Abstract
Background
Reducing the transmission of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a global priority. Contact tracing identifies people who were recently in contact with an infected individual, in order to isolate them and reduce further transmission. Digital technology could be implemented to augment and accelerate manual contact tracing. Digital tools for contact tracing may be grouped into three areas: 1) outbreak response; 2) proximity tracing; and 3) symptom tracking. We conducted a rapid review on the effectiveness of digital solutions to contact tracing during infectious disease outbreaks.
Objectives
To assess the benefits, harms, and acceptability of personal digital contact tracing solutions for identifying contacts of an identified positive case of an infectious disease.
Search methods
An information specialist searched the literature from 1 January 2000 to 5 May 2020 in CENTRAL, MEDLINE, and Embase. Additionally, we screened the Cochrane COVID‐19 Study Register.
Selection criteria
We included randomised controlled trials (RCTs), cluster‐RCTs, quasi‐RCTs, cohort studies, cross‐sectional studies and modelling studies, in general populations. We preferentially included studies of contact tracing during infectious disease outbreaks (including COVID‐19, Ebola, tuberculosis, severe acute respiratory syndrome virus, and Middle East respiratory syndrome) as direct evidence, but considered comparative studies of contact tracing outside an outbreak as indirect evidence.
The digital solutions varied but typically included software (or firmware) for users to install on their devices or to be uploaded to devices provided by governments or third parties. Control measures included traditional or manual contact tracing, self‐reported diaries and surveys, interviews, other standard methods for determining close contacts, and other technologies compared to digital solutions (e.g. electronic medical records).
Data collection and analysis
Two review authors independently screened records and all potentially relevant full‐text publications. One review author extracted data for 50% of the included studies, another extracted data for the remaining 50%; the second review author checked all the extracted data. One review author assessed quality of included studies and a second checked the assessments. Our outcomes were identification of secondary cases and close contacts, time to complete contact tracing, acceptability and accessibility issues, privacy and safety concerns, and any other ethical issue identified. Though modelling studies will predict estimates of the effects of different contact tracing solutions on outcomes of interest, cohort studies provide empirically measured estimates of the effects of different contact tracing solutions on outcomes of interest. We used GRADE‐CERQual to describe certainty of evidence from qualitative data and GRADE for modelling and cohort studies.
Main results
We identified six cohort studies reporting quantitative data and six modelling studies reporting simulations of digital solutions for contact tracing. Two cohort studies also provided qualitative data. Three cohort studies looked at contact tracing during an outbreak, whilst three emulated an outbreak in non‐outbreak settings (schools). Of the six modelling studies, four evaluated digital solutions for contact tracing in simulated COVID‐19 scenarios, while two simulated close contacts in non‐specific outbreak settings.
Modelling studies
Two modelling studies provided low‐certainty evidence of a reduction in secondary cases using digital contact tracing (measured as average number of secondary cases per index case ‐ effective reproductive number (Reff)). One study estimated an 18% reduction in Reff with digital contact tracing compared to self‐isolation alone, and a 35% reduction with manual contact‐tracing. Another found a reduction in Reff for digital contact tracing compared to self‐isolation alone (26% reduction) and a reduction in Reff for manual contact tracing compared to self‐isolation alone (53% reduction). However, the certainty of evidence was reduced by unclear specifications of their models, and assumptions about the effectiveness of manual contact tracing (assumed 95% to 100% of contacts traced), and the proportion of the population who would have the app (53%).
Cohort studies
Two cohort studies provided very low‐certainty evidence of a benefit of digital over manual contact tracing. During an Ebola outbreak, contact tracers using an app found twice as many close contacts per case on average than those using paper forms. Similarly, after a pertussis outbreak in a US hospital, researchers found that radio‐frequency identification identified 45 close contacts but searches of electronic medical records found 13. The certainty of evidence was reduced by concerns about imprecision, and serious risk of bias due to the inability of contact tracing study designs to identify the true number of close contacts.
One cohort study provided very low‐certainty evidence that an app could reduce the time to complete a set of close contacts. The certainty of evidence for this outcome was affected by imprecision and serious risk of bias. Contact tracing teams reported that digital data entry and management systems were faster to use than paper systems and possibly less prone to data loss.
Two studies from lower‐ or middle‐income countries, reported that contact tracing teams found digital systems simpler to use and generally preferred them over paper systems; they saved personnel time, reportedly improved accuracy with large data sets, and were easier to transport compared with paper forms. However, personnel faced increased costs and internet access problems with digital compared to paper systems.
Devices in the cohort studies appeared to have privacy from contacts regarding the exposed or diagnosed users. However, there were risks of privacy breaches from snoopers if linkage attacks occurred, particularly for wearable devices.
Authors' conclusions
The effectiveness of digital solutions is largely unproven as there are very few published data in real‐world outbreak settings. Modelling studies provide low‐certainty evidence of a reduction in secondary cases if digital contact tracing is used together with other public health measures such as self‐isolation. Cohort studies provide very low‐certainty evidence that digital contact tracing may produce more reliable counts of contacts and reduce time to complete contact tracing. Digital solutions may have equity implications for at‐risk populations with poor internet access and poor access to digital technology.
Stronger primary research on the effectiveness of contact tracing technologies is needed, including research into use of digital solutions in conjunction with manual systems, as digital solutions are unlikely to be used alone in real‐world settings. Future studies should consider access to and acceptability of digital solutions, and the resultant impact on equity. Studies should also make acceptability and uptake a primary research question, as privacy concerns can prevent uptake and effectiveness of these technologies.
Plain language summary
Are digital contact tracing technologies effective during infectious disease outbreaks?
Why is this question important?
The global COVID‐19 pandemic highlights the importance of accurate and timely contact tracing. Contact tracing tells people that they may have been near someone with ‐ or showing symptoms of ‐ an infectious disease, allowing them to self‐isolate and helping to stop the spread of infection. Traditionally, contact tracing begins with notification that someone has an infectious disease. They are asked to recall their contacts, going back two to three days before symptom onset. This is time‐consuming and may not always give a complete picture, so digital aids could help contact tracers.
Digital contact tracing uses technology to track and trace contacts. Individuals download an app onto their smartphones and record location and symptom information, or their devices might use location‐finding technology, like Bluetooth or GPS (global positioning system). If the user is infected, the technology identifies close contacts and/or secondary infections (people to whom they passed the disease), and informs people whom they have been near. The technology identifies where the infection was passed on and its duration (the context).
However, problems may occur where access to technology is limited, in low‐income settings or for elderly people, for example. Also, some people see it as an invasion of privacy and are suspicious of how their data will be used.
We wanted to know whether digital contact tracing, compared to manual contact tracing, is effective in reducing the spread of infection, as measured by secondary infections, identifying close contacts, tracing a complete set of contacts, and identifying the context of infection.
What did we do?
We searched medical databases for studies that assessed digital contact tracing. We preferred studies set during infectious disease outbreaks, which assessed real people in real time, but we included studies in any setting and of any design.
To answer our question quickly, we shortened some steps of the Cochrane review process, however, we are confident in our conclusions.
What we found
We found 12 relevant studies. Six assessed the effectiveness of digital contact tracing on specific groups (cohorts) of people: three during an outbreak (Ebola in Sierra Leone; tuberculosis in Botswana; and whooping cough (pertussis) in USA); and three replicated an outbreak in schools to assess systems for identifying close contacts of participants. The remaining six were modelling studies, which simulated digital contact tracing.
Main results
Digital contact tracing with self‐isolation probably reduces the number of secondary infections, but not as much as manual contact tracing with self‐isolation (2 modelling studies).
Digital contact tracing found more close contacts in two outbreaks than manual (2 studies in USA and Sierra Leone). Devices in non‐outbreak settings can identify more close contacts than self‐reported diaries or surveys.
An app may reduce the time to complete a set of close contacts (1 study). Digital systems were faster to use than paper systems for recording new contacts and monitoring known contacts, and possibly less prone to data loss.
Problems with system access (2 studies) included patchy network coverage, lack of data, technical problems and higher staff training needs. Contact tracers' personal expenses increased (1 study) due to travel and recharging phone batteries. Devices all appeared to protect diagnosed users from contacts, snoopers and authorities but one app's users were members of public health agencies. Studies recorded stolen hardware (second‐hand mobile phones); reported that paper forms were "often lost", and that digital data were password protected (2 studies) and encrypted (1 study).
We found no evidence on contextual information and acceptability.
What this means
It is unlikely that digital technologies would be the sole method of contact tracing during an outbreak; they would probably be used alongside manual methods. Unfortunately, the technology is largely unproven in real‐world outbreak settings and none of our included studies assessed digital plus manual contact tracing with digital contact tracing alone. Our included studies assessed different technologies and used different methods from each other, so we are uncertain about their evidence.
Governments that implement digital contact tracing should ensure that at‐risk populations are not disadvantaged and take privacy concerns into account.
This review is up to date to May 2020.
Background
As of 28 July 2020, the coronavirus (COVID‐19) pandemic had resulted in 16,341,920 confirmed cases and 650,805 deaths globally (WHO 2020a). As the international health community struggles to cope with healthcare systems working beyond their capacities, the World Health Organization (WHO) and several countries are exploring how technology may help to address this public health crisis.
Contact tracing, identifying people who were in contact with an infected individual, is a key component in preventing the spread of infectious diseases. Although settings and disease types determine the length of time that contact tracers will follow‐up with cases, contact tracing and subsequent isolation has been shown to reduce transmission of infectious diseases (Faye 2015). Traditional contact tracing typically begins upon notification of a case of an infectious disease. For example, with COVID‐19 a patient would be notified of their positive test result and then interviewed so that they could recall their contacts (going back typically two to three days before symptom onset). Then, the contacts are notified that they have been exposed to an active case and informed that they should self‐isolate and possibly test themselves.
Currently, the scale of COVID‐19 infections has outstripped governments’ capacities to conduct manual contact tracing (Ferretti 2020). Existing contact tracing practices are resource‐intensive, slow and often subject to recall bias (Kretzschmar 2020). For example, in New Zealand, contact tracing for a single individual took a team of three to five contact tracers several days to complete and the contact tracing system was overwhelmed with only 80 daily cases (Verrall 2020). Manual contact tracing processes are also limited to the recall of participants, who may not remember every clinically relevant interaction nor know identifiable information for their close contacts (e.g. names, phone numbers).
In response, many countries are investigating and deploying technology solutions to augment and accelerate manual contact tracing (Ferretti 2020). The WHO categorises digital tools for contact tracing into three areas:
outbreak response tools;
proximity tracing tools; and
symptom tracking tools (WHO 2020b).
Outbreak response tools relate to the management of cases and their contacts through electronic data entry of case and contact information. Proximity tracing tools focus on tracing the movements of individuals to identify people who may have been exposed to an infected person. Symptom tracking tools typically rely on routinely collecting self‐reported signs and symptoms to assess the prevalence of the disease by time and place that can help inform contact tracing processes. For each type of digital solution, the process can be manual or automatic or a blend of both. For example, manual proximity tracing tools can rely on users scanning on entry into stores using QR codes, while automated tools may automatically register your visit using Bluetooth or GPS technology.
The weigh‐up in effectiveness and privacy that takes place when an individual decides whether to download and use an app is clearly demonstrated in research conducted by Kaptchuk and colleagues, which reported that 70% to 80% of respondents would be willing to install a hypothetical COVID‐19 contact tracing app that is 100% private and 100% accurate in identifying contacts of cases (Kaptchuk 2020). This proportion decreases (50% to 60%) when either the accuracy or privacy of the app is not specified to individuals. Further, when weighing up the required level of app effectiveness (both considering public health benefit and individual benefit), for every 1% reduction in infection rate offered by an app, respondents were 5% more likely to adopt such an app. Such findings speak to the combined impact that effectiveness and privacy will play in the future success of digital contact tracing technologies.
Digital contact tracing may have advantages in terms of accuracy or speed of contact tracing, but also present real risks if poorly designed or if introduced without the appropriate safeguards (Grundy 2019a; Grundy 2019b; WHO 2020c). For example, digital solutions could exacerbate existing health inequities as people living in high deprivation, the elderly and ethnic minorities experience a disproportionate COVID‐19‐related burden of disease (Van Dorn 2020; Vaughan 2020), and also tend to have poor access to smartphones and live in areas of low connectivity (WHO 2020c). Further, they may have low levels of technical expertise to actively engage with digital solutions. In Singapore, these inequities played out as migrant workers were effectively excluded from access to the TraceTogether smartphone app and this is where a new wave of the disease took hold (Baharudin 2020).
Description of the intervention
This review focuses on digital solutions that are compared to traditional contact tracing methods. We considered digital solutions that needed to be maintained by an individual (i.e. a user or public health contact tracer) through a device, through an app, or through some other locally‐maintained technology, and not through broad surveillance technology (e.g. CCTV or credit card usage).
For contact tracing to be effective, accurate records of the places or people an individual has visited need to be kept. This can be a diary or guest book at places visited. Alternatively, location surveillance technologies have been employed in some regions, and these solutions can include mobile phone location data, CCTV, or even credit card usage.
The technologies that we aim to review are devices or apps, individually maintained by members of the public or public health workers (e.g. contact tracers). These can be apps (software) installed on their mobile phones or firmware installed on other devices (e.g. wearables) that use some form of proximity tracing technology, such as Bluetooth, GPS or manual input. In the case of automatic proximity tracing technologies, the device registers when a person is near other devices (e.g. when a user meets someone face‐to‐face or when a user stands near someone in the supermarket queue).
Some common technological solutions for improvement of contact tracing include:
automatic solutions, which are downloaded onto a person’s smartphone and automatically record when the user is near to other devices;
manual solutions, which would also be downloaded to a smartphone but would require the user to scan a barcode as they enter a store (for example) or to manually enter the identifications of people they have been near on that day, or both. These can also be apps maintained by public health workers to aid in contact tracing data management.
In this review, the digital solutions we include are app‐based technologies, software‐based approaches, wearable smart devices, and other hardware‐based solutions for contact tracing.
How the intervention might work
Digital solutions are developed to reduce the time and improve the accuracy of follow‐up with all close contacts of a case of an infectious disease. Traditional contact tracing protocols rely on an extensive network of personnel who obtain a contact history from the index case and follow‐up with possibly infected individuals. Contact tracing personnel collect information on who an infected individual has been in close proximity to, as well as contextual details about the interaction, such as the setting and duration of contact. Digital solutions are designed to improve data management, and analyse the contact list and contextual information for the contact tracers so that the contacts can be quickly notified about their potential exposure to a newly diagnosed case. Rapidly identifying close contacts allows for more rapid isolation. Though not often practiced as it relies heavily on very accurate and timely contact tracing, recursive contact tracing has the potential to also be implemented as a result of digital contact tracing. For example, in Harbin, China, public health authorities began a strategy to quarantine not only close contacts, but the close contacts of close contacts (i.e. recursive contact tracing; Reuters 2020). An additional benefit of digital solutions is that they may also incorporate automatic data entry technology, which can potentially identify unknown or anonymous contacts that the case would normally not be able to recall.
Objectives
To assess the benefits and harms of digital solutions for identifying contacts of an identified positive case of an infectious disease and to assess acceptability of this approach from qualitative studies.
The population of interest is any population with and without any infectious diseases, for example COVID‐19, Ebola, and tuberculosis. The intervention of interest is digital contact tracing solutions, including (but not limited to) smartphone apps, wearable devices, and hardware‐ and software‐based solutions. The comparison of interest is traditional or manual contact tracing techniques, including interviews and diaries, or other digital solutions. Outcomes of interest are summarised in the Methods. The key questions (KQs) we aimed to answer are below.
Primary research questions
Key question 1: how effective are digital solutions in identifying the secondary cases from index cases when compared to traditional contact tracing methods?
Key question 2: how effective are digital solutions in identifying the close contacts from an index case when compared to traditional contact tracing methods?
Key question 3: how long does contact tracing take to construct a complete set of close contacts with and without digital solutions?
Key question 4: how effective are digital solutions in identifying contextual information about contacts (i.e. setting, duration) compared to traditional contact tracing methods?
Secondary research questions
Key question 5: how effective are different types of digital solutions in identifying secondary cases from index cases when compared to each other?
Key question 6: how effective are different types of digital solutions in identifying the close contacts from an index case when compared to each other?
Key question 7: what is the acceptability and accessibility of the digital solution in a given setting and population?
Key question 8: what privacy or safety concerns for the different contact tracing approaches have been identified?
Key question 9: what other ethical concerns have been identified (e.g. equity issues, harms to the individual from high false positives)?
Methods
We employed the following amendments to common Cochrane Reviews to allow for a rapid development and dissemination of data (Anglemyer 2020 PROSPERO registration: CRD42020188946).
We restricted search dates to publications after 1 January 2000, partly due to time constraints and partly due to the fact that in the rapidly changing field of digital health technology, it seems likely that studies older than 2000 will be less relevant.
For data extraction, we had dual data extraction, with the second review author checking all the extracted data. For assessment of risk of bias, we employed dual assessment, with the second review author checking all judgements.
Criteria for considering studies for this review
Types of studies
For the quantitative research studies, we included cohort studies, cross‐sectional studies, modelling studies, controlled trials with non‐randomised means of allocation (quasi‐randomised controlled trials), randomised controlled trials (RCTs), including cluster‐RCTs. Cohort studies and cross‐sectional studies are likely the most common designs used in real‐world pragmatic studies evaluating an infectious disease in real‐time. In fact, even cross‐sectional studies simply capturing the method by which close contacts were identified (i.e. traditional contact tracing or another tech‐based method) could provide useful information. Cross‐sectional studies had to capture at least two different contact tracing strategies to be included. We excluded cross‐sectional studies without a comparison group.
Much of the published literature discussing digital solutions includes simulations, therefore we included modelling studies that evaluated different types of contact tracing. We only included modelling studies that were at least in preprint, prior to peer‐review. As we aimed to evaluate empirical evidence together with modelling evidence in its advanced stages, we did not include many incomplete, open‐source models or white papers that we were unable to locate. Though we did not anticipate cluster‐RCTs, in theory these would be feasible in a contact‐tracing setting if two different settings/areas were allocated two different types of contact tracing and compared. Similarly, we did not anticipate quasi‐RCTs, but in theory a study could be designed as a quasi‐RCT and compare two different contact tracing strategies. We found no RCTs of any design that met our inclusion criteria. As a result, our methods focus primarily on cohort and modelling study designs.
We excluded case reports and systematic reviews (though we used them for cross‐referencing citations).
There is no classic exposure or intervention in this review, so there is no minimum duration of follow‐up or exposure. We assumed that the maximum time to trace all close contacts using traditional and digital methods was the duration of the outcome measurement.
We included qualitative research studies, including surveys or mainly quantitative studies that also contained descriptive free‐text data. We originally intended to only include qualitative studies that employed qualitative methods for both data collection and analysis. However, given the sparsity of qualitative studies on this topic, we included all studies that contained qualitative data even if qualitative analysis was minimal.
Types of interventions
We included any digital solution to contact tracing that could fall under any one of the following WHO categories:
outbreak response tools;
proximity tracing tools; and
symptom tracking tools (WHO 2020b).
These digital solutions could take a variety of forms that cannot be easily categorised but typically include developing software (or firmware) for contact tracing management systems, for users to install on their devices or to be uploaded to devices provided by governments or third‐parties. For example, an outbreak response intervention could develop software used to log and maintain contact tracing clusters digitally. Proximity tracing or symptom tracing interventions could be software developed in the form of a smartphone app or firmware for a wearable device (e.g. Fitbit) that is installed by users onto their personal devices. Equally, an intervention could distribute hardware (e.g. phone, card or wearable device) with preloaded firmware for symptom tracking.
Control measures include:
traditional or manual contact tracing;
self‐reported diaries;
self‐reported surveys;
interviews;
other standard methods for determining close contacts;
other technologies compared to digital solutions (e.g. electronic medical records).
We did not include interventions employed through broad surveillance technology (e.g. CCTV or credit card usage), as these are not maintained by the individual user.
Types of outcome measures
Primary outcomes
The number of secondary cases identified from contact tracing procedures. This can be measured with counts of secondary cases, or with the average number of secondary cases per index case (i.e. effective reproductive number, Reff).
The number of close contacts identified from contact tracing procedures
The average length of time to complete contact tracing for a case (end point would be the last day of follow‐up in the study and, if available, at seven days after case notification). Complete contact tracing is contextually dependent and could mean different things in different settings. For example, the time to complete contact tracing for a case in a study using an app designed to aid data management for contact tracers may mean the time to complete contact tracing with the end point of interest being how long it took to collect all the close contact details. However, a study evaluating an app maintained by the user may evaluate the time to complete contact tracing from the time of notification of the index case to the time of notification of the close contacts. Lastly, a study evaluating an app maintained by the user may evaluate the time to complete contact tracing from the time of notification of the index case to the time of isolation of the close contacts.
Acceptability and accessibility
Privacy issues (whether theoretical or realised)
Safety concerns
Other ethical issues
We included studies in the review irrespective of whether measured outcome data were reported in a way that we could analyse them.
Search methods for identification of studies
An information specialist conducted our search of the literature from 1 January 2000 to 5 May 2020 in CENTRAL, Ovid MEDLINE, and Embase (Embase.com). We selected this date range partly due to time constraints and partly because, in the rapidly changing field of digital health technology, it seems likely that studies older that 2000 will be less relevant. Additionally, we screened the Cochrane COVID‐19 Study Register (covid-19.cochrane.org/). Lastly, we screened reference lists of included studies and identified reviews.
For qualitative research studies, we adopted an iterative approach based on the studies identified through the electronic bibliographic database searching. We searched reference lists of all the included quantitative and qualitative studies and searched for citations to the included qualitative studies.
For empirical qualitative research studies, we included published articles or reports that had undergone some level of peer review, and preprints.
For all studies, we made no restriction on language or design. See Appendix 1 for detailed search strategies.
Data collection and analysis
Selection of studies
Two review authors screened all titles and abstracts and compared them to our defined inclusion and exclusion criteria. Additionally, two review authors independently screened all potentially relevant full‐text publications. We sought consensus or a senior review author’s feedback, if needed, to resolve discrepancies.
Characteristics of excluded studies
The majority of studies excluded (n = 7) had no comparison groups (Chen 2020; Eisenkraft 2018; Menon‐Johansson 2018; Tom‐Aba 2018; Tom‐Aba 2020; Voirin 2015; White 2018; ), one was case identification only, not contact tracing (Van Hest 2016), and one was a case study (Sacks 2015).
Data extraction and management
LP and TM independently performed data extraction from non‐randomised studies of interventions and from qualitative research papers. One review author extracted data for 50% of the included studies; another review author extracted data for the remaining 50%. AA and TC performed data extraction from modelling and simulation studies. We extracted data using data extraction forms in Covidence, Microsoft Excel (Microsoft 2020a) and Microsoft Word (Microsoft 2020b).
Data extracted
The data extracted from all studies included:
study dates;
setting (disease type, severity and duration of infectious outbreak, country location and income);
study design (including methods, location, sites, sample size, methods of data collection, groups and aspects needed to assess risk of bias);
population uptake of intervention, (if relevant);
intervention/exposure characteristics (type of digital technology used, data entry method (e.g. manual by professional contract tracers or index cases, automatic by tracking of device locations or interactions), cost to public health service, cost (if any) to participant population, internet requirements, whether or not traditional contact tracing was continued alongside intervention);
comparator characteristics (traditional contact tracing alone, other technologies);
numerical data for outcomes of interest (number of secondary cases identified, number of contacts identified);
effect estimates comparing counts of secondary cases and counts of contacts identified;
tests comparing length of time to complete contact tracing;
and measures of epidemic growth.
For non‐randomised studies of interventions specifically, the data extracted included:
participant characteristics of population undergoing contact tracing (age, clinical risk factors, identified epidemiological risk factors);
participant characteristics of personnel involved in contact tracing if different from above (number, profession, training requirements);
information on all confounding factors reported by authors; and contact tracing definitions used in the specific setting.
Importantly, the definition of a close contact is contextually specific and will vary between countries, between jurisdictions, between diseases, and between settings. In general, for droplet‐borne diseases, a close contact is defined as within two metres' proximity for 10 to 15 minutes. For other diseases, such as Ebola, the close contact is more defined by physical contact with bodily fluids. Additionally, though modelling studies will predict estimates of the effects of different contact tracing solutions on outcomes of interest, cohort studies provide empirically measured estimates of the effects of different contact tracing solutions on outcomes of interest.
We extracted qualitative data using a purpose‐built tool that included study characteristics and methods (including date, aims of paper, description of participants, sample strategy and size, data collection methods, analytic methods, qualitative research theory, type of intervention) along with qualitative data relevant to the review objectives. We extracted primary data such as quotations, lists of identified concerns and secondary data such as author interpretations and overarching themes.
For contact tracing, we collected data until the last reported time and, if reported, after seven days of contact tracing. We preferentially included adjusted estimates over unadjusted estimates, if available.
Assessment of risk of bias in included studies
We assessed all outcomes for risk of bias. We used the ROBINS‐I risk of bias tool for non‐randomised studies of interventions (Sterne 2016). In the context of contact tracing, there is not only a lack of RCTs evaluating solutions, but there is no standard, optimal randomised study design that would be able to compare the two interventions accurately. In turn, a 'target trial' not only does not exist, but it likely could not exist in the real world. In theory, an RCT could be designed in which a prespecified population is followed closely all day and night with cameras, enumerating every close contact each member in the population has. Then, if a member of the population is identified as an index case, then all their close contacts from up to five days before their diagnosis would be identified. The list of these close contacts would be compared to the close contacts the index case recalls using manual tracing methods with case intake forms and interviews. The specific domains we used for assessing the cohort studies using ROBINS‐I included: pre‐intervention domains (bias due to confounding; bias in selection of participants into the study), at‐intervention domain (bias in classification of interventions), and post‐intervention domains (bias due to deviations from intended interventions; bias due to missing data; bias in measurement of the outcome; bias in selection of the reported result).
For modelling studies, we applied a similar approach that was previously employed in a recent COVID‐19 rapid review (Nussbaumer‐Striet 2020). We assessed whether the modelling and reporting followed the Society for Medical Decision Making (SMDM) recommendations and the International Society for Pharmacoeconomics and Outcomes (ISPOR) (Nussbaumer‐Striet 2020; Pitman 2012). There were three key areas we used to assess the risk of bias in modelling or simulation studies: we assessed:
whether they were dynamic (a dynamic transmission model can estimate direct and indirect effects of public health and control measures on infectious diseases and allows for trends in risk changes; Pitman 2012);
whether the study authors evaluated the uncertainty on important model assumptions and parameters; and
whether the study authors transparently present infectious diseases models (e.g. differential equation or behaviour of agents specified).
Two review authors rated the modelling studies. The second review author adjudicated disagreements and checked assessments. If a modelling study satisfied all three criteria, we assessed it as 'no or minor concerns only'; if we determined that a study was unclear in any of the three criteria, we assessed it as 'moderate concerns'; and we labelled studies that failed to meet any of the three criteria as 'major concerns'.
For qualitative data, we used the Critical Appraisal Skills Programme (CASP) tool to assess the methodological strengths and limitations of included studies (CASP 2020; Noyes 2019a; Noyes 2019b). The tool included questions on: clarity of aims; appropriateness of methodology and methods including research design, recruitment strategy, data collection methods; reflexivity of authors; ethical considerations; rigour of data analysis; clarity of results; relevance and usefulness of the study to our review. Two review authors (LP, TM) completed independent CASP assessments of each study and resolved any discrepancies by discussion. We did not exclude studies based on quality concerns, but quality assessment was part of our reasoning when considering the contribution of each study and our confidence in the findings of the synthesis.
Data synthesis
If we determined that it was not possible to pool data, we followed SWiM guidelines for data synthesis without meta‐analysis (Campbell 2020). In the case of sparse or heterogeneous data, we narratively reviewed the studies and did not include them in a meta‐analysis. We displayed the data in a table by study ID (with quantitative results stratified by key research questions, and the direction of the effect. We intended to construct forest plots (without pooling estimates), using SWiM guidelines, but the data did not yield themselves to be displayed as effect estimates (McKenzie 2019). We grouped similar study types together, if we determined that they were similar enough (e.g. studies in infectious diseases environments together, and separately, non‐epidemic studies together).
For continuous outcomes, we anticipated estimating the mean difference (MD) and 95% confidence intervals (CIs). For dichotomous outcomes, we planned to estimate relative risks (RR) and 95% CIs. However, the data reported by individual studies were dependent counts, that is to say, the same observations seen from one method of contact tracing were likely also seen in the other method. We determined that presenting these as simple counts was an appropriate method to avoid dependency issues. Specifically, for contact detection, we anticipated that studies would report a proportion of close contacts identified using one method and compare those with the number identified using a comparator or second method (where a gold standard is known). When we evaluated the counts of close contacts identified using two different contact tracing methods, we qualitatively compared these counts to determine if a greater number of close contacts was identified using one method when compared to another method.
Reporting results
For each comparison and outcome, we describe the number of participants in each study and in what settings. Further, we describe the number of studies addressing each outcome of interest and evaluate the findings by using vote tallies of the direction of effect. We also performed qualitative comparisons of studies with regards to epidemic growth (predicted or realised), including Reff (effective reproductive number), R0 (basic reproduction number), growth rate, and doubling time. Where necessary, we standardised outcome data to the same unit of measurement. If these required scaling/conversion factors, these were sourced, and their use confirmed (a priori) with a content expert (TC).
Description of, and rationale for, groupings of studies
We grouped non‐randomised studies of interventions by setting (during an infectious disease outbreak or in a benign environment with a general population and no current outbreak). Studies investigating an infectious disease in an epidemic setting would provide real‐world context to contact tracing methods, but the choice of method could risk missing important true close contacts. Whereas studies focused on the methods of contact tracing, but employed in settings where there is no outbreak, allow the researcher to observe true exposures to true close contacts, depending on the close contact definition used.
Standardised metric and synthesis method
Because effect estimates were not commonly reported we were unable to calculate summary statistics. Therefore, our synthesis was based on the direction of effect of the intervention compared to the control group. We used vote counting to synthesise the evidence as there were no other options and vote counting was based on these directions of effect (McKenzie 2019).
Criteria used to prioritise results for summary
We prioritised studies of wearable devices or apps that were maintained by the user over devices or apps maintained by healthcare systems. This is because we believe the effectiveness in contact tracing lies with identifying those close contacts who would not normally be identified, and any system that is not on the person will invariably miss some of those close contacts.
If there was a conflict between data reported across multiple sources for a single study (e.g. between a published article and a trial registry record), we contacted the study authors for clarification. Otherwise, we reported the data as published.
We managed and analysed qualitative data using Word (Microsoft 2020b). Our synthesis was informed by thematic analysis (Braun 2020). We sorted (‘coded’) the data into categories using a coding tree that was informed by the data and our research questions. We analysed the coded data, looking for patterns and insights that were relevant to our stated questions. Although we had only two studies that contained qualitative data, one of which contained only very ‘thin’ data with very limited qualitative analysis, we were still able to obtain useful information that was pertinent to some of our research questions.
Assessment of heterogeneity
We assessed the clinical variability of the studies in terms of setting, and type of technology used. Had we identified data suitable for pooled analyses we planned to assess heterogeneity by visually inspecting forest plots and by using the I2 statistic (Higgins 2003), where 0% to 40%: might not be important; 30% to 60%: moderate heterogeneity; 50% to 90%: substantial heterogeneity; and 75% to 100%: considerable heterogeneity (Deeks 2019).
Subgroup analysis
We performed subgroup analyses for disease type (e.g. COVID‐19, Ebola, tuberculosis (TB), severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS)), and infectious disease outbreak setting versus non‐outbreak setting. Additionally, we intended to perform a subgroup analysis by stratifying by income of the country in which the study was performed (e.g. high, medium, low). However, in the absence of a pooled analysis, we have discussed our results in the context of evidence seen in outbreak settings versus non‐outbreak settings. We anticipated stratifying by studies performed in low‐income countries versus middle‐/high‐income countries, as access to care and healthcare systems can dramatically affect the spread of any infectious disease. Further, in low‐income countries, the ability of the contact tracing technology to reduce the burden of disease spread may be the result of the technology’s limitations, but could also be the effect of disease dynamics in specific communities. However, due to a lack of data we were unable to perform this subgroup analysis. Lastly, we stratified our discussions of the results by types of technology (e.g. automatic versus manual solutions, or apps versus device, high uptake levels (e.g. 60% or higher uptake in the target population) versus not high uptake levels), though much of this discussion is largely driven by literature not included in the systematic review.
Sensitivity analysis
If we had found that some studies did not exactly fit our PICO question (population, intervention, comparison, outcome), but they still could have been informative to contact tracing more broadly, we would have performed sensitivity analyses whereby we would have evaluated the impact of their exclusion on the pooled analyses.
Assessment of certainty of the evidence
We used GRADE for all quantitative synthesis outcomes and we present the results in 'Summary of findings' tables. One review author assessed evidence quality of included studies and a second review author checked the assessments. The specific domains we used to assess the certainty of evidence from cohort and modelling studies included risk of bias, inconsistency, indirectness, and imprecision. For modelling studies, we used guidance on assessing the certainty of evidence outlined by Brozek and colleagues (Brozek 2020).
For qualitative synthesis outcomes, we used GRADE‐CERQual (Confidence in the Evidence from Reviews of Qualitative Research) to assess confidence in each of the findings (Lewin 2015; Lewin 2018). In line with this approach, LP and TM independently assessed four key issues in relation to each finding and their supporting studies:
methodological quality of the studies contributing data to this finding;
adequacy or ‘richness’ of the supporting data;
relevance of the supporting studies to the review question (e.g. with regard to intervention of interest, population, setting); and finally
how coherent the finding was, in other words, how likely it was that the finding explained the patterns seen in the qualitative data; this was determined from assessing how well grounded the finding was in the data, and reflecting on how convincing the explanatory finding was for the observed patterns.
Consideration on these issues fed into an overall judgement about how confident we were that our findings were a reasonable representation of our topics of interest. We considered the default to be a high degree of confidence in our findings, and downgraded that confidence according to our assessments on the four domains described above. LP and TM discussed their judgements and resolved any differences by mutual agreement.
Qualitative review author reflexivity
The review authors involved in the qualitative synthesis (LP and TM) had experience in qualitative research. LP is a medical clinician and experienced qualitative researcher with expertise in health ethics and health technology. TM has prepared systematic reviews of qualitative research. LP (from Australia) and TM (from England) are both living through a pandemic of COVID‐19. LP’s government is encouraging citizens to use an automatic mobile phone app for contact tracing and TM’s government is investigating a similar product. We reflected on our past experiences, including with digital health technologies, and talked together about how these might inform our interpretations of the data.
Results
Description of studies
We identified 181 studies from our initial search (see Figure 1; Moher 2009). After removing 144 studies that were clearly not relevant, we screened 37 full‐text articles and excluded 18 as they were editorials or commentaries, and excluded an additional nine full‐text studies for various reasons (see Characteristics of excluded studies). We identified two further studies through cross‐referencing and included 12 studies in our review in total.
1.
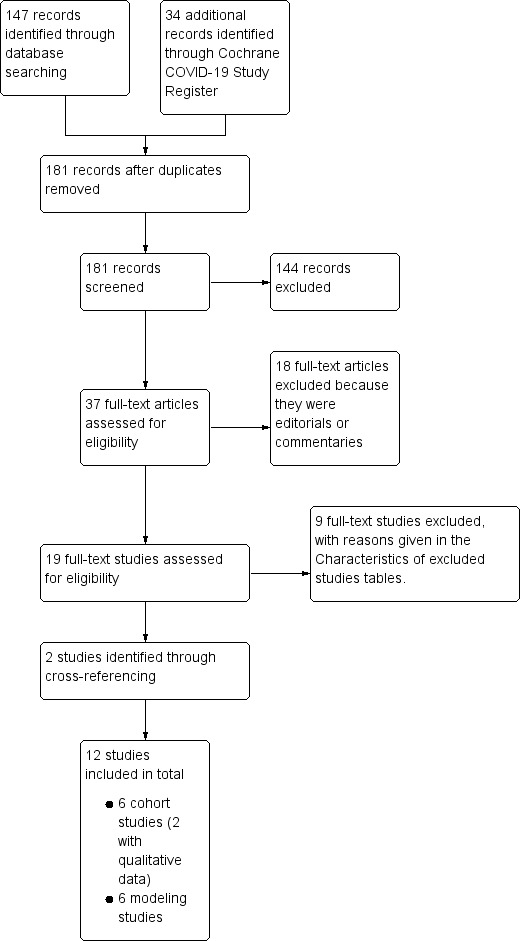
Study flow diagram
We provide an overview of the included studies in Characteristics of included studies. We identified six cohort studies reporting quantitative data (Danquah 2019; Ha 2016; Helmich 2017; Leecaster 2016; Mastrandrea 2015; Smieszek 2014), and six simulation studies reporting modelling of digital solutions for contact tracing (Farrahi 2014; Ferretti 2020; Fournet 2016; Hinch 2020; Kucharski 2020; Yasaka 2020). Two of the cohort studies provided qualitative research data as well (Danquah 2019; Ha 2016).
Of the six cohort studies, three were used for contact tracing during an outbreak: one was used during an Ebola outbreak in Sierra Leone (Danquah 2019); one was used to identify close contact of TB patients in Botswana (Ha 2016); and one was used during pertussis outbreak in the USA (Helmich 2017), while three were used to identify close contacts of participants in non‐outbreak settings (i.e. school environments) in an attempt to emulate an outbreak (Leecaster 2016; Mastrandrea 2015; Smieszek 2014).
Of the six modelling studies, four evaluated digital solutions for contact tracing in COVID‐19‐simulated scenarios in the UK or in non‐specific settings (Ferretti 2020; Hinch 2020; Kucharski 2020; Yasaka 2020), while two simulated close contacts in non‐specific outbreak settings (Farrahi 2014; Fournet 2016).
Characteristics of cohort studies
We identified three cohort studies of digital solutions to contact tracing within active disease outbreak settings. One study evaluated a contact tracing app used by public health contact tracers of 18 Ebola cases in Sierra Leone over four months amid an Ebola outbreak in 2015. Contact tracing intake information collected using the app was compared to traditional paper case intake forms used by public health contact tracers of 25 cases (Danquah 2019). The app was a manual solution developed to augment paper‐based contact tracing and monitoring efforts during data collection and management phases; it was not maintained by the cases or contacts, but by the contact tracers. Specifically, it streamlined data entry processes and prevented critical delays in gathering information regarding close contacts.
Similarly, a contact tracing app was developed for contact tracers investigating TB in Botswana over six months in 2012 to 2013 (Ha 2016). The aim of the app was to improve data management of close contact follow‐up and to minimise data entry errors or missing data. Again, this was a manual solution developed to be used by public health workers, not the cases or close contacts of the cases.
Additionally, we identified a retrospective cohort of a small outbreak of pertussis in the USA within a healthcare setting (Helmich 2017). The study authors quantified the number of close contacts that were found with a more advanced device (a radio‐frequency identification‐enabled (RFID) badge) compared to the standard electronic medical records search to determine which healthcare workers were likely within close proximity for a sustained amount of time (e.g. face‐to‐face contact with the case in an exam room or triage area).
Two of these cohort studies also provided qualitative data for this review. We highlight the characteristics of these two cohort studies in Appendix 2. Of note, one study employed semi‐structured interviews for three contact tracing workers in the healthcare system during an Ebola outbreak in Sierra Leone, while the other cohort collected data from free‐text entry within a survey of contact tracing health workers following up with TB patients in Botswana.
Lastly, there were three cohort studies of children in schools in the USA and France, not during an active outbreak (Leecaster 2016; Mastrandrea 2015; Smieszek 2014). Each of these studies evaluated network dynamics between active participants and aimed to determine whether passive digital devices could better capture close encounters than self‐recall. Self‐recall was captured through surveys and diaries.
Characteristics of modelling studies
Of the four modelling studies specifically evaluating contact tracing solutions during the COVID‐19 outbreak, one modelled scenarios within the UK, and three were modelled in non‐specific geographic areas with COVID‐19.
One study developed a general mathematical model evaluating the effects of a non‐specific, passive smartphone app on contact tracing efforts in a non‐specific area (Ferretti 2020).
All of the modelling studies specifically set in COVID‐19 environments evaluated non‐specific, automatic smartphone apps to aid in contact tracing. Further, an adapted disease transmission model (susceptible, infectious, or recovered (SIR)) was built by Yasaka and colleagues to evaluate an unnamed smartphone app in an unspecified geographic area (Yasaka 2020). Two additional modelling studies used individual‐based models to evaluate non‐specific, passive digital contact tracing apps in the UK (Kucharski 2020), or in an unspecified area (Hinch 2020). Hinch and colleagues were the only researchers to evaluate the impact of added benefits from recursive contact tracing from digital contact tracing (Hinch 2020).
Two modelling studies were performed outside the context of COVID‐19 (Farrahi 2014; Fournet 2016), and both studies used adapted SIR models. Farrahi 2014 used generic dual‐network topology in a non‐specific setting. Fournet 2016 used empirical data collected from passive RFID sensors during a study within a school in France to further model network dynamics.
Characteristics of studies including qualitative data
Two included cohort studies provided qualitative data from contact tracers in the healthcare worker setting (Danquah 2019; Ha 2016). For both studies, the intervention was a smartphone‐based app to assist contact tracers with managing data.
Risk of bias in included studies
ROBINS‐I for non‐randomised studies of interventions
We determined that three cohort studies had a moderate risk of bias and three had a serious risk of bias (see Table 1). However, the risk of bias from confounding, specifically, was low for all studies. Not many factors will affect both the number of contacts, or any other primary outcome of contact tracing, and the method of contact tracing employed. As a result, primary studies did not make many adjustments for confounding bias. Four of six studies were at serious risk of bias as a result of self‐selecting volunteers or enrolment of only a few participants over a short period of time. In studies in which participants self‐reported their close contacts, some participants chose to not participate in self‐reports, and as such were at risk of bias from deviations from intended interventions. In general, in studies in which the researchers hope to quantify close contacts, there is a serious risk of bias due to missing data because the true number of close contacts will rarely be known. Lastly, five of six studies were at risk of bias from the measurement of outcomes, resulting from participants knowing they would have to report the number of close contacts throughout the study. As a result, they may have been more likely to report differently than they would have had they not known.
1. Summary of ROBINS‐I signalling questions for included cohort studies.
| Author | Confounding | Selection of participants | Classification of interventions | Deviations from intended interventions | Missing data | Measurement of outcomes | Selection of reported result | Overall risk of bias |
| Danquah 2019 | No | No | No | No | Probably Yesa | Probably Yesb | No | Moderate |
| Helmich 2017 | No | Yesc | No | No | Probably Yesa | No | No | Moderate |
| Ha 2016 | No | No | No | No | Probably Yesa | Probably Yesb | No | Moderate |
| Leecaster 2016 | No | Yesd | No | Yese | Probably Yesa | Probably Yesb | No | Serious |
| Mastrandrea 2015 | No | Yesd | No | Yese | Probably Yesa | Probably Yesb | No | Serious |
| Smieszek 2014 | No | Yesd | No | Yese | Probably Yesa | Probably Yesb | No | Serious |
aMissing data risk will always be a serious threat in contact tracing as the true number of close contacts is rarely known. bParticipants knew they would have to report the number of close contacts throughout the study. As a result, they could possibly report differently than they would have had they not known. cStudy of successive patients over only two months, very few cases. dAs students were volunteers, their desire to participate may have biased their results. eNot all participants reported close contacts on a daily basis, and not all of those wearing devices self‐reported contacts.
In Table 2, we highlight the 'Risk of bias' assessments for outcomes from cohort studies in which we had direct evidence of an effect of digital contact tracing. For identifying close contacts, we included two cohort studies (Danquah 2019; Helmich 2017), and the overall risk of bias was serious due primarily to selection of participants, potentially missing data, and measurement of outcomes (participants may have been more likely to report differently if they knew they had to later quantify their close contacts). For time spent for data management, we identified one cohort study (Ha 2016), which addressed this outcome and it was at serious risk of bias due to missing data and measurement of outcomes.
2. 'Risk of bias' assessments for cohort studies (using ROBINS‐I).
| Outcome | Number of studies | Confounding | Selection of participants | Classification of interventions | Deviations from intended interventions | Missing data | Measurement of outcomes | Selection of reported result | Overall risk of bias |
| Identifying close contacts | 2 | Low | Moderatea | Low | Low | Moderateb | Moderatec | Low | Serious |
| Time spent for data management | 1 | Low | Low | Low | Low | Moderateb | Moderated | Low | Serious |
aOne of two studies included only successive patients over only two months. bMissing data risk will always be a serious threat in contact tracing as the true number of close contacts is rarely known. cIn one of two studies, participants knew they would have to report the number of close contacts throughout the study. As a result, they could possibly report differently than they would have had they not known. dIn this study, participants knew that they would have to report the number of close contacts throughout the study. As a result, they could possibly report differently than they would have had they not known.
Risk of bias for qualitative data
In Table 3, we provide an overview of the risk of bias from the qualitative data using an adapted CASP tool. Danquah 2019 provided qualitative data that we determined to be useful, primarily due to the fact that the researchers employed appropriate qualitative research methods and stated their findings clearly. Ha 2016 provided qualitative data that we determined to be marginally valuable. We found that the qualitative research methods employed by Ha and colleagues did not fully address the research issue, the data analysis was not rigorous, and the findings were not clearly stated.
3. Assessment of methodological limitations (adapted CASP).
| Study | Was there a clear statement of aims? | Was a qualitative methodology appropriate? | Was the research design appropriate? | Was the recruitment strategy appropriate? | Were data collected in a way that addressed the research issue? | Was the relationship between researcher and participants adequately considered? | Were ethical issues taken into consideration? | Was data analysis sufficiently rigorous? | Was there a clear state ment of findings? | How valuable is the research? | Overall assessment of methodological limitations |
| Danquah 2019 | Yes | Yes | Yes | Unclear | Yes | No | Yes | Unclear | Yes | Useful | Qualitative research methods used, but little information on how the analysis was done and very brief statement of data in the paper. Little information on respondents |
| Ha 2016 | Yes | Yes | No | Unclear | No | No | Yes | No | No | Marginal | This study did not use a robust qualitative method. They analysed free‐text responses in a survey. No discussion of respondents' relationship to those providing the survey or methods of analysis. |
| CASP: Critical Appraisal Skills Programme (CASP 2020) | |||||||||||
Risk of bias for modelling studies
Using recommendations from ISPOR‐SMDM, which were previously used by another recent Cochrane rapid review (Nussbaumer‐Striet 2020), we broadly summarised the risks of bias for the six included modelling studies in Table 4. All the models used a dynamic transmission model. Further, all studies performed additional analyses exploring the effects of changing assumptions. The presentation of the models was not always transparent; study authors did not specify the behaviour of the agents, show differential equations, or the model presentation was simply not clear in three of the six modelling studies. Overall, we determined that three of the six studies had no or minor concerns only, while two had moderate concerns, and one had major concerns (all resulting from transparency of models).
4. 'Risk of bias' assessments for modelling studies (using ISPOR‐SMDM recommendations).
| Study | Was the model a dynamic transmission model? | Were uncertainty analyses on main assumptions performed? | Is there a transparent presentation of infectious diseases models (e.g. differential equation or behaviour of agents specified)? | Quality |
| Farrahi 2014 | Yes | Yes | Yes | No or minor concerns only |
| Ferretti 2020 | Yes | Yes | Yes | No or minor concerns only |
| Fournet 2016 | Yes | Yes | Yes | No or minor concerns only |
| Hinch 2020 | Yes | Yes | No | Major concerns |
| Kucharski 2020 | Yes | Yes | Unclear | Moderate concerns |
| Yasaka 2020 | Yes | Yes | Unclear | Moderate concerns |
| ISPOR: International Society for Pharmacoeconomics and Outcomes; SMDM: Society for Medical Decision Making | ||||
Effects of interventions
We hoped to be able to pool studies employing similar contact tracing solutions, however, there was significant heterogeneity between Bluetooth apps (somewhat expected as they are not standardised) and a lack of reported effect estimates. Instead, we narratively summarise the effects of digital solutions on contact tracing during outbreaks. We determined direct evidence of the effects to be from studies specifically in a COVID‐19 or other outbreak settings. We determined indirect evidence of the effects to be from studies in non‐outbreak settings.
Identifying the secondary cases from index cases when compared to traditional contact tracing solutions (key question 1)
Direct evidence
Though multiple modelling studies evaluated the impact of digital contact tracing, only two specifically provide low‐certainty evidence on reducing secondary cases from index cases (see Table 5). Both of these modelling studies found some evidence of harm with digital contact tracing when compared to manual contact tracing, though the comparisons are in combination with other public health measures and not an additive effect. For example, Kucharski 2020 estimated that digital contact tracing would achieve an 18% reduction in Reff compared to self‐isolation alone (i.e. 1 − (Reff,DCT = 1.4/Reff,SI = 1.7) = 18%), while manual contact tracing would achieve a 35% reduction (i.e. 1 − (Reff,MCT = 1.1/Reff,SI = 1.7) = 35%). The study authors do not estimate the impact of combining digital and manual contact tracing. Similarly, using Ferretti 2020's model, we found a reduction in Reff for digital contact tracing when compared to self‐isolation alone (26% reduction) and a reduction in Reff for manual contact tracing compared to self‐isolation alone (53% reduction) under the same scenarios described by Kucharski 2020. It is important to note that these models do not model the likely scenario of digital solutions PLUS manual contact tracing (i.e. augmenting what is already occurring within public health units). These models instead evaluate digital solutions with other public health measures and then evaluate manual contact tracing solutions with other public health measures separately. We have made relative comparisons between solutions to get the reductions in Reff. Digital solutions in these models do not perform as well as manual contract tracing when compared to each other, however an additional important note is that there are quite strong assumptions about the effectiveness of manual contact tracing (95% to 100% of acquaintances would be traced), and assumptions about the proportion of the population who would have the app (53%).
5. Modelling summaries/assumptions.
| Outputs | Inputs | |||||||||
|
Modelling Paper |
Parameters | Reff | Reff reduction | Daily growth rate | R0 |
Lag: symptoms → test |
Lag: test → contact quarantine |
Fraction true contacts traced (= uptake² * sensitivity) |
Effectiveness of contact quarantine | Effectiveness of case isolation |
| Kucharski 2020a | Baseline | 2.6 | 0% | 2.6 | 0% | 0% | 0% | |||
| Kucharski 2020a | SI | 1.7 | −35% | 2.6 | 2.6 | 0 | 0% | 0% | 90% | |
| Kucharski 2020a | SI + manual trace 100% contacts | 1.1 | −58% | 2.6 | 2.6 | 0 | 100% | 100% | 90% | |
| Kucharski 2020a | SI + app‐based trace 53% contacts | 1.4 | −44% | 2.6 | 2.6 | 0 | 53% | 100% | 90% | |
| Ferretti 2020 | Baseline | 2.0 | 0% | 0.14 | 2 | 0% | 0% | 0% | ||
| Ferretti 2020 | SI | 1.9 | −7% | 0.12 | 2 | 2.6 | 0 | 0% | 0% | 90% |
| Ferretti 2020 | SI + manual trace 100% contacts | 0.9 | −57% | −0.02 | 2 | 2.6 | 0 | 100% | 100% | 90% |
| Ferretti 2020 | SI + app‐based trace 53% contacts | 1.4 | −32% | 0.05 | 2 | 2.6 | 0 | 53% | 100% | 90% |
| Hinch 2020 | ||||||||||
| Yasaka 2020b | 50% | 100% | ||||||||
| Reff: effective reproductive number; R0: basic reproduction number; SI: self isolation | ||||||||||
aContact network based on BBC Pandemic dataset; includes asymptomatic spread. bDefault values given. Simple SIR (susceptible‐infected‐recovered) model; all parameters can be varied; curves of population proportion of infection given with/without tracing tyleryasaka.shinyapps.io/covidwatch/.
Indirect evidence
We found no indirect evidence addressing the effectiveness of digital solutions in identifying secondary cases.
Qualitative evidence
There were no qualitative data directly addressing this question. One of the two studies that compared digital data entry and management systems to paper‐based systems (Danquah 2019), reported on the impact of this intervention on regular (twice‐daily) monitoring of known contacts of patients with Ebola. Contact monitoring teams found digital systems to be generally faster and more efficient even though they were prone to problems with network coverage, technical faults, user errors or a combination of two or all of these. More efficient monitoring of contacts might mean that identification of secondary cases amongst those contacts is more effective.
Identifying the close contacts from index cases when compared to traditional contact tracing solutions (key question 2)
Direct evidence
We identified two cohort studies that provided very low‐certainty evidence for identifying close contacts in a real‐world epidemic setting (see Table 6). From both cohort studies, the digital solution provided an increased benefit over manual contact tracing approaches. In an Ebola outbreak setting, contact tracers using an app to record close contacts of Ebola cases found twice as many close contacts per case on average than using paper intake forms (Danquah 2019). Similarly, after a pertussis outbreak in a hospital in the USA, Helmich 2017 found that RFID could identify more close contacts than searches of electronic medical records for direct contact with medical personnel.
6. Direction of effects in cohort studies.
| Study | Intervention | Control |
Direction of effect (↑: increased benefit; ↔ equivocal; ↓ decreased benefit) |
| Non‐outbreak setting | |||
| Number of close contacts identified | |||
| Leecaster 2016 | 5592a | 711b | ↑ |
| Mastrandrea 2015 | 488 | 287 | ↑ |
| Smieszek 2014 | 1074c | 392d | ↑ |
| Outbreak setting | |||
| Number of close contacts identified | |||
| Danquah 2019 | 36e | 16e | ↑ |
| Mean duration of time from case registration to contact assignment | |||
| Danquah 2019 | 4.3 hours | 23.4 hours | ↑ |
| Mean duration of total time from case registration to first visit of contacts | |||
| Danquah 2019 | 73.2 hours | 70.2 hours | ↓ |
| Median time spent for data management per close contact | |||
| Ha 2016 | 2.8 minutesf | 5.0 minutesf | ↑ |
| Helmich 2017 | 45a | 13b | ↑ |
aNumber of recorded contacts by device/intervention only. bNumber of recorded contacts by self‐report/control only. cThe number of close contacts 15 minutes or more identified with device. dThe number of close contacts 15 minutes or more identified through self‐report. eAverage contacts per case. fMedian time spent with contacts of adult cases.
Indirect evidence
Overall, in non‐outbreak settings, we found added benefits from digital contact tracing solutions, as devices identified more close contacts than self‐reported diaries or surveys (Leecaster 2016; Mastrandrea 2015; Smieszek 2014 see Table 6).
Qualitative evidence
There were no qualitative data directly addressing this question. One of the two studies that compared digital data entry and management systems to paper‐based systems (from Botswana) reported on the impact of this intervention on the recording and collating of data about close contacts of people with TB (Ha 2016). Contact tracers said that digital systems avoided the need to carry papers, "which are often lost". Avoidance of lost data would suggest that effectiveness of contact tracing is improved.
Time to complete a set of close contacts with and without digital solutions (key question 3)
Direct evidence
We identified one cohort study that provided very low‐certainty evidence that an app could provide a benefit by reducing the time to complete a set of close contacts. Specifically, in a TB setting, the median time spent for data management was lower per close contact when an app was used by contact tracers when compared to a paper‐based data management system (Ha 2016).
Indirect evidence
Though not directly addressing the key question regarding time to complete contact tracing, we found added benefit from an app for case management, as the time from an Ebola case registration to close contacts being assigned was nearly six times longer if the traditional paper system was used (Danquah 2019; see Table 6). However, the total time from case registration to visiting close contacts was slightly longer for app‐based tracking than for paper‐based tracking systems (Danquah 2019), indicating a potential harm, as it would lengthen the time to visit contacts.
Qualitative evidence
There were no qualitative data directly addressing this question. However, contact tracing teams reported that digital data entry and management systems were faster to use than paper systems for recording of new contacts and monitoring of known contacts and possibly less prone to data loss (Danquah 2019; Ha 2016).
Identifying contextual information about the contact (i.e. setting, duration) compared to traditional contact tracing methods (key question 4)
Direct evidence
To directly answer this question, the study would have to be able to evaluate the context in which the close contact was exposed to the case. Most digital solutions lack the ability to capture information about the context (e.g. within close quarters). Some apps/devices have the ability to evaluate the density of other app/device users in their immediate surrounding. This, in turn, would provide valuable feedback about the environment in which users were exposed. However, we found no published data on this capability within the COVID‐19 epidemic.
Indirect evidence
We found no indirect evidence addressing the effectiveness of digital solutions in identifying contextual information about the contact.
Qualitative evidence
There were no qualitative data addressing this question.
Effectiveness of different types of digital solutions in identifying the secondary cases from index cases when compared to each other (key question 5)
Direct evidence
We found no direct evidence addressing the effectiveness of digital solutions in identifying the secondary cases from index cases when comparing alternative digital contact tracing solutions.
Indirect evidence
We identified one modelling study (Hinch 2020), which provided indirect evidence comparing digital contact tracing with and without recursive contact tracing. The researchers found that contact tracing with an app can only quell epidemic growth rates if strong assumptions are made regarding the doubling time, while a contact tracing app with recursive contact tracing could control the epidemic even with much more relaxed assumptions. This provided indirect evidence because the study authors only compared a hypothetical app with another hypothetical app (or the same app with an added feature or broadened use), and these were not necessarily different types of apps, but the same apps used in different ways.
Qualitative evidence
There were no qualitative data addressing this question.
Effectiveness of different types of digital solutions in identifying the close contacts from an index case when compared to each other (key question 6)
There were no data addressing this question.
Acceptability and accessibility of the digital solution in a given setting and population (key question 7)
Qualitative evidence
The two studies on digital data entry and management systems (Danquah 2019; Ha 2016), both from lower‐ or middle‐income countries, contained qualitative data relevant to this question. Both studies reported that contact tracing teams were positive about the digital systems, being simpler to use and generally preferred over traditional paper systems. Digital systems saved personnel time, particularly in locations where contact tracer personnel otherwise faced several hours of travel to and from central headquarter teams to collect and deliver paper‐based data entry forms. Digital systems reportedly improved accuracy with large data sets, and were easier to transport compared with paper forms.
Both studies reported that contact tracing teams faced some issues with accessibility of digital systems compared to paper systems. Problems with system access included patchy network coverage, lack of data, technical problems with hardware or software that were unable to be resolved by local technical teams, and higher staff training needs, including the need for refresher training. Other accessibility issues related to financial costs; contact training personnel in Sierra Leone were concerned at increased personal costs resulting from a need for them to travel and recharge phone batteries in the field using commercial tele‐charging stations. There may also be financial issues for the local districts due to costs associated with the necessary hardware. The hardware used for the digital system evaluated in Sierra Leone (Danquah 2019), were second‐hand phones donated by the United Nations.
Privacy or safety concerns for the different contact tracing approaches (key question 8)
We summarise privacy and safety concerns in Table 7. Prior research has evaluated specific types of apps with regards to their privacy (Cho 2020). Adopting their approach, we briefly summarise privacy concerns with regards to snoopers (i.e. unapproved, passive collection of data), contacts, and authorities. Briefly, a breach of privacy from snoopers would occur if an app publicly broadcasts the identifications of contacts or cases, or both, and someone purposefully passively collects those data and tracks individuals. A breach of privacy from contacts would occur if two app users’ phones exchange data due to proximity (and contact definitions), but the case status of the app user is known in that data exchange (i.e. an app user could identify which of his contacts were a case based on the data exchanged between their phones). Lastly, a privacy breach from authorities would occur if the case status or contact history, or both, of an app’s user were known to governmental agencies or large companies. The devices identified in the cohort studies all appeared to have privacy from contacts with regards to the exposed or diagnosed users. Privacy from snoopers was possibly breached if linkage attacks occurred, particularly for the wearable devices (the contact tracing app used by contact tracers was used for data management only and password protected ‐ it is unclear if data were ever broadcast). Privacy from authorities or leading agencies was mostly achieved with the wearable devices, as these studies were mostly field trials and not conducted by public health agencies. However, the app used by contact tracers for data management was not private from authorities as the contact tracers were members of the public health agency and knew the exposure and diagnosis status of the participants.
7. Privacy/security summaries among solutions included in reviewa.
| App/digital solution | Privacy from snoopers | Privacy from contacts | Privacy from authorities | ||
| Exposed user | Diagnosed user | Exposed user | Diagnosed user | ||
| Contact tracing app used by contact tracers for data management | Password protected only | Yes | Yes | No. Exposure status known to tracers | No. Diagnosis status known to tracers |
| Wearable RFID‐enabled badge, wireless linkage to readers | Linkage attacks possible | Yes | Yes | Yes. Local affiliated researcher access only | Yes. Local affiliated researcher access only |
| Wireless ranging enabled node‐device | Linkage attacks possible | Yes | Yes | Yes. Local affiliated researcher access only | Yes. Local affiliated researcher access only |
| RFID‐enabled sensors | Linkage attacks possible | Yes | Yes | Yes. Local affiliated researcher access only | Yes. Local affiliated researcher access only |
| RFID: radio‐frequency identification‐enabled | |||||
aAdapted from Cho 2020. There are dozens of apps and digital solutions currently in use or being considered, but none with published data.
Qualitative evidence
One of the two studies on digital data entry and management systems recorded an issue with stolen hardware (second‐hand mobile phones; Danquah 2019). The other study (Ha 2016), included a comment from contact tracer personnel that paper data entry forms were ‘often lost’. Neither study contained any further text about privacy issues in relation to these malign/accidental data loss scenarios. Both studies recorded that the digital system under study was password‐protected and Ha 2016 noted that data were encrypted although only provided limited technical detail about this.
Ethical concerns identified (e.g. equity issues, harms to the individual from high false positives) (key question 9)
There were no data addressing this question.
Quality of evidence
We summarise the CERQual assessments for the qualitative findings in Table 8. Two studies provided data for these assessments (Danquah 2019; Ha 2016). We had moderate or substantial concerns about adequacy of data, the quality of the study methods, relevance to the research questions, and coherence for three qualitative findings within the review: use of digital data management systems may be more effective for identifying secondary cases than paper systems since they allow more efficient contact monitoring; use of digital data management systems may be more effective for identifying close contacts than paper systems because paper forms may be prone to loss; and use of digital data management systems may enable faster identification of a set of close contacts than paper systems because they enable greater efficiency in data entry and management. In turn, we have low confidence in the evidence for these outcomes. For three additional qualitative outcomes (contact tracing teams prefer digital data management systems over paper systems; digital data management systems are less accessible than paper systems in certain settings; and both digital and paper data management systems raise concerns about loss of privacy due to accidental or malign data loss), we have moderate confidence in the evidence. For these outcomes, our primary concerns were regarding the adequacy of the data.
8. Summary of qualitative findings and CERQual assessment.
| Review finding | CERQual assessment of confidence in the evidence | Explanation of CERQual assessment | Studies contributing to the review finding |
| Use of digital data management systems may be more effective for identifying secondary cases than paper systems since they allow more efficient contact monitoring | Low | We had moderate concerns about the adequacy of data and methodological quality, and substantial concerns about relevance and coherence | Danquah 2019 |
| Use of digital data management systems may be more effective for identifying close contacts than paper systems because paper forms may be prone to loss | Low | We had substantial concerns about adequacy of data and methodological quality, and moderate concerns about relevance and coherence | Ha 2016 |
| Use of digital data management systems may enable faster identification of a set of close contacts than paper systems because they enable greater efficiency in data entry and management | Low | We had substantial concerns about adequacy of data, methodological quality, relevance and coherence | Danquah 2019; Ha 2016 |
| Contact tracing teams prefer digital data management systems over paper systems | Moderate | We had moderate concerns about methodological quality and adequacy of data | Danquah 2019; Ha 2016 |
| Digital data management systems are less accessible than paper systems in certain settings | Moderate | We had minor concerns about adequacy of data | Danquah 2019; Ha 2016 |
| Both digital and paper data management systems raise concerns about loss of privacy due to accidental or malign data loss | Moderate | We had moderate concerns about adequacy of data | Danquah 2019; Ha 2016 |
| CERQual: Confidence in the Evidence from Reviews of Qualitative Research | |||
In Table 9, we summarise the certainty of direct evidence from the identified cohort and modelling studies with regards to our primary outcomes. Specifically, two cohort studies provided very low‐certainty evidence regarding identifying close contacts within an epidemic setting. Similarly, two modelling studies provided low‐certainty evidence in identifying secondary cases. We determined that the two cohort studies (Danquah 2019; Helmich 2017), provided very low‐certainty evidence as a result of serious risk of bias (missing data risk, as the true number of close contacts is unknown), indirectness (studies in dissimilar epidemic settings may not be comparable or generalisable), and imprecision (one cohort had very few index cases to perform contact tracing on). The summary direction of effect from these two cohort studies suggested an increased benefit from the digital solution to contact tracing. Two modelling studies provided low‐certainty evidence for identifying secondary cases (Ferretti 2020; Kucharski 2020), finding reductions in Reff between 18% to 26% when digital contact tracing is used, compared to self‐isolation alone, though they estimate that greater reductions are possible with manual contact tracing (35% to 53% reduction). The modelling studies did not make direct comparisons between a digital contact tracing solution and manual contact tracing. Additionally, the modelling studies did not make direct comparisons under more likely adoption scenarios (e.g. digital contact tracing PLUS manual contact tracing vs manual contact tracing alone). As with any modelling study, the results are dependent on the assumptions, and in the context of contact tracing we are simply unsure about the true number of close contacts, which can lead to increased ascertainment biases. Additionally, one cohort provided very low‐certainty evidence for the time spent for close contact data management (Ha 2016). This study found an increased benefit with the use of an app for data management of close contacts, though its ability to reduce the amount of time to complete a follow‐up of all close contacts is unknown. Further, the study had few index cases on which the researchers could perform close contact histories. For all other research questions (key question 4 to key question 9), we found no direct evidence.
9. Certainty of evidence ratings for the effectiveness of digital contact tracing for cases in an epidemic setting.
| Outcome | Number of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Summary direction of effect/outcome (↑: increased benefit; ↔ equivocal; ↓ decreased benefit) | Certainty of the evidence |
| Identifying secondary cases | 2 modelling studies estimating epidemic growth | Seriousa | Consistent | Indirectc | Precise | None |
Ferretti 2020 model shows 26% Reff reduction if digital solution AND SI used, compared to 53% Reff reduction if manual contact tracing used Kucharski 2020 model shows 18% Reff reduction if digital solution AND SI used, compared to 35% Reff reduction if manual contact tracing used |
Low |
| Identifying close contacts | 2 cohort studies | Seriousa,b | Consistent | Indirectd | Imprecisee | None | Danquah 2019 ↑ Helmich 2017 ↑ | Very low |
| Time spent for data management | 1 cohort study | Seriousa | Consistent | Direct | Imprecisee | None | Ha 2016 ↑ | Very low |
| Reff: effective reproductive number; SI: self‐isolation | ||||||||
aMissing data risk will always be a serious threat in contact tracing as the true number of close contacts is rarely known. bStudy of successive patients over only two months, very few cases.
cModelling studies did not make direct comparisons between digital contact tracing and manual contact tracing, instead making indirect comparisons with dissimilar parameters. Further, the modelling studies did not consider more pragmatic comparisons (e.g., digital solution PLUS manual contact tracing vs manual contact tracing alone). Lastly, the assumptions regarding the effectiveness of manual contact tracing (90‐100%) and the uptake of digital contact tracing solutions (53%) were strong and influential on the effect estimates. dStudies not conducted in similar disease settings and may not be comparable or generalisable. eFew cases were identified to get information regarding close contacts.
Discussion
Effectiveness of digital solutions in real‐world settings is largely unproven. The certainty of evidence is very low from several cohort studies, which found that digital solutions can identify more close contacts than traditional contact tracing. Further, modelling studies provide low‐certainty evidence of a reduction in secondary cases if digital contact tracing solutions are used together with other public health measures, although the relative reduction in secondary cases was not as good as with manual contact tracing if certain, strong assumptions are made. Importantly, there is very little empirical evidence evaluating the effectiveness of digital solutions for contact tracing during an outbreak.
We found no published, direct evidence evaluating the effectiveness of digital contact tracing solutions on identifying contextual information about contacts, identifying the acceptability and accessibility of the digital solution, identifying privacy or safety concerns for different digital contact tracing solutions, or identifying ethical concerns from different contact tracing solutions when compared to traditional contact tracing methods or when compared to other digital contact tracing solutions. Our findings closely align with another recent review evaluating automated contact tracing (Braithwaite 2020). Researchers similarly found that contact tracing apps could identify a more complete contact history and follow‐up, and potentially reduce times to isolation, but the evidence largely rested with modelling studies and empirical evidence outside of the COVID‐19 setting.
As automatic contact tracing solutions aim to identify more close contacts, the consequences this may have on contact tracing teams within public health agencies need to be carefully considered. In fact, as the number of close contacts increases for an index case, so too will the amount of time needed to complete contact tracing for that index case. The accuracy of the tools used to identify close contacts is paramount, as to avoid over‐burdening the contact tracing teams with clinically unimportant contacts identified with digital solutions. Further, negative unintended consequences that could be realised with these solutions could be the very large number of uninfected people quarantined (mirroring a small‐scale lockdown). For example, Hinch and colleagues found that recursive contact tracing could suppress COVID‐19 epidemic growth even with pessimistic assumptions, but they also found that the largest number of uninfected people would be quarantined (Hinch 2020).
Most studies do not define or often even discuss the implications of centralised compared to decentralised digital contact tracing systems. The different systems relate to where contact data are stored. Apple and Google’s exposure notification system is the most publicly discussed decentralised system whereby contact data are stored on individual users’ phones and individuals are notified of their potential exposure independent of the government contact tracing infrastructure. While providing additional privacy to individuals from governments, the lack of integration with contact tracing infrastructure would likely undermine the effectiveness of decentralised systems due to the reduced likelihood of people isolating without manual follow‐up from contact tracers and reducing the potential effectiveness of a contact tracer’s case investigations. The trade‐offs between decentralised and centralised digital contact tracing systems are not sufficiently discussed in the included papers, but are a central consideration in the development and implementation of digital solutions.
Agreements and disagreements with other studies and reviews
It is important to note that we are unaware of any published (preprint or otherwise) study of empirical, individual‐level data evaluating the effectiveness of any automatic digital solution for contact tracing conducted during an active epidemic. Helmich and colleagues, however, used RFID to retrospectively identify close contacts to pertussis patients in a hospital setting (Helmich 2017). We know of at least one preprint study that attempted to assess a digital tool in an active COVID‐19 outbreak in the UK (Kendall 2020). However, this study relied on aggregated incidence data and adopted an ecological study design so that the observed reduction in cases could not be attributed to the digital tool. In the absence of publicly available evaluations, early media reports have concluded that national‐based digital solutions, like those implemented in Australia and Singapore, have had limited effect on the pandemic. For example, not one additional contact was found by the digital contact tracing tool during the recent outbreak in Victoria, Australia (Taylor 2020). Future research needs to look at individual‐level data to determine the effectiveness of the digital solution, and there is an urgent need for governments to enable robust and independent evaluations of their digital tools. We are aware of at least two additional modelling studies that were not yet in preprint form at the time of our search and have not yet been peer‐reviewed (Cencetti 2020; Lambert 2020). These modelling studies evaluate either generic contact tracing strategies or overview an agnostic digital proximity tracing app in the context of COVID‐19. Further, there is an ongoing mobile health interventional study in Uganda which is evaluating home‐based TB contact investigations (Ayakaka 2020). This ongoing study may provide further evidence regarding the effectiveness of digital contact tracing solutions in specific settings, though to date we are unaware of any published data from this study. We plan to update this review as new evidence becomes available.
Completeness and applicability of evidence
As with any healthcare intervention, the decision on whether, and how, to use contact tracing technologies in epidemics needs to be based on evidence. The effectiveness of these technologies is largely unproven in real‐world scenarios, so the impact on controlling COVID‐19 infection rates is unclear. The technologies are reliant on accurately detecting close contacts and secondary cases, evidence for which is not yet available. National government social distancing guidance can also differ between countries (generally 1 metre versus 2 metres), which will have an impact on the accuracy of the technologies to capture all true transmissions. One modelling study using a close contact definition of 2 metres for 15 minutes or more estimated that to detect 4 out of 5 true secondary cases from an infected individual, 36 contacts would need to be traced (Keeling 2020).
The implications of false positives (reports of exposure when no close contact event occurred or the interaction was not clinically relevant) and false negatives (when exposure should have been reported but it was not) must be understood in real‐world scenarios. False positives could burden communities with repeated requests to isolate, in addition to the contact tracing teams. Those in high‐density communities could be more at risk of repeated false positives, which would affect an individual’s ability to work and live. Living in high‐density communities is generally associated with those with lower socio‐economic status, which could exacerbate societal inequities.
Analyses have shown that the majority of a population would need to enrol and actively participate in contact tracing for it to be effective (Kim 2020). To maximise enrolment and participation, especially for voluntary technologies, national governments need to understand the impact of different personal preferences, sociodemographic characteristics and public sentiment. The majority of the public appears to support app‐based contact tracing solutions, according to a large survey recently conducted in the UK, USA, France, Germany and Italy (Altmann 2020). The researchers found that there were high levels of support, with approximately three‐quarters of those surveyed from each country saying they would probably or definitely install an app onto their phone (Altmann 2020). However, real‐world attempts to implement digital technologies suggest a disconnect between intent and action. Apps in Singapore and Australia have received less than 25% uptake, of which, at least half are likely to be non‐compliant users.
There is a need for consensus and evidence‐based standards that consider what personal data to collect, how data are collected (automatic versus manual), how data are stored and secured (centrally storing data or storing data on individual’s devices), how long data are held, what tests are used within the technologies (these should be validated and consistent across technologies), among other considerations (Nature 2020). The need for standards is even more important as national responses to COVID‐19 include many countries developing their own, slightly different, contact tracing technologies. Developers will need to be transparent about the limitations of these technologies, otherwise it will erode public trust. For example, in Belgium, the government has already paused implementation of contact tracing technologies as part of its COVID‐19 responses based on some of these concerns (Vandamme 2020).
South Korea has been regarded as a country to have benefited from including contact tracing technologies as part of their national responses to COVID‐19. However, South Korea’s response also includes a comprehensive testing strategy, an established, nationwide network of contact tracers, and the social license for surveillance of individuals in the population that other countries would be unlikely to accept, for example, accessing confidential records, such as credit‐card transactions (Korea CDC 2020).
Most studies in this review failed to explore even one equity element, in particular, those modelling studies utilising individual‐based technologies such as smartphone apps. Any proposed solution must place equity at the center of its development, testing and implementation (WHO 2013). At‐risk communities need to be engaged early in the process, invited as co‐developers and be integrated into the dissemination and communication strategies. COVID‐19 has required unprecedented acceleration of policy to fight the virus, however, health equity cannot be a casualty of the need for rapid response measures. Failing to actively address health equity in the COVID‐19 response will result in ever‐widening inequalities that governments will be paying for in the decades to come.
Privacy concerns regarding data access by snoopers, contacts and the authorities have been identified, with a range of suggested options for enhancing the privacy of user’s data (Cho 2020). A number of methods for such contact tracing apps to identify cases and contacts exist ‐ primarily location data or proximity data, both of which come with pros and cons when weighing up the potential efficacy of contact tracing apps versus user privacy concerns (Hart 2020). For example, the advantage of mobile GPS location data over Bluetooth proximity data is that they can be used to identify and warn users of high‐risk areas to reduce surface transmission. However, they are unable to identify whether two people have been at the same place at the same time in close proximity to be defined as a contact (Hart 2020). On the other hand, using Bluetooth proximity data may be innately error‐prone in identifying exposure to a case, with an approximate error rate of 7% to 15% of both false positive and false negative rates (UAB 2020).
In the context of technology use, privacy often refers to the individual’s right to safeguard and control access to themselves and their information. While manual contact tracing technologies also present risks to individual privacy, the privacy risks from the use of contact tracing technologies can occur at a larger scale. Compared with manual methods, a larger number of entities (ranging from individual users, to unauthorised parties/hackers, technology developers and owners, third party services, public health units, and government entities) have the potential to access a greater amount of sensitive user information (user personal details, anonymous IDs of those in proximity to users, disease and exposure status, other health information, movement and location information, social graph, device information; Redmiles 2020). For example, the use of digital technologies allows for the potential of data aggregation across multiple sources and subsequent user re‐identification, especially if third party services are involved in the operation of the technology (Grundy 2019b).
There are multiple points in which privacy issues from contact tracing technologies can occur (FPF 2020). These include:
data collection (what is collected and how it is collected)
data access (who can access and aggregate data)
data use (who can use it and for what purposes)
data storage and retention (where it is stored, duration of retention)
data deletion (whether it is deleted, and if so, how it is deleted and by whom)
As a moderately high uptake of contact tracing technologies by the population is required for the technology to be effective at reducing epidemic growth, it is important to consider the impact that real and perceived privacy risks have on the public’s trust in the institutions developing and implementing these policies, and their subsequent willingness to use these strategies. The requirements for public trust and confidence will differ between individuals and between socio‐political contexts. For example, in states with democratic systems, this will likely include publishing ethical principles or frameworks to guide the use of contact tracing technologies, and collected user data; providing explicit and justifiable answers to the above questions on data protection; establishing oversight systems that are transparent and accountable, effective, and inclusive; developing and using technologies that adhere to data protection laws and the highest standards of security; and the availability of application protocols and application programming interfaces (APIs) for public and transparent auditing (Access Now 2020).
Our goal in this review was to evaluate the evidence of effectiveness of digital contact tracing solutions, not to evaluate all apps currently in use. There are a number of resources currently qualitatively evaluating the privacy and security of digital contact tracing solutions (CCC 2020; O'Neill 2020). In brief, at the time of this review, there are currently 29 automatic contact tracing apps used that are supported by just as many countries’ governments. None of these apps, to our knowledge, have published any empirical evidence of effectiveness.
Potential biases in the review process
There were very few qualitative data available in our identified studies despite using citation searching on included studies, reference list checking of the wider pool of potentially relevant full‐text studies and amending our protocol slightly to include studies with any qualitative data at all (i.e. waiving our previously stated exclusion of studies that provided qualitative data without qualitative analysis). The quantitative evidence we identified was not adequate for synthesising data. As such, our results and conclusions are potentially more subjective to our interpretations. However, we made an attempt to be very transparent with regards to the evidence, to allow for the reader to make their own interpretations. More extensive and iterative searching for key texts might yield additional studies, for example, looking for grey literature, contacting experts, and searching for apps in use by name (Booth 2016). This would be an important aspect of our methods to develop for future updates of this review. Lastly, there were some aspects of the rapid review that may have impacted our review. We used single review authors for data extraction, 'Risk of bias' assessments, and certainty of evidence assessments. While a second review author checked these assessments, the lack of dual, independent data extraction and analyses may have introduced some error in our review. Regardless, as we double‐checked our work and collaborated as a team, we feel that this review’s conclusions and findings are unlikely to be affected by any limitation introduced from the rapid review process.
Authors' conclusions
Implications for practice
We found that the effectiveness of digital solutions is largely unproven in real‐world outbreak settings. However, multiple mathematical models using different assumptions and different scenarios agree that digital solutions have the capability of reducing the epidemic growth if there is high utilisation together with strong public health efforts, and several cohort studies provide very low certainty evidence that technology can produce more reliable counts of contacts and reduce the time to complete contact tracing in real‐world epidemic settings.
When implementing contact tracing digital technologies, governments should consider issues of privacy and equity. The current COVID‐19 pandemic is disproportionately affecting ethnic minorities, the elderly, and people living in high deprivation. These health inequities could be magnified with the introduction of digital solutions that do not consider these at‐risk populations, who are likely to have poor access to smartphones with full connectivity. The design and implementation of health policies regarding digital solutions for contact tracing should account for this to ensure that digital tracing strategies are inclusive.
Implications for research
Stronger primary research on effectiveness of contact tracing technologies is needed, including studies that provide appropriately analysed numerical estimates of effectiveness, which are at low risk of bias and conducted in real‐world epidemic settings. This includes research into how digital solutions may be used in conjunction with manual systems, as digital solutions are unlikely to be used in isolation. The current body of literature also fails to explore equity issues adequately, and future studies of digital solutions to contact tracing, including modelling studies of individual‐based technologies, should highlight the importance of equity and differences in acceptability between subpopulations. Lastly, future studies examining the effectiveness of digital contact tracing must make privacy concerns a primary research question, as risks of privacy breaches are an important concern and barrier to participation in digital contact tracing interventions during an outbreak.
What's new
| Date | Event | Description |
|---|---|---|
| 18 August 2020 | Amended | Minor typo fixed |
History
Review first published: Issue 8, 2020
Acknowledgements
We would like to acknowledge our information specialist, Michael Smalle, at University of Limerick for his assistance with the search. His prompt adjustments and thorough searches significantly improved our systematic review.
The editorial process for this review was managed by Cochrane's EMD Editorial Service in collaboration with Cochrane Public Health. We thank Anne‐Marie Stephani for editorial management, and Helen Wakeford, Toby Lasserson and Rachel Richardson for comments on the manuscript. We thank Robin Featherstone and Ruth Foxlee for comments on the search and Robert Boyle for sign‐off comments. We thank Denise Mitchell for her efforts in copy‐editing this review.
Thank you also to peer referees Hajo Zeeb, Luca Ferreti and Meshari Alwashmi, and methodological referee Miranda Cumpston for their insights.
We would also like to thanks Tom Cunningham for his insightful feedback on our interpretations of the modelling studies.
We would also like to thank Tom Cunningham (Core Data Scientist, Facebook) for his insightful feedback on the interpretation of the included modelling studies. This contribution was checked by an independent referee, Ian Shemilt.
Appendices
Appendix 1. Search strategies
Database(s): Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) 2000 to 5 May 2020
Search strategy:
| # | Searches | Results |
| 1 | exp Coronavirus/ | 13132 |
| 2 | exp Coronavirus Infections/ | 11636 |
| 3 | (coronavirus* or 2019‐nCoV or 2019 ncov or nCov or covid‐19 or covid19 or sars‐cov‐2 or sars or sarscov2 or sarscov‐2 or sars‐coronavirus‐2 or sars corona virus or sars‐like coronavirus novel coronavirus or novel corona virus or covid* or pneumonia or severe acute respiratory syndrome or coronavirus 2 or coronavirus infection* or coronavirus disease or corona virus disease or new coronavirus or new corona virus or new coronaviruses or novel coronaviruses or severe acute respiratory syndrome coronavirus‐2 or respiratory infectious disease* or acute respiratory disease* or middle‐east respiratory syndrome or mers or tuberculosis or influenza or pandemic* or epidemic* or zika or ebola).tw,kf. | 551732 |
| 4 | (COVID‐19 or COVID‐19 drug treatment or COVID‐19 diagnostic testing or COVID‐19 serotherapy or COVID‐19 vaccine or severe acute respiratory syndrome coronavirus 2).ps. | 301 |
| 5 | exp Communicable Diseases/ | 35098 |
| 6 | exp Epidemics/ | 16708 |
| 7 | exp Respiratory Tract Infections/ | 355428 |
| 8 | or/1‐7 | 765481 |
| 9 | exp Contact Tracing/ | 4269 |
| 10 | (contact adj3 (trac* or examination or screening or management or investigation)).tw,kf. | 3945 |
| 11 | transmission dynamics.tw,kf. | 3284 |
| 12 | or/9‐11 | 10380 |
| 13 | exp Wearable Electronic Devices/ | 11301 |
| 14 | exp computer communication networks/ | 91431 |
| 15 | exp Telecommunications/ | 90466 |
| 16 | exp Mobile Applications/ | 5615 |
| 17 | *Smartphone/ | 2876 |
| 18 | (Smartphone application* or wearable electronic device* or wearable* or smartphone* or smart phone* or cell phone* or cellular phone* or mobile phone* or digital solution* or GPS device* or GPS track* or ICTs or Bluetooth or health apps or health application* or eHealth or mHealth or mobile app*or movement mapping or digital technolog* or digital health or smart watch* or application programming interface* or telemedicine or digital contact tracing or automated data entry or automated software or data capture).tw,kf. | 57842 |
| 19 | or/13‐18 | 219289 |
| 20 | 8 and 12 and 19 | 67 |
| 21 | animals/ not (humans/ and animals/) | 4662726 |
| 22 | 20 not 21 | 64 |
| 23 | limit 22 to yr="2000 ‐Current" | 64 |
Database(s): Embase
Search strategy:
No.
Query
Results
90
#20
#7 AND #11 AND #18 AND [2000‐2020]/py
91
#19
#7 AND #11 AND #18
664,425
#18
#12 OR #13 OR #14 OR #15 OR #16 OR #17
622,133
#17
'wearable electronic device*':ab,ti OR wearable*:ab,ti OR smartphone*:ab,ti OR 'smart phone*':ab,ti OR 'cell phone*':ab,ti OR 'cellular phone*':ab,ti OR 'mobile phone*':ab,ti OR 'digital solution*':ab,ti OR 'gps device*':ab,ti OR 'gps track*':ab,ti OR icts:ab,ti OR bluetooth:ab,ti OR 'health apps':ab,ti OR 'health application*':ab,ti OR ehealth:ab,ti OR mhealth:ab,ti OR 'mobile app*':ab,ti OR 'movement mapping':ab,ti OR digital* OR 'smart watch*':ab,ti OR 'application programming interface*':ab,ti OR telemedicine:ab,ti OR automat*:ab,ti OR 'data capture':ab,ti OR api:ab,ti OR apis:ab,ti OR iphone*:ab,ti OR ipad*:ab,ti OR 'mobile device*':ab,ti OR tracetogether:ab,ti OR covidsafe:ab,ti
14,719
#16
'computer network'/exp
43,855
#15
'telehealth'/exp
3,549
#14
'wearable computer'/exp
11,808
#13
'smartphone'/de
11,219
#12
'mobile application'/exp
11,043
#11
#8 OR #9 OR #10
3,663
#10
'transmission dynamics':ab,ti
4,910
#9
(contact NEAR/3 (trac* OR examination OR screening OR management OR investigation)):ab,ti
3,831
#8
'contact examination'/de
964,149
#7
#1 OR #2 OR #3 OR #4 OR #5 OR #6
455,953
#6
'respiratory tract infection'/exp
31,434
#5
'communicable disease'/exp
107,280
#4
'epidemic'/de
639,567
#3
coronavirus*:ab,ti OR '2019 ncov':ab,ti OR ncov:ab,ti OR 'covid 19':ab,ti OR covid19:ab,ti OR 'sars cov 2':ab,ti OR sars:ab,ti OR sarscov2:ab,ti OR 'sarscov 2':ab,ti OR 'sars coronavirus 2':ab,ti OR 'sars corona virus':ab,ti OR 'sars‐like coronavirus':ab,ti OR 'novel coronavirus':ab,ti OR 'novel corona virus':ab,ti OR covid*:ab,ti OR pneumonia:ab,ti OR 'severe acute respiratory syndrome':ab,ti OR 'coronavirus 2':ab,ti OR 'coronavirus infection*':ab,ti OR 'coronavirus disease':ab,ti OR 'corona virus disease':ab,ti OR 'new coronavirus':ab,ti OR 'new corona virus':ab,ti OR 'new coronaviruses':ab,ti OR 'novel coronaviruses':ab,ti OR 'severe acute respiratory syndrome coronavirus‐2':ab,ti OR 'middle‐east respiratory syndrome':ab,ti OR mers:ab,ti OR tuberculosis:ab,ti OR influenza:ab,ti OR pandemic*:ab,ti OR epidemic*:ab,ti OR zika:ab,ti OR ebola:ab,ti
3,638
#2
'coronavirus infection'/de
20,607
#1
'coronavirinae'/exp
Database(s): CENTRAL
#1 MeSH descriptor: [Coronavirus] explode all trees 27
#2 MeSH descriptor: [Coronavirus Infections] explode all trees 240
#3 (coronavirus* or "2019‐ncov" or nCov or "covid 19" or covid19 or "sars cov 2" or sars or "sarscov‐2" or "sarscov‐2" or "sars coronavirus‐2" or "sars corona virus" or "sars‐like coronavirus" or "novel coronavirus" or "novel corona virus" or covid* or pneumonia or "severe acute respiratory syndrome" or "coronavirus 2" or (coronavirus NEXT infection*) or "coronavirus disease" or "corona virus disease" or "new coronavirus" or "new corona virus" or "new coronaviruses" or "novel coronaviruses" or "severe acute respiratory syndrome coronavirus‐2" or (respiratory NEXT infectious NEXT disease*) or (acute NEXT respiratory NEXT disease*) or "middle east respiratory syndrome" or mers or tuberculosis or influenza or pandemic* or epidemic* or zika or ebola):ti,ab,kw 31474
#4 MeSH descriptor: [Communicable Diseases] explode all trees 551
#5 MeSH descriptor: [Epidemics] explode all trees 89
#6 MeSH descriptor: [Respiratory Tract Infections] explode all trees 14596
#7 {OR #1‐#6} 38759
#8 MeSH descriptor: [Contact Tracing] explode all trees 73
#9 (contact NEAR/3 (trac* or examination or screening or management or investigation)):ti,ab,kw 328
#10 "transmission dynamics":ti,ab,kw 43
#11 {OR #8‐#10} 371
#12 MeSH descriptor: [Wearable Electronic Devices] explode all trees 405
#13 MeSH descriptor: [Computer Communication Networks] explode all trees 3920
#14 MeSH descriptor: [Telecommunications] explode all trees 6212
#15 MeSH descriptor: [Mobile Applications] explode all trees 580
#16 MeSH descriptor: [Smartphone] explode all trees 345
#17 ((smartphone NEXT application*) or (wearable NEXT electronic NEXT device*) or wearable* or smartphone* or (smart NEXT phone*) or (cell NEXT phone*) or (cellular NEXT phone*) or (mobile NEXT phone*) or (digital NEXT solution*) or (GPS NEXT device*) or (GPS NEXT track*) or ICTs or Bluetooth or "health apps" or (health NET application*) or eHealth or mHealth or (mobile NEXT app*) or "movement mapping" or (digital NEXT technolog*) or "digital health" or (smart NEXT watch*) or (application NEXT programming NEXT interface*) or telemedicine or "digital contact tracing" or "automated data"):ti,ab,kw 13084
#18 {OR #12‐#17} 19708
#19 #7 and #11 and #18 14
Appendix 2. Characteristics of studies with qualitative data
| Study ID | Country | Respondents | Characteristics of respondents | Intervention | Disease | Setting | Data collection method | Method of analysis |
| Danquah 2019 | Sierra Leone | Contact tracing workers in the healthcare system | n = 3 Gender: not stated Socioeconomic status: not stated |
Smart phone‐based data collection app | Ebola | Real‐world epidemic | Semi‐structured interviews with topic guide. Focus group discussion | Thematic analysis |
| Ha 2016 | Botswana | Contact tracing workers in the healthcare system | n = 2 Gender: male Socioeconomic status: not stated |
Smartphone‐based data collection app | Tuberculosis | Real‐world epidemic | Free‐text boxes within a survey | Not stated |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Danquah 2019.
| Study characteristics | ||
| Study design | Cohort study | |
| Intervention | Contact tracing app used by contact tracers for data management | |
| Disease/setting | Ebola in Sierra Leone | |
| Physical contact with Ebola case (alive or dead) in previous 3 weeks | ||
| Notes | ||
Farrahi 2014.
| Study characteristics | ||
| Study design | Modelling study Adapted SIR model |
|
| Intervention | Generic dual‐network topology | |
| Disease/setting | Non‐specific | |
| Not stated | ||
| Notes | ||
Ferretti 2020.
| Study characteristics | ||
| Study design | Modelling study General mathematical model |
|
| Intervention | Non‐specific contact tracing app | |
| Disease/setting | COVID‐19/non‐specific | |
| Standard COVID‐19 close contact definition | ||
| Notes | ||
Fournet 2016.
| Study characteristics | ||
| Study design | Modelling study Adapted SIR model |
|
| Intervention | RFID‐enabled sensors | |
| Disease/setting | School in France | |
| Not stated | ||
| Notes | ||
Ha 2016.
| Study characteristics | ||
| Study design | Cohort study | |
| Intervention | Contact tracing app used by contact tracers for data management | |
| Disease/setting | TB in Botswana | |
| Not stated | ||
| Notes | ||
Helmich 2017.
| Study characteristics | ||
| Study design | Cohort study | |
| Intervention | Wearable RFID‐enabled badge, wireless linkage to readers | |
| Disease/setting | Pertussis in USA | |
| Healthcare worker with face‐to‐face contact with case in exam room or triage area | ||
| Notes | ||
Hinch 2020.
| Study characteristics | ||
| Study design | Modelling study Individual‐based model |
|
| Intervention | Non‐specific contact tracing app with and without recursive | |
| Disease/setting | COVID‐19/non‐specific | |
| Standard COVID‐19 close contact definition | ||
| Notes | ||
Kucharski 2020.
| Study characteristics | ||
| Study design | Modelling study Individual‐based model |
|
| Intervention | Non‐specific contact tracing app | |
| Disease/setting | COVID‐19 in UK | |
| Standard COVID‐19 close contact definition | ||
| Notes | ||
Leecaster 2016.
| Study characteristics | ||
| Study design | Cohort study | |
| Intervention | Wireless ranging‐enabled node‐device | |
| Disease/setting | School in USA | |
| Device: face‐to‐face, within 6 feet, (183 cm) at least 20 seconds Diary: record initials of the person they talked to or touched |
||
| Notes | ||
Mastrandrea 2015.
| Study characteristics | ||
| Study design | Cohort study | |
| Intervention | RFID‐enabled sensors | |
| Disease/setting | School in France | |
| Device: face‐to‐face, within 6 feet (183 cm), at least 20 seconds Diary: record initials of the person they talked to or touched and duration of interaction |
||
| Notes | ||
Smieszek 2014.
| Study characteristics | ||
| Study design | Cohort study | |
| Intervention | RFID‐enabled sensors | |
| Disease/setting | School in USA | |
| Device: face‐to‐face < 2 metres Survey: 1‐day recall of contacts who were maximum of 2 arm‐lengths apart, at least 10 words spoken, and at school |
||
| Notes | ||
Yasaka 2020.
| Study characteristics | ||
| Study design | Modelling study Adapted SIR model |
|
| Intervention | Generic dual‐network topology | |
| Disease/setting | Non‐specific | |
| Not stated | ||
| Notes | ||
RFID: radio‐frequency identification‐enabled; SIR: susceptible‐infected‐recovered; TB: tuberculosis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 2020 | No comparisons |
| Eisenkraft 2018 | No comparisons |
| Menon‐Johansson 2018 | No comparisons |
| Sacks 2015 | Case study |
| Tom‐Aba 2018 | No comparisons |
| Tom‐Aba 2020 | No comparisons |
| Van Hest 2016 | Case identification rather than contact tracing |
| Voirin 2015 | No comparisons |
| White 2018 | No comparisons |
Characteristics of studies awaiting classification [ordered by study ID]
Kendall 2020.
| Notes | This is an ecologic investigation of the impact of a Test, Trace, Isolate programme in the UK. |
Characteristics of ongoing studies [ordered by study ID]
Ayakaka 2020.
| Study name | Mobile health for implementation of home‐based TB contact investigation in Uganda |
| Starting date | 27 August 2014 |
| Contact information | ayakaka@gmail.com Irene Ayaka‐‐Project Manager |
| Notes | This is an ongoing interventional study. |
Cencetti 2020.
| Study name | n/a This is a modelling study of an agnostic digital proximity tracing app |
| Starting date | First available on 2 July 2020. Not yet peer‐reviewed |
| Contact information | gcencetti@fbk.eu Giulia Cencetti is the lead author of this not‐yet published modelling study |
| Notes |
Lambert 2020.
| Study name | n/a This is a modelling study of generic contact tracing strategies in addressing COVID‐19 |
| Starting date | First available on 8 May 2020. Not yet peer‐reviewed |
| Contact information | amaury.lambert@college‐de‐france.fr Amaury Lambert is the lead author on this not‐yet published modelling study |
| Notes |
Differences between protocol and review
We amended our protocol (PROSPERO Protocol CRD42020188946; Anglemyer 2020), slightly to include studies with any qualitative data (i.e. waiving our previously stated exclusion of studies that provided qualitative data without qualitative analysis). Additionally, though not explicitly stated, we used Reff to address outcomes related to identifying secondary cases, as Reff refers directly to enumerating secondary cases from index cases.
The published protocol with PROSPERO does not specifically list the Embase databases as a possible source of identified studies. Further, how the data were extracted (i.e. the number of data extractors, whether they were dual, independent extractions or split) was not included in the published PROSPERO protocol.
Contributions of authors
Anglemyer A conceived of the review and oversaw all edits and analyses. Moore THM and Parker L performed data extraction for the cohort studies and performed the qualitative data analysis. Chambers T and Anglemyer A extracted data from modelling studies. Parry M interpreted the results from the modelling studies, analysed the modelling data, and performed any additional analyses with the modelling results. Grady A and Bero L performed the full‐text screening and wrote the discussion and edited the manuscript. Flemyng E contributed to the manuscript's editing and discussion. Chiu K and Wilczynska M initially screened the titles for eligibility and contributed to the manuscript's writing and editing.
Declarations of interest
Andrew Anglemyer is a consultant for New Zealand’s national digital contact tracing solutions team and receives remuneration from Cochrane as a member of the Methods Support Unit. Theresa Moore is partly funded by the National Institute for Health Research Collaboration West (NIHR ARC West) at University Hospitals Bristol and Weston NHS Foundation Trust, partly funded by the University of Bristol, UK, and partly funded by the Cochrane Collaboration as Methodological Editor. Lisa Parker: none Tim Chambers is a consultant for New Zealand’s national digital contact tracing solutions team. Alice Grady: none Kellia Chiu: none Matthew Parry: none Magdalena Wilczynska is funded by nib Health. Ella Flemyng is employed by and receives a salary from Cochrane though has no financial interest in the review’s findings. Lisa Bero receives remuneration from Cochrane as Senior Editor Public Health and Health Systems Network and Research Integrity.
Edited (no change to conclusions)
References
References to studies included in this review
Danquah 2019 {published data only}
- Danquah L, Hasham N, MacFarlane M, Conteh FE, Momoh F, Tedesco AA, et al. Use of a mobile application for Ebola contact tracing and monitoring in northern Sierra Leone: a proof-of-concept study. BMC Infectious Diseases 2019;19:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farrahi 2014 {published data only}
- Farrahi K, Emonet R, Cebrian M. Epidemic contact tracing via communication traces. PLoS One 2014;9(5):e95133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferretti 2020 {published data only}
- Ferretti L, Wymant C, Kendall M, Zhao L, Nurtay A, Abeler-Dörner L, et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science 2020;368(6491):eabb6936. [DOI: 10.1126/science.abb6936] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fournet 2016 {published data only}
- Fournet J, Barrat A. Epidemic risk from friendship network data: an equivalence with a non-uniform sampling of contact networks. Nature Scientific Reports 2016;6:24593. [DOI: 10.1038/srep24593] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ha 2016 {published data only}
- Ha Y, Littman-Quinn R, Antwi C, Seropola G, Green R, Tesfalul MA, et al. A mobile health approach to tuberculosis contact tracing in resource-limited settings.. Studies in Health Technology and Informatics 2013;192:1188. [PubMed] [Google Scholar]
- Ha YP, Tesfalul MA, Littman-Quinn R, Antwi C, Green RS, Mapila TO, et al. Evaluation of a mobile health approach to tuberculosis contact tracing in Botswana. Journal of Health Communication 2016;21(10):1115-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Helmich 2017 {published data only}
- Helmich T, Clements C, El-Sherif N, Pasupathy KS, Nestler DM, Boggust A, et al. Contact tracing with a real-time location system: a case study of increasing relative effectiveness in an emergency department. American Journal of Infection Control 2017;45:1308-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hinch 2020 {published data only}
- Hinch R, Probert W, Nurtay A, Kendall M, Wymant C, Hall M, et al. Effective configurations of a digital contact tracing app: a report to NHSX. Available at cdn.theconversation.com/static_files/files/1009/Report_-_Effective_App_Configurations.pdf?1587531217 (accessed prior to 14 July 2020).
Kucharski 2020 {published data only}
- Kucharski A, Klepac P, Conlan A, Kissler SM, Tang M, Fry H, et al. Effectiveness of isolation, testing, contact tracing and physical distancing on reducing transmission of SARS-CoV-2 in different settings. Lancet Infectious Diseases 2020. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leecaster 2016 {published data only}
- Leecaster M, Toth D, Pettey W, Rainey JJ, Gao H, Uzicanin A, et al. Estimates of social contact in a middle school based on self-report and wireless sensor data. PLoS One 2016. [DOI: 10.1371/journal.pone.0153690] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mastrandrea 2015 {published data only}
- Mastrandrea R, Fournet J, Barrat A. Contact patterns in a high school: a comparison between data collected using wearable sensors, contact diaries and friendship surveys. PLoS One 2015. [DOI: 10.1371/journal.pone.0136497] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smieszek 2014 {published data only}
- Smieszek T, Barclay V, Seeni I, Rainey JJ, Gao H, Uzicanin A, et al. How should social mixing be measured: comparing web-based survey and sensor-based methods. BMC Infectious Diseases 2014;14:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yasaka 2020 {published data only}
- Yasaka T, Lehrich B, Sahyouni R. Peer-to-peer contact tracing: development of a privacy-preserving smartphone app. JMIR mHealth and uHealth 2020;4:e18936. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Chen 2020 {published data only}
- Chen C, Jyan H, Chien S, Jen H, Hsu C, Lee P-C, et al. Containing COVID-19 among 627,386 persons in contact with the Diamond Princess cruise ship passengers who disembarked in Taiwan: big data analytics. Journal of Medical Internet Research 2020;22(5):e19540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisenkraft 2018 {published data only}
- Eisenkraft A, Afriat A, Hubary Y, Lev R, Shaul H, Balicer RD. Using cell phone technology to investigate a deliberate Bacillus anthracis release scenario. Health Security 2018;16(1):22-9. [DOI] [PubMed] [Google Scholar]
Menon‐Johansson 2018 {published data only}
- Menon-Johansson A. Innovative digital contact tracing tool data indicates that only one in three contactable contacts were seen and tested in England in 2016. Sexually Transmitted Diseases 2018;45 Suppl 2:POS 46-T. [Google Scholar]
Sacks 2015 {published data only}
- Sacks J, Zehe E, Redick C, Bah A, Cowger K, Camara M, et al. Introduction of mobile health tools to support Ebola surveillance and contact tracing in Guinea. Global Health: Science and Practice 2015;3(4):646-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tom‐Aba 2018 {published data only}
- Tom-Aba D, Toikkanen S, Gloeckner S, Adeoye O, Mall S, Fänrich C, et al. User evaluation indicates high quality of the surveillance outbreak response management and analysis system (SORMAS) after field deployment in Nigeria in 2015 and 2018. Studies in Health Technology and Informatics 2018;253:233-7. [PubMed] [Google Scholar]
Tom‐Aba 2020 {published data only}
- Tom-Aba D, Silenou BC, Doerrbecker J, Fourie C, Leitner C, Wahnschaffe M, et al. The surveillance outbreak response management and analysis system (SORMAS): digital health global goods maturity assessment. JMIR Public Health and Surveillance 2020;6(2):e15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Hest 2016 {published data only}
- Van Hest R, De Vries G. Active tuberculosis case-finding among drug-users and homeless persons: after the outbreak. European Respiratory Journal 2016;48:269-71. [DOI] [PubMed] [Google Scholar]
Voirin 2015 {published data only}
- Voirin N, Payet C, Barrat A, Cattuto C, Khanafer N, Régis C, et al. Combining high-resolution contact data with virological data to investigate influenza transmission in a tertiary care hospital. Infection Control and Hospital Epidemiology 2015;36(3):254-60. [DOI] [PubMed] [Google Scholar]
White 2018 {published data only}
- White E, Meyer A, Ggita J, Babirye D, Mark D, Ayakaka I, et al. Feasibility, acceptability, and adoption of digital fingerprinting during contact investigation for tuberculosis in Kampala, Uganda: a parallel-convergent mixed-methods analysis. Journal of Medical Internet Research 2018;20(11):e11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kendall 2020 {published data only}
- Kendall M, Milsom L, Abeler-Dorner L, Wymant C, Ferretti L, Briers M, et al. COVID-19 incidence and R decreased on the Isle of Wight after the launch of the Test, Trace, Isolate programme. medRxiv [Preprint] 2020. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Ayakaka 2020 {published data only}
- Ayakaka I, Katamba A. Mobile health for implementation of home-based TB contact investigation in Uganda. apps.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201509000877140 2020.
Cencetti 2020 {published data only}
- Cencetti G, Santin G, Longa A, Pigani E, Barrat A, Cattuto C, et al. Digital proximity tracing in the COVID-19 pandemic on empirical contact networks. medRxiv [Preprint] 2020. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lambert 2020 {published data only}
- Lambert A. A mathematical assessment of the efficiency of quarantining and contact tracing in curbing the COVID-19 epidemic. medRxiv [Preprint] 2020. [DOI: ] [Google Scholar]
Additional references
Access Now 2020
- Access Now. Privacy and public health: the dos and don’ts for COVID-19 contact tracing apps. Available at www.accessnow.org/privacy-and-public-health-the-dos-and-donts-for-covid-19-contact-tracing-apps/ (accessed prior to 15 July 2020).
Altmann 2020
- Altmann S, Milsom L, Zillessen H, Blasone R, Gerdon F, Bach R, et al. Acceptability of app-based contact tracing for COVID-19: cross-country survey evidence. Lancet [Preprint] 2020. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baharudin 2020
- Baharudin H. Wearable device for COVID-19 contact tracing to be rolled out soon, may be issued to everyone in Singapore. Straits Times 2020.
Booth 2016
- Booth A. Searching for qualitative research for inclusion in systematic reviews: a structured methodological review. Systematic Reviews 2016;5:74. [DOI: 10.1186/s13643-016-0249-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Braithwaite 2020
- Braithwaite I, Calendar T, Bullock M, Aldridge R. Automated and partially-automated contact tracing: a rapid systematic review to inform the control of COVID-19. medRxiv [Preprint] 2020. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Braun 2020
- Braun V, Clarke V. Guidelines for reviewers and editors evaluating thematic analysis manuscripts. Available at cdn.auckland.ac.nz/assets/psych/about/our-research/documents/Checklist%20for%20reviewers%20and%20editors%20evaluating%20thematic%20analysis%20manuscripts.pdf (accessed prior to 14 July 2020).
Brozek 2020
- Brozek J, Canelo-Aybar C, Akl E, et al. GRADE Guidelines 30: The GRADE approach to assessing the certainty of modelled evidence - an overview in the context of health decision-making. Unpublished article.
Campbell 2020
- Campbell M, McKenzie J, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368:I6890. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CASP 2020
- Critical Appraisal Skills Programme UK . CASP checklists. Available at: casp-uk.net/casp-tools-checklists/ (accessed prior to 14 July 2020).
CCC 2020
- Chaos Computer Club (CCC). 10 requirements for the evaluation of "Contact Tracing" apps. Available at www.ccc.de/en/updates/2020/contact-tracing-requirements (accessed prior to 15 July 2020).
Cho 2020
- Cho H, Ippolito D, Yu Y. Contact tracing mobile apps for COVID-19: privacy considerations and related trade-offs. arXiv [Preprint] 2020. [arXiv:2003.11511] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed prior to 14 July 2020. Melbourne, Australia: Veritas Health Innovation. Availabe at covidence.org.
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Faye 2015
- Faye O, Boelle P-Y, Heleze E, Faye O, Loucoubar C, Magassouba N, et al. Chains of transmission and control of Ebola virus disease in Conakry, Guinea, in 2014: an observational study. Lancet Infectious Diseases 2015;15(3):320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
FPF 2020
- Future of Privacy Forum (FPF). Privacy & pandemics: the role of mobile apps. Available at fpf.org/wp-content/uploads/2020/04/editPrivacy-Pandemics_-The-Role-of-Mobile-Apps-Chart-11.pdf (accessed prior to 15 July 2020).
Grundy 2019a
- Grundy Q, Chiu K, Bero L. Commercialization of user data by developers of medicines-related apps: a content analysis. Journal of General Internal Medicine 2019;34:2833-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grundy 2019b
- Grundy Q, Chiu K, Held F, Continella A, Bero L, Holz R, et al. Data sharing practices of medicines related apps and the mobile ecosystem: traffic, content, and network analysis. BMJ 2019;364:I920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hart 2020
- Hart V, Siddarth D, Cantrell B, Tretikov L, Eckersley P, Langford J, et al. Outpacing the virus: digital response to containing the spread of COVID-19 while mitigating privacy risks. Center for Ethics, Harvard University. Available at ethics.harvard.edu/outpacing-virus 2020.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaptchuk 2020
- Kaptchuk G, Goldstein D, Hargittai E, Hoffman J, Redmiles EM. How good is good enough for COVID19 apps? The influence of benefits, accuracy, and privacy on willingness to adopt. aRxiv [Preprint] 2020. [arXiv:2005.04343 [cs.CY]] [Google Scholar]
Keeling 2020
- Keeling M, Hollingsworth T, Read J. The efficacy of contact tracing for the containment of the 2019 novel Coronavirus (COVID-19). Journal of Epidemioloy and Community Health 2020:jech-2020-214051. [DOI: 10.1136/jech-2020-214051] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2020
- Kim H, Paul A. Contact tracing: a game of big numbers in the time of COVID-19. aRxiv [Preprint] 2020. [arXiv:2004.10762 [q-bio.PE]] [DOI] [PMC free article] [PubMed] [Google Scholar]
Korea CDC 2020
- COVID-19 National Emergency Response Center, Epidemiology & Case Management Team, Korea Centers for Disease Control & Prevention. Contact transmission of COVID-19 in South Korea: novel investigation techniques for tracing contacts. Osong Public Health and Research Perspectives 2020;11(1):60-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kretzschmar 2020
- Kretzschmar, M, Rozhnova G, Van Boven M. Effectiveness of isolation and contact tracing for containment and slowing down a COVID-19 epidemic: a modelling study. Available at papers.ssrn.com/sol3/papers.cfm?abstract_id=3551343 [Preprint] 2020. [DOI: ] [Google Scholar]
Lewin 2015
- Lewin S, Glenton C, Munthe-Kaas H, Carlsen B, Colvin CJ, Gülmezoglu M, et al. Using qualitative evidence in decision making for health and social interventions: an approach to assess confidence in findings from qualitative evidence syntheses (GRADE-CERQual). PLoS Medicine 2015;12:e1001895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewin 2018
- Lewin S, Booth A, Glenton C, Munthe-Kaas H, Rashidian A, Wainwright M, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings: introduction to the series. Implementation Science 2018;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKenzie 2019
- McKenzie JE, Brennan SE. Chapter 12: Synthesizing and presenting findings using other methods. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Microsoft 2020a [Computer program]
- Microsoft Excel. Microsoft Corporation. Microsoft Corporation, 2020. office.microsoft.com/excel.
Microsoft 2020b [Computer program]
- Microsoft Word. Microsoft Corporation. Microsoft Corporation, 2020. products.office.com/word.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nature 2020
- Nature. Show evidence that apps for COVID-19 contact-tracing are secure and effective. Available at www.nature.com/articles/d41586-020-01264-1 2020;580:563. [DOI: 10.1038/d41586-020-01264-1] [DOI] [PubMed] [Google Scholar]
Noyes 2019a
- Noyes J, Booth A, Moore G, Flemming K, Tunçalp Ö, Shakibazadeh E. Synthesising quantitative and qualitative evidence to inform guidelines on complex interventions: clarifying the purposes, designs and outlining some methods. BMJ Global Health 2019;4 (Suppl 1):e000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noyes 2019b
- Noyes J, Booth A, Cargo M, Flemming K, Harden A, Harris J, et al. Chapter 21: Qualitative evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Nussbaumer‐Striet 2020
- Nussbaumer-Streit B, Mayr V, Dobrescu A, Chapman A, Persad E, Klerings I, et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013574. [DOI: 10.1002/14651858.CD013574] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Neill 2020
- O’Neill P, Ryan-Mosley T, Johnson B. A flood of coronavirus apps are tracking us. Now it’s time to keep track of them. MIT Technology Review. MIT Technology Review. Available at www.technologyreview.com/2020/05/07/1000961/launching-mittr-covid-tracing-tracker/ 2020. [Google Scholar]
Pitman 2012
- Pitman R, Fisman D, Zaric G, Postma M, Kretzschmar M, Edmunds J, et al. Dynamic transmission modelling: a report of the ISPOR-SMDM modelling Good Practices Task Force-5. Value in Health 2012;16:828-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Redmiles 2020
- Redmiles E. User concerns & tradeoffs in technology-facilitated contact tracing. arXiv [Preprint] 2020. [arxiv.org/pdf/2004.13219.pdf] [Google Scholar]
Reuters 2020
- Reuters. Chinese city tightens coronavirus travel curbs in biggest outbreak. Available at www.reuters.com/article/us-health-coronavirus-china-harbin/chinese-city-tightens-coronavirus-travel-curbs-in-biggest-outbreak-idUSKCN22409D (accessed prior to 14 July 2020).
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016;355:i4919. [DOI: 10.1136/bmj.i4919] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2020
- Taylor J. Victoria contact tracers access Covidsafe data 99 times but no identified close contacts reported. www.theguardian.com/world/2020/jul/04/victoria-contact-tracers-access-covidsafe-data-99-times-but-no-identified-close-contacts-reported 2020.
UAB 2020
- University of Alabama, Birmingham. Smartphone-based automated contact tracing: is it possible to balance privacy, accuracy and security? Newswise 2020. Available at www.newswise.com/coronavirus/smartphone-based-automated-contact-tracing-is-it-possible-to-balance-privacy-accuracy-and-security/?article_id=731270 2020.
Van Dorn 2020
- Van Dorn A, Cooney R, Sabin M. COVID-19 exacerbating inequalities in the US. Lancet 2020;395(10232):1243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vandamme 2020
- Vandamme A, Nguyen T. Belgium-concerns about coronavirus contact-tracing apps. Available at www.nature.com/articles/d41586-020-01552-w?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+nature%2Frss%2Fcurrent+%28Nature+-+Issue%29 2020;581:384. [DOI: 10.1038/d41586-020-01552-w] [DOI] [PubMed] [Google Scholar]
Vaughan 2020
- Vaughan A. There are many reasons why COVID-19 contact-tracing apps may not workA. Available at www.newscientist.com/article/2241041-there-are-many-reasons-why-covid-19-contact-tracing-apps-may-not-work/ 2020. [Google Scholar]
Verrall 2020
- Verrall A. Rapid audit of contact tracing for COVID-19 in New Zealand. Available at www.health.govt.nz/system/files/documents/publications/contact_tracing_report_verrall.pdf (accessed prior to 14 July 2020).
WHO 2013
- World Health Organization. Closing the health equity gap: policy options and opportunities for action. World Health Organization 2013. Available at apps.who.int/iris/bitstream/handle/10665/78335/9789241505178_eng.pdf;jsessionid=11DEC66F0522C49572821873BD7ED228?sequence=1 (accessed prior to 15 July 2020).
WHO 2020a
- World Health Organization. Coronavirus disease 2019 (COVID-19) situation report 190. Available from www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed prior to 29 July 2020).
WHO 2020b
- World Health Organization. Digital tools for COVID-19 contact tracing. Annex: Contact tracing in the context of COVID-19. Available at www.who.int/publications/i/item/WHO-2019-nCoV-Contact_Tracing-Tools_Annex-2020.1 (accessed prior to 14 July 2020).
WHO 2020c
- World Health Organization. Ethical considerations to guide the use of digital proximity tracking technologies for COVID-19 contact tracing: interim guidance. Available at www.who.int/publications/i/item/WHO-2019-nCoV-Ethics_Contact_tracing_apps-2020.1 (accessed prior to 14 July 2020).
References to other published versions of this review
Anglemyer 2020
- Anglemyer A, Moore TH, Parker L, Chambers T, Grady A, Chiu K, et al. Contact tracing technologies in epidemics: a rapid review. PROSPERO. Available from www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020188946 2020;(CRD42020188946). [CRD42020188946] [DOI] [PMC free article] [PubMed]


