Abstract
Familial communication of pathogenic genetic variants is necessary to maximize the clinical utility of genetic testing and its public health benefits. Insights to family communication considerations may be obtained from existing clinical documentation available in medical records. The goal of this study was to describe and characterize information about family communication of pathogenic variants and cascade genetic testing from genetic counseling summary notes. We completed structured content analysis of 656 summary notes describing pathogenic variants in breast cancer genes, for patients seen at a tertiary cancer center. Patients were 89.5% female, median age of 49 years, 32.6% non-White, and were counseled by 23 unique genetic counselors (GCs) with mean post-certification experience of 3.7 years. Cascade genetic testing was documented in 92.2% of all notes. Specific relatives (i.e., relationship to patient) who would benefit from genetic counseling and cascade testing were referenced in 33.1% of notes. Specific risk messaging was 2.5 times more likely to be present in notes of high- compared to moderate-risk genes (OR=2.53, 95% CI: 1.71-3.80), and when summary notes indicated the presence of a friend or relative (OR=2.29, 95% CI: 1.50-3.48). Summary notes frequently attempted to contextualize the patients’ familial relationships by referencing positive family communication patterns (41.6%) or negative communication issues (2.4%) and included various strategies to address barriers to communication and assist relatives with cascade testing. Overall, GCs consistently documented family communication recommendations when pathogenic variants are identified on patients’ genetic testing, albeit with heterogeneous use of specific communication prompts.
Keywords: Family communication, Pathogenic variants, Breast cancer, Summary notes, Genetic counseling
INTRODUCTION
Genetic testing for hereditary predisposition is routinely performed during breast cancer diagnosis and treatment. When a hereditary predisposition to cancer is identified, cascade genetic testing for relatives can guide genetically targeted cancer screening and prevention, maximizing public health benefits of genetic testing (Roberts et al. 2018). Although the process of family communication of genetic risk information has been studied extensively, rate of patients’ communication with first-degree relatives and uptake of cascade genetic testing remain low (Samadder et al. 2020; Lee et al. 2019). Practical and effective strategies for promoting family communication are needed to improve cascade testing uptake. Family communication and cascade genetic testing have been studied mainly using patient interview and questionnaire data (Mendes et al. 2016) rather than through analysis of clinical encounters. There is a notable lack of empirical real-world evidence of how family communication is addressed by GCs while disclosing pathogenic genetic rest results. Little is known about how genetic counselors (GCs) address family communication, and it remains unclear if and how they encourage and engage in the process of cascade genetic testing in practice (Roberts et al. 2018). Genetic test result disclosure appointments present an ideal opportunity for intervention to increase family communication through counseling patients about the importance of family communication and dissemination of genetic information to relatives.
Globally, several strategies to encourage and support familial dissemination of genetic information have been explored. These include directly contacting relatives about patients’ results (Sermijn et al. 2016), sending letters encouraging patients to share information with at-risk relatives, assisting the communication process through psychoeducational guidance (i.e., helping probands’ recognize the importance of genetic information as well as its impact on their lives), and written information aids (Mendes et al. 2016). Some GCs employ a relational approach to encourage disclosure of genetic information—one that upholds patients’ wishes while also being family-centered (Forbes Shepherd et al. 2017). Discussing family dynamics is another commonly reported strategy to better understand and consequently assist with family communication (Young et al. 2019). Other factors that may impact family communication strategies and approaches may include gene-specific considerations (associated cancer risks, inheritance patterns, and penetrance), patients’ and relatives’ age (legal adult status, reproductive considerations), the number of pathogenic results being disclosed, and the presence of accompanying variant of uncertain significance (VUS) results (Dattilo et al. 2020; Srinivasan et al. 2020; Li et al. 2018; Finlay et al. 2008).
Summary notes provide a detailed, personalized post-visit summary of the genetic counseling that was provided as well as a permanent record of the discussion and has been considered an important part of the genetic counseling process for decades. In the era of electronic medical records (EMR), these summary notes are uploaded to patients’ EMR and are also accessible to patients via patient portals. These notes offer a window into the patient-GC conversations, although the degree to which the notes represent actual conversation is not known. Better understanding of the family communication content of these notes will expand our understanding of provider roles in prompting family communication and may ultimately inform the design of interventions to improve family communication of genetic information. The goal of this study was to gain insight into GC’s discussion of family communication by (1) describing the family communication content of summary notes generated by GCs following disclosure of pathogenic results on breast cancer susceptibility genes and (2) examining how GCs assess and document patients’ family communication issues. Moreover, this study attempted to (3) explore associations between GCs’ background characteristics and their family communication.
METHODS
We conducted a structured content analysis to evaluate and compare the content of genetic summary notes written by GCs secondary to the identification of patients’ pathogenic variants in breast cancer susceptibility genes. We employed an approach to content analysis that was partly deductive (where initial codes are derived from observations reported in previous research) and partly inductive (where codes arose from features of the summary notes we examined).
Sample
All patients with pathogenic variants in breast cancer susceptibility genes seen between 2013 and May 2019 at The University of Texas MD Anderson Cancer were identified from a prospectively maintained research registry in the department of clinical cancer genetics. Genetic summary notes were abstracted from the EMR from eligible patients who met the following eligibility criteria: (1) underwent germline genetic testing for two or more breast cancer susceptibility genes (ATM, BRCA1, BRCA2, CDH1, CHEK2, NBN, NF1, PALB2, PTEN, STK11, and TP53), (2) received a pathogenic test result, and 3) had results (new or existing) disclosed to them by a GC. We excluded patients who underwent single-gene testing as this testing approach is primarily used for targeted familial testing which has systematically different familial communication implications. Results that identified only VUS, benign, or likely benign results were excluded, due to differences in family communication implications and recommendations. We chose the summary note associated with disclosure of genetic test results regardless of whether it occurred via a telephone or in-person encounter. If multiple summary notes were available for a given patient, we chose the most comprehensive note (i.e., with the highest word count) for analysis. This study was approved by the MD Anderson institutional review board with a waiver of informed consent for retrospective data collection.
Data Collection
Patient and GC characteristics
Characteristics of counselled patients including age, sex, race, and genetic test result (gene and variant) were abstracted from their EMR. GC’s estimated years of experience at the time of clinic documentation was calculated using the difference between their date of first certification as noted by the American Board of Genetic Counseling(Counseling, ABGC n.d.) and date of genetic counseling for each summary note. GCs who were not certified at the time the note was written were excluded from the analysis. Characteristics of summary notes were abstracted using a codebook.
Codebook
Three main features of the discussion of familial implications of pathogenic results in summary notes were captured by the codebook: specificity of familial risk-messaging, assessment of family dynamics, and strategies to encourage cascade testing. Choice of codes was directed in part by literature on family communication of genetic information (Dheensa et al. 2018; Baker et al. 2002; Brown et al. 2016; VandenBoom et al. 2018); example codes and sub-codes from the codebook are shown in Table 1. For example, identifying specific at-risk relatives who would benefit from cascade genetic testing, exploring family dynamics and patterns of communication, possible causes of poor communication and targeted support for addressing communication barriers. We captured reference to specific interventions to assist in communication with at-risk relatives. For example, supportive informational aids including the stated purpose and recipient of these aids. Relational manners used to counsel patients about family communication were classified as 1) covert – subtle and indirect encouragement to disseminate results (e.g., “it would be important to share … ”, “should consider discussing … ”); 2) overt – active and obvious encouragement for disclosure (e.g., “we encouraged patient to share”); or 3) authoritative – highly direct (e.g., “must be shared”). Additional codes were used to note the presence or absence of a friend/relative accompanying the patient to the counseling appointment, presence of an interpreter, the duration of the counseling session (as documented on the note), and word count of the note including headers and footers. The remaining codes were inductively generated from the themes identified in the summary notes.
Table 1.
Example codes and subcodes from the consensus codebook.
| Domain | Codes | Example quotes/sub-codes |
|---|---|---|
| Cascade genetic testing | No purpose documented | We discussed that first degree family members (children, full siblings, parents) have a 50% (1 in 2) likelihood of also carrying the BRCA2 mutation. Predictive genetic testing would be indicated for at-risk family members |
| Purpose: To determine variant’s inheritance pattern | We discussed that it is unknown whether the CHEK2 mutation was maternally or paternally inherited. Specific site testing should be considered for [patient's] maternal relatives as neither of her parents are available for testing. | |
| Purpose: To inform individualized screening recommendations | It was discussed that for anyone who tests negative for a familial mutation they would not be at increased risk for Lynch syndrome and would not have to undergo Lynch syndrome screening recommendations. | |
| Purpose: For early detection of cancer | [Patient's] maternal family would be at increased risk for breast and ovarian cancer and should undergo genetic testing and any subsequent cancer prevention that would be indicated | |
| Specificity of familial risk messaging | Identifies specific relatives for genetic testing | We reviewed that it would be important to share her genetic test results with her full siblings and maternal half siblings |
| Provides generic description for family testing | We discussed that first degree family members have a 50% (1 in 2) likelihood of also carrying the BRCA1 mutation. Therefore close family members should consider genetic counseling and predictive genetic testing | |
| Assessment of family dynamics | Negative communication | … however, the patient reports that she is not in contact with her mother and suspects her mother would not pursue genetic testing |
| Positive communication | Patient seemed motivated to share these results with her family members | |
| Barriers to cascade testing | Estranged relatives, deceased or underage relatives, uninterested in testing, cost/insurance concerns, etc. | |
| Strategies used to encourage cascade genetic testing | Specifies purpose and intended recipient of genetic test report | Family members wishing to undergo predictive genetic testing would need to have a copy of [patient’s] results because the information contained on this report would allow their healthcare providers to order the appropriate site-specific and therefore significantly less expensive genetic test. |
| Specifies purpose and intended recipient of family letter | I mailed to them our family letter that briefly describes the implications of the genetic test results for family members, and encouraged them to distribute the family letter along with a copy of the results to at risk relatives | |
| Mentions lower cost of targeted testing | Since this specific mutation has been identified in [patient’s] family, the cost of the testing should be less than 500 dollars per person | |
| Offers logistical genetic counseling assistance | I have recommended that he pursue genetic testing through UT Tyler. There is a nurse practitioner there who provides genetic counseling and testing services. I encouraged [patient's] son to contact them to set up an appointment. | |
| Relational manner used to encourage family communication | Covert | Therefore family members such as their children may wish to consider predictive genetic testing. |
| Overt | I did recommend that patient also share the information from the genetic test results with her half-sister and with the children of her half-brother | |
| Authoritative | N/A |
Interrater Reliability
Two independent coders (L.T. and A.B.) began by test coding five summary notes and edited the codebook by consensus. The process of iterative refinement of the codebook continued as we coded more notes until no new themes were identified and acceptable inter-coder reliability ratings were achieved. Discrepancies were either resolved through discussion or through an independent third coder (SM). The final codebook was used by two by the coders (L.T. and A.B.) to code a random sample of 18 notes, interrater reliability of coded summary notes was calculated. Since the inter-rater reliability was above excellent (κ=0.86), it was decided to code all remaining notes independently. We performed one random sample (n=10) inter-coder reliability testing but observed no evidence of coder drift(Carey, and G.a.M. Guest, K. M 2008).
Statistical Analysis
We summarized characteristics of the patients, GCs, and summary notes using mean (SD) or number (percentage). Differences between groups were compared using chi squared analysis for categorical variables or t-tests for continuous variables. We used ANOVA to compare practice variations among individual GCs. !!!We compared family communication patterns noted within summary notes of variants identified in high-risk gene breast cancer genes (BRCA1, BRCA2, PALB2, PTEN, and TP53) compared to moderate-risk gene genes (ATM, CDH1, CHEK2, NBN, NF1, STK11), by GCs post-certification experience (modeled continuously), and by the presence vs. absence of an accompanying relative/friend to the genetic counseling appointments using multivariate logistic regressions. Prior to March 4, 2016, the EMR system at MD Anderson was Clinic Station, which was developed in-house and had limited capability for note customization, and was replaced by Epic EMR systems (Epic, Verona, Wisconsin). Therefore, the regression model was also adjusted for notes written before and after Epic implementation. Two-sided p ≤ 0.05 values were considered to be statistically significant. Because all analyses were considered exploratory, no adjustment for multiplicity was made. We used R software (Version 3.4.4) for all statistical analyses.
RESULTS
Patient and GC characteristics
In total, 656 summary notes were included in the analysis. Table 2 summarizes the characteristics of patients and GCs who correspond to these notes. Patients were predominantly female (89.5%), White (67.4%), with a median age of 49 years (range 14-81 years), and were counseled by 23 unique GCs with mean post-board certification time of 3.7 years (range 1.25-14.33 years). Half of the notes were written in response to a pathogenic result in BRCA1 or BRCA2 (49.4%), whereas the remaining were written in response to other breast cancer susceptibility genes. The vast majority of patients had one or more pathogenic results (80.0%), and 20.0% had a VUS result accompanying their pathogenic result.
Table 2.
Characteristics of summary notes (N=656), the corresponding patients, and genetic counselors who wrote them (one summary note per patient).
| Variable | Category | N (%) | |
|---|---|---|---|
|
Genetic summary note (n=656) |
Patient sex | Female | 587 (89.5) |
| Male | 69 (10.5) | ||
| Patient race | White | 442 (67.4) | |
| Black | 56 (8.5) | ||
| Asian | 39 (5.9) | ||
| Other | 79 (12.0) | ||
| Patient age (years) | Mean | 49.7 | |
| Median | 49 | ||
| Range | 14-81 | ||
| Gene with pathogenic test result: | BRCA | 324 (49.4) | |
| Non-BRCA | 332 (50.6) | ||
| Type of genetic test result: | Pathogenic only | 525 (80.0) | |
| Pathogenic and VUS | 131 (20.0) | ||
| Summary note word count | Mean | 950.9 | |
| Median | 902.5 | ||
| Range | 733-3082 | ||
| Genetic Counselors (n=23) | Summary note per genetic counselor | Mean | 30.7 |
| Median | 30 | ||
| Range | 1-80 | ||
| GC Post-certification experience (years) | Mean | 3.7 | |
| Median | 2.5 | ||
| Range | 1.25-14.33 |
VUS: variant of uncertain significance; GC: Genetic Counselor
Genetic summary notes
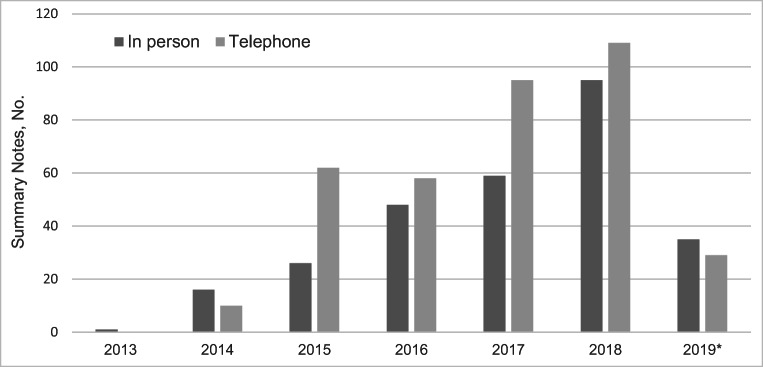
Overall, 54.6% of all summary notes described the initial disclosure of the genetic test result to the patient, 38.7% documented a follow-up appointment for a genetic test result that had been previously disclosed to the patient, and disclosure type was not documented in the remaining 6.4% of notes. Over the 7-year study period, 43.0% of all appointments occurred in-person and 57% occurred over telephone with little variation in practice over time (Figure 1). 45.9% of in-person appointments and 2.7% of telephone appointments were attended by a family member or friend of the patient. A translator was documented as present in 3.6% of all appointments.
Figure 1.
Distribution of summary notes documenting counseling appointments conducted over telephone and in-person between 2013 and May 2019 (N=656).
Table 3 shows factors associated with length of summary note assessed using multivariable regression. Summary notes describing the first disclosure of test results were on average 127 words longer than follow-up disclosures (p = 0.003) which support the detailed discussion that occur during result disclosure appointments. Similarly, notes from in-person disclosure of results were on average 304 words longer than telephone disclosures (p = < 0.001); summary notes from disclosure of results from BRCA1/2 genes were 74 words longer than non-BRCA genes (p = < 0.001); and notes disclosing multiple pathogenic results were 400 words longer than those disclosing a single pathogenic result (p = < 0.001).
Table 3.
Factors associated with of length of summary note (word count) in bivariate and multivariable regression models (N=656).
| Variable | Category | Bivariate | Multivariate | ||
|---|---|---|---|---|---|
| β | p-value | β | p-value | ||
| Summary note context | Initial disclosure | ref | ref | ||
| Follow-up disclosure | -127.03 | <0.001 | -63.37 | 0.003 | |
| Encounter type | Over telephone | ref | ref | ||
| In person | 304.22 | <0.001 | 272.3 | <0.001 | |
| Gene with pathogenic variant | Non-BRCA genes | Ref | ref | ||
| BRCA1 or BRCA2 | 74.08 | 0.002 | 93.07 | <0.001 | |
| Number of pathogenic variants | 1 pathogenic result | Ref | ref | ||
| 2 pathogenic results | 400.01 | <0.001 | 315.78 | <0.001 | |
| No. of VUS results accompanying pathogenic results | VUS present | Ref | ref | ||
| VUS absent | 157 | <0.001 | 103.77 | <0.001 | |
VUS: Variant of uncertain significance
Cascade testing
Cascade genetic testing is documented in the vast majority of all summary notes n = 605 (92.2%), usually through the use of non-specific statements (Table 1). Of the notes that mentioned cascade genetic testing, 292 (48.3%) also documented one or more of the following purposes for cascade testing: to determine the variant’s inheritance pattern, to inform individualized screening recommendations, or for early detection of cancer. Notes commonly documented use of covert (91.0%) rather than overt (11.7%) relational manners to encourage dissemination of genetic test results and never used authoritative manners. Cascade genetic testing was not discussed in the remaining 7.8% of summary notes.
Strategies to encourage cascade testing
GCs documented several strategies to address barriers to family communication and to assist relatives to undergo genetic counseling. GCs documented facilitating family communication by providing family letters (n=182) and informing patients about reduced price targeted testing for relatives (n=20). Summary notes commonly stated that a copy of the genetic test report would be given to the patient 85.8% (563/656). Only 12.1% (68/563) of these notes specified the implication of the genetic test report for relatives—i.e., patients should share the test report with their relatives as it contains information that is required for them to undergo single-site targeted testing. Logistical genetic counseling assistance for relatives was also documented including instructions for scheduling a GC appointments (n = 17), assistance for out of state relatives to locate a GC near their residence (n = 22), and resources for underinsured relatives to undergo genetic counseling (n = 2).
Specificity of familial risk messaging
Specific relatives (by relationship to patient, e.g., mother) who would benefit from genetic counseling and cascade genetic testing were named in 33.1% of notes, whereas 59.1% described the benefits of genetic counseling and cascade genetic testing for relatives in general (e.g., all first-degree relatives). There was significant variation among GCs with regard to noting specific relatives who would benefit from genetic testing (F (22, 683) = 1.99, ηp2 = 0.05, p = 0.04).
As shown in Table 4 such specific risk messaging is 2.5 times more likely to be present in notes for high-risk breast cancer genes compared to moderate-risk genes (OR = 2.53, 95% CI: 1.71–3.80). Specific risk messaging was also more often documented when counseling occurred in the presence of a friend or relative (OR = 2.29, 95% CI: 1.50–3.48). There was no difference in specific risk messaging by GC’s professional experience or when single vs. multiple pathogenic variants or VUS results were disclosed.
Table 4.
Factors associated with specific familial risk messaging documented in genetic summary notes (n=656) assessed using multivariable regression models.
| Predictor variable | Specifying at risk relatives | Implications of family letter | Familial implications of genetic test report | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| High-risk BC gene* | 2.53 | 1.71-3.80 | 3.24 | 1.56-6.85 | 15.05 | 5.42-62.58 |
| No. of pathogenic results | 1.40 | 0.48-3.48 | NA | NA | 1.57 | 0.23-6.73 |
| No. of VUS results accompanying pathogenic results | 0.86 | 0.59-1.21 | 1.19 | 0.60-2.54 | 0.76 | 0.38-1.35 |
| GC experience (years) | 1.00 | 0.95-1.05 | 0.91 | 0.82-1.00 | 0.96 | 0.87-1.03 |
| Patient accompanied by relative/friend | 2.29 | 1.50-3.48 | 1.33 | 0.59-3.12 | 1.66 | 0.82-3.25 |
|
Epic EMR Systema (post March 4, 2016) |
0.67 | 0.43-1.07 | 1.99 | 0.83-4.74 | 2.00 | 0.91-5.08 |
*compared with moderate-risk genes; acompared to Clinic Station EMR system used before March 4, 2016; significant results are in bold
BC: breast cancer; VUS: variant of uncertain significance; GC: genetic counselor
Of the 182 summary notes that discussed a family letter, 46.7% specified both the intended recipient and purpose of these letters, 9.3% only specified the intended recipient, and 12.1% only specified the intended purpose. Implications of family letter were specified more often in summary notes discussing high-risk breast cancer genes compared to moderate-risk breast cancer genes (OR=3.24, 95%CI: 1.56-6.85). Documentation of these implications was marginally associated with GC’s years of post-certification experience (OR = 0.91, 95% CI: 0.82–1.00). Summary notes discussing high-risk gene variants are significantly more likely to report the familial implication of genetic test reports compared to notes discussing variants in moderate-risk genes (OR = 15.05, 95% CI: 5.42–62.58) (Table 4).
Assessment of family communication and dynamics
GC’s frequently documented patients’ familial relationships by making notes of positive family communication patterns (41.6%) or negative communication issues (2.4%). Positive communication patterns noted in summary notes include patients who were open or eager to disclose results to relatives (n = 37) or patients who had already shared results with all relatives (n = 16). On rare occasions, patients were documented as having refused or were hesitant to share information with relatives (n = 4). Barriers to cascade genetic testing were noted in 60 (9.1%) summary notes including concerns about relatives who were estranged (n = 10), deceased (n = 11), underage (n = 14), not interested in pursuing cascade testing (n = 11), unable to get tested due to cost/insurance concerns (n = 8), or not available for testing due to other reasons (n = 6).
DISCUSSION
This observational study describes and characterizes genetic summary notes from patient encounters for hereditary breast cancer in order to understand how family communication of genetic information and cascade genetic testing are addressed in genetic counseling. We find that, although family communication of genetic information is actively encouraged and supported in genetic counseling documentation, there is considerable variation in the use of specific communication prompts. Overall, cascade genetic testing and assessment of family dynamics are frequently documented. There may be additional opportunity for improving family communication in genetic counseling summary notes through specific familial risk-messaging, providing logistical help for relatives to undergo testing, explaining the clinical implication of cascade genetic testing for family members, and by clarifying the intended recipients of family letters.
Nearly all summary notes reviewed in this study mentioned the need for cascade genetic testing, but they utilized different prompts. Family communication is a complex multifactorial phenomenon, and what prompts/interventions are needed to maximize communication depend largely on the individual family and their family communication norms, barriers, and needs (Gaff et al. 2007). Specific familial risk messaging for communication compared to generic ones may facilitate the process of communication in families. In addition, reconsideration of family communication of moderate- and high-risk variants may be necessary to accurately measure and ultimately improve family communication and cascade testing rates. We find that summary notes of moderate-risk gene variants were significantly less likely to specify at risk relatives, familial implications of family letters, and genetic test reports compared to summary notes of high-risk genes, which reflects the limited clinical utility of cascade testing for moderate-risk variants(Tung et al. 2016). However, high- and moderate-risk variants are often confounded in family communication literature, which primarily distinguishes variant classification rather than variant risk or penetrance (Gaff et al. 2007). Therefore, the suboptimal family communication rates may be partly explained by the reduced encouragement patients receive to communicate moderate-risk variants.
Most summary notes documented the use of covert rather than overt relational manners to counsel patients about family communication—a practice likely representing the non-directive approach in the field of genetic counseling. However, some summary notes documented use of overt relational manners which may indicate the evolving nature of the practice of genetic counseling. Direct advice to engage in family communication may result in better family communication outcomes and can be considered as “appropriate directiveness” (Clarke 1997) in genetic counseling. We also find that GCs frequently assessed patients’ family dynamics in an attempt to facilitate communication—a practice that is consistent with previous qualitative (Young et al. 2019) and quantitative studies (Forrest et al. 2010). Similarly, although determining the inheritance pattern of a family variant is necessary to find at risk relatives and to guide cascade genetic testing, greater emphasis on the potential cancer prevention benefits for relatives may be necessary to persuade patients to engage in family communication.
Logistics of cascade genetic testing including cost and insurance coverage for testing, and limited familiarity with the genetic counseling process, are significant barriers to test uptake that were frequently addressed by GCs (Kne et al. 2017). For example, cost of genetic testing is a commonly cited and significant barrier to cascade testing uptake even though single-site targeted testing for a familial variant is significantly less expensive than panel genetic testing and certain laboratories offer it free of charge when performed within 6 months of patient’s genetic test. Proactive communication of these financial considerations, referred to as “genesurance counseling” (Brown et al. 2018), may be important to improve cascade testing rates. In addition, counseling should specify that relatives will require a copy of the patient’s genetic test report in order to undergo cascade testing, as it allows providers’ to order the cheaper, targeted test. Assistance with scheduling GC appointments for relatives and locating genetic counseling services near relatives’ residence—both nationally and internationally—were other proactive strategies documented in this study. Future studies of the impact of these strategies on family communication and cascade genetic testing are warranted. In the absence of sweeping practice reform to address cascade testing (such as providers directly contacting relatives with patients’ results), consistent implementation of these small strategies in current practice may bring the incremental change necessary to improve family communication rates.
Summary notes are standard clinical practice tools in the field of genetic counseling (Baker et al. 2002) as patients’ knowledge about what information to share with relatives and whom to inform remain suboptimal (Eijzenga et al. 2018). While summary notes containing specific information about relatives who should be prioritized to undergo cascade genetic testing may help with family communication of genetic risk information, the extent to which patients access and read summary notes through patient portals remains unknown. Heterogeneity in content of summary notes written by different GCs may either reflect genuine practice differences or it may reflect GCs’ differing philosophies of the utility of writing detailed and specific notes. Additional investigation of the degree to which these notes accurately reflect the dialogue of the counseling session, varying styles, and their impact on familial communication is warranted.
There are limitations to consider in the current study. All summary notes in this study were obtained from a large well-resourced tertiary cancer hospital specialized in breast cancer, and findings may not generalize to other clinical settings. In order to better understand practice variations about family communication, we plan to replicate this study in four other geographically diverse community cancer hospitals that serve different patient populations. Although our analysis of summary note characteristics suggests that despite using templates, copying, or importing text, summary notes are somewhat representative of the genetic counseling appointments. However, there may still be discordance between the content of notes as documented within EMR and the content of actual GC-patient counseling encounters. Future studies using audio-recorded genetic counseling interactions are needed to explore these possible differences. Such audio-/video-recorded data may also provide insight into strategies (e.g., subtle nudges) that GCs use to address barriers to familial communication. We also plan to conduct follow-up analyses to determine the provenance of characters within a summary note (i.e., whether the character was manually entered, copied, or imported) to understand the tailoring of specific content within summary notes. By choosing the most comprehensive document for analysis and not the cumulative recorded documentation for a given patient, we may have missed some documented family communication. In addition, patient-GC communication documented within other databases and shadow charts may contain additional information about family communication which were missed in this study.
In conclusion, we sought to characterize family communication content of breast cancer genetic counseling summary notes and found that GCs consistently document family communication recommendations when pathogenic variants are identified on patients’ genetic testing, albeit with heterogeneous use of specific communication prompts. We identified several factors that have the potential to impact family communication including specificity of familial risk messaging, use of overt vs. covert relational manner, and high- vs. moderate-risk genes. These findings may inform design of interventions to improve the ease of familial communication of cancer genetic risk and ultimately cascade testing. Patients often find the onus of communicating genetic test result placed on them to be burdensome (Leenen et al. 2016), and intensified effort is needed to find provider-mediated strategies that may alleviate this burden from patients.
Author’s contributions
Concept and design: SM, HSS, SKP
Acquisition, analysis, or interpretation of data: SM, AB, LT
Drafting of the manuscript: All authors
Critical revision of the manuscript for important intellectual content: SM, EMB, HSS, RJV, SKP
Statistical analysis: SM
Administrative, technical, or material support: SM, BA, SKP
Supervision: RJV, BA, SKP
Funding
SM’s work is supported by a research training grant award from the Cancer Prevention and Research Institute of Texas – CPRIT (Award# RP170259). Erica M. Bednar’s work is supported by philanthropic contributions to The University of Texas MD Anderson Cancer Center Moon Shots Program, Cancer Prevention & Control Platform. This project was supported in part by a grant from NIH/NCI under award number P30CA016672 and used the Shared Decision Making Core.
Data Availability
Data is available from the corresponding author upon reasonable request.
Compliance with ethical standards
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The study was approved by the ethics committee of UT MD Anderson Cancer Center. This research study was conducted retrospectively from data obtained for clinical purposes.
Consent to participate
We obtained a waiver of informed consent for the study.
Code availability
No custom code or software was used for this study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Baker DL, Eash T, Schuette JL, Uhlmann WR. Guidelines for Writing Letters to Patients. J Genet Couns. 2002;11(5):399–418. doi: 10.1023/A:1016841731426. [DOI] [PubMed] [Google Scholar]
- Brown E, Skinner M, Ashley S, Reed K, Dixon SD. Assessment of the Readability of Genetic Counseling Patient Letters. J Genet Couns. 2016;25(3):454–460. doi: 10.1007/s10897-015-9890-0. [DOI] [PubMed] [Google Scholar]
- Brown S, Puumala S, Leonhard J, Bell M, Flanagan J, Dean LW, Stein Q. Genesurance Counseling: Genetic Counselors' Roles and Responsibilities in Regards to Genetic Insurance and Financial Topics. J Genet Couns. 2018;27(4):800–813. doi: 10.1007/s10897-017-0180-x. [DOI] [PubMed] [Google Scholar]
- Carey JWaG. D Systematic Methods for Collecting and Analyzing Multidisciplinary Team-Based Qualitative Data. In: G.a.M. Guest, K. M, editor. Handbook for Team-Based Qualitative Research. Lanham, MD: Altamira Press; 2008. pp. 227–274. [Google Scholar]
- Clarke AJ. Genetics, Society and Clinical Practice. Oxford: Bios Scientific Publishers; 1997. The process of genetic counselling: Beyond nondirectiveness; pp. 179–200. [Google Scholar]
- Counseling, A.B.o.G. (n.d.) Find a certified genetic counselor. [cited 2020 November 11]; Available from: https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor.aspx/.
- Dattilo TM, Lipak KG, Clark OE, Gehred A, Sampson A, Quinn G et al (2020) Parent-Child Communication and Reproductive Considerations in Families with Genetic Cancer Predisposition Syndromes: A Systematic Review. J Adolesc Young Adult Oncol [DOI] [PMC free article] [PubMed]
- Dheensa S, Lucassen A, Fenwick A. Limitations and Pitfalls of Using Family Letters to Communicate Genetic Risk: a Qualitative Study with Patients and Healthcare Professionals. J Genet Couns. 2018;27(3):689–701. doi: 10.1007/s10897-017-0164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijzenga W, de Geus E, Aalfs CM, Menko FH, Sijmons RH, de Haes H, et al. How to support cancer genetics counselees in informing at-risk relatives? Lessons from a randomized controlled trial. Patient Educ Couns. 2018;101(9):1611–1619. doi: 10.1016/j.pec.2018.05.009. [DOI] [PubMed] [Google Scholar]
- Finlay E, Stopfer JE, Burlingame E, Evans KG, Nathanson KL, Weber BL, Armstrong K, Rebbeck TR, Domchek SM. Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genet Test. 2008;12(1):81–91. doi: 10.1089/gte.2007.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes Shepherd R, Browne TK, Warwick L. A Relational Approach to Genetic Counseling for Hereditary Breast and Ovarian Cancer. J Genet Couns. 2017;26(2):283–299. doi: 10.1007/s10897-016-0022-2. [DOI] [PubMed] [Google Scholar]
- Forrest LE, Delatycki MB, Curnow L, Skene L, Aitken M. Genetic health professionals and the communication of genetic information in families: Practice during and after a genetic consultation. Am J Med Genet A. 2010;152a(6):1458–1466. doi: 10.1002/ajmg.a.33385. [DOI] [PubMed] [Google Scholar]
- Gaff CL, Clarke AJ, Atkinson P, Sivell S, Elwyn G, Iredale R, Thornton H, Dundon J, Shaw C, Edwards A. Process and outcome in communication of genetic information within families: a systematic review. Eur J Hum Genet. 2007;15(10):999–1011. doi: 10.1038/sj.ejhg.5201883. [DOI] [PubMed] [Google Scholar]
- Kne A, Zierhut H, Baldinger S, Swenson KK, Mink P, Veach PM, Tsai ML. Why Is Cancer Genetic Counseling Underutilized by Women Identified as at Risk for Hereditary Breast Cancer? Patient Perceptions of Barriers Following a Referral Letter. J Genet Couns. 2017;26(4):697–715. doi: 10.1007/s10897-016-0040-0. [DOI] [PubMed] [Google Scholar]
- Lee C, Rivera-Valerio M, Bangash H, Prokop L, Kullo IJ. New Case Detection by Cascade Testing in Familial Hypercholesterolemia: A Systematic Review of the Literature. Circ Genom Precis Med. 2019;12(11):e002723. doi: 10.1161/CIRCGEN.119.002723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenen CH, Heijer M, van der Meer C, Kuipers EJ, van Leerdam ME, Wagner A. Genetic testing for Lynch syndrome: family communication and motivation. Fam Cancer. 2016;15(1):63–73. doi: 10.1007/s10689-015-9842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ST, Sun S, Lie D, Met-Domestici M, Courtney E, Menon S, Lim GH, Ngeow J. Factors influencing the decision to share cancer genetic results among family members: An in-depth interview study of women in an Asian setting. Psychooncology. 2018;27(3):998–1004. doi: 10.1002/pon.4627. [DOI] [PubMed] [Google Scholar]
- Mendes Á, Paneque M, Sousa L, Clarke A, Sequeiros J. How communication of genetic information within the family is addressed in genetic counselling: a systematic review of research evidence. Eur J Hum Genet. 2016;24(3):315–325. doi: 10.1038/ejhg.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MC, Dotson WD, DeVore CS, Bednar EM, Bowen DJ, Ganiats TG, Green RF, Hurst GM, Philp AR, Ricker CN, Sturm AC, Trepanier AM, Williams JL, Zierhut HA, Wilemon KA, Hampel H. Delivery Of Cascade Screening For Hereditary Conditions: A Scoping Review Of The Literature. Health Aff (Millwood) 2018;37(5):801–808. doi: 10.1377/hlthaff.2017.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadder NJ, Riegert-Johnson D, Boardman L, Rhodes D, Wick M, Okuno S et al (2020) Comparison of Universal Genetic Testing vs Guideline-Directed Targeted Testing for Patients With Hereditary Cancer Syndrome. JAMA Oncol [DOI] [PMC free article] [PubMed]
- Sermijn E, Delesie L, Deschepper E, Pauwels I, Bonduelle M, Teugels E, de Grève J. The impact of an interventional counselling procedure in families with a BRCA1/2 gene mutation: efficacy and safety. Fam Cancer. 2016;15(2):155–162. doi: 10.1007/s10689-015-9854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Won NY, Dotson WD, Wright ST, Roberts MC. Barriers and facilitators for cascade testing in genetic conditions: a systematic review. Eur J Hum Genet. 2020;28:1631–1644. doi: 10.1038/s41431-020-00725-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung N, Domchek SM, Stadler Z, Nathanson KL, Couch F, Garber JE, Offit K, Robson ME. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588. doi: 10.1038/nrclinonc.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandenBoom E, Trepanier AM, Carmany EP. Assessment of Current Genetic Counselor Practices in Post-Visit Written Communications to Patients. J Genet Couns. 2018;27(3):681–688. doi: 10.1007/s10897-017-0163-y. [DOI] [PubMed] [Google Scholar]
- Young AL, Butow PN, Tucker KM, Wakefield CE, Healey E, Williams R. Challenges and strategies proposed by genetic health professionals to assist with family communication. Eur J Hum Genet. 2019;27(11):1630–1638. doi: 10.1038/s41431-019-0447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available from the corresponding author upon reasonable request.