Abstract
The reticuloendotheliosis virus (REV) and the Marek's disease virus (MDV) cause reticuloendotheliosis (RE) and Marek's disease (MD) in poultry, respectively. According to epidemiological results obtained in our laboratory from 2010 to 2017, the positive rates of REV and MDV co-infection remained at low levels. In the present study, during the period of October 2018 to July 2020, 4 clinical cases with high morbidity (5%-20%) and mortality (2%-10%), caused by the co-infection of REV and vv+ MDV-like strains, were diagnosed and analyzed by histopathological observation, cell cultures and detection with ELISA and IFA, and the PCR and by sequencing of the isolates’ genes. Sequencing and the sequence analysis on the complete genomes of the REV strains and the meq genes of the MDV strains were performed. The results, based on the complete genome, LTR, gag, pol, and env genes’ nucleotide sequences of the REV strains, showed that the REV isolates and 68.0 % (17/25) of the reference strains were in a same branch, and all had a high sequence similarity (>99.0%). The similarities between the four isolates and a vv+MDV strain GX18NNM4 were very high, up to 99.3-99.8%. Also, the amino acid residuals at locations 71, 77, 80, 115, 139, 176, and 217 were all the same as A, E, Y, A, A, R, and A, respectively, in the meq gene of the four MDV isolates. In addition, the substitutes at P176R and P217A interrupted the stretches of the proline-rich repeat PPPP, indicating that these strains belonged to the vv+ MDV-like category. Our findings indicated that the more recent and frequent reemergence of REV and the subsequent co-infection with vv+ MDV-like strain has become one of the causes of the clinical outbreaks of tumors and is undoubtedly a threat to the poultry industry in southern China.
Key words: reticuloendotheliosis virus, marek's disease virus, reemergence, co-infection, yellow-chickens
INTRODUCTION
Reticuloendotheliosis virus (REV) is an oncogenic and immunosuppressive retrovirus that causes reticuloendotheliosis (RE) (Xu et al., 2020). REV can cause neoplasia and immunosuppression (Sun et al., 2017b), as well as runting stunting syndrome (RSS) in many avian hosts, including chickens, turkeys, ducks, geese, pigeon, and many other wild birds (Zhai et al., 2016; Thontiravong et al., 2019; Alfaki et al., 2020; Caleiro et al., 2020; Liu et al., 2020). The initial REV strain T was isolated from a turkey with visceral lymphomas in 1957 (Robinson and Twiehaus, 1974). In China, REV was first isolated in 1986 (Jiang et al., 2014), and spread rapidly throughout the country, causing great loss to the poultry industry (Yang et al., 2017). The full-length of the genome sequences of REV was 8.0-9.0 kb, including the LTR, gag, pol, and env genes (Jiang et al., 2012).
Marek's disease virus (MDV) is an oncogenic alphaherpesvirus that causes Marek's disease (MD) (He et al., 2019). MDV induces lymphomas and immunosuppression in chickens (Faiz et al., 2018) and the clinical signs include paralysis, skin leukosis, depression and death (Gergen et al., 2019). According to the virulence of the virus, Species 1 MDV (MDV-1) can be clustered into mild (m), virulent (v), very virulent (vv) and very virulent plus (vv+) strains (Teng et al., 2011). The full-length of the genome sequences of MDV was 160-180 kb (He et al., 2019) and encodes a large number of genes. Among these genes the meq gene was one of the genes most associated with viral oncogenicity and pathogenicity (Shamblin et al., 2004).
Clinically, few cases were caused by REV infection alone as most of them were the co-infection of REV and MDV or other tumor viruses (Li et al., 2019b). A previous study showed that the co-infection of REV and MDV can increases illness severity and reduces MD vaccine efficacy (Sun et al., 2017b). According to epidemiological results obtained in our laboratory from 2010 to 2017, the positive rate of the co-infection of REV and MDV had remained at a low level (Li et al., 2019a). However, during a period from October 2018 to July 2020, 4 clinical cases of different breeds of Yellow-chickens with high morbidity and mortality were sent to our laboratory for diagnosis and the results showed that all these cases were the co-infection of REV and MDV.
MATERIALS AND METHODS
Case History
In the present study, during the period October 2018 to July 2020, diseased chickens of different breeds with emaciation, white combs and depression from clinical disease cases were sent to our laboratory for diagnosis. The ages of the diseased birds ranged from 78 to 120 day-old. The affected birds exhibited morbidities of 5% to20% and mortalities of 2% to 10% (Supplementary Table S1) at the dates of delivery to the laboratory for diagnosis. All birds had been vaccinated with the commercial MD vaccine CVI988/Rispens at hatching.
Sample Collection and Processing
Tissue samples of significant tumor-like lesions were collected from all submitted birds, and the anticoagulant treated blood samples were collected also from the same birds. One portion of each tissue was fixed in 10% buffered formalin and used for histopathological observations according to routine procedures (Wang et al., 2019). Another portion of the tissue was homogenized and made into a 1:3 (v/v) suspension with phosphate buffered saline, freeze-thawed three times at -80 ℃ and room temperature, and then filtrated through a 0.22 μm filter, and stored at -80 ℃ until used. Lymphocytes were separated from the blood samples by using the lymphocyte separation medium according to the routine procedure (Zhang et al., 2019).
Virus Isolation and Identification
DF-1 cells was used to isolate ALV as recently described (Wang et al., 2020). Briefly, the filtrated tissue suspension was inoculated into DF-1 cells for 2 hs, then replenished with DMEM containing 1% fetal calf serum (Life, Australia) and passed for three serial passages (7 days for each passage). Chicken embryo fibroblasts (CEFs) were used to isolate REV and MDV as recently described (Zhang et al., 2019). Briefly, the harvested lymphocytes were inoculated onto the primary CEFs grown in the 24-well plate (two wells per each sample at least) and the cultures were incubated at 37 ℃ in 5% CO2 and passed for three serial passages (5 days per passage). The DF-1 cell cultures were used for the detection of ALV P27 antigen with the Avian Leukosis Virus Antigen Test Kit (Biochek, Holland) according to the routine procedure (Wang et al., 2020). The CEFs cultures, two wells from each sample, were used for the identification of REV and MDV by IFA using the monoclonal antibodies (mAbs) 11B118 against REV and BA4 against MDV, respectively (Cui et al., 2016). In order to detect the viral isolates’ genes, DNA samples from the cell cultures were extracted by using a commercial DNA extraction kit (Tiangen, Beijng). The primers for the PCR detections are listed in Table 1, and the PCR procedure was performed as described previously (Li et al., 2019a).
Table 1.
Primer sequences used for detection or amplification of REV and MDV isolates.
| Name | Sequence (5′-3′) | Expected size (bp) |
|---|---|---|
| MDV meq F | CCGTCTAGAAGGCGGGCACGGTAC | 1113 |
| MDV meq R | CGGAAGCTTAAACATGGGGCATAGACG | |
| MDV 132bpr F | TGCGATGAAAGTGCTATGGAGG | 185, 317 449, 581 |
| MDV 132bpr R | GAGAATCCCTATGAGAAAGCGC | |
| REV LTR F | CATACTGGAGCCAATGGT | 291 |
| REV LTR R | AATGTTGTAGCGAAGTACT | |
| REV-1-F | GCTATATAAGCCAGGTGC | 1520 |
| REV-1-R | ACTGGGCTTCTATTTCTCGCT | |
| REV-2-F | GTGGATTAGAGTTCGGAGCA | 3300 |
| REV-2-R | CCGTCGGTTACCAGTTGA | |
| REV-3-F | TGATCTACAGAGAAAGGGG | 1102 |
| REV-3-R | CTTAACGAAGACGAGGTCAC | |
| REV-4-F | GGTCTCAAGCCTCCTGTA | 600 |
| REV-4-R | CCACTGTTGTCTAAATCCC | |
| REV-5-F | CTCCTGACAACCAAGAAG | 1825 |
| REV-5-R | AGCTCCCTCCCACATTC | |
| REV-6-F | AATGTGGGAGGGAGCTCC | 800 |
| REV-6-R | GTAACAAACAAACACACAAACCAC | |
| REV-7-F | GTGCATACTGGCATCAATCG | 1243 |
| REV-7-R | CCCCCAAATGTTGTACCGAAAT |
Sequencing and Analysis of the Isolates’ Gene and Genome
Seven primer-sets for the amplification of the full genome of REV and one primer-set for the amplification of the MDV meq gene were designed by our team with Primer Premier software version 5, listed in Table 1. The conditions for PCR were as follows: 95 ℃, 5 min; 95 ℃ 15 s; 50-60℃, 15 s; 72 ℃, 15s/kb (30 cycles), 72 ℃, 10 min. The PCR products were purified using a Universal DNA Purification Kit (TIANGEN, Beijing, China), and then cloned into the vector pMD18-T (TaKaRa, Dalian, China), respectively. Three independent clones of each of the strains were sequenced by Beijing Genomics Institute (BGI, Guangzhou, China).
The nucleotide (nt) sequences were retrieved, edited using the EditSeq modules of DNASTAR (ver. 7.1.0). All the nt and amino acid (aa) sequences (including the isolates and reference strains) were analyzed using the ClustalW software program with DNASTARR (ver. 7.1.0). Phylogenetic analysis of the REV complete genome and MDV meg sequences were performed with the maximum-likelihood (ML) method using MEGA X (ver.10.1.7).
Ethics Statement
All the experimental animals of this study were cared for and maintained throughout the experiments strictly following the ethics and biosecurity guidelines approved by the Institutional Animal Care and Use Committee of Guangxi University (NO.2018-GXU-168).
RESULTS
Gross and Histological Observations
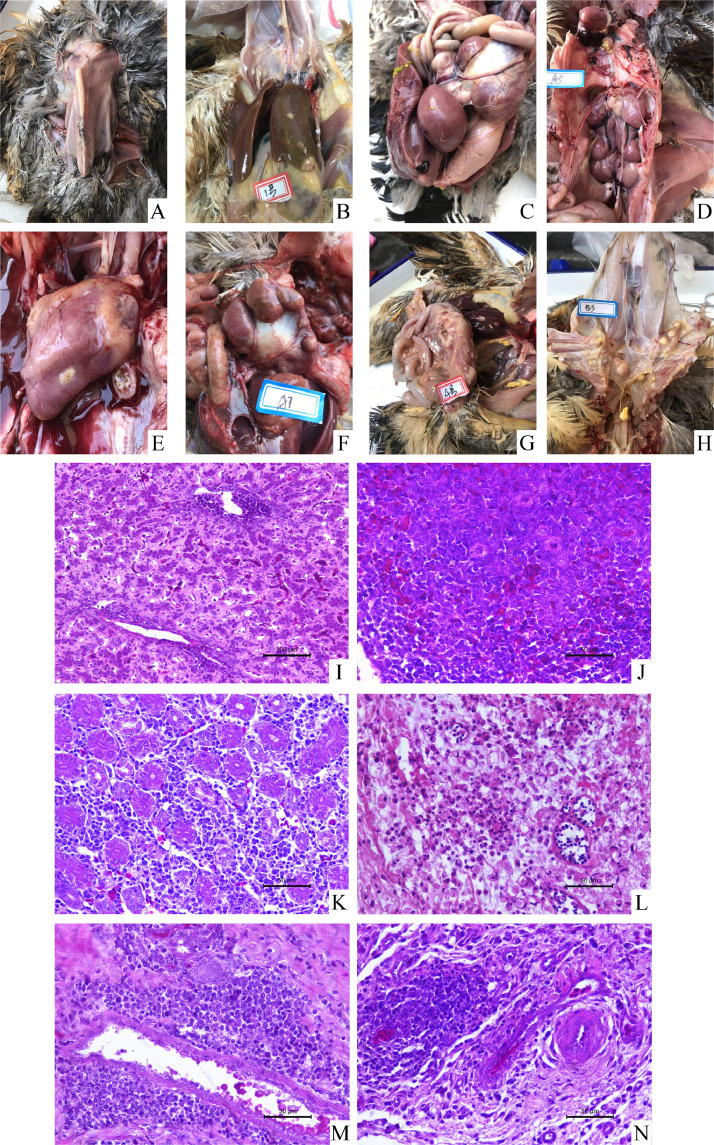
Postmortem examinations were performed on 4 to 6 diseased birds per case. The birds showed stunted growth, and prominent sternums (Figure 1A). Birds in case 1 had white nodules in the liver, spleen and kidney and some birds had marked hepatosplenomegaly (Figure 1B, 1C, 1D); Birds in case 2 had white nodules in the heart, proventriculus and cecum (Figure 1E, 1F, 1G); Birds in case 3 had white nodules in the chest wall (Figure 1H); Birds in case 4 had hyperplasia in the lung (detailed information is shown in Table 2). The histopathological observations of the tissue sections indicated that a large area of neoplastic lymphocytes was observed in the liver, spleen, kidney, heart, proventriculus, and cecum (Table 2, Figure 1I–1N). Specifically, the spleen, kidney, and proventriculus had been infiltrated by both neoplastic lymphocytes and primitive reticular cells (Table 2, Figure 1J, 1K, 1M).
Figure 1.
Gross and histopathological changes. The birds showed stunted growth, and prominent sternums (A). Birds in case 1 had white nodules in the liver, spleen and kidney (B–D). Birds in case 2 had white nodules in the heart, proventriculus and cecum (E–G). Birds in case 3 had white nodules in the chest wall (H). A large area of neoplastic lymphocytes was observed in the liver, spleen, kidney, heart, proventriculus, and cecum (I–N).
Table 2.
Histopathologic findings in the 4 clinical cases.
| Case | Breed | Age (day) | Postmortem examination (4-6 chickens) | Microscopic examination |
|---|---|---|---|---|
| 1 | Native Chicken | 120 | Liver: hepatomegaly or tumor nodule Spleen: splenomegaly or tumor nodule Kidney: none or tumor nodule |
Neoplastic lymphocytes, and primitive reticular cells Neoplastic lymphocytes, and primitive reticular cells Neoplastic lymphocytes |
| 2 | Three- Yellow chicken |
95 | Heart: none or tumor nodule Liver: hepatomegaly or tumor nodule Spleen: splenomegaly or tumor nodule Kidney: none or tumor nodule Proventriculus: swollen proventriculuor or tumor nodule Cecum: none or tumor nodule |
None or neoplastic lymphocytes Neoplastic lymphocytes, and primitive reticular cells Neoplastic lymphocytes, and primitive reticular cells Neoplastic lymphocytes Neoplastic lymphocytes |
| 3 | Native Chicken | 100 | Heart: none or pericardial effusion or tumor nodule Liver: hepatomegaly or tumor nodule Chest wall: none or tumor nodule Spleen: none or tumor nodule Kidney: none or tumor nodule |
Neoplastic lymphocytes Neoplastic lymphocytes, and primitive reticular cells Neoplastic lymphocytes Neoplastic lymphocytes, and primitive reticular cells Neoplastic lymphocytes |
| 4 | Qingyuan-Ma Chicken | 78 | Heart: none or pericardial effusion or tumor nodule Liver: bleeding point or hepatomegaly or tumor nodule Spleen: none or splenomegaly or tumor nodule Lung: none or hyperplasia Proventriculus: swollen proventriculuor or tumor nodule |
Neoplastic lymphocytes Neoplastic lymphocytes, and primitive reticular cells Neoplastic lymphocytes, and primitive reticular cells None or Neoplastic lymphocytes Neoplastic lymphocytes |
Detection and Identification of the Isolates
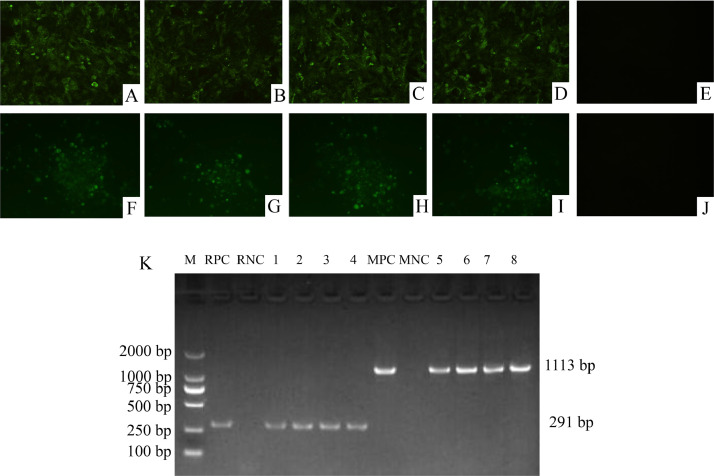
The results of ELISA applied on the DF-1 cultures showed that all samples were negative for ALV (data not shown). The IFA applied on the CEF cultures using the MDV-specific and REV-specific mAbs, respectively, showed that specific immunofluorescence signals were both detected in two wells of cultures from every sample (Figure 2A–2D, 2E–2H). The PCR detection results using the cell cultures also showed every well from each sample was positive for both MDV and REV (Figure 2K).
Figure 2.
IFA and PCR results of CEF cell cultures infected with the samples. Positive fluorescence was observed in the GX18NNR1, GX19YLR1, GX19NNR1, and GD20R1 infected cells by the IFA with REV-specific monoclonal antibody 11B118 (A–D), while no specific fluorescence was observed in the non-infected cells (E). Positive fluorescence was observed in the GX18NNM7, GX19YLM5, GX19NMNM3 and GD20M1 infected cells by the IFA with MDV-1 gB-specific monoclonal antibody BA4 (F–I). No specific fluorescence was observed in the non-infected cells (J). (K) PCR was used to identify the REV and MDV positive samples. M, Marker, DL2000; RPC, CEF cells infected with GD1210 as positive control; RNC, uninfected CEF cells used as a negative control; 1-4, positive PCR products obtained using the primers specific for REV LTR of GX18NNR1, GX19YLR1, GX19NNR1 and GD20R1, respectively; MPC, CEF cells infected with GX18NNM4 as positive control; MNC, uninfected CEF cells used as a negative control; 5-8, positive PCR products obtained using the primers specific for MDV meq of GX18NNM7, GX19YLM5, GX19NMNM3 and GD20M1, respectively.
Sequencing Results of the Provirus Genome of REV and the MegGene of MDV Isolates
For the samples of each case, one REV isolate and one MDV isolate were used for the sequencing. Four REV provirus genomes (GX18NNR1, MW046297; GX19YLR1, MW046296; GX19NNR1, MW046295; GD20R1, MW046294) and four MDV meq genes (GX18NNM8, MW046300; GX19YLM5, MW046293; GX19NNM3, MW046292; GD20M1, MW046291) were successfully sequenced and submitted to GenBank. The complete genomes of the four REV isolates all had a length of 8284 bp with the typical replicative structure of γ-retroviruses. The nt-sequence similarities of the complete genome, LTR, gag, pol and env genes among the four isolates were 99.6-99.8%, 99.4-99.8%, 99.7-99.8%, and 99.7-99.9%, respectively (Supplementary Table S2). The meq genes of the four MDV isolate all had a length of 1020 bp and show 99.3-100% similarities among the four isolates (Supplementary Table S3).
Phylogenetic Analysis of the REV and MDV Isolates
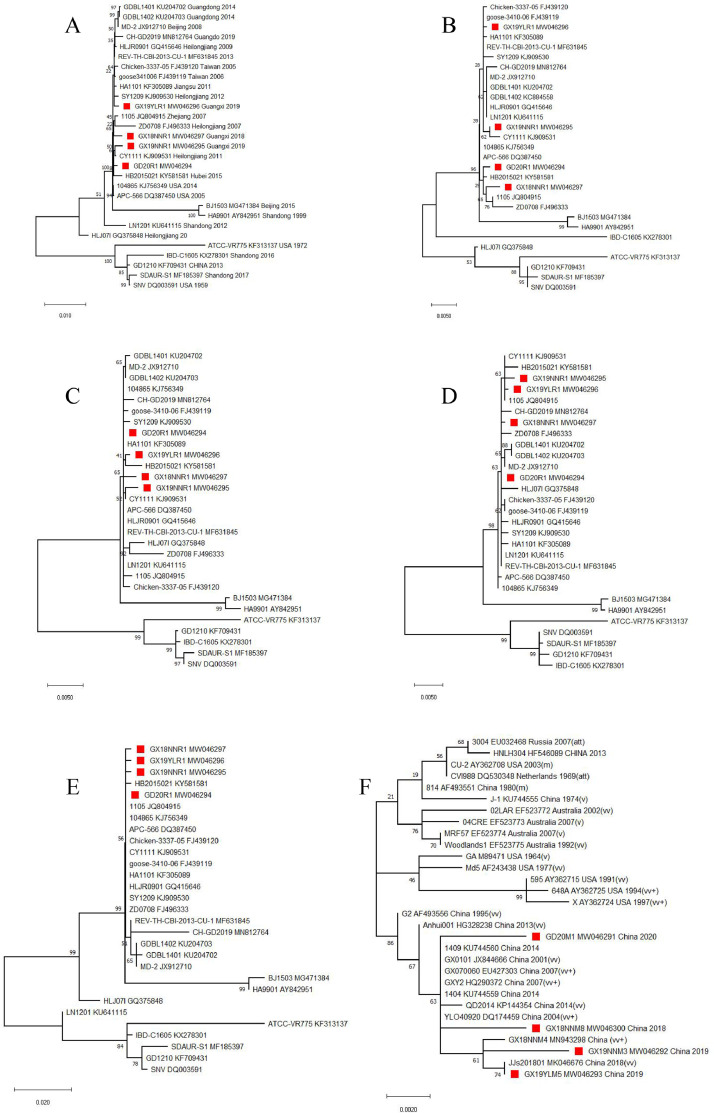
The complete genome sequences of the four REV isolates and 25 other references strains (isolated from chickens, geese, ducks, and wild birds in different countries and regions) from GenBank were aligned, and the identities of different regions of the genes (LTR, gag, pol, env) were assessed (Supplementary Table S2). The results showed that the sequence similarities between the four REV isolates complete genome and the 25 reference strains were 91.0-99.9 %, and those of LTR, gag, pol, and env genes between the four REV isolates and all the reference strains were 91.0% to 100.0%, 95.7% to 99.9%, 95.9% to 100%, and 94.5% to100%, respectively (Supplementary Table S2). All of the phylogenetic trees, which were based on the complete genome, LTR, gag, pol, and env genes, showed that 68.9% (20/29, including the four isolates in the study) of the REV strains were in the same branch, and almost all showed high similarities of more than 99.00% (Figure. 3A–3E, and Supplementary Table S2) .
Figure 3.
Phylogenetic trees based on the sequences of the complete-genome (A), LTR (B), gag (C), pol (D) and env (E) of REV strains, and meq gene of MDV strains (F). The phylogenetic trees were constructed by using the Maximum Likelihood method based on nucleotide alignment with the GTR Substitution Model with 1000 bootstrap replicates using the software MEGA X 10.1.7.
The comparisons of meq on the nt and aa sequences between the four MDV isolates and 26 other reference strains were assessed (Supplementary Table S3) and the similarities were 98.3-99.8% (Supplementary Table S2). It is noteworthy that the similarities between the four isolates and GX18NNM4, a new vv+MDV strain just identified by our group in 2020 (Shi et al., 2020), were high, up to 99.3% to 99.8%. The phylogenetic tree showed that the four MDV isolates and 11 of the reference strains (including the new vv+MDV strain GX18NNM4) were in a same branch (Figure 3F). In addition, the aa residuals of the four MDV isolates at positions 71, 77, 80, 115, 139, 176, and 217 were all the same as A, E, Y, A, A, R, and A, respectively (Table 3), consistent with the characteristics of the vv+ MDV strains like GX18NNM4. Also, the substitutes of P176R and P217A interrupted the stretches of the proline-rich repeat (PRR) PPPP, which might result in an increase in virulence (Mescolini et al., 2019).
Table 3.
Amino acid substitutions in the MEQ proteins of the MDV strains.
| Strains | 71 | 77 | 80 | 115 | 139 | 176 | 217 | Number of PPPP repeats |
|---|---|---|---|---|---|---|---|---|
| CVI988 (att) | S | E | D | V | T | P | P | 8 |
| CU-2 (m) | S | E | D | v | T | P | P | 7 |
| 814 (m) | S | E | D | a | T | P | P | 4 |
| GA (v) | A | K | D | V | T | P | P | 5 |
| J-1 (v) | A | K | D | V | T | P | P | 5 |
| Md5 (vv) | A | K | D | V | T | P | P | 4 |
| G2 (vv) | A | E | Y | A | T | P | A | 4 |
| QD2014 (vv) | A | E | Y | A | A | R | A | 3 |
| GXY2 (vv+) | A | E | Y | A | A | R | A | 3 |
| YLO40920 (vv+) | A | E | Y | A | A | R | A | 3 |
| GX070060 (vv+) | A | E | Y | A | A | R | A | 3 |
| GX18NNM4 | A | E | Y | A | A | R | A | 3 |
| GX18NNM8 | A | E | Y | A | A | R | A | 3 |
| GX19YLM5 | A | E | Y | A | A | R | A | 3 |
| GX19NNM3 | A | E | Y | A | A | R | A | 3 |
| GX20M1 | A | E | Y | A | A | R | A | 3 |
Boldface represents the virus strains isolated in the study.
DISCUSSION
REV can be transmitted vertically in eggs, horizontally by contact with infected birds (Xu et al., 2020), and also could disperse through the contamination of attenuated virus live vaccines (Schat and Erb, 2014). Previous studies have shown that some attenuated live vaccines against IBDV, MDV, HTV, and FPV are contaminated by REV (Fadly et al., 1996; Li et al., 2013; Li et al., 2015; Li et al., 2016), and we believe that this is one of the most common reasons for the high positive rate of REV infection in commercial chickens. A previous study showed that during the years 2005-2015, the seroprevalence in individual chickens in China ranged from 7.49% to 22.83% (Yang et al., 2017), indicting REV infection was common in China in this period. Since the first description of MD in 1907, MD has spread rapidly all over the world (Eschke et al., 2018). At present, dramatic success has been achieved through the extensive use of the CVI988/Rispens and 814 vaccines (Ralapanawe et al., 2016; Reddy et al., 2017). Nevertheless, MD outbreaks have been continually reported in southern China in recent years (Zhang et al., 2015; Sun et al., 2017a; Li et al., 2019a), which is likely due to MDV evolution to increased virulence (Shi et al., 2020) and the co-infection with other viruses (Cui et al., 2016; Li et al., 2019b). According to our previous study, MDV and ALV-J co-infection was about 15% responsible for the clinical tumor outbreaks during 2010 to 2016 and jumped to 57.14% (8/14) in 2017 (Li et al., 2019a). Interestingly, there were few reports of co-infection of REV and MDV in clinical cases during January 2016 to September 2018 in southern China
In the present study, four clinical cases with high morbidity and mortality due to tumors were diagnosed by histopathological observation, cell cultures and the detection with ELISA and IFA, and with PCR and the sequencing of the viral isolate gene and/or genome. The results showed that all the cases exhibited co-infection of REV and MDV. To our knowledge, this is the re-isolation of the REV in clinical cases since 2015 from Yellow-chickens in southern China. Previous study showed that compared with the mono-infection of REV, MDV or ALV, co-infection with different tumor viruses (REV and/or MDV and/or ALV) has more potent pathogenicity: the co-infection with MDV and REV could increase the severity of the diseases and reduce MD vaccine efficacy (Sun et al., 2017b); the co-infection of ALV-J and REV could cause more serious synergistic pathogenic effects (Dong et al., 2015); the ALV and MDV co-infection could cause greater pathogenic effects than the MDV single infections (Wang et al., 2020).
In order to further characterize the pathogens isolated from the diseased birds, the complete genome of the four REV strains and the oncogenic meq gene of four MDV strains were sequenced and analyzed. Based on the phylogenetic tree, 68.9% (20/29) REV strains included the four REV isolates and 16 other reference strains were in the same branch and shared a high similarity (>99.00%), indicating that they may have the same origin. However, we cannot identify the exact source of the four REV strains here, since there are two transmission routes, vertical or horizontal, of REV to the chickens including through the contaminated live vaccine (Schat and Erb, 2014; Li et al., 2016; Xu et al., 2020). The sequence analysis based on meq gene indicated that these four MDV strains belong to vv+ MDV-like strains. A previous study has shown that the vvMDV strain can break through the protection of the vaccine (Eschke et al., 2018) and a recent study by our group also showed that the vv+MDV strain can cause severe immunosuppression to the challenged chickens and break through the protections provided by the current used commercial vaccines CVI988/Rispens and 814 (Shi et al., 2020).
In the present study, four clinical cases with high morbidity and mortality caused by the co-infection of REV and vvMDV in Yellow-chickens in southern China were diagnosed and the isolated pathogens were further characterized as to gene/genome. Our finding indicated that the frequent reemergence of REV and the subsequent co-infection with vv+MDV have become one of the causes of the clinical outbreaks of tumors and undoubtedly become a threat to the poultry industry in southern China. Since the pathogenicity of the co-infection cases is much higher than that of the mono-infection cases, eradication programs, elimination of the contamination of the live vaccines, strict biosecurity practices, and vaccinations plus the resistance breeding measures should be universally implemented in the effective prevention and control programs against these clinical tumor diseases.
ACKNOWLEDGEMENTS
This work was supported by the Guangxi Special Funding on Science and Technology Research [AA17204057], the Guangxi Program for Modern Agricultural Industry Technical System Construction-Chicken Industry [nycytxgxcxtd-19-03], and the Shandong Provincial Natural Science Foundation Project, China [ZR2019BC047]. The manuscript was kindly reviewed by Dr. Richard Roberts, Aurora, CO 80014, USA. The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary.
DISCLOSURES
The authors declare that they have no competing interests.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi: https://doi.org/10.1016/j.psj.2021.101099.
Appendix. Supplementary materials
REFERENCES
- Alfaki S.H., Hussien M.O., Osman N.A., Enan K.A., El Hussein A.R.M. First report onmolecular characterization and phylogenetic analysis of Reticuloendotheliosis virus in Sudan. Trop. Anim. Health. Prod. 2020;52:2073–2078. doi: 10.1007/s11250-020-02235-4. [DOI] [PubMed] [Google Scholar]
- Caleiro G.S., Nunes C.F., Urbano P.R., Kirchgatter K., de Araujo J., Durigon E.L., Thomazelli L.M., Stewart B.M., Edwards D.C., Romano C.M. Detection of reticuloendotheliosis virus in muscovy ducks, wild turkeys, and chickens in Brazil. J. Wildl. Dis. 2020;56:631–635. doi: 10.7589/2019-04-088. [DOI] [PubMed] [Google Scholar]
- Cui N., Su S., Sun P., Zhang Y.K., Han N., Cui Z.Z. Isolation and pathogenic analysis of virulent Marek's disease virus field strain in China. Poult. Sci. 2016;95:1521–1528. doi: 10.3382/ps/pew073. [DOI] [PubMed] [Google Scholar]
- Dong X., Zhao P., Chang S., Ju S., Li Y., Meng F., Sun P., Cui Z. Synergistic pathogenic effects of co-infection of subgroup J avian leukosis virus and reticuloendotheliosis virus in broiler chickens. Avian Pathol. 2015;44:43–49. doi: 10.1080/03079457.2014.993359. [DOI] [PubMed] [Google Scholar]
- Eschke K., Trimpert J., Osterrieder N., Kunec D. Attenuation of a very virulent Marek's disease herpesvirus (MDV) by codon pair bias deoptimization. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadly A.M., Witter R.L., Smith E.J., Silva R.F., Reed W.M., Hoerr F.J., Putnam M.R. An outbreak of lymphomas in commercial broiler breeder chickens vaccinated with a fowlpox vaccine contaminated with reticuloendotheliosis virus. Avian Pathol. 1996;25:35–47. doi: 10.1080/03079459608419118. [DOI] [PubMed] [Google Scholar]
- Faiz N.M., Cortes A.L., Guy J.S., Reddy S.M., Gimeno I.M. Differential attenuation of Marek's disease virus-induced tumours and late-Marek's disease virus-induced immunosuppression. J. Gen. Virol. 2018;99:927–936. doi: 10.1099/jgv.0.001076. [DOI] [PubMed] [Google Scholar]
- Gergen L., Cook S., Ledesma B., Cress W., Higuchi D., Counts D., Cruz-Coy J., Crouch C., Davis P., Tarpey I., Morsey M. A double recombinant herpes virus of turkeys for the protection of chickens against Newcastle, infectious laryngotracheitis and Marek's diseases. Avian Pathol. 2019;48:45–56. doi: 10.1080/03079457.2018.1546376. [DOI] [PubMed] [Google Scholar]
- He L., Li J., Peng P., Nie J., Luo J., Cao Y., Xue C. Genomic analysis of a Chinese MDV strain derived from vaccine strain CVI988 through recombination. Infect. Genet. Evol. 2019;78 doi: 10.1016/j.meegid.2019.104045. [DOI] [PubMed] [Google Scholar]
- Jiang L., Deng X., Gao Y., Li K., Chai H., Fan Z., Ren X., Wang Q., Zhang L., Yun B., Yin C., Chen Y., Qin L., Gao H., Wang Y., Hua Y., Wang X. First isolation of reticuloendotheliosis virus from mallards in China. Arch. Virol. 2014;159:2051–2057. doi: 10.1007/s00705-013-1821-5. [DOI] [PubMed] [Google Scholar]
- Jiang T., Lu X., Yuan Y., Zheng L., Shi J., Zhang D. Complete genomic sequence of a Muscovy duck-origin reticuloendotheliosis virus from China. Comparative Study J. Virol. 2012;86:13140–13141. doi: 10.1128/JVI.02531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wang P., Lin L., Shi M., Gu Z., Huang T., Mo M.L., Wei T., Zhang H., Wei P. The emergence of the infection of subgroup J avian leucosis virus escalated the tumour incidence in commercial Yellow chickens in Southern China in recent years. Transbound. Emerg. Dis. 2019;66:312–316. doi: 10.1111/tbed.13023. [DOI] [PubMed] [Google Scholar]
- Li J., Dong X., Yang C., Li Q., Cui Z., Chang S., Zhao P., Yu K., Yang H. Isolation, identification, and whole genome sequencing of reticuloendotheliosis virus from a vaccine against Marek's disease. Poult. Sci. 2015;94:643–649. doi: 10.3382/ps/pev034. [DOI] [PubMed] [Google Scholar]
- Li J., Yang C., Li Q., Li H., Xia Y., Liu D., Yu K., Yang H. Complete genome sequence of reticuloendotheliosis virus strain MD-2, isolated from a contaminated turkey herpesvirus vaccine. Genome. Announc. 2013;1 doi: 10.1128/genomeA.00785-13. e00785-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Xie J., Liang G., Ren D., Sun S., Lv L., Xie Q., Shao H., Gao W., Qin A., Ye J. Co-infection of vvMDV with multiple subgroups of avian leukosis viruses in indigenous chicken flocks in China. BMC Vet. Res. 2019;15:288. doi: 10.1186/s12917-019-2041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Cui S., Cui Z., Chang S., Zhao P. Genome analysis and pathogenicity of reticuloendotheliosis virus isolated from a contaminated vaccine seed against infectious bursal disease virus: first report in China. J. Gen. Virol. 2016;97:2809–2815. doi: 10.1099/jgv.0.000588. [DOI] [PubMed] [Google Scholar]
- Liu J., Li H., Liu B., Zhao B., Zhang P., Yu X., Ning Z. Emergence of spontaneously occurring neoplastic disease caused by reticuloendotheliosis virus in breeding Muscovy ducks in China, 2019. Transbound. Emerg. Dis. 2020;67:1442–1446. doi: 10.1111/tbed.13519. [DOI] [PubMed] [Google Scholar]
- Mescolini G., Lupini C., Felice V., Guerrini A., Silveira F., Cecchinato M., Catelli E. Molecular characterization of the meq gene of Marek's disease viruses detected in unvaccinated backyard chickens reveals the circulation of low- and high-virulence strains. Poult. Sci. 2019;98:3130–3137. doi: 10.3382/ps/pez095. [DOI] [PubMed] [Google Scholar]
- Ralapanawe S., Walkden-Brown S.W., Renz K.G., Islam A.F. Protection provided by Rispens CVI988 vaccine against Marek's disease virus isolates of different pathotypes and early prediction of vaccine take and MD outcome. Avian Pathol. 2016;45:26–37. doi: 10.1080/03079457.2015.1110850. [DOI] [PubMed] [Google Scholar]
- Reddy S.M., Izumiya Y., Lupiani B. Marek's disease vaccines: current status, and strategies for improvement and development of vector vaccines. Vet. Microbiol. 2017;206:113–120. doi: 10.1016/j.vetmic.2016.11.024. [DOI] [PubMed] [Google Scholar]
- Robinson F.R., Twiehaus M.J. Isolation of tha avian reticuloendothelial virus (strain T) Avian Dis. 1974;18:278–288. [PubMed] [Google Scholar]
- Schat K.A., Erb. H.N. Lack of evidence that avian oncogenic viruses are infectious for humans: a review. Avian Dis. 2014;58:345–358. doi: 10.1637/10847-041514-Review.1. [DOI] [PubMed] [Google Scholar]
- Shamblin C.E., Greene N., Arumugaswami V., Dienglewicz R.L., Parcells M.S. Comparative analysis of Marek's disease virus (MDV) glycoprotein-, lytic antigen pp38- and transformation antigen Meq-encoding genes: association of meq mutations with MDVs of high virulence. Vet. Microbiol. 2004;102:147–167. doi: 10.1016/j.vetmic.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Shi M.Y., Li M., Wang W.W., Deng Q.M., Li Q.H., Gao Y.L., Wang P.K., Huang T., Wei P. The emergence of a vv+MDV can break through the protections provided by the current vaccines. Viruses. 2020;12:E1048. doi: 10.3390/v12091048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G.R., Zhang Y.P., Lv H.C., Zhou L.Y., Cui H.Y., Gao Y.L., Qi X.L., Wang Y.Q., Li K., Gao L., Pan Q., Wang X.M., Liu C.J. A chinese variant marek's disease virus strain with divergence between virulence and vaccine resistance. Viruses. 2017;9:71. doi: 10.3390/v9040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G.R., Zhang Y.P., Zhou L.Y., Lv H.C., Zhang F., Li K., Gao Y.L., Qi X.L., Cui H.Y., Wang Y.Q., Gao L., Pan Q., Wang X.M., Liu C.J. Co-infection with marek's disease virus and reticuloendotheliosis virus increases illness severity and reduces marek's disease vaccine efficacy. Viruses. 2017;9:158. doi: 10.3390/v9060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng L.Q., Wei P., Song Z.B., He J.J., Cui Z.Z. Molecular epidemiological investigation of marek's disease virus from Guangxi. China. Arch. Virol. 2011;156:203–206. doi: 10.1007/s00705-010-0840-8. [DOI] [PubMed] [Google Scholar]
- Thontiravong A., Wannaratana S., Sasipreeyajan J. Genetic characterization of reticuloendotheliosis virus in chickens in Thailand. Poult. Sci. 2019;98:2432–2438. doi: 10.3382/ps/pez025. [DOI] [PubMed] [Google Scholar]
- Wang P., Lin L., Shi M., Li H., Gu Z., Li M., Gao Y., Teng H., Mo M., Wei T., Wei P. Vertical transmission of ALV from ALV-J positive parents caused severe immunosuppression and significantly reduced marek's disease vaccine efficacy in three-yellow chickens. Vet. Microbiol. 2020;244 doi: 10.1016/j.vetmic.2020.108683. [DOI] [PubMed] [Google Scholar]
- Wang P., Shi M., He C., Lin L., Li H., Gu Z., Li M., Gao Y., Huang T., Mo M., Wei T., Wei P. A novel recombinant avian leukosis virus isolated from gamecocks induced pathogenicity in Three-Yellow chickens: a potential infection source of avian leukosis virus to the commercial chickens. Poult. Sci. 2019;98:6497–6504. doi: 10.3382/ps/pez548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A., Huo C., Zhong Q., Xu M., Yang Y., Tian H., Zhang G., Hu Y. Isolation and pathogenicity testing of avian reticuloendotheliosis virus from layer chickens in China. J. Vet. Diagn. Invest. 2020;32:389–393. doi: 10.1177/1040638720914881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Zhao J., Ma Z., Xu M., Xue J., Zhang G. Serological survey of Reticuloendotheliosis virus infection in chickens in China in 2005 to 2015. Poult. Sci. 2017;96:3893–3895. doi: 10.3382/ps/pex209. [DOI] [PubMed] [Google Scholar]
- Zhai S.L., Chen S.N., Lin T., Wen X.H., Wei W.K., Lv D.H., Chen R.A. Emergence of reticuloendotheliosis virus in pigeons in Guangdong Province, Southern China. Arch. Virol. 2016;161:2007–2011. doi: 10.1007/s00705-016-2870-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yu Z., Lan X., Zhang F., Wang Q., Li K., Pan Q., Gao Y., Qi X., Cui H.Y., Wang Y., Gao L., Wang X., Liu C. A high frequency of Gallid herpesvirus-2 co-infection with Reticuloendotheliosis virusis associated with high tumor rates in Chinese chicken farms. Vet. Microbiol. 2019;237 doi: 10.1016/j.vetmic.2019.108418. [DOI] [PubMed] [Google Scholar]
- Zhang Y.P., Li Z.J., Bao K.Y., Lv H.C., Gao Y.L., Gao H.L., Qi X.L., Cui H.Y., Wang Y.Q., Ren X.G., Wang X.M., Liu C.J. Pathogenic characteristics of Marek's disease virus field strains prevalent in China and the effectiveness of existing vaccines against them. Vet. Microbiol. 2015;77:62–68. doi: 10.1016/j.vetmic.2014.12.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.