Abstract
Objectives
To assess the prevalence and antibiotic resistance profile of Mycoplasma genitalium detected from urogenital/rectal swab samples obtained from MSM in Tokyo, Japan.
Methods
We performed PCR-based screening for M. genitalium urogenital/rectal infection in 982 asymptomatic MSM between 1 January 2019 and 5 November 2020. Mutations in the antibiotic resistance-associated genes gyrA and parC and the 23S rRNA of M. genitalium were analysed.
Results
The prevalence of M. genitalium infection was 6.1%: the prevalence of rectal and urogenital infection was 4.7% and 1.4%, respectively. Among the cases, 48 were successfully analysed for 23S rRNA, 41 for parC mutations and 37 for gyrA mutations. Macrolide- and quinolone-resistance associated mutations (23S rRNA and parC mutations) were observed in 43 (89.6%) and 28 (68.3%) cases, respectively. The quinolone-resistance associated mutation-harbouring variants also harboured macrolide-resistance associated mutations. The S83I mutation in the parC gene was most commonly identified (24 cases, 58.5%), and its combination with M95I or D99N mutation in the gyrA gene was observed in 9 of 36 successfully analysed cases (25.0%). No significant association was observed between the presence of antibiotic resistance and antibiotic exposure for either macrolides or fluoroquinolones (P = 0.785 and 0.402, respectively).
Conclusions
In Tokyo, there is an alarmingly high prevalence of M. genitalium harbouring macrolide and/or quinolone resistance-associated mutations in MSM, irrespective of antibiotic exposure. The high prevalence of M. genitalium strains with both parC and gyrA mutations limits the efficacy of sitafloxacin. Therefore, suitable alternatives are required to treat such M. genitalium infections.
Introduction
Mycoplasma genitalium causes a sexually transmitted infection (STI). This pathogen was initially identified in a case of non-gonococcal urethritis and it is associated with other urogenital conditions in both men and women.1,2 Frequent reports of asymptomatic M. genitalium infections1,3 have led to speculations that the rectum may be an important reservoir of M. genitalium.3 However, routine screening for M. genitalium is not recommended in asymptomatic individuals, because screening has not been shown to prevent the infection or related complications.4 Moreover, empirical antibiotic treatment for M. genitalium infection or concurrent STIs, like those caused by Chlamydia trachomatis, raises concerns about the spread of M. genitalium strains harbouring macrolide and quinolone resistance-associated mutations.3,5 Azithromycin failure is associated with mutations at positions 2071 or 2072 of the 23S rRNA gene;6 fluoroquinolone failure, especially that of moxifloxacin, is associated with the S83I, S83R, D87N or D87Y mutations in parC and might be associated with the M95I or D99N mutations in gyrA.7,8 Combined mutations in the parC and gyrA genes, such as S83I plus D99N, G93C or M95I, are associated with increased MIC values for quinolones9 and S83I combined with M95I or D99 are more strongly associated with quinolone failure, compared with the S83I mono-mutations.10 In fact, multidrug resistance in M. genitalium has become a global threat. A recent meta-analysis by Machalek et al.11 reported that the rate of macrolide resistance-associated mutations (MRM; in the 23S rRNA gene) in M. genitalium was approximately 10.0%, while that of quinolone resistance-associated mutations (QRM; in parC) was 4.8% before 2010; these rates increased to 51.4% and 9.3% for macrolide and quinolone resistance, respectively, in 2016–17.11 The prevalence of MRM and QRM is higher in the Western Pacific region, including Australia and Japan, compared with that in European countries.11 A more recent study from Belgium on samples collected in 2015–18 showed higher frequencies of 74.3% and 39.7% for MRM and QRM, respectively; 17.8% of the samples harboured S83I mutations.12 However, in individuals with asymptomatic rectal infections, the prevalence of antibiotic resistance-associated mutations remains unclear,3 and rectal infections, rather than urethral infections, may help to better detect circulating strains due to higher prevalence.3 Here, we report the current epidemiology of M. genitalium rectal and urethral infections among MSM in Tokyo, Japan, an endemic area of MDR M. genitalium.
Materials and methods
Study population
We conducted two prospective cohort studies at two outpatient clinics at the AIDS Clinical Center (ACC) and the Sexual Health Clinic (SHC), National Centre for Global Health and Medicine (NCGM), Tokyo, Japan, between 1 January 2019 and 5 November 2020. MSM who regularly visited the ACC (age >20 years) or the SHC (age >16 years) were eligible to participate. The ACC cohort comprised HIV-positive MSM, while the SHC cohort comprised HIV-negative but high-risk MSM who regularly tested for HIV and other STIs. All participants provided written informed consent and completed a questionnaire regarding their recent sexual behaviour. Data on participant demographics were obtained from the medical records. This study was approved by the Human Research Ethics Committee of NCGM (NCGM-G-003350-00; NCGM-G-002091-00) and conducted according to the principles expressed in the Declaration of Helsinki of 1964 and the later amendments.
Detection of M. genitalium and macrolide and quinolone resistance
Ten millilitres of first void urine and/or a rectal swab were collected from each participant and screened for M. genitalium using a commercial PCR assay kit (LSI Medience Co., Tokyo, Japan).13M. genitalium-positive samples were used for resistance detection. Resistance-associated mutations were detected by targeted amplification of the 23S rRNA V domain and of the relevant regions of the gyrA and parC genes, as previously described,10 followed by Sanger sequencing, performed using the ABI PRISM BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Japan) on the Applied Biosystems 3500 Genetic Analyzer (Thermo Fisher Scientific). The sequences were compared with the corresponding target regions of the M. genitalium G37 complete genome (GenBank: L43967.2) to detect and identify mutations.
Statistical analysis
The prevalence of M. genitalium infection was estimated from the number of infected individuals among the study population. Statistical analyses were conducted using STATA V.16 (StataCorp, College Station, TX, USA) and GraphPad Prism V9 (GraphPad Software, CA, USA). The χ2 or Mann–Whitney U tests and the Kruskal–Wallis test were used for the categorical and continuous variables, respectively; P < 0.05 was considered statistically significant.
Results
Participants’ demographic and clinical characteristics
A total of 982 MSM were analysed. The median age was 39 (31–47) years. Among them, 49.2% (483/982) and 50.8% (499/982) were HIV-positive and -negative, respectively. All study participants were asymptomatic, as per the questionnaires or direct examination. The median CD4+ T cell count in patients with HIV (PLWH) was 585.5 (452.5–758) cells/mm3; 94.4% of PLWH showed well-controlled viral replication (HIV-1 RNA < 200 copies/mL).
Prevalence of M. genitalium infection
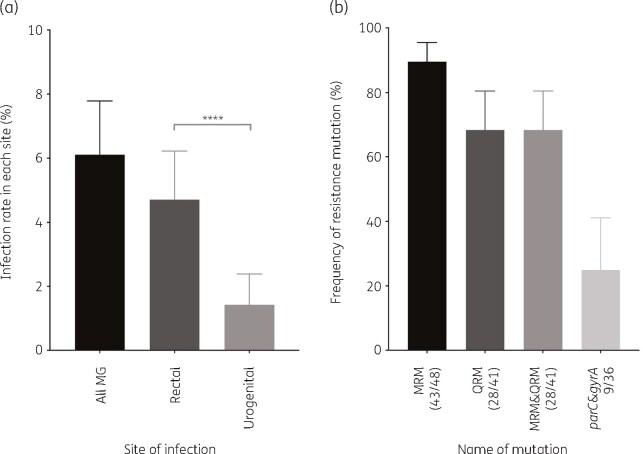
The prevalence of M. genitalium infection among the study participants was 6.1% (60/982). M. genitalium was significantly more prevalent in rectal samples compared with that in urogenital samples (4.7% [46/977] versus 1.4% [14/980], P < 0.0001; Figure 1). No cases of urogenital and anorectal coinfection were observed. The difference in the prevalence of M. genitalium infection between HIV-positive (7.5% [36/483]) and -negative MSM (4.8% [24/499]) was not statistically significant (P = 0.083). The prevalence of rectal and urogenital M. genitalium infections in PLWH was 6.0% (29/482) and 1.4% (7/483), respectively, while that in HIV-negative participants was 3.4% (17/495) and 1.4% (7/497), respectively; the differences were not significant (P = 0.057 and 0.957, respectively).
Figure 1.
(a) Asymptomatic M. genitalium infection rate; ****P < 0.0001. (b) The rate of mutations associated with macrolide and/or fluoroquinolone resistance (MRM or QRM, respectively), defined as parC (excluding D82N) or gyrA mutations. MG, Mycoplasma genitalium.
Resistance
Among the 60 isolates, 48 were successfully analysed for MRM, 41 for parC mutations, and 37 for gyrA mutations (Figure 1). MRM, including A2071G, A2071T, or A2072G, were detected in 89.6% (43/48) of samples. MRM were detected in 89.5% (34/38) and 90% (9/10) of rectal and urogenital samples, respectively. Regarding QRM, 68.3% (28/41) isolates harboured parC mutations: 63.6% (21/33) and 87.5% (7/8) in rectal and urogenital samples, respectively (Table S1, available as Supplementary data at JAC-AMR Online). Most of them (58.5% [24/41]) harboured the S83I mutation. In contrast, gyrA mutations were detected in 27.0% (10/37) of the isolates: 25% (7/28) and 33.3% (3/9) in rectal and urogenital samples, respectively. The S83I in the parC combined with the M95I or D99N mutations in the gyrA were detected in 25.0% (9/36) of the successfully analysed cases. Among the study participants, 28.3% (17/60) with M. genitalium infections had a history of azithromycin therapy, while 6.7% (4/60) had undergone fluoroquinolone therapy in the 90 days before enrolment. However, no significant associations were observed between antibiotic exposure and resistance to either of these antibiotics (P = 0.785 and 0.402, respectively).
Discussion
We found a high prevalence of M. genitalium carrying MRM (89.6%), QRM (68.3%) and dual-class resistance-associated mutations (68.3%) in asymptomatic MSM in Tokyo, irrespective of antibiotic exposure.
Almost all M. genitalium carried MRM, and 68.3% of the detected parC mutations, including S83I, S83R and D87N, limit moxifloxacin-based therapy.7–10 The use of sitafloxacin, an alternative antibiotic for the treatment of resistant M. genitalium strains carrying parC mono-mutations, is approved in some countries, including Japan.9,10,14 However, sitafloxacin-based treatment may not be as effective against the strains co-harbouring the parC and gyrA mutations reported here (25.0%). To the best of our knowledge, this is the highest ever reported prevalence of strains with mutations associated with dual-class resistance. One study has reported a prevalence of 6.1% of dual-class resistance,15 whereas other studies have reported a prevalence of 7.4%–10.4% in the context of triple mutations in 23S rRNA, parC and gyrA.5,16 Another alternative is to focus on quinolone resistance-based treatment strategies; however, the availability of commercial diagnostic tools to investigate quinolone resistance-associated mutations is a major challenge.17 Importantly, both parC and gyrA mutations should be monitored as potential markers of fluoroquinolone treatment failure.
Doxycycline and sitafloxacin combination therapy and pristinamycin exhibit high efficacy in the treatment of resistant M. genitalium.14,18 Therefore, further research on combination therapy using doxycycline, which is a low-cost antibiotic, and new potential drugs such as zoliflodacin, lefamulin and gepotidacin, which are effective against resistant M. genitalium in vitro,19–21 is essential for establishing effective alternate treatments for M. genitalium.
Here, we report M. genitalium infection rates of 4.7% and 1.7% in the rectum and urethra of MSM in Japan. These results are in line with reports from Australia3,22 and Japan,23 with rectal and urethral infection rates of 7.0%–8.5% and 2.7%–4.0%, respectively. The high prevalence of M. genitalium in asymptomatic MSM necessitates considering routine screening in high-risk MSM. However, there are no consistently effective treatments against resistant strains of M. genitalium. Unlike in females, in whom pelvic inflammatory diseases and infertility are linked to infections, there is no sufficient evidence of sequelae to justify routine screening in MSM. Moreover, evidence that macrolides induce selective pressure for resistance-associated strains is limited. Therefore, at present, routine screening of M. genitalium in asymptomatic MSM is not fully justified.4
Urogenital and anorectal M. genitalium infections are more prevalent in HIV patients, compared with those in non-HIV patients;24,25 this was not observed in this study, probably because of the small sample size in this study. Additionally, in contrast to the results of a previous study on symptomatic patients from China,5 no association between resistant strains and antibiotic exposure in asymptomatic patients was observed in this study. Our results imply that MDR M. genitalium strains are already in circulation in Japan. Therefore, there is an urgent need to optimize antibiotic use based on antimicrobial stewardship programmes to prevent the spread of MDR M. genitalium strains.
This study is not without limitations. Not all samples were successfully sequenced; therefore, the reported rates may be slightly different from the actual ones.
In conclusion, we report an alarmingly high prevalence of dual-class resistant M. genitalium in asymptomatic MSM from Tokyo. Moxifloxacin-based therapy may not be sufficient in this context. Therefore, alternative approaches are needed to treat infections caused by MDR M. genitalium.
Supplementary Material
Acknowledgements
We thank Teruya Katsuji, Kunihisa Tsukada, Junko Tanuma and Daisuke Shiojiri for data collection and Yoshimi Deguchi and Mikiko Ogata, the study coordinators, for their assistance.
Funding
This work was supported by the National Center for Global Health and Medicine (grant numbers 19A1002 and 20A1020) and by ViiV Healthcare Supported International Clinical Research (ViiV Ref. 209459).
Transparency declarations
None to declare.
Supplementary data
Table S1 is available as Supplementary data at JAC-AMR Online.
References
- 1. Taylor-Robinson D, Jensen JS.. Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24: 498–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lis R, Rowhani-Rahbar A, Manhart LE.. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 2015; 61: 418–26. [DOI] [PubMed] [Google Scholar]
- 3. Read TRH, Murray GL, Danielewski JA. et al. Symptoms, sites, and significance of Mycoplasma genitalium in men who have sex with men. Emerg Infect Dis 2019; 25: 719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Golden MR, Workowski KA, Bolan G.. Developing a public health response to Mycoplasma genitalium. J Infect Dis 2017; 216 Suppl 2: S420–S426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Y, Su X, Le W. et al. Mycoplasma genitalium in symptomatic male urethritis: macrolide use is associated with increased resistance. Clin Infect Dis 2020; 70: 805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jensen JS, Bradshaw CS, Tabrizi SN. et al. Azithromycin treatment failure in Mycoplasma genitalium-positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin Infect Dis 2008; 47: 1546–53. [DOI] [PubMed] [Google Scholar]
- 7. Murray GL, Bradshaw CS, Bissessor M. et al. Increasing macrolide and fluoroquinolone resistance in Mycoplasma genitalium. Emerg Infect Dis 2017; 23: 809–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Couldwell DL, Tagg KA, Jeoffreys NJ. et al. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS 2013; 24: 822–8. [DOI] [PubMed] [Google Scholar]
- 9. Hamasuna R, Le PT, Kutsuna S. et al. Mutations in ParC and GyrA of moxifloxacin-resistant and susceptible Mycoplasma genitalium strains. PLoS One 2018; 13: e0198355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murray GL, Bodiyabadu K, Danielewski J. et al. Moxifloxacin and sitafloxacin treatment failure in Mycoplasma genitalium infection: association with parC mutation G248T (S83I) and concurrent gyrA mutations. J Infect Dis 2020; 221: 1017–24. [DOI] [PubMed] [Google Scholar]
- 11. Machalek DA, Tao Y, Shilling H. et al. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis. Lancet Infect Dis 2020; 20: 1302–14. [DOI] [PubMed] [Google Scholar]
- 12. De Baetselier I, Kenyon C, Vanden Berghe W. et al. An alarming high prevalence of resistance-associated mutations to macrolides and fluoroquinolones in Mycoplasma genitalium in Belgium: results from samples collected between 2015 and 2018. Sex Transm Infect 2021; 97: 297–303. [DOI] [PubMed] [Google Scholar]
- 13. Takanashi M, Ito S, Kaneto H. et al. Development and clinical application of an InvaderPlus assay for the detection of genital mycoplasmas. J Infect Chemother 2015; 21: 516–9. [DOI] [PubMed] [Google Scholar]
- 14. Durukan D, Doyle M, Murray G. et al. Doxycycline and sitafloxacin combination therapy for treating highly resistant Mycoplasma genitalium. Emerg Infect Dis 2020; 26: 1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deguchi T, Ito S, Yasuda M. et al. Surveillance of the prevalence of macrolide and/or fluoroquinolone resistance-associated mutations in Mycoplasma genitalium in Japan. J Infect Chemother 2018; 24: 861–7. [DOI] [PubMed] [Google Scholar]
- 16. Vesty A, McAuliffe G, Roberts S. et al. Mycoplasma genitalium antimicrobial resistance in community and sexual health clinic patients, Auckland, New Zealand. Emerg Infect Dis 2020; 26: 332–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernández-Huerta M, Bodiyabadu K, Esperalba J. et al. Multicenter clinical evaluation of a novel multiplex real-time PCR (qPCR) assay for detection of fluoroquinolone resistance in Mycoplasma genitalium. J Clin Microbiol 2019; 57: e00886-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Read TRH, Jensen JS, Fairley CK. et al. Use of pristinamycin for macrolide-resistant Mycoplasma genitalium infection. Emerg Infect Dis 2018; 24: 328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Damião Gouveia AC, Unemo M, Jensen JS.. In vitro activity of zoliflodacin (ETX0914) against macrolide-resistant, fluoroquinolone-resistant and antimicrobial-susceptible Mycoplasma genitalium strains. J Antimicrob Chemother 2018; 73: 1291–4. [DOI] [PubMed] [Google Scholar]
- 20. Veve MP, Wagner JL.. Lefamulin: review of a promising novel pleuromutilin antibiotic. Pharmacotherapy 2018; 38: 935–46. [DOI] [PubMed] [Google Scholar]
- 21. Jensen JS, Nørgaard C, Scangarella-Oman N. et al. In vitro activity of the first-in-class triazaacenaphthylene gepotidacin alone and in combination with doxycycline against drug-resistant and –susceptible Mycoplasma genitalium. Emerg Microbes Infect 2020; 9: 1388–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Couldwell DL, Jalocon D, Power M. et al. Mycoplasma genitalium: high prevalence of resistance to macrolides and frequent anorectal infection in men who have sex with men in western Sydney. Sex Transm Infect 2018; 94: 406–10. [DOI] [PubMed] [Google Scholar]
- 23. Kato Y, Kawaguchi S, Shigehara K. et al. Prevalence of N. gonorrhoeae, C. trachomatis, M. genitalium, M. hominis and Ureaplasma spp. in the anus and urine among Japanese HIV-infected men who have sex with men. J Infect Chemother 2020; 26: 403–6. [DOI] [PubMed] [Google Scholar]
- 24. Mavedzenge SN, Weiss HA.. Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis. AIDS 2009; 13: 611–20. [DOI] [PubMed] [Google Scholar]
- 25. Crowell TA, Lawlor J, Lombardi K. et al. Anorectal and urogenital Mycoplasma genitalium in Nigerian men who have sex with men and transgender women: prevalence, incidence, and association with HIV. Sex Transm Dis 2020; 47: 202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.