Abstract
This study evaluated the effects of muscle fiber characteristics on meat quality traits in 45 female fast- and slow-growing ducks. Three duck breeds at typical market ages were selected and slaughtered, including fast-growing ducks (Cherry Valley duck) and slow-growing ducks (Small-sized Beijing duck and Liancheng White duck). M. pectoralis major (PM), m. soleus (SOL), m. gastrocnemius (GAS) and m. extensor digitorum longus (EDL) were used to assess muscle fiber characteristics as well as meat quality properties. The results showed that the fiber compositions in PM, GAS, and EDL muscles only consisted of fast-twitch fibers irrespective of the breeds, while a low percentage of slow-twitch fibers were observed in slow-growing ducks (17.03% and 29.14%). The significant clear differences of fiber diameter, fiber density and fiber cross-sectional area (CSA) was observed among three duck breeds. Small-sized Beijing ducks had the highest diameter and cross-sectional fiber area coupled with a dramatically lowest fiber density when compared to other 2 breeds both in breast and leg muscles. In addition, the meat quality traits such as moisture content, release water, and intramuscular fat content were significantly affected by the breeds. Slow-growing ducks, especially Liancheng White ducks, exhibited higher release water, intramuscular fat content, as well as lower moisture content (P < 0.05) compared to the fast-growing ducks. The lower pH24 h value and shear force tended to be present in breast of Liancheng White ducks (P < 0.05). The higher protein content and collagen content were detected in breast of Liancheng White ducks and the leg muscle of Small-sized Beijing ducks (P < 0.05), respectively. Finally, the correlation coefficients between muscle fiber characteristics and meat quality showed that the diameter, density and CSA of fibers had a moderate or significant correlation with pH, shear force value, moisture content, and protein content of meat in fast-growing ducks. In slow-growing ducks, muscle fiber characteristics had a moderate or significant correlation with pH, shear force value, release water, protein content, and intramuscular fat content of meat. These results indicated that muscle fiber characteristics is a useful parameter to explain in parts the variation of meat quality including pH, shear force value, and protein content of meat, both in slow-growing ducks and fast-growing ducks.
Key words: fast-growing ducks, slow-growing ducks, muscle fiber, meat quality
INTRODUCTION
In the 21st century, meat quality has always been important to the consumer, and it is an especially critical issue for the meat industry (England et al., 2013; Listrat et al., 2016). Breed is an important factor that can influence meat quality in some ways including total number of fibers, fiber cross-sectional area (CSA) and fiber type composition of a given muscle within a species. For example, the longissimus dorsi muscle of Berkshire pigs has a larger percentage of slow-twitch fiber compared to Landrace and Yorkshire pigs (Ryu et al., 2008). Increasing the proportion of slow-twitch fibers in muscle is known to increase the redness and decreases the rate of pH decline (Choi et al., 2007). Lee et al. (2012) showed that significant differences in pork quality traits and muscle fiber characteristics exist among various breeds, and the variation in muscle fiber characteristics can partially explain the variation in meat quality, both across and within breeds. However, little or no information is available on the existence of relationships between various meat quality traits and muscle fiber characteristics in different duck breeds.
There are numerous duck breeds in China including 3 main types of meat ducks are raised: native breeds (also called slow-growing ducks or high-quality ducks), standard ducks (also called fast-growing ducks) and crossbred ducks (Zhang et al., 2019). Slow-growing ducks have an inferior growth rate with a poor feed conversion rate and are reared for a longer period as compared to fast-growing breeds, but the meat of slow-growing ducks has unique meat flavor and texture (Kwon et al., 2014). Earlier Dransfield and Sosnicki (1999) reported higher growth rates may induce morphological abnormalities, induce larger fiber diameters and a higher proportion of glycolytic (white) fibers in poultry. Although certain studies on muscle fiber characteristics, carcass traits and meat quality in ducks were reported by Kisiel and Książkiewicz (2004); Witkiewicz et al. (2004); Bernacki et al. (2008) and Kokoszyński et al. (2020), extensive information on factors determinant of meat quality in slow- and fast-growing ducks is not available. Therefore, the purpose of this study was evaluated the effects of muscle fiber characteristics on meat quality traits in fast- and slow-growing ducks, and to determine how muscle fiber structure influences meat quality in different duck breeds. These data could find out the effect of breeds on meat quality through muscle fiber characteristics in duck, which might provide alternatives to further improve the meat quality in the duck production.
MATERIALS AND METHODS
Ethics Statement
All animal experimental procedures were approved and guided by the Institutional Animal Care and Use Committee of the School of Animal Science and Technology, Yangzhou University (Permit Number: YZUDWSY, Government of Jiangsu Province, China).
Birds, Slaughter Procedures, and Sample Collection
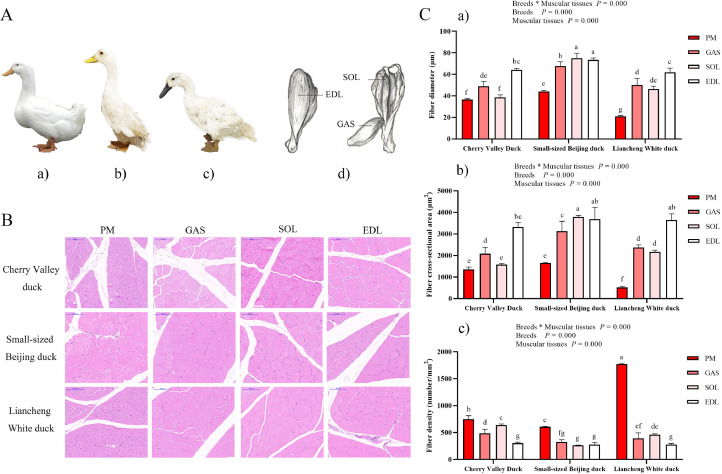
A total of 45 female ducks, including fast-growing ducks (37 d for Cherry Valley ducks, SM3 heavy hybrid, n = 15) and slow-growing ducks (70 d for Small-sized Beijing ducks and 65 d for Liancheng White ducks, n = 15 respectively) were obtained (Figure 1A–1C) from LiHua Farming Co., Jiangsu, China. Individuals within each breed had the same genetic background. The birds were electrically stunned in a water bath (240 mA, 120 V, 5 s), killed by neck cut. After slaughter, the carcasses were cooled in a chilling room (4°C). At 45 min postmortem, the m. pectoralis major (PM) and the leg muscle were taken from the right side of the carcass. Form the leg muscle of carcasses, m. soleus (SOL), m. gastrocnemius (GAS) and m. extensor digitorum longus (EDL) were excised (anatomical locations are shown in Figure 1A–1D). Part of the samples were cut in pieces (0.5 × 0.5 × 1.0 cm3 pieces), stored at 4% paraformaldehyde until analyzed for hematoxylin-eosin (H&E) staining and immunohistochemistry analysis. Another part of the samples were frozen in liquid nitrogen and kept at –80°C until subsequent analyses. Thereafter the breast muscle and leg muscle from the left side of the carcass were excised, and the skin, visible fat and excessive connective tissues were removed, vacuum packed, and stored at 4°C for meat quality analysis.
Figure 1.
Schematic view of muscle fiber and bundle in three distinct commercial breeds of ducks. (A) Appearance of the Cherry Valley duck (a), Small-sized Beijing duck (b) and Liancheng White duck (c) at market age; (d) Schematic diagram of leg muscle anatomical locations. (B) Hematoxylin and eosin staining. Scale bar = 100 μm. (C) Comparison of histological characteristics of muscle fiber of the three duck breeds. a) muscle fiber diameter; b) muscle fiber cross-sectional area; c) fiber density. Vertical bars represent mean ± SD (n = 6). The different superscripts on the bars indicate significant (P < 0.05) differences. Abbreviations: EDL, m. extensor digitorum longus; PM, m. pectoralis major; SOL, m. soleus; GAS, m. gastrocnemius.
H&E Staining and Immunohistochemistry
To evaluate the characteristics of muscle fiber, each muscle section was stained with H&E staining based on the method Cardiff et al. (2014). In brief, muscle samples were fixed in 4% paraformaldehyde for 24 h at room temperature, embedded in paraffin, cut at thicknesses of 5 microns on a cryostat (CM 1860, Leica Biosystems, Wetzlar, Germany), and stained with H&E. Representative areas were photographed under a Nikon 90i microscope (Nikon, Tokyo, Japan) at a magnification of 200 × .
The immunohistochemistry staining protocol was performed as previously described (Kim et al., 2016) with minor modifications. Each muscle sample was fixed in 4% paraformaldehyde for 24 h and paraffin-embedded. Serial muscle sections (10 µm in thickness) were sliced from each sample with a cryostat (CM 1860, Leica Biosystems, Wetzlar, Germany). The sections were blocked with 10% normal goat serum (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Two primary antibodies, anti-fast myosin skeletal heavy chain (MYH1A, 1:1,000, #ab51263; Abcam, Cambridge, UK) and anti-slow myosin skeletal heavy chain (MYH7B, 1:4,000, #ab11083; Abcam, Cambridge, UK) were used, respectively. Sections were incubated overnight at 37°C with the primary antibodies. The secondary anti-bodies, goat anti-mouse IgG H&L (HRP) (1:5,000, # ab205719; Abcam, Cambridge, UK) was applied for 30 min at 37°C. An image analysis system (Image-Pro plus 5.1; Media Cybernetics Inc., Rockville, MD) was used to examine the stained sections. For each muscle, three different points on three images containing a total of about 300 muscle fibers without signs of tissue disruption and freeze damage were estimate. Fiber density (number/mm2), fiber proportions (area %), CSA (μm2) and fiber diameter (μm) were calculated. Mean CSA of each fiber was estimated from about 300 fibers and fiber density was presented as the number of individual fibers per 1 mm2 of the total fiber CSA. Muscle fiber area percentage (%) was defined as the total CSA of each fiber type to the total fiber area.
Meat Quality Measurements
The pH24h was measured 24 h after slaughter by using a portable pH meter (HI9125 portable water proof pH/oxidation reduction potential meter, HANNA Instruments, Cluj-Napoca, Romania). The pH meter was standardized by a two-point method against standard buffers of pH 4.0 and pH 7.0. The average pH value was defined by 3 measurements at different points on the same muscle samples.
Release water analysis was carried out with a dilatometer (C-LM3B, Tenovo, Beijing, China) according to the method of Joo (2018) with adaptations as follows. Briefly, samples (about 1 g, W1) of the breast muscle and the leg muscle were weighed at 24 h postmortem. Then, 16 layers of filter papers were placed on the top and bottom of the sample. This sandwich was placed between hard plastic plates on the platform of the dilatometer. The meat sample was pressurized (68.66 kPa) for 5 min and weighed the meat sample again (W2). The release water of meat was calculated based on the difference in weight before and after the test. The percentage of release water % was calculate as follow: Release water (%) = (W1-W2) / W1 × 100%.
Shear force was evaluated according to the method of Baublits et al. (2005). Cores with a diameter of 1 cm were removed from the breast muscle and leg muscle at different positions and parallel to fiber orientation. The samples were sheared perpendicular to the long axis of the core with a Digital Meat Tenderness Instrument (model C-LM3B, Northeast Agricultural University, Harbin, China; probe model XL1155). The results are expressed in Newton (N). Each value was an average of 3 measurements on the same muscle samples.
Ground meat composition (protein, intramuscular fat, collagen and moisture) was analyzed with a FoodScan Analyzer (Delta 320, Mettler-Toledo Group) using the methods described by Song et al. (2017). The results were given in percent (g/100 g × 100%) of intramuscular fat, moisture, collagen and protein.
mRNA Expression Analysis
The mRNA expression levels of selected genes were determined using quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis. Total RNA was extracted from the each sample using the TRIZOL reagent (Invitrogen Corp, Carlsbad, CA) with DNase I (Takara Biotechnology Co. Ltd., Dalian, China) to remove DNA. Assessment of RNA quality was performed on a 1.0% agarose-gel electrophoresis and photographed. An aliquot of each extract was used for spectrophotometry to determine RNA quality and concentration. RNA with a 260/280 ratio between 1.95 and 2.2 and a 260/230 ratio > 1 and < 3 was considered satisfactory and was used in this study. Each RNA extract was assayed in triplicate and an average value was determined. Reverse transcription was performed using a PrimeScript RT Master Mix kit (Takara Biotechnology Co. Ltd., China) according to the manufacturer's instructions. qRT-PCR was carried out in optical 96-well plates on an ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using SYBR Premix Ex Taq Kits (Takara Biotechnology Co. Ltd., China). Primer sequences for qRT-PCR were designed using Primer Express 5.0 software (Applied Biosystems) based on the National Center for Biotechnology Information published sequences (www.ncbi.nlm.nih.gov, Table 1). All primer pairs generated specific amplicons with expected size. All primers were evaluated for amplification efficiency before use. Efficiency of target genes was within 5% of the reference gene (β-actin). A melting curve analysis was performed after all reactions to ensure amplification specificity. MYH7B, MYH1A, and MYH1B were normalized to internal control gene. Relative expression levels of the target mRNAs were calculated using the 2−ΔΔCt method as described by Livak & Schmittgen (2001).
Table 1.
Primers for qRT-PCR of MyHC-related genes.
| Gene | GenBank accession | Primer sequence (5′→3′) | Length (bp) | Product size (bp) | Annealing temperature (°C) |
|---|---|---|---|---|---|
| MYH7B | NM_204587.2 | F: GCTGCGGTGTAACGGTGTC | 19 | 119 | 60 |
| R: CTGGAATGGCTGCTGGGT | 18 | ||||
| MYH1A | NM_001013396.1 | F: GAACCCTCCCAAGTATGA | 18 | 124 | 60 |
| R: GAGACCCGAGTAGGTGTAG | 19 | ||||
| MYH1B | NM_204228.3 | F: GGGAGACCTGAATGAAATGGAG | 22 | 140 | 60 |
| R: CTTCCTGTGACCTGAGAGCATC | 22 | ||||
| β-actin | EF667345.1 | F: GAGAAATTGTGCGTGACATCA | 21 | 152 | 60 |
| R: CCTGAACCTCTCATTGCCA | 19 |
Note: All the primers are designed with Primer 5.0 software and synthesized by Nanjing Qingke bioengineering company. F, forward primer. R, reverse primer.
Abbreviations: MyHC, myosin heavy-chain; MYH7B, (type I) slow-twitch myosin heavy-chain; MYH1A, (type IIb) fast-twitch myosin heavy-chain; MYH1B, (type IIa) fast-twitch myosin heavy-chain.
Statistical Analysis
The error terms used throughout this study are standard deviation (SD). The experimental data were presented as means ± SD. Statistical analysis was performed using SPSS statistical software (SPSS, Ver. 18.0). A two-way ANOVA was performed in order to evaluate the main effect. Duncan's multiple range test was used to analysis of the main effect of each independent variable and any significant differences between them. A level of P < 0.05 was set as the criterion for statistical significance. The relationships between muscle fiber characteristics and meat quality in different duck breeds were analyzed using Pearson correlation coefficients. Besides, descriptive statistics based on the Guillford scale was applied (Guillford, 1942).
RESULTS
Comparison of Meat Quality Traits Among Breeds
To compare the meat quality traits among breeds, the pH24 h, release water, shear force, moisture content, protein content, intramuscular fat content and collagen content were measured. Some significant main effects were observed for different breeds and muscular tissues on the contents of water and intramuscular fat (Table 2). The higher intramuscular fat content and the lower moisture content were observed in Liancheng White ducks (P < 0.05), while the highest moisture content and the lowest intramuscular fat content in Cherry Valley ducks (P < 0.05). Also, the intramuscular fat content was greater in leg muscle than that in the breast muscle, and less release water in leg muscles (P < 0.05). In addition, there was a significant interaction between factors (breeds and muscular tissues) for the meat pH24 h value, shear force, protein content and collagen content (P < 0.05). The lower pH24 h value and shear force tended to be present in breast muscle of Liancheng White ducks (P < 0.05). The greater protein contents were detected in breast muscle of Liancheng White ducks and Small-sized Beijing ducks (P < 0.05). Also, the highest collagen content was found in leg muscle of Small-sized Beijing ducks (P < 0.05). Collectively, these results indicated that the breed was the more important influencing factor in meat quality traits tested, and the Liancheng White ducks had greater protein content, intramuscular fat content, as well as fewer shear force and moisture content.
Table 2.
Meat quality traits of the Cherry Valley duck, Small-sized Beijing duck and Liancheng White duck at market age.
| Items | pH24 h | Release water (%) | Shear force (N) | Moisture content (%) | Protein content (%) | IMF content (%) | Collagen content (%) |
|---|---|---|---|---|---|---|---|
| Cherry Valley duck | |||||||
| Breast | 5.99 ± 0.11b | 15.97 ± 4.45 | 36.08 ± 2.94c | 75.61 ± 0.12 | 23.28 ± 0.57b | 1.75 ± 0.17 | 1.32 ± 0.22b |
| Leg | 6.39 ± 0.11a | 9.88 ± 2.68 | 54.33 ± 0.61a | 74.48 ± 1.24 | 22.41 ± 0.45c | 2.50 ± 0.58 | 1.51 ± 0.20ab |
| Small-sized Beijing duck | |||||||
| Breast | 6.30 ± 0.11a | 11.34 ± 2.97 | 46.64 ± 1.14b | 73.34 ± 0.97 | 23.67 ± 0.09a | 2.37 ± 0.40 | 1.44 ± 0.08b |
| Leg | 6.33 ± 0.14a | 8.13 ± 3.78 | 57.83 ± 2.53a | 72.97 ± 0.81 | 21.77 ± 0.10d | 3.28 ± 0.02 | 1.63 ± 0.16a |
| Liancheng White duck | |||||||
| Breast | 5.76 ± 0.04c | 15.98 ± 0.69 | 31.57 ± 5.18c | 72.78 ± 0.60 | 23.76 ± 0.14a | 2.41 ± 0.30 | 1.44 ± 0.10b |
| Leg | 5.96 ± 0.08b | 13.26 ± 2.54 | 55.68 ± 1.56a | 73.23 ± 1.12 | 21.97 ± 0.07d | 3.20 ± 0.44 | 1.31 ± 0.07b |
| Breeds | |||||||
| Cherry Valley duck | 6.20 ± 0.23 | 12.49 ± 4.56a | 45.20 ± 10.17 | 75.04 ± 1.04a | 22.84 ± 0.67 | 2.13 ± 0.57b | 1.41 ± 0.22 |
| Small-sized Beijing duck | 6.33 ± 0.12 | 9.97 ± 3.48b | 52.23 ± 6.38 | 73.15 ± 0.88b | 22.72 ± 0.99 | 2.83 ± 0.54a | 1.54 ± 0.15 |
| Liancheng White duck | 5.86 ± 0.12 | 14.43 ± 2.34a | 43.63 ± 13.64 | 73.00 ± 0.95b | 22.87 ± 0.94 | 2.80 ± 0.56a | 1.37 ± 0.11 |
| Muscular tissues | |||||||
| Breast | 5.99 ± 0.23 | 14.12 ± 3.63a | 38.09 ± 7.35 | 74.15 ± 1.43 | 23.53 ± 0.43 | 2.12 ± 0.42b | 1.39 ± 0.16 |
| Leg | 6.23 ± 0.22 | 10.63 ± 3.44b | 55.95 ± 2.16 | 73.69 ± 1.29 | 22.10 ± 0.40 | 2.92 ± 0.57a | 1.49 ± 0.20 |
| P-value (two-way ANOVA) | |||||||
| Breeds | 0.000 | 0.027 | 0.000 | 0.000 | 0.545 | 0.000 | 0.052 |
| Muscular tissues | 0.000 | 0.009 | 0.000 | 0.228 | 0.000 | 0.000 | 0.121 |
| Breeds * Muscular tissues | 0.000 | 0.547 | 0.006 | 0.080 | 0.000 | 0.867 | 0.029 |
Note: Results are reported as means ± SD (n = 6).
a-dSuperscripted letters within a row indicate significant differences (P < 0.05).
Abbreviation: IMF, intramuscular fat.
Comparison of Muscle Fiber Morphology Traits Among Breeds
To compare the morphology traits of muscle fibers in ducks, representative characteristics of 4 muscular tissues were investigated (Figure 1B). There was significant interaction between factors (breeds and muscular tissues) for the fiber diameter, CSA, and fiber density (Figure 1C, Supplementary Table 1). The diameter of breast muscle fibers was significantly smaller than that of other muscular tissues in all three duck breeds, especially when compared to EDL (P < 0.05) (Figure 1C-a). The largest muscle fiber CSA was observed in SOL and EDL of Small-sized Beijing duck (Figure 1C-b). As expected, the fiber density showed an opposite trend from the fiber diameter and the CSA (Figure 1C-c). The highest fiber density (1770/μm2) was observed in PM of Liancheng White duck.
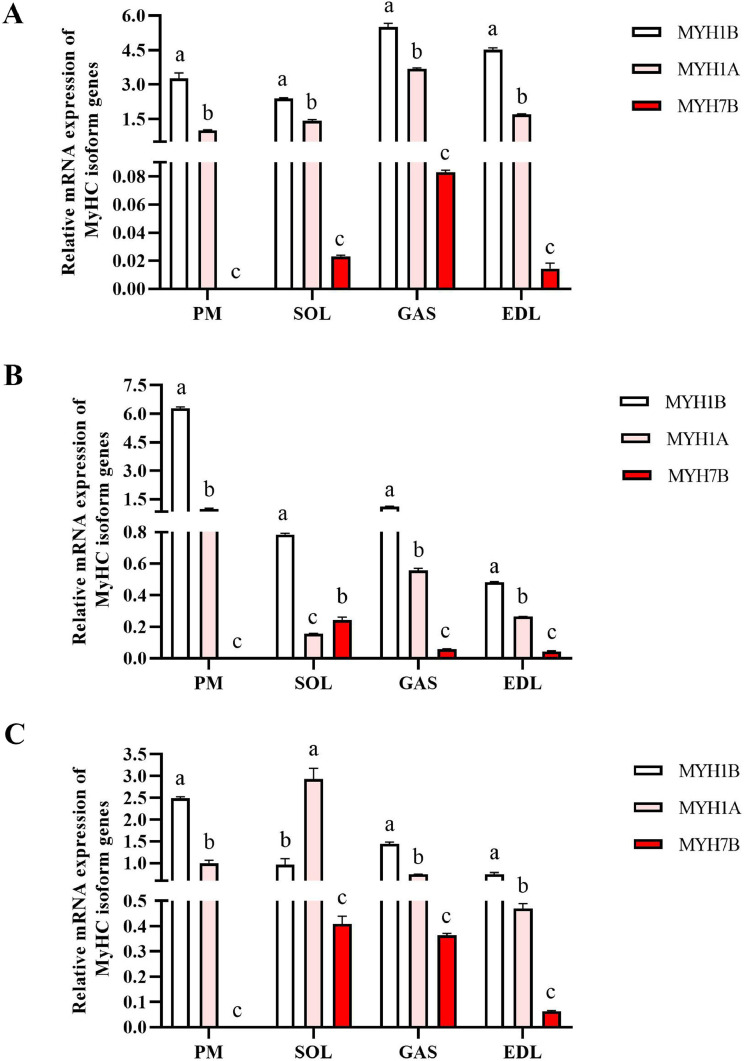
Comparison of Myosin Heavy Chain-Based Fiber Characteristics Among Breeds
Next, the mRNA expression of MyHC isoform MYH7B (type I, slow-twitch), MYH1A (type IIb, fast-twitch), and MYH1B (type IIa, fast-twitch) in three duck breeds were detected by qRT-PCR. As shown in Figure 2, Cherry Valley duck, Small-sized Beijing duck and Liancheng White duck exhibited higher expression levels of MYH1B and MYH1A, but MYH7B was barely expressed in PM (P < 0.05). Although the MyHC genes were expressed in all leg muscles, the expression patterns were different in GAS, SOL, and EDL. Generally, the expression levels of MYH1B was the highest, and the expression levels of MYH7B was the lowest. However, the Small-sized Beijing duck exhibited higher expression levels of MYH7B than MYH1B in SOL (P < 0.05) (Figure 2B), and the Liancheng White duck exhibited higher expression levels of MYH7B than MYH1A in SOL (P < 0.05) (Figure 2C).
Figure 2.
Relative mRNA expressions of myosin heavy-chain (MyHC) isoform genes in the muscles of the Cherry Valley duck (A), Small-sized Beijing duck (B) and Liancheng White duck (C). mRNA expression was normalized to β-actin gene expression. Data are expressed as means ± SD (n = 3). Statistically significant differences are indicated by different letters (P < 0.05). Abbreviations: PEDL, m. extensor digitorum longus; M, m. pectoralis major; GAS, m. gastrocnemius; SOL, m. soleus.
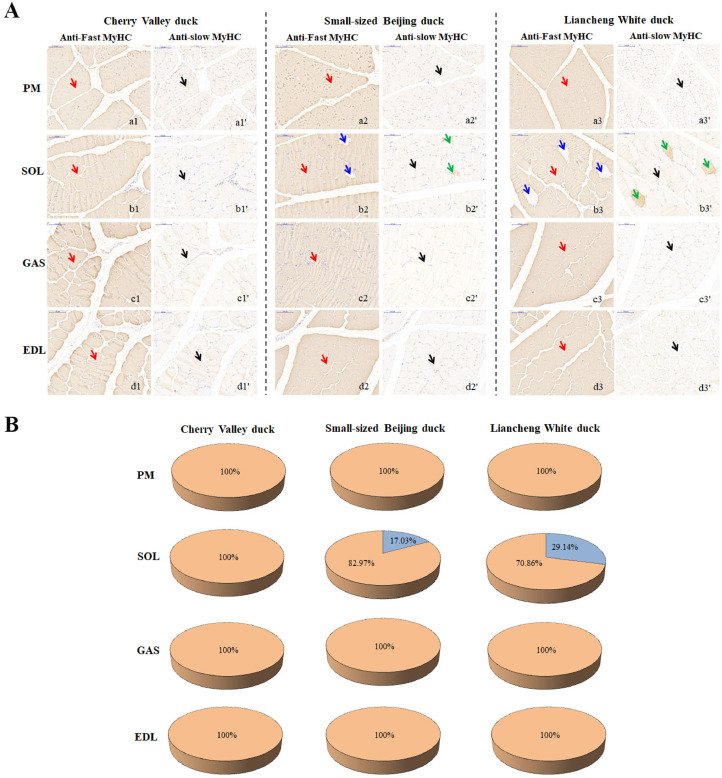
We also performed immunohistochemistry analysis of anti-MyHC antibody to investigate the fiber type composition. As shown in Figure 3A, fiber type composition within muscles showed a similar trend to qRT-PCR results. Fast-reactive fibers tended to be distributed more evenly throughout the sections while slow-reactive fibers tended to be more specific. Anti-slow MHC, which corresponds to type I, did not stain any muscles in Cherry Valley duck (Figure 3a1-a1 and a1′-d1′). The three major muscles (PM, GAS and EDL) observed were pure types (consisting of one isoform, type II), whereas hybrid types (consisting of 2 isoforms, type I and type II) were only found in the SOL skeletal muscles in Small-sized Beijing duck and Liancheng White duck (Figure 3b2-b3 and b2′-b3′). However, the proportions of the fiber types varied greatly between the SOL muscles among the breeds. As shown in Figure 3B, the area percentage of type I fiber in SOL muscle was highest in Liancheng White ducks (29.14%) when compared to Small-sized Beijing ducks (17.03%) and Cherry Valley duck (0%). There were no differences in the percentage of fibers between breed groups in PM, GO, and EDL, which comprise only fast-twitch fibers. Together, the fiber compositions of PM, GAS and EDL muscles only consisted of fast-twitch fibers irrespective of the breeds, while little slow-twitch fibers could be identified in SOL in Liancheng White ducks and Small-sized Beijing ducks.
Figure 3.
Comparison of myosin heavy-chain (MyHC) based muscle fiber characteristics. (A) Immunohistochemical analysis for four separate muscles using an anti-MyHC antibody in three duck breeds at market age. Scale bars: 100 µm. Red arrows are pointing to examples of fibers showing positive reactivity for fast-myosin staining (type II); blue arrows are pointing to examples of fibers that were nonreactive. Green arrows are pointing to examples of fibers showing positive reactivity for slow-myosin staining (type I); black arrows are pointing to examples of fibers that were non-reactive. (B) Fiber area composition of four separate muscles in three duck breeds at market age. Results are mean values. type I = blue; type II = orange. Abbreviations: EDL, m. extensor digitorum longus; GAS, m. gastrocnemius; PM, m. pectoralis major; SOL, m. soleus.
The Relationship of Muscle Fiber Characteristics and Meat Quality Traits in Duck Breeds
To estimate the relationships of muscle fiber characteristics and meat quality traits in duck breeds, the Pearson correlation coefficients (r) were evaluated. Correlation coefficients of muscle fiber characteristics including fiber diameter, CAS, and fiber density with meat quality traits for fast- and slow-growing ducks are presented in Table 3. For moisture content with the muscle fiber diameter, obtained value R was sufficient for evaluating the relationship as poor (r = −0.081), and for collagen content of values R = 0.489, correlations could be regarded as moderate and the value of correlation factor as real in slow-growing ducks. Significant correlations of meat quality traits with fiber density were observed for pH24 h (r = 0.610), shear force value (r = 0.809), intramuscular fat content (r = 0.593), collagen content (r = 0.489), release water (r = −0.696) and protein content (r = −0.769). Consistent with these observations, significant correlations with fiber CSA were observed for shear force value (r = 0.808), intramuscular fat content (r = 0.606), release water (r = −0.528) and protein content (r = −0.814). For fiber density parameters, significant correlations were observed with pH24 h (r = −0.569), shear force value(r = -0.936), intramuscular fat content (r = -0.574), release water (r = 0.505) and protein content (r = 0.796). Although a similar relationship between fiber diameter and meat quality was observed in fast-growing ducks, there were poor relationships between fiber diameter and release water (r = −0.393), collagen content (r = −0.025) in fast-growing ducks. For pH24 h (r = 0.497), shear force value (r = 0.54) and moisture content (r = −0.469) with the fiber CSA, correlations could be regarded as moderate, and for protein content of values r = −0.594, correlations could be regarded as significant. In addition, there was poor relationship between fiber density and collagen content (r = −0.034). For other items with the muscle fiber density, an r as large as 0.469 or larger, either positive or negative, correlations could be regarded as moderate or significant.
Table 3.
Correlation coefficients (r) between muscle fiber characteristics and meat quality traits in fast- and slow-growing ducks.
| Slow-growing ducks |
Fast-growing ducks |
|||||
|---|---|---|---|---|---|---|
| Items | Fiber diameter | Fiber cross-sectional area | Fiber density | Fiber diameter | Fiber cross-sectional area | Fiber density |
| pH24 h | 0.610⁎⁎ | 0.444* | −0.569⁎⁎ | 0.574* | 0.497* | −0.604⁎⁎ |
| Shear force value (N) | 0.809⁎⁎ | 0.808⁎⁎ | −0.936⁎⁎ | 0.56* | 0.54* | −0.671⁎⁎ |
| Release water (%) | −0.696⁎⁎ | −0.528⁎⁎ | 0.505⁎⁎ | −0.393 | −0.393 | 0.532* |
| Moisture content (%) | −0.081 | −0.066 | −0.034 | −0.571* | −0.469* | 0.594⁎⁎ |
| Protein content (%) | −0.769⁎⁎ | −0.814⁎⁎ | 0.796⁎⁎ | 0.525* | −0.594⁎⁎ | 0.469* |
| IMF content (%) | 0.593⁎⁎ | 0.606⁎⁎ | −0.574⁎⁎ | 0.517* | 0.407 | −0.542* |
| Collagen content (%) | 0.489* | 0.319 | −0.127 | −0.025 | 0.036 | −0.034 |
Note: Levels of significance: *P < 0.05; **P < 0.01.
Abbreviation: IMF, Intramuscular fat.
DISCUSSION
China is the most productive duck meat producer in the world (Zeng et al., 2016), and duck meat plays an important role in the Chinese poultry market (Bai et al., 2020). Consumers are increasingly demanding duck meat products which are of high quality 100% of the time, particularly in affluent countries. In present study, we evaluated the effects of muscle fiber characteristics on meat quality traits in fast- and slow-growing ducks, and to determine how muscle fiber structure influences meat quality in different duck breeds.
Intramuscular fat content is discussed as a target trait for breeding schemes because it is a major concern for both the meat industry and consumers, increasing the intramuscular fat content to improve the meat quality is the most important part of the breeding work (Chen et al., 2018). In present study, results showed a lower intramuscular fat content of fast-growing ducks compared to slow-growing ducks. The shear force values were used to assess the tenderness, which is another meat trait of importance to consumers and processors (Huda et al., 2011). In our study, the lower shear force tended to be present in the breast muscle of slow-growing ducks. Also, the greater protein contents were detected in breast muscle of slow-growing ducks and fewest in the leg muscle of fast-growing ducks. To concluded, these indicated that the breed was the more important influencing factor in meat quality traits tested, and the slow-growing ducks, particularly the Liancheng White ducks had greater protein content and intramuscular fat content, as well as fewer shear force and moisture content.
In general, muscle fiber type is responsible for the variation in meat quality within and between muscles (Kirchofer et al., 2002; Choi and Kim, 2009). Kim et al. (2016) reported that the clearly different characteristics of the muscle fiber type in bovine muscles are basically a result of the differences in their physiological functions, and are consequently related to beef quality. Ishamri and Joo (2017) reported that the main fiber types are type IIB, IIA and type I in poultry. In this study, we investigated the expression of myosin heavy chain-related genes (MYH1A, MYH1B, and MYH7B) and the myosin heavy chain-related proteins. We distinguished the fast-twitch fibers (type II) and slow-twitch fibers (type I) in fast- and slow-growing ducks. However, we were unable to distinguish fast-twitch fibers (IIB and IIA) at the protein level in breast muscle and leg muscle, due to the lack of antibody. Our results showed that the breast muscle in three duck breeds is entirely composed of fast-twitch fibers, which inconsistent with the previous studies demonstrated the duck has higher red muscle fiber (approximately 16% fast-twitch fibers and 84% slow-twitch fibers) in Pekin duckling breast (Smith et al., 1993). It seems that the variations between the abovementioned 3 studies possibly caused by the different breed and different age of the experimental animal (ducklings vs. ducks at typical market ages). In our study, all of the GAS and EDL muscles in 3 duck breeds were entirely composed of fast-twitch fibers, while little slow-twitch fibers could be identified in SOL in slow-growing ducks. So, given this reality, it is unlikely that muscle fiber type directly explains differences in duck meat quality. Therefore, in the following section publications are considered that analyzed the different morphological characteristics of muscle fiber, as we speculated that the muscle fiber morphology traits had greater impacts on meat quality traits compared to the fiber types.
It is generally accepted that low muscle fiber number correlates with fibers that exhibit greater hypertrophy, and these large muscle fibers seem to have a larger fiber CSA tend to have poorer meat quality than muscles having a smaller fiber CSA (Gentry et al., 2002). One possible explanation is that the size of muscle fibers affects muscle growth potential and the size of the fiber bundle, resulting in the visible coarseness of transverse sections of meats (Chen et al., 2007; Zhao et al., 2011; Kokoszyński et al., 2019). That is confirmed in the present study, given that the close relationship between the muscle fiber characteristics (including muscle fiber diameter, fiber density and fiber CSA) and tenderness of meat: thinner muscle, smaller CSA, more density, lower shear force (better tenderness). The assumption that muscle fiber size also influences the water-holding capacity is supported by the results of Berri et al. (2007) who showed that breast muscles with larger fiber size exhibited and lower drip loss values. Our results indicated that muscle fiber size and CAS was inversely correlated with release water in slow-growing ducks. However, the correlations between fiber characteristics and release water were no significant correlation in fast-growing ducks. Notably, our experiment also indicated that moisture content was significant correlated with the fiber diameter, CSA and density in fast-growing ducks. Therefore, we conclude that differences in meat quality traits and muscle fiber characteristics exist among various breeds, and the variation in muscle fiber characteristics can partially explain the variation in meat quality, both across and within breeds.
CONCLUSIONS
In conclusion, the results showed that the significant differences in meat quality traits and muscle fiber characteristics exist among duck breeds, although all ducks evaluated in this research were assigned a normal quality class. Slow-growing ducks, especially Liancheng White ducks, exhibited higher release water, intramuscular fat content, as well as lower moisture content. Muscle fiber characteristics is a useful parameter to explain in parts the variation of meat quality including pH, shear force value, and protein content of meat, both in slow-growing ducks and fast-growing ducks. Our continued researches will focus on manipulating muscle fiber characteristics to improve meat quality traits in the duck production.
Acknowledgments
ACKNOWLEDGMENTS
This work was financially supported by the earmarked fund for Modern Agro-industry Technology Research System (CARS-42-3) and the Plant and Animal Breeding Project of Jiangsu province (PZCZ201735).
DISCLOSURES
The authors declare there was no conflict of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2021.101264.
Appendix. Supplementary materials
REFERENCES
- Bai H., Bao Q., Zhang Y., Song Q., Liu B., Zhong L., Zhang X., Wang Z., Jiang Y., Xu Q., Chang G., Chen G. Research Note: Effects of the rearing method and stocking density on carcass traits and proximate composition of meat in small-sized meat ducks. Poult. Sci. 2020;99:2011–2016. doi: 10.1016/j.psj.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baublits R.T., Pohlman F.W., Brown A.H., Johnson Z.B. Effects of sodium chloride, phosphate type and concentration, and pump rate on beef biceps femoris quality and sensory characteristics. Meat Sci. 2005;70:205–214. doi: 10.1016/j.meatsci.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Bernacki Z., Kokoszyński D., Mallek T. Evaluation of selected meat traits in seven-week-old duck broilers. Anim. Sci. Pap. Rep. 2008;26:165–174. [Google Scholar]
- Berri C., Bihan-Duval E.Le, Debut M., Santé-Lhoutellier V., Baéza E., Gigaud V., Jégo Y., Duclos M.J. Consequence of muscle hypertrophy on characteristics of Pectoralis major muscle and breast meat quality of broiler chickens. J. Anim. Sci. 2007;85:2005–2011. doi: 10.2527/jas.2006-398. [DOI] [PubMed] [Google Scholar]
- Cardiff R., Miller C., Munn R. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014;2014:655–658. doi: 10.1101/pdb.prot073411. [DOI] [PubMed] [Google Scholar]
- Chen G.S., Sui Y.N. Production, performance, slaughter characteristics, and meat quality of Ziwuling wild crossbred pigs. Trop. Anim. Health. Pro. 2018;50:365–372. doi: 10.1007/s11250-017-1441-2. [DOI] [PubMed] [Google Scholar]
- Chen X.D., Ma Q.G., Tang M.Y., Ji C. Development of breast muscle and meat quality in Arbor Acres broilers, Jingxing 100 crossbred chickens and Beijing fatty chickens. Meat Sci. 2007;77:220–227. doi: 10.1016/j.meatsci.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Choi Y.M., Kim B.C. Muscle fibre charactiristics, myofibrilar protein isoforms, and meat quality. Livest. Sci. 2009;122:105–118. [Google Scholar]
- Choi Y.M., Ryu Y.C., Kim B.C. Influence of myosin heavy- and light chain isoforms on early postmortem glycolytic rate and pork quality. Meat Sci. 2007;76:281–288. doi: 10.1016/j.meatsci.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Dransfield E., Sośnicki A. Relationship between muscle growth and poultry meat quality. Poult. Sci. 1999;78:743–746. doi: 10.1093/ps/78.5.743. [DOI] [PubMed] [Google Scholar]
- England E.M., Scheffler T.L., Kasten S.C., Matarneh S.K., Gerrard D.E. Exploring the unknowns involved in the transformation of muscle to meat. Meat Sci. 2013;95:837–843. doi: 10.1016/j.meatsci.2013.04.031. [DOI] [PubMed] [Google Scholar]
- Gentry J.G., McGlone J.J., Blanton J.R., Miller M.F. Impact of spontaneous exercise on performance, meat quality, and muscle fiber characteristics of growing/finishing pigs. J. Anim. Sci. 2002;80:2833–2839. doi: 10.2527/2002.80112833x. [DOI] [PubMed] [Google Scholar]
- Guilford J.P. McGraw-Hill Book, Inc.; New York, NY: 1942. Fundamental Statistics in Psychology and Education. [Google Scholar]
- Huda N., Putra A.A., Ahmad R. Proximate and physicochemical properties of Peking and Muscovy duck breasts and thighs for further processing. J. Food Agric. Environ. 2011;9:82–88. [Google Scholar]
- Ishamri I., Joo S.-T. Poultry meat quality in relation to muscle growth and muscle fiber characteristics. Korean J. Food Sci. Anim. Resour. 2017;37:873–883. doi: 10.5851/kosfa.2017.37.6.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo S.T. Determination of water-holding capacity of porcine musculature based on released water method using optimal load. Korean J. Food Sci. Anim. Resour. 2018;38:823. doi: 10.5851/kosfa.2018.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.D., Yang H.S., Jeong J.Y. Comparison of characteristics of myosin heavy chain-based fiber and meat quality among four bovine skeletal muscles. Korean J. Food Sci. Anim. Resour. 2016;36:819–828. doi: 10.5851/kosfa.2016.36.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchofer K.S., Calkins C.B., Gwartney B.L. Fiber-type composition of muscles of the beef chuck and round. J. Anim. Sci. 2002;80:2872–2878. doi: 10.2527/2002.80112872x. [DOI] [PubMed] [Google Scholar]
- Kisiel T., Książkiewicz J. Comparison of physical and qualitative traits of meat of two polish conservative flocks of ducks. Arch. Tierz. 2004;47:367–373. [Google Scholar]
- Kokoszyński D., Piwczyński D., Arpášova H., Hrnčár C., Saleh M., Wasilewski R. A comparative study of carcass characteristics and meat quality in genetic resources Pekin ducks and commercial crossbreds. Asian-Australas J. Anim. Sci. 2019;32:1753–1762. doi: 10.5713/ajas.18.0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoszyński D., Bernacki Z., Biegniewska M., Saleh M., Stęczny K., Zwierzyński R., Kotowicz M., Sobczak M., Żochowska-Kujawska J., Wasilewski P.D., Bucek T., Kmiecik M. Carcass composition, physicochemical and sensory characteristics of meat from genetic reserve ducks. S. Afric. J. Anim. Sci. 2020;50:55–68. [Google Scholar]
- Kwon H.J., Choo Y.K., Choi Y.I., Kim E.J., Kim H.K., Heo K.N., Choi H.C., Lee S.K., Kim C.J., Kim B.G., Kang C.W., An B.K. Carcass characteristics and meat quality of Korean native ducks and commercial meat-type ducks raised under same feeding and rearing conditions. Asian-Australas J. Anim. Sci. 2014;27:1638–1643. doi: 10.5713/ajas.2014.14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Choe J.H., Choi Y.M., Jung K.C., Rhee M.S., Hong K.C., Lee S.K., Ryu Y.C., Kim B.C. The influence of pork quality traits and muscle fiber characteristics on the eating quality of pork from various breeds. Meat Sci. 2012;90:284–291. doi: 10.1016/j.meatsci.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Listrat A., Lebret B., Louveau I., Astruc T., Bonnet M., Lefaucheur L., Picard B., Bugeon J. How muscle structure and composition influence meat and flesh Quality. Sci. World J. 2016;2016 doi: 10.1155/2016/3182746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ryu Y.C., Choi Y.M., Lee S.H., Shin H.G., Choe J.H., Kim J.M., Hong K.C., Kim B.C. Comparing the histochemical characteristics and meat quality traits of different pig breeds. Meat. Sci. 2008;80:363–369. doi: 10.1016/j.meatsci.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Smith D.P., Fletcher D.L., Buhr R., Beyer S. Pekin duckling and broiler chicken pectoralis muscle structure and composition. Poult. Sci. 1993;72:202–208. [Google Scholar]
- Song Y., Li Y., Zheng S., Dai W., Shen X., Zhang Y., Zhao W., Chang G., Xu Q., Chen G. Effects of forage feeding versus grain feeding on the growth performance and meat quality of Yangzhou geese. Br. Poult. Sci. 2017;58:397–401. doi: 10.1080/00071668.2017.1307942. [DOI] [PubMed] [Google Scholar]
- Witkiewicz K., Kontecka H., Książkiewicz J., Szwaczkowski T., Perz W. Carcass composition and breast microstructure in selected vs non-selected ducks. Anim. Sci. Pap. Rep. 2004;22:65–73. [Google Scholar]
- Zeng T., Chen L., Du X., Lai S.J., Huang S.P., Liu Y.L., Lu L.Z. Association analysis between feed efficiency studies and expression of hypothalamic neuropeptide genes in laying ducks. Anim. Genet. 2016;47:606–609. doi: 10.1111/age.12457. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang L., Bian Y., Wang Z., Xu Q., Chang G., Chen G. Marginal diversity analysis of conservation of Chinese domestic duck breeds. Sci. Rep. 2019;9:13141. doi: 10.1038/s41598-019-49652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Cui H., Liu R., Zheng M., Chen J., Wen J. Comparison of breast muscle meat quality in 2 broiler breeds. Poult. Sci. 2011;90:2355–2359. doi: 10.3382/ps.2011-01432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.