Abstract
With the promotion of the intensive breeding model, the incidence of leg diseases has risen in fast-growing commercial broilers with higher body weight, seriously affecting their feed efficiency and causing animal welfare problems. Femoral head necrosis (FHN) is the most common leg disease in broilers. Previous studies reported that hormone-induced FHN is related to endoplasmic reticulum (ER) stress, apoptosis, and oxidative stress, but no detailed study has been conducted in broilers with spontaneous FHN. In the study, the articular cartilage of 5-wk-old Ross 308 broilers with spontaneous FHN was used to investigate the pathogenesis of the disease. According to the degree of femoral head injury, the birds participating in the experiment were divided into 3 groups, namely a control group, femoral head separation group and femoral head separation with growth plate lacerations group. The morphological changes in articular cartilage were observed by hematoxylin and eosin, toluidine blue, alcian blue and safranine O-solid green staining, and the expressions of genes related to cartilage homeostasis, ER stress, autophagy, apoptosis and oxidative stress was detected using Real-Time Quantitative PCR. In the results, the expression of aggrecan and collagen-2 mRNA levels decreased in the articular cartilage of spontaneous FHN broilers, and the same changes were observed in the tissue staining results, indicating the disordered nature of articular cartilage homeostasis. At the same time, FHN in broilers causes ER stress in articular chondrocytes and regulates oxidative stress by activating the nuclear factor erythroid 2-related factor 2/antioxidant response element pathway through protein kinase RNA-like ER kinase. Autophagy can be activated through the protein kinase RNA-like ER kinase-activating transcription factor-4 pathway, and apoptosis can even be activated through CCAAT-enhancer-binding protein homologous protein. Therefore, the secretory activity of articular chondrocytes in spontaneous FHN broilers is negatively affected, which leads to the disorder of cartilage homeostasis and results in FHN due to ER-stress-mediated chondrocyte apoptosis and oxidative stress.
Key words: femoral head necrosis, endoplasmic reticulum stress, apoptosis, oxidative stress, broiler
INTRODUCTION
With the increasing demand for poultry meat products, broiler breeding has gradually changed from a traditional free-range model to an intensive model (Kuttappan et al., 2016; Dinev et al., 2019). Intensive breeding has greatly improved production efficiency, but the incidence rate of broiler leg disease has increased because of the high intensity and rapid growth rate associated with this model, which has exerted a significantly negative impact on economic success and animal welfare in the broiler industry (Julian, 2005; Olkowski et al., 2011; Sanchez-Rodriguez et al., 2019). FHN is one of the most common leg disorders, in fast growing broilers, and the highest incidence rate is found at 4 to 6 wk of age(Okimoto et al., 2009; Li et al., 2015; Liu et al., 2020b; Yu et al., 2020). Zhang et al., 2017; Zhang et al., 2019 reported that FHN is related to apoptosis of chondrocytes and ER stress. The ER is a highly dynamic membrane network and consists of cisternae, linear tubules, and junctions that can be constantly rearranged (Rowland and Voeltz, 2012; Marchi et al., 2014). ER stress is sensed by three upstream signaling proteins: inositol requiring protein-1α (IRE1-α), activating transcription factor-6 (ATF6) and PERK. Under physiological conditions, these transmembrane proteins bind to the chaperone glucose-regulated protein 78 (GRP78)/BiP in the ER. When unfolded proteins accumulate in the ER, GRP78/BiP is released from these complexes to assist with the folding of accumulated proteins, and then PERK, IRE1α and ATF6 activate their respective sensors (Gardner and Walter, 2011). The activation of these 3 signaling pathways induces apoptosis (Morishima et al., 2011; Tabas and Ron, 2011). The ER stress response mediated by the PERK-eukaryotic initiation factor 2α (eIF2α)-ATF4 pathway is involved in the regulation of the expression of several target genes such as CHOP (Su and Kilberg, 2008; Brewer, 2014). Under chronic or overwhelming ER stress, the normal functions of the ER cannot be recovered, resulting in cellular dysfunction and apoptosis (Ayaub et al., 2016; Hu et al., 2018). The PERK/ATF4/CHOP signaling pathway is considered to play a pivotal role in inducing cell apoptosis (Cao et al., 2012; Chen et al., 2014; Liu et al., 2016). Apoptosis is an active mode of cell death under physiological or pathological conditions (Zhai et al., 2019; Hou et al., 2020). It is typically characterized by nuclear fragmentation, DNA degradation in the early stage, and formation of apoptotic bodies (Dong et al., 2019).
ER stress also leads to the induction of autophagy (Yorimitsu et al., 2006; Chen et al., 2019; Liu et al., 2020a). ATF4 induces autophagy through the transcriptional regulation of autophagy-related genes (ATG), such as Atg5, Atg7 and Atg10 (B'Chir et al., 2013). Autophagy is a cellular degradation process, initiated in response to stress, which attempts to restore metabolic homeostasis through the catabolic lysis of aggregated proteins, unfolded/misfolded proteins or damaged subcellular organelles (Mizushima, 2007; Glick et al., 2010). Beclin1 is one of the key proteins required to initiate autophagy. At the maturation stage of autophagy, LC3 acts as an LC3-II by binding to phosphatidylethanolamine (PE, LC3–PE) to form autophagic vacuoles via a ubiquitination-like enzymatic reaction(Yang et al., 2015).
During ER stress, Nrf-2 is activated by PERK, which promotes the expression of genes related to oxidative stress (Brewer, 2014). Nrf-2 plays a role in regulating the oxidative stress conditions. The Nrf-2 protein naturally binds to cytoplasmic proteins and, through various stresses, progresses to the nucleus of the cell, which causes the expression of several genes, including antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and enzymes involved in detoxification and drug processing (Ma, 2013; Suzuki et al., 2013). SOD and GSH-Px are the most important enzymes in this pathway, which reduces the toxicological effects of ROS (Ighodaro and Akinloye, 2017; Afshari-Kaveh et al., 2020), improves the antioxidant capacity and helps to maintain the body's overall health.
In a previous report (Liu et al., 2020b), we confirmed the presence of spontaneous FHN broiler chondrocyte apoptosis, therefore, in the present study, the articular cartilage of 5-wk-old broilers with spontaneous FHN was used to investigate the underlying mechanisms involved in chondrocyte apoptosis.
MATERIALS AND METHODS
Sample Collection
The experiment was performed in accordance with the “Guidelines for Laboratory Animals” issued by the Ministry of Science and Technology (2006, Beijing, China), and it was approved by the Animal Protection and Utilization Committee of Nanjing Agricultural University (#NJAU-Poult-2019102205, approved on October 22, 2019). Broilers (Ross 308 birds) of both sexes were acquired from a farm in Jiangsu Province. Birds were fed a two-phase commercial diet ad libitum: a starter ration (21.00% crude protein, 1.00% Ca, 0.52% total P, 0.45% methionine, 0.46% available P, 3000 IU/kg Vitamin D3, 750FTU/kg phytase) from 0 to 21 d and a grower ration (19.00% crude protein, 0.95% Ca, 0.47% total P, 0.38% methionine, 0.39% available P, 3000 IU/kg Vitamin D3, 750FTU/kg phytase) from 22 to 35 d. At the 5th wk of age, the birds with lameness were selected for tissue samples collection. After euthanasia, femoral head tissue was collected from FHN broilers immediately, washed with saline and stored in 4% paraformaldehyde or at −80°C for histological or genetic analysis. In accordance with the FHN score standard (Okimoto et al., 2009), the broilers were divided into 3 groups (Control, FHS and FHSL groups). In each group, 8 broilers were used for analysis.
Histopathological Analysis
The femoral head was fixed with 4% paraformaldehyde, washed overnight with flowing water, and decalcified in 10% EDTA for 2 wk (Liu et al., 2020b; Yu et al., 2020). The decalcified cartilage tissue was dehydrated in ethanol, made transparent in xylene and embedded in paraffin. The cartilage tissue was stained with H&E, alcian blue, toluidine blue and safranine O-solid green for histological observation.
RNA Extraction and Real-Time Quantitative PCR
The femoral head tissue was ground into powder in a liquid nitrogen environment and treated with Trizol (Nanjing Angle Gene Biotechnology Co. Ltd., Nanjing, China) to extract the total RNA. The complementary DNA (cDNA) was synthesized via reverse transcription utilizing HiScript II QRT SuperMix for qPCR (+gDNA wiper; Zazyme, Nanjing, China). The expression of the related genes was detected by quantitative real-time PCR (qRT-PCR), carried out on the ABI PRISM 7300 HT sequence-detection system (Applied Biosystems, Inc., Foster City, CA), which was repeated 3 times. The genes selected were PERK, ATF-4, ATF-6, IRE1-α, GRP78, CHOP, B-cell lymphoma-2 (Bcl-2), Caspase-3, Caspase-8, Caspase-9, Bcl-2 homology interacting-domain death agonist (Bid), LC3-I, LC3-II, Beclin1, Atg-5, protein kinase B (Akt), mammalian target of rapamycin (mTOR), collagen-2, collagen-10, aggrecan, hypoxia inducible factor-1 (HIF-1α), HIF-2α, Nrf-2, kelch-like ECH-associated protein 1 (Keap-1), heme Oxygenase-1 (HO-1), NADPH quinineoxidoreductase-1 (NQO-1), catalase (CAT), SOD-1, SOD-2, SOD-3, GSH-Px1, GSH-Px2, GSH-Px7. Quantitative data were normalized relative to the housekeeping GAPDH. The genes’ primer sequences as described above are listed in Table 1. All PCR operations were performed in triplicate. The results were analyzed as the relative fold change (2−ΔΔCT) (Livak and Schmittgen, 2002).
Table 1.
Sequences of primers used to amplify specific mRNAs by qRT-PCR.
| Target gene | Primer sequence (5′-3′) |
|---|---|
| mTOR | Forward: AACCACTGCTCGCCACAATGC Reverse: GATCGCCACACGGATTAGCTCTTC |
| PERK | Forward: TCGAGCTGCTTTACCCTTTC Reverse: CTCATTGTCCGTGACCTCTG |
| ATF4 | Forward: GAGGAGAACCATTCCGATGA Reverse: CACCTTTGCTGACGCTACCT |
| ATF6 | Forward: GTCCCTTCTCCGTCCTCTG Reverse: CGCCCACAATCGGTTTC |
| IRE1-α | Forward: CCCAAAGCATCAAACCATTC Reverse: CAACGTCGCGGTTATCAAAT |
| GRP78 | Forward: TGCCAATGACCAGGGGAACC Reverse: GAGGGGTCATTCCAGGTGCG |
| CHOP | Forward: CAGGAAGAAGAGCTGGCCCCACT Reverse: TGCTGTGCTCGCCGTGCTGT |
| Bcl-2 | Forward: CGACTGGGATGACAGGAAAG Reverse: GGAGCGCACAGGTGAGACA |
| Caspase-3 | Forward: AAGGCTCCTGGTTTATTCA Reverse: TCTGCCACTCTGCGATTT |
| Caspase-8 | Forward: CCTCTTGGGCATGGCTA Reverse: TGCTGCTCACCTCTTGATT |
| Caspase-9 | Forward: CGAAGGAGCAAGCACGAC Reverse: CGCAGCCCTCATCTAGCAT |
| Bid | Forward: GCCTGACCCTGAGGTAAATG Reverse: ACAGGCACCGTGTTATCTCC |
| LC3-I | Forward: GCTGCCAGTGCTGGACAAGAC Reverse: TCCTCATCCTTCTCCTGCTCGTAG |
| LC3-II | Forward: CCTGGTGCCAGATCACGTCAAC Reverse: AAGCCGTCCTCGTCCTTCTCG |
| Beclin1 | Forward: ACCGCAAGATTGTGGCTGAAGAC Reverse: TGAGCATAACGCATCTGGTTCTCC |
| ATG5 | Forward: GGCACCGACCGATTTAGT Reverse: GCTGATGGGTTTGCTTTT |
| Akt | Forward: GGCTACAAGGAACGACCGCAAG Reverse: TACTGTGGTCCACTGGAGGCATC |
| mTOR | Forward: AACCACTGCTCGCCACAATGC Reverse: GATCGCCACACGGATTAGCTCTTC |
| Collagen-2 | Forward: ACCTACAGCGTCTTGGAGGA Reverse: ATATCCACGCCAAACTCCTG |
| Collagen-10 | Forward: GCCTTCCAGGTCAGCCAGGTAT Reverse: TTGCCGATGCCAACTTCTCCAG |
| Aggrecan | Forward: TGCAAGGCAAAGTCTTCTACG Reverse: GGCAGGGTTCAGGTAAACG |
| HIF-1α | Forward: CAGCCAGGTGCCGAAGAAGC Reverse: ATGGTCAGCCTCATAATGGATGCC |
| HIF-2α | Forward: CTGTTGACGATGAGCAGTGCCT Reverse: CCAGGTGTTGGAGCCAGTTGTG |
| Nrf-2 | Forward: CTGCTAGTGGATGGCGAGAC Reverse: CTCCGAGTTCTCCCCGAAAG |
| Keap-1 | Forward: ACTTCGCTGAGGTCTCCAAG Reverse: CAGTCGTACTGCACCCAGTT |
| HO-1 | Forward: AGCTTCGCACAAGGAGTGTT Reverse: GGAGAGGTGGTCAGCATGTC |
| NQO-1 | Forward: CTCCGAGTGCTTTGTCTACGA Reverse: ATGGCTGGCATCTCAAACC |
| CAT | Forward: GTTGGCGGTAGGAGTCTGGTCT Reverse: GTGGTCAAGGCATCTGGCTTCTG |
| SOD-1 | Forward: TTGTCTGATGGAGATCATGGCTTC Reverse: TGCTTGCCTTCAGGATTAAAGTGAG |
| SOD-2 | Forward: CAGATAGCAGCCTGTGCAAATCA Reverse: GCATGTTCCCATACATCGATTCC |
| SOD-3 | Forward: TTTTCTCCTAAAGATGGCAAG Reverse: CTTCCTGCTCATGGATCACAA |
| GSH-Px1 | Forward: TCACCATGTTCGAGAAGTGC Reverse: ATGTACTGCGGGTTGGTCAT |
| GSH-Px2 | Forward: AGGGGGAGAAGGTGGACTT Reverse: TCCTGGTAGCCGAACTGGT |
| GSH-Px7 | Forward: TTGCAATTACAGCACTCCTGCTC Reverse: TGCAACGTTGACAACTAACGACA |
| GAPDH | Forward: GAACATCATCCCAGCGTCCA Reverse: CGGCAGGTCAGGTCAACAAC |
Statistical Analysis
One-way ANOVA was used to analyze the differences between normal broilers and spontaneous FHN broilers, using SPSS Statistics 25.0 software (SPSS Software Inc., USA). A histogram was created using by GraphPad Prism 7.0 (GraphPad Software Inc., USA). All values were expressed as mean ± SD. All measurements were repeated 3 times. The level of significance was as follows: * indicated P < 0.05 (significant), ** indicated P < 0.01 (extremely significant).
RESULTS
Changes in Cartilage Homeostasis-Related Level of mRNA Expression Between Spontaneous FHN and Normal Broilers
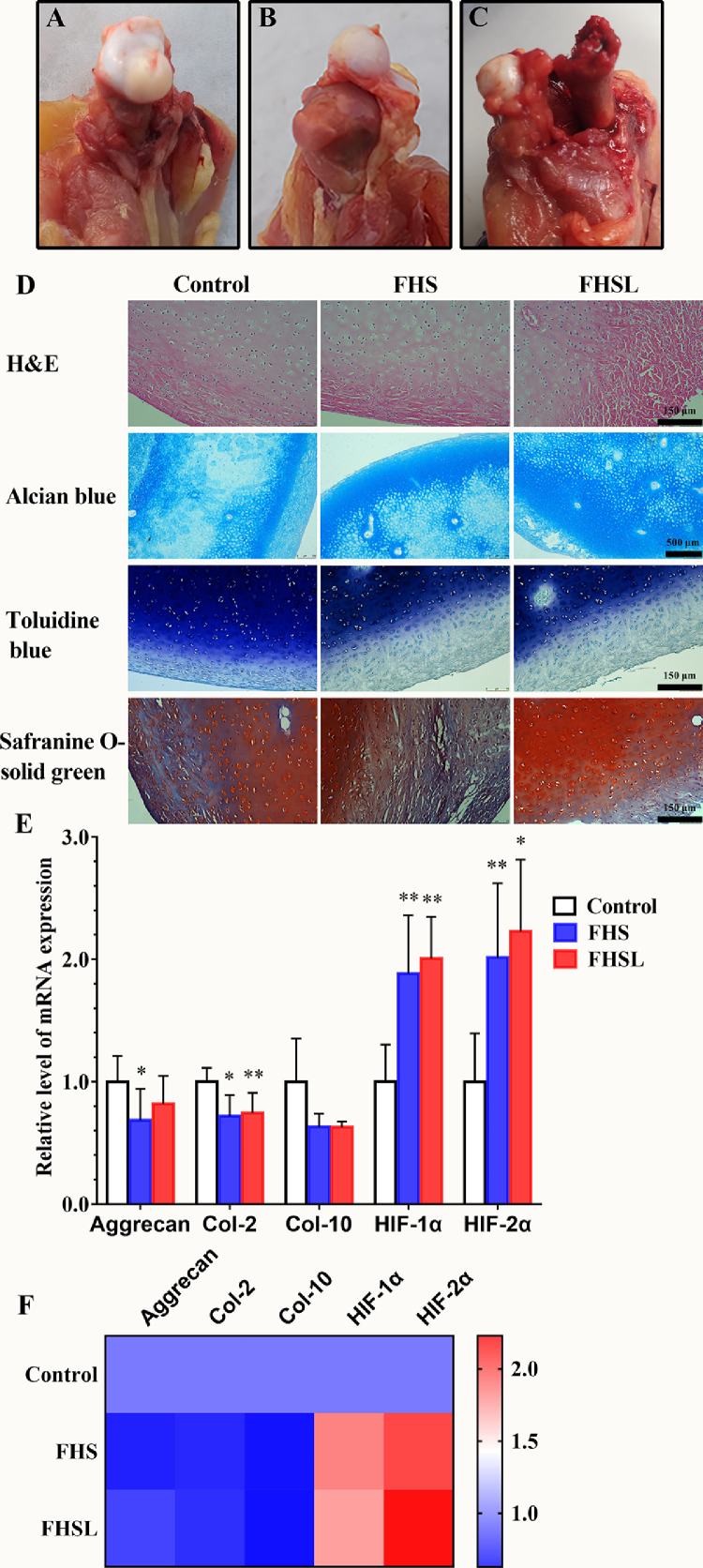
The related markers of cartilage homeostasis were detected in the articular cartilage of broilers with spontaneous FHN. Chondrocytes are the only cells involved in the synthesis of the extracellular matrix (ECM) in the articular cartilage. Cartilage homeostasis requires full synthesis of ECM in order to fulfill its biomechanical function. Aggrecan and collagen-II are the specific markers of chondrocyte activities, and their expression reflects the physiological function of chondrocytes. As shown in Figure 1B, the cartilage of the femoral head was separated from the growth plate, but there was novisible lesion in the growth plate, which was named FHS if the growth plate was damaged, and if the epiphysis was broken, it was named FHSL (Figure 1C). The results (Figure 1E and 1F) showed that in the FHS group, the mRNA level of aggrecan was significantly lower than that in the control (P < 0.05), and the mRNA level of collagen-2 was extremely significantly lower (P < 0.01). Furthermore, in the FHSL group, compared with the control group, the mRNA level of collagen-2 was significantly decreased (P < 0.05). The levels of HIF-1α and HIF-2α were extremely significantly increased in the FHS group. The level of HIF-1α in the FHSL group was extremely significantly increased (P < 0.01), and the level of HIF-2α was significantly increased (P < 0.05), which indicate that cartilage homeostasis was disturbed. Additionally, the cartilage histomorphology (Figure 1D) showed that the cartilage matrix was destroyed, which was confirmed by the results presented in Figure 1E and 1F.
Figure 1.
The anatomical manifestations and comparison of cartilage homeostasis between spontaneous FHN and normal broilers. (A) Normal femoral head. (B) Femoral head Incomplete separation of articular cartilage and growth plate. (C) Fracture of the epiphysis. (D) The histomorphology of articular cartilage was observed by H&E, alcian blue, toluidine blue and safranine O-solid green staining. (E) Cartilage homeostasis related level of mRNA expression. (F) Heatmap of cartilage homeostasis related genes mRNA level. Date was presented as mean ± SD (n = 8). *P < 0.05, **P < 0.01. Abbreviations: Col-2, collagen 2; Col-10, collagen-10.
Changes in ER Stress-Related Level of mRNA Expression Between Spontaneous FHN and Normal Broilers
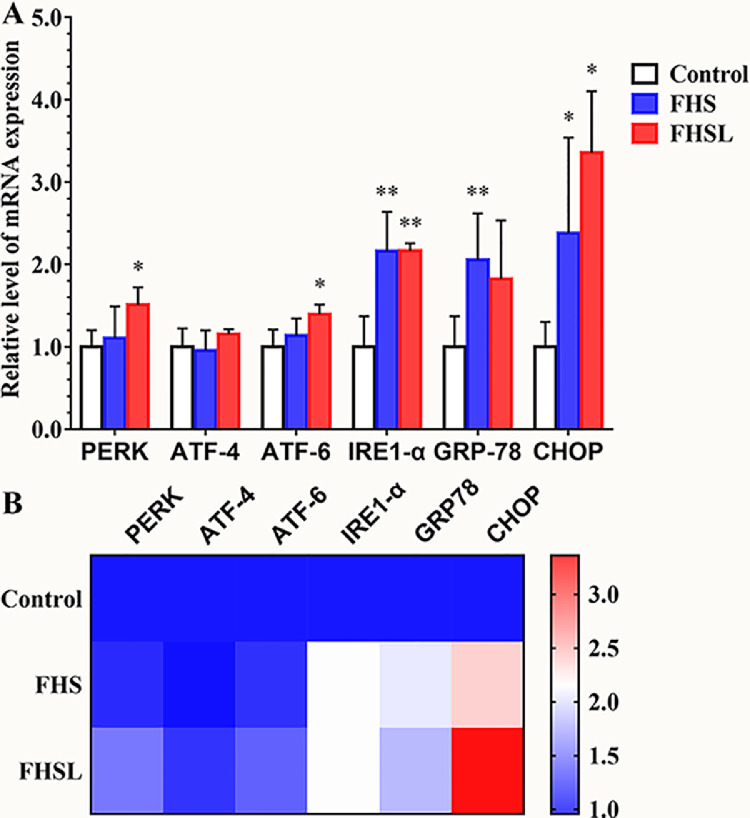
ER stress can increase GRP78/BiP expression in chondrocytes, and regulate unfolded protein responses in the ER through 3 classical pathways (PERK, ATF-6, IRE1-α). In this study (Figure 2), compared with the control group, the mRNA levels of PERK and ATF-6 in the FHSL group were significantly increased (P < 0.05), and the mRNA level of IRE1-α was extremely significantly increased (P < 0.01); the mRNA levels of IRE1-α and GRP78/Bip in the FHS group were extremely significantly increased (P < 0.01). Furthermore, related studies showed that ER stress can regulate apoptosis through the PERK-ATF4-CHOP pathway, therefore, the mRNA level of CHOP was measured, and the result (Figure 2) showed that it was significantly increased in the FHS and FHSL groups compared with the normal group, which indicated that chondrocyte apoptosis may have been promoted. Therefore, the related indexes of ER stress, autophagy and apoptosis were calculated.
Figure 2.
The mRNA levels of ER stress related genes in spontaneous FHN and normal broilers were compared. (A) ER stress related level of mRNA expression. (B) Heatmap of ER stress related genes mRNA level. Date was presented as mean ± SD (n = 8). *P < 0.05, **P < 0.01. Abbreviations: PERK, protein kinase RNA-like ER kinase; ATF-4, activating transcription factor-4; ATF-6, activating transcription factor-6; IRE1-α, inositol requiring protein-1α; GRP78, glucose-regulated protein 78; CHOP, CCAAT-enhancer-binding protein homologous protein.
Changes in Autophagy-Related Level of mRNA Expression Between Spontaneous FHN and Normal Broilers
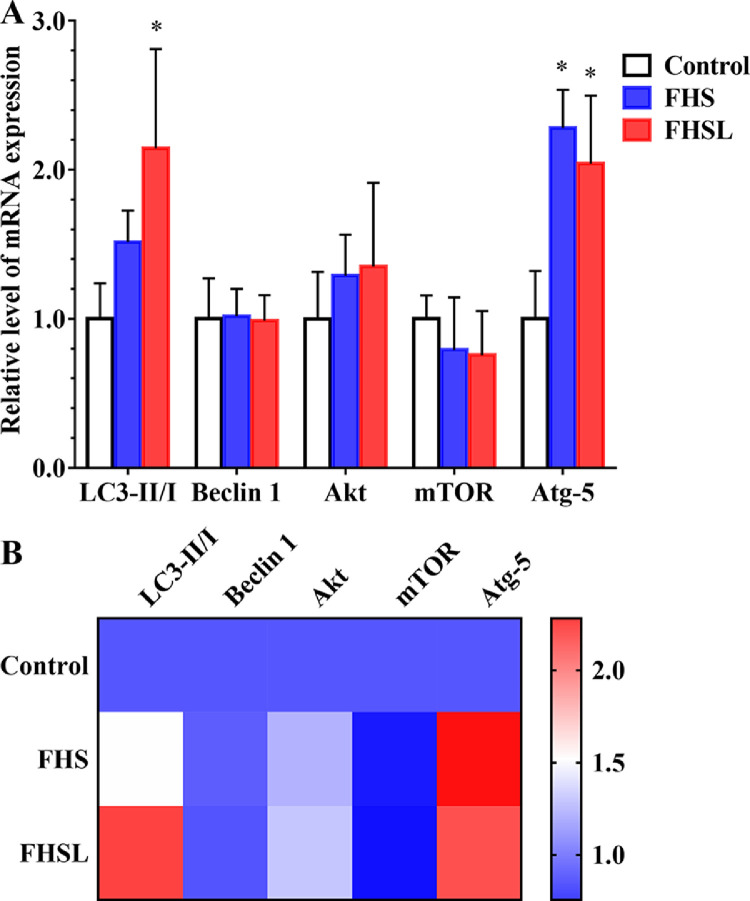
As shown in Figure 3, the mRNA levels of autophagy related genes such as LC3-II / I and Atg-5 in the FHSL group were significantly higher than those in the normal group (P < 0.05), while the mRNA level of Beclin1 did not change significantly (P > 0.05), but showed an upward trend. The mRNA levels of Akt and mTOR did not change significantly (P > 0.05), which indicated that autophagy in spontaneous FHN may not be regulated by the Akt-mTOR pathway.
Figure 3.
The mRNA levels of autophagy related genes in spontaneous FHN and normal broilers were compared. (A) Autophagy related level of mRNA expression. (B) Heatmap of autophagy related genes mRNA level. Date was presented as mean ± SD (n = 8). *P < 0.05, **P < 0.01. Abbreviations: Akt, protein kinase B; ATG, autophagy-related gene.
Changes in Apoptosis-Related Level of mRNA Expression Between Spontaneous FHN and Normal Broilers
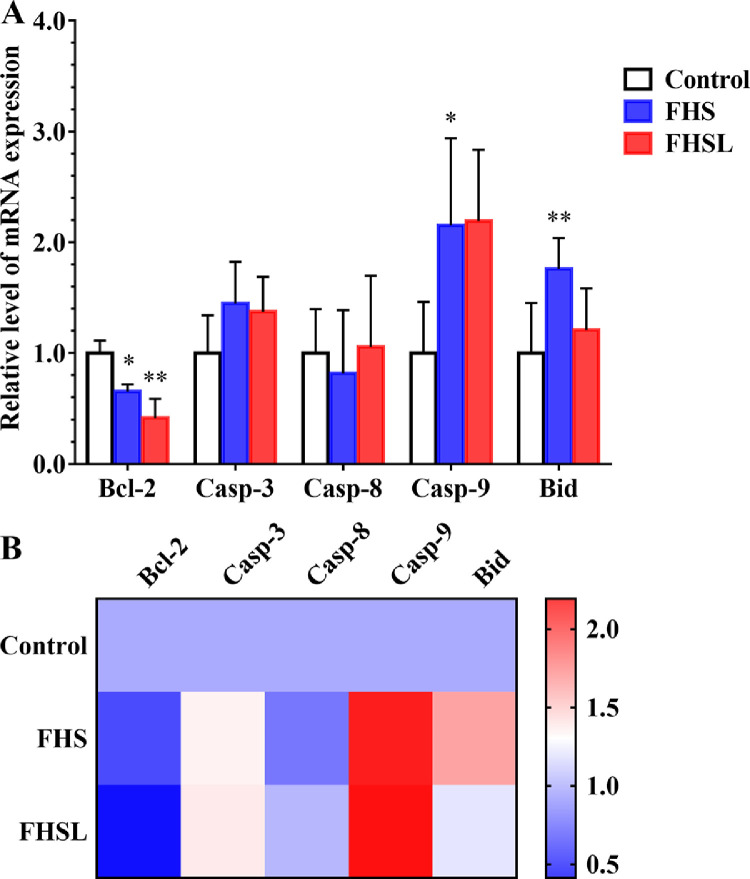
The levels of apoptosis-related genes such as Bcl-2, caspase-3, caspase-8, caspase-9 and Bid were detected. The mRNA level of Bcl-2, an antiapoptotic gene, was extremely significantly decreased in the FHSL group compared with the control group (P < 0.01). Moreover, in the FHS group, compared with the control group, the mRNA levels of Bcl-2 and caspase-9 were significantly increased (P < 0.05), and the mRNA level of Bid was extremely significantly increased (P < 0.01). The results (Figure 4) indicate that apoptosis occurred in the chondrocytes of broilers with spontaneous FHN.
Figure 4.
The mRNA levels of apoptosis related genes in spontaneous FHN and normal broilers were compared. (A) Apoptosis related level of mRNA expression. (B) Heatmap of apoptosis related genes mRNA level. Date was presented as mean ± SD (n = 8). *P < 0.05, **P < 0.01. Abbreviations: Bcl-2, B-cell lymphoma-2; Casp, caspase; Bid, Bcl-2 homology interacting-domain death agonist.
Changes in Antioxidant-Related Level of mRNA Expression Between Spontaneous FHN and Normal Broilers
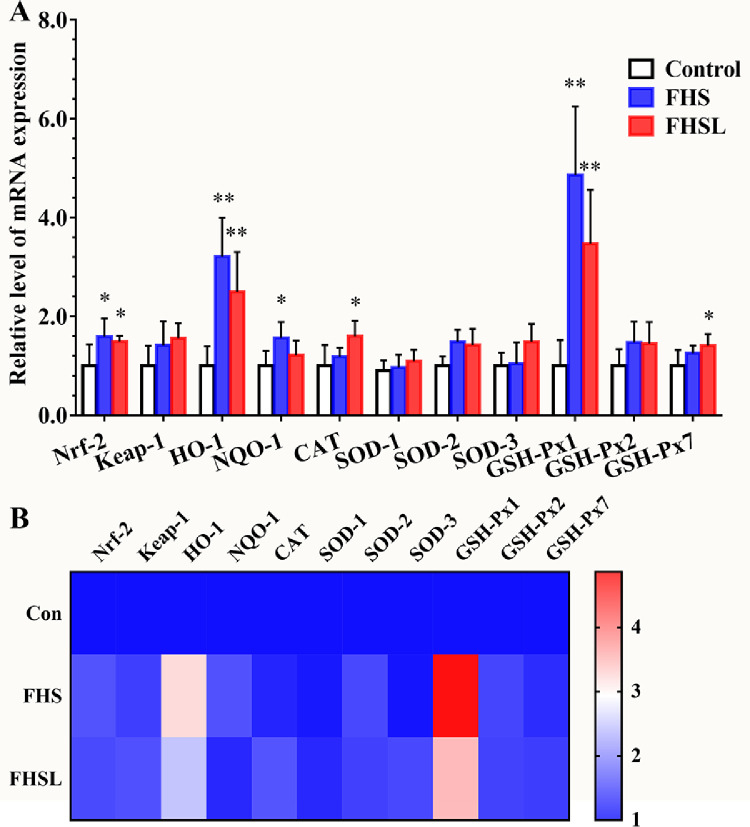
Some studies have shown that PERK can regulate the body's antioxidant levels by activating the Nrf-2 pathway. Therefore, the related indicators of oxidative stress were measured in the present study. The results are presented in Figure 5. The mRNA levels of Nrf-2 and NQO-1 in the FHS group were significantly increased compared with the control group (P < 0.05), and the level of HO-1 was extremely significantly increased (P < 0.01). In the FHSL group, compared with control group, Nrf-2 was significantly higher (P < 0.05), and HO-1 was extremely significantly increased (P < 0.01). At the same time, the levels of CAT and GSH-Px7 in the FHSL group, which are markers of antioxidant levels, were also significantly increased (P < 0.05). Furthermore, the level of GSH-Px1 in the FHS and FHSL groups was extremely significantly increased (P < 0.01), which indicates that oxidative stress occurs in spontaneous FHN, thus activating the collective antioxidant system and improving the antioxidant level.
Figure 6.
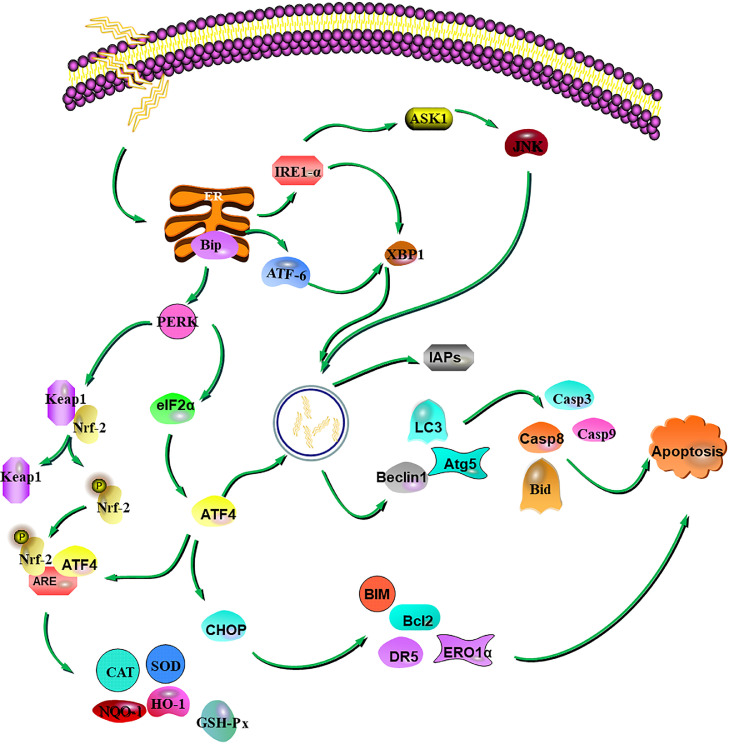
Regulatory mechanism of ER stress, apoptosis and oxidative stress in articular cartilage of broilers with spontaneous FHN. When broilers were stimulated by external environment, ER stress was activated, and through IRE1-α, ATF-6 and PERK pathways regulate UPR to maintain cell homeostasis. However, a series of reactions may occur, such as activating Nrf-2/ARE pathway through PERK to exert antioxidant function, or inducing autophagy or even apoptosis, may occur when the duration or degree of ER stress reaches the limit of cellular adaptive. Abbreviations: ATF-6, activating transcription factor-6; ER, endoplasmic reticulum; FHN, Femoral head necrosis; IRE1-α, inositol requiring protein; PERK, protein kinase RNA-like ER kinase; UPR, unfolded protein response.
Figure 5.
The mRNA levels of oxidative stress related genes in spontaneous FHN and normal broilers were compared. (A) Oxidative stress related level of mRNA expression. (B) Heatmap of oxidative stress related genes mRNA level. Date was presented as mean ± SD (n = 8). *P < 0.05, **P < 0.01. Abbreviations: Nrf-2, nuclear factor erythroid 2-related factor 2; Keap-1, kelch-like ECH-associated protein 1; HO-1, heme Oxygenase-1; NQO-1, NADPH quinineoxidoreductase-1; CAT, catalase; SOD: superoxide dismutase; GSH-Px, glutathione peroxidase.
DISCUSSION
In fast-growing broilers, the highest incidence of FHN occurs between 4 and 6 wk of age, and related studies have found that the occurrence of FHN is related to articular cartilage homeostasis (Zhang et al., 2017; Zhang et al., 2019; Yu et al., 2020). The main components of the ECM in articular cartilage are collagen-2 and aggrecan, which are synthesized and secreted by chondrocytes (Qi and Scully, 2000), thus, the death of chondrocytes is related to the destruction of cartilage (Mobasheri, 2002; Lires-Deán et al., 2008; Tang et al., 2017). Collagen-2 is a typical marker of nonproliferative chondrocytes (Mendler et al., 1989). Aggrecan provides osmotic resistance, allowing cartilage to resist external pressure (McDevitt, 1973). Their expression determines whether the physiological functioning of cells is abnormal (McDevitt, 1973; Mendler et al., 1989). Yu et al. have reported that broilers with FHN induced by methylprednisolone showed more pronounced production of catabolic factors and suppressed anabolic factors. The expression of collagen-2 and aggrecan in FHN-affected broilers caused chondrocyte levels to decrease significantly (Figure 1D-F), indicating that the secretory activity of cells was seriously damaged, a finding which is accordance those of with Zhang et al., (2019).
Regarding the destruction of chondrocyte homeostasis, related studies have reported that ER stress is linked with the occurrence of FHN (Tao et al., 2017; Zhang et al., 2019), thus, the relevant indicators of ER stress in cartilage tissue in broilers with spontaneous FHN were valuated. Sato et al., (2015) reported that GC-induced osteoblast/osteocyte apoptosis is mediated by ER stress and ROS. Gao et al., 2020 found that glucocorticoid upregulated the expression of ER stress-related proteins, indicating that ER stress plays an important role in the occurrence of FHN, which is in line with our results (Figure 2). However, the mRNA level of ATF-4 did not increase significantly, which may be due to the combination of ATF-4 and Nrf-2, which activates the antioxidant mechanism (Brewer, 2014). Nrf-2 is well known as a classic antioxidant regulator, and the Nrf-2/ARE route plays an important role in antioxidant defense in the body (Ma, 2013; Suzuki et al., 2013). When oxidative stress occurs, Nrf-2 is activated and enters the nucleus from the cytoplasm, increasing the transcription of antioxidant enzymes such as SOD, GSH-Px and CAT (Afshari-Kaveh et al., 2020), which is consistent with our results (Figure 5). Furthermore, the level of ATF-4 did not change significantly during ER stress, suggesting that ATF-4 may be involved in the activation of the Nrf-2 pathway. Therefore, it is speculated that oxidative stress may be mediated by ATF-4.
It is now apparent that ER stress is a potent trigger of autophagy, a self-degradative process that has an adaptive function (Lee et al., 2015). At present, autophagy is a well-established protective mechanism of cartilage itself (Zhou et al., 2020). Zhang et al., 2012 found that mesenchymal stem cells can promote their survival by increasing the level of autophagy under hypoxia and serum-free conditions. Our work showed that (Figure 3) the mRNA of LC3-II/I and Atg-5 was significantly higher than that of normal broilers, indicating that autophagy was triggered, but Akt-mTOR did not change significantly, indicating that ER stress in the cartilage tissue of FHN may not activate autophagy through the Akt-mTOR pathway.
When the duration or degree of ER stress reaches the limit of cellular adaptive mechanisms including autophagy, cell death programs are activated. Simultaneously, related studies show that under prolonged stress, CHOP initiates the apoptotic response by activating BIM and death receptor 5 and decreasing Bcl-2 antiapoptotic protein (McCullough et al., 2001; Yamaguchi and Wang, 2004; Puthalakath et al., 2007). Caspase-8 is the principal initiator of the extrinsic apoptotic pathway (Mandal et al., 2020). Procaspase-8 is activated through 2 cleavage events in the death‐inducing signaling complex (Chang et al., 2003). Activated caspase-8 cleaves a proapoptotic Bid into truncated Bid, and it promotes the formation of apoptosome and activates caspase-9 and subsequently caspase-3v (Baliga and Kumar, 2003). Compared with the control group, the mRNA level of antiapoptotic protein Bcl-2 was significantly decreased, while the mRNA levels of caspase-9 and Bid were significantly increased (Figure 4). Although caspase-3 and caspase-8 did not change significantly, they still showed an upward trend, indicating that cartilage tissue apoptosis was promoted.
In conclusion, the secretory activity of articular chondrocytes in broilers with spontaneous FHN was extinguished, which affected the synthesis of ECM. FHN in broilers causes ER stress in articular chondrocytes and regulates oxidative stress by activating the Nrf-2/ARE pathway through PERK (Figure 6). When the duration or degree of ER stress reaches the limit of cellular adaptive mechanisms, autophagy can be activated through the PERK-ATF4 pathway, and apoptosis can even be activated through CHOP. This study provides a theoretical basis for the prevention and treatment of spontaneous femoral head necrosis in broilers.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant number 32072936).
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Afshari-Kaveh M., Abbasalipourkabir R., Nourian A., Ziamajidi N. The protective effects of vitamins A and E on Titanium Dioxide Nanoparticles (nTiO2)-induced oxidative stress in the spleen tissues of male wistar rats. Biol. Trace Elem. Res. 2020;12:1–11. doi: 10.1007/s12011-020-02487-z. [DOI] [PubMed] [Google Scholar]
- Ayaub E.A., Kolb P.S., Mohammed-Ali Z., Tat V., Murphy J., Bellaye P.S., Shimbori C., Boivin F.J., Lai R., Lynn E.G., Lhoták Š., Bridgewater D., Kolb M.R., Inman M.D., Dickhout J.G., Austin R.C., Ask K. GRP78 and CHOP modulate macrophage apoptosis and the development of bleomycin-induced pulmonary fibrosis. J. Pathol. 2016;239:411–425. doi: 10.1002/path.4738. [DOI] [PubMed] [Google Scholar]
- B'Chir W., Maurin A.C., Carraro V., Averous J., Jousse C., Muranishi Y., Parry L., Stepien G., Fafournoux P., Bruhat A. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic. Acids. Res. 2013;41:7683–7699. doi: 10.1093/nar/gkt563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliga B., Kumar S. Apaf-1/cytochrome c apoptosome: an essential initiator of caspase activation or just a sideshow? Cell Death Differ. 2003;10:16–18. doi: 10.1038/sj.cdd.4401166. [DOI] [PubMed] [Google Scholar]
- Brewer J.W. Regulatory crosstalk within the mammalian unfolded protein response. Cell. Mol. Life Sci. 2014;71:1067–1079. doi: 10.1007/s00018-013-1490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Dai D.L., Yao L., Yu H.H., Ning B., Zhang Q., Chen J., Cheng W.H., Shen W., Yang Z.X. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol. Cell. Biochem. 2012;364:115–129. doi: 10.1007/s11010-011-1211-9. [DOI] [PubMed] [Google Scholar]
- Chang D.W., Xing Z., Capacio V.L., Peter M.E., Yang X. Interdimer processing mechanism of procaspase-8 activation. EMBO J. 2003;22:4132–4142. doi: 10.1093/emboj/cdg414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Geng N., Zhou D., Zhu Y., Xu Y., Liu K., Liu Y., Liu J. The regulatory role of COX-2 in the interaction between Cr(VI)-induced endoplasmic reticulum stress and autophagy in DF-1 cells. Ecotoxicol. Environ. Saf. 2019;170:112–119. doi: 10.1016/j.ecoenv.2018.11.120. [DOI] [PubMed] [Google Scholar]
- Chen Y., Gui D., Chen J., He D., Luo Y., Wang N. Down-regulation of PERK-ATF4-CHOP pathway by Astragaloside IV is associated with the inhibition of endoplasmic reticulum stress-induced podocyte apoptosis in diabetic rats. Cell. Physiol. Biochem. 2014;33:1975–1987. doi: 10.1159/000362974. [DOI] [PubMed] [Google Scholar]
- Dinev I., Kanakov D., Kalkanov I., Nikolov S., Denev S. Comparative pathomorphologic studies on the incidence of fractures associated with leg skeletal pathology in commercial broiler chickens. Avian Dis. 2019;63:641–650. doi: 10.1637/aviandiseases-D-19-00108. [DOI] [PubMed] [Google Scholar]
- Dong X., Zhai R., Liu Z., Lin X., Wang Z., Hu Z. The effect of intravenous infusions of glutamine on duodenal cell autophagy and apoptosis in early-weaned calves. Animals (Basel) 2019;9:404. doi: 10.3390/ani9070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Zhu H., Wang Q., Feng Y., Zhang C. Inhibition of PERK signaling prevents against glucocorticoid-induced endotheliocyte apoptosis and osteonecrosis of the femoral head. Int. J. Biol. Sci. 2020;16:543–552. doi: 10.7150/ijbs.35256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner B.M., Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011;333:1891–1894. doi: 10.1126/science.1209126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick D., Barth S., Macleod K.F. Autophagy: cellular and molecular mechanisms. J. Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Liu G., Zhang H., Hu X., Zhang X., Han F., Cui H., Luo J., Guo R., Li R., Li N., Wei L. High-mobility group box 1 protein (HMGB1) from Cherry Valley duck mediates signaling pathways and antiviral activity. Vet. Res. 2020;51:12. doi: 10.1186/s13567-020-00742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Tian M., Ding C., Yu S. The C/EBP Homologous Protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front. Immunol. 2018;9:3083. doi: 10.3389/fimmu.2018.03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2017;54:287–293. [Google Scholar]
- Julian R.J. Production and growth related disorders and other metabolic diseases of poultry–a review. Vet. J. 2005;169:350–369. doi: 10.1016/j.tvjl.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Hargis B.M., Owens C.M. White striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 2016;95:2724–2733. doi: 10.3382/ps/pew216. [DOI] [PubMed] [Google Scholar]
- Lee W.S., Yoo W.H., Chae H.J. ER stress and autophagy. Curr. Mol. Med. 2015;15:735–745. doi: 10.2174/1566524015666150921105453. [DOI] [PubMed] [Google Scholar]
- Li P.F., Zhou Z.L., Shi C.Y., Hou J.F. Downregulation of basic fibroblast growth factor is associated with femoral head necrosis in broilers. Poult. Sci. 2015;94:1052–1059. doi: 10.3382/ps/pev071. [DOI] [PubMed] [Google Scholar]
- Lires-Deán M., Caramés B., Cillero-Pastor B., Galdo F., López-Armada M.J., Blanco F.J. Anti-apoptotic effect of transforming growth factor-beta1 on human articular chondrocytes: role of protein phosphatase 2A. Osteoarthritis Cartilage. 2008;16:1370–1378. doi: 10.1016/j.joca.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Liu K., Chen P., Lu J., Zhu Y., Xu Y., Liu Y., Liu J. Protective effect of purple tomato anthocyanidin on chromium(VI)-induced autophagy in LMH Cells by inhibiting endoplasmic reticulum stress. Biol. Trace Elem. Res. 2020;194:570–580. doi: 10.1007/s12011-019-01795-3. [DOI] [PubMed] [Google Scholar]
- Liu K., Wang K., Wang L., Zhou Z. Changes of lipid and bone metabolism in broilers with spontaneous femoral head necrosis. Poult Sci. 2020;100 doi: 10.1016/j.psj.2020.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Shi Q., Song X., Wang Y., Wang Y., Song E., Song Y. Activating transcription factor 4 (ATF4)-ATF3-C/EBP Homologous Protein (CHOP) cascade shows an essential role in the ER stress-induced sensitization of tetrachlorobenzoquinone-challenged PC12 cells to ROS-Mediated APOPTOSIS via Death Receptor 5 (DR5) signaling. Chem. Res. Toxicol. 2016;29:1510–1518. doi: 10.1021/acs.chemrestox.6b00181. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR. Methods. 2002;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal R., Barrón J.C., Kostova I., Becker S., Strebhardt K. Caspase-8: the double-edged sword. Biochim. Biophys. Acta Rev. Cancer. 2020;1873 doi: 10.1016/j.bbcan.2020.188357. [DOI] [PubMed] [Google Scholar]
- Marchi S., Patergnani S., Pinton P. The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochim. Biophys. Acta. 2014;1837:461–469. doi: 10.1016/j.bbabio.2013.10.015. [DOI] [PubMed] [Google Scholar]
- McCullough K.D., Martindale J.L., Klotz L.O., Aw T.Y., Holbrook N.J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt C.A. Biochemistry of articular cartilage. Nature of proteoglycans and collagen of articular cartilage and their role in ageing and in osteoarthrosis. Ann. Rheum. Dis. 1973;32:364–378. doi: 10.1136/ard.32.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendler M., Eich-Bender S.G., Vaughan L., Winterhalter K.H., Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J. Cell Biol. 1989;108:191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Mobasheri A. Role of chondrocyte death and hypocellularity in ageing human articular cartilage and the pathogenesis of osteoarthritis. Med. Hypotheses. 2002;58:193–197. doi: 10.1054/mehy.2000.1180. [DOI] [PubMed] [Google Scholar]
- Morishima N., Nakanishi K., Nakano A. Activating transcription factor-6 (ATF6) mediates apoptosis with reduction of myeloid cell leukemia sequence 1 (Mcl-1) protein via induction of WW domain binding protein 1. J. Biol. Chem. 2011;286:35227–35235. doi: 10.1074/jbc.M111.233502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okimoto R., Rasaputra K., Clark F.D., Rath N.C. Histopathology and serum clinical chemistry evaluation of broilers with femoral head separation disorder. Avian Dis. 2009;53:21–25. doi: 10.1637/8367-051908-Reg.1. [DOI] [PubMed] [Google Scholar]
- Olkowski A.A., Laarveld B., Wojnarowicz C., Chirino-Trejo M., Chapman D., Wysokinski T.W., Quaroni L. Biochemical and physiological weaknesses associated with the pathogenesis of femoral bone degeneration in broiler chickens. Avian Pathol. 2011;40:639–650. doi: 10.1080/03079457.2011.626017. [DOI] [PubMed] [Google Scholar]
- Puthalakath H., O'Reilly L.A., Gunn P., Lee L., Kelly P.N., Huntington N.D., Hughes P.D., Michalak E.M., McKimm-Breschkin J., Motoyama N., Gotoh T., Akira S., Bouillet P., Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Qi W.N., Scully S.P. Extracellular collagen regulates expression of transforming growth factor-beta1 gene. J. Orthop. Res. 2000;18:928–932. doi: 10.1002/jor.1100180612. [DOI] [PubMed] [Google Scholar]
- Rowland A.A., Voeltz G.K. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat. Rev. Mol. Cell Biol. 2012;13:607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Rodriguez E., Benavides-Reyes C., Torres C., Dominguez-Gasca N., Garcia-Ruiz A.I., Gonzalez-Lopez S., Rodriguez-Navarro A.B. Changes with age (from 0 to 37 D) in tibiae bone mineralization, chemical composition and structural organization in broiler chickens. Poult. Sci. 2019;98:5215–5225. doi: 10.3382/ps/pez363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A.Y., Tu X., Mcandrews K.A., Plotkin L.I., Bellido T. Prevention of glucocorticoid induced-apoptosis of osteoblasts and osteocytes by protecting against endoplasmic reticulum (ER) stress in vitro and in vivo in female mice. Bone. 2015;73:60–68. doi: 10.1016/j.bone.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su N., Kilberg M.S. C/EBP homology protein (CHOP) interacts with activating transcription factor 4 (ATF4) and negatively regulates the stress-dependent induction of the asparagine synthetase gene. J. Biol. Chem. 2008;283:35106–35117. doi: 10.1074/jbc.M806874200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Motohashi H., Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci. 2013;34:340–346. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Tabas I., Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Zheng G., Feng Z., Chen Y., Lou Y., Wang C., Zhang X., Zhang Y., Xu H., Shang P., Liu H. Trehalose ameliorates oxidative stress-mediated mitochondrial dysfunction and ER stress via selective autophagy stimulation and autophagic flux restoration in osteoarthritis development. Cell Death. Dis. 2017;8:e3081. doi: 10.1038/cddis.2017.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S.C., Yuan T., Rui B.Y., Zhu Z.Z., Guo S.C., Zhang C.Q. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics. 2017;7:733–750. doi: 10.7150/thno.17450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Wang H.G. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- Yang Z., Wilkie-Grantham R.P., Yanagi T., Shu C.W., Matsuzawa S., Reed J.C. ATG4B (Autophagin-1) phosphorylation modulates autophagy. J. Biol. Chem. 2015;290:26549–26561. doi: 10.1074/jbc.M115.658088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu T., Nair U., Yang Z., Klionsky D.J. Endoplasmic reticulum stress triggers autophagy. J. Biol. Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Wang S., Zhou Z. Cartilage homeostasis affects femoral head necrosis induced by methylprednisolone in broilers. Int. J. Mol. Sci. 2020;21:4841. doi: 10.3390/ijms21144841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai R., Dong X., Feng L., Li S., Hu Z. The effect of heat stress on autophagy and apoptosis of rumen, abomasum, duodenum, liver and kidney cells in calves. Animals (Basel) 2019;9:854. doi: 10.3390/ani9100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Li S., Pang K., Zhou Z. Endoplasmic reticulum stress affected chondrocyte apoptosis in femoral head necrosis induced by glucocorticoid in broilers. Poult. Sci. 2019;98:1111–1120. doi: 10.3382/ps/pey474. [DOI] [PubMed] [Google Scholar]
- Zhang M., Shi C.Y., Zhou Z.L., Hou J.F. Bone characteristics, histopathology, and chondrocyte apoptosis in femoral head necrosis induced by glucocorticoid in broilers. Poult. Sci. 2017;96:1609–1614. doi: 10.3382/ps/pew466. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Yang Y.J., Wang H., Dong Q.T., Wang T.J., Qian H.Y., Xu H. Autophagy activation: a novel mechanism of atorvastatin to protect mesenchymal stem cells from hypoxia and serum deprivation via AMP-activated protein kinase/mammalian target of rapamycin pathway. Stem Cells Dev. 2012;21:1321–1332. doi: 10.1089/scd.2011.0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Liu L., Xu Y., Jiang J., Liu G., Zhai C. Effects of osteoblast autophagy on glucocorticoid-induced femoral head necrosis. Jt. Dis. Relat. Surg. 2020;31:411–418. doi: 10.5606/ehc.2020.73036. [DOI] [PMC free article] [PubMed] [Google Scholar]