Abstract
At the time of oviposition, the chicken embryo is in its blastodermal stage. The blastoderm displays the unique ability to undergo developmental arrest at low temperatures in a process called “embryonic diapause.” In the wild, diapause occurs in freshly laid eggs until the last egg of the clutch has been laid, providing an evolutionary advantage to hens that can synchronously hatch their eggs. The poultry industry utilizes the diapause phenomenon to store eggs before incubation, thereby mitigating their logistic problems. The embryos can only be stored at particular embryonic stages—termed “diapause developmental window” (DW)—if they are to continue to develop normally thereafter. Both cellular and molecular mechanisms define the limits of this DW which broadly comply with onset of blastulation to early gastrulation. Storage conditions affect the cellular and molecular characteristics of the embryo during this window and their ability to successfully resume development (SRD). At storage temperatures of ~12°C to 18°C, embryos can undergo diapause for a short period (up to 7 days (d)) without affecting SRD. However, following longer period of diapause (up to 28 d), embryo stored at ~12°C, but not at ~18°C, can resume development normally. Moreover, eggs can be heated before or during the storage period which will lead to their commencing in development; however, unlike the non-heated embryos, the storage temperature for heated embryos, which are more advance in developing, is not clear. Thus, based on SRD, this review brings evidence supporting the notion that a lower storage temperature is beneficial for early-stage blastoderms whereas a higher storage temperature is favorable for later-stage/gastrulating embryos. Our understanding of the molecular mechanisms underlying the relationship between storage temperature and development stage within the DW is rather limited. However, it is expected to become relevant in light of the effect of selective breeding of modern avian birds on the advancement of embryonic development stage. Thus, this review discusses parameters that are regulated during the DW and affect SRD, and presents the need to adopt new storage techniques. The pre-managerial decision of required duration of storage with manipulation of storage temperature in the currently used storage techniques may improve SRD characteristics.
Key words: embryonic diapause, diapause developmental window, egg storage, blastoderm, broiler
INTRODUCTION
From the time of fertilization until oviposition, for about 20 to 22 hours (h), the embryo develops within the oviduct. Intrauterine embryo stages are classified by the EG&K normal development table (Eyal-Giladi and Kochav, 1976; Kochav et al., 1980), and from stage VII EG&K to blastulation, the embryo is referred to as the blastoderm. After the first egg of a clutch is laid, the blastoderm has the ability to undergo a resting process that is manifested by reduced metabolism and entry into developmental arrest. The ability of avian blastoderms, including those of chickens, to undergo a temporary suspension of overt metabolic activity or development soon after oviposition is termed diapause (Fasenko, 2007). In the wild, the diapause phenomenon is exhibited by 8 to 12 eggs in a clutch (Odula Olwande et al., 2010). The hen begins incubating the eggs after the last egg of the clutch is laid to achieve synchronous hatching. As an alternative to this strategy, several hens lay eggs in a communal nest, thereby improve their synchronous hatching. Therefore, avian embryo diapause is a unique phenomenon in which the rapidly developing embryo—a living organism which, under normal conditions, has an extremely rapid cell cycle (Pokhrel et al., 2017)—almost completely stops the developmental process for a long time following exposure to low temperature, in some avian potentially up to 7 or 8 weeks (wk) (Arora and Kosin, 1966b). Similarly, other organisms have adapted to variability in environmental conditions at the beginning of embryogenesis by reducing metabolic rate and slowing development (Calow and Forbes, 1998; Chapuis and Ferdy, 2012; Brunner et al., 2014; Buryanov, 2015); in these latter organisms, this is best described as a dormancy mechanism (Desmarais and Murphy, 2002; Marijuán, Navarro, and del Moral, 2010; Squeglia et al., 2015; Sturm and Dworkin, 2015). Adoption of this mechanism allows the organisms to wait for improved conditions to resume their life activities, such as development and reproduction (Calow, 1983; Reed and Clark, 2011; Clark et al., 2012).
Aside from its evolutionary advantage, the phenomenon of diapause is relevant to the poultry industry for egg storage (Brake et al., 1997). In practice, the hatcheries can decide whether to incubate or store eggs, depending on commercial requirements such as market demand, incubator capacity, egg transport needs, and hatching synchronization (Bakst and Akuffo, 2002). Therefore, egg storage is a managerial decision commonly practiced by hatchery managers to ensure that high-quality fertilized eggs are available at all times.
Eggs can only be stored during a particular development period of the blastoderm, which is described in this review as the diapause developmental window (DW). There are several milestone criteria in the blastoderm that define the DW: stage of development, cell count, pluripotency state, and blastoderm cytoarchitecture. According to the developmental stage, the DW is limited from blastulation to early gastrulation (32–34 h of embryo development after fertilization). The developmental stage is also related to changes in cell count, pluripotent state, and cellular organization of the blastoderm, which altogether affect the ability to store eggs for prolonged periods with successful resumption of development (SRD) thereafter. In addition to the milestone DW criteria, other factors affecting the DW are characteristics of the albumin and egg yolk, the vitelline membrane, and the eggshell.
As soon as the eggs are laid, the ambient temperature of about 25°C starts to affect the DW. Edwards (1902) suggested that embryos only stop developing at 20.5°C, and termed this as the “physiological zero.” This pioneering study in the field led to the practice of cooling freshly laid eggs within 6 h, to obtain a better-synchronized hatching rate (Schulte-Drüggelte, 2011). The practice of maintaining blastoderms in DW is exploited by commercial management in hatcheries through the provision of adequate storage durations and conditions, mainly temperature and RH (Brake et al., 1997).
The storage conditions for a blastoderm during its DW can only be adequate if the embryos can successfully resume development after diapause. Thus, SRD relies largely on the storage conditions that maintain the embryo within the DW by retaining milestone criteria. Therefore, the storage conditions are considered beneficial only when they result in less advancement in development, retain higher number of live cells, maintain cell pluripotency, and conserve blastoderm morphology and cytoarchitecture. In addition, some changes in egg characteristics at later stages of storage, such as interference with albumin pH (Karoui et al., 2006), can affect SRD and subsequently negatively affect hatchability (Akhlaghi et al., 2013). The role of other physical characteristics of eggs, including the vitelline membrane and eggshell in gas exchange and ion transfer has also been reported (reviewed in Reijrink et al., 2008), but there is a lack of direct evidence for their contribution to SRD. Therefore, the milestone criteria which demonstrate biological significance are considered essential for SRD. In this review, we aim to define appropriate storage conditions for embryos based on their ability to maintain the DW criteria during diapause without affecting their SRD characteristics in the post-diapause period.
EARLY DEVELOPMENTAL STAGES OF THE CHICK EMBRYO AND THE DW
The stage of the embryo is an important factor affecting SRD. Since there is a narrow and restricted DW during which the embryo can be stored, the changes in developmental stage before and during storage are crucial (Fasenko et al., 2001; Pokhrel et al., 2017, 2018). The blastoderm developmental stage at oviposition and prior to storage advances with flock age, due to inadequate prestorage conditions and modern genetic selection. During storage, higher storage temperature and short period of incubation advance the developmental stage. Therefore, proper considerations need to be taken to retain the development stage within the DW.
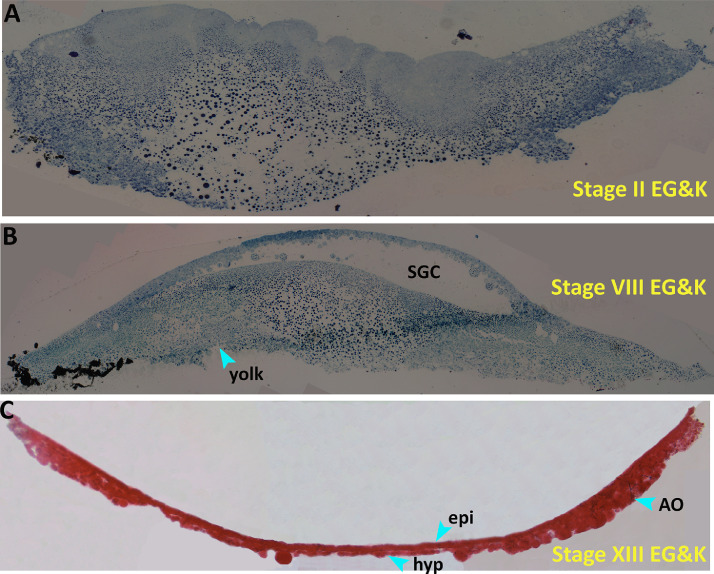
Based on the DW, early embryogenesis events can be categorized into 3 periods: 1) before the DW opens, 2) at DW opening, and 3) when the DW closes. The developmental stages before DW opening include the intrauterine stages (Figure 1A and 1B), when the eggs are unavailable and are therefore not stored. The DW opens only after the egg is laid, lasting from blastulation to early gastrulation (Figures 1C and 2A–E), and closes thereafter.
Figure 1.
Representative images of blastoderm from intrauterine stages and following oviposition. (A) Stage II EG&K – meroblastic cleavage phase. (B) Stage VIII EG&K – area pellucida formation phase. (A) and (B) are intrauterine stages; images obtained from preserved samples of Kochav et al. (1980). (C) Stage XIII EG&K – hypoblast formation phase. Hypoblast is fully covering the ventral region of the epiblast. Image obtained from a freshly oviposited egg. Abbreviations: AO, area opaca; epi, epiblast; hyp, hypoblast; SGC, subgerminal cavity.
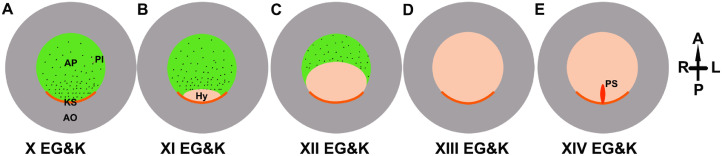
Figure 2.
Schematic diagram (ventral view) of developmental stages during the blastulation process. (A) Stage X EG&K: the embryo is composed of a single-epithelial layer disk – the area pellucida (AP, light green), surrounded by a multilayer ring – the area opaca (AO, light gray). At the posterior border between these lies Koller's sickle (KS, orange). At this stage, some polyingressing cells (PI, black dots) are evident on the ventral surface of the area pellucida. (B) Stage XI EG&K: formation of the hypoblast (Hy, pink); the second layer advances from Koller's sickle anteriorly (black arrow) and covers ~⅓ of the overlying epiblast. (C) Stage XII EG&K: the hypoblast layer further extends anteriorly and covers ~⅔ of the area pellucida. (D) Stage XIII EG&K: the end of the blastulation process; the hypoblast fully covers the overlying epiblast, forming a cavity between the 2 layers, termed blastocoel. (E) Stage XIV EG&K, initiation of the gastrulation process; the formation of the Primitive streak (PS) appears at the center of the posterior border between the area pellucida and the area opaca. Position of embryo is showed in black arrows and denoted by letters: A-Anterior, P-Posterior, L-Left, R-Right.
Eyal-Giladi and Kochav (1976) and Kochav et al. (1980) studied the earliest intrauterine stages of embryogenesis, starting from fertilization until 12 h after oviposition, marking the end of the blastulation process. These studies defined 14 successive developmental stages, starting from fertilization, through the intrauterine period, until 12 h of incubation (marked in roman numerals as I to XIV EG&K), and preceding into stage 2 of Hamburger and Hamilton (1951) (H&H). Eyal-Giladi and Kochav (1976) subdivided the intrauterine stage into 3 developmental periods: the meroblastic cleavage phase (stages I–VI EG&K, Figure 1A), area pellucida (AP) formation (stages VII–IX EG&K, Figure 1B), and hypoblast formation, which marks the blastulation process (stages X–XIV EG&K, Figures 1C and 2). At the beginning of the cleavage period, the first blastomeres are formed by slow syncytial meroblastic cleavage, which is then followed by rapid cell cycles, in which the cells' size is drastically reduced, thus forming the germinal disk. This intrauterine phase lasts ~11 h. These 2 phases are crucial for embryonic axis patterning (Kochav and Eyal-Giladi, 1971). At stage VII EG&K, posterior germinal disc cells start to dissociate and shed ventrally into the subgerminal cavity (Figure 1B). This shedding process progresses anteriorly until stage IX, when a demarcated border between the AP and the surrounding disk, the area opaca, is formed. At stage X, which marks the opening of the DW, the formation of AP is complete and a horseshoe-like cell ridge termed Koller's sickle is formed on the posterior side at the marginal zone between the AP and area opaca. The Koller's sickle indicates a clear anteroposterior axis of the embryo. Starting at stage X, some AP cells start to delaminate in a polyingression process and form ventral cell clusters. Some of these cells are the first sign of hypoblast formation, and among them are cells that give rise to primordial germ cells (Karagenç et al., 1996). In parallel, the underlying hypoblast layer starts to form and extend from the Koller's sickle, advancing anteriorly until it completely covers the overlying disk of the epiblast within the next 12 h (stages X to XIII EG&K, Figure 2A–D). Stage XIII EG&K marks the end of the blastulation process (Figures 1C and 2D), where the epiblast and hypoblast are fully formed and the blastocoel cavity between them becomes apparent. Freshly oviposited blastoderms are in blastulation stage X to XIII EG&K, although the predominant development stage during oviposition in modern poultry is shifting to more advanced stages of development. For instance, in Eyal-Giladi and Kochav (1976), Kochav et al. (1980) and numerous subsequent studies (Table 1), the predominant stage of the embryo in the freshly laid eggs was X EG&K, at the beginning of the blastulation process. However, we and others have found that in modern broiler breeds, the predominant stage is more advanced, XI and XII EG&K for young and old flocks, respectively (Bakst et al., 1997; Pokhrel et al., 2017). This highlights a critical issue regarding narrowing of the DW during which the eggs can be stored in modern breeds.
Table 1.
Total number of cells in freshly laid blastoderm.
| Total number of cells | Developmental stages (EG&K) | Methods used to count the cells | References |
|---|---|---|---|
| 40,000 | X | (Eyal-Giladi and Kochav, 1976) | |
| 30,000 to 40,000 | X | Noted in Petitte et al. (1990) | |
| 40,000 to 60,000 | NT | Noted in Etches et al. (1997) | |
| 30,000 to 50,000 | X, XI, XII, XIII | Microscopic slide and imaging | (Bloom et al., 1998) |
| 32,000 ± 3,000 | VII | Hemocytometer | (Bakst and Akuffo, 2002) |
| 49,317 | VIII, IX, X, XI, XII | Double Bürker Türk counting chamber and inverted microscope | (Reijrink et al., 2010) |
| 60,000, 90,000, 110,000, and 130,000 | X, XI, XII, and XIII, respectively | Confocal laser scanning microscope | (Pokhrel et al., 2017) |
Abbreviation: NT, not determined.
Advancement in developmental stage may also occur due to storage methods and hatchery practices. Developmental stage can advance due to incubation of eggs before storage (PSI, prestorage incubation) or during storage (SPIDES, short period of incubation during egg storage). PSI and SPIDES are aimed to increase the total cell number prior to storage or to reactivate biological mechanisms during storage, respectively (Meir and Ar, 1998; Fasenko et al., 2001a), in order to support the embryos to resume development. However, thorough investigation of their effect on total cell count has not been demonstrated. The notion that the proportion of live cells declines during long-term storage led researchers to study whether increasing the cell number, using 12 h of PSI, would facilitate embryo survival and SRD. To study the effect of PSI, eggs were incubated for 12 h, until stage 5 H&H (Reijrink et al., 2010). However, this was inconsistent with the time frame of normal development, in which 12 h of incubation will only advance the freshly laid embryo to stage XIII EG&K (Eyal-Giladi and Kochav, 1976), thus reflecting an approximate 12 h gap in development. Thus, to start the storage period as early as possible, with the widest DW, the eggs must be transported at low temperature to obtain the desired stage. In addition, while advancing the development stage, it is important to consider that the DW closes at the early gastrulation stages (Olsen and Haynes, 1948; Olsen, 1949; Kosin and St. Pierre, 1956; Reijrink et al., 2010). It should be noted from these studies that the change in the developmental stage criterion will become critical for the industry if the resultant changes also compromise SRD characteristics. In summary, it is still unknown how embryo diapause does not affect SRD characteristics during the DW between blastulation and early gastrulation. As the answer to this question is likely to rely on the crosstalk between morphogenesis, cell-number maintenance and the pluripotent state of the blastoderm; uncovering the connection between DW criteria, and the effects of storage conditions on each criterion, are relevant for poultry research and industry.
Developmental Stage and Cytoarchitecture
Several studies have shown that storage conditions influence blastoderm cytoarchitecture. For instance, under storage conditions of 26°C for less than 7 d, the chicken blastoderm continues to undergo structural changes in gross morphology (Funk and Biellier, 1944), whereas blastoderms stored under lower temperature conditions (10°C–18°C) show cessation of the developmental process (Funk and Biellier, 1944; Arora and Kosin, 1966a; Arora and Kosin, 1968). Yet, we have recently found that also during storage the blastoderm undergoes remarkable changes (Pokhrel et al., 2018). These changes, which are reflected in the developmental progression of the stored embryos, are slower at 12°C, compared to 18°C (Fasenko et al., 2001; Pokhrel et al., 2017; 2018). The morphological changes observed after prolonged storage at around 18°C, including the recesses formation, appear as an abnormal development processes, which is not similar to normal development (as described in previous chapter) under standard incubation condition. In essence, cellular changes that occur in embryos during diapause are correlated with embryonic mortality during storage or short time after incubation, for instance, are more pronounced at higher (18°C) vs. lower (12°C) storage temperature (Pokhrel et al., 2018). Therefore, the lower storage temperature of 12°C, which slows down the developmental progression of the blastoderm for a longer storage duration, as opposed to higher storage temperature, is highly correlated with better SRD characteristics. Taken together, these studies show that cellular changes occur in the DW and are affected by the advancement in the developmental stage at oviposition (due to selective breeding), increased flock age, increased diapause duration, and higher storage temperature.
Moreover, different storage practices, such as PSI, also result in cytoarchitectural changes. However, these effects are not well-defined in the range of recommended storage temperatures (11.5°C–18°C) (Fasenko et al., 2001a; Kgwatalala et al., 2013). Storing blastulation-staged embryos at 12°C improves cell viability and SRD characteristics of embryos under prolonged storage (Table 2); therefore, storage at 12°C was used to study the effect of PSI on cellular changes (Fasenko et al., 2001a). A critical aspect of this study showed a drastic decrease in hatchability when eggs were treated with PSI for 18 h and stored at 12°C for 14 d, compared to eggs that were stored immediately after being laid (9% vs. 70%, respectively, following 14 d at 12°C). Yet, 6 h of PSI resulted in improved hatchability compared to non-PSI-treated embryos (79% vs. 70%, respectively) (Faseko et al., 2001a). In contrast, in another study, 7 h of PSI showed no difference between PSI-treated (86.5%) and non-PSI-treated (control; 86.5%) embryos (Reijrink et al., 2010). In addition, a discrepancy was observed in embryonic developmental staging between the studies, despite using the same PSI protocol (stages XI to XIII EG&K, and stage X EG&K to stage 3 H&H in Fasenko et al., 2001a, and Reijrink et al., 2010, respectively). Notably, in both studies, the embryonic advancement was not in accordance with the expected development as published in the normal development tables (Hamburger and Hamilton, 1951; Eyal-Giladi and Kochav, 1976). During blastulation, the embryo advances one stage every 4 h. Therefore, freshly laid eggs, predominantly at stage XI EG&K, following 6 h of incubation, are expected to reach between stages XII and XIII EG&K and when incubated for 18 h, they are expected to advance to stages 2 to 3 H&H (Hamburger and Hamilton, 1951; Eyal-Giladi and Kochav, 1976). Thus, accurate staging is highly important to define the DW borders. Taken together, increasing PSI duration from 6 to 18 h has a negative effect on hatchability due to a shifting of the embryonic stage from blastulation to gastrulation. Under normal developmental conditions, starting from fertilization, it takes about 32 to 34 h of incubation for the embryo to reach gastrulation. Since embryonic development can advance while they are being held for an extended time in a nest or cage, and during transportation, this development should also be taken into account before modifying the storage technique. Therefore, the above inconsistencies in hatchability resulting from modified storage conditions can be due to embryonic stages that have advanced beyond the DW. Hence, understanding the DW is highly relevant when modifying storage techniques. Moreover, these studies show that embryos that are within the DW can be stored for prolonged duration at 12°C, but once the gastrulation stage starts, embryos cannot be stored at 12°C (Fasenko et al., 2001a). Studies showing beneficial effects on hatchability following heating of guinea fowl eggs and storing them at storage temperature above 12°C (18 °C) insight that storing advanced stage embryo at higher storage temperature may perhaps be beneficial (Kgwatalala et al., 2013). An investigation of whether commercial poultry bird embryos that have advanced to the gastrulation stage can be stored at 18°C without damaging hatchability is warranted. Taken together, freshly laid blastoderm consisting of a normal cytoarchitecture of single layered epithelial sheet within AP, and hypoblast and polyingressing cells ventrally, are greatly influenced by the storage temperature resulting epithelial cell topological changes and recesses formation and these cytoarchitectural changes coupled with embryonic development advancement prior to incubation may narrow the borders of the DW.
Table 2.
Effects of egg storage on blastoderm cell death via apoptosis and necrosis.
| Storage duration (d) | Storage temperature (°C) | Apoptotic/ necrotic cells (%) | Methods used | References |
|---|---|---|---|---|
| 0, 14 | 12 | 3.1±1.8, 13.9±3.6 | Microscopic slide and imaging | (Bloom et al., 1998) |
| 2, 4, and 14 | 18 | 32, 40, 34 | Hemocytometer counting | (Bakst and Akuffo, 2002) |
| 4 and 14 | 16 | 83.58 ± 2.15, 71.42 ± 3.36 | Flow cytometry | (Hamidu et al., 2010) |
| 0, 4 and 14 | 16 | Necrotic cells 0 d, 73.56 ± 8.93; 4 d, 3.56 ± 1.64; 14 d, 16.75 ± 1.73 | Flow cytometry | (Hamidu et al., 2010) |
| 0, 7, 14, 21, and 28 | 18 | 1.33,7, 16.39, 26.76, 39.44 | Hemocytometer counting and microscopic imaging | (Pokhrel et al., 2018) |
| 0, 7, 14, 21, and 28 | 12 | 1.33, 3.69, 7.95, 15.25, 20.42 | Hemocytometer counting and microscopic imaging | (Pokhrel et al., 2018) |
State of Pluripotency
Previous studies have suggested that early-staged embryos during blastulation have remarkable tolerance to large fluctuations in temperature, but during gastrulation stages, they lose their resilience to cooling (Olsen and Haynes, 1948; Marlow W. Olsen, 1949; Kosin and St. Pierre, 1956). Therefore, while stage X EG&K blastoderms can be stored at low temperature for potentially up to 28 d and still allow SRD (Pokhrel et al., 2018), advanced-staged embryos, up to stage 5 H&H, can only be stored for up to 14 d (Reijrink et al., 2010). Previous studies have shown that chick embryos at the blastulation stage activate pluripotency-specific pathways resulting in the appearance of pluripotent cells (Jean et al., 2015). Pluripotent blastodermal cells can give rise to both somatic and germline lineages (Petitte et al., 1990). However, pluripotency of the somatic cells is lost when gastrulation is initiated, and cells become committed to giving rise to the three germ layers: the ectoderm, mesoderm, and endoderm. This transition to become committed leads to loss of SRD ability of the embryo following diapause. Hence, as the DW is restricted to a narrow developmental period from blastulation to early gastrulation. Cellular markers of pluripotency (Nanog, cPOUV, Jean et al., 2015) and cellular markers for labeling the different cell commitment states (mesoderm- Brachyury, endoderm- Sox17, neuronal-SOX2, Noggin, and extra-embryonic region-GATA4) can predict the embryonic ability to resume development after storage.
Total Cell Count in the Blastoderm
The embryonic ability to resume normal development after storage depends largely on a sufficient number of live precursor cells surviving cell death during storage (Fasenko et al., 2001; Fasenko et al., 2001a). The total number of cells in freshly laid embryos has been a matter of debate for many years. The total cell number determined in different studies can be affected by counting technique, chicken strain, and the precise blastoderm stage in freshly laid egg (Table 1). Spratt and Haas (1960, 1967) were the first to report that the total number of blastoderm cells was approximately 60,000. Kochav et al. (1980) provided further data by observing that while at the initial embryonic stages (I–III EG&K), the total cell number increases slowly, a significant increase in cell number occurs between stages IV and IX EG&K. Furthermore, between stages IX and X EG&K, the number of cells decreases by about one-fifth, due to cell death and shedding, and then it increases again. Although the absolute number of cells was not provided, this study highlighted the dynamic changes in cell number from fertilization to the onset of gastrulation. Collectively, these and other studies, which are summarized in Table 1, showed that 22 to 34 h after fertilization, at the time of egg-laying, the total cell count ranges between 30,000 and 130,000, depending on the embryonic stage and counting method. Notably, the maximal number of live cells at oviposition is achieved after massive cell death (70%) that occurs in the late intrauterine stages between VII and X EG&K, only 6 to 8 h before oviposition (Kochav et al., 1980). This remarkable recovery in cell number is likely to rely on an accelerated rate of cell cycle, which is estimated to range between 60 and 100 min (Pokhrel et al., 2017). This extraordinarily rapid cell cycle highlights a possible evolutionary mechanism that can produce a sufficient number of cells in a short period to enable embryo survival following cell death that occurs during prolonged diapause. However, the exact number of live cells required for SRD following prolonged diapause remains unknown.
Regulating Blastoderm Cell Number During Storage
Derived by the amount of proliferating cells (as indicated by their mitotic index) and apoptotic/necrotic cells, the total cell number during storage is an important factor affecting the embryo's SRD ability following storage. Interestingly, we have recently found that despite overt metabolic differences at distinct storage temperatures—with lower metabolism at lower temperature (Cai et al., 2019), embryos which were stored at the lower temperature of 12°C demonstrated a higher mitotic index than those stored at 18°C (Pokhrel et al., 2018). Similar findings were described in turkey blastoderms, stored at 13°C vs. 18°C (Arora and Kosin, 1967). This indicate that higher mitotic index at lower temperatures is mainly due to accumulated mitotically arrested cells (Pokhrel et al., 2018). Recently, Ko et al. (2017) showed that cells are arrested in the G2 phase during diapause (at 16°C). However, based on mitotic index calculations, our data suggests that some of the cells are able to cross the G2–M checkpoint, enter the mitotic phase, and arrest at the metaphase-plate stage (Pokhrel et al., 2018). This finding might be explained by the cells' inability to cross the spindle-assembly checkpoint, which requires the assembly of kinetochore mitotic spindles (Cinnamon et al., 2009; Varetti et al., 2011). When these fail to form due to the instability of microtubules at lower temperature, the cells seize up in mitosis at the metaphase–anaphase transition (Zhai et al., 1995). At variance, at higher storage temperature, the microtubules may be stabilized and form the kinetochore mitotic spindles, allowing the cells to cross the metaphase–anaphase transition and terminate mitosis.
Mitotically arrested cells are likely to be protected from apoptosis and serve as a reservoir of healthy cells for when the temperature rises and incubation initiates. As such, although a small increase in cell number can be found during diapause, this increase is minute compared to the higher cell death during diapause, mainly due to activation of apoptosis (Hamidu et al., 2011). Yet, cell death is accelerated when storage temperatures are higher. Therefore, storage at a temperature lower than 18°C benefits the embryo through both higher mitotic arrest of cells and lower cell death. The regulation of cell death via apoptosis or necrosis in different avian species and storage conditions is summarized in Table 2.
The common denominator in all of these studies is the notion that various storage conditions affect cell viability, which in turn affects hatchability and chick quality. Although many studies demonstrate and agree that egg storage results in increased cell death, the actual number of live cells required for SRD is still unclear. Nevertheless, the data summarized in Table 2 strongly suggests that adequate number of viable cells can be achieved by manipulating the storage temperature within the DW.
The number of live cells can also be increased as previously described, using the PSI and SPIDES manipulations before or during storage, respectively, thereby facilitating embryonic survival following prolonged storage (Meir and Ar, 1998; Fasenko et al., 2001a). However, these practices also promote embryonic development and loss of pluripotency. As a consequence, the embryo exits the DW at an advanced stage and its SRD characteristics are compromised. Clearly, increasing the number of live cells has beneficial effects, but several other DW criteria should also be taken into account while heating the eggs before or during storage.
Taken together, the above studies provide evidence of cell-number regulation during the DW being a crucial factor for SRD, and show that the negative effect of cell death can be minimized by manipulating either the storage temperature or developmental stage of the blastoderm.
STORAGE CONDITIONS DURING DW – A PRACTICAL VIEW
Previous works has shown massive cell death during prolonged storage of eggs. However, this effect can be minimized by storing blastulation-staged embryos at a lower temperatures (Table 2). Thus, the same storage conditions can be applied to more advanced developmental stages or even early-gastrulation stage embryos as well. Here, we discuss the effects of storage conditions of embryos at the blastulation stage on their subsequent development.
Although immediate incubation of freshly laid egg will override the undesired outcomes of egg storage on the embryonic SRD following diapause (Brake et al., 1997; Fasenko, 2007; Hamidu et al., 2011), it is not commercially possible. Moreover, such practice is not highly advantageous, since a few days of storage have been shown to be beneficial in improving hatchability (Asmundson, 1947; Mayes and Takeballi, 1984; Brake et al., 1997; Pokhrel et al., 2018). Nevertheless, an increase in storage duration causes early, mid, and late embryonic mortality during 3 to 5 d, 12 to 14 d, and 18 to 20 d of incubation, respectively (Romanoff, 1949; Pokhrel et al., 2018; Cai et al., 2019). An increase in embryonic mortality is already evident after 7 d of storage, followed by a rapid increase after 13 d (Scott, 1933; Mayes and Takeballi, 1984). Thus, proper storage conditions are of the utmost importance to the poultry industry. Proper egg-storage conditions rely on several factors, the most important being storage temperature, storage duration, and RH. These inter-related factors influence SRD characteristics and as a consequence, hatchability.
Storage Temperature
Early avian embryos are poikilothermic (Cai et al., 2019); the development and maintenance of metabolic functions rely mainly on the external source of heat to which the eggs are exposed. There are 3 main temperature phases for embryonic development: 1) the body temperature of the hen from fertilization until oviposition, 2) the storage temperature, and 3) the incubation temperature until hatch. During the intrauterine period, the hen's body temperature supplies the heating energy to maintain the earliest stages of embryogenesis. Notably, variations in intrauterine duration or metabolic temperature, or both, may lead to differences in embryonic stage at oviposition. Consequently, in modern broiler breeds, the predominant stage at oviposition differs between young and old flocks—XI and XII EG&K, respectively (Reijrink et al., 2010; Dymond et al., 2013; Pokhrel et al., 2018). The variation in reproductive organ metabolism, and hence, developmental stage at oviposition, might be related to selective breeding because poultry birds have achieved significant changes in growth performance in the last 40 yr.
Practically speaking, the question of how long the fertile eggs can be held at a particular temperature before incubation and yet be capable of developing into normal chicks is of economic importance to the poultry industry and has been the subject of many studies throughout the years; these are summarized in Table 3.
Table 3.
Relationship between storage duration and storage temperature.
| Storage duration (d) | Storage temperature (°C) | References |
|---|---|---|
| 21 to 34 | 7.3 to 10 | Moran, 1925 |
| 21 to 34 | 10.0 to 12.8 | Scott, 1933; Olsen and Haynes, 1948; Funk and Forward, 1960 |
| 7 and 21 | 15.5 ± 0.5 and 10.5 ± 0.5 | Proudfoot, 1968 |
| 2, 8 and beyond 14 | 18, 15, and 12 | Kirk et al., 1980; Olsen and Haynes, 1948; Funk and Forward, 1960 |
| short- and long-term | 15 to 16 and 10 to 11 | Reinhart and Hurnik, 1976 |
| 1 to 3, 4 to 7, >7, and >13 | 20 to 23, 15 to 18, 12 to 15, and 12 | Tullett, 2009 |
| short- and long-term | 18 and 12 | Pokhrel et al., 2018 |
These studies have led to the common notion that lower storage temperature slows down embryo deterioration through a slower advancement of development and maintained cell viability (Table 2), which in turn makes them more suitable for longer storage periods with better SRD and hence, improved hatchability.
Storage Duration
In parallel to storage temperature, storage duration from oviposition to incubation is also paramount for embryonic SRD. Asmundson (1947) demonstrated that the hatchability of eggs that are cooled prior to incubation is higher than for eggs that are incubated immediately after laying. Several other studies have also shown that a few days of storage is advantageous in terms of hatchability (Funk et al., 1950; Ishaq et al., 2014), although the reason for this is not entirely understood. It might be due to changes in the embryonic microenvironment following storage: while the embryo is in the uterus, the environment is hypoxic, and after a few days of storage, eggs adjust to normoxic conditions. The transition of the egg from hypoxic to normoxic conditions is reflected in changes in albumin pH. In general, due to the release of carbon dioxide from hypoxic eggs during the first few days of storage, the buffering capacity of albumin is at its weakest between pH 7.5 and 8.5, leading to a rapid increase in pH (Lapao et al., 1997; Karoui et al., 2006). However, injection of buffer into the albumin maintained the pH at 8.2, and the hatchability of fertile eggs was found to increase (Akhlaghi et al., 2013). These studies demonstrated that a few days of storage is sufficient to achieve pH 8.2, and a further increase in pH accompanied by additional days of storage correlated with a negative effect on hatchability. In agreement with this, several studies have mentioned optimal hatchability between 4 and 7 d, with a slight decrease up to 10 d and a rapid decline beyond that (Mayes and Takeballi, 1984; Fasenko et al., 1992; reviewed by Brake et al., 1997; Mayes and Takeballi, 1984; reviewed by Brake et al., 1997; Ishaq et al., 2014). However, in addition to the changes in albumin pH, changes in the biological state of the embryos following prolonged duration of diapause are also needed to be uncovered and associated with the hatchability trend.
To maintain hatchability even after an extended period of diapause, it is recommended to store eggs at a lower temperature (Brake et al., 1997; Table 3). For instance, for fertile broiler breeder eggs, the optimal hatchability (89.7%) of eggs stored for 4 d declined to 72.2% when stored for 14 d (Mather and Laughlin, 1976; reviewed in Fasenko, 2007). Accordingly, a recent study in young broiler flocks (32 wk) showed 79% hatchability for fresh eggs that increased to 90.78% and 88.23% after 7 d of storage at 18°C and 12°C, respectively (Pokhrel et al., 2018). However, beyond 7 d of storage at 18°C, hatchability decreased to 75%, 55%, and 17% after 14, 21, and 28 d, respectively, whereas for the same duration of storage at 12°C, hatchability was maintained at 88%, 78%, and 71%, respectively. Similar results were obtained for an old flock (63 wk of age) (Pokhrel et al., 2018). These data show that hatchability increases following short-duration storage and decreases as the length of the holding period is further increased, especially beyond 1 wk. Moreover, there is a general relationship between storage temperature and time; higher and lower storage temperatures favor short and longer duration storage, respectively (Olsen and Haynes, 1948; Proudfoot, 1968). The underlying mechanism for this relationship may be reduced cell death (Table 2), maintenance of cellular architecture, and less advancement of development during prolonged storage at lower temperature, as opposed to storage at a higher temperature.
The main aim of prolong storage duration is to maintain SRD characteristics. However, hatchability decreases following prolonged storage within the DW, highlighting alterations in the egg's physical characteristics and biological state during and after storage. Importantly, the solution to this storage-related problem may be the acquisition of proper managerial decision of egg storage based on specific purpose; if hatchery requires egg storage for short-period, then a decision of storing eggs at higher or lower temperature is beneficial, while for longer period, storing them at lower temperature is advantageous. Considerably, results from previous studies have already provided solid evidence that the managerial decision of calibrating the storage temperature to a required storage duration (Table 3) can maximize vitality of post-diapause embryos.
RH
Although clearly important in preventing water loss, RH is not considered to be as important as temperature, because its impact on hatchability is limited (Reijrink et al., 2008). Dehydration, due to loss of water through evaporation, may occur if the eggs are stored under insufficiently high RH conditions for a prolonged period, and this might increase the osmolarity of the embryonic microenvironment. A recent study recommended 50 to 60% RH for up to 10 d of egg storage (Schulte-Drüggelte, 2011). Moreover, storage at higher humidity, up to 80%, is not harmful (Lapao et al., 1997), but above this level, the growth and spread of bacteria and mold are likely to become a problem (Schulte-Drüggelte, 2011). For long storage duration, relatively higher humidity conditions (70–80%) are advantageous, as moisture loss is decreased and hatchability increases (Brake et al., 1997). Importantly, water loss due to suboptimal RH is not only detrimental during egg storage but might also affect normal embryogenesis during the post-diapause period, resulting in embryonic mortality (Noiva et al., 2014).
CONCLUSIONS
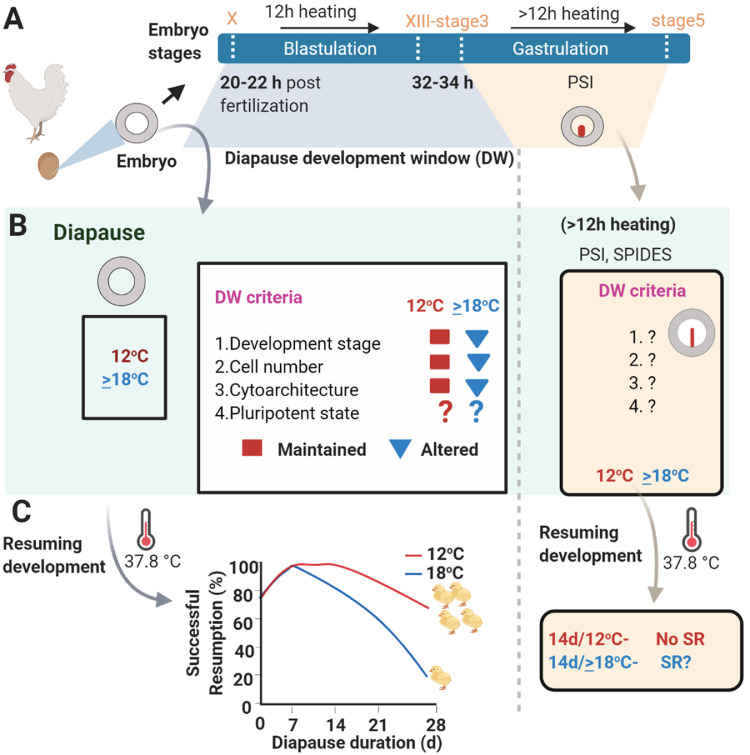
Early stages of avian embryos have the potential to undergo diapause at low temperature. Based on the evidences provided in this review, the DW is limited to 12 h of embryonic development following oviposition, which is approximately 32 to 34 h of embryonic development from fertilization (Figure 3A). Specifically, embryos during this period are within blastulation to early-gastrulation stages, and these stages are relevant to the poultry industry for defining appropriate storage conditions. For embryos that are at this window range, the storage temperature of ~12°C benefits the short or long durational storage (Figure 3B) because the DW criteria are maintained at this temperature, that is less development advancement (within blastulation to early gastrulation), maintained cellular architecture, arrested G2 and M phase cell cycle, reduced cell death, and perhaps retained pluripotent state. As a result of these preserved DW criteria, the SRD characteristics of embryos are unaffected. Conversely, for embryos at advanced development stage beyond the DW, the storage temperature of 12°C does not preserve the SRD characteristics. Temperatures around 18°C is beneficial to higher staged embryos because their SRD characteristics are not negatively affected at this temperature (Figure 3C). Future investigations are needed to explore the changes in DW criteria that benefits SRD characteristics of higher stage embryo at higher temperature. Based on the changes in DW criteria that do not compromise SRD characteristics (Figure 3C), future studies would allow calibrating the existing and modified storage techniques to obtain adequate storage conditions.
Figure 3.
Schematic representation showing the effects of diapause conditions on diapause developmental window (DW) criteria and successful resumption of development post-diapause. (A) Diapause development window. Following 20–22 h after fertilization (haf), eggs are laid. Freshly laid eggs are at blastulation stage between X-XIII EG&K. After 12 h of heating the eggs, i.e. 32–34 haf, embryo are at stage between XIII EG&K-stage 3. Altogether DW is open up to 12 h of development after oviposition. Due to PSI treatment for more than 12 h, embryos are at mid to late gastrulation stage and DW closes. (B) Effects of diapause condition on DW criteria. The important DW criteria include the development stage (X EG&K-stage3), cell number (30,000 to 130,000), cytoarchitecture (single epithelial layer with hypoblast and PI cells) and pluripotent state. During diapause at 12°C, embryo developmental stage is less progressing, cell viability is maintained, cytoarchitecture is preserved, yet to date, pluripotent state of embryo is unknown. Whereas at ≥18°C, these corresponding DW criteria are negatively affected. However, following heating the eggs for more than 12 h, the effect of these temperatures on above criteria are yet to be discovered. (C) Successful resumption of development post-diapause. Embryos that are within the diapause window can successfully recover development (SRD) following prolonged diapause at 12°C, compared with 18°C. Whereas, after the DW is closed, embryo diapause at 12°C negatively affect SRD. SRD characteristics of advanced stage embryo following their diapause at 18°C is yet to be evaluated.
DISCLOSURES
The authors do not have any conflicts of interest to declare.
REFERENCES
- Akhlaghi A., Jafari Ahangari Y., Hashemi S.R., Navidshad B., Ansari Pirsaraei Z., Deldar H., Liang J.B. Prestorage In ovo injection of biological buffers: an approach to improve Hatchability in long-term stored eggs. Poult. Sci. 2013;92:874–881. doi: 10.3382/ps.2012-02610. [DOI] [PubMed] [Google Scholar]
- Arora K.L., Kosin I.L. Changes in the gross morphological appearance of Chicken and Turkey blastoderms during preincubation storage. Poult. Sci. 1966;45:819–825. doi: 10.3382/ps.0450819. [DOI] [PubMed] [Google Scholar]
- Arora K.L., Kosin I.L. Developmental responses of early Turkey and chicken embryos to preincubation holding of eggs: inter- and intra-species differences. Poult. Sci. 1966;45:958–970. doi: 10.3382/ps.0450958. [DOI] [PubMed] [Google Scholar]
- Arora K.L., Kosin I.L. Autoradiographic and cytological study of blastodermal cells in turkey eggs subjected to extended pre-incubation storage. Biol. Bull. 1967;133:303–309. doi: 10.2307/1539826. [DOI] [PubMed] [Google Scholar]
- Arora K.L., Kosin I.L. The response of the early chicken embryo to preincubation temperature as evidenced from its gross morphology and mitotic pattern. Physiol. Zool. 1968;41:104–112. [Google Scholar]
- Asmundson V.S. Time held prior to incubation and hatchability of Turkey eggs. Poult. Sci. 1947;26:305–307. [Google Scholar]
- Bakst M.R., Akuffo V. Impact of egg storage on embryo development. Avian Poult. Biol. Rev. 2002;13:125–131. [Google Scholar]
- Bakst M.R., Gupta S.K., Akuffo V. Comparative development of the Turkey and chicken embryo from cleavage through hypoblast formation. Poult Sci. 1997;76:83–90. doi: 10.1093/ps/76.1.83. [DOI] [PubMed] [Google Scholar]
- Bloom S.E., Muscarella D.E., Lee M.Y., Rachlinski M. Cell death in the avian blastoderm : resistance to stress- induced apoptosis and expression of anti-apoptotic genes. Cell Death Differ. 1998;5:529–538. doi: 10.1038/sj.cdd.4400381. [DOI] [PubMed] [Google Scholar]
- Brake J., Walsh T.J., Benton C.E., Petitte J.N., Meijerhof R., Pen G. Egg handling and storage. Poult Sci. 1997;76:144–151. doi: 10.1093/ps/76.1.144. [DOI] [PubMed] [Google Scholar]
- Brunner A.M., Evans L.M., Hsu C., Sheng X., Horvath D., States U. Vernalization and the chilling requirement to exit bud dormancy : shared or separate regulation? Front Plant Sci. 2014;5:1–7. doi: 10.3389/fpls.2014.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buryanov Y.I. Adaptive epibiochemistry and epigenetics. Biochemistry (Mosc). 2015;80:1145–1156. doi: 10.1134/S0006297915090059. [DOI] [PubMed] [Google Scholar]
- Cai J.H., Yeh T.F., Wei H.W., Liu I.H. Temperature-induced embryonic diapause in blue-breasted quail (Coturnix chinensis) correlates with decreased mitochondrial-respiratory network and increased stress-response network. Poult Sci. 2019;98:2977–2988. doi: 10.3382/ps/pez116. [DOI] [PubMed] [Google Scholar]
- Calow P. Energetics of reproduction and its evolutionary implications. Biol. J. Linnean Soc. 1983;20:153–165. [Google Scholar]
- Calow P., Forbes V.E. How do physiological responses to stress translate into ecological and evolutionary processes? Comparative Biochem. Physiol. Part A. 1998;120:11–16. [Google Scholar]
- Chapuis E., Ferdy J. Life history traits variation in heterogeneous environment : the case of a freshwater snail resistance to pond drying. Ecol. Evol. 2012;2:218–226. doi: 10.1002/ece3.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinnamon Y., Feine O., Hochegger H., Bershadsky A., Brandeis M. Cellular contractility requires ubiquitin mediated proteolysis. PLoS One. 2009;4:1–13. doi: 10.1371/journal.pone.0006155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M.S., Denekamp N.Y., Thorne M.A.S., Reinhardt R., Drungowski M., Albrecht M.W., Lubzens E. Long-term survival of hydrated resting eggs from brachionus plicatilis. PloS One. 2012;7:1–12. doi: 10.1371/journal.pone.0029365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarais A., Murphy B.D. Embryonic diapause and its regulation. Reproduction. 2002;128:669–678. doi: 10.1530/rep.1.00444. [DOI] [PubMed] [Google Scholar]
- Dymond J., Vinyard B., Nicholson A.D., French N.A., Bakst M.R. Short periods of incubation during egg storage increase hatchability and chick quality in long-stored broiler eggs. Poult. Sci. 2013;92:2977–2987. doi: 10.3382/ps.2012-02816. [DOI] [PubMed] [Google Scholar]
- Edwards C. The physiological zero and the index of development for the egg of the domestic fowl, Gallus domesticus. Am. J. Physiol. 1902;6:351–397. [Google Scholar]
- Etches R.J., Clark M.E., Zajchowski L., Speksnijder G., Verrinder Gibbins a M., Kino K., Samarut J. Manipulation of blastodermal cells. Poult. Sci. 1997;76:1075–1083. doi: 10.1093/ps/76.8.1075. [DOI] [PubMed] [Google Scholar]
- Eyal-Giladi H., Kochav S. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev. Biol. 1976;49:321–337. doi: 10.1016/0012-1606(76)90178-0. [DOI] [PubMed] [Google Scholar]
- Proudfoot F.G. Hatching egg storage effects on hatchability and subsequent performance of the domestic fowl. Poult. Sci. 1968;47:1497–1500. [Google Scholar]
- Fasenko G.M. Egg storage and the embryo. Poult. Sci. 2007;86:1020–1024. doi: 10.1093/ps/86.5.1020. [DOI] [PubMed] [Google Scholar]
- Fasenko G.M., Christensen V.L., Wineland M.J., Petitte J.N. Examining the effects of prestorage incubation of Turkey breeder eggs on embryonic development and hatchability of eggs stored for four or fourteen days. Poult Sci. 2001;80:132–138. doi: 10.1093/ps/80.2.132. [DOI] [PubMed] [Google Scholar]
- Fasenko G.M., Robinson F.E., Whelan A.I., Kremeniuk K.M., Walker J.A. Prestorage incubation of long-term stored broiler breeder eggs: 1. Effects on hatchability. Poult. Sci. 2001;80:1406–1411. doi: 10.1093/ps/80.10.1406. [DOI] [PubMed] [Google Scholar]
- Funk E.M., Biellier H.V. The minimum temperature for embryonic development in the domestic fowl (Gallus domesticus) Poult. Sci. 1944;23:538–540. [Google Scholar]
- Funk E.M., Forward J.E. Effect of holding temperatures on hatchability of chicken eggs. Res. Bull. Missouri Agric Exp. Station. 1960 [Google Scholar]
- Funk E.M., Forward J., Kempster H.L. Effect of holding temperatures on hatchability of eggs. Bull. Mo. Agric. Exp. Stn. 1950;539:18. (539) [Google Scholar]
- Fasenko G.M., Robinson F.E., Hardin R.T., W J.L. Variability in preincubation embryonic development in domestic fowl. 2. Effects of duration of egg storage period. Poult. Sci. 1992;70:1876–1881. doi: 10.3382/ps.0712129. [DOI] [PubMed] [Google Scholar]
- Hamburger V., Hamilton. H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;81:49–92. [PubMed] [Google Scholar]
- Hamidu J.a, Rieger a M., Fasenko G.M., Barreda D.R. Dissociation of chicken blastoderm for examination of apoptosis and necrosis by flow cytometry. Poult. Sci. 2010;89:901–909. doi: 10.3382/ps.2009-00552. [DOI] [PubMed] [Google Scholar]
- Hamidu J.A, Uddin Z., Li M., Fasenko G.M., Guan L.L., Barreda D.R. Broiler egg storage induces cell death and influences embryo quality. Poult. Sci. 2011;90:1749–1757. doi: 10.3382/ps.2011-01361. [DOI] [PubMed] [Google Scholar]
- Ishaq H.M., Akram M., Baber M.E., Jatoi A.S., Sahota A.W., Javed K., Husnain F. Embryonic mortality in cobb broiler breeder strain with three egg weight and storage periods at four production phases. J. Anim. Plant Sci. 2014;24:1623–1628. [Google Scholar]
- Jean C., Oliveira N.M.M., Intarapat S., Fuet A., Mazoyer C., De Almeida I., Pain B. Transcriptome analysis of chicken ES, blastodermal and germ cells reveals that chick ES cells are equivalent to mouse ES cells rather than EpiSC. Stem Cell Res. 2015;14:54–67. doi: 10.1016/j.scr.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagenç L., Cinnamon Y., Ginsburg M., Petitte J.N. Origin of primordial germ cells in the prestreak chick embryo. Dev. Genet. 1996;19:290–301. doi: 10.1002/(SICI)1520-6408(1996)19:4<290::AID-DVG2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Karoui R., Kemps B., Bamelis F., De Ketelaere B., Decuypere E., De Baerdemaeker J. Methods to evaluate egg freshness in research and industry: a review. Eur. Food Res. Technol. 2006;222:727–732. [Google Scholar]
- Kgwatalala P.M., Faki O., Nsoso S.J. Influence of prestorage incubation on the hatchability of Guinea Fowl eggs stored for fourteen days. J. Anim. Sci. Adv. 2013;3:304–309. [Google Scholar]
- Kirk S., Emmans G.C., McDonald R., Arnot D. Factors affecting the hatchability of eggs from Broiler breeders. Br. Poult. Sci. 1980;21:37–53. [Google Scholar]
- Ko M.H., Hwang Y.S., Rim J.S., Han H.J., Han J.Y. Avian blastoderm dormancy arrests cells in G2 and suppresses apoptosis. FASEB J. 2017;31:3240–3250. doi: 10.1096/fj.201601051RR. [DOI] [PubMed] [Google Scholar]
- Kochav S, Eyal-Giladi H. Bilateral symmetry in chick embryo determination by gravity. Science. 1971;171:1027–1029. doi: 10.1126/science.171.3975.1027. [DOI] [PubMed] [Google Scholar]
- Kochav S, Ginsburg M., Eyal-Giladi H. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. II. Microscopic anatomy and cell population dynamics. Dev. Biol. 1980;79:296–308. doi: 10.1016/0012-1606(80)90117-7. [DOI] [PubMed] [Google Scholar]
- Kosin I.L., St. Pierre E. Studies on pre-incubation warming of chicken and Turkey eggs. Poult. Sci. 1956;35:1384–1392. [Google Scholar]
- Lapao C., Gama L.T., Soares M.C. Effects of broiler breeder age and length of egg storage on albumen characteristics and hatchability. Poult Sci. 1997;78:640–645. doi: 10.1093/ps/78.5.640. [DOI] [PubMed] [Google Scholar]
- Marijuán P.C., Navarro J., del Moral R. On prokaryotic intelligence: strategies for sensing the environment. Bio. Syst. 2010;99:94–103. doi: 10.1016/j.biosystems.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Mather C.M., Laughlin K.F. Storage of hatching eggs: the effect on total incubation period. Br. Poult. Sci. 1976;17:471–479. [Google Scholar]
- Mayes F.J., Takeballi M.A. Storage of the eggs of the fowl (Gallus domesticus) before incubation: a review. Worlds Poult. Sci. J. 1984;40:131–140. [Google Scholar]
- Meir M., Ar A. Proc. 10th European Poultry Conference. 1998. Pre-incubation warming as a means of lengthening storage time of fertile eggs Pages 825–829. [Google Scholar]
- Moran T. The effects of low temperature on hens’ eggs. Roy. Soc. (London), Proc. 1925;98:436–456. [Google Scholar]
- Noiva R.M., Menezes A.C., Peleteiro M.C. Influence of temperature and humidity manipulation on chicken embryonic development. BMC Vet. Res. 2014;10:234. doi: 10.1186/s12917-014-0234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odula Olwande P., Ogara W.O., Okuthe S.O., Muchemi G., Okoth E., Odindo M.O., Adhiambo R.F. Assessing the productivity of indigenous chickens in an extensive management system in southern Nyanza, Kenya. Trop. Anim. Health Prod. 2010;42:283–288. doi: 10.1007/s11250-009-9418-4. [DOI] [PubMed] [Google Scholar]
- Olsen M.W., Haynes S.K. The effect of different holding temperatures on the hatchability of Hens’ eggs. Poult Sci. 1948;27:420–426. [Google Scholar]
- Olsen M.W. Effect of shipment on pre-incubated fertile eggs. Poult. Sci. 1949;28:731–738. [Google Scholar]
- Petitte J.N., Clark M.E., Liu G., Gibbins A.M.V., Etches R.J. Production of somatic and germline chimeras in the chicken by transfer of early blastodermal cells. Development. 1990;108:185–189. doi: 10.1242/dev.108.1.185. [DOI] [PubMed] [Google Scholar]
- Pokhrel N., Cohen E.B.-T., Genin O., Ruzal M., Sela-Donenfeld D., Cinnamon Y. Effects of storage conditions on hatchability, embryonic survival and cytoarchitectural properties in broiler from young and old flocks. Poult. Sci. 2018;97:1–12. doi: 10.3382/ps/pex393. [DOI] [PubMed] [Google Scholar]
- Pokhrel N., Cohen E.B., Genin O., Cinnamon Y. Cellular and morphological characterization of blastoderms from freshly laid broiler eggs. Poult. Sci. 2017;96:4399–4408. doi: 10.3382/ps/pex242. [DOI] [PubMed] [Google Scholar]
- Reed W.L., Clark M.E. Beyond maternal effects in birds : responses of the embryo to the environment. Integr. Comp. Biol. 2011;51:73–80. doi: 10.1093/icb/icr032. [DOI] [PubMed] [Google Scholar]
- Reijrink I.A.M., Meijerhof R., Kemp B., van den Brand H. Influence of egg warming during storage and hypercapnic incubation on egg characteristics, embryonic development, hatchability, and chick quality. Poult. Sci. 2010;89:2470–2483. doi: 10.3382/ps.2010-00798. [DOI] [PubMed] [Google Scholar]
- Reijrink I.A.M., Meijerhof R., Kemp B., Van Den Brand H. The chicken embryo and its micro environment during egg storage and early incubation. Worlds Poult. Sci. J. 2008;64:581–598. [Google Scholar]
- Reinhart B.S., Hurnik J.F. The effect of temperature and storage time during the pre-incubation period 1. The influence of storage temperature changes on hatchability and first ten days chick performance. Poult. Sci. 1976;55:1632–1640. [Google Scholar]
- Romanoff A.L. Critical periods and causes of death in avian embryonic development. Am. Ornithol. Union. 1949;66:264–270. [Google Scholar]
- Schulte-Drüggelte R. Recommendations for hatching egg handling and storage. Lohmann Inform. 2011;46:55–58. [Google Scholar]
- Scott H.M. The effect of age and holding temperatures on hatchability of Turkey and chicken eggs. Poult. Sci. 1933;12:49–54. [Google Scholar]
- Spratt N.T., Haas H. Morphogenetic movements in the lower surface of the unincubated and early chick blastoderm. J. Exp. Zool. 1960;144:139–157. [Google Scholar]
- Spratt N.T., Haas H. Nutritional requirements for the realization of regulative (repair) capacities of young chick blastoderms. J.Exp. Zool. 1967;164:31–46. [Google Scholar]
- Squeglia F., Ruggiero A., Berisio R. Exit from mycobacterial dormancy: a structural perspective. Curr. Med. Chem. 2015;22:1698–1709. doi: 10.2174/0929867322666150209153027. [DOI] [PubMed] [Google Scholar]
- Sturm A., Dworkin J. Phenotypic diversity as a mechanism to exit cellular dormancy. Curr. Biol. 2015;25:2272–2277. doi: 10.1016/j.cub.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullett S. Ross tech: investigating hatchery practice. Aviagen, Ross Tech. 2009:14–15. Accessed July 2020. http://en.aviagen.com/assets/Tech_Center/Ross_Tech_Articles/RossTechInvestigatingHatcheryPractice.pdf. [Google Scholar]
- Varetti G., Guida C., Santaguida S., Chiroli E., Musacchio A. Homeostatic control of mitotic arrest. Mol. Cell. 2011;44:710–720. doi: 10.1016/j.molcel.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Zhai Y., Kronebusch P.J., Borisy G.G. Kinetochore microtubule dynamics and the metaphase-anaphase transition. J. Cell Biol. 1995;131:721–734. doi: 10.1083/jcb.131.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]