26.27 ± 0.25z |
| X | 27.66 ± 0.39y,z | 24.52 ± 0.46z,a | 25.59 ± 0.57y |
| F | 27.11 ± 0.32y | 25.29 ± 0.36y | 26.96 ± 0.42y |
| XF | 27.96 ± 0.34y | 26.07 ± 0.37y,a | 26.73 ± 0.51 |
C, control group; X, xylene group; F, flaxseed group; XF, combined group with addition of flaxseed and xylene; Day 1 – 14 = weighing days; Data are means (g) ± SEM; a,y,z Mean values with same superscript letters differ significantly (a = p < 0.05, y = p < 0.01, z = p < 0.001). Superscript letter a indicates differences between the groups on selected day of the experiment and superscript letters y,z indicate dynamic changes within the single group of mice.
3.2. Enzyme activities
Activities of enzymes in tissue extracts of the liver and kidney are summarised in Table 3. The activities of AST and ALT in X group was higher in comparison with F group (p < 0.05). The AST to ALT ratio was significqantly higher in X group compared to other groups (C and XF, both at p < 0.05, and F at p < 0.01).
Table 3.
Activity of hepatic and renal enzymes in the experimental mice.
| Organ | Parameter (U/g) | Groups | |||
|---|---|---|---|---|---|
| C | X | F | XF | ||
| Liver | AST | 1679 ± 99.7 | 1852 ± 67.48a | 1499 ± 45.1a | 1592 ± 11.7 |
| ALT | 998.9 ± 26.82 | 1121 ± 64.2a | 958.7 ± 28.5a | 1055 ± 38.34 | |
| AST/ALT ratio | 1.29 ± 0.02a | 1.87 ± 0.1a,b | 1.25 ± 0.07b | 1.38 ± 0.03a | |
| TLDH | 2281 ± 192.1 | 2482 ± 149.9 | 2459 ± 286.7 | 2486 ± 173 | |
| LDH-1 | 79.98 ± 26.84 | 78.08 ± 19.26 | 47.25 ± 9.7 | 93.03 ± 59.65 | |
| LDH-2 | 172 ± 30.41 | 230.2 ± 45.12 | 175 ± 28.11 | 165.2 ± 67.07 | |
| LDH-3 | 900 ± 192.7a | 906.8 ± 68.29a | 1256 ± 195.6a,b | 657 ± 354.6b | |
| LDH-4 | 443.4 ± 106.8 | 492.9 ± 42.27 | 350.2 ± 27.77 | 418.5 ± 181.9 | |
| LDH-5 | 685.9 ± 126.2 | 773.9 ± 44.85a | 631.2 ± 63.53 | 485.2 ± 154.4a | |
| Kidney | TLDH | 1462 ± 433b | 3866 ± 413.6b | 1936 ± 382.4b | 3711 ± 562.1 |
| LDH-1 | 316 ± 82.23b | 782.6 ± 112.5b | 377.6 ± 23.5b | 894.6 ± 138.6b | |
| LDH-2 | 306.9 ± 95.15b | 816.2 ± 90.73b | 353.9 ± 16.25b | 724.1 ± 97.3b | |
| LDH-3 | 292 ± 91.61b | 793.4 ± 76.93b | 376.9 ± 49.3b | 752 ± 157.8b | |
| LDH-4 | 252.1 ± 79.76a | 689.5 ± 60.87a | 303.8 ± 46.35a | 597.6 ± 60.17a | |
| LDH-5 | 295.4 ± 84.44b | 784.2 ± 75.77b | 424 ± 76.25b | 743.3 ± 115b | |
C, control group; X, xylene group; F, flaxseed group; XF, combined group with addition of flaxseed and xylene; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TLDH, total lactate dehydrogenase; LDH-1 – LDH-5, isoenzymes of LDH; Data are means (U/g) ± SEM; a,b Mean values in rows with same superscript letters differ significantly (a = p < 0.05, b = p < 0.01).
In liver tissue extract, the concentration of LDH-5 isoenzyme was markedly decreased in XF group compared to X group (p < 0.05). Higher activity of LDH-3 isoenzyme in F group compared to groups C and X (both p < 0.05), and XF (p < 0.01) was observed. Neither flaxseed nor xylene influenced the activity of TLDH, LDH-1, 2, and 4 in the liver. In renal tissue extract, higher activities of TLDH, LDH-1, 2, 3, and 5 (p < 0.01 for all), and LDH-4 (p < 0.05) in X and XF groups compared to C and F groups were observed.
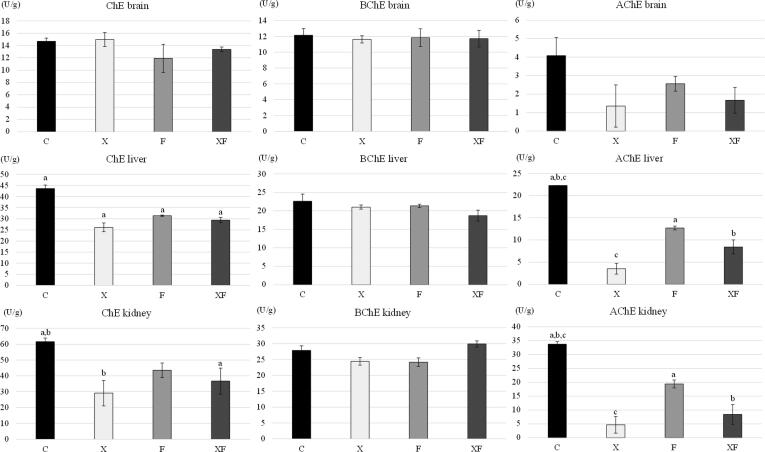
Activities of cholinesterases in selected organs of mice are shown in Fig. 1. Total cholinesterase activity was decreased in the liver of X, F, and XF group mice (p < 0.05 for all) and in the kidney of X (p < 0.01) and XF (p < 0.05) groups, compared to control animals (C), while it was not affected significantly in F group. Neither xylene nor flaxseed additives affected activity of any type of cholinesterase in the brain tissue. BChE in the liver and kidney was also unaffected. Acetylcholinesterase activity in liver and renal tissue extracts decreased in X (p < 0.001), XF (p < 0.01), and F (p < 0.05) group compared to control mice (C). Moreover, we recorded that BChE is dominant enzyme compared to AChE in the brain and liver, while not in the kidneys of mice. Testing correlations between the activity of TLDH and AChE of mice of XF group revealed high negative correlation in the liver (r = −0.98; p < 0.001) and low negative correlation in the kidneys (r = -0.13; p > 0.05).
Fig. 1.
Activities of cholinesterases in selected organs of the experimental mice. C, control group; X, xylene group; F, flaxseed group; XF, combined group with addition of flaxseed and xylene; ChE, total cholinesterases activity; BChE, butyrylcholinesterase activity; AChE, acetylcholinesterase activity; Data are means (U/g) ± SEM; a,b,c Mean values in columns with same superscript letters differ significantly (a = p < 0.05, b = p < 0.01, c = p < 0.001).
3.3. Transmission electron microscopy of the liver
Ultrastructure of the liver tissue are shown in Fig. 2. In the control group, the liver had a classic morphological appearance. Hepatocytes of polyhedral shape with centrally located euchromatic nucleus were arranged in anastomosing cords. Hepatocyte cytoplasm contained numerous mitochondria of oval or round shape, few lysosomes were scattered randomly throughout the cytoplasm, with a well-developed rough endoplasmic reticulum. At the vascular pole of the hepatocytes, the cytoplasmic membrane formed numerous microvilli that protruded into the space of Disse. The livers of mice in group X did not reveal any significant morphological changes in the ultrastructure of hepatocytes. The mitochondria and rough endoplasmic reticulum were intact. The cytoplasmic membrane at both vascular and bile poles remained unchanged. However, large electron-lucid areas in the cytoplasm with often homogeneous appearance were observed. The liver sinusoids were not dilated or infiltrated with inflammatory cells. The space of Disse was unchanged with numerous microvilli. In the group F, lipid droplets of various sizes were observed in the cytoplasm of some hepatocytes. Ultrastructure of the liver of combined group (XF) was identical to the xylene group (X); the ultrastructure of hepatocytes was preserved, but the electron-lucid areas in the cytoplasm were visible.
Fig. 2.
Histological observation of the liver, kidney, and jejunum of the experimental mice. C, control group; X, xylene group; F, flaxseed group; XF, combined group with addition of flaxseed and xylene; Liver, magnification C − 4800×, X − 3600×; F − 2400×, XF − 5600×; S, sinusoid; N, nucleus of hepatocyte; Ec, erythrocyte; thin arrows, lysosomes; thick arrows, space of Disse; L, electron-lucid areas; l, lipid droplets; R, rough endoplasmic reticulum; Kidney, magnification 500×; scale bar 20 µm; Rc, renal corpuscule with regular shape; Pt, proximal tubule; Dt, distal tubule; Rci, renal corpuscule with irregular shape and enlarged urinary space; aRc, atrophic renal corpuscule; v, vacuoles in the cytoplasm; n, necrotizing cells; a, affected brush border; Rce, renal corpuscule with enlarged urinary space; Jejunum, magnification 400 ×; scale bar 50 µm; *, positive reaction in crypts and lamina propria; incorporated image is an example of negative reaction (without primary antibody).
3.4. Light microscopy of the kidneys
Microstructure of the renal tissue are also shown in Fig. 2. The renal corpuscles of the C and F mice were round to oval shaped and consisted of well formed glomeruli, coherent Bowman's capsules and regular urinary spaces. The uriniferous tubules, collecting tubules, and interstitial components demonstrated typical structural organization. The kidney parenchyma of the X group revealed severe diffuse degenerative changes in all nephron components, collecting tubules, and interstitium. The renal corpuscule had constantly irregular shape and size. The parietal layer of the Bowman's capsule was often discontinuous and urinary spaces were dilated. Most of the renal corpuscule often contained necrotizing cells with dark picnotic nucleus. Several renal corpuscule revealed strongly disintegrated structure and atrophy. Proximal tubules (Pt) had irregular shapes with necrotizing cells oftenly seen. The Pt lining epithelial cells had constantly vacuolar cytoplasm and affected brush borders. Lining epithelium of the distal tubules (Dt) revealed strong apical vacuolation and scattered necrotizing epithelial cells. In both the Pt and Dt, the lumens often contained groups of desquamed epithelial cells. The epithelial cells of the collecting tubules contained numerous, small, pale vacuoles in their cytoplasm. The interstitium did not show any morphological changes or signs of inflammation. The kidney parenchyma of XF group showed moderate diffuse degenerative features. The renal corpuscule were mostly regular in shape and size, but their urinary spaces often contained irregular dilatations in some sections. Atrophic renal corpuscule did not occur. The Pt and Dt were typical, however cytoplasm of lining epithelial cells contained vacuoles and several segments of the Pt revealed uneven brush border. The cellular necrotizations were much less frequent compared to X group. The collecting tubules and interstitium was typical and unchained.
3.5. Immunohistochemical determination of caspase-3 in the jejunum of mice
Routine histological observation of the jejunum did not reveal any significant structural changes in all groups of mice (data not shown). The mucous layer of intestinal villi consisted of a single layer of columnar epithelial enterocytes and goblet cells. In the apical part of intestinal villi, small groups of dying enterocytes of irregular shape and with dark cytoplasm were present. Goblet cells did not show significant morphological changes.
To determine the extent to which dietary flaxseed modulates xylene-induced mucosal injury, the level of epithelial apoptosis (caspase-3) was quantified within the sections of jejunum by immunohistochemistry. As shown in Fig. 2, apoptotic cells were detected along the luminal surface of the crypts and lamina propria in the jejunum of group X. Increased intensity (relative optical density, ROD, Fig. 3) of caspase-3 was observed in group X compared to C and XF (p < 0.001 for both), and in group F compared to C and XF (p < 0.05 for both).
Fig. 3.
Intensity of immunohistochemical staining of caspase-3 in the jejunum of the experimental mice. C, control group; X, xylene group; F, flaxseed group; XF, combined group with addition of flaxseed and xylene; ROD, relative optical density; Data are means ± SEM; a,c Mean values in columns with same superscript letters differ significantly (a = p < 0.05, c = p < 0.001).
3.6. Western blot analysis of caspse-3
Expression of caspase-3 as a marker of apoptosis was measured by using western blot analysis in the brain, liver, and jejunum (only X and XF) of mice (Fig. 4). In the brain, increased expression of caspase-3 was observed in X group compared to F and XF (p < 0.05 for both), and C (p < 0.01) groups. The livers of X group mice revealed increased expression of caspase-3 compared to groups C and XF (p < 0.01 for both). Significantly higher expression of caspase-3 was also observed in F group when compared to C group (p < 0.05). Flaxseed feeding decreased the expression of caspase-3 in the jejunum of mice exposed to xylene when compared to X group mice (p < 0.01).
Fig. 4.
Western blot analysis of caspase-3 expression in selected organs of the experimental mice. C, control group; X, xylene group; F, flaxseed group; XF, combined group with addition of flaxseed and xylene; Protein level was determined by Western blot; the diagrams represent the results of densitometry; the bands were normalized by β-actin; Data are means ± SEM; a,b Mean values in columns with same superscript letters differ significantly (a = p < 0.05, b = p < 0.01).
4. Discussion
Helping the public living and working near hazardous waste sites or oil wells, including scientists and hospital lab workers, people need to be familiar with variety of health effects of xylene and potential possibilities of the prevention and/or moderation of its adverse effects. Currently, experimental research is still needed in regions with high exposure to xylene including histopathology laboratories, leather and rubber industries, petrochemicals and steel manufacturing in order to collect more information related to the health hazards of xylene. New experimental studies were carried out to help in understanding of molecular mechanism and toxicity of xylene (Niaz et al., 2015, Salimi et al., 2017). Based on some studies (Bhatia et al., 2007, Zhang et al., 2017, Lee et al., 2018), the application of flaxseed to the feed of animals could prevent harmful effects of some toxic substances.
According to US Environmental Protection Agency (US EPA, 2003), 10 ppm (10 mg/l) is a legally enforceable maximum level of xylene in a public water system. Minimal risk level of 10 mg/kg/day has been derived for acute-duration (≤14 days) oral exposure to mixed xylene. In experimental studies, oral exposure to xylene at levels in a range of 1000 to 2000 mg/kg/day mixed xylenes resulted in reduced body weight and impaired respiration, and level of ≥4000 mg/kg caused serious neurological impairment in rats (ATSDR, 2007). We found increased hypersensitivity and restlessness of mice exposed to oral mixed xylenes in a dose of 400 mg/kg/day. Xylene in high concentrations acts as a narcotic, inducing neuropsychological and neural dysfunction (Salimi et al., 2017). Overally, there is lack of studies available concerning real exposure doses of xylene in humans and animals.
Both the flaxseed and xylene additives lowered body weights in all groups mice, with the highest weight loss in the group X, those exposed to xylene. This is in line with the study of US EPA (2003), in which mice exposed to xylene lost their weights by 15%. In our study, flaxseed added to the diet suppressed the negative effect of xylene on the weight of mice. Dietary flaxseed improved body condition score in pigs receiving 5, 10, or 15% concentration (Juárez et al., 2010), piglets (Andrejčáková et al., 2016, Sopková et al., 2017, Vlčková et al., 2018) and mice (Andrejčáková et al., 2020) with gut infections receiving 10% flaxseed in the diet. On the other hand, high portion of flaxseed in the diet fed for a long time period (more then 6 weeks) could cause lose weight in mice (Andrejčáková et al., 2020, Pourjafari et al., 2019) and humans (Mohammadi-Sartang et al., 2018) or had no effect on body condition score (Rodriguez-Leyva et al., 2013).
Harmful effect of xylene exposure depends on the route, the dose, and the length of exposure. Results of animal studies indicate that large amounts of xylene can cause changes in the liver, and harm the nervous system and kidneys at long-term oral exposure (ATSDR, 2007). To monitor harmfull effects of pollutants, which dissolve lipid membranes (Riihimaki, 1979), testing the integrity and fluidity of cell membrane may be used. One way to obtain cell membrane integrity is to determine the activity of enzymes in various tissues with tissue-specific or non-specific indication. Liver transaminases, including AST and ALT that catalyse the transfer of amino groups to produce products in gluconeogenesis and amino acid metabolism. Elevated serum levels of these aminotransferases indicate acute or chronic liver damage. Increasing evidence suggest that baseline serum levels of these enzymes may be associated with the development of a wide range of diseases (García Ferrera, 2013). Aspartate aminotransferase is found in a variety of tissues including the liver, brain, pancreas, heart, kidney, lung, and skeletal muscle. If any of these tissues was damaged, increased serum level of AST indicates non-specific tissue damage. Conversely, any increase in ALT is a direct indication of the liver damage. The higher the AST/ALT ratio (De Rites coefficient), the greater the damage to the liver parenchyma. We found the highest activities of both AST and ALT enzymes, and AST/ALT ratio in the liver tissue extracts of mice exposed to xylene, and the lowest such values in mice fed flaxseed. Moreover, administration of flaxseed stabilised the levels of liver enzymes in mice exposed to xylene, which indicates protective effect of flaxseed consumption. This result is in agreement with the study of Zhang et al. (2017), in which flaxseed oil rich in ALA and other PUFAs in diet reduced abnormally elevated AST and ALT levels in mice with artificially induced chronic ethanol consumption. Positive effects of flax-enriched diet (10%) applied for 8 weeks on AST and ALT activities were also observed in hypertensive rats (Al-Bishri, 2013) and piglets (Andrejčáková et al., 2016). Flaxseed also revaeled radioprotective and antioxidant potential, which was seen in alleviated levels of hepatal transaminases and glutathion peroxidase (Bhatia et al., 2007). The De Rites coefficient indicates the rate of necrotic or another liver cell damage. This coefficient shall not exceed value 1.0 in humans (Martín and Molina, 2020) and should be kept at 1.5 in mice (Envigo, 2015). In our study, adverse effect of xylene exposure was observed at the level of AST/ALT ratio of approximately 1.87, while in flaxseed group at the level of approximately 1.25 and in combined group 1.38. These results point at the protective effect of flaxseed consumption on mice.
Another valuable marker of the cell integrity and cell survival (Legrand et al., 1992) is LDH present in all organs and tissues (Kending and Tarloff, 2007, Andrejčáková et al., 2016, Andrejčáková et al., 2020). We found that xylene increased the release of isoenzyme LDH-5 specific for the liver, TLDH and all isoenzymes in the kidney, while dietary flaxseed suppressed these levels in mice exposed to xylene (X). These results indicate that dietary flaxseed downregulates LDH release from hepatocytes and renal cells. High release of LDH indicates nephron damage by action of xylene. The evidence that flaxseed application to the diet had lowering effect on LDH release from the cells was previously determined in our (Andrejčáková et al., 2016, Andrejčáková et al., 2020) and others (Nussinovitch et al., 2009, Crémet et al., 2015) studies. This effect could be associated with ALA of n-3 PUFAs series originated from flaxseed, whose incorporation in the cell membrane modulates its fluidity (Hussein et al., 2016, Lee et al., 2018).
Another possibility of observing cell membrane integrity is to measure the activity of acetylcholine in affected organs usually decreasing markedly after toxic damage (Liao et al., 2019). Xylene exposure for 14 days on tested mice was considered to have acute adverse effects on the organism (ATSDR, 2007). There are insufficient studies to address the effect of xylene application and no studies dealing with its application combined with flaxseed as a protector, on modulation of cholinesterase activity. We found reduced concentrations of cholinesterases in intoxicated groups as well as in flaxseed group in the liver and kidneys, while not in the brain. However, flaxseed mildly prevented the negative effect of xylene. Our findings are in line with ATSDR (2007) and study by Savoleinen and Pfaffli (1980). Although, these studies claimed that toxic effect of xylene on the brain might not be caused by xylene itself, but also by benzaldehyde, a product of xylene oxidation. Inhibited AChE activity results in accumulation of acetylcholine at nerve endings attacking the brain and other parts of the nervous system (Tallat et al., 2020). A decrease in cholinesterase concentration was also observed after exposure to anticholinergic drugs, organophosphate insecticides, and nerve gases, which can contain xylenes (Wallig et al., 2018). Our results, for the first time, suggest that toxic effect of xylenes downregulating activities of cholinesterases, mainly AChE, could be partially mitigated by dietary flaxseed.
Flaxseed revealed chemoprotective effect on many body organs, including the intestine, liver, and kidney (Salim et al., 2011, Al-Bishri, 2013, Shaikh Omar, 2018). We found that exposure of xylene did not reveal any adverse effect on the ultrastructure of the hepatal tissue in mice, although in mice fed flaxseed, the cytoplasm of some hepatocytes contained lipid droplets of various sizes. Only few studies dealed with the effects of xylene on changes in the liver revealing only increased activity of liver enzymes with no histopathological changes in the liver tissue (Condie et al., 1988, Ungváry, 1990). Our result is in line with the studies in mice (Wang et al., 2016) and hens (Aziza et al., 2019), in which mild accummulation of lipids in hepatocytes is common after flaxseed feeding due to high content of ALA. Nevertheless, we found that acute xylene exposure to mice at high dose had adverse effect on the kidneys showing atrophic and necrotic changes in the cortex. Dietary flaxseed did not markedly affect the structure of the kidneys, although when applied to xylene-exposed mice, flaxseed protected cortical structures against or mitigated adverse effects of, xylenes. Most of the studies used mixed xylene for intermediate or chronic durations showing no adverse effects on the kidneys (ATSDR, 2007), although Condie et al. (1988) observed increased early nephropathy in females exposed to 1500 mg/kg/day xylenes for 90 days. Even though, there is lack of studies aimed at the protective effects of flaxseed against xylene exposure, Shaikh Omar (2018) observed similar histopathological changes showing severe alterations in the structure of renal corpuscles after thioacetamide exposure of rats, which were protected by the application of flaxseed oil.
Apoptosis, or programmed cell death, follows either extrinsic pathways associated with one of the members of the tumour necrosis factor receptor family, or intrinsic pathways, associated with cytochrome C release from mitochondria (Elmore, 2016). Caspases take part in both the extrinsic and intrinsic apoptosis pathways. Caspase-3, also known as the executioner caspase, is responsible for the morphological changes such as chromatin condensation, DNA breakdown laddering, and breakdown of membrane protein (D’Amelio et al., 2010). Therefore, caspase-3 is considered a universal indicator of an apoptotic cell death (Ostapchenko et al., 2019).
Similarly to study of Seydi et al. (2015), our results showed that xylene induced caspase-3 activation, the final mediator of apoptosis signaling. These findings contribute to a better understanding underlying mechanisms involved in xylene toxicity originating from the oxidative stress and ending in mitochondrial/lysosomal damage and cell death signaling pathways.
Many organic solvents increase activity of hepatal caspase-3 (Srilaxmi et al., 2010, Seydi et al., 2015), which could be used as prognostic marker of the liver damage after organophosphate poisoning in humans (Tallat et al., 2020). In our study, dietary flaxseed elevated caspase-3 expression in the brains, livers, and jejunums of mice, although down regulated its activity in xylene-exposed mice. Apoptosis-inducing effect of xylene via activation of oxidative stress in isolated human lymphocytes (Salimi et al., 2017) and porcine proximal tubular cells (Al –Ghamdi et al., 2004) is evident. There are a few studies concerning the protective effect of natural additives to organic solvents. Shaffie and Shabana (2019) revealed that citicoline treatment could decrease caspase-3 in rats with toluene-induced toxicity, which is comparable with xylene. Flaxseed was proven to have pro-apoptotic effect in various tissues, including ovarian tissue (Kádasi et al., 2015, Vlčková et al., 2018), and colon cancer cells (Bommareddy et al., 2010, Hernández-Salazar et al., 2013). In terms of the gut physiology, apoptosis can be defined as a mechanism where excess or redundant cells are degenerated and removed during development and restricted tissue size is maintained as a normal and controlled process of an organism growth or development (Otles and Ozgoz, 2014). The increase in the apoptosis could have been a compensatory mechanism to open the way for replenishing mucosal cells following the damage. This mechanism may be affected by viscous properties of the non-starch polysaccharides mucilage (Stanogias and Pearce, 1985) and high content of ALA, eicosapentaenoic, docosahexaenoic acid, enterolactone, and enterodiol (Ndou et al., 2018) originated from flaxseed.
5. Conclusion
As the mechanism of action and the functional relationship between environmental pollutants, medicinal plants, and metabolism are still unknown, further research in this area is required for elucidation. Nevertheless, our observations represent the first demonstration that acute highly-dosed xylene oral exposure had an adverse effect on animal behaviour, led to weight loss, damaged renal cortex and cell membranes of the brain, liver, and kidney of mice reflecting in increased LDH, transaminases and decreased AChE activities, and it had a pro-apoptotic effect on the cells of the brain, liver, and jejunum. On the other hand, dietary flaxseed helped to improve animal behaviour, weight gains, maintained cell membrane integrity of selected organs alleviating the symptoms of xylene intoxication by reducing the release of liver transaminases and LDH, increasing activity of AChE, as well as suppressing apoptosis. These results point at the protective effect of flaxseed consumption on mice.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to thank Paul McEvilly, DVM for English corrections. This work was financially supported by the projects of the Ministry of Education, Science, Research and Sport of the Slovak Republic VEGA No. 1/0204/20 and 1/0392/17.
Footnotes
Peer review under responsibility of King Saud University.
References
- ATSDR (Agency for Toxic Substances and Disease Registry, 2007). A Toxicological Profile for Xylene, U.S. Department of health and human services, https://www.atsdr.cdc.gov/ToxProfiles/tp71.pdf. Accessed 10 September 2007.
- Al-Bishri W.M. Favorable effects of flaxseed supplemented diet on liver and kidney functions in hypertensive wistar rats. J. Oleo Sci. 2013;62:709–715. doi: 10.5650/jos.62.709. [DOI] [PubMed] [Google Scholar]
- Al –Ghamdi, S.S., Raftery, M.J., Yaqoob, M.M., 2004. Toluene and p-xylene induced LLC-PK1 apoptosis. Drug Chem Toxicol 27, 425–432. [DOI] [PubMed]
- Andrejčáková Z., Sopková D., Vlčková R., Kulichová L., Gancarčíková S., Almášiová V., Holovská K., Petrilla V., Krešáková L. Synbiotics suppress the release of lactate dehydrogenase, promote non-specific immunity and integrity of jejunum mucosa in piglets. Anim. Sci. J. 2016;87:1157–1166. doi: 10.1111/asj.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrejčáková Z., Sopková D., Vlčková R., Hertelyová Z., Gancarčíková S., Nemcová R. The application of Lactobacillus reuteri CCM 8617 and flaxseed positively improved the health of mice challenged with enterotoxigenic E. coli O149:F4. Probiotics Antimicro. Prot. 2020;12:937–951. doi: 10.1007/s12602-019-09578-x. [DOI] [PubMed] [Google Scholar]
- Aziza A.E., Awadin W., Cherian G. Impact of choline supplementation on hepatic histopathology, phospholipid content, and tocopherol status in layer hens fed flaxseed. J. Appl. Poult. Res. 2019;28:679–687. [Google Scholar]
- Bhatia A.L., Sharma A., Patni S., Sharma A.L. Prophylactic effect of flaxseed oil against radiation-induced hepatotoxicity in mice. Phytother Res. 2007;21:852–859. doi: 10.1002/ptr.2169. [DOI] [PubMed] [Google Scholar]
- Bommareddy A., Zhang X.Y., Kaushik R.S., Dwivedi C. Effects of components present in flaxseed on human colon adenocarcinoma Caco-2 cells: possible mechanisms of flaxseed on colon cancer development in animals. Drug Discoveries & Therapeutics. 2010;4:184–189. [PubMed] [Google Scholar]
- Bomba A., Brandeburová A., Ričanyová J., Strojný L., Chmelárová A., Szabadosová V., Pramuková P., Žofčáková J., Salaj R., Supuková A., Čokášová D. The role of probiotics and natural bioactive compounds in modulation of the common molecular pathways in pathogenesis of atherosclerosis and cancer. Biologia. 2012;67:1–13. [Google Scholar]
- Calado A., Neves P.M., Santos T., Ravasco P. The effect of flaxseed in breast cancer: a literature review. Front Nutr. 2018;5:4. doi: 10.3389/fnut.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel T., Henderson G., Alpert C.-A., Philippe C., Rigottier-Gois L., Dore J., Blaut M. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl. Environ. Microb. 2005;71:6077–6085. doi: 10.1128/AEM.71.10.6077-6085.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condie L.W., Hill J.R., Borzelleca J.F. Oral toxicology studies with xylene isomers and mixed xylenes. Drug. Chem. Toxicol. 1988;11:329–354. doi: 10.3109/01480548809018107. [DOI] [PubMed] [Google Scholar]
- Crémet L., Broquet A., Brulin B., Jacqueline C., Dauvergne S., Brion R., Asehnoune K., Corvec S., Heymann D., Caroff N. Pathogenic potential of Escherichia coli clinical strains from orthopedic implant infections towards human osteoblastic cells. Pathog. Dis. 2015;73:ftv065. doi: 10.1093/femspd/ftv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amelio M., Cavallucci V., Cecconi F. Neuronal caspase-3 signaling: not only cell death. Cell Death Differ. 2010;17:1104–1114. doi: 10.1038/cdd.2009.180. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2016;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Envigo, 2015. Hematology and Clinical Chemistry — Female ICR (CD-1®) Outbred Mice. https://insights.envigo.com/hubfs/resources/data-sheets/icr-cd-1-7-8-wk-females-cbc.pdf. Accessed 16 September 2015.
- García Ferrera W.O. ¿Cómo evaluar la elevación de las enzimas hepáticas en personas aparentemente sanas? Su importancia para el médico general. Revista de Gastroenterología del Perú. 2013;33:262–264. [PubMed] [Google Scholar]
- Heinová D., Rosival I., Avidar Y., Bogin E. Lactate dehydrogenase isoenzyme distribution and patterns in chicken organs. Res Vet Sci. 1999;67:309–312. doi: 10.1053/rvsc.1999.0317. [DOI] [PubMed] [Google Scholar]
- Hernández-Salazar M., Guevara-González R.G., Cruz-Hernández A., Guevara-Olvera L., Bello-Pérez L.A., Castaño-Tostado E., Loarca-Piña G. Flaxseed (Linum usitatissimum L.) and its total non-digestible fraction influence the expression of genes involved in azoxymethane-induced colon cancer in rats. Plant Foods Hum Nutr. 2013;68:259–267. doi: 10.1007/s11130-013-0372-y. [DOI] [PubMed] [Google Scholar]
- Hussein S.A., El Senosi Y.A.F., Hassanien M.R., Hammad M.-M.F. Evaluation of the protective role of flaxseed oil on inflammatory mediators, antioxidant defense system and oxidative stress of liver tissue in hypercholesterolemic rats. Int. J. Pharm. Sci. 2016;6:1480–1489. [Google Scholar]
- Juárez M., Dugan M.E., Aldai N., Aalhus J.L., Patience J.F., Zijlstra R.T., Beaulieu A.D. Feeding co-extruded flaxseed to pigs: Effects of duration and feeding level on growth performance and backfat fatty acid composition of grower–finisher pigs. Meat. Sci. 2010;84:578–584. doi: 10.1016/j.meatsci.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Kandyala R., Raghavendra S.P., Rajasekharan S.T. Xylene: An overview of its health hazards and preventive measures. J. Oral. Maxillofac Pathol. 2010;14:1–15. doi: 10.4103/0973-029X.64299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kádasi A., Štochmaľová A., Maruniaková N., Kolesárová A., Grossman R., Sirotkin A.V. Effect of natural plant extracts on porcine functions. J. Microbiol. Biotech. Food Sci. 2015;4(specialissue 2):45–48. [Google Scholar]
- Kending D.M., Tarloff J.B. Inactivation of lactate dehydrogenase by several chemicals: Implications for in vitro toxicology studies. Toxicol. In Vitro. 2007;21:125–132. doi: 10.1016/j.tiv.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuráňová E., Andrejčáková Z., Sopková D., Vlčková R. Protective effect of flaxseed on the health of experimental animals exposed to xylene. Folia Veterinaria. 2020;64:38–45. [Google Scholar]
- Langman J.M. Xylene: its toxicity, measurement of exposure levels, absorption, metabolism and clearance. Pathology. 1994;26:301–309. doi: 10.1080/00313029400169711. [DOI] [PubMed] [Google Scholar]
- Laurino C., Palmieri B., Vadalà M. Gastrointestinal activity of dietary flaxseed lignans, omega-3 fatty acids and fbres. Nutrafoods. 2017;16:9. [Google Scholar]
- Lee A.Y., Choi J.M., Lee M.H., Lee J., Lee S., Cho E.J. Protective effects of perilla oil and alpha linolenic acid on SH-SY5Y neuronal cell death induced by hydrogen peroxide. Nutr. Res. Pract. 2018;12:93–100. doi: 10.4162/nrp.2018.12.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand C., Bour J.M., Capiaumont J., Martial A., Marc A., Wudtke M., Kretzmer G., Demangel C., Duval D., Hache J. Lactate dehydrogenase (LDH) activity of the number of dead cells in the medium of cultured eukaryotic cells as marker. J. Biotechnol. 1992;25:231–243. doi: 10.1016/0168-1656(92)90158-6. [DOI] [PubMed] [Google Scholar]
- Liao Z., Jaular L.M., Soueidi E., Jouve M., Muth D.C., Schøyen T.H., Seale T., Haughey N.J., Ostrowski M., Théry C., Witwer K.W. Acetylcholinesterase is not a generic marker of extracellular vesicles. J. Extracell Vesicles. 2019;8:1628592. doi: 10.1080/20013078.2019.1628592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín M., Molina A. Protocolos diagnóstico-terapéuticos de Gastroenterología; Hepatología y Nutrición Pediátrica SEGHNP-AEP: 2020. Transaminases: Valoración y significación clínica; pp. 267–275. [Google Scholar]
- Mohammadi-Sartang M., Sohrabi Z., Barati-Boldaji R., Raeisi-Dehkordi H., Mazloom Z. Flaxseed supplementation on glucose control and insulin sensitivity: a systematic review and meta-analysis of 25 randomized, placebo-controlled trials. Nutr. Rev. 2018;76:125–139. doi: 10.1093/nutrit/nux052. [DOI] [PubMed] [Google Scholar]
- Ndou S.P., Tun H.M., Kiarie E., Walsh M.C., Khafipour E., Nyachoti C.M. Dietary supplementation with flaxseed meal and oat hulls modulates intestinal histomorphometric characteristics, digesta- and mucosa-associated microbiota in pigs. Sci Rep. 2018;8:5880. doi: 10.1038/s41598-018-24043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaz K., Bahadar H., Maqbool F., Abdollahi M. A review of environmental and occupational exposure to xylene and its health concerns. EXCLI J. 2015;14:1167–1186. doi: 10.17179/excli2015-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussinovitch M., Finkelstein Y., Elishkevitz K.P., Volovitz B., Harel D., Klinger G., Razon Y., Nussinovitch U., Nussinovitch N. Cerebrospinal fluid lactate dehydrogenase isoenzymes in children with bacterial and aseptic meningitis. Transl. Res. 2009;154:214–218. doi: 10.1016/j.trsl.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Ostapchenko, V.G., Snir, J., Suchy, M., Fan, J., Cobb, M.R., Chronik, B.A., Kovacs, M., Prado, V.F., Hudson, R.H.E., Pasternak, S.H., Prado, M.A.M., Bartha. R., 2019. Detection of active caspase-3 in mouse models of stroke and Alzheimer’s disease with a novel dual positron emission tomography/fluorescent tracer [68Ga]Ga-TC3-OGDOTA. Contrast Media Mol Imaging 6403274. [DOI] [PMC free article] [PubMed]
- Otles S., Ozgoz S. Health efects of dietary fibre. Acta Sci. Pol. Technol. Aliment. 2014;13:191–202. [PubMed] [Google Scholar]
- Pourjafari F., Haghpanah T., Sharififar F., Nematollahi-Mahani S.N., Afgar A., Karam G.A., Ezzatabadipour M. Protective effects of hydro-alcoholic extract of foeniculum vulgare and linum usitatissimum on ovarian follicle reserve in the first-generation mouse pups. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K., Jadhav A. Prevention and treatment of atherosclerosis with flaxseed-derived compound secoisolariciresinol diglucoside. Curr. Pharm. Des. 2016;22:214–220. doi: 10.2174/1381612822666151112151130. [DOI] [PubMed] [Google Scholar]
- Rietjens I.M., Sotoca A.M., Vervoort J., Louisse J. Mechanisms underlying the dualistic mode of action of major soy isoflavones in relation to cell proliferation and cancer risks. Mol. Nutr. Food Res. 2013;57:100–113. doi: 10.1002/mnfr.201200439. [DOI] [PubMed] [Google Scholar]
- Riihimaki V. Percutaneous absorption of m-xylene from a mixture of m-xylene and isobutyl alcohol in man. Scand. J. Work Environ. Health. 1979;5:143–150. doi: 10.5271/sjweh.2658. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Leyva D., Weighell W., Edel A.L., La Vallee R., Dibrov E., Pinneker R., Maddaford T.G., Ramjiawan B., Aliani M., Guzman R., Pierce G.N. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension. 2013;62:1081–1089. doi: 10.1161/HYPERTENSIONAHA.113.02094. [DOI] [PubMed] [Google Scholar]
- Salim E.I., Abou-Shafey A.E., Masoud A.A., Elgendy S.A. Cancer chemopreventive potential of the egyptian flaxseed oil in a rat colon carcinogenesis bioassay – implications for its mechanism of action. Asian Pacifc J. Can. Prev. 2011;12:2385–2392. [PubMed] [Google Scholar]
- Salimi A., Talatappe B.S., Pourahmad J. Xylene induces oxidative stress and mitochondria damage in isolated human lymphocytes. Toxicol. Res. 2017;33:233–238. doi: 10.5487/TR.2017.33.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoleinen H., Pfaffli P. Dose-dependent neurochemical changes during short-term inhalation exposure to m-xylene. Arch. Toxicol. 1980;45:117–122. doi: 10.1007/BF01270909. [DOI] [PubMed] [Google Scholar]
- Seydi E., Rajabi M., Salimi A., Pourahmad J. Involvement of mitochondrial-mediated caspase-3 activation and lysosomal labilization in acrylamide-induced liver toxicity. Toxicol. Environ. Chem. 2015;97:563–575. [Google Scholar]
- Shaffie N., Shabana M.E. Role of citicoline as a protective agent on toluene-induced toxicity in rats. J. Arab. Soc. Med. Res. 2019;14:14–24. [Google Scholar]
- Shaikh Omar A.M. The potential protective influence of flaxseed oil against renal toxicity induced by thioacetamide in rats. Saudi J. Biol. Sci. 2018;25:1696–1702. doi: 10.1016/j.sjbs.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen A.J. Image analytic techniques for quantification of immunocytochemical staining in the nervous system. In: Conn P.M., editor. Methods in Neurosciences. Academic Press; New York: 1990. pp. 208–229. [Google Scholar]
- Sopková D., Hertelyová Z., Andrejčáková Z., Vlčková R., Gancarčíková S., Petrilla V., Ondrašovičová S., Krešáková L. The application of probiotics and flaxseed promotes metabolism of n-3 polyunsaturated fatty acids in pigs. J. Appl. Anim. Res. 2017;45:93–98. [Google Scholar]
- Srilaxmi P., Sareddy G.R., Kavi Kishor P.B., Setty O.H., Babu P.P. Protective efficacy of natansnin, a dibenzoyl glycoside from Salvinia natans against CCl4 induced oxidative stress and cellular degeneration in rat liver. BMC Pharmacol. 2010;12:13. doi: 10.1186/1471-2210-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanogias G., Pearce G.R. The digestion of fibre by pigs. 2. Volatile fatty acid concentrations in large intestine digesta. Br J. Nutr. 1985;53:513–536. doi: 10.1079/bjn19850062. [DOI] [PubMed] [Google Scholar]
- Strojný L., Štofilová J., Hijová E., Szabadosová V., Salaj R., Bertková I., Chmelárová A., Čokášová D., Pramuková B., Brandeburová A., Bomba A., Bobrov N., Suchánek P. Effect of Lactobacillus plantarum LS/07 in combination with flaxseed oil on the microflora, enzymatic activity, and histological changes in the development of chemically induced precancerous growth in the rat colon. Czech J. Anim. Sci. 2014;59:268–277. [Google Scholar]
- Tallat S., Hussien R., Mohamed R.H., Abd El Wahab M.B., Mahmoud M. Caspases as prognostic markers and mortality predictors in acute organophosphorus poisoning. J. Genet. Eng. Biotechnol. 2020;18:10. doi: 10.1186/s43141-020-00024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungváry G. The effect of xylene exposure on the liver. Acta Morphol Hung. 1990;38:245–258. [PubMed] [Google Scholar]
- US EPA (United States Environmental Protection Agency), 2003. Toxicological Review of Xylenes (CAS No. 1330-20-7), Support of Summary Information on the Integrated Risk Information System (IRIS) 2003, p.26. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0270tr.pdf Accessed January 2003.
- Vlčková R., Andrejčáková Z., Sopková D., Hertelyová Z., Kozioł K., Koziorowski M., Gancarčíková S. Supplemental flaxseed modulates ovarian functions of weanling gilts via the action of selected fatty acids. Anim. Reprod. Sci. 2018;193:171–181. doi: 10.1016/j.anireprosci.2018.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallig, M.A., Rousseaux, C.G., Haschek, W.M., Bolon, B., 2018. Fundamentals of toxicologic pathology (3rd Edition), Chapter – 15, 395–442.
- Wang H., Wang J., Guo X., Brennan Ch.S., Li T., Fu X., Chen G., Liu R.H. Effect of germination on lignan biosynthesis, and antioxidant and antiproliferative activities in flaxseed (Linum Usitatissimum L.) Food Chem. 2016;205:170–177. doi: 10.1016/j.foodchem.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Yanagihara N., Zhang H., Toyohira Y., Takahashi K., Ueno S., Tsuitsui M., Takahashi K. New insights into the pharmacological potential of plant flavonoids in the catecholamine system. J. Pharmacol. Sci. 2014;124:123–128. doi: 10.1254/jphs.13r17cp. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang H., Yin P., Fan H., Sun L., Liu Y. Flaxseed oil ameliorates alcoholic liver disease via anti-inflammation and modulating gut microbiota in mice. Lipids Health Dis. 2017;16:44. doi: 10.1186/s12944-017-0431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]