Abstract
We present the initial results of a neonatal screening program in part of the public health system in Honduras, that is, the Honduran Social Security Institute. The program design includes steps from neonatal bloodspot in the first newborn days to evaluation and treatment when necessary. In 2018 and 2019, 19,911 newborns were tested for hypothyroidism, cystic fibrosis, galactosemia, phenylketonuria, and adrenal hyperplasia. Abnormalities were identified in 18 newborns, corresponding to a prevalence of 9:10,000. Considering all births in Honduras, the estimated coverage of screening ranged between 4.4 and 5.7%. These results reinforce the need to expand and consolidate neonatal screening.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12687-021-00506-7.
Keywords: Neonatal screening, Birth defects, Inborn errors of metabolism, Honduras
Introduction
Neonatal bloodspot screening is based on laboratory tests in the first days of a newborn’s life, which, if done at the correct time and in the right way, prevents morbidity and mortality (Botler et al. 2010). Currently, screening newborns for up to dozens of congenital diseases in the first hours or days of life is a routine in many developed countries (Therrell and Padilla 2018), which has been considered as one of the ten major public health achievements in the first decade of the twenty-first century (CDC 2011).
In developing countries, as infant mortality rates decrease, neonatal screening becomes important in public health (Therrell and Padilla 2018). In Latin America, neonatal screening began in the 1970s, based on initiatives in Mexico and Brazil. Despite the many financial, political, cultural, and logistical difficulties, neonatal screening has expanded to most countries in the region (Borrajo 2007; Borrajo 2012; Therrell and Padilla 2018).
In September 2016, the National Congress approved the “Mandatory Neonatal Screening Law” in Honduras. As a result of this Law, it was decided that a screening plan should be implemented in the Secretariat of Health (in Spanish, Secretaría de Salud, SESAL) and the Honduran Social Security Institute (Instituto Hondureño de Seguridad Social, IHSS) progressively (Tiempo 2016). Both SESAL and IHSS are service providers in the public health system. SESAL is the main provider of public health services for the entire population in its own facilities staffed by its own physicians and nurses, but it is estimated that only 50 to 60% of Hondurans regularly use these services. IHSS manages fiscal resources and compulsory contributions from workers and employers and offers services to formal sector workers, who have an employment contract, their dependents and pensioners, using its own and contracted health facilities and professionals. Thus, IHSS covers 40% of actively employed individuals, that is, about 12 to 18% of the total population. The private sector serves some 10 to 15% of the population, of whom are those who can afford to pay or are covered by private insurance. An estimated 17% of Hondurans do not have regular access to health services (Bermúdez-Madriz et al. 2011; Carmenate-Milián et al. 2017; PAHO 2017).
After approving the Law, the IHSS began designing a model to implement neonatal screening, with budget forecast and actions to provide personnel, facilities, and technical equipment for screening. Five diseases were chosen to start the screening: hypothyroidism, cystic fibrosis, galactosemia, phenylketonuria, and adrenal hyperplasia. The choice of these diseases was based on the experience of other countries in the region, such as Costa Rica and Cuba (Borrajo 2007), and the technical conditions available for detecting, confirming, and treating these five illnesses were considered. According to the operating procedures manual established by the IHSS in April 2018 (Supplementary Information), blood collection for screening should be done ideally between the third and tenth day of life of the newborn, the samples should be sent to the Central Laboratory of Specialties at the IHSS between the fourth and the eleventh day, and laboratory tests should be performed until the baby’s eighteenth day of life. At the end of this process, the test results are handed over to the parents, and the child must have started treatment with a specialist, when necessary.
This study presents the initial results of the neonatal screening program in IHSS, that is, in part of the public health system in Honduras.
Methods
Setting
Honduras is a country in Central America, which has a population of about 9 million inhabitants (INE 2020). The country became independent in 1821 and has since been a republic, although it has consistently endured a great deal of social strife and political instability and remains one of the poorest countries in Latin America. It has a Human Development Index of 0.634, classifying it as a nation with medium development. When the Index is adjusted for income inequality, its inequality-adjusted Human Development Index is 0.472 (UNPD 2019). Honduras is divided politically into 18 departments and 298 municipalities. Indigenous and Afro-descendant people make up 8.6% of its population, and there are nine indigenous groups in the country. Per capita gross domestic product was US$ 2495 in 2015. Moreover, Honduras has been transitioning from a mainly agricultural to an industrial economy, in which industry already represents 20% of the gross domestic product (PAHO 2017).
In 2007–2012, the infant mortality rate was 24 deaths per 1000 live births, and the under-five mortality rate was 29 deaths per 1000 live births. The leading causes of death were perinatal disorders, congenital malformations, pneumonia, diarrhea, and child malnutrition (PAHO 2017). The Government’s Strategic Plan developed from 2014 established objectives to increase employment and reduce poverty, stabilize the national economic situation, and shore up the country’s infrastructure and logistical development, strengthening democratic governance and protecting citizen security. In this context, several of the policies emerged over the past 5 years were designed to have an impact on health determinants and equity (PAHO 2017). In 2019, the infant mortality rate in Honduras was estimated at about 14 deaths per 1000 live births and the under-five mortality rate at about 17 deaths per 1000 live births (UNICEF 2020).
In March 2017, the Honduran neonatal screening program started as a pilot project on the premises of the IHSS Specialty Hospital (Hospital de Especialidades del IHSS) in Tegucigalpa, the country’s capital (Proceso Digital 2017). In September 2017, screening was expanded to the Northern Regional Hospital (Hospital Regional del Norte) in San Pedro Sula, in the north of the country. In 2018, the screening was expanded to Peripheral Clinic No. 2 (Clínica Periférica N° 2) in the Santa Fe suburb, also in Tegucigalpa. Neonatal screening is not mandatory. After a child is born, the health team, mainly the pediatrician, informs the parents about what neonatal screening is and what it is for, and they decide whether to perform the test in the next 3 to 28 days (personal communication). To date, IHSS remains the only public provider that performs neonatal screening (El Heraldo 2018), detecting the five congenital selected diseases (La Prensa 2018).
Study design and data collection
This is a retrospective, cross-sectional study which was approved by the Bioethics Committee of the Honduran Social Security Institute (protocol 133-CB-HE) and the Human Research Ethics Committee at the Federal University of São Carlos (process 4,105,553, CAAE 31257020.3.0000.5504).
Information on the neonatal screening program carried out in Honduras in 2018 and 2019 was obtained directly from the Central Laboratory of Specialties at the IHSS. Data was uncovered about municipality of collection, age of the newborn when the screening was performed, methodology used in each laboratory assay, and results of the program (numbers of newborn screening performed, missing samples, and disease detected).
Additionally, the Statistics Department of IHSS (personal communication) and the National Statistics Institute (INE 2020) and the Knoema Enterprise (Knoema 2020) websites were consulted to obtain demographic data, such as the number of births in the IHSS, the total Honduran population, and the national birth rate. These data allowed us to estimate the coverage of neonatal screening by IHSS, the number of births in the country, and the national coverage of neonatal screening.
Laboratory analyses
To carry out the screening tests, capillary blood samples were collected on filter paper in three IHSS health centers: (1) the Specialty Hospital; (2) the Peripheral Clinic No. 2; and (3) the Northern Regional Hospital. The inclusion criteria were newborns aged between 3 and 28 days, born inside or outside these three IHSS health centers, and from parents affiliated with the IHSS. The collected samples were sent to the Central Laboratory of Specialties at the IHSS for analysis.
The methodology used in the screening testing included direct immunofluorescence for phenylketonuria and galactosemia and indirect for cystic fibrosis, hypothyroidism, and adrenal hyperplasia, performed in a VICTOR2D fluorometer using Perkin-Elmer kits, as part of a semi-automatic system for performing DELFIA assays. Cutoff values were as follows: for hypothyroidism, negative test TSH < 20 μL/mL, indeterminate test TSH 20–40 μL/mL, and positive test TSH > 40 μL/mL; for cystic fibrosis, negative test IRT < 63 ng/mL, indeterminate test IRT 63–70 ng/mL, and positive test IRT > 70 ng/mL; for adrenal hyperplasia, negative test 17-OHP < 30 nmol/L, indeterminate test 17-OHP 30–90 nmol/L, and positive test 17-OHP > 90 nmol/L; for galactosemia, negative test total galactose < 7.2 mg/dL, indeterminate test total galactose 7.2–9.7 mg/dL, and positive test total galactose > 9.7 mg/dL; and for phenylketonuria, negative test phenylalanine 2.1 mg/dL, indeterminate test phenylalanine 2.1–3.0 mg/dL, and positive test phenylalanine > 3.0 mg/dL.
Screen-positive or indeterminate results were confirmed by a second test, carried out using the same methodology and with the same sample. If newborns had these first two screening assays with abnormal values, a second sample was required to perform a third test. This second sample was used to perform confirmatory testing. Confirmatory tests for congenital hypothyroidism were performed at the Central Laboratory of Specialties at the IHSS by electrochemiluminescent immunoassay for serum TSH, T4 and T3. Until May 2019, confirmatory tests for cystic fibrosis, galactosemia, phenylketonuria, and adrenal hyperplasia were carried out using the same laboratory methodology as the screening assays at the Central Laboratory of Specialties at the IHSS. From May 2019, confirmatory testing for these four diseases began to be carried out using another methodology in private national and foreign laboratories with which IHSS have agreements. For phenylketonuria and galactosemia, confirmatory tests were performed by molecular biology using the real-time PCR technique. To confirm adrenal hyperplasia, 17-hydroxyprogesterone was measured by chemiluminescence. To confirm cystic fibrosis, a sweat electrolyte test was carried out.
Results
Between January 2018 and December 2019, 19,911 newborns were tested, 10,670 (53.6%) in Tegucigalpa and 9241 (46.4%) in San Pedro Sula. In this period, 214 samples were missing from both cities, and the parents of these children were called to recollect another sample. In 2018, 11 cases of abnormal screening were identified, in which there were 2 cases of cystic fibrosis, 2 of hypothyroidism, 1 of adrenal hyperplasia, 5 of galactosemia, and 1 of phenylketonuria. In 2019, 7 cases of abnormal screening were identified, among which there were 5 cases of hypothyroidism and 2 cases of adrenal hyperplasia.
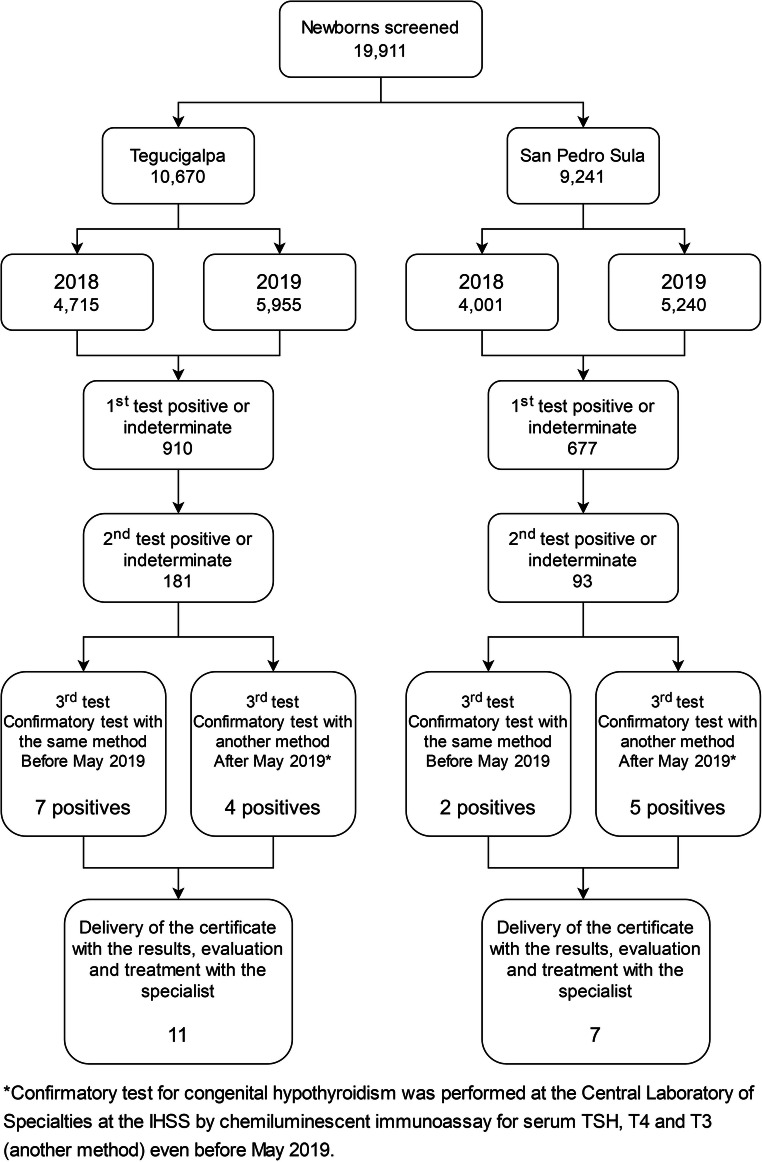
Figure 1 presents a flow chart of neonatal screening process and its results at each step in Tegucigalpa and San Pedro Sula. After a positive screen result, the newborn was located to reach the Laboratory, and there the guidelines were given to enter a medical consultation with a pediatric endocrinologist. Table 1 shows the prevalence of the five diseases investigated by the neonatal screening in 2018 and 2019 in the IHSS, compared with the overall prevalence.
Fig. 1.
Flow chart of neonatal screening process and its results at each step in San Pedro Sula and Tegucigalpa, Honduras
Table 1.
Prevalence of diseases detected by neonatal screening by the IHSS in 2018 and 2019, compared to the global prevalence
| Congenital defects investigated | Tegucigalpa | San Pedro Sula | Total cases | Prevalence in IHSS | Global prevalence* | ||
|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | ||||
| Congenital hypothyroidism | 1 | 1 | 1 | 4 | 7 | 3.52/10,000 | 2.5–5/10,000 |
| Cystic fibrosis | 1 | – | 1 | – | 2 | 1.00/10,000 | 1–1.25/10,000 |
| Adrenal hyperplasia | 1 | 2 | – | – | 3 | 1.51/10,000 | 0.75–1/10,000 |
| Phenylketonuria | 1 | – | – | – | 1 | 0.50/10,000 | 0.6–1/10,000 |
| Galactosemia | 4 | – | 1 | – | 5 | 2.51/10,000 | Unknown |
The coverage of neonatal screening in the IHSS was 60.8% in 2018 and 80.4% in 2019. When the total births that occurred in the country were considered, and not just those that occurred in the IHSS, the estimated neonatal screening coverage varied between 4.4 and 5.7% (Table 2).
Table 2.
Coverage of neonatal screening carried out by the IHSS considering births in the IHSS and the total number of births in the country in 2018 and 2019
| Period | Number of births by IHSS* | Number of neonatal screening by IHSS** | Coverage of newborn screening by IHSS | Total Honduras population*** | National birth rate**** (live births/1000 inhabitants) | Estimated number of births in the country | National coverage of newborn screening |
|---|---|---|---|---|---|---|---|
| 2018 | 14,326 | 8716 | 60.8% | 9,023,838 | 21.6 | 194,915 | 4.4% |
| 2019 | 13,919 | 11,195 | 80.4% | 9,158,345 | 21.3 | 195,073 | 5.7% |
Data source: *Statistics Department of IHSS 2019; **Central Laboratory of Specialties at the IHSS 2019; ***National Institute of Statistics 2020; ****Knoema 2020
Among the 18 cases with abnormal screening results, 15 (83.3%) were collected in the first 3 to 7 days of life, and 2 (11.1%) were collected from the eighth to the twenty-eighth day of life. In one case (5.8%), it was not possible to determine the age of the newborn at the time of bloodspot due to a lack of information.
Discussion
The neonatal screening program in Honduras started in 2017; however, due to the lack of centralization of information in the surveillance system, it was not possible to analyze the data from the initial year of implementation. Over the years, it was observed that there was an evolution of the program in terms of coverage, with the expansion of the collection centers, as well as in the laboratory techniques for the confirmation of the investigated diseases, which were modified from May 2019. However, there is still a great deal of work to be done to implement a robust and sustainable neonatal screening program in Honduras.
Latin American countries can be grouped into six groups, according to the degree of implementation and coverage of the respective neonatal screening programs (Borrajo 2007). At the moment, Honduras is in group IV, comprising countries whose newborn screening activities have started to be organized in the last 2 years and whose coverage is between 4 and 6% (Borrajo 2007). To progress to group III, Honduras will need to expand the coverage of neonatal screening.
The diseases investigated in neonatal screening in Honduras do not differ much from other countries in Latin America (Queiruga et al. 2010; Larrandaburu et al. 2019). Whether or not a particular disease is included in the neonatal screening program is at the discretion of each country (Martínez Montes and Cepeda Nieto 2018; Castiñeras et al. 2019), taking into account the recommendations of the World Health Organization that are as follows: the condition to be screened must be an important health problem; the natural history of the disease must be well-known; there must be an identifiable early stage; early treatment must provide greater benefits than at later stages; an appropriate test must be developed for the early stage; the test must be acceptable to the population; intervals must be defined for repeating the test; healthcare service provision must be adequate for the extra clinical work resulting from screening; and the risks, both physical and psychological, must be less than the benefits (Wilson et al. 1968). Considering these recommendations and also the ethnographic composition of the Honduran population, we suggest the investigation for hemoglobinopathies in the neonatal screening program. In Latin America, national population-based newborn screening for sickle cell disease and other hemoglobinopathies is carried out in Brazil, Cuba, Costa Rica, Panama, Dominican Republic, and Puerto Rico (Bandeira et al. 2007; Borrajo 2007; Queiruga et al. 2010; Huttle et al. 2015; Echeverry-Coral et al. 2016).
In the population analyzed, the prevalence of each one of the pathologies included in neonatal screening was similar to the international literature (Larrandaburu et al. 2019). Considering all five diseases, 9 out of every 10,000 newborns submitted to neonatal screening had some illness. So far, only two of the 18 departments in Honduras, Tegucigalpa (Francisco Morazán), and San Pedro Sula (Cortés) perform neonatal screening. Between 2018 and 2019, there was an increase in screening coverage at IHSS facilities. Despite that, the coverage is still low at the national level.
Concerning private laboratories, only in the last decade have they excelled in conducting neonatal screening tests in Honduras. Until 2017, the only laboratory reported in carrying out these tests was the “Centro de Diagnóstico Clínico” (CDC) and at that time the laboratory had already been doing tests for 5 years (La Prensa 2017). There are currently other laboratories and clinics offering these services, especially in the capital, Tegucigalpa, but they are not mapped across the country, and the testing prices are inaccessible to most of the Honduran population.
In Honduras, a sample collection is carried out on the newborns between 3 and 28 days of age, which differs from some recommendations that indicate the first 3 to 7 days of life as ideal for collection, allowing for early identification and treatment (Martínez Montes and Cepeda Nieto 2018). The IHSS operating procedures manual itself recommends that the collection be made until the tenth day of life of the newborn, although this age limit cannot always be obeyed. Despite this, most cases with altered screening results were collected during the first week after the baby was born. It is important to note that, according to the “Mandatory Neonatal Screening Law,” in addition to performing the screening, each health center must issue a certificate with the test results and provide recommendations and appropriate treatment when necessary (Tiempo 2016). The IHSS does this. The certificate with the results of the tests is given to the parents of the newborn. In case of being negative, it is delivered immediately, and if it is positive, then it is delivered until the moment the disease is confirmed or ruled out. Evaluation and treatment will require ongoing clinical visits with the specialist doctor of the IHSS staff, as provided for in the program.
A limitation of this study is that we did not evaluate the implementation process, considering aspects such as outreach to parents and the longitudinal follow-up of the children identified with illnesses by neonatal screening. Therefore, we cannot analyze how the treatment and follow-up are being conducted.
This is the first report on neonatal screening carried out in the public health system in Honduras, more specifically in the IHSS. These initial results reinforce the need to expand and consolidate the neonatal screening program in the country. Thus, we recommend (1) expanding neonatal screening to the clinics and hospitals linked to SESAL and to other health centers linked to the IHSS; (2) expanding screening to all 18 Honduran departments; (3) collecting samples in the first week of newborns’ lives; (4) considering incorporate hemoglobinopathies in screening; (5) organizing a national program that includes genetic counseling in the relevant situations; and (6) organizing an accessible information system on neonatal screening.
Supplementary information
(PDF 1466 kb)
Acknowledgments
We would like to thank the Honduran Institute of Social Security (IHSS), particularly the Bioethics Committee and the Statistics and Laboratory team, for their valuable help and cooperation.
Funding
This survey was supported by CAPES (Coordination for the Improvement of Higher Education Personnel, Ministry of Education of Brazil), through a master’s scholarship to M.M.M. Buckley.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Studies with human subjects
The research was approved by the Bioethics Committee of the Honduran Social Security Institute (protocol 133-CB-HE) and the Human Research Ethics Committee at the Federal University of São Carlos (process 4,105,553, CAAE 31257020.3.0000.5504).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bandeira FMGC, Bezerra MAC, Santos MNN, Gomes YM, Araújo AS, Abath FGC. Importance of screening programs of the hemoglobin S gene. Rev Bras Hematol Hemoter. 2007;29(2):179–184. doi: 10.1590/S1516-84842007000200017. [DOI] [Google Scholar]
- Bermúdez-Madriz JL, Sáenz M d R, Muiser J, Acosta M. Sistema de salud de Honduras. Salud pública Méx. 2011;53(suppl. 2):s209–s219. [PubMed] [Google Scholar]
- Borrajo GJC. Newborn screening in Latin America at the beginning of the 21st century. J Inherit Metab Dis. 2007;30(4):466–481. doi: 10.1007/s10545-007-0669-9. [DOI] [PubMed] [Google Scholar]
- Borrajo GJC. Panorama epidemiológico de la fenilcetonuria (PKU) en Latinoamérica. Acta Pediatr Mex. 2012;33(6):279–287. [Google Scholar]
- Botler J, Camacho LAB, da Cruz MM, George P. Neonatal screening - the challenge of a universal and effective coverage. Ciênc saúde coletiva. 2010;15(2):493–508. doi: 10.1590/S1413-81232010000200026. [DOI] [PubMed] [Google Scholar]
- Carmenate-Milián L, Herrera-Ramos A, Ramos-Cáceres D, Lagos-Ordoñez K, Lagos-Ordoñez T, Samoza-Vallares C. Situation of the health system in Honduras and the new proposed health model. Arch Med. 2017;9(1–8):10.21767/1989-5216.1000222. [Google Scholar]
- Castiñeras DE, Couce ML, Marin JL, González-Lamuño D, Rocha H. Situación actual del cribado neonatal de enfermedades metabólicas en España y en el mundo. Anales de Pediatría. 2019;91(2):128.e1–128.e14. doi: 10.1016/j.anpedi.2019.05.007. [DOI] [PubMed] [Google Scholar]
- CDC, Centers for Disease Control and Prevention Ten great public health achievements — United States, 2001–2010. MMWR. 2011;60(19):619–623. [PubMed] [Google Scholar]
- Echeverry-Coral SJ, Colmenares-Mejía CC, Yepes-Molina ZX, Martínez-Nieto O, Isaza-Ruget MA. Hemoglobinopathy detection through an institutional neonatal screening program in Colombia. Jornal Brasileiro de Patologia e Medicina Laboratorial. 2016;52(5):299–306. doi: 10.5935/1676-2444.20160050. [DOI] [Google Scholar]
- El Heraldo (2018) Buscan aplicar tamizaje neonatal en todo Honduras. In: Diario El Heraldo. https://www.elheraldo.hn/pais/1157650-466/buscan-aplicar-tamizaje-neonatal-en-todo-honduras. Accessed 19 Aug 2020
- Huttle A, Maestre GE, Lantigua R, Green NS. Sickle cell in Latin America and the United States. Pediatr Blood Cancer. 2015;62(7):1131–1136. doi: 10.1002/pbc.25450. [DOI] [PubMed] [Google Scholar]
- INE, Instituto Nacional de Estadística (2020) Poblacion Honduras. In: Instituto Nacional de Estadisticas. https://www.ine.gob.hn/V3/. Accessed 16 Aug 2020
- Knoema (2020) Atlas Mundial de Datos - Honduras. In: Knoema. https://knoema.es/atlas/Honduras. Accessed 22 Jun 2020
- La Prensa (2017) Tamiz neonatal, una prueba que salva la vida de bebés. In: Diario La Prensa. https://www.laprensa.hn/honduras/1060706-410/tamiz-neonatal-una-prueba-que-salva-la-vida-de-beb%C3%A9s Accessed 17 Dec 2020
- La Prensa (2018) El Seguro Social hará tamizaje en sus clínicas periféricas. In: Diario La Prensa. https://www.laprensa.hn/honduras/1156598-410/seguro_social-tamizaje-clinicas-enfermedades-bebes. Accessed 13 Aug 2020
- Larrandaburu M, Vianna FLS, Griot K, Queijo C, Monzón G, Ugarte C, Nacul L, Schuler-Faccini L, Sanseverino MTV. Rare diseases in Uruguay: focus on infants with abnormal newborn screening. J Inborn Errors Metab Screen. 2019;7:e20190002. doi: 10.1590/2326-4594-jiems-2019-0002. [DOI] [Google Scholar]
- Martínez Montes ÁE, Cepeda Nieto AC. Tamiz neonatal en México. Universidad Autonoma de Coahuila Cordinacion General de Estudios de Posgrado e Investigacion. 2018;52:1–6. [Google Scholar]
- PAHO, Pan American Health Organization (2017) Health in the Americas – Honduras. https://wwwpahoorg/salud-en-las-americas-2017/?p=4280 Accessed 31 Oct 2020
- Proceso Digital (2017) En vigencia en Honduras el tamizaje neonatal. In: Proceso Digital. https://proceso.hn/salud-examenes-sangre-menores-honduras/. Accessed 20 Aug 2020
- Queiruga G, Lemes A, Ferolla C, Machado M, Queijo C, Garlo P, Parallada G (2010) Pesquisa neonatal: lo que puede prevenir una gota de sangre: Jornada Actualidad y Nuevas Metas en la Implementación del Sistema Nacional de Pesquisa Neonatal (SNPN). BPS, Centro de Estudios en Seguridad Social, Salud y Administración, Montevideo https://www.bps.gub.uy/bps/file/6484/1/pesquisa-neonatal.pdf
- Rubio-Gozalbo ME (2011) Orphanet: Galactosemia. https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=352. Accessed 16 Jun 2020
- Therrell BL, Padilla CD. Newborn screening in the developing countries. Curr Opin Pediatr. 2018;30(6):734–739. doi: 10.1097/MOP.0000000000000683. [DOI] [PubMed] [Google Scholar]
- Tiempo D (2016) Diputados aprueban ley que protegerá niños desde recién nacidos. In: Diario Tiempo Digital Honduras. https://tiempo.hn/aprueban-ley-ninos-recien-nacidos/ Accessed 15 Aug 2020
- UNICEF, The United Nations Children's Fund (2020) UNICEF data: monitoring the situation of children and women – Honduras. https://datauniceforg/country/hnd/ Accessed 31 Oct 2020
- UNPD, United Nations Development Programme (2019) Human development reports – Honduras. http://hdrundporg/sites/all/themes/hdr_theme/country-notes/HNDpdf Accessed 31 Oct 2020
- Wilson JM, Jungner YG, World Health Organization (1968) Principles and practice of screening for disease. In: Public Health Papers No 34. https://apps.who.int/iris/handle/10665/37650 Accessed 31 Oct 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1466 kb)