Abstract
Aim: As Coronavirus Disease-2019 (COVID-19) pandemic continues to evolve, the search for safe and effective therapeutic interventions remain essential.
Methods: We conducted a retrospective cohort study on patients hospitalized with laboratory confirmed severe acute respiratory syndrome coronavirus-2 infection, comparing standard of care along with Convalescent Plasma with or without Tocilizumab (CP vs. CPT).
Results: A total of 110 patients were enrolled with an overall mean age of 50 ± 16 years. Patients on CPT were more likely to have had acute respiratory distress syndrome (77% vs. 42%; p < 0.001), sepsis (9.7% vs. 0; p = 0.036), chest X-ray abnormalities (71% vs. 44%; p = 0.004), intensive care unit admission (84% vs. 56%; p = 0.001) as well as being on mechanical ventilation (79% vs. 48%; p = 0.001). After CPT treatment, all measured inflammatory markers, except interleukine-6, showed an overall steady decline over time (all p-values <0.05) and the ventilatory parameters showed significant improvement of PaO2/FiO2 ratio from 127 to 188 within 7 days (p < 0.001). Additionally, 52% (32/62) of the patients had favorable outcome, either as improvement of ventilatory parameters or extubation within 14 days of hospitalization. However, mortality rate in those on CPT was higher than those who received CP alone (24% vs. 8.3%; p = 0.041).
Conclusion: In patients with severe COVID-19 infection, using tocilizumab with convalescent plasma is associated with improvement in inflammatory and ventilatory parameters but no effect on mortality. These findings require validation from randomized clinical trials.
Keywords: COVID-19, convalescent plasma, tocilizumab, mortality, cytokine storm, Oman
1. INTRODUCTION
Since December, 2019, a novel coronavirus named Severe Acute Respiratory Syndrome-2 (SARS-CoV-2) causing Coronavirus Disease-2019 (COVID-19) has been spreading rapidly causing significant morbidity and mortality worldwide. The clinical presentation of COVID-19 is highly variable, ranging from asymptomatic to severe pneumonia, Acute Respiratory Distress Syndrome (ARDS) that may progress rapidly to respiratory failure, and even requiring invasive Mechanical Ventilation (MV). To date, 66,252,020 cases and 1,524,768 deaths have been reported globally with a fatality rate around 3.0% [1]. Therefore, identifying risk factors for mortality and developing vaccines and novel therapeutics of severe COVID-19 is extremely important now more than ever as the pandemic is still ongoing despite global efforts.
Patients admitted with severe COVID-19 infection in the Intensive Care Units (ICU) tend to have higher levels of inflammatory cytokines, including: Tumor Necrosis Factor α (TNF-α), Interleukins (IL) 2, 6, 7, and 10, Granulocyte-Colony Stimulating Factor (G-CSF), monocyte chemoattractant protein 1, macrophage inflammatory protein 1 alpha, and interferon-γ-inducible protein 10; an aberrant host immune response suggesting a “cytokine storm” that causes increased alveolar exudate hindering the alveolar gas exchange [2,3] and contributes to the high mortality associated with severe COVID-19 infection [4].
In the absence of specific antiviral drugs against SARS-CoV-2, existing immune modulating therapies have been used to target this excessive inflammatory response [5,6]. A recombinant humanized monoclonal antibody of the IgG1 class, which is directed against both the soluble and membrane-bound forms of the IL-6 receptor, tocilizumab, has been postulated as a potential blocking target to “calm” the cytokine storm in critically ill patients [7–9]. Tocilizumab has shown effectiveness and safety in several case reports and small retrospective studies in severe SARS-CoV-2 [10–17], but to date no published randomized clinical trials. To solidify the evidence on the effectiveness and safety of immunomodulating therapies, 110 patients diagnosed with severe COVID-19 from the Royal Hospital (RH) in Oman were recruited and allocated either to standard care and Convalescent Plasma (CP) or standard care and combination CP with Tocilizumab therapy (CPT). The aim of this study was to assess the role of tocilizumab in improving outcomes including mortality, in a cohort of patients with severe COVID-19 pneumonia who received standard of care treatment and CP.
2. MATERIALS AND METHODS
2.1. Study Design and Participants
We conducted a retrospective observational cohort study at a tertiary care hospital in Muscat, Oman, from April 17th to June 20th, 2020, comparing two intervention groups: standard of care and CP versus standard of care and CPT. Both interventions were given to patients ≥13 years of age, with COVID-19 infection confirmed by the real time-polymerase-chain-reaction (RT-PCR). All the patients were hospitalized with confirmed or imminent respiratory failure and any one of the following conditions:
-
1.
ARDS defined as an acute-onset hypoxemia (the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen [PaO2:FiO2] <300, with >50% bilateral pulmonary opacities on chest imaging within 24–48 h, that were not fully explained by congestive heart failure [18].
-
2.
Severe pneumonia in adults was defined as respiratory infection with fever and one of the following: respiratory rate of >30 breaths/min, severe respiratory distress and SpO2 of <90% on room air [World Health Organization (WHO)/ 2019 – nCoV/clinical/2020.5].
-
3.
Pneumonia in adults was defined as evidence of lower respiratory tract infection, including difficulty in breathing, fast breathing >20 breaths/min, crackles on examination, or new infiltrates on chest X-ray.
-
4.
Critical respiratory condition requiring high-flow nasal cannula, or Non-invasive Ventilation (NIV), MV, or rapidly increasing oxygen requirement.
-
5.
Sepsis was defined as as life-threatening organ dysfunction caused by a dysregulated host response to infection.
-
6.
Septic shock in adults was defined as: persisting hypotension despite volume resuscitation, requiring vasopressors to maintain mean arterial pressure of ≥65 mmHg, and serum lactate level of >2 mmol/L.
-
7.
Multiple Organ Dysfunction Syndrome (MODS) was defined as the progressive, potentially reversible dysfunction of two or more organ systems following acute, life-threatening disruption of systemic homeostasis.
We collected the following data: demographics, baseline characteristics, risk factors, Sequential Organ Failure Assessment (SOFA) score, respiratory parameters (fraction of inspired oxygen (FiO2), Positive End-Expiratory Pressure (PEEP), partial pressure of arterial oxygen (PaO2), and (PaO2)/FiO2) pre-intervention on day 0, and on post-intervention days 3, 7 and 14. Similarly we collected laboratory parameters [Absolute Lymphocytic Count (ALC), C-Reactive Protein (CRP), Lactate Dehydrogenase (LDH), ferritin, d-dimer, IL-6, pH and lactate] pre-intervention on day 0, and on post-intervention days 3, 7 and 14, together with radiological features and clinical outcomes including extubation, improvement without intubation and mortality rates.
Both intervention groups received the standard of care as per the National Guidelines [Ministry of Health, Oman. Human infection with novel corona virus (COVID-19)-Interim guidelines for hospitals, primary care and private healthcare, 2020] and CP as per the RH protocol.
The study was approved by the RH Research and Ethics Committee (SRC#36/2020). We obtained a written informed consent from the patient or (if intubated) through the health proxy. Data availability, after local health authority approval, can be provided upon request.
2.2. CP Protocol
The CP group was part of another clinical study conducted at the RH “Convalescent plasma in the treatment of COVID-19: An Open Label Controlled Clinical Trial”. The study included patients admitted with PCR confirmed COVID-19 pneumonia with one of the following high-risk criteria:
-
•
Critical respiratory condition or rapidly increasing oxygen requirement.
-
•
Signs of severe pneumonia with one of the following additional risk factors for complicated disease: age ≥ 60 years, immunodeficiency, hypertension, diabetes mellitus, Coronary Heart Disease (CHD), Chronic Obstructive Pulmonary Disease (COPD), lymphocyte count <0.8 × 109/L, LDH > 250 U/L, d-dimer >1 μg/mL, serum ferritin > 300 μg/L.
The study exclusion criteria included: known IgA deficiency, hypersensitivity reaction to blood or blood products, past history of severe transfusion reactions, patient’s rejection of plasma therapy, unavailability of matching plasma and more than 14 days of illness. The patients received 200 mL of CP at enrollment (day 0). A second dose was given 24–48 h after the first dose in the following circumstances:
-
(a)
The patient continues to have severe pneumonia and is not showing significant improvement (i.e. persistently febrile, high oxygen requirement, difficulty in breathing).
-
(b)
The patient is in a critical respiratory condition.
2.3. Tocilizumab Protocol
We gave tocilizumab as per the ‘Royal Hospital Protocol for Use of IL-6 Inhibitor Tocilizumab in Severe COVID-19 with Suspected Hyperinflammation’. The clinician initiated tocilizumab after consent from the patient or a health proxy and in consultation with Infectious Diseases Team as indicated below:
-
•
Hospitalized with confirmed critical respiratory condition or rapidly increasing oxygen requirements or severe COVID-19 pneumonia as evidenced with chest X-ray or CT scan and more than of the following: blood oxygen saturation ≤93% and/or PaO2/FiO2 ratio <300 mmHg, and
-
•Established presence of hyperinflammation as per serial monitoring of serum ferritin, CRP, fibrinogen, d-dimer, LDH and IL-6
-
•Ferritin > 300 μg/L (or surrogate) and doubling within 24 h
-
•Ferritin > 600 μg/L at presentation and LDH >250 U/L
-
•Elevated d-dimer (>1 μg/mL)
-
•IL-6 > 80 pg/mL
-
•
We excluded the following conditions from the use of tocilizumab: coexistent infection other than COVID-19; history of severe allergic reactions to monoclonal antibodies, long-term oral medication of anti-rejection drugs or immunoregulatory drugs, neutrophils <500 per μL or platelets <50 × 109; active diverticulitis, inflammatory bowel disease, or another symptomatic gastrointestinal tract condition that might predispose patients to bowel perforation; severe hematological, renal, or liver function impairment [Alanine Aminotransferase (ALT)/aspartate Aminotransferase (AST) ratio >5 upper limit of normal]. Patients with active tuberculosis and other active infection were excluded.
A dose of 4–8 mg/kg of tocilizumab was administered followed by an additional dose after 12 h if no clinical response without exceeding a total of 800 mg.
2.4. Outcomes
We measured the following primary outcomes: extubation rates, discharges from hospital and death rates.
2.5. Statistical Analysis
We used descriptive statistics to describe the data. For categorical variables, we reported the frequencies and percentages and analyzed the differences between groups using Pearson’s χ2 tests (or Fisher’s exact tests for expected cells of <5). For continuous variables, we used mean and standard deviation to summarize the data while performed analyses using Student’s t-test. Laboratory investigations and ventilatory parameters of the CP cohort with and without tocilizumab (CPT) over the course of the hospital admission, as presented in Table 1 as well as in Figures 1–3, were analysed using the repeated measures analysis of variance and the p-values for the differences over time were corrected using the Greenhouse–Geisser correction factor. We considered p < 0.05 as statistically significant. Statistical analyses were conducted using STATA version 16.1 (STATA Corporation, College Station, TX, USA).
Table 1.
Laboratory investigations and ventilatory parameters of the convalescent plasma cohort with and without tocilizumab
| Investigation, mean ± SD | Day 0 | Day 3 | Day 7 | Day 14 | Overall p-value over time | Overall p-value between groups |
|---|---|---|---|---|---|---|
| CP vs. CPT | CP vs. CPT | CP vs. CPT | CP vs. CPT | |||
| WBC count, ×109/L | 8.0 vs. 10.2 | 8.3 vs. 10.4 | 9.5 vs. 12.3 | 10.9 vs. 13.6 | <0.001 | <0.001 |
| ALC, ×109/L | 1.0 vs. 1.3 | 0.9 vs. 1.2 | 1.5 vs. 1.2 | 1.8 vs. 1.9 | <0.001 | 0.027 |
| Hb, g/dL | 12.4 vs. 12.8 | 12.0 vs. 11.7 | 12.0 vs. 11.3 | 10.7 vs. 10.4 | <0.001 | 0.071 |
| Platelets, ×109/L | 264 vs. 299 | 374 vs. 344 | 440 vs. 340 | 427 vs. 297 | <0.001 | 0.471 |
| CRP, mg/dL | 128 vs. 169 | 104 vs. 111 | 89 vs. 45 | 36 vs. 12 | <0.001 | 0.532 |
| Creatinine, μg/L | 81 vs. 96 | 87 vs. 134 | 91 vs. 161 | 77 vs. 132 | <0.001 | 0.009 |
| ALT, U/L | 65 vs. 95 | 79 vs. 98 | 106 vs. 152 | 56 vs. 94 | 0.003 | 0.034 |
| AST, U/L | 82 vs. 91 | 64 vs. 114 | 63 vs. 170 | 32 vs. 66 | 0.176 | 0.026 |
| Total bilirubin, mmol/L | 11 vs. 292 | 23 vs. 47 | 15 vs. 13 | 9 vs. 11 | 0.321 | 0.335 |
| Ferritin, μg/L | 1112 vs. 3090 | 1047 vs. 1531 | 892 vs. 1642 | 634 vs. 857 | 0.029 | 0.042 |
| Corrected calcium, mmol/L | 2.1 vs. 2.0 | 5.8 vs. 3.2 | 2.2 vs. 2.2 | 2.2 vs. 2.2 | 0.050 | 0.822 |
| PO4, mg/dL | 1.4 vs. 1.4 | 1.1 vs. 1.2 | 1.2 vs. 1.5 | 4.2 vs. 5.4 | <0.001 | 0.102 |
| PEEP, cm H2O | 12 vs. 13 | 10 vs. 12 | 10 vs. 11 | 9 vs. 11 | <0.001 | 0.019 |
| FiO2, mmHg | 63 vs. 73 | 47 vs. 56 | 46 vs. 49 | 43 vs. 56 | <0.001 | 0.022 |
| PaO2, mmHg | 88 vs. 79 | 89 vs. 90 | 78 vs. 83 | 79 vs. 90 | <0.001 | 0.499 |
| pCO2, kPa | 39 vs. 47 | 44 vs. 47 | 41 vs. 47 | 48 vs. 47 | <0.001 | 0.027 |
| SpO2, mmHg | 96 vs. 93 | 97 vs. 91 | 94 vs. 95 | 97 vs. 81 | <0.001 | 0.827 |
The analyses were performed using the repeated measures Analysis of Variance (ANOVA) and the p-values for the differences over time were corrected using the Greenhouse–Geisser correction factor.: WBC, white blood cell.
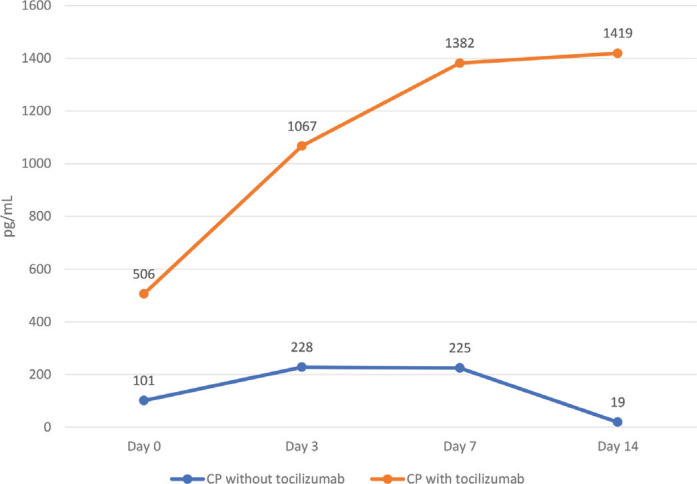
Figure 1.
Interleukin 6 (IL-6) throughout admission in the convalescent plasma (CP) cohort with and without tocilizumab. Notes: The p-value denotes significant differences in IL-6 levels between those on CP alone versus those on CP and tocilizumab over the admission period.
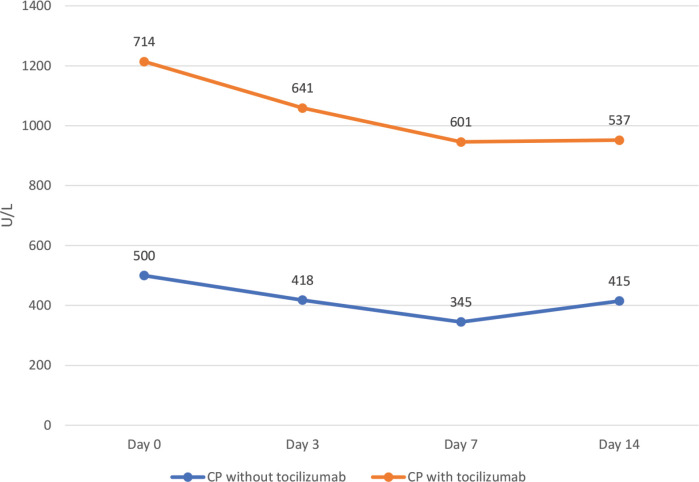
Figure 3.
Lactate Dehydrogenase (LDH) levels throughout admission in the convalescent plasma (CP) cohort with and without tocilizumab.
3. RESULTS
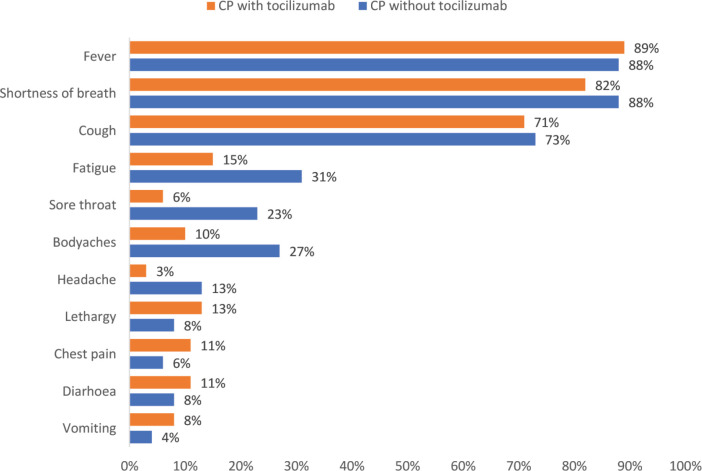
We enrolled a total of 110 COVID-19 patients that were treated, among other standard therapies, with CP, with an overall mean age of 50 ± 16 years and 85% (n = 93) were males. A total of 56% (n = 62) of the patients had tocilizumab added to their medical management. Fever (88%; n = 97), shortness of breath (85%; n = 93) and cough (72%; n = 79), were the three most prominent symptoms observed. Other signs and symptoms, as reported by the patients, are shown in Figure 4.
Figure 4.
Signs and symptoms of the Convalescent Plasma (CP) cohort with and without tocilizumab.
The three most prevalent comorbidities were hypertension (38%; n = 42), diabetes mellitus (31%; n = 34), and chronic heart disease (8.2%; n = 9). The overall median Sequential Organ Failure Assessment (SOFA) score was 2 (1–3) and 62% (n = 68) of the patients had ARDS. A further 10% (n = 11) and 5.5% (n = 5) of the patients had severe pneumonia and sepsis, respectively. Chest X-ray findings indicated major abnormalities in 59% (n = 65) of the patients while 65% (n = 72) of the cohort required MV. Patients that received tocilizumab were more likely males (94% vs. 73%; p = 0.006), having ARDS (77% vs. 42%; p < 0.001), sepsis (9.7% vs. 0; p = 0.036), major abnormalities in their chest X-ray (71% vs. 44%; p = 0.004), ICU care (84% vs. 56%; p = 0.001) as well as on MV (79% vs. 48%; p = 0.001). Other demographic and clinical characteristics are presented in Table 2.
Table 2.
Demographic and clinical characteristics of the convalescent plasma cohort with and without tocilizumab
| Characteristic, n (%) unless specified otherwise | All (N = 110) | Tocilizumab | p-value | |
|---|---|---|---|---|
| No (n = 48) | Yes (n = 62) | |||
| Age, mean ± SD, years | 50 ± 16 | 48 ± 14 | 51 ± 18 | 0.472 |
| Male gender | 93 (85%) | 35 (73%) | 58 (94%) | 0.006 |
| Smoking, past/present | 4 (3.6%) | 2 (4.2%) | 2 (3.2%) | 1.000 |
| Hypertension | 42 (38%) | 19 (40%) | 23 (37%) | 0.790 |
| Diabetes mellitus | 34 (31%) | 14 (29%) | 20 (32%) | 0.728 |
| Chronic lung disease | 2 (1.8%) | 0 | 2 (3.2%) | 0.504 |
| Chronic heart disease | 9 (8.2%) | 4 (8.3%) | 5 (8.1%) | 1.000 |
| Chronic neurological disease | 4 (3.6%) | 3 (6.3%) | 1 (1.6%) | 0.316 |
| Chronic renal disease | 3 (2.7%) | 1 (2.1%) | 2 (3.2%) | 1.000 |
| Asthma | 5 (4.6%) | 2 (4.2%) | 3 (4.8%) | 1.000 |
| ARDS | 68 (62%) | 20 (42%) | 48 (77%) | <0.001 |
| Pneumonia | 46 (42%) | 29 (60%) | 17 (27%) | 0.001 |
| Severe pneumonia | 11 (10%) | 4 (8.3%) | 7 (11%) | 0.753 |
| Sepsis | 5 (5.5%) | 0 | 6 (9.7%) | 0.036 |
| Septic shock | 4 (3.6%) | 1 (2.1%) | 3 (4.8%) | 0.631 |
| Required oxygen therapy | 110 (100%) | 48 (100%) | 62 (100%) | 1.000 |
| X-ray findings | ||||
| Major X-ray abnormality | 65 (59%) | 21 (44%) | 44 (71%) | 0.004 |
| Infiltrations/patchy shadowing | 41 (37%) | 25 (42%) | 16 (26%) | 0.005 |
| ICU care | 79 (72%) | 27 (56%) | 52 (84%) | 0.001 |
| Required mechanical ventilation | 72 (65%) | 23 (48%) | 49 (79%) | 0.001 |
| SOFA score | 2 (1–3) | 2 (1.5–3) | 2 (1–3) | 0.860 |
SD, standard deviation.
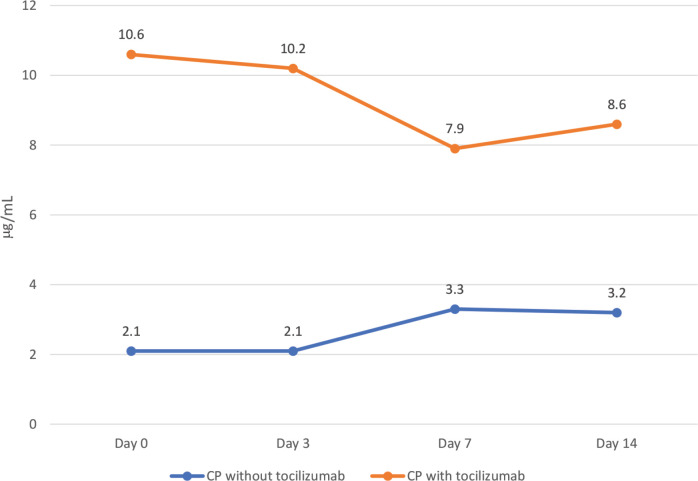
Table 1 outlines laboratory investigations and ventilatory parameters of the cohort. Those patients that received tocilizumab had higher white blood cell counts (p < 0.001), creatinine (p = 0.009), ALT (p = 0.034), AST (p = 0.026) and ferritin (p = 0.042) levels. They also had higher ventilatory parameters: PEEP (p = 0.019), fraction of inspired oxygen (FiO2) (p = 0.022), and partial pressure of carbon dioxide (pCO2) (p = 0.027), when we compared them to those that did not receive tocilizumab. As shown in Figures 2–4, patients treated with tocilizumab had significantly higher baseline levels of the inflammatory markers [IL-6 (p < 0.001), d-dimer (p < 0.001) and LDH (p < 0.001), respectively], however, with the exception of IL-6, all the other inflammatory markers, including CRP, showed an overall steady significant decline over time (with all the p-values <0.05).
Figure 2.
d-Dimer levels throughout admission in the convalescent plasma (CP) cohort with and without tocilizumab. Notes: The p-value denotes significant differences in d-dimer levels between those on CP alone versus those on CP and tocilizumab over the admission period.
Table 3 shows medications and clinical outcomes of the cohort with and without tocilizumab. Patients on tocilizumab were also more likely to be receiving antibiotics; piperacillin-tazobactam (76% vs. 48%; p = 0.003) and meropenem (42% vs. 8.3%; p < 0.001). Furthermore, patients on tocilizumab had longer hospital stay and were less likely to be discharged home (34% vs. 56%; p = 0.019). However, we noted significant improvements in ventilatory parameters as PaO2/FiO2 ratio increased from 127 to 188 within 7 days after CPT therapy (p < 0.001). The mortality rate in patients who received CPT was higher than those who received CP alone (24% vs. 8.3%; p = 0.041).
Table 3.
Medications and clinical outcomes of the convalescent plasma cohort with and without tocilizumab
| Characteristic, n (%) unless specified otherwise | All (N = 110) (%) | Tocilizumab | p-value | |
|---|---|---|---|---|
| No (n = 48) (%) | Yes (n = 62) (%) | |||
| Antibiotic | ||||
| Ceftriaxone | 77 (70) | 37 (77) | 40 (65) | 0.154 |
| Piperacillin | 70 (64) | 23 (48) | 47 (76) | 0.003 |
| Meropenem | 30 (27) | 4 (8.3) | 26 (42) | <0.001 |
| Azithromycin | 5 (4.6) | 1 (2.1) | 4 (6.5) | 0.384 |
| Antiviral | ||||
| Lopinavir/Ritonavir | 89 (81) | 37 (77) | 52 (84) | 0.369 |
| Antimalarial | ||||
| Hydroxychloroquine | 87 (79) | 39 (81) | 48 (77) | 0.624 |
| Intravenous steroids | 63 (57) | 23 (48) | 40 (65) | 0.081 |
| Interferon | ||||
| Interferon Beta 1B | 7 (6.4) | 5 (10) | 2 (3.2) | 0.125 |
| Mechanical ventilation | ||||
| Extubated | 42 (38) | 16 (33) | 26 (42) | 0.430 |
| Improved but still intubated | 8 (7.3) | 2 (4.2) | 6 (9.7) | 0.462 |
| Outcomes (N = 103) | ||||
| Remains hospitalized | 36 (33) | 14 (29) | 22 (35) | 0.542 |
| Discharged home | 48 (44) | 27 (56) | 21 (34) | 0.019 |
| Death | 19 (17) | 4 (8.3) | 15 (24) | 0.041 |
Out of the 110 patients, seven patients were transferred to another health facility or discharged to institutional quarantine.
4. DISCUSSION
We have conducted a retrospective study of 110 patients with severe COVID-19 disease who received CP or CPT in addition to the standard care treatment. All patients required supplemental oxygen and 65% (n = 72) were on MV. Both treatment groups had similar SOFA scores, but patients in the CPT group had significantly more severe disease, particularly more frequently having ARDS and sepsis. Our study showed that male patients with major bilateral chest X-ray changes tend to be at a higher risk of developing ARDS, requiring mechanical ventilation and receiving CPT. Patients with pneumonia and patchy X-ray changes were less likely to develop cytokine storm and probably the combination treatment was not warranted.
In this study, ventilatory and laboratory parameters at baseline were generally very high particularly in the CPT group indicating more severe COVID-19 disease. In fact, during that time of the pandemic, our hospital was receiving mostly the severe and critical COVID-19 infected patients. Concentrations of LDH and CRP in plasma were high, but showed progressive steady decline within 72 h after starting the treatment, and this decline was even more significant by day 14 in both groups. The downtrend of acute phase reactants correlates with patients’ clinical improvement. In contrary, IL-6 levels increased after tocilizumab administration but decreased in patients who received CP. This is because tocilizumab competitively blocks IL-6 receptors and leaves free IL-6 in plasma which remains in circulation for some time [19]. There is no established cutoff value for IL-6 level to predict the likelihood of cytokine release syndrome [10,20]. Our cut off for starting tocilizumab was 80 pg/mL. Longer monitoring and larger sample sizes are required for better understanding of the prognostic role of IL-6 concentrations and other biomarkers in COVID-19 pneumonia treated with tocilizumab.
The overall favorable clinical outcome observed in this cohort could be attributed to several interventions including CP, tocilizumab or steroids. The COVID-19 disease has an initial phase of viral replication along with a second phase determined by the host inflammatory response. In addition to respiratory failure and circulatory shock, SARS-CoV-2 infection induces a dysregulated immune response, so-called cytokine storm, characterized by increased production of many cytokines such as IL-6 and other inflammatory bio-markers that causes lung damage and fibrosis [2,3]. In this study, the combined CPT treatment group did not show the same outcomes as reported in some other studies. This might be attributed to the timing of the intervention and the severity of illness in our cohort. Tocilizumab together with CP can be considered as one of the immunomodulatory interventions to be used at an early stage of the storm which might prevent such complications [21–24].
In our study, all patients received standard treatment according to the (National Protocol for the Management of COVID-19 Infection), including hydroxychloroquine, lopinavir/ritonavir and intravenous steroids as well as oxygen therapy. The results of CPT treatment in terms of inflammatory and ventilatory parameters were encouraging. With the exception of IL-6, the inflammatory markers of almost all patients showed a steady decline. The ventilatory parameters improved as PaO2/FiO2 ratio increased significantly from 127 to 188 within 7 days after the CPT therapy.
In this study, 52% of patients, in those on CPT, had favorable outcomes that included either clinical improvement of ventilatory parameters or extubation within 14 days of hospitalization. Tocilizumab was administered after a median average of 8 (6–10) days after COVID-19 symptom onset, after failure of standard care treatment, in critically ill patients with ARDS and mostly those on MV. The CP group had similar SOFA scores but a higher baseline PaO2/FiO2 values than did the CPT group. Thus, the magnitude of the beneficial effects associated with the use of tocilizumab could have been underestimated. Further studies are needed to evaluate the optimal timing of tocilizumab initiation on the basis of PaO2/FiO2 values and severity of disease stage.
The crude fatality rate in our patients diagnosed with pneumonia and requiring oxygen therapy was 17%. The multicentre cohort study from China reported the fatality rate of 28–52% among hospitalized patients [25]. Moreover, in a study from Italy conducted among 1591 patients admitted to ICU, the fatality rate was 26% [26]. Compared with the CP group (which included pneumonia and severe pneumonia), the mortality rate in our CPT group was higher (24% vs. 8%; p = 0.041) as they were more likely to die from the severity of their illness. However, the mortality rate of CPT group is consistent with those of a smaller, retrospective, case-controlled tocilizumab studies [27,28]. Klopfenstein et al. [29], reported a death rate of 25% in patients who received tocilizumab [29]. A recent report on a randomized clinical trial comparing tocilizumab alone with placebo showed no improvement in clinical status or patient mortality, although showed decrease in length of hospital stay by 1 week which could have a significant clinical impact in resource limited settings. Full results of the trial are not yet published but the key factors that could have influenced the outcomes are the timing of the immunomodulating treatment and the presence of hyper inflammatory response [30].
In this study, 57% of the patients in both groups received intravenous corticosteroids. This could have resulted in the lower mortality rates that was observed. Some studies recommended using the combination of immunomodulatory drugs with anti-inflammatory drugs for the treatment of severe COVID-19 [16]. However, a major concern is the risk of infection, particularly in this critically ill vulnerable group with ARDS and severe pneumonia. An increased incidence of bacterial and fungal superinfections has been reported in some studies with an odds ratio of 3.96 (95% confidence interval: 1.35–11.61: p = 0.003) [31]. Similar to other studies, we noticed a significantly higher rate of sepsis in the CPT than in the CP group, reflected on a high rate of broad-spectrum antibiotics use and may also explain the mortality rate in our cohort. A careful monitoring of infections is extremely important particularly if more than one dose of tocilizumab is given.
Our study has several limitations. First, the study was retrospective with a non-randomized control group, therefore unmeasured confounding factors cannot be ruled out. Second, the participants who received CPT had more severe illness with lower PaO2/FiO2 ratios than the CP group, therefore were at a higher baseline risk of complications and death, though the overall outcomes were similar to other studies. Third, we had a relatively short follow-up duration. As a result, clinical outcomes of some patients who did improve but were still intubated and the outcomes of a number of patients that were still in ICU remains to be known. Fourth, the impact of confounding variables on the outcome in each intervention group, although both cohorts received similar interventions, concomitant use of other drugs may also have influenced the outcomes.
Despite the limitations, our findings have important clinical implications. Our sample size was larger, though no formal analysis was performed, than most published studies and we had lower mortality rates than previous reports [32]. Additionally, the key confounding factors were collected daily in a standardized way on day 0, day 3, day 7 and day 14. It would also have been more appropriate to perform a multivariable Cox regression or logistic regression; however, the sample was smaller for such multivariable modelling.
5. CONCLUSION
Immunomodulatory drugs, have been proposed for managing severe COVID-19 infection. In this study, patients with very severe course of disease and who received tocilizumab showed steady reductions in LDH and d-dimer levels as well as improvement in PaO2/FiO2 ratio. The clinical benefit could have been even more pronounced if tocilizumab was given earlier. These findings require validation from ongoing randomized clinical trials of tocilizumab in COVID-19 patients.
ACKNOWLEDGEMENTS
The authors would like to thank study participants for their corporation and in providing informed consent and other necessary information. We also acknowledge staff at the Royal Hospital who assisted us in the study.
Footnotes
Data availability statement: The data that support the findings of this study are available from the corresponding author [FK], upon reasonable request.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
FK, MAB and HAN participated in the study concepts and design of the study. DAR, ASD, SC, FAF and MAY collected and entered the data. HAN and IAZ analyzed the data. FK and IAZ drafted the manuscript. NP, ZAB, ZAA, IAS and SAH reviewed the findings and contributed to the draft of the manuscript. ZM participated in the intellectual content, reviewing edited and finalized the draft. All authors reviewed, made comments and contributed to the overall study design, implementation, and draft of the manuscript. FK takes the responsibility of the integrity of the work as a whole and he is the point of correspondence.
FUNDING
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
REFERENCES
- [1].World Health Organization (WHO) WHO Coronavirus Disease (COVID-19) Dashboard. Available from: https://covid19.who.int/?gclid=EAIaIQobChMIrJvClL2V7AIVGoBQBh2bDQEcEAAYASAAEgIam_D_BwE (accessed October 2, 2020).
- [2].Zhou Y, Fu B, Zheng X, Wang D, Zhao C, qi Y, et al. Pathogenic T–cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:327–31. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- [4].Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–8. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, Richardson P. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–2. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18:164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–9. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hennigan S, Kavanaugh A. Interleukin-6 inhibitors in the treatment of rheumatoid arthritis. Ther Clin Risk Manag. 2008;4:767–75. doi: 10.2147/tcrm.s3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. Swerdlow DI, Holmes MV, Kuchenbaecher KB, Engmann JE, Shah T, et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214–24. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102568. 102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970–5. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e84. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kewan T, Covut F, Al-Jaghbeer MJ, Rose L, Gopalakrishna KV, Akbik B. Tocilizumab for treatment of patients with severe COVID-19: a retrospective cohort study. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100418. 100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92:814–18. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Radbel J, Narayanan N, Bhatt PJ. Use of tocilizumab for COVID-19-induced cytokine release syndrome: a cautionary case report. Chest. 2020;158:e15–e19. doi: 10.1016/j.chest.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sciascia S, Aprà F, Baffa A, Baldovino S, Boaro D, Boero R, et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020;38:529–32. https://pubmed.ncbi.nlm.nih.gov/32359035/ [PubMed] [Google Scholar]
- [17].Mady A, Aletreby W, Abdulrahman B, Lhmdi M, Noor AM, Alqahtani SA, et al. Tocilizumab in the treatment of rapidly evolving COVID-19 pneumonia and multifaceted critical illness: a retrospective case series. Ann Med Surg (Lond) 2020;60:417–24. doi: 10.1016/j.amsu.2020.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].The ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- [19].Zhang X, Georgy A, Rowell L. Pharmacokinetics and pharmacodynamics of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, following single-dose administration by subcutaneous and intravenous routes to healthy subjects. Int J Clin Pharmacol Ther. 2013;51:443–55. doi: 10.5414/CP201819. [DOI] [PubMed] [Google Scholar]
- [20].Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, et al. Detectable serum Severe Acute Respiratory Syndrome Coronavirus 2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:1937–42. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020;39:2085–94. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Colaneri M, Bogliolo L, Valsecchi P, Sacchi P, Zuccaro V, Brandolino F, et al. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE) Microorganisms. 2020;8:695. doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Issa N, Dumery M, Guisset O, Mourissoux G, Bonnet F, Camou F. Feasibility of tocilizumab in ICU patients with COVID-19. J Med Virol. 2020;93:46–7. doi: 10.1002/jmv.26110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Karekadavath N, Pullichola SB, Muhammed Afsal E, Prabhu ASP, Abdulla MC, Ebrahim SH. Convalescent plasma therapy in first four critically ill COVID-19 patients in Kerala, India. Dr. Sulaiman Al Habib Med J. 2020;2:87–91. doi: 10.2991/dsahmj.k.200908.001. [DOI] [Google Scholar]
- [25].Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. https://pubmed.ncbi.nlm.nih.gov/32171076/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–81. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Capra R, De Rossi N, Mattioli F, Romanelli G, Scarpazza C, Sormani MP, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med. 2020;76:31–5. doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. 105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Klopfenstein T, Zayet S, Lohse A, Balblanc JC, Badie J, Royer PY, et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Méd Mal Infect. 2020;50:397–400. doi: 10.1016/j.medmal.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Furlow B. COVACTA trial raises questions about tocilizumab’s benefit in COVID-19. Lancet Rheumatol. 2020;2:e592. doi: 10.1016/s2665-9913(20)30313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kimmig LM, Wu D, Gold M, Pettit NN, Pitrak D, Mueller J, et al. IL6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections. medRxiv. 2020 doi: 10.1101/2020.05.15.20103531. 2020.05.15.20103531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Quah P, Li A, Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature. Crit Care. 2020;24:285. doi: 10.1186/s13054-020-03006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]