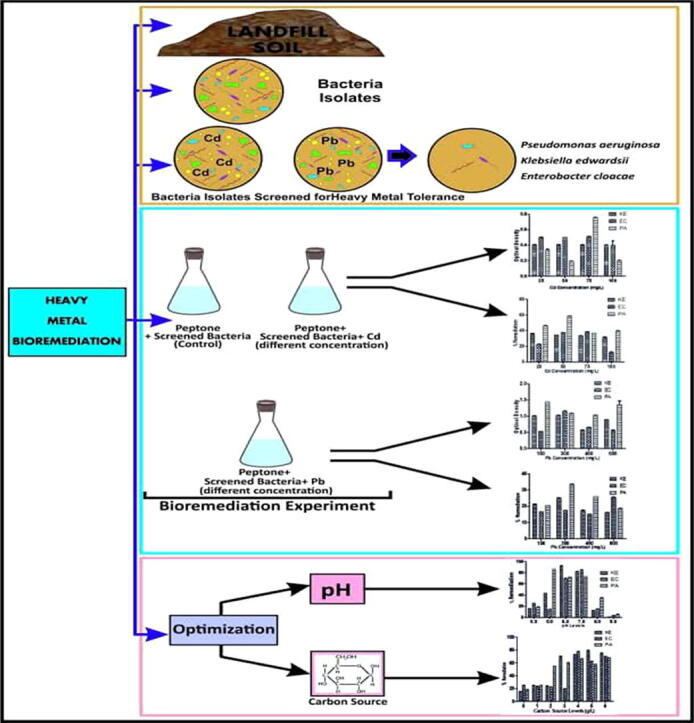
Graphical abstract
Keywords: Landfill soil, Heavy metal contamination, Cadmium, Lead, Indigenous bacteria, Soil reclamation
Abstract
Indiscriminate disposal of wastes on landfills has led to increase in heavy metal contamination in landfill soils. However, the ability of the indigenous microorganisms to remediate the polluted environment can be of great influence in reclamation of such soils. The objectives of this study were to assess the bioremediation potential of the screened indigenous bacteria and evaluate the effects of carbon source and pH in the enhancement of the bioremediation process. Bacterial isolates from landfill sites were screened for their capability to utilize heavy metal (Cd and Pb). Nutrient Agar was supplemented with five different concentrations of each metal (25 to 600 mgL-1). Viable counts of the isolates were taken four times at two days interval. Pseudomonas aeruginosa, Klebsiella edwardsii and Enterobacter cloacae were selected based on their tolerance to heavy metal for remediation process. Peptone broth was also supplemented using different concentrations of heavy metals. The remediation process was assessed by monitoring the growth of biomass using UV spectrophotometer at 600 nm and the residual heavy metal was evaluated after 8 days of incubation using AAS. Pseudomonas aeruginosa exhibited the highest bioremediation potential among the bacterial isolates with 58.80 and 33.67 remediation percentage in 50 mg Cd L-1 and 300 mg Pb L-1 . However, higher remediation percentage (79.87 and 92.41) was observed by Klebsiella edwardsii through addition of carbon source (5 g/L) and varying the pH (6) of the media in the heavy metal contaminated medium. The results of this study indicate that the effectiveness of the indigenous bacteria in remediation process can be enhanced through the addition of carbon source and increase pH for effective reclamation of contaminated soil.
1. Introduction
Generally considered to be the most available economic means of waste disposal, landfills are estimated to constitute about 95% of solid waste generated worldwide (Scott et al., 2005). The World Bank reports of Hoornweg and Bhada-Tata, (2012) posted a cumulative 1,300 million tonnes per year (1.2 kg/capita/day) of wastes generated from urban centres of the world, which is expected to rise to 2,200 million tonnes per year by 2025. Within the sub-Saharan Africa, Nigeria’s waste generation is approximately 62 million tonnes a year with each person generating an average of 0.65 kg/day, and is projected to rise to 161.27 million tonnes by 2025, at about 0.85 kg of waste/capita/day.
In developing countries, landfilling is virtually the only approach of waste management (Rubinos and Spagnoli 2018). Unfortunately, in major cities of Nigeria, these largely unsegregated and heterogenous in composition wastes are dumped in open grounds which are not engineered to prevent serious contamination of the underlying soil, ground and surface waters by toxic materials leached from the wastes (Misra et al., 2018). The submission of Karak et al. (2012) is that whatsoever be the source of these wastes, its impact on environment and quality of life is mainly related to air, water, and soil contaminations, in addition to space consumption, odours and aesthetic prejudice.
Furthermore, with the 2012 World Bank report also posting a 5% presence of metals in the wastes generated in Nigeria, several authors have documented the presence of heavy metals like, arsenic, cadmium, chromium, nickel, lead copper, mercury and zinc (Kamunda et al., 2016, Samlafo, 2017, Gbadamosi et al., 2018). These constitute an ill-defined group of inorganic hazards (Wuana and Okieimen, 2011) that render landfills unsafe to the local environment. By this, they present high ecological risk linked with the ability to harm the living organisms through their accumulative and detrimental effects on the ecology and food chains. These lead to carcinogenic effects, acute toxicity, and genotoxicity among human beings (Mukherjee et al., 2015, Xia et al., 2019).
Literature suggests that the specific type of metal contaminants in a contaminated soil is a direct indication of the operation and several anthropogenic activities of man occurring at the site, resulting in heavy metal accumulations through emissions from the rapidly expanding industrial areas, disposal of high metal wastes, land application of fertilizers, animal manures, sewage sludge, accidental spillage of petrochemicals and atmospheric deposition (Khan et al., 2008, Zhang et al., 2010). The range of contaminant concentrations and the physical and chemical forms of contaminants will also depend on activities and disposal patterns contributing to their persistence, non-degradability and microbial cell toxicity, thus, robbing the soil of its conditioning properties (Ling et al., 2007, Raulinaitis et al., 2012, Okoro et al., 2020).
Hence, the specific accumulation of cadmium and lead will inhibit soil enzymatic activities which will inadvertently have adverse effect on the soil, plants, and then humans/animals (Liu et al., 2017, Alexander et al., 2006) via the food chain or oral bioavailability of contaminated soils. This increased associated risk via phytotoxicity make the land resource unavailable for agricultural production causing food insecurity and land tenure problems (McLaughlin et al., 2000). However, the cleaning up of the polluted soils is necessary to reclaim the area and minimize the entry of toxic elements into the food chain. In an attempt to remediate the heavy metal content in the soil, different engineering-based methods namely; soil excavation, soil washing pumps and treatment systems are already being used. Nevertheless, these non-biological techniques are only partially accepted as they damage the biotic structure of the soil; and are more expensive, requiring technical manpower and expertise to be successfully executed.
Bioremediation has emerged as a new low-technological cheap technology that uses plants and their associated microbial flora for environmental cleanup (Raskin et al., 1994, Salt et al., 1995, Oladipo et al., 2014). It is a biological treatment that offers outstanding environmentally friendly method for remediating heavy metal polluted soil; as it utilizes the ability of the indigenous microorganisms in the soil environment to break down the heavy metals into their harmless components.
Several studies had been carried out on landfill sites reporting various microorganisms efficient for bioremediation (Morris et al., 2018, Koshlaf et al., 2019, Hassan et al., 2020) but there is less information on enhancement of the bioremediation potential of the indigenous bacterial isolates from the landfill soil. Therefore, the aim of this study was to determine the effect of carbon source and pH on the heavy metal (Cd and Pb) remediation potentials of the screened indigenous bacterial isolates obtained from selected landfills in Lagos State, Nigeria, for maximum remediation effects.
2. Materials and methods
2.1. Geographical location of the study area
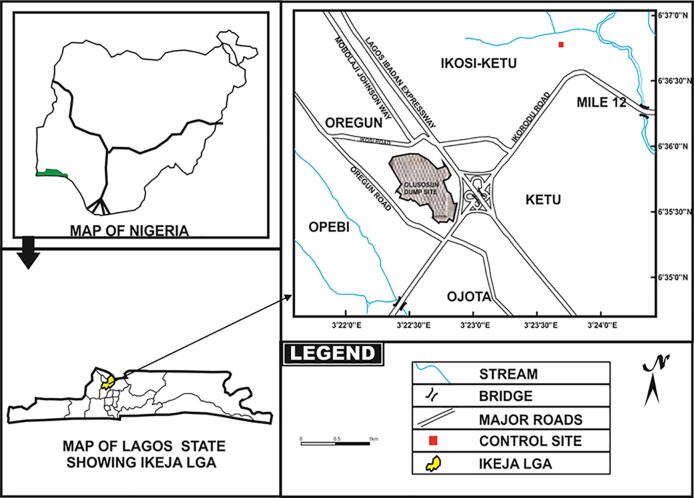
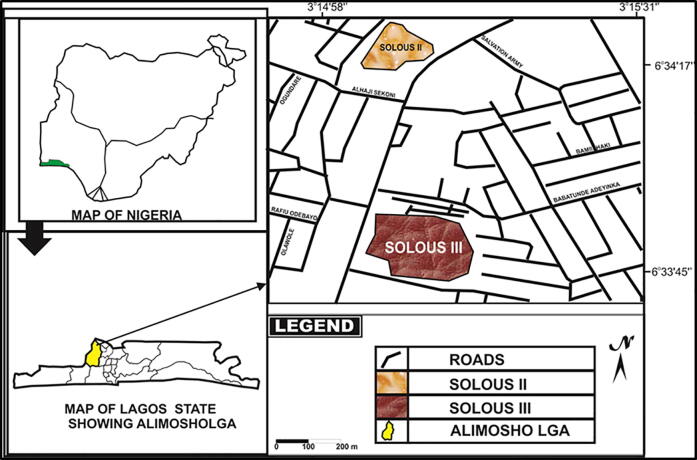
The study covered three landfills in Lagos State, Nigeria (Fig. 1). They were Olusosun (Latitude 6° 35′50′’N- 6° 36′30′’N; Longitude 3° 22′45′’E − 3° 23′30′’E), Solous II (Latitude 6° 34′15′’N- 6° 34′24′’N; Longitude 3o15′02′’E − 3o15′12′’E) and Solous III (Latitude 6° 33′42′’N- 6° 33′58′’N; Longitude 3o15′02′’E − 3o15′15′’E) (Fig. 2).
Fig. 1.
Map of Olusosun landfill site.
Fig. 2.
Map of Solous II and III landfill sites.
2.2. Geology of the study
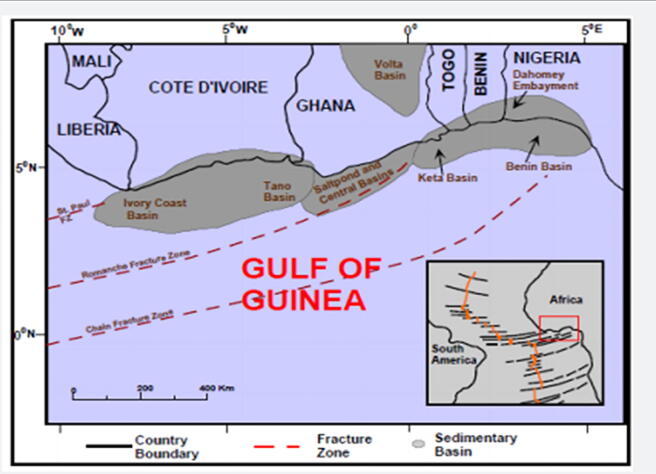
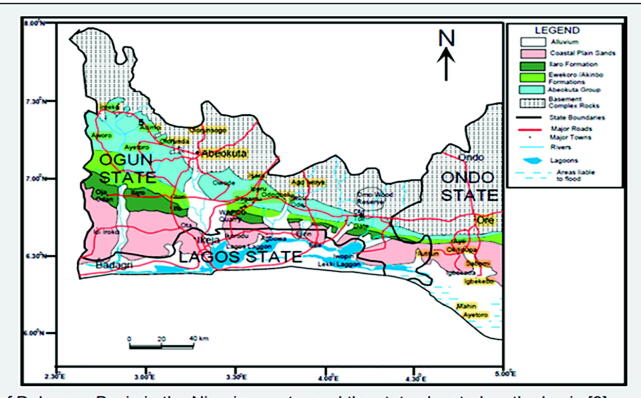
Lagos is made up of the sedimentary terrain which is an extension of the Dahomey Basin. The Dahomey Basin is a Basin that stretches from southeastern Ghana through Togo, Benin Republic to southwestern Nigeria (Fig. 3). Lagos State is one of the three states located in the Dahomey basin part of Nigeria (Fig. 4), the other States are Ogun and Ondo. Olusosun landfill receives approximately 40% of the total waste from Lagos. The size is 47.2 ha and it has a residual life span of 20 years (Olorunfemi, 2011). Solous II is on 7.8 ha of land with average life span of 5 years, while Solous III is on approximately 5 ha of land with average life span of 5 years. Each of the Solous sites receives an average of about 2,250 m3 of waste per day.
Fig. 3.
Regional map of the Gulf of Guinea showing the location of Benin (Dahomey) Basin in relation to other basins (Brownfield and Charpentier, 2006).
Fig. 4.
Geological map of Dahomey Basin in the Nigerian sector and the states located on the basin (Olabode, and Mohammed, 2016).
2.3. Soil samples
Soil samples were collected at 0–15 cm depth randomly from the landfill site divided into different plots to obtain representative samples. The control soil sample was obtained from the soil of undisturbed site within the radius of about 3 km from the landfill site. The soil samples were air-dried, mixed thoroughly and passed through a 2 mm sieve to remove debris. The bacterial isolates were isolated and biochemically characterized. API kit 20E was used for the identification and the organisms were screened for heavy metal (Cd and Pb) tolerance (Oladipo et al., 2018).
2.4. Bioremediation experiment
Subsequently, P. aeruginosa, K. edwardsii and E. cloacae isolated from the landfill soil were reactivated. Peptone supplemented with different concentrations of Cd (0, 25, 50, 75, and 100 mgL-1) and Pb (0, 150, 300, 450 and 600 mgL-1) were used for this experiment. The broth without the heavy metal served as the control. The broth was autoclaved at 121 °C for 15 min and allowed to cool. Each of the test organisms (Pseudomonas aeruginosa, Klebsiella edwardsii and Enterobacter cloacae) was inoculated into the heavy metal supplemented and control broth. It was then incubated at 35 °C. The growth and the remediation potential were monitored at two days interval for 8 days. The remediation potential of the selected organisms was evaluated by monitoring the growth of biomass using ultra violet (UV) spectrophotometer at 600 nm. The residual heavy metal in the broth was determined using atomic absorption spectrophotometer (AAS). Remediation percentage was calculated using the formula according to Nandish, 2005, Kaczorek, 2012;
2.5. Maximum remediation enhancement
Different levels of carbon source and pH were used to assess the maximum remediation of the heavy metals by the selected organisms. The broth medium with low remediation efficiency was supplemented with different levels of carbon source of 0, 1, 2, 3, 4, 5 and 6 (g/l) while the pH was varied (unadjusted pH, 5, 6, 7, 8 and 9). All the supplemented broth was inoculated with the efficient heavy metal degrader and incubated for 8 days (Loh and Wang, 1998). The growth and remediation potential was monitored at two days interval for 8 days. The optimum carbon source and pH value were determined based on their remediation percentage.
2.6. Statistical Analysis
Analysis of variance (ANOVA) and separation of means were done using Duncan’s New Multiple Range Test (p < 0.05).
3. Results and discussion
3.1. Bioremediation process
The bioremediation capabilities of three indigenous gram-negative bacteria isolates (Pseudomonas aeruginosa, Klebsiella edwardsii and Enterobacter cloacae) were evaluated using Cd and Pb supplemented medium (Tables 1). Bennisse et al., 2004, Mounaouer et al., 2014, Giovanella et al., 2017 also, indicated in their studies that Gram-negative bacteria are more tolerant to heavy metals than Gram-positive. However, the study conducted by Ndeddy Aka and Babalola (2017), discovered that most of the heavy metal tolerant organisms were gram positive, especially Bacillus. In addition, the isolated indigenous organisms showed different response to heavy metal type and concentrations in this study as confirmed by Hassen et al., 1998, Ndeddy Aka and Babalola, 2017, Abioye et al., 2018. Mostly, organisms that are able to survive and remain active in extreme environments can be identified and potentially targeted for bioremediation purposes (Akob et al., 2007, Jain et al., 2011).
Table 1.
Effect of selected bacterial isolates on heavy metal remediation.
| Cd | Pb | |||
|---|---|---|---|---|
| Isolate Code | Optical density | Remediation % | Optical density | Remediation % |
| KE | 0.562c | NA | 0.562c | NA |
| EC | 0.594b | NA | 0.594b | NA |
| PA | 0.744a | NA | 0.744a | NA |
| KEA1 | 0.409b | 36.40b | 1.013b | 21.36a |
| ECA1 | 0.504a | 22.90c | 0.544c | 16.75c |
| PAA1 | 0.350c | 46.49a | 1.440a | 20.54b |
| KEA2 | 0.409b | 34.89c | 1.032c | 25.17b |
| ECA2 | 0.506a | 37.83b | 1.161a | 17.58c |
| PAA2 | 0.195c | 58.80a | 1.093b | 33.67a |
| KEA3 | 0.405c | 33.67c | 0.578c | 17.50b |
| ECA3 | 0.515b | 38.70a | 0.663b | 15.14c |
| PAA3 | 0.759a | 37.26b | 1.036a | 25.99a |
| KEA4 | 0.403a | 31.57b | 0.891b | 16.13c |
| ECA4 | 0.400a | 12.52c | 0.573c | 25.63a |
| PAA4 | 0.206b | 39.90a | 1.147a | 18.73b |
Values in the same group followed by the same letter did not differ significantly at p < 0.05 according to Duncan’s New Multiple Range Test
KE- Klebsiella edwardsii, EC- Enterobacter cloacae
PA- Pseudomonas aeruginosa
A1- 25 mgL-1 (Cd), 150 mgL-1 (Pb)
A2- 50 mgL-1 (Cd), 300 mgL-1 (Pb)
A3- 75 mgL-1(Cd), 450 mgL-1 (Pb)
A4- 100 mgL-1(Cd), 600 mgL-1 (Pb)
NA- Not Applicable
Optical Density (OD) was used to monitor the microbial growth using pH and carbon source to ascertain the bioremediation potential of the test organisms (Nwinyi et al., 2014). The OD of the control medium throughout the days of incubation was significantly higher (p < 0.05) than Cd supplemented medium at all levels of concentration except at 75 mg Cd L-1 in P. aeruginosa inoculated medium (Table 1). Pseudomonas aeruginosa has been reported severally on its ability to adapt to polluted environment as well as its tolerance to heavy metal (Singh et al., 2013, Das et al., 2016, Rizvi and Khan, 2017, Tang et al., 2018, Pourfadakari et al., 2019, Jia et al., 2020, Varjani et al., 2020, Chen et al., 2021) due to its biosorption capability (Al-Dhabi et al., 2019). In Cd supplemented medium, P. aeruginosa has the highest remediation percentage of 58.80% at 50 mg Cd L-1 and highest optical density (0.759) at 75 mg Cd L-1. The variation of the influence of P. aeruginosa at 50 mg Cd L-1 and 75 mg Cd L-1 in this study might be due to the of rate enzymatic activity at each of the Cd concentrations. Some studies have shown that P. aeruginosa exhibits some enzymatic pathways through which pollutant are attacked and converted to harmless products. (Choudhary and Sar, 2011, Nwinyi et al., 2014, Yin et al., 2016, Giovanella et al., 2017, Ojewunmi, 2018, Zhang et al., 2020). Basically, the rate of bioremediation process depends on the concentration of the contaminant and the expression of some specific enzymes by the organisms.
The effect of bacterial isolates on the remediation of Pb as shown in Table 1. E. cloacae had the lowest remediation percentage at all levels of Pb supplementation except at 600 mg Pb L-1 which exhibited high significance (p < 0.05) in remediation percentage of 25.63. This shows that the tolerance and utilization of the heavy metal was not only dependent on the heavy metal concentration but the days of incubation of the organisms. Similar findings on the ability of Bacillus megaterium and Rhizopus stolonifer to enhance the removal of Pb, Cd and Ni from contaminated broths with increase in incubation time were reported by Njoku et al., 2020. Nikhil et al. (2013) also confirmed that incubation period of the test organisms increases the rate of degradation of diesel engine oil in soil indicating that, the organisms could still be at the exponential phase of their growth. The genera Klebsiella and Pseudomonas were most tolerant to Pb and were able to grow in all Pb concentrations investigated. Although, Pseudomonas aeruginosa had the highest remediation percentage of 33.67. The variability of the test organisms towards heavy metal tolerance in this study confirmed that the test organisms exhibited several mechanisms to reduce the elevated concentrations of heavy metals which could be specific for one or a few metals (Nies, 2003, Piddock, 2006). In addition, the concentration of heavy metal with the highest biomass of the test organisms as shown by the OD did not correspond to the concentration at which the maximum remediation occurs except at 450 mg Pb L-1. This could be as a result of different mechanisms used by the organisms to remove heavy metal as well as the ability of the heavy metal to induce oxidative damage which denatures and reduce remediation potential of the organisms. (Liu et al., 2017, Priyadarshanee and Das, 2020, Tarekegn et al., 2020, Shuaib et al., 2021). However, this study shows that most of the heavy metal with the highest concentration (100 mg Cd L -1 and 600 mg Pb L-1) had low remediation percentage. To enhance the remediation potential of the test organisms, further study with these organisms was carried out.
3.2. Bioremediation enhancement
The use of the indigenous microorganisms alone in bioremediation could limit their potential as a result of competition and elevated heavy metal concentrations. Bioremediation process could be improved using different approaches, depending on the state of the contaminated environment. One of these approaches is biostimulation, which involves promoting the growth of microorganisms at the contaminated site, in order to introduce pH-correction substances, nutrients, surfactants and oxygen (Ranđelović et al., 2015, Raimondo et al., 2020). Thus, nutrient addition and modification of environmental parameters allow microbial growth and accelerate bioremediation processes (Mehrzad et al., 2015, Nwinyi and Akinmulewo, 2019). The result of this study revealed varying effect of the carbon source concentration and pH on the microbial biomass and remediation potential. Considering, the importance of nutrients in growth and cultivation of microorganisms, the quantity of the nutrient is also imperative. In this study, the Optical Density and remediation percentage were higher for all the test organisms in the media supplemented with 1 and 2 g L-1 carbon source than the control medium of 100 mg Cd L-1 (Table 2). Klebsiella edwarsii Optical Density was significantly (p < 0.05) higher than other selected bacteria in 3 and 6 g L-1 of carbon source supplemented media all through the days of incubation. The minimum Optical Density (0.206) and remediation percentage (8.29) were exhibited by Pseudomonas aeruginosa at 0 and 6 g L-1 carbon source supplementation in 100 mg Cd L-1 medium respectively (Table 2). In contrast, the maximum Optical Density (1.176) was by K. edwarsii while maximum remediation percentage (53.18) was exhibited by P. aeruginosa in 100 mg Cd L-1 medium as shown in Table 2. This confirms that the microbial biomass and specie possess varied biosorptive capabilities which depend on treatments and experimental conditions (Malik, 2004, Fomina and Gadd, 2014). Also, levels of carbon source in 600 mg Pb L-1 media did not have any positive effect on the Optical Density of the investigated isolates. Hence, the organisms exhibited a lower Optical Density at all levels of carbon supplementation which is not in support of the findings of Teng et al., 2010 in the use of carbon as supplement in the bioremediation of PAH contaminated soil. Although, the same study (Teng et al., 2010) supports this study as the bacterial isolates exhibited increase in remediation percentage especially at 4 and 5 g L-1 carbon supplement. K. edwarsii has the highest remediation percentage of 79.87 at 5 g L-1 carbon supplement. The solubility of heavy metal in bioremediation could be specific as heavy metals used in this study exhibit varied effect on the test microorganisms. Zahoor and Rehman (2009) reported reduction of hexavalent chromium by Bacillus sp. JDM-2–1 and Staphylococcus capitis, to trivalent form as aided by the solubility of Cr (VI). Furthermore, Jin et al, (2018) describe the variation in the solubility of different heavy metal as Zn, Ni, and Cu dissolved easily while Pb and Cr are less soluble.
Table 2.
Effect of carbon source levels of the medium on the remediation of 100 mg Cd L-1 and 600 mg Pb L-1 by bacterial isolates.
| Cd | Pb | |||
|---|---|---|---|---|
| Isolate Code | Optical density | Remediation % | Optical density | Remediation % |
| KEA4 | 0.403a | 31.57b | 0.891b | 16.13c |
| ECA4 | 0.400a | 12.52c | 0.573c | 25.63a |
| PAA4 | 0.206b | 39.90a | 1.147a | 18.73b |
| KEA5 | 0.504b | 32.61b | 0.399c | 25.02b |
| ECA5 | 0.627a | 14.53c | 0.512a | 23.51c |
| PAA5 | 0.210c | 38.86a | 0.478b | 25.66a |
| KEA6 | 0.613b | 33.98b | 0.373c | 24.77b |
| ECA6 | 0.729a | 16.17c | 0.492b | 23.28c |
| PAA6 | 0.319c | 40.18a | 0.511a | 55.16a |
| KEA7 | 0.636a | 14.40b | 0.468c | 70.87a |
| ECA7 | 0.625b | 12.14c | 0.511b | 20.13c |
| PAA7 | 0.554c | 16.14a | 0.566a | 60.17b |
| KEA8 | 0.574b | 13.70b | 0.435c | 73.62b |
| ECA8 | 0.630a | 8.01c | 0.531a | 78.39a |
| PAA8 | 0.631a | 53.18a | 0.461b | 66.42c |
| KEA9 | 0.638a | 15.03c | 0.345c | 79.87a |
| ECA9 | 0.680a | 16.04b | 0.431a | 63.03b |
| PAA9 | 0.630a | 16.14a | 0.355b | 57.42c |
| KEA10 | 1.176a | 18.01a | 0.429b | 75.42a |
| ECA10 | 0.618c | 12.73b | 0.552a | 70.44b |
| PAA10 | 0.717b | 8.29c | 0.422c | 68.64c |
Values in the same group followed by the same letter did not differ significantly at p < 0.05 according to Duncan’s New Multiple Range Test
Legend: KE – Klebsiella edwarsii, EC – Enterobacter cloacae, PA – Pseudomonas aeruginosa, A4 - Unadjusted carbon source (Control),
A5 − 1 g/L carbon source, A6 − 2 g/L carbon source, A7 − 3 g/L carbon source, A8 − 4 g/L of carbon source, A9 − 5 g /L carbon source, A10 − 6 g /L carbon source
The pH also has significant effect on heavy metal ions solubility and cell surface charge, which affects the heavy metal removal processes (Guibal et al., 1994, Jin et al., 2018). Table 3 showed the effect of varied pH levels on the remediation of 100 mg L-1Cd and 600 mg L-1 Pb by bacterial isolates. The maximum optical density (0.414) was by E. cloacae at pH 8 while the maximum remediation percentage (81%) was by K. edwarsii at pH 5. In addition, the test organisms had the least optical density (0.026, 0.079 and 0.060) and remediation percentage (8.47%, 9.05% and 4.42%) value at pH 9 in 100 mg L-1Cd remediation. The adjusted pH did not have significant (P < 0.05) improvement on the optical density of the bacterial isolates for both heavy metals. There was a remarkable improvement in the remediation percentage at pH 5 and 7 in 600 mg L-1 Pb supplemented medium (Table 3). Moreover, K. edwarsii had the highest (92.41%) and lowest (1.38%) remediation percentage at pH 6 and 9 respectively. It was observed in this study, that the remediation potential and biomass of the organisms can be optimized within the pH range of 5 to 8. This shows that microbial biosorption, and optimum pH usually vary for different microorganisms. This supported the findings of Wang and Chen, 2009, Machado et al., 2008, Sánchez-Clemente et al., 2020 that pH range of 4 to 8 enhance biosorption of heavy metals for almost all types of biomass. Ashokkumar et al. (2017) recorded 48%, 75% and 52% for removal of Cu, Pb, and Cr respectively by Sphaerotilus natansin at pH 7. Also, Joshi and Lee (1995) indicated that bacteria can efficiently degrade majority of the soil contaminants within the pH range of 5.0 and 9.0. Ultimately, the study reveals that all the test organisms had the lowest remediation potential and biomass at pH 9. This confirms that heavy metals tend to be insoluble at high pH and soluble at low pH. A study conducted by Ameen et al, (2020) recorded maximum uptake of Cd2+ and Pb2+ by Lactobacillus plantarum at pH 2 while Franklin et al., 2000, Olaniran et al., 2013 reported that heavy metals are more toxic at high pH. Hence, the rate of heavy metal remediation by microorganisms increases with increase in pH levels and decreases as it gets to its limit.
Table 3.
Effect of pH of the medium on the remediation of 100 mg Cd L-1 and 600 mg Pb L-1 by bacterial isolates.
| Cd | Pb | |||
|---|---|---|---|---|
| Isolate Code | Optical density | Remediation % | Optical density | Remediation % |
| KEA4 | 0.403a | 31.57b | 0.891b | 16.13c |
| ECA4 | 0.400a | 12.52a | 0.573c | 25.63a |
| PAA4 | 0.206b | 39.90c | 1.470a | 18.73b |
| KEA11 | 0.236b | 81.56a | 0.456b | 43.54b |
| ECA11 | 0.232b | 26.62c | 0.660a | 14.87c |
| PAA11 | 0.263a | 31.48b | 0.620a | 86.38a |
| KEA12 | 0.147c | 9.81c | 0.511b | 92.41a |
| ECA12 | 0.180b | 17.22b | 0.698a | 70.15c |
| PAA12 | 0.272a | 18.31a | 0.495c | 72.93b |
| KEA13 | 0.090b | 14.40b | 0.482c | 82.61b |
| ECA13 | 0.125a | 59.03a | 0.666a | 85.43a |
| PAA13 | 0.150a | 12.60c | 0.494b | 73.39c |
| KEA14 | 0.172c | 24.19c | 0.501c | 12.26c |
| ECA14 | 0.414a | 25.85b | 0.707a | 15.57b |
| PAA14 | 0.240b | 40.56a | 0.580b | 35.42a |
| KEA15 | 0.026b | 8.47a | 0.158b | 1.38c |
| ECA15 | 0.079a | 9.05a | 0.206a | 3.89b |
| PAA15 | 0.060a | 4.42b | 0.127c | 5.40a |
Values in the same group followed by the same letter did not differ significantly at p < 0.05 according to Duncan’s New Multiple Range Test
Legend: KE – Klebsiella edwarsii, EC – Enterobacter cloacae, PA – Pseudomonas aeruginosa, A4 - Unadjusted pH (Control),
A11 - pH 5, A12 - pH 6, A13 - pH 7, A14 - pH 8, A15 - pH 9
4. Conclusion
The environmental and economic impacts of heavy metal pollution on soils are enormous; eliciting changes capable of affecting nutrient cycling, impeding nutrient uptake by plant roots and leading to reduction in crop yield. This causes serious damages to vegetation, soil fertility and soil-borne microorganisms. In the present study, the results showed that 4–6 g/L carbon source supplement at pH 5–8 enhanced the heavy metal bioremediation potential of the Pseudomonas aeruginosa, Enterobacter cloacae and Klebsiella edwardsii isolated from landfill sites in Lagos, Nigeria. The reported effectiveness of the indigenous bacteria in the remediation process can be optimized and utilized for the standardization of in-situ bioremediation as well as establishment of biodegradation protocols. The over-reaching benefits could provide effective reclamation of contaminated soils through the improvement of soil health and crop yield.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors are immensely grateful to Covenant University for the financial support. The authors also appreciate the Central Science Laboratory of Obafemi Awolowo University for all the assistance rendered during the remediation experiment.
Authors’ Contribution
OO and OAO conceived and designed the study. OO, OAO, OEJ, AEF and OJS performed experiments, drafted and edited the manuscript. All authors read and approved the final manuscript.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abioye O.P., Oyewole O.A., Oyeleke S.B., Adeyemi M.O., Orukotan A.A. Biosorption of lead, chromium and cadmium in tannery effluent using indigenous microorganisms. Brazilian Journal of Biological Sciences. 2018;5(9):25–32. [Google Scholar]
- Akob D.M., Mills H.J., Kostka J.E. Metabolically active microbial communities in uranium-contaminated subsurface sediments. FEMS Microbiol. Ecol. 2007;59(1):95–107. doi: 10.1111/j.1574-6941.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- Al-Dhabi N.A., Esmail G.A., Mohammed Ghilan A.K., Valan Arasu M. Optimizing the management of cadmium bioremediation capacity of metal-resistant Pseudomonas sp. strain Al-Dhabi-126 isolated from the industrial city of saudi arabian environment. Int. J. Environ. Res. Public Health. 2019;16(23),4788 doi: 10.3390/ijerph16234788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander P.D., Alloway B.J., Dourado A.M. Genotypic variations in the accumulation of Cd, Cu, Pb and Zn exhibited by six commonly grown vegetables. Environ. Pollut. 2006;144(3):736–745. doi: 10.1016/j.envpol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Ameen F.A., Hamdan A.M., El-Naggar M.Y. Assessment of the heavy metal bioremediation efficiency of the novel marine lactic acid bacterium, Lactobacillus plantarum MF042018. Sci. Rep. 2020;10(1):1–11. doi: 10.1038/s41598-019-57210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashokkumar P., Loashini V.M., Bhavya V. Effect of pH, Temperature and biomass on biosorption of heavy metals by Sphaerotilus natans. International Journal of Microbiology and Mycology. 2017;6(1):32–38. [Google Scholar]
- Bennisse R., Labat M., Elasli A., Brhada F., Chandad F., Liegbott P.P., Hibti M., Qatibi A.I. Rhizosphere bacterial populations of metallophyte plants in heavy metalcontaminated soils from mining areas in semiarid climate. World J. Microbiol. Biotechnol. 2004;20:759–766. [Google Scholar]
- Brownfield M.E., Charpentier R.R. No. 2207-C. US Geological Survey; 2006. (Geology and total petroleum systems of the Gulf of Guinea Province of West Africa). [Google Scholar]
- Chen Q., Li Y., Liu M., Zhu B., Mu J., Chen Z. Removal of Pb and Hg from marine intertidal sediment by using rhamnolipid biosurfactant produced by a Pseudomonas aeruginosa strain. Environ. Technol. Innovation. 2021;101456 [Google Scholar]
- Choudhary S., Sar P. Uranium biomineralization by a metal resistant Pseudomonas aeruginosa strain isolated from contaminated mine waste. J. Hazard. Mater. 2011;186(1):336–343. doi: 10.1016/j.jhazmat.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Das S., Dash H.R., Chakraborty J. Genetic basis and importance of metal resistant genes in bacteria for bioremediation of contaminated environments with toxic metal pollutants. Appl. Microbiol. Biotechnol. 2016;100(7):2967–2984. doi: 10.1007/s00253-016-7364-4. [DOI] [PubMed] [Google Scholar]
- Fomina M., Gadd G.M. Biosorption: current perspectives on concept, definition and application. Bioresour. Technol. 2014;160:3–14. doi: 10.1016/j.biortech.2013.12.102. [DOI] [PubMed] [Google Scholar]
- Franklin N.M., Stauber J.L., Markich S.J., Lim R.P. pH-dependent toxicity of copper and uranium to a tropical freshwater alga (Chlorella sp.) Aquat. Toxicol. 2000;48:275–289. doi: 10.1016/s0166-445x(99)00042-9. [DOI] [PubMed] [Google Scholar]
- Gbadamosi M.R., Afolabi T.A., Ogunneye A.L., Ogunbanjo O.O., Omotola E.O., Kadiri T.M., Akinsipo O.B., Jegede D.O. Distribution of radionuclides and heavy metals in the bituminous sand deposit in Ogun State, Nigeria – A multi-dimensional pollution, health and radiological risk assessment. J. Geochem. Expl. 2018;190:187–199. [Google Scholar]
- Giovanella P., Cabral L., Costa A.P., de Oliveira Camargo F.A., Gianello C., Bento F.M. Metal resistance mechanisms in Gram-negative bacteria and their potential to remove Hg in the presence of other metals. Ecotoxicol. Environ. Saf. 2017;140:162–169. doi: 10.1016/j.ecoenv.2017.02.010. [DOI] [PubMed] [Google Scholar]
- Guibal E., Sancedo I., Roussy J., LeCloiree P. Uptake of uranyl ions by new sorbing polymers: discussion of adsorption isotherms and pH effect. Reaction. Polymer. 1994;23:147–156. [Google Scholar]
- Hassan A., Periathamby A., Ahmed A., Innocent O., Hamid F.S. Effective bioremediation of heavy metal–contaminated landfill soil through bioaugmentation using consortia of fungi. J. Soils Sediments. 2020;20(1):66–80. [Google Scholar]
- Hassen A., Saidi N., Cherif M., Boudabous A. Resistance of environmental bacterial to heavy metals. Bioresour. Technol. 1998;64:7–15. [Google Scholar]
- Hoornweg, D., and Bhada-Tata, P. 2012. What a Waste: A global review of solid waste management. Urban development series; knowledge papers no. 15. World Bank. Washington, DC: ©World Bank.
- Jain P.K., Gupta V.K., Guar R.K., Lowry M., Jaroli D.P., Chauhan U.K. Bioremediation of Petroleum Oil Contaminated Soil and Water. Research Journal of Environmental Toxicology. 2011;5(1):1–26. [Google Scholar]
- Jia L., Zhou J., Cao J., Wu Z., Liu W., Yang C. Foam fractionation for promoting rhamnolipids production by Pseudomonas aeruginosa D1 using animal fat hydrolysate as carbon source and its application in intensifying phytoremediation. Chemical Engineering and Processing-Process Intensification. 2020;158 [Google Scholar]
- Jin Y., Luan Y., Ning Y., Wang L. Effects and mechanisms of microbial remediation of heavy metals in soil: a critical review. Applied Sciences. 2018;8(8):1336. [Google Scholar]
- Joshi M.M., Lee S. A novel treatment train for remediation of PAH contaminated soils. Fresenius Environ. Bull. 1995;4(10):617–623. [Google Scholar]
- Kaczorek E. Effect of External Addition of Rhamnolipids Biosurfactant on the Modification of Gram Positive and Gram Negative Bacteria Cell Surfaces during Biodegradation of Hydrocarbon Fuel Contamination. Polish Journal of Environmental Studies. 2012;21(4) [Google Scholar]
- Kamunda C., Mathuthu M., Madhuku M. Health Risk Assessment of Heavy Metals in Soils from Witwatersrand Gold Mining Basin, South Africa. Int. J. Environ. Res. Pub. Health. 2016;13:1–11. doi: 10.3390/ijerph13070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karak T., Bhagat R.M., Bhattacharyya P. Municipal solid waste generation, composition, and management: The world scenario. Critical Reviews in Environmental Science and Technology. 2012;42(15):1509–1630. [Google Scholar]
- Khan S., Cao Q., Zheng Y.M., Huang Y.Z., Zhu Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing. China. Environmental Pollution. 2008;152(3):686–692. doi: 10.1016/j.envpol.2007.06.056. [DOI] [PubMed] [Google Scholar]
- Koshlaf E., Shahsavari E., Haleyur N., Osborn A.M., Ball A.S. Effect of biostimulation on the distribution and composition of the microbial community of a polycyclic aromatic hydrocarbon-contaminated landfill soil during bioremediation. Geoderma. 2019;338:216–225. [Google Scholar]
- Ling W., Shen Q., Gao Y., Gu X., Yang Z. Use of bentonite to control the release of copper from contaminated soils. Aust. J. Soil Res. 2007;45(8):618–623. [Google Scholar]
- Liu S.H., Zeng G.M., Niu Q.Y., Liu Y., Zhou L., Jiang L.H., Tan X.F., Xu P., Zhang C., Cheng M. Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review. Bioresour. Technol. 2017;224:25–33. doi: 10.1016/j.biortech.2016.11.095. [DOI] [PubMed] [Google Scholar]
- Loh K.C., Wang S.J. Enhancement of biodegradation of phenol and a non-growth substrate 4- chlorophenol by medium augmentation with conventional carbon sources. Biodegradation. 1998;8:329–338. doi: 10.1023/a:1008267607634. [DOI] [PubMed] [Google Scholar]
- Malik A. Metal bioremediation through growing cells. Environ. Int. 2004;30(2):261–278. doi: 10.1016/j.envint.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Machado M.D., Santos M.S., Gouvei A.C., Soares H.M., Soares E.V. Removal of heavy metals using a brewar’s yeast strain of Saccharomyces cerevisiae: the flocculation as a separation process. Bioresources and Technology. 2008;99:2107–2115. doi: 10.1016/j.biortech.2007.05.047. [DOI] [PubMed] [Google Scholar]
- McLaughlin M.J., McLaren R.E.R.G., Speir T.W., Rogers S.L. Review: a bioavailability-based rationale for controlling metal and metalloid contamination of agricultural land in Australia and New Zealand. Aust. J. Soil Res. 2000;38(6):1037–1086. [Google Scholar]
- Mehrzad F., Fataei E., Rad S.N., Imani A.A. The investigation of nutrient addition impact on bioremediation capability of gasoil by Alcaligenes faecalis. Journal of Pure and Applied Microbiology. 2015;9(3):2185–2191. [Google Scholar]
- Misra G.P., Kaushal P., Bhaskarwar A.K., Grover P.D. Requirement of pre-processing in a waste to energy (wte) plant based on indian municipal solid waste (MSW) Journal of Solid Waste Technology and Management. 2018;44:130–141. [Google Scholar]
- Morris S., Garcia-Cabellos G., Enright D., Ryan D., Enright A.M. Bioremediation of landfill leachate using isolated bacterial strains. International Journal. 2018;6(1):26–35. [Google Scholar]
- Mounaouer B., Nesrine A., Abdennaceur H. Identification and characterization of heavy metal-resistant bacteria selected from different polluted sources. Desalin. Water Treat. 2014;52:7037–7052. [Google Scholar]
- Mukherjee S., Mukhopadhyay S., Hashim M.A., Gupta B.S. Contemporary environmental issues of landfill leachate: assessment and Remedies. Critical Reviews in Environmental Science and Technology. 2015;45(5):472–590. [Google Scholar]
- Nandish, M.S., 2005. Microbial degradation of phenol and pentachlorophenol (Doctoral dissertation, University of Agricultural Sciences GKVK, Banglore).
- Ndeddy Aka R.J., Babalola O.O. Identification and characterization of Cr-, Cd-, and Ni-tolerant bacteria isolated from mine tailings. Biorem. J. 2017;21(1):1–19. [Google Scholar]
- Nies D.H. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 2003;27:313–339. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]
- Nikhil T., Deepa V., Rohan G., Satish B. Isolation, characterization and identification of diesel engine oil degrading bacteria from garage soil and comparison of their bioremediation potential. International Research Journal of Environment Sciences. 2013;2(2):48–52. [Google Scholar]
- Njoku K.L., Akinyede O.R., Obidi O.F. Microbial Remediation of Heavy Metals Contaminated Media by Bacillus megaterium and Rhizopus stolonifer. Scientific African. 2020;10:e00545. [Google Scholar]
- Nwinyi, O.C. and Akinmulewo, B.A., 2019. Remediation of soil polluted with spent oil using cow dung. In IOP Conference Series: Earth and Environmental Science (Vol. 331, No. 1, p. 012058). IOP Publishing.
- Nwinyi O.C., Kanu I.A., Tunde A., Ajanaku K.O. Characterization of diesel degrading bacterial species from contaminated tropical ecosystem. Brazilian Archives of Biology and Technology. 2014;57(5):789–796. [Google Scholar]
- Ojewunmi M.E. A bioremediation study of raw and treated crude petroleum oil polluted soil with Aspergillus niger and Pseudomonas aeruginosa. Journal of Ecological Engineering. 2018;19(2):226–235. [Google Scholar]
- Okoro E.E., Okolie A.G., Sanni S.E., Omeje M. Toxicology of heavy metals to subsurface lithofacies and drillers during drilling of hydrocarbon wells. Sci. Rep. 2020 doi: 10.1038/s41598-020-63107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olabode S.O., Mohammed M.Z. Depositional facies and sequence stratigraphic study in parts of Benin (Dahomey) Basin SW Nigeria: implications on the re-interpretation of tertiary sedimentary successions. International Journal of Geosciences. 2016;7(2):210–228. [Google Scholar]
- Oladipo O.G., Olayinka A., Awotoye O.O. Ecological impact of mining on soils of southwestern Nigeria. Environmental and Experimental Biology. 2014;12:179–186. [Google Scholar]
- Oladipo O.G., Ezeokoli O.T., Maboeta M.S., Bezuidenhout J.J., Tiedt L.R., Jordaan A., Bezuidenhout C.C. Tolerance and growth kinetics of bacteria isolated from gold and gemstone mining sites in response to heavy metal concentrations. J. Environ. Manage. 2018;212:357–366. doi: 10.1016/j.jenvman.2018.01.038. [DOI] [PubMed] [Google Scholar]
- Olaniran A.O., Balgobind A., Pillay B. Bioavailability of heavy metals in soil: impact on microbial biodegradation of organic compounds and possible improvement strategies. Int. J. Mol. Sci. 2013;14(5):10197–10228. doi: 10.3390/ijms140510197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olorunfemi F.B. Landfill Development and Current Practices in Lagos Metropolis, Nigeria. Journal of Geography and Regional Planning. 2011;4(12):656–663. [Google Scholar]
- Piddock L.J. Multidrug-resistance efflux pumps–not just for resistance. Natural Rev. Microbiology. 2006;4:629–636. doi: 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- Pourfadakari S., Ahmadi M., Jaafarzadeh N., Takdastan A., Ghafari S., Jorfi S. Remediation of PAHs contaminated soil using a sequence of soil washing with biosurfactant produced by Pseudomonas aeruginosa strain PF2 and electrokinetic oxidation of desorbed solution, effect of electrode modification with Fe3O4 nanoparticles. J. Hazard. Mater. 2019;379:120839. doi: 10.1016/j.jhazmat.2019.120839. [DOI] [PubMed] [Google Scholar]
- Priyadarshanee M., Das S. Biosorption and removal of toxic heavy metals by metal tolerating bacteria for bioremediation of metal contamination: A comprehensive review. Journal of Environmental. Chem. Eng. 2020;104686 [Google Scholar]
- Raimondo E.E., Saez J.M., Aparicio J.D., Fuentes M.S., Benimeli C.S. Bioremediation of lindane-contaminated soils by combining of bioaugmentation and biostimulation: Effective scaling-up from microcosms to mesocosms. J. Environ. Manage. 2020;276:111309. doi: 10.1016/j.jenvman.2020.111309. [DOI] [PubMed] [Google Scholar]
- Ranđelović D., Stanković S., Mihailović N., Leštan D. Remediation of Copper from Copper Mine Wastes and Contaminated Soils Using (S, S)-Ethylenediaminedisuccinic Acid and Acidophilic Bacteria. Biorem. J. 2015;19(3):231–238. [Google Scholar]
- Raskin I., Kumar P.B.A.N., Dushenkov S., Salt D.E. Bio concentration of heavy metals by plants. Curriculum. Opinion and Biotechnology. 1994;5:285–290. [Google Scholar]
- Raulinaitis M., Ignatavicius G., Sinkevicius S., Oskinis V. Assessment of heavy metal contamination and spatial distribution in surface and subsurface sediment layers in the northern part of Lake Babrukas. Ekologija. 2012;58(1):33–43. [Google Scholar]
- Rizvi A., Khan M.S. Biotoxic impact of heavy metals on growth, oxidative stress and morphological changes in root structure of wheat (Triticum aestivum L.) and stress alleviation by Pseudomonas aeruginosa strain CPSB1. Chemosphere. 2017;185:942–952. doi: 10.1016/j.chemosphere.2017.07.088. [DOI] [PubMed] [Google Scholar]
- Rubinos D.A., Spagnoli G. Utilization of waste products as alternative landfill liner and cover materials – A critical review. Critical Reviews in Environmental Science and Technology. 2018:1–63. [Google Scholar]
- Salt D.E., Blaylock M., Kumar P.B.A.N., Dushenkov V., Ensley B.D., Chet I., Raskin I. Phytoremediation: A novel strategy for the removal of toxic metals from the environment using plants. Biotechnology. 1995;13:468–475. doi: 10.1038/nbt0595-468. [DOI] [PubMed] [Google Scholar]
- Samlafo V.B. Comparative analysis of leachable heavy metals in earthenware clay deposits in the Central and Volta regions of Ghana. American J. Chem. 2017;7(4):145–151. [Google Scholar]
- Sánchez-Clemente R., Guijo M.I., Nogales J., Blasco R. Carbon Source Influence on Extracellular pH Changes along Bacterial Cell-Growth. Genes. 2020;11(11):1292. doi: 10.3390/genes11111292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J., Beydoun D., Amal R., Low G., Cattle J. Landfill management, leachate generation, and leach testing of solid wastes in australia and overseas. Critical Reviews in Environmental Science and Technology. 2005;35(3):239–332. [Google Scholar]
- Shuaib M., Azam N., Bahadur S., Romman M., Yu Q., Xuexiu C. Variation and succession of microbial communities under the conditions of persistent heavy metal and their survival mechanism. Microb. Pathog. 2021;150:104713. doi: 10.1016/j.micpath.2020.104713. [DOI] [PubMed] [Google Scholar]
- Singh R., Bishnoi N.R., Kirrolia A. Evaluation of Pseudomonas aeruginosa an innovative bioremediation tool in multi metals ions from simulated system using multi response methodology. Bioresour. Technol. 2013;138:222–234. doi: 10.1016/j.biortech.2013.03.100. [DOI] [PubMed] [Google Scholar]
- Tang X., Zeng G., Fan C., Zhou M., Tang L., Zhu J., Wan J., Huang D., Chen M., Xu P., Zhang C. Chromosomal expression of CadR on Pseudomonas aeruginosa for the removal of Cd (II) from aqueous solutions. Sci. Total Environ. 2018;636:1355–1361. doi: 10.1016/j.scitotenv.2018.04.229. [DOI] [PubMed] [Google Scholar]
- Teng Y., Luo Y., Ping L., Zou D., Li Z., Christie P. Effects of soil amendment with different carbon sources and other factors on the bioremediation of an aged PAH-contaminated soil. Biodegradation. 2010;21(2):167–178. doi: 10.1007/s10532-009-9291-x. [DOI] [PubMed] [Google Scholar]
- Tarekegn M.M., Salilih F.Z., Ishetu A.I. Microbes used as a tool for bioremediation of heavy metal from the environment. Cogent Food & Agriculture. 2020;6(1):1783174. [Google Scholar]
- Varjani S., Upasani V.N., Pandey A. Bioremediation of oily sludge polluted soil employing a novel strain of Pseudomonas aeruginosa and phytotoxicity of petroleum hydrocarbons for seed germination. Sci. Total Environ. 2020;737:139766. doi: 10.1016/j.scitotenv.2020.139766. [DOI] [PubMed] [Google Scholar]
- Wang J., Chen C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009;27:195–226. doi: 10.1016/j.biotechadv.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Wuana R.A., Okieimen F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Scholarly Res. Network Ecology. 2011:1–20. [Google Scholar]
- Xia S., Song Z., Jeyakumar P., Shaheen S.M., Rinklebe J., Ok Y.S., Bolan N., Wang H. A critical review on bioremediation technologies for Cr (VI)-contaminated soils and wastewater. Critical Reviews in Environmental Science and Technology. 2019 [Google Scholar]
- Yin K., Lv M., Wang Q., Wu Y., Liao C., Zhang W., Chen L. Simultaneous bioremediation and biodetection of mercury ion through surface display of carboxylesterase E2 from Pseudomonas aeruginosa PA1. Water Res. 2016;103:383–390. doi: 10.1016/j.watres.2016.07.053. [DOI] [PubMed] [Google Scholar]
- Zahoor A., Rehman A. Isolation of Cr (VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. J. Environ. Sci. 2009;21(6):814–820. doi: 10.1016/s1001-0742(08)62346-3. [DOI] [PubMed] [Google Scholar]
- Zhang M.K., Liu Z.Y., Wang H. Use of single extraction methods to predict bioavailability of heavy metals in polluted soils to rice. Commun. Soil Sci. Plant Anal. 2010;41(7):820–831. [Google Scholar]
- Zhang X., Yan J., Luo X., Zhu Y., Xia L., Luo L. Simultaneous ammonia and Cr (VI) removal by Pseudomonas aeruginosa LX in wastewater. Biochem. Eng. J. 2020;157 [Google Scholar]