Abstract
Lupin cultivation worldwide is threatened by anthracnose, a destructive disease caused by the seed- and air-borne fungal pathogen Colletotrichum lupini. In this study we explored the intraspecific diversity of 39 C. lupini isolates collected from different lupin cultivating regions around the world, and representative isolates were screened for their pathogenicity and virulence on white and Andean lupin. Multi-locus phylogeny and morphological characterizations showed intraspecific diversity to be greater than previously shown, distinguishing a total of six genetic groups and ten distinct morphotypes. Highest diversity was found across South America, indicating it as the center of origin of C. lupini. The isolates that correspond to the current pandemic belong to a genetic and morphological uniform group, were globally widespread, and showed high virulence on tested white and Andean lupin accessions. Isolates belonging to the other five genetic groups were mostly found locally and showed distinct virulence patterns. Two highly virulent strains were shown to overcome resistance of advanced white lupin breeding material. This stresses the need to be careful with international seed transports in order to prevent spread of currently confined but potentially highly virulent strains. This study improves our understanding of the diversity, phylogeography and pathogenicity of a member of one of the world’s top 10 plant pathogen genera, providing valuable information for breeding programs and future disease management.
Subject terms: Agricultural genetics, Genetic interaction, Genotype, Microbial genetics, Plant breeding, Sequencing, Fungi, Microbial genetics, Pathogens, Plant breeding
Introduction
The fungal genus Colletotrichum contains many important plant pathogenic species that cause anthracnose and other pre- and post-harvest diseases in a wide variety of hosts1–4. Among potential hosts are important fruit, cereal and legume crops such as strawberry5, maize6 and soybean7,8. Besides being of economic importance, Colletotrichum spp. have been widely used as model species to study plant-fungus interactions because of the diversity of lifestyles within this genus9–12. Colletotrichum is listed in the top 10 of most important fungal plant pathogens worldwide13. Within the genus, members of the Colletotrichum acutatum species complex are notorious and cause disease in many important crops14,15. The most important morphological characteristic for members of this species complex are the acute ends of its conidia14. Discrimination of Colletotrichum species solely based on morphological traits, however, is deemed unreliable due to the few and highly variable characteristics, the strong influence of environmental conditions and the high overlap between species16. Therefore, a polyphasic approach, combining morphological and genetic data is recommended17,18. Multi-locus phylogeny revealed a high diversity within the C. acutatum species complex, showing at least 32 different species divided among five clades14. Although many species within the C. acutatum species complex have a broad host range, Colletotrichum lupini, belonging to clade 1, appears to be highly host specific on lupins (Lupinus)19,20.
Lupin anthracnose caused by C. lupini is the most important disease in lupin cultivation worldwide, affecting all economically important lupin species such as blue (Lupinus angustifolius L.), white (L. albus L.), Andean (L. mutabilis Sweet.), yellow (L. luteus L.) and ornamental lupin (L. polyphyllus Lindl.)20. The disease was first reported in 1912 in Brazil21, but the fungal pathogen was identified much later22. A first outbreak was reported in the 1940–1950s in North America and was followed by a more severe and globally widespread outbreak around the 1980s which is still persisting until this day20. The disease is mainly dispersed via seeds, facilitating rapid spread through international seed transports, and within the crop by rain splash during the growing season23. Even low amounts of initial inoculum can cause total yield losses making this disease highly destructive24,25. Typical symptoms are stem twisting and necrotic lesions on stems and pods (Fig. 1)26. Current disease management is focused on planting certified disease-free seed and chemical protection23,27. However, crop resistance could offer a more sustainable alternative. In blue lupin, anthracnose resistance is controlled by single resistance genes28–30, whereas in white, Andean and yellow lupin no such single gene resistance is known and the observed quantitative resistance is considered to be polygenic31–33. The increasing demand for plant-based protein is renewing the interest for lupins as a high quality protein crop34–36, the current anthracnose pandemic, however, severely hampers cultivation.
Figure 1.
Symptoms on lupin tissue associated with Collletotrichum lupini. (A) typical stem twisting (Lupinus mutabilis); (B) on the leaves (L. albus); (C) on the main stem (L. albus); (D–F) on the pods (L. albus). Photos by Alkemade JA.
The pathogen was first described as Gloesporium lupini, followed by C. gloeosporioides and C. acutatum until it was fully described as C. lupini14,37,38. Currently two genetic groups (I and II) are distinguished within C. lupini based on vegetative compatibility groups (VCG)38, the ITS (internal transcribed spacer) region37 and multi-locus phylogeny of the ITS, GAPDH (glyceraldehyde-3-phosphate dehydrogenase), CHS-1 (chitin synthase), HIS3 (histone), ACT (actin), TUB2 (β-tubulin 2), HMG (HMG box region) and APN/MAT1 (Apn2-Mat1-2-1 intergenic) loci39. The TUB2 and GAPDH loci were shown to be the most informative within the C. acutatum species complex and APN/MAT1 the most informative within C. lupini, whereas classification based on the ITS region can be problematic due to low resolution within the complex14,39. Although only two groups within C. lupini have been distinguished, with most of the reported strains belonging to group II39, intraspecific diversity is thought to be greater as a high diversity was found in a Chilean C. lupini collection using random amplified polymorphic DNA (RAPD) markers40 and a distinct lupin infecting C. acutatum group was identified in Ecuador based on the ITS region41. This suggests that highest intraspecific diversity is found in South America, which is believed to be the center of origin of members belonging to clade 1 of the C. acutatum species complex10,15.
The overall aim of this study was to assess a worldwide collection of lupin-infecting Colletotrichum isolates through (i) multi-locus phylogeny, (ii) morphology and (iii) virulence on white and Andean lupin. Insights into C. lupini diversity, phylogeography and plant-C. lupini interactions will improve our understanding of the current lupin anthracnose pandemic and support future disease management strategies and lupin breeding programs.
Results
Colletotrichum lupini comprises of six genetic groups supported by morphology
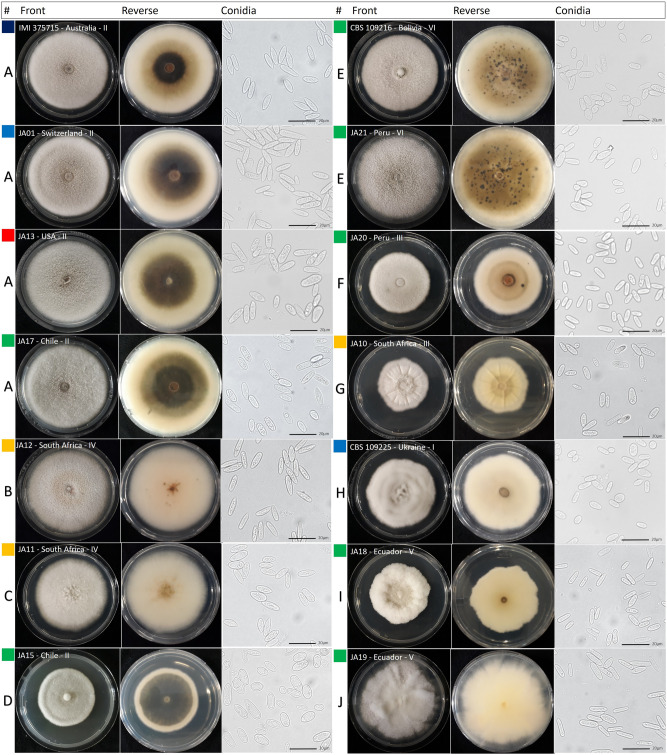
From the 50 sequenced isolates, 39 belonged to C. lupini (Table 1). A globally representative subset of 28 C. lupini isolates was characterized based colony morphology (form, aerial mycelium, margin type and color of the reverse side) and 18 of those were further characterized for growth rate and conidial shape and size, revealing ten distinct morphotypes (A–J; Fig. 2, Table 2, Supplementary Figs. S1, S2). Despite certain variability, all observed conidia shared features typical for C. lupini (hyaline, smooth-walled, aseptate, straight and with one acute end) as described by Damm et al.14. Morphotype A was the most common and was observed for isolates from across the world (Europe, Australia, North- and South America), all belonging to genetic group II. Morphotypes B, C and G were observed for isolates from South Africa and morphotypes D, E, G, I and J were observed for isolates from South America.
Table 1.
Isolation details and GenBank accessions of Colletotrichum strains used in this study.
| Straina | Alternative code(s) | Species | Host | Origin | Year | GenBank no.b | |||
|---|---|---|---|---|---|---|---|---|---|
| ITS | GAPDH | TUB2 | APN/MAT1 | ||||||
| JA01 | Colletotrichum lupini | Lupinus albus | Switzerland, Melikon | 2018 | MT741840 | MW342515 | MW342537 | MW342559 | |
| JA02 | C. lupini | L. albus | Switzerland, Feldbach | 2019 | MW342494 | MW342516 | MW342538 | MW342560 | |
| JA03 | C. lupini | L. albus | Germany, Hattenhofen | 2019 | MW342495 | MW342517 | MW342539 | MW342561 | |
| JA04 | C. lupini | L. albus | Germany, Witzenhausen | 2018 | MW342496 | MW342518 | MW342540 | MW342562 | |
| JA05 | C. lupini | L. albus | Germany, Westerau | 2018 | MW342497 | MW342519 | MW342541 | MW342563 | |
| JA06 | C. lupini | L. albus | Russia, Saint Petersburg | 2018 | MW342498 | MW342520 | MW342542 | MW342564 | |
| JA07 | BRIP 63850, WAC 12994 | C. lupini | L. angustifolius | Australia, WA, Dongara | 2004 | MW342499 | MW342521 | MW342543 | MW342565 |
| JA08 | BRIP 63851, WAC 12995 | C. lupini | L. luteus | Australia, WA, Mingenew | 2004 | MW342500 | MW342522 | MW342544 | MW342566 |
| JA09 | BRIP 63857, WAC 13001 | C. lupini | L. albus | Australia, WA, Yandanooka | 2004 | MW342501 | MW342523 | MW342545 | MW342567 |
| JA10 | CMW 9930, SHK 788 | C. lupini | L. albus | South Africa, Bethlehem | 1994 | MW342502 | MW342524 | MW342546 | MW342568 |
| JA11 | CMW 9931, SHK 1033 | C. lupini | L. albus | South Africa, Stellenbosch | 1995 | MW342503 | MW342525 | MW342547 | MW342569 |
| JA12 | CMW 9933, SHK 2148 | C. lupini | L. albus | South Africa, Malmesbury | 1999 | MW342504 | MW342526 | MW342548 | MW342570 |
| JA13 | C. lupini | L. mutabilis | USA, Florida, Martin County | 2013 | MW342505 | MW342527 | MW342549 | MW342571 | |
| JA14 | C. lupini | L. hartwegii | USA, Florida, Martin County | 2013 | MW342506 | MW342528 | MW342550 | MW342572 | |
| JA15 | A-02 | C. lupini | L. albus | Chile, Cajón | 2009 | MW342507 | MW342529 | MW342551 | MW342573 |
| JA16 | A-10 | C. lupini | L. angustifolius | Chile, Cajón | 2009 | MW342508 | MW342530 | MW342552 | MW342574 |
| JA17 | A-24 | C. lupini | L. albus | Chile, Temuco | 2015 | MW342509 | MW342531 | MW342553 | MW342575 |
| JA18 | Lup1 | C. lupini | L. mutabilis | Ecuador, Juan Montalvo | 2007 | MW342510 | MW342532 | MW342554 | MW342576 |
| JA19 | Lup18 | C. lupini | L. mutabilis | Ecuador, Pujili | 2007 | MW342511 | MW342533 | MW342555 | MW342577 |
| JA20 | C. lupini | L. mutabilis | Peru, Carhuaz | 2019 | MW342512 | MW342534 | MW342556 | MW342578 | |
| JA21 | C. lupini | L. mutabilis | Peru, Carhuaz | 2019 | MW342513 | MW342535 | MW342557 | MW342579 | |
| JA22 | C. lupini | L. mutabilis | Peru, Carhuaz | 2019 | MW342514 | MW342536 | MW342558 | MW342580 | |
| CBS 109216 | BBA 63879 | C. lupini | L. mutabilis | Bolivia | JQ948156 | JQ948486 | JQ949807 | MW342581 | |
| CBS 109221 | BBA 70352, RB172 | C. lupini | L. albus | Germany | JQ948169 | JQ948499 | JQ949820 | MK478328 | |
| CBS 109225 | BBA 70884 | C. Lupini | L. albus | Ukraine | JQ948155 | JQ948485 | JQ949806 | MK478329 | |
| CBS 109226 | RB121, IMI 504884, HY09, BBA 71249 | C. lupini | L. albus | Canada, Nova Scotia | JQ948158 | JQ948488 | MK478189 | MK478316 | |
| CBS 509.97 | RB235, LARS 178 | C. lupini | L. albus | France | JQ948159 | JQ948489 | JQ949810 | MK478355 | |
| IMI 375715 | 96A4 | C. lupini | L. albus | Australia, WA, Perth | 1997 | JQ948161 | JQ948491 | JQ949812 | MK478341 |
| RB020 | PT30 | C. lupini | L. albus | Portugal, Azores | 1999 | MK463722 | KM252117 | MK478186 | MK478308 |
| RB042 | CBS 129944, CMG12 | C. lupini | Cinnamonium zeylanicum | Portugal, Lisbon | 1996 | MH865693 | JQ948508 | JQ949829 | MK478310 |
| RB116 | CSL 1294 | C. lupini | L. polyphyllus | UK, York | MK463723 | KM252194 | KM251944 | MK478313 | |
| RB122 | BBA 71310, C3 | C. lupini | L. luteus | Poland | MK463726 | MK463750 | MK478190 | MK478317 | |
| RB123 | IMI 504885, SHK788 | C. lupini | L. albus | South Africa, Bethlehem | 1994 | MK463727 | MK463751 | MK478191 | MK478318 |
| RB124 | BBA 70555 | C. lupini | L. albus | Chile | MK463728 | MK463752 | MK478192 | MK478319 | |
| RB125 | CBS 109224, BBA 70399 | C. lupini | L. albus | Austria | JQ948172 | JQ948502 | JQ949823 | MK478320 | |
| RB127 | PT702 | C. lupini | Olea europaea | Spain | MK463729 | MK463753 | MK478193 | MK478321 | |
| RB147 | IMI 350308 | C. lupini | Lupinus sp. | UK, Kent | 1991 | MK463730 | KM252203 | KM251951 | MK478322 |
| RB221 | IMI 504893 | C. lupini | Lupinus sp. | France, Brittany | 2016 | MK463733 | MK463756 | MK478196 | MK478345 |
| RB226 | C. lupini | Lupinus sp. | France, Brittany | 2016 | MK463738 | MK463761 | MK478201 | MK478350 | |
| CBS 129814 | T.A6 | C. tamarilloi | Solanum betaceum | Colombia, Gundinamarca | 2012 | JQ948184 | JQ948514 | JQ949835 | MW342584 |
| CBS 129955 | RB018, Tom-12 | C. tamarilloi | Solanum betaceum | Colombia, Antioquia, Santa Rosa | 1998 | JQ948189 | JQ948519 | JQ949840 | MK478307 |
| CBS 211.78 | IMI 309622, RB184 | C. costaricensis | Coffea sp. | Costa Rica, Turrialba | JQ948181 | JQ948511 | JQ949832 | MK478333 | |
| CBS 134730 | RB237 | C. melonis | Malus domestica | Brazil, Rio Grande do Brazil | KC204997 | KC205031 | KC205065 | MK478357 | |
| IMI 304,802 | RB216 | C. cuscutae | Cuscuta sp. | Dominica | JQ948195 | JQ948525 | JQ949846 | MK478340 | |
| IMI 384185 | CPC 18937, RB218 | C. paranaense | Caryocar brasiliense | Brazil | JQ948191 | JQ948521 | JQ949842 | MK478342 | |
| CBS 130239 | Frag NL-1 | C. nymphaeae | Fragaria x ananassa | The Netherlands | 2011 | JQ948250 | JQ948580 | JQ949901 | MW342583 |
| IMI 360928 | CPC 18926, RB163 | C. nymphaeae | Fragaria x ananassa | Switzerland, Zürich | 1993 | JQ948243 | JQ948573 | JQ949894 | MK478326 |
| CBS 122122 | BRIP28519, RB179 | C. simmondsii | Carica papaya | Australia | 1987 | JQ948276 | JQ948606 | JQ949927 | MK478332 |
| CBS 369.73 | NRCC 10081 | C. acutatum | L. angustifolius | New Zealand, Kumeu | 1968 | JQ948350 | JQ948681 | JQ950001 | MW342582 |
| CBS 370.73 | NRCC 10088, RB187 | C. acutatum | Pinus Aridata | New Zealand, Tokoroa | 1965 | JQ948351 | JQ948682 | JQ950002 | MK478335 |
JA strains from the FiBL culture collection characterized in this study, RB personal collection of Riccardo Baroncelli described in Dubrulle et al.39, CBS collection of the Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands, IMI Culture collection of CABI Europe UK Centre, Egham, UK, ITS internal transcribed spacers 1 and 2 together with 5.8S nrDNA, GAPDH glyceraldehyde-3-phosphate dehydrogenase, TUB2 β-tubulin 2, APN/MAT1 Apn2-Mat1-2-1 intergenic.
Codes in bold were used for morphology analysis in this study.
Accession numbers in bold are newly determined in this study.
Figure 2.
Colletotrichum lupini morphology. Capital letters (A–J) indicate the different morphology types based on conidia shape and size and colony growth rate and morphology (see Table 2). Strain codes are followed by country of origin and roman numbers (I–VI) indicate genetic groups. Plates show the front and reverse of 14 day old colonies on PDA. Scale bars indicate 20 µm. Colors indicate strain origin: blue = Europe, green = South America, red = North America, orange = Southern Africa, dark blue = Australia.
Table 2.
Growth rate, conidial size and shape, and colony morphology for the different morphotypes observed within Colletotrichum lupini.
| Strain | Morphotype | Genetic group | Growth rate (mm/day)a | Conidia L × W (µm)a |
Conidia shapebc | Colony morphologyc |
|---|---|---|---|---|---|---|
| IMI 375715, JA01, -06, -07, -13, -16, -17 | A | II | 6.2 ± 0.1 | 12 ± 2.1 × 4 ± 0.7 | Cylindrical to elliptical, occasionally clavate | Flat, circular, with entire margins, white-greyish cottony aerial mycelium, pale to orange on reverse, dark in center |
| JA12 | B | IV | 5.6 ± 0.1 | 13.3 ± 1.4 × 3.4 ± 0.5 | Cylindrical to elliptical, occasionally clavate | Flat, circular, with entire margins, white-brownish cottony aerial mycelium, pale on reverse |
| JA11 | C | IV | 5.5 ± 0.1 | 12 ± 1.7 × 4.5 ± 0.7 | Cylindrical to elliptical, occasionally clavate | Flat, circular, slightly filiform margins, white-greyish cottony aerial mycelium, pale on reverse, orange in center |
| JA15 | D | II | 5 ± 0 | 9.7 ± 2.4 × 4.2 ± 1.0 | Cylindrical, occasionally roundish | Flat, circular, with entire margins, white-greyish cottony aerial mycelium, dark on reverse, pale at margins |
| CBS 109216, JA21, -22 | E | VI | 5.4 ± 0.3 | 8.5 ± 2.1 × 3.5 ± 0.7 | Cylindrical to clavate | Flat, circular, with entire margins, white-greyish cottony aerial mycelium, pale orange on reverse with black dots |
| JA20 | F | III | 4.2 ± 0.3 | 8.7 ± 1.1 × 3.2 ± 0.6 | Cylindrical, occasionally clavate | Flat, circular, with entire margins , sparse white-greyish aerial mycelium, pale on reverse |
| JA10 | G | III | 4.9 ± 0.2 | 9.2 ± 1.7 × 3.6 ± 0.7 | Cylindrical to elliptical, occasionally clavate | Irregular and radially sulcate with aerial mycelia growth in the center, pale on reverse |
| CBS 109225 | H | I | 5.2 ± 0.1 | 8.5 ± 1.7 × 3.8 ± 0.8 | Cylindrical to clavate | Slightly irregular and thickly covered with wooly white-greyish aerial mycelia, pale on reverse |
| JA18 | I | V | 4.1 ± 0 | 10 ± 1.8 × 2.9 ± 0.7 | Cylindrical | Irregular, wooly white areal mycelia on the margins, pale on reverse |
| JA19 | J | V | 6 ± 0.2 | 12.1 ± 1.8 × 2.4 ± 0.7 | Cylindrical | Irregular, white-greyish wooly aerial mycelium, pale on reverse with occasional black/orange dots |
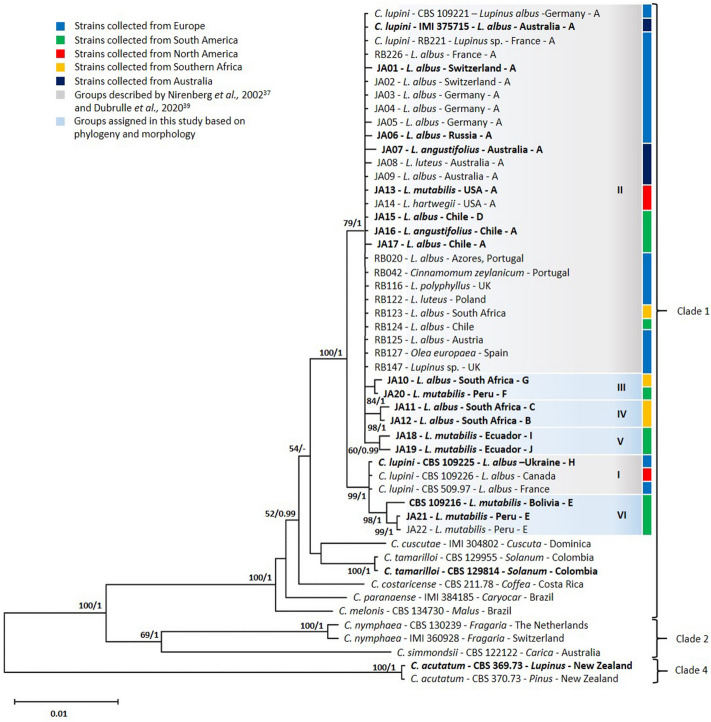
Multi-locus phylogenetic analyses of 50 Colletotrichum isolates identified six distinct genetic groups within C. lupini (I–VI; Fig. 3, Supplementary Fig. S3). The combined sequence dataset contained 2251 characters (ITS: 1–496, GAPDH: 497–745, TUB2: 746–1200, APN/MAT1: 1201–2251) including alignment gaps. The APN/MAT1 locus showed the highest variability across the nucleotide data set, with 75.8% conserved sites for the whole data set (including out-groups) and 97.4% within C. lupini (Supplementary Table S1). The TUB2 and GAPDH loci showed 89.9% and 81.1% identical sites for the entire dataset and 97.8% and 98.4% identity within C. lupini, respectively. The ITS region showed the lowest variability with 97% identical sites across the whole dataset and 99.2% within C. lupini. As shown in Fig. 3, most C. lupini strains clustered with a high bootstrap support (BS) value of 79 and posterior probability (PP) of 1 with reference strains representing genetic group II (CBS 109221, IMI 375715 and RB221). Strains within group II showed a high identity among each other (> 99.9%) and showed morphotype A, except for Chilean strain JA15 showing morphotype D (Fig. 2). South African strain JA10 and Peruvian strain JA20, with morphotypes G and F, respectively, clustered together with a BS of 84 and PP of 1, forming a highly supported group (III). South African strains JA11 and JA12, with morphotypes C and B, respectively, clustered together with a BS of 98 and PP of 1, forming a highly supported group (IV). Ecuadorian strains JA18 and JA19 with distinct morphotypes I and J, respectively, showed 99.7% identity with reference strains of group II and clustered together with a BS of 60 (Fig. 3, Supplementary Fig. S3) and a PP of 1 in (Fig. 3), forming a distinct group (V). The reference strains for group I (CBS 109225 with morphotype H, CBS 109226 and CBS 509.97) are clustered together with a BS of 99 and PP of 1 and show 100% identity with each other and 99.6% identity with reference strains of group II. South American strains JA21, JA22 and CBS 109216, with morphotype E, cluster together with a BS of 98 and PP of 1 (Fig. 3) and a BS of 54 (Supplementary Fig. S3) forming a highly supported group (VI). JA21 and JA22 showed 99.8% and CBS 109216 showed 99.7% identity with reference strains of group I and 99.4% and 99.2% identity with references strains of group II, respectively.
Figure 3.
Multi-locus phylogeny of Colletotrichum lupini. Bayesian analysis tree inferred from the combined ITS, TUB2, GAPDH and APN/MAT1 sequence datasets of 50 Colletotrichum strains used in this study. Bootstrap support values (> 50) and Bayesian posterior probabilities (> 0.95) are given at each node. The tree is rooted to C. acutatum (CBS 369.73 and CBS 370.73). Strain codes are followed by host, country of origin and morphology (A–J). Grouping (I–VI) is based on phylogeny and morphology. Strains used for virulence assays are highlighted in bold. Clades indicate the different clades within the C. acutatum species complex.
Distinct virulence patterns on white and Andean lupin
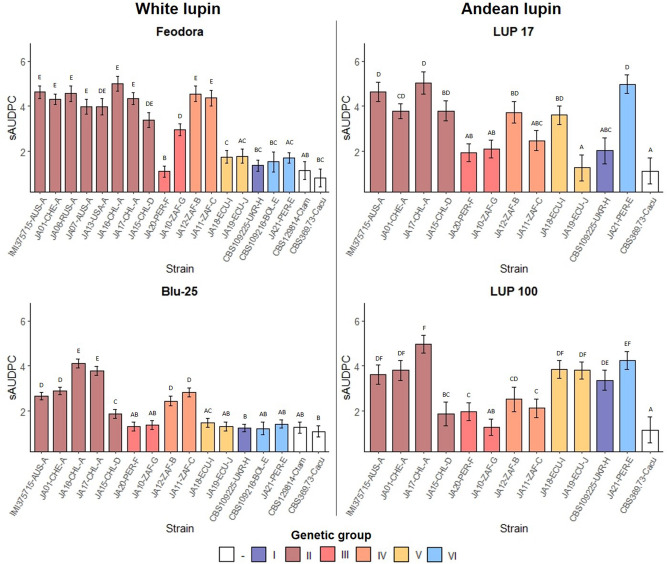
Virulence assays performed on two white lupin (L. albus L.) accessions (Feodora and Blu-25) and two Andean lupin (L. mutabilis Sweet.) accessions (LUP 17 and LUP 100) with strains representing the different morphotypes and genetic groups indicated in Fig. 3, revealed strong strain (p < 0.0001), lupin species (p < 0.0001) and strain × lupin species interaction effects (p < 0.0001). A strong accession effect was found within white lupin (p < 0.0001), whereas for Andean lupin there was no significant accession effect (p = 0.43). Strain (p < 0.0001) and strain × accession (p < 0.0001) interaction effects were found for both species. Strains belonging to genetic group II with morphotype A, caused severe disease on white lupin accession Feodora and both Andean lupin accessions (Supplementary Fig. S4), showing standardized area under the disease progress curve (sAUDPC) means ranging from 3.95 to 5 (Fig. 4). On the more tolerant white lupin accession Blu-25, sAUDPC means for strains of group II with morphology A were more variable, with JA01 and IMI 375715 showing moderate (2.7–2.9) and Chilean strains JA16 and 17 showing high (3.8–4.1) virulence. Chilean strain JA15, also belonging to genetic group II but with a different morphology (D), caused low disease on LUP 100 and Blu-25 (1.9), showing a different virulence spectrum compared to the other tested strains of genetic group II. South African strains JA11 and JA12, belonging to genetic group IV with morphotypes C and B, respectively, showed a similar virulence spectrum on white lupin as strains of group II. JA10 and JA20, representing group III and morphotype G and F, respectively, were overall avirulent (< 2), with the exception of JA10 on Feodora, showing moderate virulence (2.95). Peruvian strain JA21, representing genetic group VI and morphotype E, caused low disease on white lupin (1.4–1.8), but severe disease on Andean lupin (4.25–5). A similar observation was found for the two Ecuadorian strains JA18 and JA19 of genetic group V and morphotypes I and J, respectively. These two strains caused low disease on white lupin and high disease on Andean lupin LUP 100. On Andean lupin LUP 17, however, a severe disease phenotype was only found for JA18 (3.6), whereas JA19 barely caused any disease symptoms (1.25). Similar to the observations for JA19, the Ukrainian strain CBS 109225 (genetic group I, morphotype H) caused severe disease on Andean lupin LUP 100 (3.36) and low disease on Andean lupin LUP 17 and white lupin (1.2–2). The C. tamarilloi and C. acutatum strains were avirulent across the lupin accessions (< 1.26).
Figure 4.
Virulence of Colletotrichum lupini strains on white (Lupinus albus) and Andean lupin (L. mutabilis). Anthracnose severity is expressed in standardized area under the disease progress curve (sAUDPC) and estimated means are shown. Strain codes are followed by abbreviated country of origin and morphotype (A–J). Different capital letters above bars indicate significant differences between strains (Tuckey-HSD, p < 0.05). Error bars indicate the standard error of the estimated mean.
Discussion
This study compared 39 C. lupini and 11 Colletotrichum spp. isolates collected from across the world to explore intraspecific diversity of C. lupini and to better understand the dynamics of the current lupin anthracnose pandemic and potential implications of further migrations of distinct pathogenic strains. Based on multi-locus phylogeny supported by isolate morphology, we identified four distinct genetic groups additional to previously described genetic groups I and II. Highest intraspecific diversity was identified among C. lupini isolates collected from across the South American Andes region. This is in line with reports of Falconí et al.41 and Riegel et al.40 showing high diversity in Ecuador and Chile, respectively. In those regions, Andean lupin has been cultivated for more than 2000 years42 growing alongside numerous wild lupin species43. Isolates collected in South Africa showed a distinct morphology and virulence spectrum, indicating higher diversity than previously shown44. Although lupins form a significant part of the local agriculture and have been researched there since at least 189745, they are not native to South Africa and lupin anthracnose was not reported in South Africa until 199346. Taking into account the relatively recent reports of anthracnose in South Africa, the low diversity in Europe and Australia and the center of origin for species within clade 1 of the C. acutatum species complex being in South America10,15, we consider the South American Andes to be the center of origin of C. lupini.
The majority of the C. lupini isolates (26 out of 39) belong to the highly virulent genetic group II, showing morphotype A, and were collected in Europe, Australia, South Africa, the USA and Chile. This result confirms previous reports classifying most C. lupini strains from across the world in the same genetic group14,39,47–49. The low genetic diversity among strains of group II, the uniform morphology and non-observed sexual morph14 indicates clonality as suggested by Talhinhas et al.20. Pathogenicity of group II strains has also been shown on blue28, yellow32 and various other lupin species across the world20, indicating a broad host range within the genus Lupinus. Reports from South Korea and China indicate that group II strains also cause disease in those regions50,51, highlighting that these strains are globally widespread and are the cause of the current anthracnose pandemic in lupin. The group II strain RB221 can be used as reference, as it is now fully sequenced52 and tested on both Andean and white lupin53.
The stem-wound inoculation assay used in this study was previously described to be highly reproducible and strongly correlated to field performance under natural infection pressure26. In the present study, virulence assays based on stem-wounding showed strong strain x accession interaction effects for white and Andean lupin, suggesting a strain-dependent host spectrum and the existence of different physiological races within C. lupini. Similar observations were described by Falconí et al.41, showing a C. lupini strain x Andean lupin accession interaction effect. The existence of physiological races has been observed for various Colletotrichum species, such as for C. lindemuthianum on common bean54, C. sublineola on sorghum55 and C. truncatum on lentil56, but, in general, this is not common within the genus Colletotrichum. The similar virulence levels of isolates belonging to group II observed on Andean and white lupin accessions are in line with Alkemade et al.26, in which equal virulence was observed for IMI 375715 (Australia) and JA01 (Switzerland) when inoculated on six different white lupin accessions. However, an exception within group II is Chilean strain JA15, which, besides having a distinct morphology, was less virulent on Andean lupin LUP 100 and white lupin Blu-25. Further, Chilean strains JA16 and JA17 (also group II) overcame resistance of the resistant advanced breeding line Blu-25, which has been specifically bred for anthracnose resistance in Chile and was shown resistant under Swiss field conditions26. These results indicate that new introductions of highly virulent foreign strains can have severe consequences as seen for many other crops57–59 and it should be investigated if this high virulence is also affecting other resistant (white) lupin material26,31,60. Although disease development after stem-wounding of seedlings correlated strongly to field disease scores of mature plants26, we cannot exclude the possibility that conclusions drawn on virulence level might differ for secondary infection processes (e.g. via rain splash).
This study provides first solid evidence that, based on multi-locus phylogeny and morphology, genetic diversity within C. lupini is higher than previously shown. High-resolution genome-wide sequencing and an increased sampling density from especially the South American Andes region are now necessary to increase genetic resolution and to better understand C. lupini phylogeny and phylogeography. This could provide the basis for in-depth comparative genomic studies to identify effector gene clusters within the C. lupini genome. This study confirms that the current lupin anthracnose pandemic is caused by a genetically uniform group of highly virulent strains. The identification of strains with an increased virulence on tolerant white lupin breeding material and the observation of strain-specific virulence patterns should be taken into account in lupin resistance breeding programs. Due to its seed-borne nature, caution should be taken when importing seeds, especially from South America, to prevent further introductions of potentially virulent strains across the world.
Methods
Fungal and plant material
A diverse collection of 39 Colletotrichum lupini and 11 closely related Colletotrichum spp. isolates, originating from Europe, Australia, Southern Africa and South and North America, was analyzed (Table 1). Nine isolates were collected from symptomatic lupin plants in this study, whereas the rest of the isolates was already identified as C. lupini or as other members of the C. acutatum species complex representing clades 1, 2 and 4. The C. lupini strains CBS 109225 (Ukraine), CBS 509.97 (France) and CBS 109226 (Canada) were chosen as reference for genetic group I, strains CBS 109221 (Germany), IMI 375715 (Australia) and RB221 (France) served as reference for genetic group II and the C. acutatum strains CBS 369.73 and CBS 370.73 were used as outgroup in the phylogenetic analysis. Inoculations were performed on two white lupin (Lupinus albus L.) accessions: Feodora (susceptible; breeder: Jouffrai Drillaud, France) and Blu-25 (tolerant; breeder: Semillas Baer, Chile), and two Andean lupin (L. mutabilis) accessions: LUP 17 and LUP 100 (genebank: IPK, Germany). Plant material can be requested at mentioned breeders and genebanks, who performed formal identification and gave permission to use the material for research purposes. The experimental research of the plant material used in this study complies with relevant institutional, national, and international guidelines and legislation.
Fungal isolation and culture conditions
Symptomatic (dried) lupin stem or pod tissue (Fig. 1) of 1–3 cm was surface sterilized (after rehydration in sterile ddH2O for dried samples) for 5 s with 0.25% sodium hypochlorite solution and rinsed thrice for 5 s in sterile ddH2O. Thin slices of 1 mm were cut and placed on PDA (potato dextrose agar, Carl Roth, Karlsruhe, Germany) amended with Tetracycline (0.02 g/l, Carl Roth) for 3 to 4 days at 22 °C in the dark. Single cultures were selected and grown on fresh PDA plates amended with Tetracycline for 4 to 6 days at 22 °C in the dark and suspected Colletotrichum species were sub-cultured. Single spore cultures were obtained and transferred to PDA and maintained at 22 °C in the dark as working cultures and stored at − 80 °C in 25% glycerol for long-term storage.
Morphology
A globally representative subset of 28 C. lupini isolates was characterized based on colony morphology (form, aerial mycelium, margin type and color of the reverse side). From those, a subset of 18 isolates was further characterized for growth rate (mm/day), and conidial shape and size19. Isolates were subcultured by placing a droplet of 5 μl spore suspension in the middle of three PDA plates and grown for 14 days at 22 °C in the dark. Culture diameter was recorded every 3 days. Photographs were taken from the front and reverse sides of the PDA plates after 14 days of incubation. Conidia were collected with a sterile spreader after flooding the Petri plate with 2 ml sterile ddH20, the spore suspension was filtered with sterile cheese cloth and microscopic slides were prepared with sterile ddH2O. Conidia morphology was observed using light microscopy (DM2000-LED, Leica Microsystems, Wetzlar, Germany) equipped with a high definition camera (Gryphax Subra, Jenoptik AG, Jena, Germany). A minimum of at least 50 measurements were performed to determine conidia length and width. A principal component analysis (PCA) was performed on a subset of 17 representative C. lupini isolates, based on average conidia length and width, length width ratio, colony growth rate, form (circular = 1, most irregular = 4), aerial mycelia (no aerial mycelia = 1, most aerial mycelia = 4), color (palest = 1, darkest = 4) and filiform margin (yes = 1, no = 0), using R 4.0.361 and the FactoMineR package62.
DNA extraction, PCR amplification and sequencing
Mycelium from single-spore cultures was collected after 7–10 days on PDA at 22 °C with a sterile spreader after flooding the Petri dish with 2 ml sterile ddH20. Genomic DNA was isolated with a CTAB extraction protocol63. Partial gene sequences were determined for the internal transcribed spacer (ITS) region using primers ITS5 and ITS464, the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene using primers GDF1 and GDR165, the β-tubulin 2 (TUB2) gene using primers Btub2Fd and Btub4Rd66 and the Apn2-Mat1-2-1 intergenic (APN/MAT1) spacer and partial mating type gene using Apnmat1F and Apnmat1R39. PCR was performed in a S1000 Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to conditions described in Dubrulle et al.39 PCR products were verified by gel electrophoresis, purified using the QIAquick PCR Purification Kit (Qiagen, Hilden, Germany), quantified with a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and sent to Eurofins Genomics (Ebersberg, Germany) for sequencing. The obtained DNA sequences were analyzed and consensus sequences were generated using BioEdit v. 7.2.567.
Phylogenetic analyses
Alignments for each of the four loci, including sequences obtained in this study and downloaded from GenBank (Table 1), were performed with ClustalW using MEGA X68. Obtained multiple alignments where manually corrected and trimmed to obtain comparable sequences. Best-fit substitution models were determined for each locus separately and for the concatenated multi-locus alignment (ITS, TUB2, GAPDH and APN/MAT1). Phylogenetic analyses of the multi-locus alignment were based on Maximum Likelihood (ML) and Bayesian Inference (BI). The ML analysis was performed using RAxML v. 869 through the CIPRES science gateway portal70 using default parameters and 1000 bootstrap iterations. The BI analysis was performed with MrBayes v. 3.2.771 using a Markov Chain Monte Carlo (MCMC) algorithm using four chains and starting from a random tree topology. Substitution models for each locus were included for each partition. The analysis ran for 500,000 generations with trees sampled every 1000 generations to reach average standard deviations of split frequencies below 0.01. The first 25% of saved trees were discarded at the ‘burn-in’ phase and the 50% consensus trees and posterior probabilities (PP) were determined from the remaining trees. Bootstrap support values (BS) from the ML analysis were plotted on the Bayesian phylogeny. Further phylogenetic analyses were performed with the unweighted pair group method with arithmetic mean (UPGMA) with 10,000 replicates in Mega X. All generated sequences were deposited in GenBank (Table 1) and alignments and trees in TreeBASE.
Virulence
Virulence tests were performed on white and Andean lupin with representative C. lupini strains (see Fig. 3), C. tamarilloi strain CBS 129814 and C. acutatum strain CBS 369.73 through stem-wound inoculation as described by Alkemade et al.26, which was shown to highly correspond to field performance in Switzerland (r = 0.95). Disease scores ranging from 1 (non-pathogenic), 2 (low virulence) to 9 (highly virulent) were taken 4, 7 and 10 days post inoculation (dpi) and the standardized area under the disease progress curve was calculated (sAUDPC)26. All inoculations were performed in a growth chamber (25 ± 2 °C, 16 h light and ~ 70% relative humidity) in a completely randomized block design with a minimum of six replicates per experiment.
Statistical analysis
Statistical analyses were performed with R 4.0.3 using the packages lme472, lmerTest73 and emmeans74, following a mixed model with factors of interest (i.e. strain, lupin species, lupin accession) as fixed and replicated block nested in experiment as random factor. Datasets that did not follow assumptions of normality of residuals and homogeneity of variance were log10 transformed. Data are presented as estimated least-squares means using the aforementioned mixed model. A Tukey-HSD test (p ≤ 0.05) was applied for pairwise mean comparison of the different Colletotrichum strains within each lupin accession.
Supplementary Information
Acknowledgements
The authors acknowledge Dr. César E. Falconí (ESPE, Universidad de las Fuerzas Armadas, Quito, Ecuador), Dr. Amelia W. Huaringa Joaquin (Universidad Nacional Agraria La Molina, Lima, Peru), Jocelyn Betancur & Erik von Baer (Semillas Baer, Temuco, Chile), Dr. Roger G. Shivas & Dr. Yu Pei Tan (Queensland Plant Pathology Herbarium (BRIP), Brisbane, Queensland, Australia), Dr. Erin Rosskopf (USDA-ARS, Fort Pierce, Florida, USA), Dr. Seonju Marincowitz (FABI, University of Pretoria, Pretoria, South Africa), Dr. Gaétan Le Floch (Université de Bretagne Occidentale, Brest, France) and Dr. Peter Wehling (JKI, Gross Lüsewitz, Germany) for kindly providing C. lupini strains, Christine Arncken and Simon Rosenfeld (FiBL, Frick, Switzerland) for helping collect Colletotrichum strains, Erik von Baer for supplying white lupin (Blu-25) seeds and Jan Trávníček and Katharina Bitterlich for assisting with setting up controlled condition virulence experiments at FiBL (Switzerland). This research has received funding from the European Union’s Horizon 2020 research and innovation programme LIVESEED under Grant Agreement No. 727230 and by the Swiss State Secretariat for Education, Research and Innovation (SERI) under Contract Number 17.00090, and the Federal Office for Agriculture FOAG. The information contained in this communication only reflects the author’s view. Neither the Research Executive Agency nor SERI is responsible for any use that may be made of the information provided.
Author contributions
J.A.A., P.H. and M.M.M. conceived the original idea for this study. J.A.A. conducted the experiments and took the lead in manuscript writing. J.A.A. analyzed the data with contributions from P.H. and M.M.M. All authors significantly contributed to data interpretation and provided critical feedback that shaped the final version. J.A.A. designed the figures and tables with input from P.H., M.M.M., R.T.V. and M.R.F.
Data availability
The data that support the findings of this study are shown in this manuscript or, in the case of new sequences data, are openly available in Genbank at https://www.ncbi.nlm.nih.gov/genbank/ (for reference numbers see Table 1) and in Treebase at http://purl.org/phylo/treebase/phylows/study/TB2:S27356?x-access-code=260136f8e6416a0614b93528ddbfe0ef&format=html.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-92953-y.
References
- 1.Lenné J. Some major plant diseases. In: Waller JM, Lenné JM, Waller SJ, editors. Plant Pathologist’s Pocketbook. CABI Publishing; 2002. pp. 4–18. [Google Scholar]
- 2.Cannon P, Damm U, Johnston P, Weir B. Colletotrichum—Current status and future directions. Stud. Mycol. 2012;73:181–213. doi: 10.3114/sim0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Udayanga D, Manamgoda DS, Liu X, Chukeatirote E, Hyde KD. What are the common anthracnose pathogens of tropical fruits? Fungal Divers. 2013;61:165–179. doi: 10.1007/s13225-013-0257-2. [DOI] [Google Scholar]
- 4.Shivas RG, et al. Colletotrichum species in Australia. Australas. Plant Pathol. 2016;45:447–464. doi: 10.1007/s13313-016-0443-2. [DOI] [Google Scholar]
- 5.Baroncelli R, et al. Molecular diversity of anthracnose pathogen populations associated with UK strawberry production suggests multiple introductions of three different Colletotrichum species. PLoS ONE. 2015;10:e0129140. doi: 10.1371/journal.pone.0129140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frey T, Weldekidan T, Colbert T, Wolters P, Hawk J. Fitness evaluation of Rcg1, a locus that confers resistance to Colletotrichum graminicola (Ces.) GW Wils. using near‐isogenic maize hybrids. Crop Sci. 2011;51:1551–1563. doi: 10.2135/cropsci2010.10.0613. [DOI] [Google Scholar]
- 7.Rogério F, et al. Genome sequence resources of Colletotrichum truncatum, C. plurivorum, C. musicola and C. sojae: Four species pathogenic to soybean (Glycine max) Phytopathology. 2020;110:1497–1499. doi: 10.1094/PHYTO-03-20-0102-A. [DOI] [PubMed] [Google Scholar]
- 8.Boufleur TR, et al. Soybean anthracnose caused by Colletotrichum species: Current status and future prospects. Mol. Plant Pathol. 2021;22:393–409. doi: 10.1111/mpp.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perfect SE, Hughes HB, O'Connell RJ, Green JR. Colletotrichum: a model genus for studies on pathology and fungal–plant interactions. Fungal Genet. Biol. 1999;27:186–198. doi: 10.1006/fgbi.1999.1143. [DOI] [PubMed] [Google Scholar]
- 10.Baroncelli R, et al. The Colletotrichum acutatum species complex as a model system to study evolution and host specialization in plant pathogens. Front. Microbiol. 2017;8:2001. doi: 10.3389/fmicb.2017.02001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Silva DD, Crous PW, Ades PK, Hyde KD, Taylor PW. Life styles of Colletotrichum species and implications for plant biosecurity. Fungal Biol. Rev. 2017;31:155–168. doi: 10.1016/j.fbr.2017.05.001. [DOI] [Google Scholar]
- 12.Yan Y, et al. Colletotrichum higginsianum as a model for understanding host–pathogen interactions: A review. Int. J. Mol. Sci. 2018;19:2142. doi: 10.3390/ijms19072142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean R, et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012;13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damm U, Cannon PF, Woudenberg JHC, Crous PW. The Colletotrichum acutatum species complex. Stud. Mycol. 2012;73:37–113. doi: 10.3114/sim0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bragança CA, Damm U, Baroncelli R, Júnior NSM, Crous PW. Species of the Colletotrichum acutatum complex associated with anthracnose diseases of fruit in Brazil. Fungal Biol. 2016;120:547–561. doi: 10.1016/j.funbio.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Cannon PF, Bridge P, Monte E. Linking the past, present and future of Colletotrichum systematics. In: Prusky D, Freeman S, Dickman MB, editors. Colletotrichum: Host Specificity, Pathology and Host-Pathogen Interaction. APS Press; 2000. pp. 1–20. [Google Scholar]
- 17.Johnston P. The importance of phylogeny in understanding host relationships within Colletotrichum. In: Prusky D, Freeman S, Dickman MB, editors. Colletotrichum: Host Specificity, Pathology, and Host-Pathogen Interaction. APS Press; 2000. pp. 21–28. [Google Scholar]
- 18.Cai L, et al. A polyphasic approach for studying Colletotrichum. Fungal Divers. 2009;39:183–204. [Google Scholar]
- 19.Lardner R, Johnston P, Plummer K, Pearson M. Morphological and molecular analysis of Colletotrichum acutatum sensu lato. Mycol. Res. 1999;103:275–285. doi: 10.1017/S0953756298007023. [DOI] [Google Scholar]
- 20.Talhinhas P, Baroncelli R, Le Floch G. Anthracnose of lupins caused by Colletotrichum lupini: A recent disease and a successful worldwide pathogen. J. Plant Pathol. 2016;98:5–14. [Google Scholar]
- 21.Bondar G. Tremoco branco e suas molestias. Bol. Agric. Sao Paolo. 1912;13:427–432. [Google Scholar]
- 22.Weimer J. Anthracnose of lupines. Phytopathology. 1943;33:249–252. [Google Scholar]
- 23.White P, French B, McLarty A. Grains research and development corporation. In: White P, French B, McLarty A, editors. Producing Lupins. Department of Agriculture and Food; 2008. pp. 48–49. [Google Scholar]
- 24.Thomas G, Sweetingham M. Cultivar and environment influence the development of lupin anthracnose caused by Colletotrichum lupini. Australas. Plant Pathol. 2004;33:571–577. doi: 10.1071/AP04060. [DOI] [Google Scholar]
- 25.Shea G, et al. Case study: Industry response to the lupin anthracnose incursion in Western Australia. In: Palta JA, Berger JB, et al., editors. Lupins for Health and Wealth. Proc. 12th International Lupin Conference. International Lupin Association; 2008. pp. 425–431. [Google Scholar]
- 26.Alkemade JA, et al. A high-throughput phenotyping tool to identify field-relevant anthracnose resistance in white lupin. Plant Dis. 2021 doi: 10.1094/PDIS-07-20-1531-RE. [DOI] [PubMed] [Google Scholar]
- 27.Thomas G, Sweetingham M, Adcock K. Application of fungicides to reduce yield loss in anthracnose-infected lupins. Crop Prot. 2008;27:1071–1077. doi: 10.1016/j.cropro.2007.12.012. [DOI] [Google Scholar]
- 28.Yang H, Boersma JG, You M, Buirchell BJ, Sweetingham MW. Development and implementation of a sequence-specific PCR marker linked to a gene conferring resistance to anthracnose disease in narrow-leafed lupin (Lupinus angustifolius L.) Mol. Breed. 2004;14:145–151. doi: 10.1023/B:MOLB.0000038003.49638.97. [DOI] [Google Scholar]
- 29.Yang H, Renshaw D, Thomas G, Buirchell B, Sweetingham M. A strategy to develop molecular markers applicable to a wide range of crosses for marker assisted selection in plant breeding: A case study on anthracnose disease resistance in lupin (Lupinus angustifolius L.) Mol. Breed. 2008;21:473–483. doi: 10.1007/s11032-007-9146-2. [DOI] [Google Scholar]
- 30.Fischer K, et al. Characterization and mapping of LanrBo: A locus conferring anthracnose resistance in narrow-leafed lupin (Lupinus angustifolius L.) Theor. Appl. Genet. 2015;128:2121–2130. doi: 10.1007/s00122-015-2572-3. [DOI] [PubMed] [Google Scholar]
- 31.Adhikari K, Buirchell B, Thomas GJ, Sweetingham MW, Yang H. Identification of anthracnose resistance in Lupinus albus L. and its transfer from landraces to modern cultivars. Crop Pasture Sci. 2009;60:472–479. doi: 10.1071/CP08092. [DOI] [Google Scholar]
- 32.Adhikari KN, Thomas G, Buirchell BJ, Sweetingham MW. Identification of anthracnose resistance in yellow lupin (Lupinus luteus L.) and its incorporation into breeding lines. Plant Breed. 2011;130:660–664. doi: 10.1111/j.1439-0523.2011.01880.x. [DOI] [Google Scholar]
- 33.Falconí, C. Lupinus mutabilis in Ecuador with special emphasis on anthracnose resistance. PhD thesis, Wageningen University, the Netherlands (2012).
- 34.Lucas MM, et al. The future of lupin as a protein crop in Europe. Front. Plant Sci. 2015;6:705. doi: 10.3389/fpls.2015.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van de Noort M. Lupin: An important protein and nutrient source. In: Nadathur SR, Wanasundara JPD, Scanlin L, editors. Sustainable Protein Sources. Academic Press; 2017. pp. 165–183. [Google Scholar]
- 36.Gulisano A, Alves S, Neves Martins J, Trindade LM. Genetics and breeding of Lupinus mutabilis: an emerging protein crop. Front. Plant Sci. 2019;10:1385. doi: 10.3389/fpls.2019.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nirenberg HI, Feiler U, Hagedorn G. Description of Colletotrichum lupini comb. nov. in modern terms. Mycologia. 2002;94:307–320. doi: 10.1080/15572536.2003.11833238. [DOI] [PubMed] [Google Scholar]
- 38.Shivas R, McClements J, Sweetingham M. Vegetative compatibility amongst isolates of Colletotrichum causing lupin anthracnose. Australas. Plant Pathol. 1998;27:269–273. doi: 10.1071/AP98032. [DOI] [Google Scholar]
- 39.Dubrulle G, et al. Phylogenetic diversity and effect of temperature on pathogenicity of Colletotrichum lupini. Plant Dis. 2020;104:938–950. doi: 10.1094/PDIS-02-19-0273-RE. [DOI] [PubMed] [Google Scholar]
- 40.Riegel R, Veliz D, von Baer I, Quitral Y, Munoz M. Genetic diversity and virulence of Colletotrichum lupini isolates collected in Chile. Trop. Plant Pathol. 2010;35:144–152. doi: 10.1590/S1982-56762010000300002. [DOI] [Google Scholar]
- 41.Falconí CE, Visser RG, van Heusden AW. Phenotypic, molecular, and pathological characterization of Colletotrichum acutatum associated with Andean lupine and tamarillo in the Ecuadorian Andes. Plant Dis. 2013;97:819–827. doi: 10.1094/PDIS-02-12-0175-RE. [DOI] [PubMed] [Google Scholar]
- 42.Atchison GW, et al. Lost crops of the Incas: Origins of domestication of the Andean pulse crop tarwi, Lupinus mutabilis. Am. J. Bot. 2016;103:1592–1606. doi: 10.3732/ajb.1600171. [DOI] [PubMed] [Google Scholar]
- 43.Nevado B, Atchison GW, Hughes CE, Filatov DA. Widespread adaptive evolution during repeated evolutionary radiations in New World lupins. Nat. Commun. 2016;7:1–9. doi: 10.1038/ncomms12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lotter H, Berger D. Anthracnose of lupins in South Africa is caused by Colletotrichum lupini var. setosum.Australas. Plant Pathol. 2005;34:385–392. [Google Scholar]
- 45.Van Der Mey JA. Crop development of Lupinus species in Africa. S. Afr. J. Sci. 1996;92:53–56. [Google Scholar]
- 46.Ghebremariam D, Koch S, Swart W. Susceptibility of lupin cultivars to South African isolates of Colletotrichum gloeosporioides associated with lupin anthracnose. Afr. Plant Prot. 2002;8:51–56. [Google Scholar]
- 47.Yang H, Sweetingham M. The taxonomy of Colletotrichum isolates associated with lupin anthracnose. Aust. J. Agric. Res. 1998;49:1213–1224. doi: 10.1071/A98074. [DOI] [Google Scholar]
- 48.Elmer WH, Yang HA, Sweetingham MW. Characterization of Colletotrichum gloeosporioides isolates from ornamental lupines in Connecticut. Plant Dis. 2001;85:216–219. doi: 10.1094/PDIS.2001.85.2.216. [DOI] [PubMed] [Google Scholar]
- 49.Talhinhas P, Sreenivasaprasad S, Neves-Martins J, Oliveira H. Genetic and morphological characterization of Colletotrichum acutatum causing anthracnose of lupins. Phytopathology. 2002;92:986–996. doi: 10.1094/PHYTO.2002.92.9.986. [DOI] [PubMed] [Google Scholar]
- 50.Han K, Kim B, Choi I, Park J, Shin H-D. First report of anthracnose caused by Colletotrichum lupini on yellow lupin in Korea. Plant Dis. 2014;98:1158–1158. doi: 10.1094/PDIS-02-14-0175-PDN. [DOI] [PubMed] [Google Scholar]
- 51.Zou M, et al. First report of Colletotrichum lupini causing anthracnose on lupin in China. Plant Dis. 2019;103:767. doi: 10.1094/PDIS-07-18-1270-PDN. [DOI] [Google Scholar]
- 52.Dubrulle G, et al. Deciphering the infectious process of Colletotrichum lupini in lupin through transcriptomic and proteomic analysis. Microorganisms. 2020;8:1621. doi: 10.3390/microorganisms8101621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guilengue N, Neves-Martins J, Talhinhas P. Response to anthracnose in a tarwi (Lupinus mutabilis) collection is influenced by anthocyanin pigmentation. Plants. 2020;9:583. doi: 10.3390/plants9050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Falleiros MO, Mota SF, Ferreira AN, de Souza EA. Mixture of Colletotrichum lindemuthianum races in anthracnose resistance screening and its implication for common bean breeding. Trop. Plant Pathol. 2018;43:271–277. doi: 10.1007/s40858-018-0219-z. [DOI] [Google Scholar]
- 55.Xavier K, Mizubuti E, Queiroz M, Chopra S, Vaillancourt L. Genotypic and pathogenic diversity of Colletotrichum sublineola isolates from sorghum (Sorghum bicolor) and johnsongrass (S. halepense) in the southeastern United States. Plant Dis. 2018;102:2341–2351. doi: 10.1094/PDIS-04-18-0562-RE. [DOI] [PubMed] [Google Scholar]
- 56.Armstrong-Cho C, Wang J, Wei Y, Banniza S. The infection process of two pathogenic races of Colletotrichum truncatum on lentil. Can. J. Plant Pathol. 2012;34:58–67. doi: 10.1080/07060661.2012.664565. [DOI] [Google Scholar]
- 57.Ordonez N, et al. Worse comes to worst: Bananas and Panama disease—When plant and pathogen clones meet. PLoS Pathog. 2015;11:e1005197. doi: 10.1371/journal.ppat.1005197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Godfray HCJ, Mason-D’Croz D, Robinson S. Food system consequences of a fungal disease epidemic in a major crop. Philos. Trans. R. Soc. B Biol. Sci. 2016;371:20150467. doi: 10.1098/rstb.2015.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lidwell-Durnin J, Lapthorn A. The threat to global food security from wheat rust: Ethical and historical issues in fighting crop diseases and preserving genetic diversity. Glob. Food Sec. 2020;26:100446. doi: 10.1016/j.gfs.2020.100446. [DOI] [Google Scholar]
- 60.Jacob I, Feuerstein U, Heinz M, Schott M, Urbatzka P. Evaluation of new breeding lines of white lupin with improved resistance to anthracnose. Euphytica. 2017;213:236. doi: 10.1007/s10681-017-2011-4. [DOI] [Google Scholar]
- 61.R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2020). http://www.R-project.org/. Accessed 10 Oct 2020.
- 62.Lê S, Josse J, Husson F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008;25:1–18. doi: 10.18637/jss.v025.i01. [DOI] [Google Scholar]
- 63.Minas K, McEwan NR, Newbold CJ, Scott KP. Optimization of a high-throughput CTAB-based protocol for the extraction of qPCR-grade DNA from rumen fluid, plant and bacterial pure cultures. FEMS Microbiol. Lett. 2011;325:162–169. doi: 10.1111/j.1574-6968.2011.02424.x. [DOI] [PubMed] [Google Scholar]
- 64.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols: A Guide to Methods and Applications. Elsevier; 1989. pp. 315–322. [Google Scholar]
- 65.Guerber JC, Liu B, Correll JC, Johnston PR. Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns, mtDNA and intron RFLPs, and mating compatibility. Mycologia. 2003;95:872–895. doi: 10.1080/15572536.2004.11833047. [DOI] [PubMed] [Google Scholar]
- 66.Woudenberg J, Aveskamp M, De Gruyter J, Spiers A, Crous P. Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Persoonia. 2009;22:56. doi: 10.3767/003158509X427808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hall TA. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 1999;41:95–98. [Google Scholar]
- 68.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller, M. A., Pfeiffer, W. & Schwartz, T. The CIPRES science gateway: A community resource for phylogenetic analyses. In Proc. 2011 TeraGrid Conference: Extreme Digital Discovery 1–8 (2011).
- 71.Ronquist F, et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 73.Kuznetsova A, Brockhoff PB, Christensen RH. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017;82:1–26. doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- 74.Lenth, R., Singmann, H., Love, J., Buerkner, P. & Herve, M. emmeans: Estimated Marginal Means, aka Least-Squares Means (Version 1.3. 4) (2019). https://CRAN.R-project.org/package=emmeans. Accessed 5 Oct 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are shown in this manuscript or, in the case of new sequences data, are openly available in Genbank at https://www.ncbi.nlm.nih.gov/genbank/ (for reference numbers see Table 1) and in Treebase at http://purl.org/phylo/treebase/phylows/study/TB2:S27356?x-access-code=260136f8e6416a0614b93528ddbfe0ef&format=html.