Abstract
Drug-resistance of bacteria is an ongoing problem in hospital treatment. The main mechanism of bacterial virulency in human infections is based on their adhesion ability and biofilm formation. Many approaches have been invented to overcome this problem, i.e. treatment with antibacterial biomolecules, which have some limitations e.g. enzymatic degradation and short shelf stability. Silver nanoparticles (AgNPs) may be alternative to these strategies due to their unique and high antibacterial properties. Herein, we report on yeast Saccharomyces cerevisiae extracellular-based synthesis of AgNPs. Transmission electron microscopy (TEM) revealed the morphology and structure of the metallic nanoparticles, which showed a uniform distribution and good colloid stability, measured by hydrodynamic light scattering (DLS). The energy dispersive X-ray spectroscopy (EDS) of NPs confirms the presence of silver and showed that sulfur-rich compounds act as a capping agent being adsorbed on the surface of AgNPs. Antimicrobial tests showed that AgNPs inhibit the bacteria growth, while have no impact on fungi growth. Moreover, tested NPs was characterized by high inhibitory potential of bacteria biofilm formation but also eradication of established biofilms. The cytotoxic effect of the NPs on four mammalian normal and cancer cell lines was tested through the metabolic activity, cell viability and wound-healing assays. Last, but not least, ability to deep penetration of the silver colloid to the root canal was imaged by scanning electron microscopy (SEM) to show its potential as the material for root-end filling.
Subject terms: Biotechnology, Microbiology
Introduction
Silver nanoparticles (AgNPs), due to their unique chemical, physical and biological properties, are one of the most commonly tested nanoparticles in nanobiotechnology1,2. Various routes, including physical, chemical and biological procedures, have been developed to synthesize silver nanoparticles. However, an increased demand in eco-friendly processes and using biocompatible reagents make a great of interest the biological way of synthesis of nanoparticle3. During the green synthesis, biological compounds from bacteria, microalga and yeasts are used, instead of chemical formulas, which makes the reaction more environmentally friendly. Among many organisms, Saccharomyces cerevisiae, nonpathogenic and non-toxigenic, generally recognized as safe (GRAS) organism should be considered as a promising “tool” for green silver nanoparticles synthesis. Indeed, Niknejad et al. demonstrated the potential of S. cerevisiae for extracellular synthesis of fairly monodisperse silver nanoparticles and proved their antifungal activity4. Furthermore, obtaining the functionalization of biogenic silver nanoparticles with a biocompatible compound, came from yeast extract, would further enhance their biological activities3. Moreover, multi-sites action of nanosilver could be a promising candidate to overcome the ongoing microbial resistance4.
Microbial adhesion, resulting in biofilm formation, is very often attributed or associated to the number of diseases. It is a significant virulence mechanism in the pathogenesis of many medically important bacterial pathogens5. Unfortunately, most currently used antimicrobial treatments are developed against planktonic bacteria6. Moreover, biofilms are 10- to 1000-fold more adaptively resistant to antimicrobials than planktonic bacteria, probably because the biofilm antibiotic tolerance is thought to involve alternative mechanisms to bacterial antimicrobial resistance5,7. According to the US Centers for Disease Control, 80% of human bacterial infections are due to biofilms, and therefore, they pose a significant problem to human health7,8. Thus, due to the failure in the prevention or eradication of microbial biofilm, it is a great demand to look for a new strategy and treatment.
Up to date, there are a few approaches to comb with recalcitrant biofilms; i.e. electrochemical treatment, use the antimicrobial compounds or biomolecules which exhibit antibiofilm activity and target the biofilm architecture, as well as drug delivery methods [review in7. In the latter one, especially engineered nanoparticles have been explored as drug delivery vehicles, where compound of interest is adsorbed to a nanomatrix or entrapped by it9. However, targeting of bacteria pathogens with nanocarriers may have some limitation, such as shelf life, stability, encapsulation efficacy, drug release/leakage, and enzymatic degradation of loaded compound10. To overcome these disadvantages, silver nanoparticles with its antimicrobial efficacy, may become an alternative strategy. Indeed, these nanostructure have been used to control bacterial colonization and infection in wound healing11. Moreover, some authors demonstrated that AgNPs can interact with mature bacterial biofilm, and showed their high toxicity against pathogens12. The strong antimicrobial effect of silver nanoparticles is indisputable. However, when they are supposed to be applied to humans, their toxic effect to eukaryotic cells must be considered. The cytotoxicity of AgNPs was noticed for human cervical cancer cells (HeLa), human lung carcinoma (A549), and human hepatocellular carcinoma (Hep-G2)13–15. Unfortunately, there is still gap in knowledge on the impact of the silver nanoparticles toward human cells when its antibacterial potential is taken into account the human application. For example, the lack of the cytotoxicity of silver nanoparticles toward eukaryotic cells, with their antibacterial potential may be very useful for endodontic treatment when the elimination of bacteria in the root canal system is required16,17. Pathogenic microorganisms are able to penetrate the root dentin up to a depth of more than 1 mm, whereas disinfecting solutions only reach a depth around 100 µm18,19. Dentin with its structural features as dentinal tubules, which are filled with pathogenic germs in dentinal tubules is an ideal object for disinfection of nanoparticles. Thus, the new devices/compounds which may help to overcome the problem of the insufficient penetration depth into the tooth are very desirable.
The aim of the study was to describe the antibacterial effect of the green-synthetized silver nanoparticles, simultaneously with the analysis of their cytotoxic effect on eukaryotic cells. The antimicrobial potential was checked through either the ability to inhibit the formation of biofilm or to eradicate already existed one, for Pseudomonas aeruginosa and Escherichia coli. Moreover, the growth was monitored also through the zone of inhibition assay for Staphylococcus aureus and Candida albicans strains. To assess the cytotoxicity, the impact of AgNPs on metabolism activity, viability and migration ability of mammalian cell lines (mouse embryonic fibroblasts, human keratinocytes, human osteosarcoma and human non-small cell lung carcinoma) was demonstrated. Bioformation of silver nanoparticles was monitored by UV–Vis spectroscopy. The structure and chemical composition of silver nanoparticles were evaluated with electron transmission microscopy imaging. The size and stability of the nanostructures were analyzed by hydrodynamic light scattering measurements. Moreover, the ability of the silver nanoparticles to penetrate the root canal has been imaging.
Material and methods
Reagents
Yeast extract Peptone Dextrose medium (YPD), Luria–Bertani (LB) and agar were purchased from BTL (Poland). Acridine orange (AO), 1-(4,5-Dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT), crystal violet (CV), ethanol were purchased from Sigma-Aldrich (USA). Dimethyl sulfoxide (DMSO) and ethidium bromide (EtBr) were purchased from Chempur and MpBio, respectively. Dulbecco's Modified Eagle Medium High Glucose (DMEM, Corning), antibiotic solution (Antibiotic Antimycotic Solution, Sigma-Aldrich), fetal bovine serum (FBS, Biowest), phosphate buffered saline w/o magnesium and calcium (PBS, Corning), 0.25% trypsin (PAA) were used.
Microorganisms and cell lines
Saccharomyces cerevisiae (10,058/69 strain) were previously isolated from patient mouth, delivered by Department of Microbiology (Medical University of Wrocław) and genetically identified20. The YPD medium (10 g/L yeast extract, 10 g/L peptone, 20 g/L glucose) was used for preculture and the yeasts were grown in flat-bottom flask at 28 °C with rotatory shaking (150 rpm). For antimicrobial assays, bacteria (Staphylococcus aureus ATCC 25,923, Pseudomonas aeruginosa ATCC 27,853, Escherichia coli PCM 2209) and fungi (Candida albicans ATCC 14,053) strains were employed.
Bacteria were precultured in LB medium at 37 °C with overnight shaking (150 rpm), while fungi in YPD medium at 30 °C. The cell lines used in this study were mouse embryonic fibroblasts (NIH 3T3, ATCC CRL-1658), human keratinocytes (HaCaT, ATCC CRL-2522), human osteosarcoma (U-2OS, ATCC HTB-96) and human non-small cell lung carcinoma (NCI-H1299, ATCC CRL-5803). They were grown and handled according to standard technique as described elsewhere21. Cultures were performed in 24- or 96-well plate (Corning) containing 1.5 mL and 200 µL of DMEM medium for wound-healing assay and MTT assay or AO/EB staining, respectively. Medium was supplemented with 1% of antibiotics solution and 10% of fetal bovine serum and cultures were incubated at 37 °C in a 5% CO2 air saturated incubators. 24 h before the experiments, cells were seeded at a density 5 × 104 and 5 × 103 cells/well for 24- or 96-well plate, respectively.
Synthesis of AgNPs
The Saccharomyces cerevisiae 10,058/69 strain was used for AgNPs synthesis due to their silver nitrate reduction properties. The production of AgNPs were performed with lower and higher concentration of AgNO3, 1 mM and 3 mM (for further analysis the abbreviations were as followed: AgNPs_L or AgNPs_H). Briefly, after 48 h of yeast culturing at optimal temperature 28 °C with shaking, the cell suspension, when reached the density atOD600 ~ 14, was centrifuged. The obtained supernatant was removed, followed the yeast biomass was resuspended in sterile distilled water and cultured for next 48 h in 28 °C with shaking. The post-culturing water was collected by centrifugation and its pH was adjusted to 10. Consequently, the silver nitrate was added to the post-culturing water in final concentration 1 mM and 3 mM and placed into 60 °C for 24 h. The specific conditions during the synthesis (i.e. silver ions concentrations, time and pH of the synthesis) were chosen based on our preliminary results (data not shown). For the evaluation of the ability to penetrate the root canal of the tooth, the colloid of silver nanoparticles was developed in collaboration with the Institute of Cell Biology of NAS, Lviv, Ukraine.
Physicochemical characterization of AgNPs
UV–Vis spectrum of AgNPs was recorded on TECAN spectrofluorometer (Infinite M200, Thermo Scientific) in the range of 330–900 nm. To determine the concentration of AgNPs, the microbalance technique using Radwag MYA 5.4Y balance was used. Briefly, 100 μL of the colloid suspension was placing on aluminum crucible with known mass and evaporating the solvent to a dry mass. Concentration values are given as mean and standard deviation of triplicate. The Dynamic Light Scattering (DLS) and the polydispersity index (PdI) were determined using the universal Nanoplus HD3 system (Particulate System/Micrometrics) equipped with 660 nm laser diode, as described elsewhere22. All the analysis were performed at 25 °C. Prior measurement, solutions of AgNPs were sonicated (310 W, 50 Hz, 100%, 10 min, Polsonic, SONIC-3). AgNPs were imaged with transmission electron microscopy (FEI Tecnai Osiris as described elsewhere23.
Antimicrobial potential of AgNPs
The antimicrobial of synthesized AgNPs activity through the zone of inhibition was tested against different pathogens such as S. aureus, P. aeruginosa, E. coli and C. albicans, according to a modified standard protocol24. Briefly, this modification consisted in punching 5-mm diameter wells in the nutrient agar instead of using a soaked disc. 100 µL of the overnight culture of the strains suspensions were spread onto the agar plates. When dried, 50 µL of AgNPs_1 and AgNPs_3 were aseptically transferred into separate wells. The plates were incubated at 30 or 37 °C for 24 h, for yeast and bacteria respectively. The inhibition zones surrounding the wells were recognized as the ability of the NPs to inhibit the growth of cells. For further analysis, P. aeruginosa and E. coli strains have been chosen to evaluate the influence of AgNPs on the bacteria ability to create biofilm in the presence of nanoparticles. Here, two type of assays have been implemented, to determine if the AgNPs can (i) prevent biofilm formation or (ii) reduce the final biofilm biomass25. (i) To evaluate the inhibition of biofilm formation the overnight bacteria inoculums (~ 108 CFU) were mixed with the colloid of AgNPs in the range of concentrations of 0.125–2 mg/mL. Cells were then incubated for 24 h at 37 °C without agitation, to allow biofilm formation. Plates were washed thrice with PBS from non-adhered cells and dried for 15 min at room temperature. Biofilms were stained for 15 min by adding 0.4% of crystal violet (CV) solution to each tested well. Then after, cells were washed with PBS to remove excess of the dye. Finally, the crystals were solubilized with 30% acetic acid, followed by absorbance measurement at 595 nm using a microplate readers (Infinite M200, Thermo Scientific). Results are reported relative to untreated biofilm biomass, as an OD values. (ii) Degradation of pre-established biofilm was tested as described above with some alterations. Cells were seeded in 96-well plate in LB without adding AgNPs and incubated at 37 °C for 24 h to allow biofilm creating. After this time, the wells were rinsed and replaced with fresh one, containing different concentrations of AgNPs. After 24 h the CV staining as described above was performed. The results were expressed as a percentage of biofilm degradation compare to non-treated cells.
Cytotoxicity of AgNPs
Cell metabolic activity
The 1-(4,5-Dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT) assay was used as an indicator of metabolism activity level of the cell after AgNPs solutions treatment. The assay was performed according to23. Briefly, cells were seeded on 96-well plate at density 5 × 103 cells/well, 24 h before the experiment. After this time, the medium was removed and replaced with the fresh one, containing different concentrations of AgNPs colloid (1 mM and 3 mM of precursor variants) in the range of 0.125–2 mg/mL. Cells were cultured for 24 h at 37 °C in a humidified incubator in a 5% of CO2 atmosphere. The medium was discarded from each well and 200 µL of fresh DMEM with MTT (0,5 mg/mL) was added into well. After an additional incubation of 2–3 h, to allow the formazans to form, 100 µL of DMSO were added per well to stop the reaction and dissolve crystals. The absorbance was finally measured at 565 nm wavelength on microplate reader (Tecan, Switzerland) and the metabolic activity results were expressed as a percentage of the control (untreated cells). All tests were performed at least in triplicate.
Cell viability
Examination of cell viability has been conducted by ethidium bromide (EB) and acridine orange (AO) staining to distinguish live and death cells, according to26. Briefly, confluent cells were incubated for 24 h in the presence of 0.5 or 1 mg/mL of AgNPs. After this time, plates were centrifuged (300 × g, 5 min) and supernatant was discarded. Cells were washed with PBS and were stained with a solution of acridine orange (100 μg/mL in PBS) and ethidium bromide (100 μg/mL in PBS) at a volume ratio of 1:1 for 5 min. Under fluorescence microscope (Olympus, Japan) living cells were visualized as green while dead cell were stained in red. For cell viability determination, a total of 100 cells from image (at least six images from different wells were examined) were counted and dead cells were expressed as a percentage of the total number of the cells.
Wound-healing assay
The impact of AgNPs on cells migration were performed using wound-healing assay (scratch assay), according to27. Briefly, the cells were seeded at a density 5 × 104 cells/well in 24-well plate and incubated until reached 90% of confluence. Wells with confluent cells were scratched by using a P10 pipette tip in the diameter of the culture. Then after, the medium was removed, and the cells were washed with PBS to discard detached cells. Followed, the 0.5 mg/mL of AgNPs solution suspended in DMEM with 1% of FBS was added to each well and cultured for 24 and 48 h. Wound closures were periodically visualized (0, 24 and 48 h intervals) under inverted microscope. The ability of cell to migrate and wound closure after AgNPs treatment was calculated with using ImageJ software according to28. The results were expressed as a percentage of the scratch closure compared to scratch surface at 0 h time in each group. All tests were performed at least in duplicate.
Electron scanning microscopy of the root canal penetration
To examine the tooth samples in an electron scanning microscope, 44 endodontically developed.
single-rooted human teeth with straight canals were taken, in which 2 μL of a solution of silver nanoparticles were injected. The tooth samples were prepared in the longitudinal direction of the root canal to visualize the lumen of the dentinal tubules. The study was performed using an electron scanning microscope (ESEM XL30, Philips, Netherlands).
Statistical analysis
The results represent the mean ± SD from at least three independent experiments. One-way ANOVA with Tuckey post-hox test, using Graph 10 software (*P < 0.05; **P < 0.01 and ***P < 0.001) was performed (*P < 0.05; **P < 0.01 and ***P < 0.001) compare to the control group. t-test was also used to determine statistical difference between scratch closure (HaCaT vs. U-2OS) (#P < 0.05).
Results and discussion
Physicochemical characterization of AgNPs
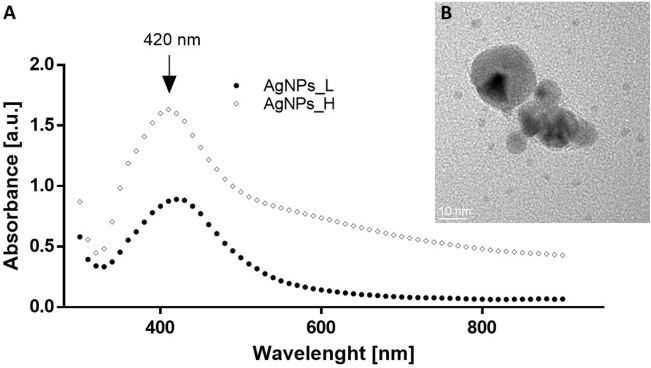
The ability of yeast water extract to reduce silver ions in the reacting solution and formed the silver nanoparticles was monitored with UV–visible spectra, where specific surface plasmon resonance (SPR) should appear during nanoparticles formation29. SPR is the absorption of the visible electromagnetic radiation of the collective oscillations of surface electrons30. Indeed, the maximum absorption peak was observed at 420 nm, for both of the samples (Fig. 1A). This wavelength was identical as reported by Elamawi et al., who obtained silver NPs synthetized from the cell-free fungal extract of Trichoderma sp.31. And was also closely matched (410 nm) to those obtained from the cell-free filtrate of Aspergillus fumigatus32. According to the Mie’s theory only a single SPR band is expected in the absorption spectra of spherical metal nanoparticles33. We present that silver nanoparticles absorb blue light and exhibit one single peak, thus, they are spherical in shape. Moreover, morphology of the synthesized NPs was confirmed with transmission electron microscopy (Fig. 1B), where predominantly spherical shape was imaged.
Figure 1.
(A) UV–Vis absorption spectra of biosynthesized AgNPs_L (circle) and AgNPs_H (diamond). (B) Example of TEM image of AgNPs.
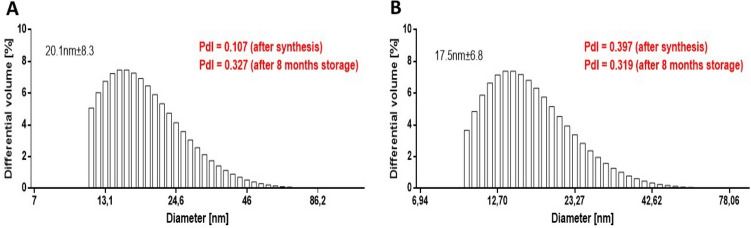
For various bioapplications, physicochemical properties determine nanomaterial cellular uptake, transport and fate34. Considering this, here we evaluated some of the most important features of the nanomaterials, primarily the size, stability and surface chemistry of biomanufactured AgNPs. The dynamic light scattering (DLS) was used to study the size distribution and colloidal stability of AgNPs35. The synthetized silver nanoparticles presented a size with median value 20.1 nm and 17.5 nm, for the sample AgNPs_L and AgNPs_H, respectively (Fig. 2). The stability of the nanoparticles as colloid is very important, as unstable NPs will not be able to disperse homogenously, which may effect on their antibacterial properties and reducing the efficacy31,36. Therefore, the polidyspersity index (PdI) was used as a value which show the stability of the nanomaterial. The higher the PdI value is, the less monodispersed are the nanoparticles37. In this study, the PdI of the materials were equal to 0.107 and 0.397 after synthesis or 0.327 and 0.319, after 8 month storage, for the AgNPs_L and AgNPs_H, respectively (Fig. 2). Thus, this suggest the nanoparticles are stable as they do not exhibit any considerable aggregation. It is worth to mention that many papers reported on difficulties in the synthesis of stable solution of NPs due to their tendency to agglomerate38–40. According to Gorham et al. PdI of AgNPs increased due to oxidation-dependent processes. The authors reported that citrate-coated AgNPs are characterized by increasing of agglomeration level during 104-day storage, despite citrate use41. Similar effect was observed by Izak-Nau et al., who show that agglomeration of citrate-coated AgNPs can be delayed effectively about 6 months since being synthesized. After this time, the hydrodiameter size of NPs in tested solutions increased significantly42. Interestingly, our results clearly showed that using post-harvested yeast water during synthesis allow to obtain stable NPs solutions, as PdI is not increased significantly, even after 8-month-storage.
Figure 2.
Size of the AgNPs_L (A) and AgNPs_H (B) evaluated by hydrodynamic light scattering analysis. PdI, polidyspersity index.
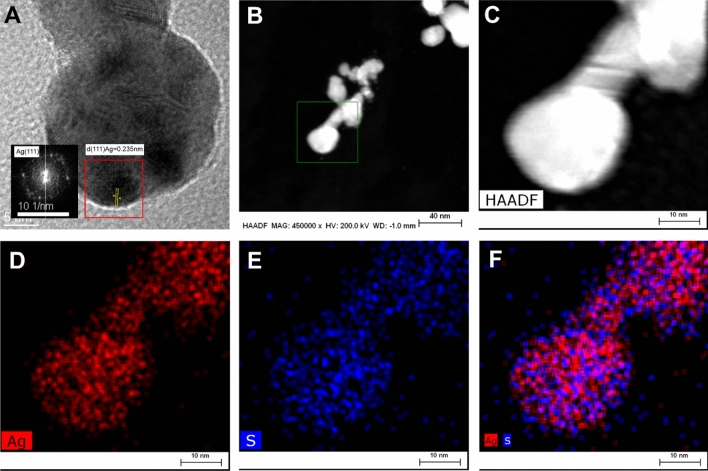
The morphology and elemental composition of the AgNPs were determined by transmission electron microscopy (TEM) and energy dispersive X-ray spectroscopy (EDS). Figure 3A depicts the HRTEM image of biosynthesized silver nanoparticles showing the lattice fringes clearly. The calculated inter planar distance was equal to 0.235 nm, confirmed occurrence of phase of Ag. According to the STEM-HAADF and EDS results (Fig. 3B-F) the synthesized nanomaterial is mainly constituted of Ag (Fig. 3D). Interestingly, the presence of sulfur precisely covering the nanoparticles was mapped (Fig. 3E). This may suggest that biocompounds reach in sulfur, which exists in the yeast water extract, may have the capping and stabilizing role. Many studies have revealed that the use of inorganic stabilizers such as citrate, PVP or PAA during synthesis allow to obtain stable NPs solutions43,44. However, some of them can influence negatively on human health e.g. PAA, which may cause the irritation of respiratory system after inhalation45. On the other hand, during the biological synthesis of nanoparticles, some biocompounds i.e. exopolysaccharides or proteins may exist as a stabilizing agent when nanoparticles are formed46,47. The sulfur which was imaged on Fig. 3E suggests the presence of some yeast proteins coating the surface of NPs. According to the above-mentioned literature, we suppose that during the synthesis, the Saccharomyces cerevisiae proteins or sulfur-rich biocompund coating the AgNPs surface and stabilize them, allowing to maintain stable while storage.
Figure 3.
(A) Representative HRTEM images of single silver nanoparticle with lattice fringes. Silver was identified by the inter-planes spacing d = 0.235 nm corresponding to the (111) plane of silver. Measurement of inter-planes spacing was done on FFT image. (B, C) HAADF STEM image of AgNPs with various magnification. (D–F) The chemical composition analysis of AgNPs. EDS elemental mapping images of silver (red), sulfur (blue), and an overlay of the Ag and S. All the EDS measurements are collected from image B (lower magn.) and C (higher magn).
Antimicrobial effect of AgNPs
Zone of inhibition
Antimicrobial potential of the silver nanoparticles is ascribed to their diverse mechanism of action, and it is believed as the multistep and multilevel process48. To evaluate this potential, the biosynthesized AgNPs (both of the tested variants) were analyzed against most pathogenic strains of bacteria: Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus and fungi Candida albicans, through the zone of inhibition assay. After 24 h of exposure, the growth was inhibited for each of the bacteria strains, whereas no inhibition was observed for yeast strain for both of the tested samples (Fig. 4). Kota et al. showed that AgNPs were characterized by high antibacterial properties, confirmed by analysis on sixteen pathological isolates from human, both Gram positive and Gram negative strains49. Moreover, the authors proved that 50 µg/µL concentration of these nanoparticles were able to increase zone of bacteria growth inhibition with the same efficiency in all tested isolates49. Interestingly, Jalal et al. showed high antifungal properties of AgNPs (extracellularly synthetized by C. glabrata supernatant) towards six Candida species50. Similarly results were presented by Perween et al., who reported on potential usefulness of AgNPs in C. albicans infections, better than well-known antifungal xenobiotics51. These conclusions are in the opposition of our results, which showed no effect on C. albicans growth inhibition of tested AgNPs solutions, which may be caused by higher diameter of tested AgNPs and/or sulfur coating of NPs surface.
Figure 4.
Antimicrobial activity of the AgNPs after 24 h of incubation against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Candida albicans.
Inhibition of biofilm formation
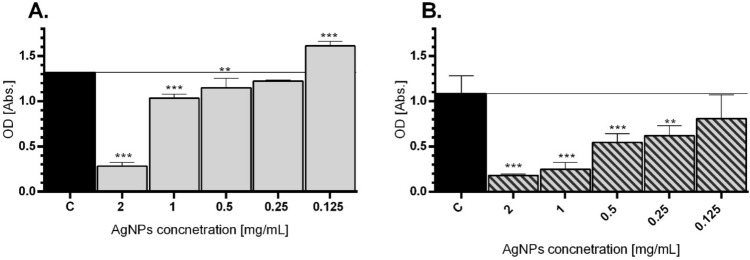
Bacterial colonization on abiotic or biotic surfaces may leads to biofilm formation and these microbial aggregates in biofilms produce a blockade that resists antimicrobial agents48. Thus, due to extremely small sizes of NPs, they may be useful for accomplishing antimicrobial actions and fighting intracellularly with bacteria52. Herein, the antibiofilm efficacy of the silver nanoparticles was evaluated with the crystal violet staining assay, in a case of ability to inhibit biofilm formation. Different biofilm percentage of reduction was detected for inhibition biofilms when treated with different concentrations of AgNPs_L, however, in the concentration dependent manner. For the E. coli strain, the best reduction was achieved at the highest concentration 2 mg/mL, which causes reducing the OD biofilm from 1.3182 (control) to 0.2806 in the tested group (Fig. 5A). While P. aeruginosa exhibits decreasing of biofilm OD up to 0.1813 (control group 1.0831) after 24 h treatment of 2 mg/mL concentration of AgNPs_L (Fig. 5B). Various mechanisms of antibacterial properties of AgNPs are described in the literature. Among them the high level of ROS production and the failure to eliminate them by P. aeruginosa after silver NPs exposition was noticed53. Thus, it is suggest that AgNPs may become an antimicrobial agent on the multidrug-resistant strain, which is an ongoing problem in the medicine53. Similarly, our results showed the high antibacterial potential of AgNPs_L against E. coli and P. aeruginosa. Moreover, the presented results revealed the potential of tested AgNPs to prevent the creation of bacterial biofilm. Masurkar et al. highlighted that AgNPs were able efficiently to inhibit biofilm of Staphylococcus aureus, comparable to antibiotic-treated group54. Martinez-Gutierrez et al. presented a comprehensive report about negative impact of AgNPs on many clinically important bacteria strains i.e. S. aureus, S. epidermidis and A. baumanni which are considered to be problematic in hospital treatment55. Our results clearly showed that AgNPs synthetized with easy, cheap, fast and cost-effective way, may have high application value to treat pathogenic strains.
Figure 5.
Biofilm inhibition after treatment of AgNPs in E. coli (A) and P. aeruginosa (B). Strains were incubated for 24 h in the presence of AgNPs. Post-treatment surface-associated biofilm was stained and the OD of biofilm biomass were presented. Mean values with standard deviation (error bars) with *, **, ***are statistically different from the respective control at P < 0.05, P < 0.01, and P < 0.001, respectively (one-way ANOVA, Tukey test).
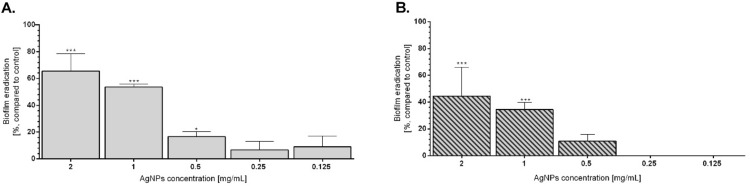
Biofilm eradication
The presence of bacterial biofilms is an emerging problem in nowadays hospital infections, due to high resistance of these structures on antibiotic56,57. Many xenobiotics were tested as a potential anti-biofilm agents, however the highest efficiency of biofilm eradication was proved by AgNPs58,59. Therefore, herein the efficiency of AgNPs ability to E. coli and P. aeruginosa biofilm eradication was evaluated (Fig. 6). In both tested bacterial strains, AgNPs caused decreasing in biofilm biomass. The highest eradication was observed for 1 mg/mL and 2 mg/mL concentrations for both tested strains and reached 65% and 53% in E. coli or 44% and 36% in P. aeruginosa, respectively (Fig. 6). The degradative effect of AgNPs in dose-dependent manner was observed, providing by higher eradication of established biofilm of 2 mg/mL and 1 mg/mL concentrations in comparison to 0.5 – 0.125 mg/mL concentrations of tested AgNPs (Fig. 6) E. coli established biofilm was more sensitive to AgNPs presence than the P. aeruginosa one (Fig. 6). Similar antibacterial effect on E. coli biofilm was also proved by Goswami et al., who showed the AgNPs (synthetized by tea leaf extract) were able to eradicate biofilm up to 100%, similar properties of this AgNPs were also observed for S. aureus biofilm60. Moreover, Ching-Yee et all. showed that citrate-coated AgNPs were characterized by high antibacterial properties against P. aeruginosa biofilm and caused its detachment61. Nevertheless, many literature data show that biofilm eradication ability of AgNPs is strict correlated with concentration – the higher AgNPs content, the more effective biofilm degradation62,63. The same tendency was observed in our results, proving that AgNPs usually act in dose-dependent manner and can be useful in treatment of antibiotic-resistant bacterial strains.
Figure 6.
Biofilm eradication after treatment of AgNPs in E. coli (A) and P. aeruginosa (B). Created biofilms were treated with AgNPs for 24 h and the percentage of biofilm eradication in comparison to untreated bacteria was calculated. Mean values with standard deviation (error bars) with *, **, ***are statistically different from the respective control at P < 0.05, P < 0.01, and P < 0.001, respectively (one-way ANOVA, Tukey test).
Biological activity
Cell metabolic activity and viability
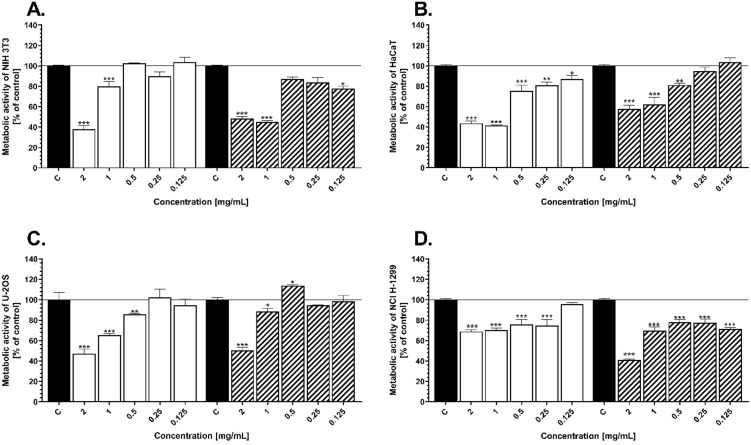
The potential effect of AgNPs on cell metabolic activity was tested using MTT assay which measures the cell mitochondrial activity through NAD(P)H-dependent cellular oxidoreductase enzyme64. The toxicity of various concentrations of the silver nanoparticles (in the range of 0.125–2 mg/mL) toward four different cell lines: mouse embryonic fibroblasts (NIH 3T3), human keratinocytes (HaCaT), human osteosarcoma (U-2OS) and human non-small cell lung carcinoma (NCI-1299) is shown on Fig. 7. Generally, cell metabolic activity was decreased in a dose-dependent manner for human fibroblasts, keratinocytes and osteosarcomas (Fig. 7A-C). While, NCI-1299 cell line exhibits similar level of toxicity no matter on the concentration of the NPs (Fig. 7D). The highest significant (***P < 0.05) decrease was obtained at 2 mg/mL for each tested line, for both type of sample (AgNPs_L and AgNPs_H) compared to the nontreated cells. Comparing the results for cancer and normal cell lines, after their exposure toward two highest concentrations of AgNPs (2 and 1 mg/mL), it is shown that the cancer cells exhibit higher level of metabolic activity in the range from 48 to 73% (compared to control), respectively for U-2OS and NCI H1299 cell lines, contrary to normal cells (HaCaT and NIH 3T3), which metabolic activity was in the range from 37 to 43%, in comparison to control (Fig. 7). It is known that AgNPs are one of the most reactive metal nanoparticles65,66. The cytotoxicity effect of these nanostructures is correlated to ROS generation, after uptake inside the cell2. According to Kumari et all. cancer cell lines are more resistant to oxidative stress generation, due to their adapting ability67. Our results showed higher toxicity of AgNPs in normal keratinocytes and fibroblasts than in cancer ones, which confirm the higher resistance of these cell types to AgNPs-dependent oxidative stress (Fig. 7). On the other hand, Garvey et al. proved higher toxicity in lung carcinoma cells in comparison to normal human keratinocytes. However, the authors used chemically synthetized AgNPs citrate-coated, which are well-known of their high toxicity68. Capping agent attached to the surface of nanomaterial may have an impact on their biological activity. Indeed, our EDS results (Fig. 3D-F) showed that silver nanoparticles have been coated with sulfur-rich molecules, which act as a stabilizers. Therefore, we supposed these compounds decrease direct contact of high-reactive AgNPs surface with cells and decrease their toxic effect. This is in line with Senthil et al. who reported on the green synthetized AgNPs and showed less cytotoxicity effect on HaCaT cells, but higher antibacterial properties69.
Figure 7.
Cell metabolic activity of the NIH 3T3 (A), HaCaT (B), U-2OS (C), and NCI-1299 (D) after 24 h exposure to various concentration of AgNPs_L and AgNPs_H. The values are the means (n = 6) with standard deviation (error bars). The statistical significance is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.005 according to one-way ANOVA, Tukey test.
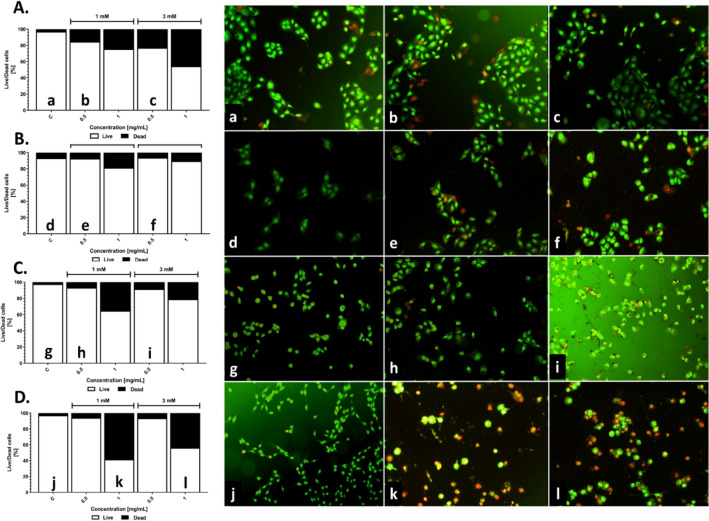
Many authors usually report on the cytotoxicity of the nanomaterials toward cells based on the only one method used for the analysis70–74. Considering only mitochondria activity, it may give the false positive results due to cells exhibit some activity even in early and late apoptosis stadium75. Therefore, in this study, either the AO/EB staining or MTT assays have been implemented to evaluate the ratio of the live and dead cells or metabolic activity, respectively. The dual acridine orange/ethidium bromide (AO/EB) staining assay was used to discriminate the live and dead cells after exposure to silver nanoparticles. Confluent cells were incubated with 0.5 and 1 mg/mL of AgNPs_L or AgNPs_H for 24 h and were labeled by AO/EB. For the higher NPs concentration (1 mg/mL), the number of dead cells was significantly increased for each type of cell line (Fig. 8A-D). Contrary, the number of live cells exposure to 0.5 mg/mL of AgNPs was still around 95%. Thus, this confirms the previous results and exhibits no or low toxicity in the range of 0.125–0.5 mg/mL of AgNPs. Representative images of the cells stained with AO/EB are shown on Fig. 8a-l, where red and green colors are dead and live cells, respectively. Based on this result, for further wound healing assay the AgNPs_L sample was used. Sambale et al. used the LDH release level as an indicator of AgNPs toxicity in lung carcinoma (A549) and proved that tested nanostructures did not significantly change the LDH level in the medium, highlighting that cytotoxicity effect of AgNPs is related to stabilizer and cells type76. Similarly, our results allow to concluded that the tested nanostructures (in the lower concentration of NPs) were more toxic for normal cell lines than for cancer ones.
Figure 8.
Cell viability of the NIH3T3 (A), HaCaT (B), U2OS (C) and NCI-1299 (D) after exposure to AgNPs_L and AgNPs_H. Cells after NPs treatment were stained with acridine orange and ethidium bromide, and were imaged with inverted fluorescence microscope (representative images—right panel). Dead cells were scored per 100 total cells analyzed and expressed as % (graphs).
Wound healing assay
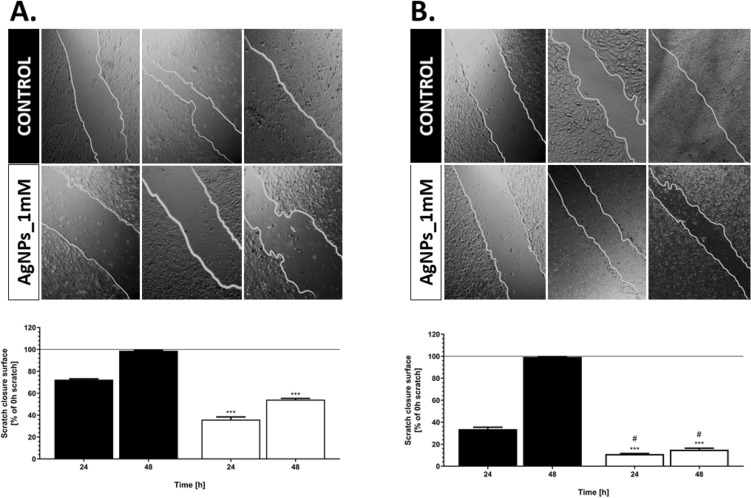
Cancer cell migration and invasion play a key role in disease progression77. Therefore, we further examine the impact of the silver nanoparticles on the behavior of the cancer and normal cells through the scratch assay. The motility capacities of the cells were performed on human keratinocytes and osteosarcoma cells. After 48 h either HaCaT or U2OS control cells (without AgNPs treatment) were able to migrate and close the scratch (Fig. 9). While, after exposure to AgNPs this ability was inhibited. Although, the migration ability of both of the tested cell line decreased after nanomaterial exposure, there is a difference among each type of cell. AgNPs inhibited more the migratory capability of the human osteosarcoma cell, compared to keratinocytes. After 48 h the % of scratch closure compare to time 0 was equal to 54% and 15% for normal and cancer cells, respectively. Many researchers emphasize on cytotoxicity of the metal NPs and highlight the migration of tumor cell and metastasis-related ability may be impacted by nanomaterials78. Herein, the strong inhibition efficacy of AgNPs on migration was observed in cancer cells, which were in line with other group79,80. Thus, this suggests that silver nanoparticles may have potential function in the inhibition of the metastasis.
Figure 9.
Cell migration ability in response to AgNPs. Comparison of migration in both HaCaT (A) and U-2OS (B) cell lines by taking images at different time intervals (0, 24, 48 h). Results are represented by marking the scratch with parallel lines and visually displaying the number of cells migrated in to the scratch area. The widths were measured using Image J software, and the data were analyzed using Prism 5.0. The values are the means with standard deviation (error bars). The statistical significance is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.005 according to one-way ANOVA, Tukey test (compared to respective control), and #P < 0.05 are statistically different from respectively tested group (HaCaT vs. U-2OS).
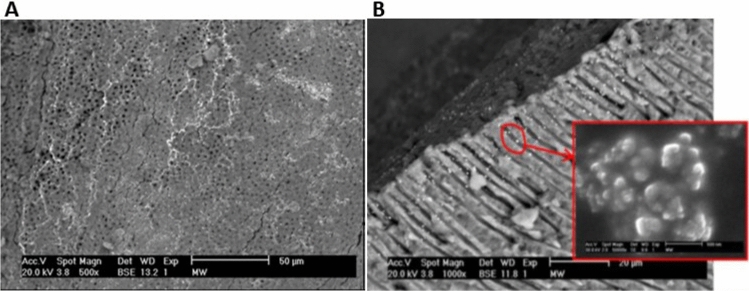
Penetration of the root canal of the tooth by AgNPs
As can be seen from the obtained photomicrographs, the walls of the root canal are covered with silver nanoparticles (Fig. 10A). In addition, the penetration of nanoparticles into the dentin structure is observed. The depth of free penetration of silver nanoparticles in the dentinal tubule is about 20 μm (Fig. 10B), which is an extremely important experimental fact81. From a physical point of view, the dentin-nanoparticle system tries to reach thermodynamic balance. Nanoparticles tend to occupy a position that corresponds to their minimum energy costs. Such conditions have been found in the developed system of dentinal microcanals, penetrating and lingering in them at a certain depth. Thereby causing a deep bactericidal effect on the pathogenic microflora82. We observe that the size of silver inclusions distributed at different depths in the microtubules is preferably slightly larger than the stated 15 nm sizes of nanoparticles (Fig. 10B). This is due to their tendency over time to agglomerations and clustering. This fact can play another, no less important role. Agglomerates of nanoparticles, the size of which acquires the measurement of the diameter of the microtubules, reliably block the return of bacteria from the periphery of the dentin to the macrocanal of the root43,83. Thus, the binary action of nanoparticles actually significantly enhances the bactericidal effect of silver.
Figure 10.
The wall of the macrocanal covered with silver nanoparticles (A) and the micrograp of the penetration of silver nanoparticles into the dentinal canal (B) with agglomerates of nanoparticles (inset).
The green synthesis of silver NPs is usually based on either the whole yeast cells or cell extract. In case of metal nanoparticles are formed with using yeast biomass, they can accumulate inside the cells in response to exposure to metal ions and additional steps are demand to extract the nanomaterials47,84. So far, there is lack of the data where the nanoparticles biomanufacturing is performed with the post-culturing water. In that way, the yeast biomass can be easy obtained as the waste product, and may be used many times for preparing the nanomaterials. Moreover, the low-cost of their production will take place in case of culturing in the huge bioreactors at the industrial scale, which does not require the complicated down-stream process for the recovery of the silver nanoparticles. What more, due to antibacterial effect these materials may be useful for various biomedical application, i.e. as antimicrobial and disinfect agent, or to prepare the antiseptic layers85–87. Simultaneously, the same nanomaterial, depends on the concentration used, could be a great platform for targeted drug delivery, as well as to combat with cancer cells88,89. Altogether, the presented method of AgNPs synthesis, is a simple, cost-effective and efficient approach to obtain the nanomaterials.
Conclusions
The presented work was focused on the physicochemical and biological characterization of biosynthesized silver nanoparticles. It was found that manufactured AgNPs had spherical shape with the size range between 17–20 nm, depends on the concentration of the silver ions. TEM, DLS and PdI analysis pointed out that AgNPs were administered as a stable and a well dispersed single particles suspension. The EDX map of sulfur located around the nanoparticles suggests that some proteins or sulfur-rich biocompund coat the AgNPs surface and stabilize them. A series of assays with bacteria and fungi strains have confirmed the antimicrobial activity of the AgNPs. Importantly from the potential biomedical application, beside the pronounced antibacterial activity, the reduced cytotoxic effect towards mammalian somatic and tumoral cells was confirmed. Moreover, the ability to deep penetration of the silver colloid to the root canal, imaged by SEM, highlight its potential as the material for root-end filling.
Author contributions
M.K.L. conceived and designed the experiments. M.K.L., B.S., U.K., A.N. performed the experiments. M.K.L. and B.S. analyzed the data and wrote the paper. A.D. and A.B. performed T.E.M. and S.E.M., respectively. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016 doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahamed M, AlSalhi MS, Siddiqui MKJ. Silver nanoparticle applications and human health. Clin. Chim. Acta. 2010 doi: 10.1016/j.cca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad A, Wei Y, Syed F, Tahir K, Rehman AU, Khan A, Ullah S, Yuan Q. The effects of bacteria-nanoparticles interface on the antibacterial activity of green synthesized silver nanoparticles. Microb. Pathog. 2017 doi: 10.1016/j.micpath.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 4.Niknejad F, Nabili M, Ghazvini RD, Moazeni M. Green synthesis of silver nanoparticles: Another honor for the yeast model Saccharomyces cerevisiae. Curr. Med. Mycol. 2015 doi: 10.18869/acadpub.cmm.1.3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verderosa AD, Totsika M, Fairfull-Smith KE. Bacterial biofilm eradication agents: a current review. Front. Chem. 2019 doi: 10.3389/fchem.2019.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebeaux D, Ghigo J-M, Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014 doi: 10.1128/mmbr.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfmeier H, Pletzer D, Mansour SC, Hancock REW. New perspectives in biofilm eradication. ACS Infect. Dis. 2018 doi: 10.1021/acsinfecdis.7b00170. [DOI] [PubMed] [Google Scholar]
- 8.Römling U, Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012 doi: 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
- 9.Singh R, Lillard JW. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009 doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeh YC, Huang TH, Yang SC, Chen CC, Fang JY. Nano-based drug delivery or targeting to eradicate bacteria for infection mitigation: a review of recent advances. Front. Chem. 2020 doi: 10.3389/fchem.2020.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paladini F, Pollini M. Antimicrobial silver nanoparticles for wound healing application: progress and future trends. Materials (Basel). 2019 doi: 10.3390/ma12162540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasithevar M, Periakaruppan P, Muthupandian S, Mohan M. Antibacterial efficacy of silver nanoparticles against multi-drug resistant clinical isolates from post-surgical wound infections. Microb. Pathog. 2017 doi: 10.1016/j.micpath.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Yeasmin S, Datta HK, Chaudhuri S, Malik D, Bandyopadhyay A. In-vitro anti-cancer activity of shape controlled silver nanoparticles (AgNPs) in various organ specific cell lines. J. Mol. Liq. 2017 doi: 10.1016/j.molliq.2017.06.047. [DOI] [Google Scholar]
- 14.De Matteis V, Cascione M, Toma CC, Leporatti S. Silver nanoparticles: synthetic routes, In Vitro toxicity and theranostic applications for cancer disease. Nanomater. 2018;8:10. doi: 10.3390/nano8050319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaba SI, Egorova EM. In vitro studies of the toxic effects of silver nanoparticles on HeLa and U937 cells. Nanotechnol. Sci. Appl. 2015 doi: 10.2147/NSA.S78134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moritz A, Gutknecht N, Goharkhay K, Schoop U, Wernisch J, Sperr W. In vitro irradiation of infected root canals with a diode laser: results of microbiologic, infrared spectrometric, and stain penetration examinations. Quintessence Int. 1997;5:63. [PubMed] [Google Scholar]
- 17.Moritz A, Gutknecht N, Schoop U, Goharkhay K, Doertbudak O, Sperr W. Irradiation of infected root canals with a diode laser in vivo: results of microbiological examinations. Lasers Surg. Med. 1997 doi: 10.1002/(SICI)1096-9101(1997)21:3<221::AID-LSM1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Gutknecht N, Van Gogswaardt D, Conrads G, Apel C, Schubert C, Lampert F. Diode laser radiation and its bactericidal effect in root canal wall dentin. J. Clin. Laser Med. Surg. 2000 doi: 10.1089/clm.2000.18.57. [DOI] [PubMed] [Google Scholar]
- 19.Kreisler M, Kohnen W, Beck M, Al Haj H, Christoffers AB, Götz H, Duschner H, Jansen B, D’hoedt B. Efficacy of NaOCl/H2O2 irrigation and GaAlAs laser in decontamination of root canals in vitro. Lasers Surg. Med. 2003 doi: 10.1002/lsm.10148. [DOI] [PubMed] [Google Scholar]
- 20.Siedlarz P, Sroka M, Dyląg M, Nawrot U, Gonchar M, Kus-Liśkiewicz M. Preliminary physiological characteristics of thermotolerant Saccharomyces cerevisiae clinical isolates identified by molecular biology techniques. Lett. Appl. Microbiol. 2016;62:277–282. doi: 10.1111/lam.12542. [DOI] [PubMed] [Google Scholar]
- 21.Freshney RI. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications. 6. Springer; 2011. [Google Scholar]
- 22.Sharma V, Tarachand CC, Ganesan V, Okram GS. Influence of particle size and dielectric environment on the dispersion behaviour and surface plasmon in nickel nanoparticles. Phys. Chem. Chem. Phys. 2017 doi: 10.1039/c7cp01769c. [DOI] [PubMed] [Google Scholar]
- 23.Tahar IB, Fickers P, Dziedzic A, Płoch D, Skóra B, Kus-Liśkiewicz M. Green pyomelanin-mediated synthesis of gold nanoparticles: Modelling and design, physico-chemical and biological characteristics. Microb. Cell Fact. 2019 doi: 10.1186/s12934-019-1254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CLSI, M02-A12 Performance Standards for Antimicrobial Disk (2015).
- 25.Segev-Zarko LA, Shai Y. Methods for investigating biofilm inhibition and degradation by antimicrobial peptides. Methods Mol. Biol. 2017 doi: 10.1007/978-1-4939-6737-7_22. [DOI] [PubMed] [Google Scholar]
- 26.Kasibhatla S. Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. Cold Spring Harb. Protoc. 2006 doi: 10.1101/pdb.prot4493. [DOI] [Google Scholar]
- 27.Tahar IB, Kus-Liśkiewicz M, Lara Y, Javaux E, Fickers P. Characterization of a nontoxic pyomelanin pigment produced by the yeast Yarrowia lipolytica. Biotechnol. Prog. 2020 doi: 10.1002/btpr.2912. [DOI] [PubMed] [Google Scholar]
- 28.Jonkman JEN, Cathcart JA, Xu F, Bartolini ME, Amon JE, Stevens KM, Colarusso P. An introduction to the wound healing assay using live-cell microscopy. Cell Adhes. Migr. 2014 doi: 10.4161/cam.36224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agnihotri M, Joshi S, Kumar AR, Zinjarde S, Kulkarni S. Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Mater. Lett. 2009 doi: 10.1016/j.matlet.2009.02.042. [DOI] [Google Scholar]
- 30.Park BK, Jeong S, Kim D, Moon J, Lim S, Kim JS. Synthesis and size control of monodisperse copper nanoparticles by polyol method. J. Colloid Interface Sci. 2007 doi: 10.1016/j.jcis.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 31.Elamawi RM, Al-Harbi RE, Hendi AA. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest Control. 2018 doi: 10.1186/s41938-018-0028-1. [DOI] [Google Scholar]
- 32.Othman AM, Elsayed MA, Al-Balakocy NG, Hassan MM, Elshafei AM. Biosynthesis and characterization of silver nanoparticles induced by fungal proteins and its application in different biological activities. J. Genet. Eng. Biotechnol. 2019 doi: 10.1186/s43141-019-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiley BJ, Im SH, Li ZY, McLellan J, Siekkinen A, Xia Y. Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J. Phys. Chem. B. 2006 doi: 10.1021/jp0608628. [DOI] [PubMed] [Google Scholar]
- 34.Zhu M, Nie G, Meng H, Xia T, Nel A, Zhao Y. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc. Chem. Res. 2013 doi: 10.1021/ar300031y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi K, Kato H, Saito T, Matsuyama S, Kinugasa S. Precise measurement of the size of nanoparticles by dynamic light scattering with uncertainty analysis. Part. Part. Syst. Charact. 2008 doi: 10.1002/ppsc.200700015. [DOI] [Google Scholar]
- 36.Vazquez-Muñoz R, Bogdanchikova N, Huerta-Saquero A. Beyond the nanomaterials approach: influence of culture conditions on the stability and antimicrobial activity of silver nanoparticles. ACS Omega. 2020;2:69. doi: 10.1021/acsomega.0c02007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roebben G, Ramirez-Garcia S, Hackley VA, Roesslein M, Klaessig F, Kestens V, Lynch I, Garner CM, Rawle A, Elder A, Colvin VL, Kreyling W, Krug HF, Lewicka ZA, McNeil S, Nel A, Patri A, Wick P, Wiesner M, Xia T, Oberdörster G, Dawson KA. Interlaboratory comparison of size and surface charge measurements on nanoparticles prior to biological impact assessment. J. Nanoparticle Res. 2011 doi: 10.1007/s11051-011-0423-y. [DOI] [Google Scholar]
- 38.Meyer DE, Curran MA, Gonzalez MA. An examination of silver nanoparticles in socks using screening-level life cycle assessment. J. Nanoparticle Res. 2011 doi: 10.1007/s11051-010-0013-4. [DOI] [Google Scholar]
- 39.Pavlin M, Bregar VB. Stability of nanoparticle suspensions in different biologically relevant media. Dig. J. Nanomater. Biostruct. 2012;2:63. [Google Scholar]
- 40.Phan HT, Haes AJ. What does nanoparticle stability mean? J. Phys. Chem. C. 2019 doi: 10.1021/acs.jpcc.9b00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorham JM, Rohlfing AB, Lippa KA, MacCuspie RI, Hemmati A, Holbrook RD. Storage wars: How citrate-capped silver nanoparticle suspensions are affected by not-so-trivial decisions. J. Nanoparticle Res. 2014 doi: 10.1007/s11051-014-2339-9. [DOI] [Google Scholar]
- 42.Izak-Nau E, Huk A, Reidy B, Uggerud H, Vadset M, Eiden S, Voetz M, Himly M, Duschl A, Dusinska M, Lynch I. Impact of storage conditions and storage time on silver nanoparticles’ physicochemical properties and implications for their biological effects. RSC Adv. 2015 doi: 10.1039/c5ra10187e. [DOI] [Google Scholar]
- 43.Panáček A, Kvítek L, Prucek R, Kolář M, Večeřová R, Pizúrová N, Sharma VK, Nevěčná T, Zbořil R. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B. 2006 doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 44.Ribeiro AI, Modic M, Cvelbar U, Dinescu G, Mitu B, Nikiforov A, Leys C, Kuchakova I, De Vrieze M, Felgueiras HP, Souto AP, Zille A. Effect of dispersion solvent on the deposition of PVP-silver nanoparticles onto DBD plasma-treated polyamide 6,6 fabric and its antimicrobial efficiency. Nanomaterials. 2020 doi: 10.3390/nano10040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lithner D, Larsson A, Dave G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011 doi: 10.1016/j.scitotenv.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 46.Escárcega-González CE, Garza-Cervantes JA, Vázquez-Rodríguez A, Morones-Ramírez JR. Bacterial exopolysaccharides as reducing and/or stabilizing agents during synthesis of metal nanoparticles with biomedical applications. Int. J. Polym. Sci. 2018 doi: 10.1155/2018/7045852. [DOI] [Google Scholar]
- 47.Korbekandi H, Mohseni S, Jouneghani RM, Pourhossein M, Iravani S. Biosynthesis of silver nanoparticles using Saccharomyces cerevisiae. Artif. Cells Nanomed. Biotechnol. 2016 doi: 10.3109/21691401.2014.937870. [DOI] [PubMed] [Google Scholar]
- 48.Shaikh S, Nazam N, Rizvi SMD, Ahmad K, Baig MH, Lee EJ, Choi I. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int. J. Mol. Sci. 2019 doi: 10.3390/ijms20102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kota S, Dumpala P, Anantha RK, Verma MK, Kandepu S. Evaluation of therapeutic potential of the silver/silver chloride nanoparticles synthesized with the aqueous leaf extract of Rumex acetosa. Sci. Rep. 2017 doi: 10.1038/s41598-017-11853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jalal M, Ansari MA, Alzohairy MA, Ali SG, Khan HM, Almatroudi A, Raees K. Biosynthesis of silver nanoparticles from oropharyngeal candida glabrata isolates and their antimicrobial activity against clinical strains of bacteria and fungi. Nanomaterials. 2018 doi: 10.3390/nano8080586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perween N, Khan HM, Fatima N. Silver nanoparticles: an upcoming therapeutic agent for the resistant Candida infections. J. Microbiol. Exp. 2019 doi: 10.15406/jmen.2019.07.00240. [DOI] [Google Scholar]
- 52.Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomedicine. 2017 doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liao S, Zhang Y, Pan X, Zhu F, Jiang C, Liu Q, Cheng Z, Dai G, Wu G, Wang L, Chen L. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant pseudomonas aeruginosa. Int. J. Nanomed. 2019 doi: 10.2147/IJN.S191340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masurkar SA, Chaudhari PR, Shidore VB, Kamble SP. Effect of biologically synthesised silver nanoparticles on Staphylococcus aureus biofilm quenching and prevention of biofilm formation. IET Nanobiotechnol. 2012 doi: 10.1049/iet-nbt.2011.0061. [DOI] [PubMed] [Google Scholar]
- 55.Martinez-Gutierrez F, Boegli L, Agostinho A, Sánchez EM, Bach H, Ruiz F, James G. Anti-biofilm activity of silver nanoparticles against different microorganisms. Biofouling. 2013 doi: 10.1080/08927014.2013.794225. [DOI] [PubMed] [Google Scholar]
- 56.Jabra-Rizk MA, Falkler WA, Meiller TF. Fungal Biofilms and Drug Resistance. Emerg. Infect. Dis. 2004 doi: 10.3201/eid1001.030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kouidhi B, Al Qurashi YMA, Chaieb K. Drug resistance of bacterial dental biofilm and the potential use of natural compounds as alternative for prevention and treatment. Microb. Pathog. 2015 doi: 10.1016/j.micpath.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 58.Top EM, Springael D. The role of mobile genetic elements in bacterial adaptation to xenobiotic organic compounds. Curr. Opin. Biotechnol. 2003 doi: 10.1016/S0958-1669(03)00066-1. [DOI] [PubMed] [Google Scholar]
- 59.Sanin SL, Sanin FD, Bryers JD. Effect of starvation on the adhesive properties of xenobiotic degrading bacteria. Process Biochem. 2003 doi: 10.1016/S0032-9592(02)00173-5. [DOI] [Google Scholar]
- 60.Goswami SR, Sahareen T, Singh M, Kumar S. Role of biogenic silver nanoparticles in disruption of cell-cell adhesion in Staphylococcus aureus and Escherichia coli biofilm. J. Ind. Eng. Chem. 2015 doi: 10.1016/j.jiec.2014.11.017. [DOI] [Google Scholar]
- 61.Loo CY, Young PM, Cavaliere R, Whitchurch CB, Lee WH, Rohanizadeh R. Silver nanoparticles enhance Pseudomonas aeruginosa PAO1 biofilm detachment. Drug Dev. Ind. Pharm. 2014 doi: 10.3109/03639045.2013.780182. [DOI] [PubMed] [Google Scholar]
- 62.Thuptimdang P, Limpiyakorn T, Khan E. Dependence of toxicity of silver nanoparticles on Pseudomonas putida biofilm structure. Chemosphere. 2017 doi: 10.1016/j.chemosphere.2017.08.147. [DOI] [PubMed] [Google Scholar]
- 63.Masák J, Čejková A, Schreiberová O, Řezanka T. Pseudomonas biofilms: possibilities of their control. FEMS Microbiol. Ecol. 2014 doi: 10.1111/1574-6941.12344. [DOI] [PubMed] [Google Scholar]
- 64.Lopez-Pascual A, Urrutia-Sarratea A, Lorente-Cebrián S, Martinez JA, González-Muniesa P. Cerium oxide nanoparticles regulate insulin sensitivity and oxidative markers in 3T3-L1 adipocytes and C2C12 myotubes. Oxid. Med. Cell. Longev. 2019 doi: 10.1155/2019/2695289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skóra B, Szychowski KA, Gmiński J. A concise review of metallic nanoparticles encapsulation methods and their potential use in anticancer therapy and medicine. Eur. J. Pharm. Biopharm. 2020;154C:153–165. doi: 10.1016/j.ejpb.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 66.Li L, Zhu YJ. High chemical reactivity of silver nanoparticles toward hydrochloric acid. J. Colloid Interface Sci. 2006 doi: 10.1016/j.jcis.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 67.Kumari S, Badana AK, Mohan GM, Shailender G, Malla RR. Reactive oxygen species: a key constituent in cancer survival. Biomark. Insights. 2018 doi: 10.1177/1177271918755391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garvey M, Padmanabhan SC, Pillai SC, Cruz-Romero MC, Kerry JP, Morris MA. Title In vitro cytotoxicity of water soluble silver (Ag) nanoparticles on HaCat and A549 cell lines. Science. 2017;2:10. [Google Scholar]
- 69.Senthil B, Devasena T, Prakash B, Rajasekar A. Non-cytotoxic effect of green synthesized silver nanoparticles and its antibacterial activity. J. Photochem. Photobiol. B Biol. 2017 doi: 10.1016/j.jphotobiol.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 70.Zanette C, Pelin M, Crosera M, Adami G, Bovenzi M, Larese FF, Florio C. Silver nanoparticles exert a long-lasting antiproliferative effect on human keratinocyte HaCaT cell line. Toxicol. Vitr. 2011 doi: 10.1016/j.tiv.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 71.Jalilian F, Chahardoli A, Sadrjavadi K, Fattahi A, Shokoohinia Y. Green synthesized silver nanoparticle from Allium ampeloprasum aqueous extract: characterization, antioxidant activities, antibacterial and cytotoxicity effects. Adv. Powder Technol. 2020 doi: 10.1016/j.apt.2020.01.011. [DOI] [Google Scholar]
- 72.Adnan M, Azad MOK, Madhusudhan A, Saravanakumar K, Hu X, Wang MH, Ha CD. Simple and cleaner system of silver nanoparticle synthesis using kenaf seed and revealing its anticancer and antimicrobial potential. Nanotechnology. 2020 doi: 10.1088/1361-6528/ab7d72. [DOI] [PubMed] [Google Scholar]
- 73.Kovács D, Igaz N, Keskeny C, Bélteky P, Tóth T, Gáspár R, Madarász D, Rázga Z, Kónya Z, Boros IM, Kiricsi M. Silver nanoparticles defeat p53-positive and p53-negative osteosarcoma cells by triggering mitochondrial stress and apoptosis. Sci. Rep. 2016;6:27902. doi: 10.1038/srep27902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu P, Gao Y, Lu Y, Zhang H, Cai C. High specific detection and near-infrared photothermal therapy of lung cancer cells with high SERS active aptamer-silver-gold shell-core nanostructures. Analyst. 2013 doi: 10.1039/c3an01375h. [DOI] [PubMed] [Google Scholar]
- 75.Henslee EA, Serrano RMT, Labeed FH, Jabr RI, Fry CH, Hughes MP, Hoettges KF. Accurate quantification of apoptosis progression and toxicity using a dielectrophoretic approach. Analyst. 2016 doi: 10.1039/c6an01596d. [DOI] [PubMed] [Google Scholar]
- 76.Sambale F, Wagner S, Stahl F, Khaydarov RR, Scheper T, Bahnemann D. Investigations of the toxic effect of silver nanoparticles on mammalian cell lines. J. Nanomater. 2015 doi: 10.1155/2015/136765. [DOI] [Google Scholar]
- 77.Kuphal S, Bauer R, Bosserhoff AK. Integrin signaling in malignant melanoma. Cancer Metastasis Rev. 2005 doi: 10.1007/s10555-005-1572-1. [DOI] [PubMed] [Google Scholar]
- 78.Pandya P, Orgaz JL, Sanz-Moreno V. Modes of invasion during tumour dissemination. Mol. Oncol. 2017 doi: 10.1002/1878-0261.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kovács D, Igaz N, Marton A, Rónavári A, Bélteky P, Bodai L, Spengler G, Tiszlavicz L, Rázga Z, Hegyi P, Vizler C, Boros IM, Kónya Z, Kiricsi M. Core-shell nanoparticles suppress metastasis and modify the tumour-supportive activity of cancer-associated fibroblasts. J. Nanobiotechnol. 2020 doi: 10.1186/s12951-020-0576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Que YM, Fan XQ, Lin XJ, Jiang XL, Hu PP, Tong XY, Tan QY. Size dependent anti-invasiveness of silver nanoparticles in lung cancer cells. RSC Adv. 2019;9:21134–21138. doi: 10.1039/C9RA03662H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lok C-N, Ho C-M, Chen R, He Q-Y, Yu W-Y, Sun H, Tam PK-H, Chiu J-F, Che C-M. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006 doi: 10.1021/pr0504079. [DOI] [PubMed] [Google Scholar]
- 82.Baker C, Pradhan A, Pakstis L, Pochan D, Shah SI. Synthesis and Antibacterial Properties Of Silver Nanoparticles. J. Nanosci. Nanotechnol. 2005 doi: 10.1166/jnn.2005.034. [DOI] [PubMed] [Google Scholar]
- 83.Dibrov P, Dzioba J, Gosink KK, Häse CC. Chemiosmotic mechanism of antimicrobial activity of Ag + in Vibrio cholerae. Antimicrob. Agents Chemother. 2002 doi: 10.1128/AAC.46.8.2668-2670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bolbanabad EM, Ashengroph M, Darvishi F. Development and evaluation of different strategies for the clean synthesis of silver nanoparticles using Yarrowia lipolytica and their antibacterial activity. Process. Biochem. 2020 doi: 10.1016/j.procbio.2020.03.024. [DOI] [Google Scholar]
- 85.Dziedzic A, Bochnowski W, Adamiak S, Szyller Ł, Cebulski J, Virt I, Kus-Liśkiewicz M, Marzec M, Potera P, Żaczek A, Zdeb B. Structure and antibacterial properties of Ag and N doped titanium dioxide coatings containing Ti2.85O4N phase, prepared by magnetron sputtering and annealing. Surf. Coat. Technol. 2020 doi: 10.1016/j.surfcoat.2020.125844. [DOI] [Google Scholar]
- 86.Rodrigues CT, de Andrade FB, de Vasconcelos LRSM, Midena RZ, Pereira TC, Kuga MC, Duarte MAH, Bernardineli N. Antibacterial properties of silver nanoparticles as a root canal irrigant against Enterococcus faecalis biofilm and infected dentinal tubules. Int. Endod. J. 2018 doi: 10.1111/iej.12904. [DOI] [PubMed] [Google Scholar]
- 87.Bhandi S, Mehta D, Mashyakhy M, Chohan H, Testarelli L, Thomas J, Dhillon H, Raj AT, Balaji TM, Varadarajan S, Patil S. Antimicrobial efficacy of silver nanoparticles as root canal irrigant’s: a systematic review. J. Clin. Med. 2021 doi: 10.3390/jcm10061152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ivanova N, Gugleva V, Dobreva M, Pehlivanov I, Stefanov S, Andonova V. Silver nanoparticles as multi-functional drug delivery systems. Nanomed. IntechOpen. 2019 doi: 10.5772/intechopen.80238. [DOI] [Google Scholar]
- 89.AshaRani PV, Mun GLK, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009 doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]