Abstract
Congenital disorders (CD) remain an unprioritized health care issue in South Africa with national surveillance underreporting by > 95%. This lack of empiric data contributes to an underestimation of the CD disease burden, resulting in a lack of services for those affected. Modelling offers estimated figures for policymakers to plan services until surveillance is improved. This study applied the Modell Global Database (MGDb) method to quantify the South African CD disease burden in 2012. The MGDb combines birth prevalence data from well-established registries with local demographic data to generate national baseline estimates (birth prevalence and outcomes) for specific early-onset, endogenous CDs. The MGBd was adapted with local South African demographic data to generate baseline (no care) and current care national and provincial estimates for a sub-set of early-onset endogenous CDs. Access to care/impact of interventions was quantified using the infant mortality rate as proxy. With available care in 2012, baseline birth prevalence (27.56 per 1000 live births, n = 32,190) decreased by 7% with 2130 less affected births, with 5400 (17%) less under-5 CD-related deaths and 3530 (11%) more survivors at 5 years, including 4720 (15%) effectively cured and 1190 (4%) less living with disability. Results indicate a higher proportion of CD-affected births than currently indicated by national surveillance. By offering evidence-based estimates, the MGDb may be considered a tool for policymakers until accurate empiric data becomes available. Further work is needed on key CD groups and costing of specific interventions.
Keywords: Community genetics, Congenital disorders, Congenital anomalies, Birth defects, Rare diseases, Infant mortality rate, Modell Global Database, South Africa
Introduction
Surveillance is crucial to enable timely and appropriate public health interventions and is an integral part of health needs assessment (HNA) (Christianson et al. 2013). Public health surveillance includes the monitoring of communicable and non-communicable diseases (NCDs), health interventions, injuries, child growth and nutrition, and occupational health (Centers for Disease Control 1986; Declich and Carter 1994; Center for Disease Control and Prevention 2012). Even where no treatment interventions are available, surveillance helps prioritize and guide research (Hall et al. 2012). Public health surveillance also includes surveillance of congenital disorders (CDs), the first NCDs experienced by people (Christianson et al. 2013).
CDs, also known as birth defects, are a critical, common and costly health issue affecting all countries globally. CDs are defined as “abnormalities in structure or function present from birth, whether evident at birth or manifesting later in life” (World Health Organization 2006). They fall into two broad groups:
disorders with mainly endogenous causes (e.g. chromosomal and single-gene disorders, congenital malformations, and disorders with multifactorial inheritance), and
disorders caused by an abnormal foetal environment, such as the thalidomide tragedy of the late 1960s or the more recent Zika outbreak. Congenital anomaly registries were established following the thalidomide tragedy to identify clusters and offer early warning of specific teratogens: their surveillance function has subsequently expanded to establishing the prevalence of congenital anomalies, monitor trends and evaluate ongoing programmes (Christianson et al. 2013).
In high-income countries where the epidemiologic transition was completed decades ago, CDs are recognized as a leading cause of death in childhood (Malherbe et al. 2015; Matthews et al. 2015; World Health Organization 2015). Although robust CD monitoring and surveillance systems have developed in many countries over the past 40 years, obtaining comprehensive, standardized data remains a challenge (Luquetti and Koifman 2011). In middle and low-income countries (MLICs) the primary focus has hitherto been upon communicable diseases, resulting in a scarcity of reliable epidemiological data on CDs, due to insufficient diagnostic capacity and resources for accurate diagnosis, and inadequate or absent surveillance systems (World Health Organization 1999; Christianson and Modell 2004; Christianson et al. 2006). The resulting data shortfall skews national and global estimates of CDs and results in a serious underestimation of their significance as a health care issue (World Health Organization 1999; Nippert et al. 2013). Without an evidence base to highlight CDs as a health care priority, policy development is impeded, preventing those affected by or at risk of CDs from receiving the care they require.
In 2006, the March of Dimes estimated the minimum global birth prevalence of endogenous CDs in the absence of intervention as approximately 40 per 1000 live births, with around half of these due to congenital anomalies (obvious structural abnormalities)1 (Czeizel and Sankaranarayanan 1984, World Health Organization 1985, Baird et al. 1988, World Health Organization 1992, Christianson et al. 2006, Moorthie et al. 2018). Updated country-specific estimates for South Africa indicate a baseline birth prevalence of 32.5 per 1000 births for endogenous, early-onset CDs (Modell et al. 2016)2. When combined with an estimate for CDs due to adverse foetal environment of 14–15 per 1000 live births in 2010–2014 (Christianson 2012), this indicates a minimum expected birth prevalence of around 49 per 1000 or one in 20 births. This translates to 55,000 births affected annually in South Africa by a serious CD3 (Christianson 2012).
Data collected via national surveillance in South Africa do not reflect these expected figures. Only 13,252 CD cases were reported between 2006 and 2014, an average of only 1472 per year, suggesting under-reporting of over 95% of annually expected cases (Malherbe et al. 2015; Lebese et al. 2016). According to the Perinatal Problem Identification Programme (PPIP), a neonatal death audit database, 2151 (12.6%) neonatal deaths for babies weighing over 1000 g were attributed to congenital anomalies from 2014 to 2016 in South Africa (National Perinatal Morbidity and Mortality Committee 2017). By including only congenital anomalies in this audit, the contribution of total CDs is underestimated, and many deaths due to invisible anomalies and functional disorders remain undiagnosed and uncounted and are misallocated to other causes of death, such as prematurity (Malherbe et al. 2018a, b). The total, as yet, undocumented, accurate burden of all CDs is likely much higher (Malherbe et al. 2018a, b). Widespread under-reporting of CDs in South Africa is attributed to failure to diagnose, misdiagnosis, inadequate surveillance systems, and the persisting parallel burden of infectious disease masking the CD burden as the country continues to transition epidemiologically (Christianson and Modell 2004; Kahn et al. 2007; Debas et al. 2015; Malherbe et al. 2015).
Observed empiric data in South Africa currently provides an insufficient basis for the development of appropriate policy and services for the diagnosis, care and prevention of CDs. Given this, evidence-based estimates may be used provisionally to quantify and communicate the represented CD health burden to policymakers - and enable appropriate services and resources to be developed and allocated in response. This study was undertaken to explore this option for South Africa.
The role of modelled epidemiologic data for public health policy
Continuous surveillance is required to follow the epidemiology of CDs with environmental causes. By contrast, it is feasible to generate evidence-based estimates for endogenous CDs since their baseline birth prevalence (i.e. prevalence in the absence of intervention) can usually be calculated from biological first principles (Malherbe et al. 2018a, b). The birth prevalence of most congenital malformations is similar in most populations; that of chromosomal disorders is related to maternal age distribution; the minimum birth prevalence of rare single-gene disorders is determined by the balance of new mutation and natural selection and so is similar globally, while that of recessive disorders is related to parental consanguinity (Moorthie et al. 2018c). Country-specific estimates are also available for haemoglobin disorders, rhesus haemolytic disease of newborns and G6PD deficiency. These rates may be combined with key demographic data to model the baseline birth prevalence of endogenous CDs for any country or population.
Baseline birth prevalence is relatively constant over time. It provides a measure of the scale of the problem, and a benchmark against which to evaluate the likely quality of available surveillance data and the impact of current interventions. Once baseline birth prevalence is known, associated mortality, disability and the effects of current interventions (care) can be modelled using (a) historical reports of outcomes with no or minimal care, (b) observed outcomes with current care in high resource settings (optimal care) and (c) a country-specific estimate of access to services (Modell et al. 2018a, b, c, Moorthie et al. 2018a, b, c, Blencowe et al. 2018a).
This “biological first principles” approach was first applied in the March of Dimes Global Report on Birth Defects (Christianson et al. 2006). This combined birth prevalence data from classical surveillance systems and demographic data to generate provisional country-specific estimates for the baseline birth prevalence of early-onset CDs with endogenous causes, and their outcomes4 in the absence of care. An updated model, the Modell Global Database of Congenital Disorders (MGDb), now also includes estimates for outcomes with optimal care5, actual outcomes, and the impact of available interventions (reduction of affected pregnancies and births through folic acid food fortification, anti-D for Rh-negative mothers, genetic risk detection and counselling, prenatal diagnosis with the option of termination of pregnancy (TOP) and early diagnosis and care) (Modell et al. 2018a, b, c; Moorthie et al. 2018a, b, c). The MGDb model shows that with full access to these interventions at least 50–70% of CDs can be prevented or effectively treated depending on the specific category of CD, confirming Czeizel’s earlier estimate (Czeizel et al. 1993; World Health Organization 1996; Alwan and Modell 2003).
The MGDb national estimates use whole-country demographic data published by United Nations World Population Prospects (WPP) (United Nations Department of Economic and Social Affairs 2019). However, using WPP data is clearly unsatisfactory for large countries with culturally and economically diverse populations and inequitable access to services such as South Africa. Therefore, a long-term intention has been to develop sub-national estimates by applying the MGDb Method to locally sourced demographic data to generate a refined national picture of annual affected births and their outcomes. Accordingly, in MGDb South Africa (MGDb-ZA) we have applied the MGDb Method to model baseline and actual birth prevalence and outcomes for early-onset, endogenous CDs at national and provincial levels for South Africa in 2012, and to assess the present effect of available interventions. We consider that these modelled estimates constitute an evidence-based tool for public health policy- and decision-makers to use in service planning, and a comparator for emerging and future empiric data.
Method
This desktop, data analysis study was conceptualized in October 2014 at a consultative meeting at the Centre for Health Informatics and Multiprofessional Education (CHIME), University College London (UCL), and was conducted in South Africa under the auspices of the University of KwaZulu Natal6. MGDb-ZA was adapted from MGDb version 2017/18 (Modell 2017) provided in Microsoft Excel spreadsheets. Integrated formulae updated automatically in the spreadsheets as South African demographic data relevant for 2012 were added, generating national and provincial estimates. Demographic data for 2012 was used in this study to generate estimates for 2012, the most recent year for which relevant data were available at study initiation, and offering an appropriate baseline for future work following the stagnation of child mortality rates in the country since 2011 (Dorrington et al. 2020).
The MGDb Method involves the following main steps:
Selection of disorders for inclusion, with estimated baseline birth prevalence;
Estimation of outcomes in the absence of care and with optimal care;
Identification of local demographic data;
Estimation of local access to services;
Calculation of actual birth prevalence and outcomes in the selected year;
Estimation of years of life lost (YLL) or years lived with disability (YLD), due to CDs.
The Guidelines for Accurate and Transparent Health Estimates Reporting were followed as far as possible (Stevens et al. 2016).
Conditions included and baseline birth prevalence
MGDb includes early-onset endogenous CDs present before the age of 20 and which cause death or disability in the absence of intervention (Moorthie et al. 2018a, b, c). Table 1 shows the disorder groups included in MGDb-ZA, their estimated baseline birth prevalence and relevant sources. The South African selection of CDs takes into account the local situation and differs somewhat from that in the original MGDb (Modell et al. 2016; Moorthie et al. 2018a, b, c). The MGDb-ZA common single-gene disorders category does not include haemoglobin disorders which are uncommon locally but does include oculocutaneous albinism, the most common single-gene disorder in South Africa (Kromberg and Jenkins 1982). Two early-onset disorders due to common risk factors are also not included: GPD6 deficiency because of low local prevalence, and rhesus haemolytic disease because of lack of adequate data available at the time of this study.
Table 1.
Endogenous congenital disorders included in MGDb-ZA, estimated baseline live birth prevalence, characteristics, proportion of disorder group and total, South Africa 2012
| Congenital disorder group | Prevalence characteristics | Source of estimated birth prevalence | Affected per1000 live births (SA) | % of total in MGDb-ZA | % of disorder group |
|---|---|---|---|---|---|
| Rare single-gene disorders | |||||
| Baseline rare single-gene disorders | Constant | (Stevenson 1959, Trimble and Doughty 1974, Carter 1977, Baird et al. 1988) | 4.27 | 16.3 | 91.0 |
| Consanguinity-associated | Population-specifica | (Bundey and Alam 1993, Bittles and Neel 1994, Blencowe et al. 2018b) | 0.17 | 0.6 | 3.6 |
| Common single-gene disorders | |||||
| Oculocutaneous Albinism | Population-specific | (Kromberg and Jenkins 1982) | 0.25 | 1.0 | 5.3 |
| Total single-gene disorders | 4.69 | 17.9 | 100 | ||
| Chromosomal disorders | |||||
| Down syndrome | Population-specificb | (Moorthie et al. 2018a, b, c) | 1.73 | 6.6 | 45.8 |
| Other trisomiesc | Population-specificd | (Moorthie et al. 2018a, b, c) | 0.33 | 1.3 | 8.7 |
| Rare chromosomal | Constant | (Wellesley et al. 2012) | 0.67 | 2.6 | 17.7 |
| Turner syndrome | Constant | (EUROCAT 2015) | 0.18 | 0.7 | 4.8 |
| Klinefelter syndrome | Constant | (Visootsak and Graham 2006, Morris et al. 2008) | 0.87 | 3.3 | 23.0 |
| Total chromosomal disorders | 3.78 | 14.4 | 100 | ||
| Isolated malformationse | |||||
| Congenital heart diseasef | Constant | (EUROCAT 2009, Tennant et al. 2010, Wren et al. 2012) | 3.30 | 12.6 | 18.7 |
| Neural tube defects | Population-specific | (Sayed et al. 2008) | 0.90 | 3.4 | 5.1 |
| Oral facial clefts | Population-specific | (Mossey and Little 2002, EUROCAT 2015) | 0.24 | 0.9 | 1.4 |
| Very severe other malformationsg | Constant | (EUROCAT 2015, Moorthie et al. 2018a, b, c) | 7.00 | 26.8 | 39.6 |
| Less severe other malformationsh | Constant | (EUROCAT 2015, Moorthie et al. 2018a, b, c) | 5.15 | 19.7 | 29.1 |
| Three additional conditionsi | Population-specific | (Modell and Modell 1992) | 1.10 | 4.2 | 6.2 |
| Total isolated malformations | 17.69 | 67.6 | 100 | ||
| Total disorders included in MGDb ZA | 26.19 | 100 | 100 | ||
aEquation: Consanguinity associated/1,000 = Population F x 100 x 6.5 (Blencowe et al. 2018b).
bEquation: (0.834 + (% mothers 35plus x 0.067)) x 1.053 (Moorthie et al. 2018a).
cEdwards and Patau syndromes (Trisomy 18 and 13) are grouped together due to similar outcomes.
dEquation: equivalent to 41% of Down syndrome /1,000 (Moorthie et al. 2018a).
eIsolated malformations i.e. not associated with a chromosomal disorder or genetic syndrome or a malformation in another system group.
fCongenital heart defects that present before 20 years of age and would cause premature death or disability in the absence of intervention (Moorthie et al. 2018c).
gPotentially fatal other malformations in absence of care: CNS not NTD, eye, ear, face and neck, respiratory, digestive, abdominal wall defects, urinary system, multiple malformations.
hPotentially non-fatal malformations in the absence of care: genital and limb.
iThree potentially lethal isolated malformations not included in most congenital anomaly registries but that are preventable or curable (thyroid aplasia/hypoplasia, prematurity-related persistent patent ductus arteriosus, pyloric stenosis) are combined as a single category due to relatively weak evidence for local birth prevalence
The CD groups not included in MGDb-ZA are largely responsible for differences between populations. Therefore, in the case of South Africa, there is little difference in baseline affected birth prevalence between provinces.
Estimation of outcomes with no care and with optimal care
Table 2 shows the birth prevalence of the selected disorders in South Africa, with MGDb-ZA estimates of the distribution of outcomes and mean life expectancy for each disorder group in the absence of care and with 100% optimal care. These outcome rates can be used together with estimated access to services to estimate actual outcomes in any given year.
Table 2.
Estimated baseline birth prevalence of endogenous congenital disorders in South Africa, with proportionate outcomes in the absence of care and with optimal care. Deaths under-5 due to other causes are included in calculations but excluded from this table. The total of the proportions does not equal 100% due to the exclusion of U5 deaths from other causes
| Congenital disorder group | SA: baseline affected/1000 births | No-care % outcomes | Liveborn mean life expectancy (years) | % outcomes with optimal care | Liveborn mean life expectancy (years) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % affected stillbirths | % U5 deaths (CDs) | % survivors @ 5 years (disabled) | % affected births prevented | % TOP | % Affected Stillbirths | % U5 deaths (CDs) | % survivors @ 5 years (cured) | % survivors @ 5 years (disabled) | ||||
| Baseline rare single-gene disorders | 4.45 | 4.14 | 71.55 | 21.32 | 4.4 | 0.00 | 8.05 | 3.66 | 32.32 | 0.00 | 52.71 | 23.6 |
| Consanguinity increment | 0.2 | 15.00 | 67.37 | 12.67 | 6.7 | 0.00 | 12.57 | 14.20 | 27.21 | 0.00 | 49.97 | 28.1 |
| Oculocutaneous Albinism | 0.25 | 0.00 | 0.00 | 94.91 | 56.5 | 0.00 | 0.00 | 0.00 | 0.63 | 0.00 | 94.90 | 73.4 |
| Total single-gene disorders | 4.9 | 4.38 | 67.73 | 24.72 | 22.53 | 0.00 | 7.83 | 3.90 | 30.50 | 0.00 | 54.75 | 41.7 |
| Down syndrome | 1.82 | 4.99 | 57.32 | 35.09 | 7.7 | 0.00 | 30.64 | 3.46 | 3.90 | 0.00 | 61.76 | 50.6 |
| Other trisomies | 0.74 | 55.09 | 44.60 | 0.00 | 0.1 | 0.00 | 83.13 | 9.42 | 7.60 | 0.00 | 0.00 | 0.1 |
| Rare chromosomal | 0.73 | 8.52 | 59.16 | 30.54 | 6.9 | 0.00 | 50.54 | 4.22 | 11.94 | 0.00 | 33.37 | 45.6 |
| Turner syndrome | 0.22 | 21.38 | 1.87 | 75.04 | 56.8 | 0.00 | 73.94 | 5.85 | 0.18 | 0.00 | 21.84 | 67.8 |
| Klinefelter syndrome | 0.9 | 2.90 | 0.84 | 91.91 | 66.4 | 0.00 | 0.00 | 2.90 | 0.84 | 0.00 | 96.23 | 66.4 |
| Total chromosomal disorders | 4.41 | 14.37 | 41.20 | 42.04 | 27.58 | 0.00 | 38.65 | 4.59 | 5.04 | 0.00 | 51.74 | 46.1 |
| Congenital heart disease | 3.33 | 0.96 | 79.47 | 17.78 | 14.2 | 4.94 | 2.74 | 0.86 | 8.30 | 65.13 | 14.19 | 57.0 |
| Neural tube defects | 1.16 | 22.33 | 73.57 | 2.96 | 0.3 | 19.73 | 41.30 | 4.72 | 10.27 | 0.00 | 22.62 | 27.5 |
| Oro-facial Clefts | 0.25 | 1.45 | 76.55 | 16.63 | 4.4 | 8.53 | 1.92 | 1.29 | 1.84 | 69.59 | 9.49 | 73.0 |
| Very severe other malformations | 7.22 | 2.87 | 78.77 | 16.50 | 9.9 | 0.00 | 15.43 | 2.25 | 15.10 | 47.80 | 16.11 | 55.1 |
| Less severe other malformations | 5.19 | 0.67 | 4.66 | 90.27 | 72.2 | 0.00 | 3.72 | 0.64 | 2.17 | 84.70 | 4.46 | 75.1 |
| Three additional conditions | 1.1 | 0.00 | 75.26 | 21.60 | 8.7 | 0.00 | 0.00 | 0.00 | 0.00 | 95.51 | 0.00 | 80.0 |
| Total isolated congenital malformations | 18.25 | 2.94 | 57.25 | 37.16 | 18.28 | 2.27 | 10.31 | 1.55 | 8.78 | 61.59 | 11.80 | 61.28 |
| Total South Africa | 27.56 | 5.03 | 56.54 | 35.73 | 22.51 | 1.51 | 14.41 | 2.45 | 12.05 | 40.79 | 25.83 | 51.66 |
Acquisition of local demographic data
Table 3 outlines the demographic data used. Identifying optimal sources of local data in South Africa required considerable effort due to incomplete vital registration data at the District level. Provincial-level data adjusted for incompleteness was therefore sourced from the CARe projection model developed by the Centre for Actuarial Research, University of Cape Town (Personal Communication (email), Prof R Dorrington, August 2016). These locally sourced country indicators used in MGDb-ZA are compared with equivalent WPP country indicators in Table 3. Though there is generally good correspondence between these data, WPP estimates for infant and under-5 mortality are significantly higher than local estimates. This is an important difference since infant mortality is used to estimate access to services7. Table 4 details locally sourced demographic input data by province. All births and under-5 deaths occurring in all nine South African provinces were included for the 2012 vital registration year.
Table 3.
Demographic data indicators required for the MGDb Method and comparison of local data with United Nations World Population Prospects (UN WPP) data indicators
| Demographic indicator | Data source | Civil division | National total/rate | WPP 2010–2014 | WPP /MGDb-ZA |
|---|---|---|---|---|---|
| Population (1000s) | CARe projection modelj | Provincial | 52,261 | 52,837 | 1.01 |
| Annual live births (1000s) | CARe projection modelj | Provincial | 1169 | 1115 | 0.95 |
| Infant mortality rate (per 1000 LB) | CARe projection modelj | Provincial | 28.3 | 38.3 | 1.35 |
| Under-5 mortality rate (per 1000 LB) | CARe projection modelj | Provincial | 46 | 50.8 | 1.10 |
| Mean life expectancy: male & female (years) | CARe projection modelj | Provincial | 62 | 57.1 | 0.92 |
| Total fertility rate | (Dorrington and Moultrie 2015) | Provincial | 2.5 | 2.4 | 0.96 |
| Sex ratio at birth | CARe projection modelj | Provincial | 1.02 | 1.03 | 1.01 |
| Stillbirth rate (per 1000 total births) | (Cousens et al. 2011) | National | 20.4 | – | – |
| Neonatal mortality rate (per 1000 LB) | Estimated at 40% of IMR | Provincial | 11.3 | 11.3 | 1.00 |
| Crude birth rate | CARe projection modelj | Provincial | 21.9 | 21.1 | 0.96 |
| Percentage urbanized | CARe projection modelj | Provincial | 63.0% | 62.2% | 0.99 |
| Percentage mothers aged 35 plusk | CARe projection modelj | Provincial | 13.3% | 11.6% | 0.87 |
| Coefficient of consanguinity (F)l | (Stevenson et al. 1966, Bundey and Alam 1993, Bittles and Black 2015, Blencowe et al. 2018a) | National | 0.00033 | – | – |
j Personal Communication (email), Prof R Dorrington, Centre for Actuarial Research, University of Cape Town, August 2016
k Percentage of mothers aged 35+ is required for calculating estimates for chromosomal disorders
l Coefficient of consanguinity and HIV/AIDS-related mortality are used to adjust the IMR for calculating access to services (Modell et al. 2016)
Table 4.
Demographic input data for South African Provinces ranked in ascending order of infant mortality rate (IMR) and estimated access to services
| Province | Population (1000s) | Births (1000s) | % of total national Births | Crude birth rate | IMR per 1000 live births | U5MR per 1000 live births | Total fertility rate | Urbanized (%) | % mothers 35 plus | Mean life expect. male & female | IMR adjusted for HIV & consanguinity | Estimated % access to services |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Western Capem | 5848 | 125.0 | 10.7 | 20.1 | 14.8 | 24.0 | 2.3 | 92.0 | 13.6% | 68.9 | 13.3 | 79% |
| Limpopo | 5533 | 138.3 | 11.8 | 25.3 | 21.5 | 36.0 | 3.0 | 18.0 | 13.4% | 67.0 | 19.3 | 44% |
| Gauteng | 12,500 | 281.8 | 24.1 | 21.0 | 22.2 | 36.0 | 2.3 | 97.0 | 14.0% | 63.9 | 19.3 | 44% |
| Northern Cape | 1121 | 22.8 | 2.0 | 20.6 | 25.4 | 37.0 | 2.5 | 76.0 | 13.0% | 64.6 | 23.6 | 32% |
| North West | 3595 | 80.0 | 6.8 | 21.9 | 25.5 | 40.0 | 2.7 | 44.0 | 13.7% | 61.9 | 22.7 | 34% |
| Mpumalanga | 4001 | 93.5 | 8.0 | 21.9 | 32.9 | 57.0 | 2.6 | 43.0 | 12.7% | 59.6 | 28.6 | 24% |
| Eastern Cape | 6598 | 131.1 | 11.2 | 20.3 | 34.8 | 55.0 | 2.5 | 46.0 | 13.4% | 57.9 | 31.5 | 20% |
| KwaZulu Natal | 10,323 | 235.9 | 20.2 | 22.5 | 38.3 | 60.0 | 2.5 | 48.0 | 12.2% | 57.7 | 34.3 | 18% |
| Free State | 2742 | 60.7 | 5.2 | 21.5 | 38.3 | 59.0 | 2.5 | 84.0 | 13.9% | 59.2 | 35.3 | 17% |
| South Africa | 52,261 | 1169.1 | 100.0 | 21.9 | 28.3 | 46.0 | 2.5 | 63.0 | 13.3% | 62.0 | 25.3 | 29% |
m Western Cape is a reference province for what could be achieved with universal equitable access to health services because of high level of access to services in the province – thus all province-specific tables are ranked in descending order of IMR, the indicator used as the basis for the access to care calculation in MGDb
Estimation of access to services
MGDb uses the infant mortality rate (IMR) as a proxy for access to relevant health services (Blencowe et al. 2018a)8. Access is estimated according to the following equation using the BETA.DIST function in Microsoft Excel:
To improve the estimate of access to care—the MDGb subtracts infant deaths due to known, unrelated additional factors such as HIV/AIDS or parental consanguinity from total infant mortality, to obtain an adjusted IMR for use in the above equation (Johnson et al. 2016; Blencowe et al. 2018a). In South Africa, the HIV/AIDS epidemic has caused significant infant mortality. Therefore, MGDb-ZA made use of HIV/AIDS-adjusted IMRs provided locally by the CARe projection model, resulting in a deduction from total IMR of an average of 2.68 per 1000 births, ranging provincially from 4.23 per 1000 (Mpumalanga) to 1.45 per 1000 (Western Cape). Due to the limited consanguinity data available, consanguinity-associated infant mortality was calculated using a national consanguinity coefficient of 0.0003: this resulted in a relatively minor deduction of 0.06 per 1000 live births from all IMR. Resulting provincial estimates of access to services for South Africa in 2012 are included in Table 4.
Calculation of actual birth prevalence and outcomes
MGDb-ZA combines estimated access to care in 2012, and estimated outcomes with no care and with optimal care to generate actual outcomes at the national level and as far as possible, at provincial levels. The difference between actual estimates and estimates for the no-care situation constitutes an assessment of the current effects of available interventions.
Calculation of years of life lost or lived with disability
Health burden is classically described in terms of years of Disability Adjusted Life Years (DALYS) which are the sum of the number of YLL due to a specific disorder plus YLD as a result of the disorder (Czeizel et al. 1990; Lopez and Mathers 2006). In MGDb-ZA, disorder-specific mean local life expectancy with no care and optimal care can be used to estimate actual YLL and YLD (Table 2) (Modell et al. 2016; Moorthie et al. 2018a, b, c).
For comparison between populations, YLL and YLD are usually expressed as rates per 100 000 population. However, use of the whole population as a denominator for disorders that are present at birth has the limitation that the result is affected by population age-distribution, so that the same affected birth prevalence results in higher rates with a young than with a mature population age distribution—i.e. leads to higher YLL and YLD per 100,000 in low resource settings. The MGDb-ZA, therefore, uses annual births as a denominator and expresses the result in terms of YLL or YLD in the relevant birth cohort. This produces a more consistent measure of the health burden of this disorder group. It may also assist the reader in grasping the implications when results are expressed in terms of the average effect on all members of the population.
Results
Estimated annual affected births and outcomes with no care and with current care
The baseline (no care) national birth prevalence of 27.56 per 1000 total births (Table 2) corresponds to an estimated 32,190 CD-affected births in South Africa 2012 (Tables 5 and 6).
Table 5.
Estimated baseline and actual outcomes with access to care and difference from no care situation for South Africa 2012 (numbers rounded to nearest multiples of 5). The total of the proportions do not equal 100% due to the exclusion of U5 deaths from other causes in this table
| Congenital disorder group | Baseline Births 2012 | Outcomes with no care | Estimated outcomes with current care 2012 | Change with current care | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Still-births | U5 Deaths (CDs) | Survivors @ 5 years (disabled) | Affected births prevented | TOP | Actual births | Still-births | U5 Deaths (CDs) | Survivors @ 5 years (cured) | Survivors @ 5 years (disabled) | Affected births | Still-births | U5 deaths (CDs) | Survivors @ 5 years (cured) | Survivors @5 years (disabled) | ||
| Single-gene disorders | ||||||||||||||||
| Baseline single-gene | 5210 | 215 | 3720 | 1110 | 0 | 145 | 5065 | 205 | 2965 | 0 | 1725 | − 145 | − 10 | − 755 | 0 | 615 |
| Consanguinity increment | 230 | 35 | 160 | 30 | 0 | 10 | 220 | 35 | 120 | 0 | 60 | − 10 | 0 | − 40 | 0 | 30 |
| Oculocutaneous albinism | 290 | 0 | 0 | 275 | 0 | 0 | 290 | 0 | 0 | 0 | 275 | 0 | 0 | 0 | 0 | 0 |
| Total single gene | 5730 | 250 | 3880 | 1415 | 0 | 155 | 5575 | 240 | 3085 | 0 | 2060 | − 155 | − 10 | − 795 | 0 | 645 |
| Chromosomal disorders | ||||||||||||||||
| Down syndrome | 2125 | 105 | 1220 | 745 | 0 | 235 | 1890 | 95 | 810 | 0 | 930 | − 235 | − 10 | − 410 | 0 | 185 |
| Other trisomies | 865 | 475 | 385 | 0 | 0 | 260 | 610 | 335 | 270 | 0 | 0 | − 255 | − 140 | − 115 | 0 | 0 |
| Rare chromosomal | 855 | 75 | 505 | 260 | 0 | 155 | 700 | 60 | 360 | 0 | 265 | − 155 | − 15 | − 145 | 0 | 5 |
| Turners syndrome | 260 | 55 | 5 | 195 | 0 | 70 | 195 | 40 | 5 | 0 | 145 | − 65 | − 15 | 0 | 0 | − 50 |
| Klinefelter syndrome | 1050 | 30 | 10 | 965 | 0 | 0 | 1050 | 30 | 10 | 0 | 965 | 0 | 0 | 0 | 0 | 0 |
| Total chromosomal | 5155 | 740 | 2,125 | 2165 | 0 | 720 | 4 445 | 560 | 1455 | 0 | 2305 | − 710 | − 180 | − 670 | 0 | 140 |
| Malformations (isolated) | ||||||||||||||||
| Congenital heart disease | 3895 | 35 | 3095 | 690 | 190 | 40 | 3660 | 35 | 2005 | 910 | 620 | − 235 | 0 | − 1090 | 910 | − 70 |
| Neural tube defects | 1355 | 305 | 1000 | 40 | 270 | 200 | 885 | 180 | 565 | 0 | 130 | − 470 | − 125 | − 435 | 0 | 90 |
| Oral facial clefts | 280 | 5 | 225 | 50 | 25 | 0 | 260 | 5 | 135 | 75 | 40 | − 20 | 0 | − 90 | 75 | − 10 |
| Very severe other | 8430 | 240 | 6650 | 1395 | 0 | 465 | 7965 | 225 | 4725 | 1450 | 1380 | − 465 | − 15 | − 1925 | 1450 | − 15 |
| Less severe other | 6060 | 40 | 285 | 5475 | 0 | 80 | 5980 | 40 | 230 | 1845 | 3605 | − 80 | 0 | − 55 | 1845 | − 1870 |
| Additional conditions | 1285 | 0 | 970 | 280 | 0 | 0 | 1290 | 0 | 630 | 440 | 180 | 5 | 0 | − 340 | 440 | − 100 |
| Total malformations | 21,305 | 625 | 12,225 | 7 930 | 485 | 785 | 20,040 | 485 | 8290 | 4720 | 5955 | − 1265 | − 140 | − 3935 | 4720 | − 1975 |
| Total CDs | 32,190 | 1615 | 18,230 | 11,510 | 485 | 1660 | 30,060 | 1285 | 12,830 | 4720 | 10,320 | − 2130 | − 330 | − 5 400 | 4720 | − 1190 |
| Proportion (%) | 100% | 5% | 57% | 36% | 2% | 5% | 93% | 4% | 40% | 15% | 32% | − 7% | − 1% | − 17% | 15% | − 4% |
Table 6.
Baseline (no care) and actual (with current care) outcomes and reduction in perceived adverse outcomes (stillbirths, U5 deaths, disability), South African provinces, 2012 (numbers rounded to nearest multiples of 5)
| Province | Baseline affected births (total) | Baseline outcome (no care) numbers | Actual outcome (with care) numbers | CDs, % of U5MR | % reduction in adverse outcomes (perceived) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stillbirths | U5 deaths (CDs) | U5 deaths (other causes) | Survivors @ 5 years (disabled) | Affected births prevented | TOP | Stillbirths | U5 deaths (CDs) | U5 Deaths (other causes) | Survivors @ 5 years (disabled) | Survivors @ 5 years (cured) | ||||
| Western Cape | 3450 | 175 | 1965 | 50 | 1260 | 50 | 390 | 100 | 725 | 60 | 975 | 1140 | 24% | 48% |
| Limpopo | 3810 | 190 | 2165 | 80 | 1375 | 55 | 245 | 145 | 1390 | 90 | 1200 | 685 | 28% | 28% |
| Gauteng | 7780 | 395 | 4415 | 165 | 2805 | 115 | 485 | 295 | 2840 | 180 | 2460 | 1400 | 28% | 28% |
| North West | 2205 | 110 | 1250 | 50 | 795 | 35 | 110 | 90 | 900 | 55 | 715 | 305 | 28% | 23% |
| Northern Cape | 625 | 30 | 355 | 15 | 225 | 10 | 30 | 25 | 260 | 15 | 205 | 80 | 31% | 22% |
| Mpumalanga | 2570 | 130 | 1450 | 85 | 905 | 40 | 85 | 110 | 1155 | 90 | 845 | 245 | 22% | 18% |
| Eastern Cape | 3610 | 180 | 2035 | 115 | 1280 | 55 | 105 | 155 | 1680 | 120 | 1200 | 295 | 23% | 16% |
| KwaZulu Natal | 6470 | 320 | 3640 | 230 | 2280 | 100 | 165 | 280 | 3070 | 235 | 2160 | 460 | 22% | 15% |
| Free State | 1675 | 85 | 940 | 60 | 590 | 25 | 40 | 75 | 800 | 60 | 560 | 115 | 22% | 14% |
| South Africa | 32,195n | 1615 | 18 215 | 850 | 11,515 | 485 | 1655 | 1275 | 12,820 | 905 | 10,320 | 4725 | 24% | 24% |
| Proportion | 100% | 5% | 57% | 3% | 36% | 2% | 5% | 4% | 40% | 3% | 32% | 15% | ||
nDifference in total from Table 5 due to rounding
Outcomes with no care (baseline)
In the absence of care (Table 5), 1615 or 5% of total affected births would be stillborn, the majority due to chromosomal disorders, particularly trisomy 13 and 18. Of affected live births, 18,230 (57%) would die under-5 from CD-related causes, of which two-thirds would be from congenital malformations. All 11,510 survivors at 5 years would live with some form of disability.
Outcomes with current care (actual)
Access to care in South Africa 2012 was estimated at 29%. This level of care is estimated to have the following effects on baseline outcomes:
A decrease of 2 130 (7%) affected births, with an estimated 485 converted to unaffected pregnancies and 1660 avoided through pre-pregnancy interventions, prenatal diagnosis (PND), genetic counselling and choice of TOP. Proportionately, the greatest reductions are for NTDs (35%) and other trisomies (30%).
A decrease of 330 (1%) stillbirths, with the greatest reductions estimated for other trisomies and NTDs.
A decrease of 5 400 (17%) in CD-related under-5 deaths, including a 30% reduction in deaths due to NTDs, OFCs and CHDs.
Approximately 15,040 survivors at age 5, an increase of 3530 (11%) compared with baseline (no care) estimates. Of these, around 4720 would be effectively cured (isolated malformations only) and 10,320 would be living with a disability.
A 1190 (4%) reduction in survivors with disability.
With access to current care, total adverse outcomes (stillbirth, disability and death under-5) are reduced by 24%, including a decrease of around 4% in survivors with a disability at age 5 compared with the no-care situation (Table 6). The increase in survivorship is largely due to improved survival for isolated malformations: the proportion of single-gene and chromosomal disorders surviving with disability either remaining unchanged or increasing in comparison with baseline estimates (Table 6).
Provincial outcomes
Provincial baseline (no care) and actual (with care) estimates are detailed in Tables 6 and 7. Little difference was observed between the provinces for baseline birth outcomes, and the provincial distribution of affected births is proportional to annual births in each province, with most occurring in Gauteng Province (GP) and least in Northern Cape (NC).
Table 7.
Difference between baseline and actual estimates, numerical and proportional in South African provinces, 2012
| Province | Difference from baseline (no-care) estimates (number) | Reduction (%) | Increase (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline affected births | Actual affected births (LB & SB) | Affected stillbirths | U5 deaths (CDs) | U5 deaths (other causes) | Survivors @5 years (disability) | Survivors @ 5 years (effective cure) | Affected births prevented | Affected stillbirths | U5 deaths (CDs) | Survivors @ 5 years (disability) | U5 deaths (other) | Survivors @ 5 years (cured) | |
| Western Cape | 3450 | − 442 | − 75 | − 1240 | 10 | − 285 | 1140 | − 13% | − 2% | − 36% | − 8% | 0% | 33% |
| Limpopo | 3810 | − 305 | − 45 | − 775 | 10 | − 175 | 685 | − 8% | − 1% | − 20% | − 5% | 0% | 18% |
| Gauteng | 7780 | − 602 | − 100 | − 1575 | 15 | − 345 | 1400 | − 8% | − 1% | − 20% | − 4% | 0% | 18% |
| North West | 2205 | − 142 | − 20 | − 350 | 5 | − 80 | 305 | − 6% | − 1% | − 16% | − 4% | 0% | 14% |
| Northern Cape | 625 | − 38 | − 5 | − 95 | 0 | − 20 | 80 | − 6% | − 1% | − 15% | − 3% | 0% | 13% |
| Mpumalanga | 2570 | − 126 | − 20 | − 295 | 5 | − 60 | 245 | − 5% | − 1% | − 11% | − 2% | 0% | 10% |
| Eastern Cape | 3610 | − 159 | − 25 | − 355 | 5 | − 80 | 295 | − 4% | − 1% | − 10% | − 2% | 0% | 8% |
| KwaZulu Natal | 6470 | − 262 | − 40 | − 570 | 5 | − 120 | 460 | − 4% | − 1% | − 9% | − 2% | 0% | 7% |
| Free State | 1675 | − 66 | − 10 | − 140 | 0 | − 30 | 115 | − 4% | − 1% | − 8% | − 2% | 0% | 7% |
| South Africa | 32,195 | − 2142 | − 340 | − 5395 | 55 | − 1195 | 4725 | − 7% | − 1% | − 17% | − 4% | 0% | 15% |
Estimated access to care in the nine provinces ranged from 79% in the Western Cape (WC) to 17% in the Free State (FS) (Table 4), resulting in unique birth outcomes in each province for the current care (actual) scenario (Tables 6 and 7). The impact of current interventions was greatest in the WC, with a 48% reduction in total adverse outcomes. In WC, 13% of affected births were converted to healthy births or avoided through pre-pregnancy care (affected pregnancies converted to healthy pregnancies), PND, genetic counselling and option of TOP. Of affected live births in WC, there was a decrease of 36% in CD-related under-5 deaths, survivors at 5 years with disability decreased by 8%, and 33% were effectively cured (Table 7).
The least impact was estimated for the FS with only a 14% decrease in adverse outcomes (Table 6). This included 4% of affected births prevented or avoided; 1% fewer stillbirths; 8% less CD-related under-5 deaths, and 7% effectively cured (Table 7).
Estimates in Table 6 indicate that in 2012, CD-related under-5 mortality accounted for 24% of total under-5 mortality in South Africa, ranging from 31% in NC to 22% in the FS, Kwa-Zulu Natal (KZN) and the Eastern Cape (EC).
Survival and disability
Proportional changes in disability and survival for actual (current care) estimates are compared with baseline estimates in Table 8. Total years of life affected by the CDs included in MGDb-ZA accounted for 3% of total years of life for all births in South Africa in 2012. With 29% access to care, total YLL for affected births decreased by 13% compared with baseline estimates. A small reduction of 4% was seen in YLD and an increase of 15% of years of life lived cured. Table 9 shows the impact of specific interventions included in MGDb-ZA on YLL and YLD. Access to folate fortification (pre-conception care) reduced YLL by 2% and PND, genetic counselling and access to medical TOP resulted in a further 5% reduction in YLL. Tertiary prevention or care, specifically surgical intervention after birth, resulted in a 15% decrease in YLL through lives effectively cured.
Table 8.
Total proportional changes in survival (YLL) and disability (YLD) for baseline and actual MGDb-ZA estimate, South Africa 2012
| Baseline (no care) | Current care (actual) | |||||||
|---|---|---|---|---|---|---|---|---|
| Total years of life (all births) | Total years life affected | Total YLL | Total YLD | Total affected years prevented | Total YLL | Total YLD | Total years lived cured | |
| Number/percent | 72,437 436 | 1,983,432 (3%) | 70% | 30% | 2% | 58% | 26% | 15% |
| Change | 2% | − 13% | − 4% | 15% | ||||
Table 9.
Estimated improved survival (proportion) due to specific primary, secondary and tertiary prevention interventions included with 29% access to care, South Africa 2012. The change in years lived due to pre-natal care (PND) and medical TOP are accounted for as years of life lost (YLL)
| Primary prevention (pre-conception) | Secondary prevention (PND & TOP) | Tertiary prevention (post-natal care) | |
|---|---|---|---|
| Change in years life lost (YLL) | − 30 145 | 106,171 | − 299,734 |
| Proportional change (YLL) | − 2% | 5% | − 15% |
Comparison of baseline, actual and optimal outcomes
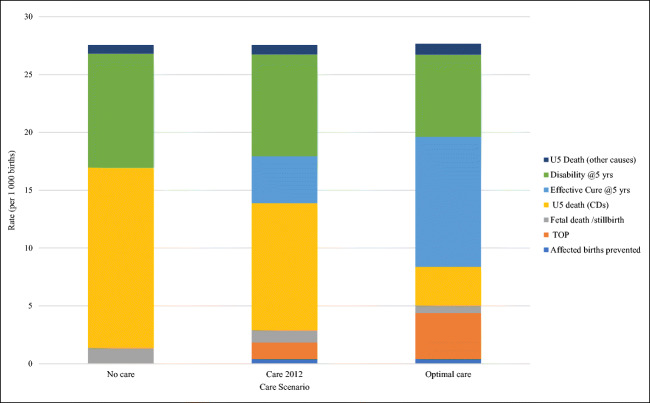
A characteristic feature of MGDb is the recognition that baseline affected births provide an “envelope” or closed system into which all birth outcomes must fit in each scenario. Figure 1 graphically summarizes outcomes for MGDb-ZA CDs in the baseline, current care (actual) and optimal care scenarios (using Table 2 rates).
Fig. 1.
Comparison of birth outcomes for baseline (no care), actual (current care) and optimal care (100% access) scenarios for CDs included in MGDb-ZA, rates per 1000 births, South Africa 2012
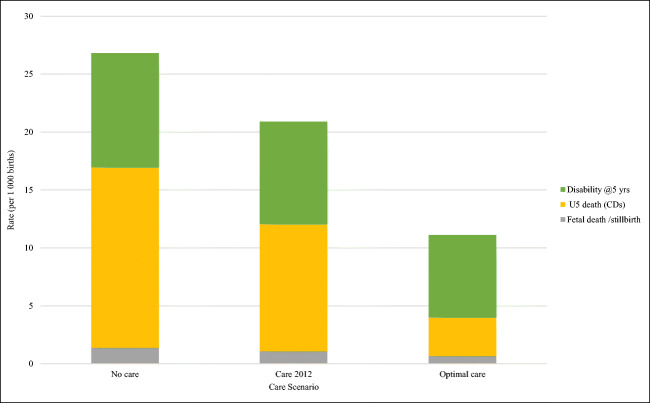
While accounting for all birth outcomes is essential, it is also necessary to identify data of most relevance to policymakers for use in decision-making around service planning. Figure 2 highlights the decrease in adverse outcomes only (stillbirths, U5 death, disability) in the different care scenarios. Births prevented, avoided and effectively cured are omitted as these will not be apparent to policymakers in vital registration mortality data or morbidity indicators and are no longer considered a component of the disease burden.
Fig. 2.
Comparison of perceived adverse birth outcomes for baseline (no care), actual current care (29% access) and optimal care (100% access) scenarios for CDs included in MGDb-ZA. Rates per 1000 births, South Africa 2012
Figure 2 highlights the potential contribution of care in reducing adverse birth outcomes (most notably under-5 deaths) possible if all South Africans had access to optimal care. There is an ostensibly smaller reduction in survivors with a disability at 5 years. However, as their life expectancy is substantially increased there is a cumulative increase in numbers with CD-related disability. Thus, as access to care increases and outcomes improve, the need to provide care for those living with disability increases rather than diminishes (Modell et al. 2018a, b, c; Moorthie et al. 2018a, b, c).
Discussion
The aims of this study were to apply the MGDb Method to assess the birth prevalence and outcomes of specific early-onset, endogenous CDs in South Africa in 2012 at a national and sub-national level; and to estimate the effect of different interventions in reducing attributable stillbirth, early death and disability.
The MGDb premise is that in the absence of any intervention, the baseline birth prevalence and outcomes of births affected by endogenous CDs are relatively constant in any given population. However, actual (with care) outcomes depend on access to available interventions, and this can be estimated using IMR as an indicator. Thus, it is possible to make country-specific estimates of outcomes in the absence of care, with full access to available interventions, and with estimated current (actual) access. While MGDb-ZA cannot claim to be fully comprehensive, the inclusion of collective estimates for rare single-gene disorders makes it more complete than other estimates to date which are limited to congenital anomalies only (Christianson et al. 2006; Global Burden of Disease Collaborative Network 2018).
The pioneering development of the MGDb-ZA in collaboration with the MGDb creators has provided a unique opportunity to identify and solve challenges in applying the Method at the sub-population level (Modell et al. 2018a, b, c). One outcome is the creation of a simple, provisional starting methodology for application by other countries wishing to develop in-country estimates. This study highlights the simplicity of the MGDb approach and how, through combining relevant demographic indicators and prevalence rates in a prescribed template, estimates may be generated by any country or population without requiring specialist input.
Several MGDb conditions are excluded from MGDB-ZA due to their lack of applicability in South Africa, but both structural and functional early-onset CDS are included within key groupings of chromosomal disorders, single-gene disorders and isolated malformations (Czeizel and Sankaranarayanan 1984; World Health Organization 1985; Baird et al. 1988; Czeizel et al. 1993; Modell et al. 2018a, b, c; Moorthie et al. 2018a, b, c). The inclusion of oculocutaneous albinism as a placeholder single-gene disorder demonstrates how the MGDb approach may be tailored to include conditions of most relevance to a specific population.
MGDb-ZA result highlights
Collectively, with an estimated 29% access to care nationally in 2012, the MGDb-ZA outputs demonstrate the impact of relevant genetic services on reducing adverse birth outcomes for the included CDs. Primary9 and secondary10 preventative interventions have the greatest impact on life-limiting CDs with severe prognoses, unlikely to benefit from interventions after birth. The 485 CD-affected births converted to healthy births (through pre-pregnancy interventions) is due to the countrywide implementation of folic acid food fortification—preventing many NTD affected births and positively impacting families and the economy with a cost-benefit ratio of 30:1 (Sayed et al. 2008). The majority of the 1660 CD-affected births avoided through PND, counselling and option of TOP—are severe CDs likely to result in miscarriage, stillbirth or early neonatal death—confirmed by the 1% reduction in stillbirths. The 5% decrease in CDaffected births in MGDb-ZA due to TOP also accounted for a reciprocal 5% decrease in YLL. Further work is required to identify how best to quantify the impact of TOP in MGDb. Comparison with empirical reported data on TOPs undertaken due to severe physical foetal abnormality/malformations is not yet possible as this TOP burden of disease remains unquantified (Republic of South Africa 1996).
Early diagnosis and access to care at birth (tertiary prevention)11 substantially improve birth outcomes, including survival and the quality of life for those affected by CDs. The MGDb-ZA estimated reduction of 17% of CD-related under-5 deaths and a third of survivors effectively cured demonstrates the effect of surgical intervention, particularly for potentially lethal isolated malformations (NTDs, OFCs and additional conditions). These extremely poor birth outcomes are converted to healthy survivors (Walani and Biermann 2017). While paediatric surgery has been historically perceived as prohibitively expensive and of little relevance for MLICs, the evidence is emerging to the contrary—offering considerable socioeconomic benefits while averting suffering (Mocumbi et al. 2011; Sitkin et al. 2015; Ozgediz et al. 2016; Sitkin and Farmer 2016). This highlights the need for investment in developing local paediatric surgical capacity in South Africa.
Within MGDb-ZA estimates for 2012, the persisting proportion of affected births surviving at 5 years with disability that cannot be effectively cured are demonstrated. “Care is an absolute, prevention the ideal” highlights the need for increased commitment, capacity and resource allocation to first care for those affected by CDs, balanced with preventative interventions to ensure the sustainability of services (Christianson et al. 2000; Christianson et al. 2006; Walani and Biermann 2017).
Estimated access to care by MGDb-ZA at the sub-national level in South Africa showed a varying impact on birth outcomes for the different CD categories. The varying access to care estimated for the nine provinces points to a relationship between access to care in the nine provinces and a decrease in perceived adverse outcomes for CD-affected births, and counters the widely-held belief that “little can be done to treat CDs”. A comparison between WC: the province with the greatest estimated access to care (79%) and the province with the least access—FS (17%) establishes the WC as a reference province for health care services in the country. Yet, while significant numbers of lives are saved through accessing currently available services and this reduces the perceived burden of disease, many additional lives of children might also be saved if optimum (100%) services were accessible to all.
Challenges
Sourcing local data
The quality of data sources used by MGDb are addressed elsewhere (Moorthie et al. 2018a, b, c.; Blencowe et al. 2018b). In MGDb-ZA the greatest source of uncertainty is the calculation of access to care based on the IMR (Blencowe et al. 2018a). Locally sourced IMR and U5MR data were used in preference to UN WPP (see “Method”) but identifying robust sources of local data was unexpectedly challenging. While vital registration (VR) reporting has improved significantly in South Africa over the past two decades with the introduction of legislated compulsory birth registration, low reporting levels persist for infants and children under-5 (Republic of South Africa 1992; Dobbie et al. 2007; Republic of South Africa 2010; Joubert et al. 2012; Garenne et al. 2016; Nannan et al. 2019). Families of poor economic status in rural areas cannot afford the expense or time away from work to transport children to hospital to die, resulting in traditional burials at home and unregistered infant deaths outside health facilities (Kabudula et al. 2014; Garenne et al. 2016). Adjusted infant and under-5 mortality rates developed locally were available at a provincial level only and not for South African Districts. Identifying relevant local data may also be a challenge for other countries applying the MGDb Method nationally, and appropriate time and effort should be allocated accordingly.
Underestimation
MGDb-ZA estimates are conservative, and likely an underestimate due to challenges in diagnosis (i.e. invisible disorders and lack of capacity and infrastructure) and misallocation of CDs to other causes of death and disability (Debas et al. 2015. Moorthie et al. 2018a, b, c). Limited coverage of single-gene conditions makes these MGDb-ZA estimates a minimum starting point as literature on this large, heterogeneous group of disorders expands (Moorthie et al. 2018a, b, c). Elsewhere, single-gene disorders are being modelled individually, and with over 7000 rare diseases already described, providing a timely estimate of the disease burden represented to inform policymakers for service planning is an implausible task.
Strengths
Sub-national estimates
The development of sub-national estimates through MGDb-ZA is particularly beneficial for informing provincial policymakers in a country as large and diverse as South Africa, where provincial IMRs range from 14 per 1000 to 38 per 1000 births. Since IMR is used to calculate access to care, the result is a unique ratio of outcomes for each province.
Evaluation of specific interventions
The MGDb-ZA method enables the specific impact of individual interventions to be determined (Table 9). The return on investment (reduced mortality and morbidity versus cost) of each care intervention, e.g. genetic counselling, TOP, paediatric surgery etc. may be evaluated individually by policymakers to enable their prioritization and progressive integration into packages of health care services. This is particularly relevant in a MLIC country such as South Africa, where implementing universal health coverage via the National Health Initiative (NHI) requires packages of services across the life course (Department of Health 2015).
Closed system approach
The founding “envelope” principle of MGDb—that the sum of all outcomes must equal the baseline prevalence—differs from other modelling approaches. Each disorder in MGDb-ZA is handled as a closed system, and each birth is accounted for by an outcome, as opposed to other approaches that account only for specific outcomes only, e.g. deaths, which may exclude a considerable portion of the burden of disease by unaccounted affected births (Modell et al. 2018a, b, c; Moorthie et al. 2018a, b, c).
Extensive peer review
The MGDb approach has undergone extensive critique by community genetic experts. Rooted in early work on haemoglobin disorders and work originating in Hungary in the early 1990s, the MGDb was initially only implemented for the Hungarian population (World Health Organization 1985; Czeizel et al. 1993; Czeizel 1997). Global applicability became clear following publication in the British Medical Journal (BMJ) in 1993 (Czeizel et al. 1993). Endorsement by the World Health Organization (WHO) of the MGDb estimates published in the 2006 Global Report on Birth Defects (Christianson et al. 2006) added to the credibility of this Method. More recently, the MGDb Method has undergone extensive peer review via publication in a special edition of the Journal of Community Genetics (Modell et al. 2018a, b, c; Moorthie et al. 2018a, b, c; Blencowe et al. 2018a; Blencowe et al. 2018b), available online via MGDB.info (Modell et al. 2016).
Limitations
Quantification of disability
In its current form, the MGDb-ZA does not quantify or qualify physical disability other than estimating the proportion of survivors at age 5 living with severe or less severe disability (Moorthie et al. 2018a, b, c). Due to the extensive variation in disability categories and scale for the CDs included (Moorthie et al. 2018a, b, c), further consideration is needed to develop this component. Several conditions included in MGDb-ZA (e.g. oculocutaneous albinism, Klinefelter and Turner syndromes, less severe other malformations) are not life-limiting under-5 in the absence of care, and further analysis solely on the quality of life is required.
Theory versus practice
The use of the IMR as a blanket health indicator in MGDb-ZA to calculate access to available care does not account for actual services available in-country, factors influencing the IMR, or IMR variation (rural/urban) within provinces. In South Africa, genetic services are not equally distributed between the nine provinces and function via a hierarchical referral network. Five tertiary clinical genetic units12 are based at academic centres in four provinces only. Specialist services for specific disorders are implemented at different centres countrywide, e.g. OFC clinics operating at 11 sites countrywide at academic centres in six provinces (Hlongwa et al. 2019). Poor health service delivery across provinces, e.g. in the Free State, is exacerbated by incomplete District-level IMR data, transportation challenges, poor service delivery in peripheral areas, over-referral to tertiary services, late presentation and other social determinants of health (Pers. Comm. Bertram Henderson) (Dobbie et al. 2007; Sartorius et al. 2011; Joubert et al. 2012; Garenne et al. 2016). This disparity between theory and practice needs to be considered when using these estimates for service planning, and further work to enhance the applicability towards practical implementation is required.
Value of the results for South Africa
Comparison with other sources
The MGDb-ZA estimates are well above those documented by national surveillance, with only 2174 (7%) of CD cases notified in 2012 compared with the 30,060 affected births estimated with access to available care, suggesting a large proportion of CD-affected births remained undiagnosed and/or unreported in 2012 (Lebese et al. 2016).
Table 10 compares MGDb-ZA under-5 death data with equivalent data sourced from South African Vital Registration (VR) and Global Burden of Disease (GBD) 2017 estimates. Comparison is only possible for congenital anomalies since GBD does not include estimates for all CDs (World Health Organization 1992, 1993, 2006; Modell et al. 2016). Proportionally, both VR and GBD 2017 under-5 death estimates for congenital anomalies are around a fifth (21% and 22% respectively) of those estimated by MGDb-ZA. Disparities between GBD and MGDb estimates have been previously noted, and as reported by Boyle et al. (2018), GBD estimates are based on the WHO Mortality Database which mainly sources death certification (VR) data and may result in significant underestimates, especially for MLIC due to inaccurate causes of death data (Christianson and Modell 2004; Liu et al. 2012; Modell et al. 2012; Boyle et al. 2018; Modell et al. 2018a, b, c; Moorthie et al. 2018a, b, c; World Health Organization 2020). This also accounts for the similarity between the VR and GBD estimates. Unlike MGDb-ZA, GBD does not include stillbirths and TOP for foetal impairment in their estimates, so excluding a substantial component of the total CD burden of disease (Boyle et al. 2018).
Table 10.
Comparison of estimated under-5 deaths per 1000 in South Africa 2012 for congenital anomalies, Global Burden of Disease (GBD) (Global Burden of Disease Collaborative Network 2018), Vital Registration (VR) data (Statistics South Africa 2012) and MGBb-ZA
| 2012 source | Under-5 deaths/1000 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Down | Unbal chrom | NTD | OFC | CHD | Other CM | Total chrom | Total cong malfns | Total cong anomalies | |
| SA VR Data | 0.13 | 0.11 | 0.10 | 0.02 | 0.48 | 0.88 | 0.24 | 1.47 | 1.71 |
| GBD 2017 | 0.12 | 0.09 | 0.14 | 0.01 | 0.55 | 0.90 | 0.21 | 1.61 | 1.82 |
| MGDb-ZA | 0.69 | 0.54 | 0.48 | 0.12 | 1.72 | 4.78 | 1.23 | 7.09 | 8.32 |
| VR % of MGDB-ZA | 18% | 20% | 20% | 14% | 28% | 18% | 19% | 21% | 21% |
| GBD % of MGDb-ZA | 17% | 17% | 30% | 11% | 32% | 19% | 17% | 23% | 22% |
Contribution to total under-5 mortality and disability
Under-5 mortality
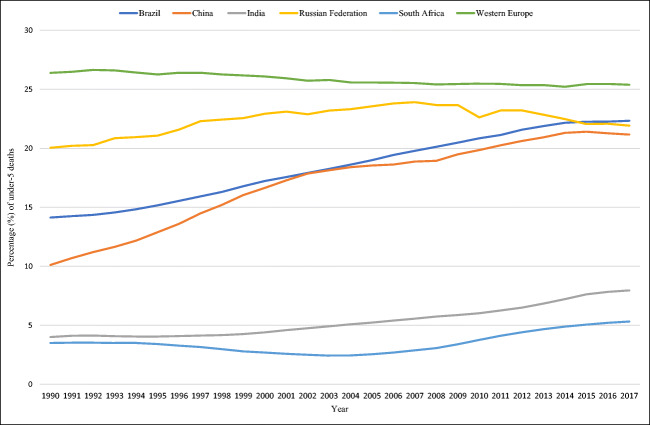
The MGDb-ZA estimate of 24% of total under-5 deaths attributed to CDs is almost an order of magnitude greater than the 3% of congenital anomalies reported through vital registration in 2012, and four times higher than the 5.9% of congenital abnormalities13 reported nationally in 2015 (Bamford et al. 2018; Nannan et al. 2019). Key reasons for this disparity include a high proportion of undiagnosed and misdiagnosed CDs due to inadequate diagnostic capacity, the masking of CDs, particularly invisible anomalies, by the persisting burden of infectious disease, and the exclusion of functional and environmental CDs, accounting for almost 50% of total CDs (Debas et al. 2015; Modell et al. 2016; Malherbe et al. 2018a, b). Globally, the proportion of under-5 deaths due to congenital anomalies alone ranges from 9–14% for upper-middle-income countries such as South Africa, and up to 30% for high-income countries (World Health Organization 2015). Figure 3 graphically compares GBD 2017 under-5 deaths attributed to congenital anomalies for the five BRICS countries (Brazil, Russia, India, China and South Africa), with Western Europe included as a reference. Under-5 deaths due to congenital anomalies have proportionally increased for all BRICS over the past 30 years due to epidemiological transition, with Brazil, Russia and China now proportionally comparable with Western Europe, India and South Africa lag significantly behind, with minimal increases in the proportion of these deaths for South Africa. This suggests that cases of even obvious congenital anomalies continue to go undiagnosed and uncounted in South Africa, and death data does not reflect the underlying (ICD-10) cause of death due to inaccurate death reporting. For South Africa to attain the Sustainable Development Goal (SDG) 3 target of U5MR of 25 per 1000 live births by 2030 (United Nations), child deaths must be significantly further reduced. In accordance with the World Health Assembly Resolution 63.17, this requires CDs to be prioritized as a health care issue (World Health Assembly 2010).
Fig. 3.
A comparison of the proportion of GBD 2017 under-5 deaths due to congenital anomalies in the BRICS countries, with Western Europe as an indicator, 1990–2017 (Global Burden of Disease Collaborative Network 2018)
Disability
Unresolved issues around definitions, measurements and methods to quantify disability, particularly for children under-5, make it challenging to compare MGDb-ZA estimates for survivors with disability and YLD with other data sources (African Children Policy Forum 2011; Statistics South Africa 2014; Maart et al. 2019). Further work is required to address these issues.
Applicability and usefulness of MGDb-ZA estimates
The MGDb-ZA is a tool that offers evidence-based estimates of births and predicted outcomes affected by selected CDs for use by health policymakers to develop a relevant health care response (Moorthie et al. 2018a, b, c). These estimates offer (1) An approximation of the actual CD burden in South Africa, and (2) An opportunity to compare these estimates with observed data using the difference between these as a measure of the shortfall in current services. This service “gap” highlights the under-estimation of CDs in the country, due to inadequate diagnostic capacity and infrastructure, preventing those affected from accessing relevant care. These estimates also provide a starting point for improving service provision for CDs by enabling cost estimation of the specific interventions included. A particularly important achievement in undertaking this study has been the development of a collaborative network, both in South Africa and further afield, required for the further improvement of this modelling method and to advocate for change.
Conclusion and recommendations
The findings of this study have
Validated the MGDb Method for generating information relevant for policymakers;
Generated and assessed the national and provincial prevalence and outcome estimates for specific early-onset, endogenous CD; and
Evaluated the impact of different interventions on birth outcomes.
Areas for further research include:
Developing estimates for early-onset examples of genetic risk disorders for South Africa, e.g. GPD6 deficiency and rhesus haemolytic disease.
Undertaking in-depth analyses of modelled estimates for specific CDs included in MGDb-ZA for South Africa 2012 and comparison with observed data where available.
Developing updated MGDb-ZA for included CDs for relevant interval years since 2012.
Clarifying the use of accurate and consistent terminology for CDs and sub-sets of CDs. Current confusion with sub-sets of CDs being reported as the total disease burden is resulting in underreporting, preventing appropriate prioritization and development of relevant genetic services, costing lives as a result (World Health Organization 1999; Christianson and Modell 2004; Christianson et al. 2006; World Health Organization 2006; Malherbe et al. 2016).
Further consideration is needed around quantifying TOP as an outcome, particularly when parents plan to conceive another, unaffected child who may not be born otherwise, thus reducing YLL overall. While the choice of medical TOP following PND and genetic counselling is currently accounted for as YLL in the MGDb-ZA, this intervention ultimately reduces the number of births affected by CDs.
Further investigating the quantification and qualification of disability within the MGDb Method.
Acknowledgements
Thank you to Prof Rob Dorrington, Centre for Actuarial Research (CARe), University of Cape Town, for providing demographic data for use in this study; Prof Debbie Bradshaw, Burden of Disease Unit, South African Medical Research Council for valuable advice; and Statistics South Africa (StatsSA) for providing 2012 vital registration data sets which, although not used in the final modelling process, were key in the decision-making process at the data-gathering stage of this study.
Code availability
Not applicable.
Author contribution
All authors contributed to the study conception and design. Methodology and source material preparation: Bernadette Modell, Helen Malherbe, Arnold Christianson and Matthew Darlison. Data collection, modelling and analysis were performed by Helen Malherbe, Bernadette Modell and Colleen Aldous. The first draft of the manuscript was written by Helen Malherbe with detailed input by Bernadette Modell with all authors providing feedback on early versions of the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by the University of KwaZulu Natal (UKZN), initially via PhD (2013–2016) and Post-Doctoral (2017–2019) bursaries awarded to HM by the College of Health Sciences, UKZN, and subsequently by UKZN APACHE Flagship Post-Doctoral Research Scholarship (2019—to date) via the KwaZulu Natal Research, Innovation and Sequencing Platform (KRISP).
Data availability
All relevant study data is included in the article.
Declarations
Ethics approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
Helen Malherbe was the Honorary Chair of Genetic Alliance South Africa (NPO: 001-029) until March 2020 and was appointed as a (Honorary) Director of Rare Diseases South Africa in April 2020. Colleen Aldous declares she has no conflict of interest. Arnold Christianson declares he has no conflict of interest. Matthew Darlison declares he has no conflict of interest. Bernadette Modell declares she has no conflict of interest.
Footnotes
Congenital anomalies are defined as macroscopic morphological anomalies present at birth and represented by chapter XVII Congenital malformations, deformations and chromosomal abnormalities of the International Statistical Classification of Diseases and Related Health Problems 10th Revision ICD-10)(World Health Organization 1992, 2006).
Supplementary file TA01-Bottom-Line-WHO-2017-04.xlsx at https://discovery.ucl.ac.uk/id/eprint/1532179/
Serious birth defects cause death or disability in the absence of intervention (Christianson et al. 2006).
Baseline outcomes include fetal deaths/still births; live births; neonatal, infant and under-5 deaths (CD related); deaths from other causes; survivors with disability at age 5; and mean life expectancy.
In the MGDb context, optimal care is defined as the standard of care available in high-income settings with equitable access to services, at any given point in time.
Within the School of Clinical Medicine 2013-2019 and with the KwaZulu Natal Research Innovation and Sequencing Platform (KRISP), School of Laboratory Medicine and Medical Sciences from 2019 to 2020.
A wider review suggested that major differences are uncommon but the possibility should be considered.
For details on this calculation see Blencowe et al. 2018a.
Primary prevention, e.g. folate fortification, genetic counselling etc. resulting in the prevention of affected conceptions.
Secondary preventions, e.g. PND, genetic counselling, option of TOP resulting in the avoidance of affected births.
Tertiary prevention (care) includes newborn screening, diagnosis, therapeutic and surgical interventions, rehabilitation and palliative care, mitigating the impact of affected births and improving outcomes.
University of Cape Town/Groote Schuur Hospital/Red Cross War Memorial Children’s Hospital; Stellenbosch University/Tygerburg Hospital; University of the Free State/Universitas Hospital; University of KwaZulu-Natal/Inkhosi Albert Luthuli Central Hospital (pending registration); National Health Laboratory Service/University of the Witwatersrand
Congenital abnormalities are considered equivalent to congenital anomalies (Pillay-Van Wyk et al. 2014).
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- African Children Policy Forum (2011) Children with disabilities in South Africa: The hidden reality. ACP Forum. https://www.africanchildforum.org/index.php/en/component/com_sobipro/Itemid,0/pid,2/sid,144/. Accessed 25 October 2011
- Alwan A, Modell B. Recommendations for introducing genetics services in developing countries. Nat Rev Genet. 2003;4(1):61–68. doi: 10.1038/nrg978. [DOI] [PubMed] [Google Scholar]
- Baird PA, Anderson TW, Newcombe HB, Lowry RB. Genetic disorders in children and young adults: a population study. Am J Med Genet. 1988;42(5):677. [PMC free article] [PubMed] [Google Scholar]
- Bamford L, McKerrow N, Barron P, Aung Y. Child mortality in South Africa: Fewer deaths, but better data are needed. S Afr Med J. 2018;108(3):25–32. doi: 10.7196/samj.2017.v108i3b.12779. [DOI] [Google Scholar]
- Bittles A, Black ML (2015) Global Patterns and Tables of Consanguinity. Table 1. Consanguineous Marriage in Africa. http://consang.net. Accessed 15 August 2017
- Bittles AH, Neel JV. The costs of human inbreeding and their implications for variations at the DNA level. Nat Genet. 1994;8(2):117–121. doi: 10.1038/ng1094-117. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Moorthie S, Darlison MW, Gibbons S, Modell B. Methods to estimate access to care and the effect of interventions on the outcomes of congenital disorders. J Community Genet. 2018;9(4):363–376. doi: 10.1007/s12687-018-0359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H, Moorthie S, Petrou M, Hamamy H, Povey S, Bittles A, Gibbons S, Darlison M, Modell B. Rare single gene disorders: estimating baseline prevalence and outcomes worldwide. J Community Genet. 2018;9(4):397–406. doi: 10.1007/s12687-018-0376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle B, Addor M-C, Arriola L, Barisic I, Bianchi F, Csáky-Szunyogh M, de Walle HE, Dias CM, Draper E, Gatt M. Estimating Global Burden of Disease due to congenital anomaly: an analysis of European data. Arch Dis Child Fetal Neonatal Ed. 2018;103(1):F22–F28. doi: 10.1136/archdischild-2016-311845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundey S, Alam H. A five-year prospective study of the health of children in different ethnic groups, with particular reference to the effect of inbreeding. Eur J Hum Genet. 1993;1(3):206–219. doi: 10.1159/000472414. [DOI] [PubMed] [Google Scholar]
- Carter C. Monogenic disorders. J Med Genet. 1977;14(5):316–320. doi: 10.1136/jmg.14.5.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention (2012) CDC's Vision for Public Health Surveillance in the 21st Century. http://www.cdc.gov/mmwr/pdf/other/su6103.pdf. Accessed 1 November 2016
- Centers for Disease Control . Comprehensive plan for epidemiologic surveillance. CDC, Atlanta: U.S. Department of Health and Human Services; 1986. [Google Scholar]
- Christianson A. Medical genetic services for the care and prevention of birth defects. Cape Town: Child Health for All. A manual for southern Africa. ed Oxford University Press Southern Africa; 2012. pp. 231–241. [Google Scholar]
- Christianson A, Modell B. Medical Genetics in Developing Countries. Ann Rev Genomics Hum Genet. 2004;5:219–265. doi: 10.1146/annurev.genom.5.061903.175935. [DOI] [PubMed] [Google Scholar]
- Christianson A, Venter P, Modiba J, Nelson M. Development of a primary health care clinical genetic service in rural South Africa–The Northern Province experience, 1990–1996. J Community Genet. 2000;3(2):77–84. doi: 10.1159/000051108. [DOI] [Google Scholar]
- Christianson A, Howson C, Modell B (2006) March of Dimes: Global Report on Birth Defects, the Hidden Toll of Dying and Disabled Children. March of Dimes Birth Defects Foundation. http://www.marchofdimes.org/materials/global-report-on-birth-defects-the-hidden-toll-of-dying-and-disabled-children-full-report.pdf Accessed 17 November 2016
- Christianson A, Zimmern R, Kristoffersson U, Schmidtke J, Kent A, Raouf R, Barreiro C, Nippert I. Health needs assessment for medical genetic services for congenital disorders in middle-and low-income nations. J Community Genet. 2013;4(3):297–308. doi: 10.1007/s12687-013-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens S, Blencowe H, Stanton C, Chou D, Ahmed S, Steinhardt L, Creanga AA, Tunçalp Ö, Balsara ZP, Gupta S. National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: a systematic analysis. The Lancet. 2011;377(9774):1319–1330. doi: 10.1016/S0140-6736(10)62310-0. [DOI] [PubMed] [Google Scholar]
- Czeizel A. First 25 years of the Hungarian congenital abnormality registry. Teratology. 1997;55(5):299–305. doi: 10.1002/(SICI)1096-9926(199705)55:5<299::AID-TERA1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Czeizel A, Sankaranarayanan K. The load of genetic and partially genetic disorders in man I. Congenital anomalies: estimates of detriment in terms of years of life lost and years of impaired life. Mutat. 1984;128(1):73–103. doi: 10.1016/0027-5107(84)90049-6. [DOI] [PubMed] [Google Scholar]
- Czeizel A, Sankaranarayanan K, Szondy M. The load of genetic and partially genetic diseases in man III. Mental retardation. Mutat Res-Fund Mol M. 1990;232(2):291–303. doi: 10.1016/0027-5107(90)90136-R. [DOI] [PubMed] [Google Scholar]
- Czeizel A, Intôdy Z, Modell B. What proportion of congenital abnormalities can be prevented? Br Med J. 1993;306:499–503. doi: 10.1136/bmj.306.6876.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debas HT, Donkor P, Gawande A, Jamison DT, Kruk ME, Mock CN. Disease Control Priorities, Third Edition : Volume 1. World Bank, Washington DC: Essential Surgery; 2015. [PubMed] [Google Scholar]
- Declich S, Carter AO. Public health surveillance: historical origins, methods and evaluation. B World Health Organ. 1994;72(2):285. [PMC free article] [PubMed] [Google Scholar]
- Department of Health National Health Insurance: Towards Universal Health Coverage. White Paper on National Health Insurance. Government Gazette (No. 1230) 11 December 2015. Pretoria 2015 Accessed 12 May 2016
- Dobbie M, Masebe L, Nhlapo M (2007) The coverage and quality of birth registration data in South Africa, 1998-2005 SS Africa. Accessed 28 October 2016
- Dorrington R, Moultrie T. 7th African Population Conference. South Africa: Johannesburg; 2015. Understanding recent fertility in South Africa. [Google Scholar]
- Dorrington R, Bradshaw D, Laubscher R, Nannan N (2020) Rapid Mortality Surveillance Report 2018. https://www.samrc.ac.za/reports/rapid-mortality-surveillance-report-2018. Accessed 18 March 2020
- EUROCAT (2009) Special Report: Congenital Heart Defects in Europe 2000-2005. http://www.eurocat-network.eu/content/Special-Report-CHD.pdf. Accessed
- EUROCAT (2015) EUROCAT Prevlence Tables. https://eu-rd-platform.jrc.ec.europa.eu/eurocat/eurocat-data_en. Accessed January 2015
- Garenne M, Collinson MA, Kabudula CW, Gómez-Olivé FX, Kahn K, Tollman S. Completeness of birth and death registration in a rural area of South Africa: the Agincourt health and demographic surveillance, 1992–2014. Global Health Action. 2016;9:32795. doi: 10.3402/gha.v9.32795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease Collaborative Network (2018) Global Burden of Disease Study 2017 (GBD 2017). Institute for Health Metrics and Evaluation (IHME). Seattle, USA.
- Hall HI, Correa A, Yoon PW, Braden CR. Lexicon, definitions, and conceptual framework for public health surveillance. MMWR CDC Surveill Summ. 2012;61(Suppl):10–14. [PubMed] [Google Scholar]
- Hlongwa P, Levin J, Rispel LC (2019) Epidemiology and clinical profile of individuals with cleft lip and palate utilising specialised academic treatment centres in South Africa. PLoS One 14(5). 10.1371/journal.pone.0215931 [DOI] [PMC free article] [PubMed]
- Johnson LF, Chiu C, Myer L, Davies M-A, Dorrington RE, Bekker L-G, Boulle A, Meyer-Rath G. Prospects for HIV control in South Africa: a model-based analysis. Global Health Action. 2016;9:1–12. doi: 10.3402/gha.v9.30314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert J, Rao C, Bradshaw D, Dorrington RE, Vos T, Lopez AD. Characteristics, availability and uses of vital registration and other mortality data sources in post-democracy South Africa. Global health action. 2012;5:1–19. doi: 10.3402/gha.v5i0.19263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabudula CW, Joubert JD, Tuoane-Nkhasi M, Kahn K, Rao C, Gmez-Oliv FX, Mee P, Tollman S, Lopez AD, Vos T. Evaluation of record linkage of mortality data between a health and demographic surveillance system and national civil registration system in South Africa. Population Health Metrics. 2014;12(1):1. doi: 10.1186/s12963-014-0023-z. [DOI] [Google Scholar]
- Kahn K, Garenne ML, Collinson MA, Tollman SM. Mortality trends in a new South Africa: Hard to make a fresh start1. Scand J Public Healt. 2007;35(69 suppl):26–34. doi: 10.1080/14034950701355668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromberg J, Jenkins T. Prevalence of albinism in the South African Negro. S Afr Med J. 1982;61:383–386. [PubMed] [Google Scholar]
- Lebese L, Aldous C, Malherbe H. South African congenital disorders data, 2006 - 2014. S Afr Med J. 2016;106(10):992–995. doi: 10.7196/samj.2016.v106i10.11314. [DOI] [PubMed] [Google Scholar]
- Liu L, Cousens S, Lawn JE, Black RE. Global regional and national causes of child mortality–Authors' reply. The Lancet. 2012;380(9853):1556–1557. doi: 10.1016/s0140-6736(12)61879-0. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD. Measuring the global burden of disease and epidemiological transitions: 2002–2030. Ann Trop Med Parasitol. 2006;100(5-6):481–499. doi: 10.1179/136485906x97417. [DOI] [PubMed] [Google Scholar]
- Luquetti DV, Koifman RJ. Surveillance of birth defects: Brazil and the US. Ciência & Saúde Coletiva. 2011;16:777–785. doi: 10.1590/s1413-81232011000700008. [DOI] [PubMed] [Google Scholar]
- Maart S, Amosun S, Jelsma J. Disability prevalence-context matters: A descriptive community-based survey. Afr J Disabil. 2019;8:1–8. doi: 10.4102/ajod.v8i0.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe HL, Christianson AL, Aldous C. Need for services for the care and prevention of congenital disorders in South Africa as the country's epidemiological transition evolves. S Afr Med J. 2015;105(3):186–188. doi: 10.7196/samj.9136. [DOI] [PubMed] [Google Scholar]
- Malherbe H, Aldous C, Woods D, Christianson A. South African Health Review 2017. ed Health Systems Trust, Durban. 2016. The contribution of congenital disorders to child mortality in South Africa; pp. 137–152. [Google Scholar]
- Malherbe HL, Christianson A, Woods D, Aldous C (2018a) Contribution congenital disorders to neonatal mortality in South Africa. S Afr Med J 108(7). 10.7196/samj.2018.v108i7.13393 [DOI] [PubMed]
- Malherbe HL, Christianson AL, Woods D, Aldous C. Under-5 mortality and the contribution of congenital disorders in South Africa. S Afr Med J. 2018;108(6):447–448. doi: 10.7196/samj.2018.v108i6.13331. [DOI] [PubMed] [Google Scholar]
- Matthews T, MacDorman M, Thoma M (2015) Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Vital National Statistics Reports; vol 64 no 9. . https://stacks.cdc.gov/view/cdc/32752. Accessed 19 September 2016 [PubMed]
- Mocumbi AO, Lameira E, Yaksh A, Paul L, Ferreira MB, Sidi D. Challenges on the management of congenital heart disease in developing countries. Int J Cardiol. 2011;148(3):285–288. doi: 10.1016/j.ijcard.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Modell B (2017) The Modell Global Database of Congenital Disorders. http://mgdb.info. Accessed 15 Jan 2018
- Modell B, Modell M. Towards a healthy baby: Congenital disorders and the new genetics in primary health care. New York: Oxford University Press; 1992. [Google Scholar]
- Modell B, Berry RJ, Boyle CA, Christianson A, Darlison M, Dolk H, Howson CP, Mastroiacovo P, Mossey P, Rankin J. Global regional and national causes of child mortality. The Lancet. 2012;380(9853):1556. doi: 10.1016/S0140-6736(12)61878-9. [DOI] [PubMed] [Google Scholar]
- Modell B, Darlison M, Moorthie S, Blencowe H, Petrou M, Lawn J (2016) Epidemiological methods in community genetics and the Modell Global Database of Congenital Disorders (MGDb).
- Modell B, Darlison M, Malherbe H, Moorthie S, Blencowe H, Mahaini R, El-Adawy M. Congenital disorders: epidemiological methods for answering calls for action. J Community Genet. 2018;9(4):335–340. doi: 10.1007/s12687-018-0390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modell B, Darlison MW, Lawn JE. Historical overview of development in methods to estimate burden of disease due to congenital disorders. J Community Genet. 2018;9(4):341–345. doi: 10.1007/s12687-018-0382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modell B, Darlison MW, Malherbe H, Moorthie S, Blencowe H, Mahaini R, El-Adawy M. Congenital disorders: epidemiological methods for answering calls for action. J Community Genet. 2018;9:335–340. doi: 10.1007/s12687-018-0390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthie S, Blencowe H, Darlison MW, Gibbons S, Lawn JE, Mastroiacovo P, Morris JK, Modell B. Chromosomal disorders: estimating baseline birth prevalence and pregnancy outcomes worldwide. J Community Genet. 2018;9(4):377–386. doi: 10.1007/s12687-017-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthie S, Blencowe H, Darlison MW, Lawn J, Morris JK, Modell B, Bittles A, Blencowe H, Christianson A, Cousens S. Estimating the birth prevalence and pregnancy outcomes of congenital malformations worldwide. J Community Genet. 2018;9(4):387–396. doi: 10.1007/s12687-018-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthie S, Blencowe H, Darlison MW, Lawn JE, Mastroiacovo P, Morris JK, Modell B. An overview of concepts and approaches used in estimating the burden of congenital disorders globally. J Community Genet. 2018;9(4):347–362. doi: 10.1007/s12687-017-0335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Alberman E, Scott C, Jacobs P. Is the prevalence of Klinefelter syndrome increasing? Eur J Hum Genet. 2008;16(2):163–170. doi: 10.1038/sj.ejhg.5201956. [DOI] [PubMed] [Google Scholar]
- Mossey PA, Little J. Cleft lip and Palate: From Origin to Treatment. Epidemiology of oral clefts: an international perspective. ed. New York: Oxford University Press; 2002. pp. 127–158. [Google Scholar]
- Nannan N, Dorrington R, Bradshaw D. Estimating completeness of birth registration in South Africa, 1996 - 2011. Bull World Health Organ. 2019;97(7):468–476. doi: 10.2471/BLT.18.222620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Perinatal Morbidity, Mortality Committee (2017) Saving Babies 2014-2016. Triennial report on perinatal mortality in South Africa. https://www.westerncape.gov.za/assets/departments/health/napemmco_triennial_report_2014-2016_saving_babies.pdf. Accessed 9 May 2015
- Nippert I, Christianson A, Gribaldo L, Harris H, Horovitz D, Randa K, Kent A, Kristoffersson U, Padilla C, Penchaszadeh V (2013) Genetic Testing in Emerging Economies (GenTEE). Summary Report. Publications Office of the European Union. https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/genetic-testing-emerging-economies-gentee. Accessed 12 May 2016
- Ozgediz D, Langer M, Kisa P, Poenaru D. Pediatric surgery as an essential component of global child health. Semin Pediatr Surg. 2016;25(1):3–9. doi: 10.1053/j.sempedsurg.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Pillay-Van Wyk V, Laubscher R, Msemburi W, Dorrington R, Groenewald P, Vos T, Matzopoulos R, Prinsloo M, Nojilana B, Nannan N, Somdyala N, Sithole N, Neethling I, Nicol E, Rossouw A, Joubert J, Bradshaw D (2014) Second South African National Burden of Disease Study: Data cleaning, validation and SA NBD List. http://www.mrc.ac.za/bod/reports.htm. Accessed 1 June 2016
- Republic of South Africa Births and Deaths Registration Act No. 51 (1992) Government Gazette 13953 6 May https://www.gov.za/documents/births-and-deaths-registration-act. Accessed 28 October 2016
- Republic of South Africa Births and Deaths Registration Amendment Act No.18 (2010) Government Gazette 33851 7 December https://www.gov.za/documents/births-and-deaths-registration-amendment-act-3. Accessed 28 October 2016
- Republic of South Africa Choice on Termination of Pregnancy Act No. 92 (1996) Government Gazette 17602 22 November http://www.gov.za/sites/www.gov.za/files/Act92of1996.pdf. Accessed 25 May 2016
- Sartorius BK, Sartorius K, Chirwa TF, Fonn S. Infant mortality in South Africa-distribution, associations and policy implications, 2007: an ecological spatial analysis. Int J Health Geogr. 2011;10(1):61. doi: 10.1186/1476-072x-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed AR, Bourne D, Pattinson R, Nixon J, Henderson B. Decline in the prevalence of neural tube defects following folic acid fortification and its cost-benefit in South Africa. Birth Defects Res A Clin Mol Teratol. 2008;82(4):211–216. doi: 10.1002/bdra.20442. [DOI] [PubMed] [Google Scholar]
- Sitkin NA, Farmer DL. Congenital anomalies in the context of global surgery. Semin Pediatr Surg. 2016;25(1):15–18. doi: 10.1053/j.sempedsurg.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Sitkin NA, Ozgediz D, Donkor P, Farmer DL. Congenital anomalies in low-and middle-income countries: the unborn child of global surgery. World J Surg. 2015;39(1):36–40. doi: 10.1007/s00268-014-2714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics South Africa (2012) Cause of death vital registration data for congenital anomalies 2012. http://nesstar.statssa.gov.za:8282/webview/. Accessed 9 July 2020
- Statistics South Africa (2014) Census 2011. Profile of persons with disabilities in South Africa. http://www.statssa.gov.za/?p=3180. Accessed 5 September 2014
- Stevens GA, Alkema L, Black RE, Boerma JT, Collins GS, Ezzati M, Grove JT, Hogan DR, Hogan MC, Horton R. Guidelines for accurate and transparent health estimates reporting: The GATHER statement. PLoS Med. 2016;13(6):e1002056. doi: 10.1371/journal.pmed.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson AC. The load of hereditary defects in human populations. Radiation Research Supplement. 1959;1:306–325. doi: 10.2307/3583647. [DOI] [Google Scholar]
- Stevenson AC, Johnston HA, Stewart M, Golding DR. Congenital malformations. A report of a study of series of consecutive births in 24 centres. B World Health. Organ. 1966;34(Suppl):9. [PMC free article] [PubMed] [Google Scholar]
- Tennant PW, Pearce MS, Bythell M, Rankin J. 20-year survival of children born with congenital anomalies: a population-based study. The lancet. 2010;375(9715):649–656. doi: 10.1016/s0140-6736(09)61922-x. [DOI] [PubMed] [Google Scholar]
- Trimble BK, Doughty JH. The amount of hereditary disease in human populations. Ann Hum Genet. 1974;38(2):199–223. doi: 10.1111/j.1469-1809.1974.tb01951.x. [DOI] [PubMed] [Google Scholar]
- United Nations Department of Economic and Social Affairs PD (2019) World Population Prospects 2019. https://population.un.org/wpp/. Accessed 7 April 2020
- United Nations Sustainable Development Goal 3: Ensure Healthy Lives and Promote Wellbeing for All and at All Ages. http://www.un.org/sustainabledevelopment/health/. Accessed 13 January 2016
- Visootsak J, Graham JM. Klinefelter syndrome and other sex chromosomal aneuploidies. Orphanet J Rare Dis. 2006;1(1):42. doi: 10.1186/1750-1172-1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walani SR, Biermann J. March of Dimes Foundation: leading the way to birth defects prevention. Public Health Rev. 2017;38:12. doi: 10.1186/s40985-017-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellesley D, Dolk H, Boyd PA, Greenlees R, Haeusler M, Nelen V, Garne E, Khoshnood B, Doray B, Rissmann A. Rare chromosome abnormalities, prevalence and prenatal diagnosis rates from population-based congenital anomaly registers in Europe. Eur J Hum Genet. 2012;20(5):521–526. doi: 10.1038/ejhg.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Assembly (2010) Sixty-Third World Health Assembly - Resolution 63.17. Birth Defects. http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_R17-en.pdf. Accessed 2 September 2014
- World Health Organization (1985) Community approaches to the control of hereditary diseases. Report of a WHO Advisory Group, Geneva, 3-5 October 1985. World Health Organization. https://apps.who.int/iris/handle/10665/62324. Accessed 12 May 2016
- World Health Organization (1992) International Statistical Classification of Diseases and Related Health Problems. 10th revision. https://icd.who.int/browse10/2016/en. Accessed 1 June 2016
- World Health Organization (1993) Guidelines for the development of national programmes for monitoring birth defects. World Health Organization. https://apps.who.int/iris/handle/10665/61536. Accessed 25 September 2016
- World Health Organization (1996) Control of hereditary diseases: report of a WHO scientific group 1993, Geneva, Switzerland. https://apps.who.int/iris/handle/10665/41846. Accessed 12 May 2016
- World Health Organization (1999) Services for the Prevention and Management of Genetic Disorders and Birth Defects in Developing Countries. Report of a Joint WHO/WAOPBD Meeting, The Hague, 5-7 January 1999. World Health Organization. https://apps.who.int/iris/handle/10665/66501. Accessed 12 May 2016
- World Health Organization (2006) Management of Birth Defects and Haemoglobin Disorders. Report of a Joint who-March of Dimes Meeting. Geneva, Switzerland, 17-19 May 2006. World Health Organization. http://www.who.int/genomics/publications/WHO-MODreport-final.pdf?ua=1. Accessed 20 February 2016
- World Health Organization (2015) World Health Statistics 2015. World Health Organization. https://www.who.int/gho/publications/world_health_statistics/2015/en/. Accessed 14 February 2016
- World Health Organization (2020) WHO Mortality Database. https://www.who.int/healthinfo/mortality_data/en/. Accessed 1 April 2020
- Wren C, Irving CA, Griffiths JA, O’Sullivan JJ, Chaudhari MP, Haynes SR, Smith JH, Hamilton JL, Hasan A. Mortality in infants with cardiovascular malformations. Eur J Pediatr. 2012;171(2):281–287. doi: 10.1007/s00431-011-1525-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant study data is included in the article.