Abstract
Tumor spread through air spaces (STAS) in non-small-cell lung cancer (NSCLC) is known to influence a poor patient outcome, even in patients presenting with early-stage disease. However, the pre-operative diagnosis of STAS remains challenging. With the progress of radiomics-based analyses several attempts have been made to predict STAS based on radiological findings. In the present study, patients with NSCLC which is located peripherally and tumors ≤ 2 cm in size on computed tomography (CT) that were potential candidates for sublobar resection were enrolled in this study. The radiologic features of the targeted tumors on thin-section CT were extracted using the PyRadiomics v3.0 software package, and a predictive model for STAS was built using the t-test and XGBoost. Thirty-five out of 226 patients had a STAS histology. The predictive model of STAS indicated an area under the receiver-operator characteristic curve (AUC) of 0.77. There was no significant difference in the overall survival (OS) for lobectomy between the predicted-STAS (+) and (−) groups (p = 0.19), but an unfavorable OS for sublobar resection was indicated in the predicted-STAS (+) group (p < 0.01). These results suggest that radiomics with machine-learning helped to develop a favorable model of STAS (+) NSCLC, which might be useful for the proper selection of candidates who should undergo sublobar resection.
Subject terms: Non-small-cell lung cancer, Image processing
Introduction
Kawakami et al. first reported the findings for tumor spread through air spaces (STAS) in pulmonary adenocarcinoma in 20081. The 4th Edition WHO Classification of Tumor of The Lung Pleura, Thymus and Heart describe STAS as a novel invasive morphology depicted as micropapillary clusters, solid nests, or single cells beyond the edge of the tumor spreading into air spaces in the surrounding lung parenchyma2. STAS is often associated with aggressive subtypes of pulmonary adenocarcinoma, such as micropapillary and solid pattern, and 30–50% of pulmonary adenocarcinomas manifest STAS findings. Even in early-stage disease, positivity for STAS is regarded as a poor prognostic characteristic3,4. In addition to adenocarcinoma histology, STAS coexists with other histologies, such as squamous cell carcinoma, pleomorphic carcinoma and neuroendocrine carcinoma. A poor postoperative outcome is a common feature for STAS-positive non-small-cell lung cancer5–8.
The standard procedure for lung cancer has been lobectomy with lymph node dissection, and patients with small lung cancer (≤ 2 cm) have shown an excellent long-term survival after lobectomy9. However, the efficacy of sublobar resection for managing small lung cancer (≤ 2 cm) remains controversial10,11. In a nonrandomized confirmatory phase III trial that assessed sublobar resection for peripheral ground-glass opacity (GGO)-dominant lung cancer, such as those with a maximum tumor diameter ≤ 2 cm and a consolidation/tumor ratio (C/T ratio) ≤ 0.25 on thin-section CT (Japan Clinical Oncology Group 0804/WJOG4507L), sublobar resection was shown to offer a sufficient degree of local control and recurrence-free survival12. We are now awaiting the results of a randomized controlled trial to confirm the noninferiority of segmentectomy to lobectomy with regard to the prognosis according to the JCOG0802/WJOG4607L trial, although the safety of segmentectomy has already been reported (JCOG0802/WJOG4607L)13.
Sublobar resection has been reported to have a poor prognosis in patients with a micropapillary component of ≥ 5%, even for small lung cancers with a maximum tumor diameter of ≤ 2 cm14. The preoperative diagnosis of aggressive histological subtypes, including STAS, is important to determine the proper operative procedure; however, making pathological and radiological diagnoses before surgery remains difficult. Regarding radiology, a larger tumor size and greater degree of solid component were reportedly correlated with the presence of STAS15,16; however, no evidence has been obtained concerning correlations for small, peripheral tumors (< 2 cm), which may be candidates for limited surgery, such as segmentectomy. It was also shown to be difficult to diagnose STAS by cytology, with a reported sensitivity of 45%, which was not sufficient for a pre-surgical diagnosis17.
Predicting aggressive histological subtypes or STAS preoperatively by imaging using novel image analysis technologies might therefore help surgeons determine the optimal surgical treatment plan.
With the introduction of radiomics-based machine-learning analyses, several reports have provided new insight into aspects such as the diagnosis of cancer and prognosis prediction18–20. Recently, two groups reported that radiomics-based analyses of CT findings could predict the existence of STAS in lung adenocarcinoma21,22.
STAS is observed in several histologies, and its existence is known to be related to the surgical outcome. We therefore retrospectively reviewed the histology and CT findings of patients who underwent surgical resection for ≤ 2 cm lung cancers. Using preoperative thin-section CT images, we developed a radiomics-based machine-learning prediction model of STAS-positive lung cancer, without using any clinical information. We also conducted a survival analysis comparing the radiomics results and actual histological STAS results to assess the utility of the prediction model as a preoperative surgical planning tool.
Methods
Patient selection
We retrospectively reviewed the patients who underwent surgical resection of lung cancer between January 2013 and December 2018 at our institution. Peripheral lung tumors with the maximum axial diameter should be ≤ 2 cm that were suitable for limited surgery were included, and cases with pre-operative or frozen sections during surgery proving the presence of small-cell lung cancer were excluded from this study. Preoperative thin-section plain CT (≤ 1 mm slice thickness) performed within 2 months prior to surgery was mandatory for eligibility.
The clinical information and follow-up data were obtained from electronic medical records. The clinicopathologic features, including the age, sex, smoking history, pulmonary function, CT images, surgical procedure, pathological diagnosis following the 7th edition of TNM classification, histologic subtype following the 2015 WHO classification, and EGFR mutation status, were examined. Perioperative surgical treatment was performed following the standard thoracic surgery guidelines23. The standard surgical procedure for lung cancer at our institute is lobectomy with systematic lymph node dissection, but limited surgery is adopted for peripheral GGO-dominant lung cancer or patients who are compromised, such as those with comorbidities, a low pulmonary function, or an older age who are indicated for palliative care.
Routine postoperative follow-up included regular physical examinations, blood tests, chest X-ray, and chest CT. Fluorodeoxyglucose-positron emission tomography (FDG-PET) and brain magnetic resonance imaging (MRI) were performed on demand according to the physician’s decision.
The histological diagnosis
The histological diagnostic information was obtained from official pathology reports made by board-certified pathologists. All formalin-fixed, paraffin-embedded 4-μm sections with hematoxylin–eosin staining were then re-evaluated by a pathology-trained physician (J.M.) to confirm the STAS diagnosis. We previously reported free tumor clusters as a risk factor for a poor prognosis24. Following the latest WHO classification2, STAS was defined as micropapillary clusters, solid nests, or single cells beyond the edge of the tumor extending into the air spaces in the surrounding lung parenchyma. To avoid false positives due to extraneous factors, such as artifacts induced by cutting though a tumor with a knife25, we developed criteria to diagnose STAS by histology. For example, haphazardly distributed fragments of a tumor cells (clusters) with sharp jagged edges on microscopy were regarded as “artifacts STAS” which meant artifactual floaters caused by the steps of making histological sections by knife. We excluded such artifacts STAS from our STAS criteria26. To avoid compromising the reproducibility of the STAS assessment, STAS was defined as > 3 clusters floating in an alveolar space > 3 mm away from the main tumor margin. Regarding intrinsic factors, the invasive mucinous adenocarcinoma manifest goblet or columnar tumor cells spread diffusely to the surrounding alveoli via the airway. Therefore, invasive mucinous adenocarcinoma usually shows a STAS-like growth pattern and thus it cannot be regarded as STAS in this study.
Chest CT and tumor segmentation
All patients underwent chest CT within two months prior to surgery. Chest CT was performed in the supine position during an inspiratory breath-hold using various multidetector row scanners: an Aquilion prime (CANON MEDICAL SYSTEMS CORPORATION, Tochigi, Japan), Aquilion ONE (CANON), Alexion (CANON), Activion16 (CANON), and Aquilion64 (CANON). All CT data were resampled at 0.5 × 0.5 × 1.0 mm resolution with trilinear interpolation.
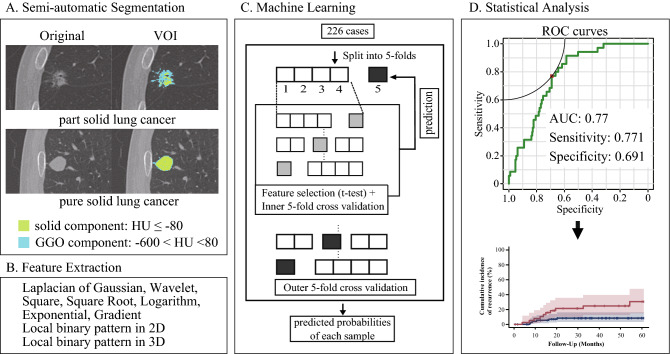
Targeted lung cancer was segmented with a semiautomated method using the 3D Slicer software program, ver. 4.10.2 (https://www.slicer.org/). Lung cancer was denoted with solid and ground-glass components. We defined solid components as those of > − 80 Hounsfield Unit (HU) and ground-glass opacity as components of > − 600 HU and < − 80 HU. The volume of interest (VOI) of lung cancer was delineated by the fusion of the solid and ground-glass parts.
Feature extraction and machine learning
Imaging features were extracted using the PyRadiomics v3.0 open-source software program (http://www.radiomics.io/pyradiomics.html27. Absolute rescaling (− 600 to 1300 HU) was applied. Pixel values between the upper and lower limits were resampled at 64 levels uniformly. The morphology, histogram, and texture features were calculated from each VOI on original and filtered images (Laplacian of Gaussian, Wavelet, Square, Square Root, Logarithm, Exponential, Gradient, local binary pattern in 2D, and local binary pattern in 3D). A total of 1874 features were thus extracted.
Several processes were performed to construct a predictive model for STAS using R version 3.5.1 (R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/). First, a t-test was used to select the features that differed significantly between cases with and without STAS. Second, feature selection with recursive feature elimination was performed in five-fold cross-validation. We tuned the hyperparameters in a training set divided so that the STAS prevalence was equivalent to a test set and assessed how well the model could be generalized to unseen data in five-fold cross validation. The model was built with XGBoost, and the probability of STAS in each patient within a test set was calculated. This method was performed five times for mutually different test sets, and the probability was calculated for all patients.
Statistical analyses
For the clinical characteristics, we constructed summary statistics and compared the patient characteristics using Fisher’s exact test and chi-square test for categorical outcomes and t-tests or Wilcoxon’s rank sum test for continuous variables, as appropriate.
Four of the patients included in the analysis predicting STAS underwent surgery 2 times during the study period. Therefore, survival curves were analyzed excluding those patients (Fig. 1). The survival curves were generated with the Kaplan–Meier method and compared with the log-rank test for STAS. The cumulative incidence of recurrence (CIR) was analyzed using a competing risk analysis with Gray’s test28. Competing risks are defined as events that prevent the outcome of interest from occurring. Censoring patients at the time of death would lead to a biased probability of the outcome of interest as estimated by the Kaplan–Meier method and would likely overestimate the probability of recurrence. Therefore, the patients were censored if they were alive without recurrence at the latest follow-up.
Figure 1.
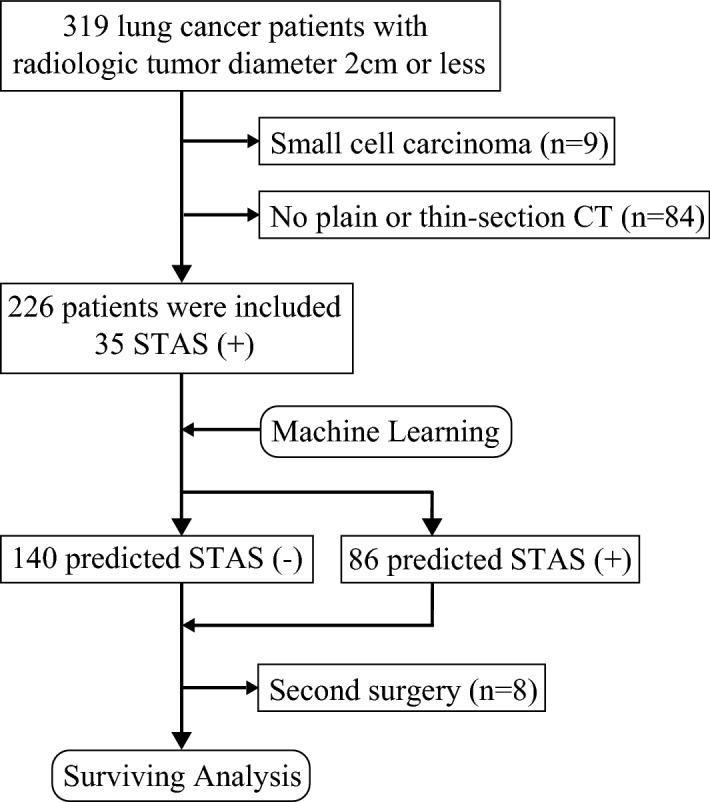
Study cohort flow chart. Peripheral tumors ≤ 2 cm in size on computed tomography that were potential candidates for sublobar resection were enrolled. Four of the patients included in the analysis predicting STAS underwent surgery 2 times during the period. Therefore, the survival curves were analyzed excluding those patients.
The machine-learning model produced probabilities for STAS that were used for the receiver operating characteristic (ROC) analysis. A threshold to binarize the model output was calculated from the point closest to the top-left part of the ROC curve. Whether the predicted STAS was positive or negative was defined using this threshold. The overall survival (OS) and CIR based on the predicted presence of STAS were analyzed (Fig. 2).
Figure 2.
The VOI of lung cancer was delineated, and imaging features were extracted. First, the t-test was used to select features between cases STAS(+)/(−). Second, feature selection with recursive feature elimination was performed. Third, the features were put into XGBoost. The cut-off value was calculated with a ROC analysis, and then the survival curves were analyzed. (D, R version 3.5.1, R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/).
All statistical tests used a 5% level of statistical significance. Statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R statistical program (The R Foundation for statistical Computing, Vienna, Austria).
The R codes of machine-learning used in this study have been uploaded to the following URL: https://github.com/hy-423/R_STAS/blob/main/R_result20200902.R.
Study approval
The study is based on the principles of the Helsinki declaration. The institutional review board (No.3809) at Chiba University Graduate School of Medicine reviewed and approved this study. The need for informed consent was waived by the institutional review board at Chiba University Graduate School of Medicine (No.3809) due to the retrospective nature of the study.
Results
Patients’ characteristics
Three hundred and nineteen patients underwent thoracotomy for lung cancer ≤ 2 cm during this study period. Of them, 226 patients ultimately met the study criteria, and 35 (15.4%) patients harbored the STAS histology.
The characteristics of the patients are summarized in Table 1. No significant differences were observed between the STAS (+) and STAS (−) groups with respect to the age, gender, or comorbidities. Regarding the chest CT findings, there was no significant difference in the tumor diameter between the groups. Regarding the consolidation/tumor ratio (C/T ratio), the STAS (+) group included a significantly larger population with a ratio > 0.25 (p = 0.03). The surgical procedures (lobectomy or sublobar resection) were equally distributed between the groups. Regarding the pathological findings, nodal metastasis was found in 9 patients (26%) (p < 0.001) in the STAS (+) group; the overall p-Stage was thus significantly higher in the STAS (+) group than in the STAS (−) group. Lymphatic invasion was more frequently observed in the STAS (+) group than in the STAS (−) group (p < 0.01). There was no significant difference in the incidence of an EGFR mutation between the groups (p = 0.70).
Table 1.
Patients’ characteristics.
| Total (N = 226) | STAS negative (n = 191) | STAS positive (n = 35) | p value |
|---|---|---|---|
| Age (y) (median, range) | 70 (39–88) | 68 (38–89) | 0.29 |
| Gender, male/female | 103 (54%)/88 (46%) | 20 (57%)/15 (43%) | 0.85 |
| Brinckmann index (median, range) | 300 (0–2760) | 500 (0–1600) | 0.33 |
| FEV1 (L) | 2.13 (0.95–4.11) | 2.08 (0.9–3.95) | 0.33 |
| FEV1 predicted (%) | 90.6 (45.9–140.5) | 90.2 (49.7–138.7) | 0.81 |
| Comorbidities | |||
| Chronic obstructive pulmonary disease | 69 (36%) | 14 (40%) | 0.71 |
| Cardiac disorder | 36 (19%) | 9 (26%) | 0.36 |
| Hypertension | 62 (32%) | 10 (29%) | 0.70 |
| Diabetes | 33 (17%) | 3 (9%) | 0.31 |
| Radiologic tumor diameter | 14.0 (5.6–2.0) | 14.2 (7.0–19.0) | 0.82 |
| C/T ratio ≦0.25/ > 0.25 | 40 (21%)/151 (79%) | 2 (6%)/33 (94%) | 0.03 |
| Surgical procedure | 0.28 | ||
| Lobectomy | 86 (45%) | 20 (57%) | |
| Segmentectomy | 70 (37%) | 8 (23%) | |
| Wedge resection | 35 (18%) | 7 (20%) | |
| Tumor histology | < 0.01 | ||
| Adenocarcinoma | 150 (79%) | 31 (89%) | |
| AIS, MIA/Lepidic/Papillary/Acinar/Solid/Micropapillary/Variant | 68 (45%)/12 (8%)/46 (31%)/10 (7%)/ 5 (3%)/0 (0%)/9 (6%) | 1 (3%)/0 (0%)/18 (58%)/4 (13%)/ 0 (0%)/4 (13%)/4 (13%) | < 0.001 |
| Squamous cell carcinoma | 34 (18%) | 1 (3%) | |
| Others | 7 (4%) | 3 (9%) | |
| Pathologic stage | |||
| T1a/T1b/T2/T3/T4 | 148 (77%)/11 (6%)/30 (16%)/2 (1%)/0(0%) | 22 (63%)/7 (20%)/6 (17%)/0 (0%)/0 (0%) | 0.07 |
| N0/N1/N2/N3/NX | 152 (79%)/1 (1%)/5 (3%)/0 (0%)/33 (17%) | 24 (69%)/5 (14%)/4 (11%)/0 (0%)/2 (6%) | < 0.001 |
| Stage IA/IB/II/III/IV | 156 (81%)/26 (14%)/4 (2%)/5 (3%)/0 (0%) | 22 (63%)/4 (11%)/5 (15%)/4 (11%)/0 (0%) | < 0.01 |
| Pathologic lymphatic invasion | 12 (6%) | 8 (23%) | < 0.01 |
| Pathologic vascular invasion | 34 (18%) | 11 (31%) | 0.07 |
| Pathologic plural invasion | 30 (16%) | 5 (14%) | 1.00 |
| EGFR Wildtype/Mutant | 8 (47%)/9 (53%) | 4 (40%)/6 (60%) | 1.00 |
The CIR and OS
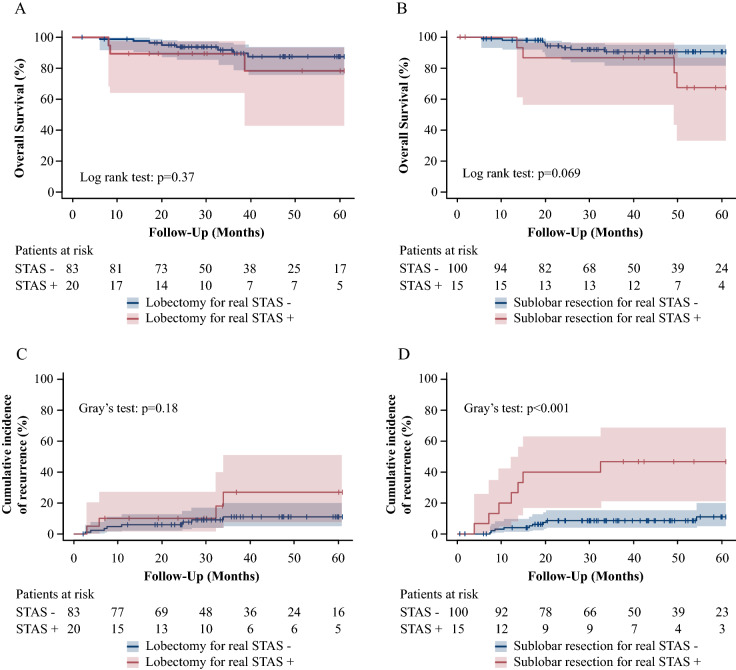
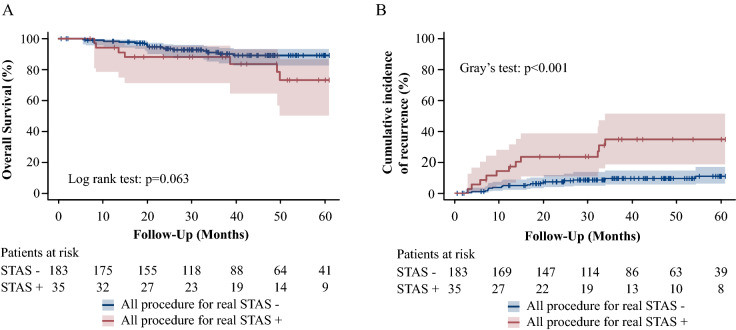
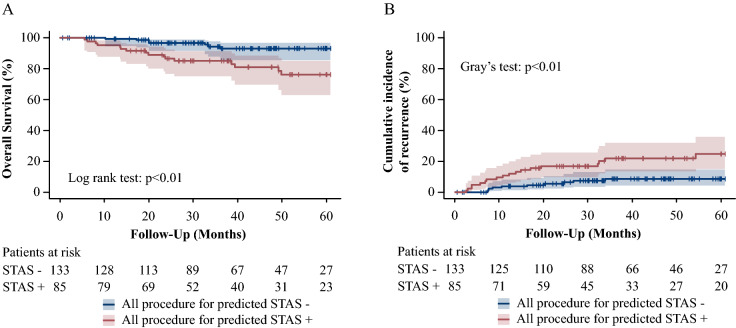
The median observation period was 36.9 months (interquartile range 24.4–60.4 months). The CIR was compared between the STAS (+) and STAS (−) groups and while there was no significant difference in the CIR among patients who underwent lobectomy (p = 0.18), the STAS (+) group showed a higher CIR after sublobar resection than the STAS (−) group (p < 0.001) (Fig. 3C,D). There was no significant difference in the OS between the STAS (+) and (−) groups among lobectomy (p = 0.37) or sublobar resection (p = 0.069) patients (Fig. 3A,B). For all procedures, the STAS (+) group showed a higher CIR (p < 0.001) than the STAS (−) group but no significant difference in the OS (p = 0.063) (Fig. 4A,B).
Figure 3.
(R version 3.5.1, R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/). The OS and CIR associated with real STAS in lobectomy and sublobar resection. Shaded areas represent 95% confidence limits. (A) The OS in patients who underwent lobectomy. (B) The OS in patients who underwent sublobar resection. (C) The CIR in patients who underwent lobectomy. (D) The CIR in patients who underwent sublobar resetion.
Figure 4.
(R version 3.5.1, R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/). The OS and CIR associated with real STAS in all procedure. Shaded areas represent 95% confidence limits. (A) The OS in all patients. (B) The CIR in all patients.
The CIR and OS based on STAS predicted by radiomics
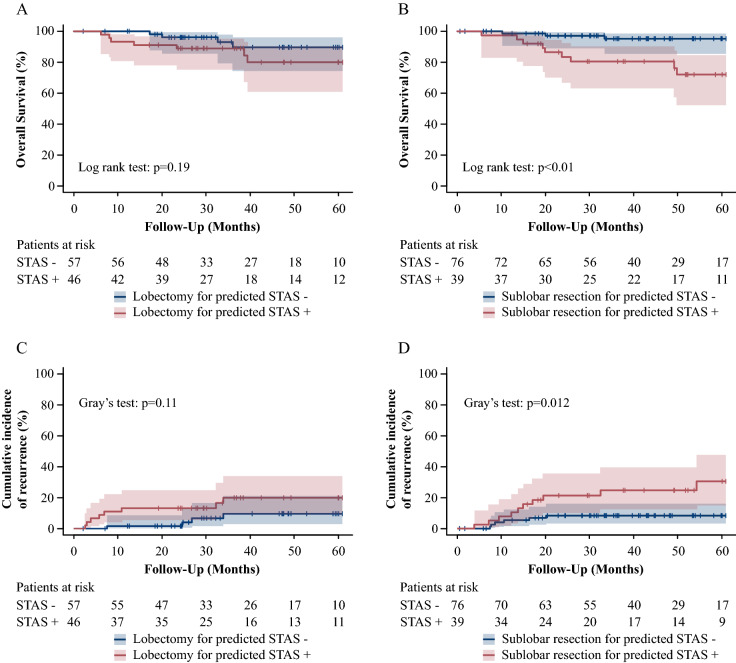
The AUC for the positivity of STAS was 0.77. The likelihood of STAS was determined based on the radiomics, and patients were then divided into predicted-STAS (+) and (−) groups. For the patients who underwent lobectomy, there was no significant difference in the CIR (p = 0.11) or OS (p = 0.19) (Fig. 5A,C) between the groups. However, for patients who underwent sublobar resection, the predicted-STAS (+) group showed a higher incidence of CIR (p = 0.012) and poorer OS (p < 0.01) than the predicted-STAS (−) group (Fig. 5B,D). For all procedures, the predicted-STAS (+) group showed a higher CIR and OS than the predicted-STAS (−) group (p < 0.01) (Fig. 6A,B).
Figure 5.
(R version 3.5.1, R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/). Survival curves based on the model predicted by radiomics. The OS and CIR associated with predicted-STAS in lobectomy and sublobar resection. Shaded areas represent 95% confidence limits. (A) The OS in patients who underwent lobectomy. (B) The OS in patients who underwent sublobar resection. (C) The CIR in patients who underwent lobectomy. (D)The CIR in patients who underwent sublobar resection.
Figure 6.
(R version 3.5.1, R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/). Survival curves based on the model predicted by radiomics in all procedure. Shaded areas represent 95% confidence limits. (A) The OS in all patients. (B) The CIR in all patients.
Discussion
Radiomics, the process of converting radiographic images into quantifiable information, helps develop prediction models that can potentially improve the accuracy of a diagnosis29. In a previous radiomics study reported by Jiang et al.21, their predicted model was built using the Random Forest algorithm with training (n = 195) and validation cohorts (n = 84) for 107 radiologic features. Their model was able to effectively predict STAS-positive tumors with an AUC of 0.7. In another study, Chen et al.22 constructed a prediction model using 88 features extracted from stage I adenocarcinomas using the Naïve Bayes algorithm, which also effectively predicted STAS positive tumors with an AUC of 0.63 for a training cohort of 233 cases and 0.69 for a validation cohort of 112 cases. In the present study, the prediction model was constructed by XGBoost for 1874 features extracted from 226 peripherally located small NSCLC and was able to predict the presence of STAS with an AUC of 0.77.
Our study focused on two clinical questions. The first one was whether or not a preoperative diagnosis and surgery procedure selection were possible using a model built by machine-learning-based radiomics. The AUC of 0.77 is regarded as moderate accuracy and is better than that obtained in previous studies; however, this accuracy will need to be improved for clinical use. The second one was whether or not the model was useful for determining the ideal type of surgery. STAS (micropapillary pattern)-positive NSCLC, especially adenocarcinoma, is reportedly associated with recurrence after segmentectomy but not after lobectomy30. Therefore, the present study focused on peripherally located small (≤ 2 cm) lesions. Our subjects also included non-adenocarcinoma patients, since they sometimes have STAS. In total, 4 (8.9%) of the 45 non-adenocarcinoma tumors possessed STAS in our study. This second question was also positively answered based on the results of a survival analysis for the predicted-STAS (+) group, who showed a higher incidence of recurrence and a worse survival than the predicted-STAS (−) group when sublobar resection was performed.
The invasive morphology of STAS, characterized by micropapillary clusters, solid nests or single cells beyond the edge of the tumor extending into the air spaces in the surrounding lung parenchyma2, resulted in poor clinical outcomes, even for early-stage disease, in patients with NSCLC3,4. Kadota et al. described an increased risk of developing CIR in STAS-positive pulmonary adenocarcinomas treated with sublobar resection; however, this risk was mitigated by lobectomy31. The preoperative and intraoperative diagnoses of STAS therefore seem important for deciding on the surgical plan (sublobar resection or lobectomy) for peripheral small-sized lung cancer. However, the detection of STAS remains challenging prior to thoracotomy by preoperative radiology and cytology, as well as by frozen section analyses during surgery. The sensitivity of diagnosing STAS by airway secretion cytology32 or frozen sections during surgery33 is not satisfactory for clinical use. STAS-positive pulmonary adenocarcinoma34 is frequently observed as solid nodules on computed tomography with a low incidence of ground-glass attenuation, showing abnormal avidity on PET35,36. We select lobectomy rather than sublobar resection for lesions deemed suspicious on radiology37. However, the sensitivity and specificity of conventional CT feature interpretation by radiologists were not satisfactory for predicting STAS. A few reports21,22,38,39 have described the utility of radiomics for predicting STAS based on the findings of radiological examinations.
Machine-learning-based radiomics are a powerful tool in thoracic oncology, using an exploratory analysis to detect complex patterns that cannot be recognized by traditional analyses. In particular, some publications reported that quantifiable radiomics features extracted from the VOI within CT images can provide more information not only for subtype but also genetic information and immune infiltration of tumors18–20. Most cancer cases are subjected to multiple rounds of CT, MRI, and PET-CT prior to thoracotomy, accumulating a large amount of data; however, modern CT, MRI and combined PET-CT units are not standardized for image acquisition and reconstruction protocols. With simple machine-learning of radiological images, overfitting cannot be completely avoided, resulting in some input data bias, with the results affected not only by the biological features, but also by the imaging protocols. In the present study, we first extracted the image feature values and then applied the data to machine-learning algorithms. To avoid showing results that overfit the partitioning of a particular data set, the cross-validated models were evaluated in a test set five times using different sets each time.
Several limitations associated with the present study warrant mention. First, our study was a retrospective analysis of CT findings based on the results of pathology, not a prospective study. Because the ground truth diagnosis associated with medical imaging data analyzes the accumulated data, it necessarily focuses on the retrospective aspects of such studies. A prospective study in which surgical procedures are controlled by the result of radiomics prediction may be warranted in the future. Second, STAS has not been defined for many years, and even pathologists may have difficulty diagnosing this entity. No clear criteria for distinguishing STAS from artifacts have been established26. We re-evaluated each diagnosis to reduce the diagnostic error. The postoperative outcomes were similar to those of previous reports, which might have ensured the quality of the STAS diagnosis. Third, this analysis included some cases of high-grade neuroendocrine tumors, such as combined small-cell lung cancer and large-cell neuroendocrine carcinoma, which were diagnosed by a final pathological evaluation. We focused on the utility of the pre-operative prediction of STAS-positive lung cancer using radiomics-based technology; these cases were therefore not excluded. Fourth, we did not set a validation cohort to confirm the prediction model, although five-times-repeated five-fold cross-validation was applied. However, the survival data clearly demonstrated the clinical usefulness of the prediction model. In our study, the presence of STAS was a strong predictor of surgical outcomes that was not restricted to a histologic type. The further accumulation of cases with a prospective cohort study should be conducted in the future.
In conclusion, radiomics-based analyses utilizing chest CT imaging findings were found to be feasible for predicting STAS as well as the postoperative outcomes. The survival curves suggested if the STAS could be predicted preoperatively by radiomics-based analyses, then we could select lobectomy to ensure better outcomes. This technology would greatly aid surgeons in selecting the optimal surgical treatment plan and it is also important for lung cancer clinics treating small nodules detected by CT screening.
Acknowledgements
We thank the Department of Diagnostic Pathology, Chiba University Hospital for their support in making the STAS diagnosis. This work was supported by JSPS KAKENHI grant number 21K08880.
Author contributions
The contributions of each author are as follows. Y.O. takes responsibility for the content of the manuscript, including data extracting and analysis. T.N. serves as the guarantor of the articled, taking responsibility for the integrity of the work. T.U., H.Y. and A.N. were responsible for the radiomics analyses with machine-learning. J.M., T.T., T.I., K.T., Y.S., H.S. and I.Y. contributed substantially to the study design, data analysis and interruption.
Competing interests
T.N. reports personal fees from Olympus, personal fees from AstraZeneca, personal fees from Johnson and Johnson, outside the submitted work. The other authors have nothing to disclose.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kawakami T, et al. Small cluster invasion: a possible link between micropapillary pattern and lymph node metastasis in pT1 lung adenocarcinomas. Virchows Arch. Int. J. Pathol. 2008;454:61–70. doi: 10.1007/s00428-008-0695-5. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, et al. The 2015 World Health Organization classification of lung tumors impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 3.Onozato ML, et al. Tumor islands in resected early-stage lung adenocarcinomas are associated with unique clinicopathologic and molecular characteristics and worse prognosis. Am. J. Surg. Pathol. 2013;37:287–294. doi: 10.1097/PAS.0b013e31826885fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warth A, et al. Prognostic impact of intra-alveolar tumor spread in pulmonary adenocarcinoma. Am. J. Surg. Pathol. 2015;39:793–801. doi: 10.1097/PAS.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 5.Lu S, et al. Spread through air spaces (STAS) is an independent predictor of recurrence and lung cancer-specific death in squamous cell carcinoma. J. Thorac. Oncol. 2017;12:223–234. doi: 10.1016/j.jtho.2016.09.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadota K, et al. Tumor spread through air spaces is an independent predictor of recurrence-free survival in patients with resected lung squamous cell carcinoma. Am. J. Surg. Pathol. 2017;41:1077–1086. doi: 10.1097/PAS.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama S, et al. Tumor spread through air spaces identifies a distinct subgroup with poor prognosis in surgically resected lung pleomorphic carcinoma. Chest. 2018;154:838–847. doi: 10.1016/j.chest.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Aly RG, et al. Spread through air spaces (STAS) is prognostic in atypical carcinoid, large cell neuroendocrine carcinoma, and small cell carcinoma of the lung. J. Thorac. Oncol. 2019;14:1583–1593. doi: 10.1016/j.jtho.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito H, et al. Long-term survival outcome after lobectomy in patients with clinical T1N0 lung cancer. J. Thorac. Cardiovasc. Surg. 2020 doi: 10.1016/j.jtcvs.2019.12.072. [DOI] [PubMed] [Google Scholar]
- 10.Ginsberg RJ, et al. Randomized trial of lobectomy versus limited resection for T1 NO non-small cell lung cancer. Ann. Thorac. Surg. 1995;60:615–623. doi: 10.1016/0003-4975(95)00537-U. [DOI] [PubMed] [Google Scholar]
- 11.Okada M, et al. Radical sublobar resection for small-sized non–small cell lung cancer: a multicenter study. J. Thorac. Cardiovasc. Surg. 2006;132:769–775. doi: 10.1016/j.jtcvs.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K, et al. A nonrandomized confirmatory phase III study of sublobar surgical resection for peripheral ground glass opacity dominant lung cancer defined with thoracic thin-section computed tomography (JCOG0804/WJOG4507L) J. Clin. Oncol. 2017;35:8561–8561. doi: 10.1200/JCO.2017.35.15_suppl.8561. [DOI] [Google Scholar]
- 13.Suzuki K, et al. Comparison of pulmonary segmentectomy and lobectomy: safety results of a randomized trial. J. Thorac. Cardiovasc. Surg. 2019;158:895–907. doi: 10.1016/j.jtcvs.2019.03.090. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida Y, et al. Micropapillary histological subtype in lung adenocarcinoma of 2 cm or less: impact on recurrence and clinical predictors. Gen. Thorac. Cardiovasc. Surg. 2017;65:273–279. doi: 10.1007/s11748-017-0747-3. [DOI] [PubMed] [Google Scholar]
- 15.Kim SK, et al. Lung adenocarcinoma: CT features associated with spread through air spaces. Radiology. 2018;289:831–840. doi: 10.1148/radiol.2018180431. [DOI] [PubMed] [Google Scholar]
- 16.Toyokawa G, et al. Computed tomography features of resected lung adenocarcinomas with spread through air spaces. J. Thorac. Cardiovasc. Surg. 2018;156:1670–1676.e4. doi: 10.1016/j.jtcvs.2018.04.126. [DOI] [PubMed] [Google Scholar]
- 17.Medina MA, et al. Preoperative bronchial cytology for the assessment of tumor spread through air spaces in lung adenocarcinoma resection specimens. Cancer. Cytopathol. 2020;128:278–286. doi: 10.1002/cncy.22243. [DOI] [PubMed] [Google Scholar]
- 18.Iwatate Y, et al. Radiogenomics for predicting p53 status, PD-L1 expression, and prognosis with machine learning in pancreatic cancer. Br. J. Cancer. 2020 doi: 10.1038/s41416-020-0997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun R, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018;19:1180–1191. doi: 10.1016/S1470-2045(18)30413-3. [DOI] [PubMed] [Google Scholar]
- 20.Liu G, et al. 3D radiomics predicts EGFR mutation, exon-19 deletion and exon-21 L858R mutation in lung adenocarcinoma. Transl. Lung Cancer Res. 2020;9:1212–1224. doi: 10.21037/tlcr-20-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang C, et al. CT-based radiomics and machine learning to predict spread through air space in lung adenocarcinoma. Eur. Radiol. 2020;30:4050–4057. doi: 10.1007/s00330-020-06694-z. [DOI] [PubMed] [Google Scholar]
- 22.Chen D, et al. Radiomics-based prediction for tumour spread through air spaces in stage I lung adenocarcinoma using machine learning. Eur. J. Cardio-Thorac. 2020;58:51–58. doi: 10.1093/ejcts/ezaa011. [DOI] [PubMed] [Google Scholar]
- 23.Crinò L, Weder W, van Meerbeeck J, Felip E. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010;21:v103–v115. doi: 10.1093/annonc/mdq207. [DOI] [PubMed] [Google Scholar]
- 24.Morimoto J, et al. Impact of free tumor clusters on prognosis after resection of pulmonary adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2016;152:64–72. doi: 10.1016/j.jtcvs.2016.03.088. [DOI] [PubMed] [Google Scholar]
- 25.Thunnissen E, Blaauwgeers HJLG, de Cuba EMV, Yick CY, Flieder DB. Ex vivo artifacts and histopathologic pitfalls in the lung. Arch. Pathol. Lab Med. 2016;140:212–220. doi: 10.5858/arpa.2015-0292-OA. [DOI] [PubMed] [Google Scholar]
- 26.Warth A. Spread through air spaces (STAS): a comprehensive update. Transl. Lung Cancer Res. 2017;6:501–507. doi: 10.21037/tlcr.2017.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Griethuysen JJM, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77:e104–e107. doi: 10.1158/0008-5472.CAN-17-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann. Stat. 1988;16:1141–1154. doi: 10.1214/aos/1176350951. [DOI] [Google Scholar]
- 29.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2015;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nitadori J, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. Jnci J. Natl. Cancer Inst. 2013;105:1212–1220. doi: 10.1093/jnci/djt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadota K, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J. Thorac. Oncol. 2015;10:806–814. doi: 10.1097/JTO.0000000000000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isaka T, et al. Detection of tumor spread through airspaces by airway secretion cytology from resected lung cancer specimens. Pathol. Int. 2017;67:487–494. doi: 10.1111/pin.12570. [DOI] [PubMed] [Google Scholar]
- 33.Kameda K, et al. MA12.05 Can tumor spread through air spaces (STAS) in lung adenocarcinomas be predicted pre- and intraoperatively? J. Thorac. Oncol. 2017;12:S411–S412. doi: 10.1016/j.jtho.2016.11.473. [DOI] [Google Scholar]
- 34.Morales-Oyarvide V, Mino-Kenudson M. Tumor islands and spread through air spaces: distinct patterns of invasion in lung adenocarcinoma. Pathol. Int. 2015;66:1–7. doi: 10.1111/pin.12368. [DOI] [PubMed] [Google Scholar]
- 35.Austin JHM, et al. Radiologic implications of the 2011 classification of adenocarcinoma of the lung. Radiology. 2012;266:62–71. doi: 10.1148/radiol.12120240. [DOI] [PubMed] [Google Scholar]
- 36.Lee HY, et al. Role of CT and PET imaging in predicting tumor recurrence and survival in patients with lung adenocarcinoma. J. Thorac. Oncol. 2015;10:1785–1794. doi: 10.1097/JTO.0000000000000689. [DOI] [PubMed] [Google Scholar]
- 37.Hattori A, Matsunaga T, Takamochi K, Oh S, Suzuki K. Indications for sublobar resection of clinical stage IA radiologic pure-solid lung adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2017;154:1100–1108. doi: 10.1016/j.jtcvs.2017.03.153. [DOI] [PubMed] [Google Scholar]
- 38.Zhuo Y, et al. Radiomics nomograms of tumors and peritumoral regions for the preoperative prediction of spread through air spaces in lung adenocarcinoma. Transl. Oncol. 2020;13:100820. doi: 10.1016/j.tranon.2020.100820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suh JW, et al. Stepwise flowchart for decision making on sublobar resection through the estimation of spread through air space in early stage lung cancer1. Lung Cancer. 2020;142:28–33. doi: 10.1016/j.lungcan.2020.02.001. [DOI] [PubMed] [Google Scholar]