Abstract
Treatment management of cancer patients in the radiation oncology departments during the current COVID-19 pandemic is challenging. A systematic review of published consensus/guidelines on the role of radiotherapy prioritization, suggested treatment protocols, and set up management was undertaken based on the PRISMA protocol and through PubMed/PMC, Scopus, Google Scholar, Web of Science databases until 01/20/2021. One hundred and sixty-eight publications or regional consensus were included. Summary of recommendations contained: (1) using hypo-fractionated (Hypo-F) regimens for therapeutic/palliative indications, (2) delaying radiotherapy for several weeks or until pandemic over, (3) omitting radiotherapy by replacement of alternative therapies or active surveillance, (4) applying safer patients' setup and preparation protocols, (5) developing telemedicine/telehealth service. To conclude, it is essential to carefully weigh the risk of exposure to COVID-19 infection and the benefit of treating cancer patients during the pandemic. Trying to have a global guideline facing this or any other probable crisis is crucial for health care service.
Keywords: Radiotherapy, COVID-19, Oncology, Pandemic
1. Introduction
The outbreak of coronavirus 2 (COVID-19) is a severe acute respiratory syndrome caused by severe acute respiratory syndrome-related coronavirus-2 (SARS-COV-2). The virus has impacted ordinary everyday life and medical approaches worldwide since about December 2019. Meanwhile, vulnerable patients such as cancerous ones are at substantial risk and need meticulous care to reduce and avoid all the possibilities of contracting the infection. Since the spread of COVID-19 is a severe and long-lasting catastrophe, termination or delay of treatment may jeopardize patient care and health. The radiation oncology centers are endeavoring to present guidelines on coping with this crisis.
There were two severe acute respiratory syndrome-related coronaviruses (SARS-CoV) and middle east respiratory syndrome-related coronavirus (MERS-CoV) in the 2002 and 2012 outbreak before this current pandemic, respectively (Saber Soltani et al., 2020; Hosseiny et al., 2020). However, the SARS outbreak has been controlled, with no human infection reported since 2003, but MERS' small epidemics continue to be notified (Hosseiny et al., 2020). World health organization indicated the initial diagnostic symptoms of this public health emergency as fever and flu-like symptoms and/or breathing difficulty with pulmonary ground-glass opacity (GGO) appearance in the computed tomography (CT) images (Novel Corona Virus Update [Online], 2021).
This rapidly expanding pandemic has impacted all daily life areas, especially the clinical routines of other life-threatening diseases such as cancer and its care in radiotherapy departments. Before the pandemic era, the radiotherapy area was categorized based on the risk of radiation exposure and contamination to controlled and uncontrolled areas (Radiation Protection in the Design of Radiothe and rapy Facilities, 2006). However, this pandemic adds other categorization based on the risk of viral infection. Many recommendations were presented by categorizing the treatment department area, room cleaning, sanitization, or disinfection protocols, staff preparation such as having a different level of protective clothing, protocols on setting treatment appointment time for the suspicious or high-risk patients, and urgent event handling (Wei et al., 2020; Starling et al., 1992).
Immunosuppression in cancer patients makes them more fragile during this crisis, and their treatment has been faced with a severe challenge. As the pandemic becomes more widespread, the population concurrently challenged by cancer and corona will increase across the world undoubtedly (Uzzo et al., 2021). Some recent multi-central studies find no meaningful associations between the COVID-19 mortality with any cancer type and anticancer therapies. In contrast, the other cohort or review ones conclude a higher prevalence and morbidity risk of COVID-19 in the cancer population. Some cohort studies reported a higher fatality rate than the other COVID-19 infected patients (Garassino et al., 2020; Zhang et al., 2020; Kuderer et al., 2020; Lee et al., 2020a; Poortmans et al., 2020; Chakraborty and Pandey, 2020).
Therefore, many departmental consensuses, original articles, rapid reviews, case/case series-reports, editorials, and national and international guidelines were presented in the last months addressing this compromised clinical condition.
Before the outbreak of this pandemic, numerous institutes and healthcare centers applied telehealth services (Parashar et al., 2020; Wright et al., 2020). Developing this service has been highlighted, and it plays an essential role in decreasing unnecessary hospital admission, specifically in the spread of the COVID-19 era (Zhao et al., 2020). This service can be used for online patient's visit and consultation, online image or lab data review (e.g., to minimize the CD handling), online/offline treatment evaluation/verification, and online patient's follow-up using real-time two-way video/audio communication mostly for the cases with low and intermediated priority (Parashar et al., 2020).
However, telemedicine is not a possible option for patients who need radiotherapy as a therapeutic/palliative treatment method. Therefore, radiotherapy (RT) resources and departments have been tried to adjust management protocols to make an optimal decision on delivering the best care to all cancer patients with radiotherapy indications (Slotman et al., 2020).
Rapid recommendations were presented by global resources such as the American Society for Radiation Oncology (ASTRO), European Society for Radiotherapy and Oncology (ESTRO), National Health Service (NHS), Cancer Core Europe (CCE), Royal College of Radiologists (RCR), European Society for Medical Oncology (ESMO), etc. on the patients and staff care and prioritizing the patient's treatment strategies. The foundation of these guidelines has been based on safety, avoidance (RT omission when there is a severe risk of infection and its related morbidity), rescheduling (deferring/delaying RT), and shortening (using hypo-fractionated RT (Hypo-F RT) schedule) (Slotman et al., 2020; Gundavda and Gundavda, 2020). However, these rapid publications of consensus can also be confusing, especially when there is not a gathered and organized schema.
Despite the improvements of cancer care and radiotherapy facilities and knowledge, there are still many limitations in the radiotherapy department centers' infrastructure that do not let them obey some of these recommendations. Therefore, to propose practical solutions, it is necessary to consider the facilities, technologies, and substructures of medical and radiotherapy centers in all countries. For categorizing the recommendations, it is essential to pay attention not only to the prioritizing of patient's cancer stage but also the national-specific RT departments practices, their reimbursement system of healthcare, scientific and experimental preparation of the treatment team, and the impact of national legislations undertaken during the crisis (Achard et al., 2020; Kochbati et al., 2020).
This study aimed to overview the presented guidelines of radiotherapy national/international organizations or individual departments' consensus during this pandemic regarding patient care. This would lead to having a compact and comprehensive radiotherapy database of recommendations for any ongoing crisis that will threaten the healthcare system. Also, any radiotherapy department can choose one of these consensuses that match his facilities and knowledge.
2. Materials and methods
2.1. Searching strategy
To perform this review searching strategy for systematic review was followed, and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) chart was designed (Moher et al., 2009, 2015). Searching was performed through the English language literature using the PubMed/PMC, Scopus, Google Scholar, Web of Science databases up to 01/20/2021.
Using Medical Subject Headings (MeSH), the following search terms were selected for coronavirus: coronavirus, SARS-CoV-2, COVID-19, COVID19, 2019-nCoV, SARS2. The search terms chosen for radiation therapy were: "radiation, radiotherapy, brachytherapy, teletherapy, and intraoperative radiation therapy". These terms were combined using the logical operator of "AND" and "OR" properly to give all relevant publications containing coronavirus in the radiation therapy field. In the Scopus database, the search was through title, abstract, and keywords. In the Pubmed/PMC, it was through the title and abstract. Through title and keyword in Google Scholar, it was through topics and titles in the Web of Science. For Web of Science and Google Scholar, the search results were restricted from 2019 to 2021. Finally, obtained search results were exported, and duplicated records were omitted after merging into EndNote™ (Clarivate Analytics, version X7) reference management software. Then, two of the researchers reviewed the results and removed irrelevant records by inspecting titles independently.
2.2. Inclusion and exclusion criteria
Articles were qualified for inclusion if they contained guidelines, consensus, or recommendations on radiotherapy standards of care for cancer patients during the COVID-19 pandemic. Single or multi-departmental consensus for the treatment of each patient's cancer type was included. Also, international radiotherapy guidelines and review articles that addressed radiotherapy and COVID-19 issue were considered. Published international/national consensus for applying different patient's preparation strategies in radiotherapy departments during the current pandemic also included. The proposed approach for delaying, continuing as pre-pandemic protocols, or deferring the RT techniques/fractionation for each discussed cancer type were addressed. Dedicated priority to choose one of these mentioned approaches confronted with each cancer patient considering his disease stage, age, performance status, and risk of infection was extracted from the published studies. To an article be excluded, both authors had to agree or consult with the third to decide if the literature was not relevant or have some unclear aspect or bias or not containing practical recommendations involving radiotherapy practice during coronavirus crisis. Moreover, publications that addressed all cancer treatment strategies, except radiotherapy, such as surgery, chemotherapy, and hormonotherapy, were excluded. The published studies in journals without peer-reviewing proceedings and the articles that just including reports of case studies or case series were also excluded.
2.3. Study screening and data collection process
A protocol was designed for data extraction following the purpose of this review by three of the authors. Besides, every independently extracted data was discussed later by two of the authors. Conflicts were resolved by referring to the third researcher. Tables and figures were designed by two authors and review by the third one, finally.
Published data were considered and presented in this review, and therefore no approval of a research ethics committee was sought.
3. Results
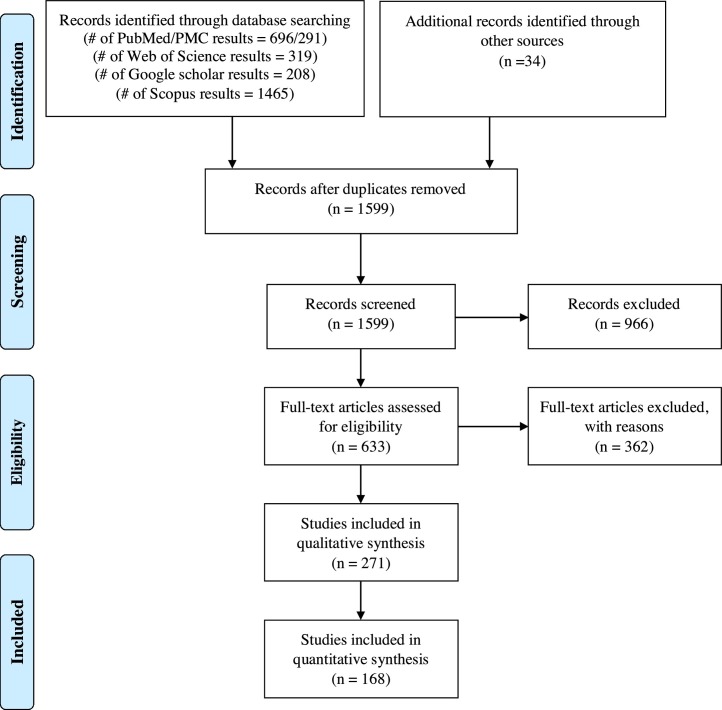
Eventually, considering the explained search, extraction strategy, and inclusion/exclusion criteria yielded 168 involved publications deemed eligible. PRISMA flowchart summarizing the results of the literature search and study selection is illustrated in Fig. 1 .
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flowchart summarizes the literature search results and study selection.
Lots of published recommendations exist to guide radio-oncology teams during the COVID-19 crisis. Recommendations support implementing standard/hypo-fractionation radiotherapy regimens, considering omission of radiotherapy for some cases with a high risk of coronavirus infection, and implementing alternatives to the previous patient's preparation/fixation techniques. Moreover, there was consensus to delay radiotherapy/chemoradiation therapy for those with lesser priority, such as the elderly or fragile case. All of the included recommendations, guidelines, and consensuses are presented in Table 1, Table 2, Table 3, Table 4 .
Table 1.
Summary of international guidelines or national multi-cancer recommendation for teletherapy prioritization during COVID-19 pandemic.
| Cancer type | Hold/Omit irradiation | Delay of radiation if required | Continue irradiation/ Treatment | |
|---|---|---|---|---|
| CNS | Re-irradiation for patients with recurrent GBM | GBM: | ||
| ||||
| GBM: fractionation type depends on KPS (Combs et al., 2020) | ||||
| Asymptomatic meningioma | Low-grade glioma | High-grade gliomas and spine tumors | ||
| Low-grade glioma | ||||
| Pituitary adenoma | ||||
| Craniopharyngioma | ||||
| Benign tumors (with progressive neurologic symptoms) (Wright et al., 2020) | ||||
| Pilocytic Astrocytoma | ||||
| Trigeminal Neuralgia | ||||
| Schwannomas | ||||
| GBM: Age > 60 yrs – methylated | Asymptomatic meningioma, Asymptomatic AVM Asymptomatic schwannoma | GBM: reduction of fractionation (Simcock et al., 2020) | ||
| Low-grade glioma | ||||
| Asymptomatic meningioma Grade I-II and AVM | ||||
| Benign CNS tumor (up to 3months from diagnosis) (Montesi et al., 2020a) | ||||
| Low-grade gliomas (Slotman et al., 2020) | ||||
| Low-grade glioma (as much as possible) | High-grade glioma (Hypo-F RT: 40·5 Gy/15 frs or 25 Gy/ 5 frs) (Starling et al., 1992) | |||
| GBM: | ||||
| Benign tumors |
|
|||
| Low‑grade gliomas | ||||
| Grade I-II meningiomas | Anaplastic astrocytoma | |||
| Recurrent meningiomas | Pineoblastoma | |||
| Schwannomas | PNET | |||
| Pituitary adenomas | Medulloblastoma | |||
| Craniopharyngiomas | Germ cell tumors | |||
| Grade II ependymoma | Anaplastic ependymoma | |||
| Brain metastasis (whole brain: 20 Gy / 5 frs) | ||||
| Oligo brain metastasis with controlled extracranial disease | ||||
| Primary CNS lymphoma (Jalali et al., 2020) | ||||
| GBM: Age > 65 yrs (esp. in poor PS) | Anaplastic oligodendroglioma (up to 4–6 month) | Continue any progressing RT: | ||
| ||||
| High-Grade Glioma: Standard of care (surgical resection followed by RT) | ||||
| Considerable tumor volume (gliomatosis) | ||||
| Involvement of brainstem/spinal cord Grade III astrocytoma | ||||
| Delicate or older patients: Hypo-F accelerated course (34 Gy /10 frs or 40.05 Gy / 15 frs and 25 Gy / 5 frs for smaller tumors) | ||||
| IDH-wild-type and IDH-mutant glioma: shorten RT courses (Vordermark, 2020a) | ||||
| Low-grade glioma | Glioblastoma, Frail/elderly (40 Gy / 15 frs or 25 Gy / 5 frs) (Kochbati et al., 2020) | |||
| asymptomatic meningioma G1–2 | ||||
| GBM: | ||||
| - Aged ≥ 65 yrs with excellent PS: Hypo-F RT (40 Gy /15 frs) | ||||
| - Aged < 65 yrs with good PS (KPS ≥ 70): standard fractionation (60 Gy / 30 frs) | ||||
| -Poor PS (KPS < 50): palliative regimens (34 Gy /10 frs or 25 Gy /5 frs) (Noticewala et al., 2020b) | ||||
| GBM: Elderly with poor KPS/unmethylated | - Grade 1, Grade 2, and Grade 3 meningiomas | - Meningioma: (Hypo-F RT) | ||
| - Schwannomas | Grade 1, Grade 2: 25 Gy / 5 frs | |||
| - Low-grade gliomas | Grade 3: 45 Gy in 15 fractions | |||
| -Schwannomas: frameless SRS/ Hypo-F RT (25 Gy / 5 frs) | ||||
| -GBM: | ||||
| Elderly with poor KPS/methylated: 34 Gy /10 frs or 5 Gy weekly × 6 weeks | ||||
| Younger patients good KPS: Hypo-F RT (60 Gy / 20 frs (SIB technique) | ||||
| -Medulloblastoma: Start with posterior fossa boost and then switch over to craniospinal RT with VMAT/IMRT | ||||
| -Cystic craniopharyngiomas: For all post-op patients, start on RT (Balakrishnan et al., 2020) | ||||
| Asymptomatic meningioma grade I-II | Grade 3 glioma (anaplastic oligodendroglioma) for 4–6 months | Non-co-deleted (anaplastic astrocytoma) | ||
| Asymptomatic AVM | Hypo-F RT: 40 Gy/15 frs or 30 Gy/6 frs (Hinduja et al., 2020) | |||
| Low-grade gliomas | Newly diagnosed glioblastoma, IDH wild-type, the lower WHO grade gliomas, | |||
| Low-grade astrocytoma and 1p/19q co-deleted tumors | IDH-mutant with relevant clinical manifestations, and adult medulloblastoma: | |||
| -Standard RT for younger fit patients with GBM (60 Gy / 30 frs) or Hypo-F RT with 60 Gy / 20 frs (SIB) | ||||
| - Hypo-F RT for poor PS and age> 70 yrs (40 Gy /15 frs or 34 Gy /10 frs) | ||||
| -For medulloblastoma: craniospinal RT (4–6 weeks after surgery) with a possible start of the posterior fossa boost (IMRT or VMAT) (Stepanović and Nikitović, 2020) | ||||
| GBM: 45 Gy/15 frs (Hypo-F RT) | ||||
| cCRT: especially for old-age patients (care of myelosuppression) (Elkhouly et al., 2020) | ||||
| Adjuvant RT: | - SRS for asymptomatic AVM by few months | - Hypo-F RT: high-grade glioma including children with diffuse intrinsic pontine glioma (40 Gy/15 frs in 3 weeks, 30–35 Gy/10 frs in 2 weeks, or even once-weekly | ||
| -Meningioma (benign and atypical) | - Adjuvant RT for primary spinal tumors in minimally symptomatic patients or patients with stable neuro-deficits | - Standard of care RT: Children with medulloblastoma, ependymoma, and intracranial germ cell tumor (Gupta et al., 2020a) | ||
| -Pituitary adenoma, schwannoma, and low-grade glioma | ||||
| Multiple brain metastases | Low grade: RT after 3 months | CNS: No changes | ||
| Hypo-F RT for glioblastoma | ||||
| Cranial Radiosurgery: No changes | ||||
| Brain metastases glioblastomas (Carvalho et al., 2020) | ||||
| Head and Neck | Adjuvant: replace alternatives (prioritize by age and other comorbidities) | Adjuvant: prioritize by age and other comorbidities | Radical: Do not defer until a rationale alternative (Simcock et al., 2020) | |
| Postop RT for salivary gland tumors (up to 12 weeks after surgery) | Definitive RT: SIB techniques (standard or accelerated) (De Felice et al., 2020) | |||
| Keloids | Low-grade unresectable salivary gland malignancies | Radical RT and High-risk postop cases (Wright et al., 2020) | ||
| Small COMS choroidal melanoma | Recurrent parotid/skull base pleomorphic adenoma | |||
| Asymptomatic glomus tumors | Medium-large COMS choroidal melanoma | |||
| Slow-growing small basal cell (with mild or no symptoms) | Symptomatic choroidal melanoma | |||
| Asymptomatic cutaneous (non-pigmented carcinomas located in low-risk anatomic regions) | Symptomatic or secretory paragangliomas | |||
| Symptomatic cutaneous non-pigmented carcinomas | ||||
| High-risk postop cutaneous non-pigmented carcinomas | ||||
| Definitive (reduction of fractionation) (Simcock et al., 2020) | ||||
| Elective priority treatments (Montesi et al., 2020a) | ||||
| COVID-19+ patients (until recovery) | HNSCC: radical RT, post-operative RT for involved margins (Thomson et al., 2020a) | |||
| COVID-19+ patients till recovery | Patients with mild respiratory symptoms | |||
| Delay but not more than 4–6 weeks: | HNSCC as radical RT and postop RT for positive margins (accelerated CRT schedules (6 frs / week), or SIB technique) (Lancia et al., 2020) | |||
| - Oropharyngeal (T2N + M0) | ||||
| - Laryngeal tumor (T3N1M0) | ||||
| - Laryngeal glottic (T1bN0M0) | ||||
| - Metastatic hypopharyngeal (T4N1M1) | ||||
| - Oral cavity (pT2pN2aM0) | ||||
| Continue the standard fractionation scheme (Starling et al., 1992) | ||||
| Palliative RT | High priority: | |||
| Adjuvant RT (lower/intermediate risk of recurrence) | - Curative treatment (Hypo-F RT (65 Gy/30 frs or 55 Gy /20 frs over four weeks rather than 70 Gy / 35 frs) | |||
| - Adjuvant treatment (positive margins): SIB in postop cases | ||||
| - Palliative: short fractionation schedules (25 Gy / 5 frs, 20 Gy / 5 frs, 30 Gy / 6 frs with IMRT, or 8 Gy / 1 fr depending upon clinical scenario) (Roques and Prestwich, 2021) | ||||
| Elderly patients (> 70 yrs): Hypo-F RT or SBRT (35−44 Gy / 5 frs every other day) | ||||
| Oropharyngeal Cancer (early stage): RT only (HPV+) / CRT if not RT alone (HPV-) | ||||
| Laryngeal Cancer: supraglottic/ subglottic, glottic cancers, hypopharyngeal cancers (RT only) | ||||
| Nasopharyngeal Cancer: preferred CRT if not RT alone | ||||
| Salivary Gland Cancer (e.g., parotid cancers): preferred primary surgery otherwise RT or SBRT | ||||
| Oral cavity: surgery if not induction of chemo, pre-op RT, or definitive RT / SBRT (35−44 Gy /5 frs) | ||||
| Postop HNC (For high-risk HNC post-resection, adjuvant RT alone (Parashar et al., 2020) | ||||
| RT plus/minus chemo if it is equal to surgery with adjuvant therapy (Vordermark, 2020a) | ||||
| Radical and postop RT for involved margins with higher priority compared to adjuvant RT for minor risk factors: Hypo-F RT (cCRT: conventional or mildly Hypo-F RT of ≤ 2.4 Gy / fr) | ||||
| Salivary glands of paranasal sinuses (Locally advanced): high-linear energy transfer carbon ions radiotherapy (CIRT): Hypo-F RT of 16 frs over 4 weeks (Ronchi et al., 2020) | ||||
| Non-surgical approach (definitive IMRT) for OSCC: | ||||
| -Accelerated conventional fractionation RT:70 Gy/35frs (over 6 weeks) | ||||
| -Accelerated Hypo-F RT: 60 Gy/25frs (over 5 weeks) | ||||
| -Accelerated HypeF-RT: 64 Gy/40frs (1.6 Gy/fr twice daily, at least 6 hours apart] over 4 weeks) (Hosni et al., 2020) | ||||
| Orbital/intraocular tumors: Frameless Hypo-F image‑guided volumetric modulated arc (stereotactic RT) 25 Gy/5frs over 1 week (Manjandavida et al., 2020) | ||||
| Adjuvant RT: R0 resection and minor risk factor | Post-op RT in patients with salivary gland tumors until 12 weeks after surgery | Curative treatment – High priority patients: | ||
| - Hypo-F RT: 65 Gy /30 frs or 55 Gy / 20 frs over 4 weeks | ||||
| -cCRT | ||||
| -Accelerated fractionation without chemotherapy (6 frs per week) / SIB (Hinduja et al., 2020) | ||||
| Recurrent nasopharyngeal carcinoma: techniques of extreme Hypo-F RT | ||||
| -SRS: 12.5 Gy; 18 Gy | ||||
| -SBRT: 24 Gy /6–8 frs; 18 Gy/3 frs; 48 Gy /6 frs; 34 Gy/2–6 frs; 54 Gy/18 frs; 33 Gy/3–5 frs; 30 Gy /5 frs (Svajdova et al., 2020) | ||||
| Short-course Hypo-F accelerated RT in non-nasopharyngeal HNSCC: | ||||
| stage II-III-IV (55 Gy/20 frs in 4weeks) (Gupta et al., 2020b) | ||||
| Intermediate sinonasal tumors: cCRT or RT | ||||
| Not to delay RT for more than 4–6 weeks (Hypo-F RT): | ||||
| -High priority for treatment: radical RT for HNSCC and adjuvant RT for HNSCC with involved margin / High-growth mass and who undergoing curative radical (chemo) RT | ||||
| -Lower priority: adjuvant RT for HNSCC with minor risk factors | ||||
| -Limited and selected cases of OSCC, T4a laryngeal SCC, and advanced sinonasal malignancy: cCRT or RT | ||||
| -Radical RT in less aggressive cancers (definitive RT or adjuvant RT in rapid proliferating cancers with residue after surgery) | ||||
| -Adjuvant RT incomplete resection patients and palliative RT (lowest priority) (Salari et al., 2020) | ||||
| Oropharynx/larynx: CRT/RT for curative intent | ||||
| Oropharynx (Early stage): RT preferred to surgery | ||||
| Oropharynx (Locally advanced): cCRT | ||||
| Locoregional advanced hypopharyngeal: cCRT (fit patients) | ||||
| Nasopharynx (stage II-IV): NACT followed by CRT (IMRT) | ||||
| Early glottic cancer: RT | ||||
| Oral cavity (early resectable) and high‑risk factors such as margin positivity and perinodal extension: cCRT (definite overall survival benefit) | ||||
| Nasopharynx (stage I): RT (Talapatra et al., 2020) | ||||
| Head‑and‑neck: RT as the main treatment (Carvalho et al., 2020) | ||||
| Hypo-F CRT for head and cancer (68–70 Gy /34–35 frs; 60–66 Gy /30 frs; 55 Gy /20 frs): | ||||
| 65 Gy/30 frs rather than standard fractionation 70 Gy/35 frs | ||||
| Locally advanced laryngeal cancer: 67.2 Gy / 28 frs | ||||
| Hypo-F RT alone: 60 Gy/25frs (T1-T3 N0-N2c HPV + and T1-T2 N0 HPV-) | ||||
| Oropharyngeal patients: 60 Gy/30 frs | ||||
| Hypo-F accelerated RT: 64 Gy / 25 frs | ||||
| Locally advanced disease: IMRT (55 Gy/20 frs) (Vreugdenhil et al., 2020) | ||||
| Breast | Age > 70 yrs: | After breast-conserving surgery | ||
| - Completely excised (margin ≥ 1 mm) | Low-intermediate risk invasive disease (pT 1-2 /pN0) | |||
| - Low-risk invasive disease (pT1/pN0, grades I-II, LVI negative, ER+, HER2-, without extensive intra-ductal component) | DCIS (Koch et al., 2020) | |||
| Age > 55 yrs: | ||||
| - DCIS < 2·5 cm, grades I-II, and margin ≥ 1 mm | ||||
| Adjuvant: replace alternatives (prioritize by age and other comorbidities) | Adjuvant: prioritize by age and other comorbidities (Samiee et al., 2020) | |||
| DCIS (except ER-negative DCIS with positive margin) | Inflammatory BC or mastectomy | Bleeding | ||
| Age > 65 yrs: | Node+: TNBC or HER2+ disease | Painful inoperable local-regional disease | ||
| - Early-stage (grade 1 or 2), less than 30 mm in tumor size, node-negative ER+/ HER2- (adjuvant endocrine therapy) | Post-mastectomy with four or more nodes+ | Symptomatic metastatic disease | ||
| Residual node + disease after NAC | Progression of disease during neoadjuvant chemotherapy (Dietz et al., 2020; Breast cancer in the COVID-19 era [Online], 2021; Luther and Agrawal, 2020) | |||
| PMRT with 1-3 tumor + nodes | ||||
| Node-: TNBC or HER2+ (BCT) Positive margin after BCT for invasive BC with no alternative | ||||
| Age <40 yrs: | ||||
| - BCT, node-negative with >1 additional high-risk features (LVI+, PNI+) | ||||
| - ER- DCIS with a positive margin | ||||
| after surgery | ||||
| Age ≥ 70 yrs | Up to 12 weeks in new patients | Post-mastectomy | ||
| Tumor < 20 mm | Nodal irradiation | |||
| Grade I | After immediate reconstruction: Hypo-F RT | |||
| No angio-lymphatic or perineural invasion | Boost: Hypo-F RT or integrated with whole-breast irradiation | |||
| ER +, PR +, HER2 negative, Ki67 < 10 % | Whole breast and node irradiation: | |||
| Low or medium grade DCIS including non-palpable tumors, | −26 Gy / 5 frs and 29 Gy at the tumor bed with an integrated boost dose of 5.8 Gy (IMRT, VMAT, IGRT) | |||
| size < 25 mm with free margins | Partial irradiation of the breast: | |||
| - Intra-operatively (30 Gy / 5 frs or 37·5 Gy /10 frs twice daily on the tumor bed with negative margin) | ||||
| Pre-op irradiation: | ||||
| −40·5 Gy / 15 frs (54 Gy concomitant boost delivered 3·6 Gy daily) | ||||
| Elderly patients without indication for surgery: | ||||
| - Weekly 6·5 Gy for five weeks for a total of 32·5 Gy (a boost of two 6·5 Gy frs) (Pardoa et al., 2020) | ||||
| Age ≤ 65 yrs (or younger with relevant co-morbidities) | Node negative tumors without boost RT (28–30 Gy in once weekly fr over five weeks or 26 Gy in 5 daily frs over one week) | |||
| An invasive tumor (up to 30 mm) | Breast/chest wall and nodal (moderate Hypo-F RT) (Coles et al., 2020) | |||
| Grade I-II, ER+, HER2- and node- (endocrine therapy) | ||||
| DCIS | ||||
| Boost RT (unless age ≤ 40 yrs, or over 40 yrs with significant risk factors for local relapse) | ||||
| Nodal RT: | ||||
| - Post-menopausal women for T1, ER+, HER2- G 1-2 tumors with 1-2 macro-metastases | ||||
| Boost RT (unless for age ≤ 60 yrs, high-grade tumors, inadequate margins) | ER+DCIS | Intact breast | ||
| Age ≥ 65 yrs: | Invasive breast cancer | Post-mastectomy and/or regional node(RT with moderate Hypo-F RT (42·5 Gy/16 frs or 40 Gy/15 frs) (Achard et al., 2020) | ||
| Invasive breast cancer < 30 mm | ||||
| Clear margins | ||||
| Grade 1-2, ER+, HER2– | ||||
| Node- (planned for endocrine therapy) | ||||
| Low-risk DCIS or active surveillance/ carcinomas (Combs et al., 2020) | ||||
| CALGB/PRIME II | All other | Non-metastatic inflammatory | ||
| ER + DCIS (esp. if take hormone) | Locoregional disease progressing via chemo (Wright et al., 2020) | |||
| Breast Conservation-DCIS | Breast conservation | Partial (APBI) RT or IORT | ||
| Invasive disease Low risk-older patients | Whole breast +/-LN | |||
| Invasive disease Genomic profile low risk, | Whole breast + LNs /Chest wall/ PMRT | |||
| Age ≥ 50 | Chest wall/whole breast/RNI | |||
| ER+, Her2- without other adverse pathologic features | Chest wall/PMRT | |||
| Post Mastectomy: T 1-2 N1 | Postmenopausal ER+/Her2- | |||
| G 1-2, T1, 1–2 SLN (mi) (reduction of fractionation) (Simcock et al., 2020) | ||||
| Early-stage | (Moderate) Hypo-F RT to the chest (Parashar et al., 2020) | |||
| Low-risk elderly breast cancer | ||||
| Boost in selected patients | ||||
| Nodal irradiation in selected patients | ||||
| Elderly patients with low risk of relapse (except for moderately or extremely Hypo-F RT) | Early breast cancer (Low-risk): Postop RT by six months | Moderately or extremely Hypo-F RT regimens (Vordermark, 2020a) | ||
| Up to 3 months from diagnosis to treatment (Montesi et al., 2020a) | ||||
| Elderly patients (underwent adjuvant endocrine therapy) | Adjuvant RT: up to 8 weeks | Moderate Hypo-F RT | ||
| FAST: Once weekly fractions over five weeks (28−30 Gy) | ||||
| FAST-Forward: five daily fractions over one week (26 Gy) (Lancia et al., 2020) | ||||
| Hormone-sensitive stage I and II | Normal fraction: young women (50−66 Gy) | |||
| Hypo-F RT protocol: elderly women (42–53·2 Gy /15–19 frs) (Amaoui et al., 2020) | ||||
| Early-stage breast cancer (Slotman et al., 2020) | ||||
| Adjuvant RT: age ≥ 65 yrs, with T1/T2N0 luminal tumors (endocrine therapy) | Early cases (in situ neoplasia, small invasive carcinomas, luminal tumors): up to 2 months after the surgery | Early cases (in situ neoplasia, small invasive carcinomas, luminal tumors): | ||
| Patients underwent chemotherapy before RT: up to 8 weeks | - IORT or accelerated partial breast RT (if available) | |||
| - Whole breast +/- LN: Hypo-F RT (5frs) (Starling et al., 1992) | ||||
| Breast conservation | ||||
| DCIS | ||||
| Invasive disease | ||||
| Low risk (esp. older patients) | ||||
| Age > 50 yrs, ER+, Her2- | ||||
| Post-mastectomy T1–2 N1 (LN + breast cancer) (Marcus and Mahajan, 2020) | ||||
| DCIS, RH+ | ||||
| Adjuvant: Age < 65 yrs (receiving hormonal therapy | ||||
| DCIS age > 65 yrs (low-risk criteria) (Ismael et al., 2020) | ||||
| Negative axilla | Emergency preop breast RT: 26 Gy / 5 frs +/- Boost (SIB:6 Gy / 5 frs or Sequential 10 Gy / 2 frs) | |||
| Complete response tumor: 26 Gy / 5 frs | ||||
| Palpable tumor: 26 Gy / 5 frs + Boost (SIB:6 Gy /5 frs totally 35 Gy / 5frs or sequential 10 Gy / 2 frs) | ||||
| Negative axilla: Not or 26 Gy / 5 frs to levels 1–4 if node-positive at presentation before primary systemic therapy | ||||
| Positive axilla (N1): 26 Gy / 5 frs to levels 1–4 | ||||
| Positive axilla (N 2-3 +IMN): Standard 3 week RT or 26 Gy / 5 frs to levels 1–4 (Brunt et al., 2021) | ||||
| - Age ≥ 65 years (younger with comorbidities) + invasive breast cancer < 3 cm with clear margins + grade 1/2 + ER + and HER2- + node- planned for endocrine therapy | Neoadjuvant RT (40 Gy in 10 fractions then 30 Gy in 5 fractions over 1 week): | |||
| - Omit boost or shift to Hypo-F RT (except in patients < 40 years age and whom with a high risk of local recurrence) | - Invasive breast cancer with no systemic therapy option | |||
| - Omit nodal RT for Postmenopausal women with T1, grade 1–2, ER+, HER2- a tumor with 1–2 macro metastases requiring WBRT following BCS and sentinel node biopsy | - Completion of all neoadjuvant therapy and triple-negative breast cancer | |||
| - Loco-regional cancer progression/poor response despite the use of all available neoadjuvant therapies | ||||
| Adjuvant RT (26 Gy in 5 daily fractions over 1 week or 28–30 Gy in 1 weekly fraction over 5 weeks): | ||||
| - Others who recognized to need whole or partial breast or chest wall: (Manoj Gowda et al., 2020) | ||||
| Boost: age > 50 yrs with HR + and/or small HER2+ | If the boost is necessary: | HR+, HER2- (Adjuvant setting): 42.6 Gy / 16 frs or 40 Gy / 15 frs (Hypo-F RT) (Raghavan et al., 2020) | ||
| RT in which survival is not affected: | - postponed up to 3 months forhigh-risk | |||
| - age ≥ 65 yrs with an early stage, HR+, HER2-, node-, grade I-II | patients and up to 6 months for low-risk patients | |||
| - after excision of a low-to-intermediate grade | Delay of definitive radiotherapy for good-risk tumors | |||
| ER + DCIS. | ||||
| Postop RT: for several weeks or even months | Adjuvant local RT in early-stage breast cancer: 26 Gy /5 frs over 1 week is non-inferior to 40 Gy / 15 frs over 3 weeks for (UK FAST-forward trial) (Upadhyay and Shankar, 2020) | |||
| boost RT in selected patients | adjuvant RT: up to 3 months after surgery | Hypo-F RT for adjuvant treatment (Ng et al., 2020a) | ||
| Certain non-invasive carcinomas with good prognosis factors (Age > 40 yrs, tumors < 2.5 cm, low and intermediate grade, and sufficient surgical margins ≥ 2 mm) | adjuvant RT: | Adjuvant RT for high-risk BC: | ||
| Age > 65 yrs (or with comorbidities) with invasive BC with good prognostic factors (grade 1–2, hormone-positive, tumors < 3 cm, Node-, HER2-) | -low-risk disease | -Stages T3 or N-positive | ||
| Boost for patients > 40 yrs without risk factors (LVI, high grade, hormone-negative, and positive surgical margins) | -In-situ carcinoma (CIS) by 3–6 months | -Stages T1/T2N0 with risk factors (LVI, high grade, margins+, and HR-) | ||
| For postmenopausal patients > 65 yrs with stage I or II and hormone-dependent disease, or patients with significant comorbidities: by 3–6 months | Hypo-F RT: 42 Gy / 15 frs | |||
| Ultra Hypo-F RT: 28/30-Gy in once weekly fractions over 5 weeks or 26- | ||||
| Gy in 5 daily fractions over 1 week as per the FAST and FAST Forward trials (N- tumors without boost). (Ismaili and El Majjaoui, 2020) | ||||
| Boost: age > 50 yrs with ER+, HER-2- invasive type tumor without other adverse pathologic features | Adjuvant RT: Hypo-F RT (42.4 Gy /16 frs or 40 Gy / 15 frs) and standard regimen (50 Gy / 25 frs) for regional lymph nodes involvement (Mahmoodzadeh et al., 2020) | |||
| Standard BCS RT: age > 70 yrs with small, grade I-II, and HR + tumor | ||||
| RT after excision for low-intermediate grade DCIS, particularly in women over 60 yrs | ||||
| After BCS: | Low-risk elderly (≥65 yrs): | -Patients already on adjuvant RT | ||
| - Low-risk elderly (≥ 65 yrs): WBRT for stage I, ER+/HER2− receiving adjuvant endocrine therapy, without impacting survival | WBRT for stage I, ER+/HER2− receiving adjuvant endocrine therapy, without impacting survival | -Adjuvant postop RT within 2–4 months post-surgery, for high-risk BC patients (inflammatory BC, N-positive, TNBC or HER2+, residual disease after neoadjuvant therapy, young age <40 yrs) | ||
| - DCIS: WBRT, especially for ER + disease receiving adjuvant endocrine therapy, without affecting overall survival. | - Adjuvant postop RT within 5–6 months post-surgery for low/intermediate-risk BC patients (age < 65 yrs and stage I–III luminal cancer, or positive margins), with starting endocrinal therapy (Elghazawy et al., 2020) | |||
| - Invasive disease with low-risk genomic profile | ||||
| -Boost: in invasive disease (except for patient ≤40 yrs or with positive margin) and in situ (except for positive margin; no survival benefit except for high-risk diseaseAfter mastectomy: T 1-2 N+ | ||||
| Breast, Elderly, N- (40 Gy / 15 fr, 28.5 / 5 frs, or 26 Gy / 5 frs) (Kochbati et al., 2020) | ||||
| Abandon RT: | Postpone RT up to 20 weeks after the completion of surgical or systemic treatment: | -Begin RT up to 8 weeks after the completion of surgical or systemic treatment: | ||
| - Patients > 65 yrs, tumors up to 30 mm, N0, ER+, HER2-, G 1-2, margins ≥ 2 mm, DCIS, especially with ER+, patients on hormone therapy. | -Tumor T1, T2, N0 hormone-sensitive, HER2, > 40 yrs, patients on hormone therapy, unfavorable prognostic factors (close margins, G3) | Inflammatory breast cancer, massive metastases to ≥4 lymph nodes, massive LVI, TNBC with N+, yp N+, and regional recurrence. | ||
| -Begin RT up to 16 weeks after the completion of surgical or systemic treatment: | ||||
| T4, TNBC, N0, yp T + and N0, LVI (NOS), Invasive cancer in patients < 40 yrs, ER + with 1–3 N + and other unfavorable prognostic factors (G3, LVI) (Łacko et al., 2020) | ||||
| Good risk DCIS: Low/intermediate grade, < 2.5 cm, margin >3 mm | DCIS: up to 12 weeks | EBC: Young premenopausal women | ||
| EBC: | EBC post BCS: delay RT without chemotherapy up to 20 weeks | Locally advanced breast cancer | ||
| -Age >70 yrs, post BCS - T1, N0, ER+, margins clear | Good risk DCIS: ER/PR+, EBC/DCIS | Boost dose for EBC: | ||
| -Age >65yrs, ER+, N0, T1/T2 (up to 3 cm), clear margins; grade 3 or LVI | ER + disease with N1a nodes (1-3 nodes)/ Node negative TNBC/Pathological N0 post-NACT / LVI | - Hypo-F RT | ||
| Boost dose for DCIS / EBC (>60 yrs) | -SIB or concomitant boost (daily or weekly) | |||
| −5.2 Gy single fraction after ultra- Hypo-F RT | ||||
| Inflammatory breast cancer/Residual nodal disease after NACT/N2 disease (4 or more nodes)/Recurrent disease/Node positive TNBC/Extensive LVI (Hinduja et al., 2020) | ||||
| Adjuvant RT (DCIS): low-risk cases (age ≥ 50 yrs with no necrosis, low grade, small tumor size, at least 2 mm margins) | Adjuvant RT (DCIS): higher-risk cases (Hypo-F RT) | |||
| Invasive breast cancers (node-negative): post-op, patients aged ≥ 65 yrs with HR + tumors | -APBI:40 Gy/10rs, 38.5 Gy/10 frs twice a day over 5–8 days | |||
| -FAST FORWARD regimen for WBI: 26 Gy / 5 daily frs | ||||
| Node negative invasive cancer: | ||||
| -Low-risk patients aged 40–64 yrs (maximum tumor size 3 cm, ER+) | ||||
| APBI: 30 Gy / 5 frs daily (IMRT) or 40 Gy / 10 frs daily (3D CRT) | ||||
| WBI: 40 Gy / 15 frs (standard Hypo-F or FAST FORWARD regimen) | ||||
| During DORSCON Red: APBI using 30 Gy / 5 frs or WBI using 26 Gy / 5 frs | ||||
| Other patients (age ≤40 yrs; or high-risk, age > 40 years; or tumors > 3 cm, high grade, ER-, HER2+ or involved margin), WBI or PMRT for tumors > 5 cm or positive margin): | ||||
| -Standard Hypo-F RT 40 Gy/15 frs or the FAST FORWARD regimen | ||||
| If the boost is indicated: simultaneously (48 Gy /15 frs or sequentially as 10.5 Gy/3 frs | ||||
| During DORSCON Red: WBI or PMRT using 26 Gy / 5 frs | ||||
| Node positive invasive cancer: | ||||
| - N1 disease: adjuvant RT to the breast/chest wall and ipsilateral supraclavicular fossa (and axilla) using standard Hypo-F RT 40 Gy / 15 frs or 26 Gy / 5 frs | ||||
| - Adjuvant RT to IMNC with N2 disease using standard Hypo-F RT 40 Gy /15 frs | ||||
| Boost: simultaneously using 48 Gy / 15 frs or sequentially 10.5 Gy /3 frs | ||||
| During DORSCON Red: adjuvant RT using 26 Gy /5 frs (Chan et al., 2020) | ||||
| All adjuvant RT except high-risk patients (T 3-4, N 2-3, TNBC or young age) | Adjuvant RT: 40 Gy / 15 frs + SIB for BCS (10 or 16 Gy / 5 or 8 frs) (Elkhouly et al., 2020) | |||
| Age ≤ 65 yrs with significant comorbidities with invasive ductal carcinoma ≤ 3 cm, ER/PR+, Her2‑, margin‑free, grade I-II, N-: RT | Breast or chest wall and nodal RT: Moderate Hypo-F RT (40 Gy / 15 frs over 3 weeks followed by boost) | |||
| Age ≤ 40 yrs with relevant comorbidities: Boost RT | Node‑negative tumors: 28–30 Gy once a week (over 5 weeks) or 26 Gy / 5 frs daily (over 1 week) (Talapatra et al., 2020) | |||
| Low-risk elderly (> 70 yrs) with favorable tumors | Postpone RT start up to 16 weeks | Selected patients (> 60 yrs, breast only RT): 26 Gy / 5 frs (Carvalho et al., 2020) | ||
| Interruption for a suspected or confirmed case of COVID-19 (15 days) | ||||
| Whenever possible: up to 12 weeks after surgery | -Foregoing RT: | |||
| Age ≥ 70 yrs, tumor size < 2 cm, grade 1, no signs of poor local prognosis, clean surgical margins, N-, HR+, and HER2-. | ||||
| -RT with ultrashort schemes: | ||||
| Age ≥ 50 yrs, tumor size < 3 cm, pN0, grade I–II, luminal A | ||||
| PBI either by IORT (at the time of lumpectomy/quadrantectomy) or by RT (30 Gy/5 frs and 6 Gy on tumor bed with margin) | ||||
| -Pre-op RT: | ||||
| For older patients: Hypo-F RT (32.5 Gy/5 frs for 5 weeks) with 13 Gy / | ||||
| 2 frs boost | ||||
| Lymph nodes: 27.5 Gy / 5 frs (Martin et al., 2020) | ||||
| Hypo-F breast RT for 1 week (Kwek et al., 2021) | ||||
| Selected patients: 28.5–6 Gy / 5 frs with DIBH over 1–2 weeks (Dong et al., 2020) | ||||
| Lung | Postop RT in NSCLC and PCI in SCLC | RT for curative treatment (stage III NSCLC | ||
| COVID-19 positive patients | LS-SCLC and palliative NSCLC | |||
| Radical RT or sequential CRT for stage III NSCLC (Hypo-F RT) | ||||
| Inoperable Stage I NSCLC: SBRT (Lancia et al., 2020) | ||||
| Consolidation of oligometastatic and oligoprogressive NSCLC (Stage I) | Limited-stage SCLC (Wright et al., 2020) | |||
| SCLC-Extensive | N0-Inoperable (T1-T2 peripheral) | |||
| NSCLC (locally advanced) | ||||
| NSCLC N+ | ||||
| SCLC (Simcock et al., 2020) | ||||
| Lung cancer: concomitant CRT (Hypo-F RT: 55 Gy / 20 frs) (Amaoui et al., 2020) | ||||
| Early-stage (non-biopsied, slow growth, advanced age, or comorbidities) | ||||
| Oligometastatic patients | ||||
| Consolidation RT or PCI in patients with SCLC and extensive disease. | ||||
| PCI in patients with SCLC with limited disease (Starling et al., 1992) | ||||
| SCLC | ||||
| Extensive disease (Marcus and Mahajan, 2020) | ||||
| NSCLC: stage I-II NSCLC (SBRT) stage II (node positive) - III NSCLC, stage IV NSCLC | ||||
| SCLC: limited-stage (stage I-III), extensive-stage (stage III-IV) | ||||
| Palliative RT (Rathod et al., 2020) | ||||
| NSCLC (curative treatment: SABR) | ||||
| Stage I-II patients (1-3 frs): | ||||
| - 30−34 Gy /1 fr for tumors < 2 cm and ≥ 1 cm from the chest wall | ||||
| - 48−54 Gy / 3 frs over one week for peripheral lesions | ||||
| - Mild Hypo-F RT (45–60 Gy / 4–8 frs) for central and ultra-central lesions | ||||
| Stage II-III patients: | ||||
| - 55 Gy / 20 frs | ||||
| Stage III inoperable: | ||||
| - Accelerated Hypo-F RT (45 Gy /15 frs) | ||||
| SCLC (curative treatment: SABR) | ||||
| Stage I-II SCLC (3–5 frs) in peripheral lesions: | ||||
| - 60 Gy /3 frs | ||||
| - 48 Gy / 4 frs | ||||
| - 50 Gy / 5 frs | ||||
| Limited-stage SCLC: | ||||
| - Early or upfront cCRT (thoracic RT / 15 days: 45 Gy / 30 twice daily 1.5 Gy frs) are comparable to the twice-daily regimen: 40−42 Gy /15 daily frs or 50−55 Gy / 20–25 daily frs) | ||||
| PCI: 25 Gy / 10 frs | ||||
| Palliative: | ||||
| - single-fraction RT (8 Gy): For patients with symptomatic (i.e., pain, hemoptysis, etc.) or medical emergency (non-brain) metastasis (SVCO or spinal cord compression) (Liao et al., 2020) | ||||
| 2. SABR for tumors within 2·5 cm of the chest wall: 54 Gy /3 frs (If PTV overlaps the chest wall: 54 Gy / 3 frs or 48 Gy / 3 frs) | ||||
| 3. SABR for moderately central tumors: 50 Gy / 5 frs | ||||
| 4. SABR for tumors >5 cm (treated with caution) | ||||
| 5. Hypo-F RT for central/ultra-central early-stage tumors not suitable for SABR: 50−60 Gy /15 frs | ||||
| Stage III NSCLC (accelerated fractionation ((55 Gy / 20 frs)/ IMRT/VMAT) | ||||
| Early-stage SCLC: SABR for T 1-2 N0M0 | ||||
| Limited-Stage (LS) SCLC (good PS): 40 Gy / 15 frs (Faivre-Finn et al., 2020) | ||||
| Curative-intent RT (reduction of the fraction) | ||||
| Early-stage NSCLC: | ||||
| 1.single-fraction SABR: 30–34 Gy for tumors ≤ 2 cm, > 1 cm from the chest wall | ||||
| Non-surgical treatment (esp. elderly patients with locoregionally advanced tumors or oligometastatic disease) (Vordermark, 2020a) | ||||
| SCLC: | ||||
| -CRT followed by PCI for limited-stage disease | ||||
| -Chemotherapy followed by RT and PCI for extensive-stage disease | ||||
| RT alone if chemotherapy is challenging. | ||||
| Peripheral stage I/IIA NSCLC (SBRT) | ||||
| Stage IIB/III NSCLC: sequential radiation and chemotherapy | ||||
| RT: definitive treatment, pre-op treatment, and postop RT, extra-capsular extension or positive margins, gross residual disease (Parashar et al., 2020) | ||||
| Peripheral early-stage NSCLC (T1-T2): Single-Fraction SBRT (34 Gy / 1 fr vs. 48 Gy / 4 frs) | ||||
| Central Lung Tumors: Multi-fraction SBRT (Sylvia et al., 2020) | ||||
| Treating lung cancer with SBRT in 1–5 frs (Upadhyay and Shankar, 2020) | ||||
| Thoracic consolidation radiotherapy extensive stage | NSCLC: | |||
| -CRT for stage III | ||||
| - Palliative or ablative radiotherapy (SBRT): compression of airways or | ||||
| bleeding | ||||
| SBRT (reduced from 8 frs to 5 or 3) and palliative RT in single or 2 frs (8–10 Gy or 17 Gy, respectively). | ||||
| SCLC: | ||||
| - CRT for limited-stage | ||||
| - Palliative or ablative radiotherapy (SBRT) (Omeroglu Simsek, 2020) | ||||
| Postpone initiation of treatment by 4 weeks: | Use less treatment sessions: | |||
| -Post-Operative Radiotherapy (PORT) NSCLC | - SABR as possible. | |||
| - Prophylactic Cranial Irradiation (PCI) SCLC | - Hypo-F RT regimens (Bakhribah et al., 2020) | |||
| Extensive-stage SCLC: PCI | - Stage I NSCLC: 45–54 Gy /3 frs or 48–50 Gy / 4 or 5 frs or 30–34 Gy /1 fr in select patients (SBRT/ablation) | |||
| - Locally advanced NSCLC (stage III): 60 Gy / 24 frs or 55 Gy / 20 frs or up to 60 Gy / 15 frs (Hypo-F RT schedule) | ||||
| - Limited-stage SCLC: twice-per-day RT (cCRT) | ||||
| PCI for age < 75 yrs (Singh et al., 2020) | ||||
| Extensive SCLC (PCI or palliative intent) | Locally advanced (palliative): | |||
| - 40 Gy / 15 frs | ||||
| - 39 Gy / 13 frs | ||||
| - 16 Gy / 2 frs (Kochbati et al., 2020) | ||||
| SCLC, Extensive: | Stage I-IIIB tumor operated: Short delay in RT if R0 resection | NSCLC, T1/2N0M0, medically inoperable; peripheral: | ||
| - PCI | -SBRT 30−34 Gy/single fr (T1 N0M0) | |||
| - Consolidation thoracic RT in extensive-stage disease | −54 Gy / 3 frs in 1.5 weeks (Eligibility includes T1, 2 (<5 cm), T3 < 5 cm, chest wall involvement positive, no mediastinal or bronchial tree invasion) | |||
| −48 Gy / 4 frs daily RT | ||||
| NSCL, T1/2N0M0, medically inoperable, central: | ||||
| - 60 Gy / 8 daily frs | ||||
| −70 Gy / 10 daily frs | ||||
| −50 Gy / 5 daily frs | ||||
| Stage III, Locally advanced NSCLC: | ||||
| −55 Gy / 20 frs with concurrent /sequential chemotherapy | ||||
| −60 Gy /15-20 frs | ||||
| NSCLC, advanced- inoperable, large for Palliative RT: 8 - 10 Gy/ 1-2 frs | ||||
| SCLC, localized: 40-42 Gy /15 daily frs (Hinduja et al., 2020) | ||||
| Curative treatment for stage III NSCLC: Hypo-F in cCRT strategy (60–66 Gy / 22–30 frs and 50 Gy / 20 frs) | ||||
| Inoperable stage II-III NSCLC | ||||
| Limited stage SCLC | ||||
| Palliative NSCLC (spinal cord compression or SVCO) | ||||
| Early-stage NSCLC: SABR:30–34 Gy /1 fr to 48–54 Gy / 3 frs | ||||
| Central tumors: Hypo-F RT (50–60 Gy /15 frs) | ||||
| Inoperable early-stage NSCLC and operable NSCLC: SBRT | ||||
| Stage II NSCLC: definitive RT (Stepanović and Nikitović, 2020) | ||||
| Adjuvant RT (pathological N2 or R1 post-op): after chemotherapy or 3 months after surgery | Early-stage disease: SBRT for tumors <2.0 cm (a single fraction of 30 − 34 Gy) | |||
| Adjuvant Hypo-F RT: 50 − 60 Gy /25–30 frs | ||||
| Locally advanced disease (clinical stage III): cCRT (mild Hypo-F:50 Gy /20frs) | ||||
| SCLC extensive disease: 45 Gy/15 frs or 30 Gy /10 frs | ||||
| SCLC limited disease: SBRT (Arrieta et al., 2020) | ||||
| Patients with known SARS-CoV-2 or active COVID-19: for a few weeks until resolving symptoms and subsiding inflammation | Lung cancer: IMRT and proton beam therapy (Hwang et al., 2020) | |||
| Delay RT for 1–2 months: sequential CRT instead of cCRT | Lung RT (palliative): 30-39 cGy / 10–13 frs (Elkhouly et al., 2020) | |||
| Delay SBRT for small, slow-growing tumors | SBRT or SABR for early-stage (<5 cm) node-negative NSCLC: | |||
| −50-70 Gy/5-10 frs for central tumors | ||||
| -A single fraction of 24-34 Gy for peripheral tumors < 2 cm | ||||
| Locally advanced lung cancer (stage III NSCLC): Hypo-F RT (55 Gy/20 frs) (Dingemans et al., 2020) | ||||
| Postponing SBRT in indolent tumors | NSCLC: | |||
| NSCLC and SCLC: Interruption for suspected or confirmed case of COVID-19 within 15 days | SBRT: no changes | |||
| Hypo-F for stage III without cC | ||||
| No Postpone RT start | ||||
| SCLC: | ||||
| Limited disease: no changes | ||||
| Extensive disease: PCI and thorax consolidation | ||||
| No Postpone RT start (Carvalho et al., 2020) | ||||
| Extensive stage SCLC: MRI active surveillance instead of PCI (after C) | Adjuvant Post-op RT for R1 resection in NSCLC: at the end of adjuvant C or delayed up to 3 months from surgery (medium priority) | High priority: | ||
| -SCLC limited disease stage I/II and III: cCRT | ||||
| -Inoperable NSCLC Stage III: CRT (Concomitant or sequential) | ||||
| -Inoperable stage II to III: RT (contraindications for C) | ||||
| -Inoperable NSCLC stage II/III and SCLC limited disease: cCRT | ||||
| Adjuvant Post-op RT N2 R0 in NSCLC: at the end of adjuvant C or delayed up to 3 months from surgery (low priority) | - SVCO or significant hemoptysis, spinal cord compression, or any threatening lesion: RT | |||
| Medium priority: | ||||
| -Stage I: SABR or SBRT | ||||
| -Limited SCLC: PCI (after C) (Passaro et al., 2020) | ||||
| ES-SCLC: MRI surveillance | NSCLC: Post-op RT | Stage I NSCLC (SBRT): | ||
| -Safe Zone: 30-34 Gy/1 fr; 54 Gy / 3 frs | ||||
| -Peripheral Lesions: 48 Gy /4 frs | ||||
| -Central Tumor: 50- 60 Gy / 5 frs vs. 60 Gy /8 frs | ||||
| Stage III NSCLC: | ||||
| - CRT: 60-66 Gy /30-33 frs | ||||
| Stage III NSCLC (RT Alone/sequential): | ||||
| - 55 Gy / 20 frs; 45 Gy / 15 frs | ||||
| LS-SCLC: | ||||
| - CRT 60-66 Gy / 30-33 frs over 6- 6.5 weeks, or 45 Gy /30 frs over 3 weeks (twice a day: 1.5 Gy) | ||||
| PCI: 25 Gy /10 frs (Counago et al., 2020) | ||||
| Gastrointestinal | Esophageal | Definitive: CRT (OSCC and OAC) if not Hypo-F RT (50 Gy/16 frs for tumors > 5 cm or 55 Gy /10 frs for tumors > 10 cm) | ||
| Neoadjuvant: Hypo-F CRT (40 Gy /15 frs) | ||||
| Palliative (8 Gy / 1 fr or 20 Gy / 5 frs) (Jones et al., 2020a) | ||||
| Neoadjuvant therapy plus surgery vs. surgery vs. dCRT (Combs et al., 2020) | ||||
| Curative-intent esophageal cancer (Wright et al., 2020) | ||||
| Locally advanced (T2N + or T3+/Nany) operable esophageal carcinoma | ||||
| Neoadjuvant CRT (41·4 Gy / 23 frs or 40 Gy /15 frs) | ||||
| Inoperable esophageal cancer: dCRT (50 Gy / 25 frs) | ||||
| Definitive RT: Hypo-F RT (50 Gy / 16 or 20 frs) | ||||
| Palliative RT (6-8 Gy /1 fr for pain or bleeding, or 20 Gy /5 frs for dysphagia) (Tchelebi et al., 2020) | ||||
| If surgery or cCRT is challenging (RT alone) | ||||
| Pre-op RT just in case of availability of surgery in a few weeks | ||||
| Definitive RT | ||||
| Post-op RT (Parashar et al., 2020) | ||||
| Gastroesophageal junction (Montesi et al., 2020a) | ||||
| Palliative: alternatives to RT | Adjuvant CRT: up to 12 weeks | Priority level 1: Rapidly proliferating tumors currently being treated with radical RT with curative intent | ||
| Priority level 2: Urgent palliative RT (malignant spinal cord compression: 8 Gy / 1 fr or 20 Gy / 5 frs) | ||||
| Priority level 3: | ||||
| - Radical RT for less aggressive tumors | ||||
| - Postop RT (determined residual disease after surgery in tumors with aggressive biology) | ||||
| Priority level 4: Palliative RT (alleviation of symptoms) | ||||
| Priority level 5: Adjuvant RT (Jones and Crosby, 2021) | ||||
| Neoadjuvant chemotherapy or CRT (Vordermark, 2020a) | ||||
| Resectable/ Unresectable (Marcus and Mahajan, 2020) | ||||
| Locally advanced (TanyNanyM0): | Palliative RT (6-8 Gy / 1 fr) (Tchelebi et al., 2020) | |||
| - Neoadjuvant CRT | ||||
| - Adjuvant (Postoperative radiation) | ||||
| Perioperative: neoadjuvant chemotherapy/CRT, adjuvant chemotherapy /CRT | ||||
| Preoperative RT to delay surgery | ||||
| Postoperative RT (Parashar et al., 2020) | ||||
| Non-surgical approach for non-urgent gastrointestinal cancer (Vordermark, 2020a) | ||||
| Adjuvant curative RT (Kochbati et al., 2020) | ||||
| cCRT: 40 Gy / 15 frs | ||||
| For tumor 5 cm in length:50 Gy / 16 frs and up to 10 cm 50-55 Gy in 20 frs (Hinduja et al., 2020) | ||||
| Postpone RT up to 3 months in indolent disease (Carvalho et al., 2020) | ||||
| Gastric | Adjuvant curative RT (Kochbati et al., 2020) | |||
| Operable and resected cases: RT may be avoided | Palliation: Short fractionation schedules (Talapatra et al., 2020) | |||
| Stomach: No neoadjuvant or adjuvant RT | Gastrointestinal: within 3 months | |||
| Stomach: up to 3 months (Carvalho et al., 2020) | ||||
| Pancreatic | Neoadjuvant/adjuvant pancreatic cancer (Wright et al., 2020) | |||
| Unresectable | Locally advanced (Simcock et al., 2020) | |||
| Unresectable (Marcus and Mahajan, 2020) | ||||
| Following resection: | Borderline resectable pancreatic cancer | |||
| - Negative margins: no role for adjuvant radiation therapy | - Neoadjuvant radiation therapy: SBRT (30-33 Gy / 5 frs) without SBRT, 25 Gy / 5 frs, or 30 Gy in 10 frs | |||
| - Positive margins: Adjuvant chemotherapy | Unresectable/locally advanced: | |||
| - Radiation therapy (SBRT/ single fraction (8-10 Gy) for palliation) (Tchelebi et al., 2020) | ||||
| Palliative: alternative non-RT procedure | Pancreatic cancer receiving dCRT (Hypo-F RT/CRT wherever feasible) | |||
| Borderline resectable / resectable patients in lack of surgery (neo-adjuvant Hypo-F RT 25-35 Gy / 5 frs or CRT: 36 Gy / 15 frs) | ||||
| LAPC: Hypo-F CRT (45 Gy / 15 frs) or RT (25-35 Gy / 5 frs) | ||||
| Palliative RT (8 Gy / 1 fr) (Mukherjee and Jones, 2021) | ||||
| CRT: prevent local recurrence (adjuvant) / decrease local progression (locally advanced) | ||||
| Unresected pancreatic adenocarcinomas: short-course SBRT (30-45 Gy / 3 frs or 25-45 Gy / 5 frs) | ||||
| Resectable preoperative CRT: 36 Gy / 2·4 Gy frs | ||||
| Resected pancreatic adenocarcinoma RT (tumor bed, surgical anastomoses, and adjacent lymph node) (Parashar et al., 2020) | ||||
| Locally advanced and borderline resectable: Multi-fraction SBRT (Sylvia et al., 2020) | ||||
| In case of the direct invasion of the bowel and stomach | Locally advanced unresectable pancreatic cancers: | |||
| - Hypo-F RT: 45 Gy/15 frs (cCRT) | ||||
| - Hypo-F RT: 25-35 Gy /5 frs (Hinduja et al., 2020) | ||||
| Palliative (Kochbati et al., 2020) | ||||
| Resected pancreatic cancer: avoided adjuvant RT | Borderline pancreatic cancers: SBRT (Talapatra et al., 2020) | |||
| No neoadjuvant or adjuvant RT | Neoadjuvant SBRT (Carvalho et al., 2020) | |||
| Liver (HCC) | Early-stage HCC, Following resection, Intermediate stage HCC, Locally advanced HCC with vascular invasion (TACE/Y90 or SBRT) | |||
| - Liver metastases: Chemotherapy then resection or RFA or SBRT (Tchelebi et al., 2020) | ||||
| BCLC 0 or BCLC A: SBRT and proton beam therapy | ||||
| BCLC B: RT (e.g., SBRT, proton beam therapy, or systemic RT | ||||
| BCLC C: | ||||
| -RT (45 Gy / 15 frs) | ||||
| -Patients with hepatocellular carcinoma and portal vein thrombosis: SBRT (Barry et al., 2020) | ||||
|
Gallbladder/ bile duct |
Curative-intent gallbladder/bile duct cancer (Wright et al., 2020) | |||
| Operable cholangiocarcinoma | Inoperable cholangiocarcinoma: Induction chemotherapy then RT (Tchelebi et al., 2020) | |||
| Colon | RT for local control and at tumor bed (high-risk diseases, e.g., T4) | |||
| Preoperative (+/- concomitant chemotherapy) or postoperative RT (Parashar et al., 2020) | ||||
| Rectal | Adjuvant: replace alternatives (prioritize by age and other comorbidities) | Adjuvant: prioritize by age and other comorbidities (Samiee et al., 2020) | ||
| Neoadjuvant treatment: Short-course RT (Achard et al., 2020) | ||||
| RT/CRT (Combs et al., 2020) | ||||
| Neoadjuvant/adjuvant | Curative-intent rectal cancer (Wright et al., 2020) | |||
| Stage I disease: | Locally advanced (T2N + or T 3-4 /Nany) operable rectal: | |||
| -Adjuvant (low risk of local failure) | - Neoadjuvant radiation (long-course CRT / short-course RT: 5 Gy / 5 frs) | |||
| - Inoperable: definitive RT | ||||
| - Preference: RT alone (52 Gy / 20 frs or 25 Gy / 5 frs) over long-course CRT (Tchelebi et al., 2020) | ||||
| Elective priority treatments (Montesi et al., 2020a) | ||||
| Locally advanced (short-course RT: (25 Gy / 5 frs) | ||||
| T3N0-2 / T4 (Lancia et al., 2020) | ||||
| Short-course preoperative RT (Starling et al., 1992) | ||||
| Early and Intermediate Rectal Cancer: | ||||
| - SCRT/ CRT | ||||
| - T1/2N0: Hypo-F RT (25 Gy/5 frs) and delay | ||||
| Locally Advanced Rectal Cancer: | ||||
| Non-margin threatening disease: SCRT instead of LCRT | ||||
| In Threatening or involving the margin or pelvic sidewall: | ||||
| -LCRT | ||||
| -SCRT | ||||
| -Delay or SCRT with a period of neoadjuvant chemotherapy (O’Cathail et al., 2020) | ||||
| Post Op RT and palliative RT (if pain controlled) | Post Op RT and palliative RT (if pain controlled) | Colorectal cancer (not elective) | ||
| Palliative RT (if possible): SRS | ||||
| Neoadjuvant treatment of rectal cancer: short-course RT (Marshall et al., 2020) | ||||
| Early / intermediate risk (Muirhead et al., 2021) | ||||
| Stage T3: 6–8 weeks | T3 and M1: a short course of pelvic RT (25 Gy / 5 frs) + surgery (one-week interval) | |||
| Conventional fractionation for postop rectal cancer (tumor bed plus boost) | ||||
| Unresectable cancer: RT alone | ||||
| Protons (Parashar et al., 2020) | ||||
| Long-course CRT (surgery: after 12 weeks) (Vordermark, 2020a) | ||||
| Early-stage: Post-op RT | Low-risk cases | Intermediate risk: SCRT where needed | ||
| Locally advanced: SCRT followed by chemotherapy | ||||
| Adjuvant RT in T4, margin positivity, N2 disease | ||||
| High-risk cases: LCRT (Lewis and Talapatra, 2020) | ||||
| T 1-2 N+/T3N ± (with > 2 mm MRF-D): SCRT (25 Gy /5 frs) | ||||
| T3N ± (with ≤ 2 mm MRF-D)/T4 disease: LCCRT (45–50.4 Gy/25–28 frs) | ||||
| Unresectable: Brachytherapy with a dose of 10–20 Gy / 2–4 frs upon SCRT (Siavashpour et al., 2020) | ||||
| LCRT for threatening margins converted to SCRT: 25 Gy/ 5 daily frs (Hinduja et al., 2020) | ||||
| Possible neoadjuvant SCRT: 25 Gy / 5 frs followed (within 1 week) by surgery (unless T4b or extension into the anal canal) (Elkhouly et al., 2020) | ||||
| Locally advanced rectal cancer: | ||||
| - SCRT (25 Gy / 5 frs) followed by delayed surgery (5–13 weeks) | ||||
| In the case of involved circumferential margin or clinical T4 disease: - LCRT (50.4–54 Gy / 28-30 frs) (De Felice and Petrucciani, 2020a) | ||||
| Neoadjuvant SCRT: 25 Gy / 5 frs (Talapatra et al., 2020) | ||||
| Neoadjuvant RT: 5 Gy / 5 frs (followed by C between RT and surgery) (Carvalho et al., 2020) | ||||
| Curative-intent anal cancer (Wright et al., 2020) | ||||
| Anal | Local or locally advanced (TanyNanyM0) | |||
| All non-metastatic cases (CRT) (Tchelebi et al., 2020) | ||||
| Elective priority treatments (Montesi et al., 2020a) | ||||
| dCRT: current standard of care | ||||
| Elderly patients (poor PS): less intensive treatment schedule: | ||||
| - Hypo-F RT 30 Gy /15 frs (cCRT) (O’Cathail et al., 2020) | ||||
| Standard radical CRT (Hypo-F RT: (30 Gy /15 frs or 30 Gy /10 frs) (Muirhead et al., 2021) | ||||
| Standard treatment: cCRT | ||||
| Low-risk/ high-risk elective nodal PTV | ||||
| T 1-2 lesions with residual disease, T 3-4 lesions, or N1 lesions | ||||
| Protons (Parashar et al., 2020) | ||||
| Non-metastatic cases: | ||||
| a) cCRT: standard fractionation schedules | ||||
| b) No cCRT: moderate Hypo-F RT (50 Gy/20 frs) (Talapatra et al., 2020) | ||||
| Where RT is the main treatment: No changes/ no postpone RT (Carvalho et al., 2020) | ||||
| Genitourinary | Renal Cell Carcinoma (RCC) | Unresectable: 26 Gy / 1 frs or 14 Gy / 3 frs | ||
| Poor surgical candidates: 25 Gy / 1 frs | ||||
| Medically inoperable: 24-48 Gy / 4 frs or 21-48 Gy / 3 frs (Parashar et al., 2020) | ||||
| Primary RCC in unresectable or comorbid patients: single-fraction SBRT (Sylvia et al., 2020) | ||||
| Bladder | Curative-intent bladder cancer (Wright et al., 2020) | |||
| Muscle invasive (CRT) (reduction of fractionation) | ||||
| Muscle invasive, N0 – Bladder only (reduction of fractionation) (Simcock et al., 2020) | ||||
| Radical RT (shorten treatment schedule: 55 Gy /20 frs) | ||||
| Palliative RT: | ||||
| - Improvement of local symptoms (21 Gy / 3 frs) | ||||
| - Good local control (36 Gy / 6 frs) | ||||
| - Bleeding or local symptom control (8-10 Gy / 1 fr) (Birtle et al., 2021) | ||||
| Unresected bladder cancers (Whole bladder +/_ pelvic nodes): | ||||
| - Conventional or accelerated Hypo-F RT +/- boost ((55 Gy / 20 frs) or SIB to gross sites) (Parashar et al., 2020) | ||||
| No changes of RT: Hypo-F RT for bladder | ||||
| No Postpone RT start | ||||
| No interruption if the patient is a suspected or confirmed case of COVID-19 (Carvalho et al., 2020) | ||||
| Prostate | If an alternative exists (Prioritize by age and other comorbidities) (Samiee et al., 2020) | |||
| Low and favorable intermediate-risk (primary setting if not detrimental) | High-risk: RT plus androgen deprivation (No shift towards increased use of extreme Hypo-F RT) (Achard et al., 2020) | |||
| Low risk (using ADT, active surveillance, or hormonal deprivation) (Combs et al., 2020) | ||||
| Low risk: Active surveillance | Intermediate and high risk: delay of radical treatment by neo-adjuvant hormonal therapy strategies. | Early salvage RT over adjuvant RT after radical | ||
| Shorter RT regimen (60 Gy / 20 frs or even 5-6 frs in total) (Lancia et al., 2020) | ||||
| All other curative-intent prostate cancers | Curative-intent high-grade prostate cancer (Wright et al., 2020) | |||
| Low- favorable intermediate-risk | Unfavorable intermediate/high/very high risk, Postop | Reduction of fractionation: | ||
| Intermediate/high risk, | ||||
| Prostate only | ||||
| High risk or M1 | ||||
| Low/intermediate risk | ||||
| Post-prostatectomy, Fossa only (Simcock et al., 2020) | ||||
| Up to 3 months (from diagnosis to treatment) (Montesi et al., 2020a) | ||||
| Low- risk and intermediate-to-low risk: active surveillance | Intermediate-to-high and high risk | Hypo-F RT: | ||
| Salvage RT up to 1 month | - 60 Gy / 20 frs | |||
| Oligometastatic patients (low-volume M1) with an indication for local RT: during hormonotherapy | - If CBCT or fiducial markers exist: 42 Gy/7 frs or 36 Gy / 6 frs, or 36·25 Gy /5 frs. | |||
| Oligometastatic patients: 36 Gy / 6 frs (Starling et al., 1992) | ||||
| Low risk; intermediate risk, high risk (Slotman et al., 2020) | ||||
| Low, favorable intermediate-risk (Marcus and Mahajan, 2020) | ||||
| Very low-/ low-/favorable intermediate-risk disease | ||||
| Unfavorable intermediate-/high-/very high-risk | ||||
| Post-prostatectomy | ||||
| Clinical node-positive | ||||
| Oligometastatic | ||||
| Low volume M1 (Zaorsky et al., 2020) | ||||
| Oligometastatic HSPC | Localized low-risk (very low-, low- and favorable-intermediate-risk) | Localized high-risk (unfavorable-intermediate-risk, high-risk, and very high-risk) | ||
| Oligometastatic HSPC | Advanced (clinical nodal involvement, BCR post-primary treatment, metastatic disease): | |||
| - Early salvage RT over adjuvant RT | ||||
| - Node-positive prostate without metastases: ADT and Hypo-F RT | ||||
| - Painful bone metastases or bone metastases at high risk of fracture (weight-bearing bone): short-course palliative RT (Kokorovic et al., 2020) | ||||
| Low/very low risk | Intermediate-risk | Localized high-risk and very high-risk diseases with positive ganglions: (neoadjuvant androgen deprivation therapy) (Ismael et al., 2020) | ||
| (No rush to initiate any prostate RT) | High priority: symptomatic palliative /radical high-risk/prostate bed | |||
| Receiving neo-adjuvant hormonal therapy and not commenced RT | Low priority: radical low/intermediate-risk prostate (Alonzi et al., 2021) | |||
| Low, Intermediate, and High-Risk Prostate Cancer: | ||||
| - Moderate Hypo-F RT: 60 Gy / 20 frs, 70·2 Gy / 26 frs, or 70 Gy / 28 frs | ||||
| - Conventional fractionation: 66·6 - 90 Gy / 37 – 45 frs | ||||
| - Ultra- Hypo-F RT: 36·25 - 40 Gy / 5 frs or 30·5/5 frs (Parashar et al., 2020) | ||||
| Moderate and extreme Hypo-F RT (Sylvia et al., 2020) | ||||
| Low risk: kept on surveillance, no urgency in therapy | ||||
| Localized prostate cancer in the primary or postop: for several weeks or even months (Upadhyay and Shankar, 2020) | ||||
| Very Low Risk -Low Risk and Intermediate Risk: | ||||
| - 78 Gy/39 frs (Conventional Fractionation) | ||||
| - 60 Gy/20 frs or 70 Gy/28 frs (Moderate Hypo-F RT) | ||||
| - 44.8 Gy/8 frs (Ultra- Hypo-F RT with MRIdian) | ||||
| - 35–40 Gy/5 frs (Ultra- Hypo-F RT with CyberKnife) | ||||
| Intermediate Risk and High - Very High Risk: | ||||
| - 78 Gy/39f (Conventional Fractionation) | ||||
| - WPRT 46 Gy/23frs or 37.5 Gy/15frs + high dose-rate interstitial brachytherapy (HDR-ISBT) boost 15 Gy/1 fr (Murakami et al., 2020) | ||||
| Brachytherapy | Low-volume metastatic: RT postponed until after the pandemic | Unfavorable intermediate-risk and High risk-Very high risk: Neoadjuvant RT (preferably Hypo-F and without fiducial marker or rectal spacer insertion) (Obek et al., 2020) | ||
| Multiple neoplasms: | Multiple neoplasms: | Multiple neoplasms: Hypo-F RT | ||
| -Omit RT in low and favorable intermediate-risk and for oligometastatic prostate cancer | -Postop RT for 2 weeks / Prostate cancer under ADT for 2 weeks | Unfavorable intermediate risk: 36.25-40 Gy / 5 frs or 60 Gy/20 frs | ||
| -Delay RT for low/intermediate-risk prostate disease | High and very high risk: 60 Gy / 20 frs or 42.7 Gy/ 7 frs every other day (if age < 75 yrs) or 36.25-40 Gy / 5 frs | |||
| Prostate: Delay RT for very low, low, and favorable intermediate-risk disease | N+: 36.25-40 Gy/5 frs or 60 Gy/20 frs | |||
| Post-prostatectomy/salvage: 52.5 Gy/20 frs (Caicedo-Martínez et al., 2020) | ||||
| Ultra- Hypo-F RT in low/low-intermediate risk: 36.25 Gy/5 frs (Griffiths et al., 2021) | ||||
| Extreme Hypo-F: 36 Gy /6 frs for elderly, frail, or metastatic patients (Martell et al., 2020) | ||||
| Very low/low risk | Favorable Intermediate risk | Unfavorable Intermediate risk/High/very high risk/N+: | ||
| -Modest Hypo-F RT: 60 Gy/20 frs | ||||
| -Ultra Hypo-F: 42·7 Gy/7 frs every other day or 36 Gy/6 frs (6 weeks) | ||||
| -SBRT: 5 frs | ||||
| -Adjuvant RT: Standard (33-35 frs) / Hypo-F RT (60 Gy/20 frs) in high-risk features | ||||
| Oligometastatic: SABR (1 or 3 frs) | ||||
| Low volume M1: 5 or 6 frs (Hinduja et al., 2020) | ||||
| Radical treatment: up to 6 months if the patient receiving hormonal therapy | Possible Hypo-F: 60 Gy / 20 frs (IMRT) (Elkhouly et al., 2020) | |||
| Very low‑risk | Low‑risk and favorable intermediate‑risk | Low‑risk and favorable intermediate‑risk; unfavorable intermediate‑risk, high‑risk, very high‑risk, and N + patients: 5 /20 frs | ||
| Adjuvant/salvage RT: 20 frs | ||||
| Oligometastatic + low volume metastatic disease: 3–5 frs RT (Talapatra et al., 2020) | ||||
| Elderly with favorable tumors | Low or intermediate-risk in hormone therapy, and high risk with only one risk factor: RT after 3 months | Favor Hypo-F (Carvalho et al., 2020) | ||
| Postpone RT start | ||||
| Interruption for a suspected or confirmed case of COVID-19 within 15 days | ||||
| 20 frs instead of the conventional 37 frs regimen (Kwek et al., 2021) | ||||
| Testicular | Seminoma, stage I (Simcock et al., 2020) | |||
| Gynecological | Cervical | Radical treatment: Do not defer until a reasonable alternative (Samiee et al., 2020) | ||
| Postop cervical cancer (up to 8 weeks) | Cervical cancer with extreme bleeding (Wright et al., 2020) | |||
| Postop vaginal brachytherapy alone (up to 4–8 weeks) | ||||
| All patients with curative intent RT: | ||||
| -Early-stage: definitive RT | ||||
| Pelvic RT remains the selective treatment (Reduction of fraction/preferred IMRT and SIB otherwise conformal RT esp. for node-negative) (Guidance for radiotherapy for gynaecological cancer and COVID-19 [Online], 2021) | ||||
| Pre-invasive, early-stage, locally-advanced disease: Hypo-F RT (Ramirez, 2020) | ||||
| Reduction of chemotherapy dose, plus RT or RT alone. | ||||
| Intact cervix: | ||||
| - Definitive RT (40 - 50 Gy) | ||||
| - IMRT or SBRT boost (Parashar et al., 2020) | ||||
| RT and cCRT (substitute surgery) (Vordermark, 2020a) | ||||
| Up to 8–12 weeks: | Locally advanced | |||
| - Inoperable cases or refuse surgery (Stage IA1, IA2) | Inoperable cases (Stage IB3-IVA or Stage IB1-IIA1) | |||
| - Postop (Stage IA1-IB2) with indication for adjuvant RT | Extreme bleeding secondary to cervical cancer (Elledge et al., 2020) | |||
| - Postop cases with positive pelvic (or PA nodes), surgical margins, or parametria (CRT) | ||||
| - Metastatic disease with annoyance oral pain or minimum bleeding (palliative RT) | ||||
| Locally advanced: standard fractionation (46-50 Gy) followed by brachytherapy (HDR) (Amaoui et al., 2020) | ||||
| Not to change or postpone the fractionation (Starling et al., 1992) | ||||
| Adjuvant therapy:12 weeks for adjuvant RT and 8 weeks for adjuvant CRT) | Early-stage disease: Radical CRT (prolonged delay of surgery >8 weeks) | |||
| Local symptomatic central or para-aortic recurrence: Salvage RT | ||||
| Locally advanced disease ((IB3-IVA) | ||||
| - Hypo-F RT (39 Gy/13frs or 39–40 Gy at > 2.5 Gy per fraction in combination with concurrent chemotherapy) | ||||
| -IMRT: 40 Gy/15 frs to the whole with 48 Gy/15frs SIB to enlarged nodes (Dewan et al., 2021) | ||||
| Postop status - the intermediate risk of recurrence: (cC)RT (up to 8 weeks after surgery) | Stage IB1, IB2, and IIA1: Neoadjuvant RT | |||
| Postop status - high risk of recurrence: cCRT | ||||
| Stage IB3 and IIA2: Hypo-F RT | ||||
| Locally advanced (IIB–IVA): Hypo-F cCRT | ||||
| Cervical stump recurrence: RT | ||||
| Local recurrence within pelvis: cCRT | ||||
| Pelvic sidewall recurrence: RT (Lee et al., 2020b) | ||||
| Patients with suspected or confirmed COVID-19 (until COVID-19 is cured) | In the case of RT is the primary treatment | |||
| For patients with minimal risk of COVID-19 | ||||
| Adjuvant RT: postponed within 12 weeks after surgery | Emergency cases (Wang et al., 2020) | |||
| Stages IB3, IIA2-IIIC2, and early IVA (cCRT): | ||||
| −50.4 Gy /28 frs (bulkier or node-positive) with 3DCRT | ||||
| −45 Gy / 25 frs with SIB to gross nodes | ||||
| −5 5-6 2.5 Gy / 25 frs with IMRT | ||||
| -RT boost (18 Gy / 10 frs) in the absence of brachytherapy | ||||
| Stages IA1, IA2, IB1, IB2, IIA1(cCRT for high-risk patients): 45 Gy / 25 frs with IMRT; if resource constraints, 3DCRT | ||||
| IVA (frank bladder or rectal infiltration) or IVB (palliative RT): 8 Gy/1 fr or 20 Gy/5frs (Hinduja et al., 2020) | ||||
| Stage II or III cervix with a radical/curative intent: Radical CRT (Talapatra et al., 2020) | ||||
| Uterine cervix: RT as the main treatment | ||||
| No changes, no postpone RT start, no interruption for suspected or confirmed case of COVID-19 (Carvalho et al., 2020) | ||||
| High priority: | ||||
| Locally advanced cervical cancer (stages IB3, IIA–IIB): Pelvic CRT | ||||
| Medium priority: | ||||
| Symptomatic localized recurrence (central, retroperitoneal lymph nodes): salvage RT (Colombo et al., 2020) | ||||
| a) Cervical dysplasia & cancer: Definitive RT over radical surgery b) Locally advanced cervical cancer: RT (without delay) (Alkatout et al., 2020) |
||||
| Endometrial | Adjuvant: if an alternative exists (prioritize by age and other comorbidities of the patient) (Samiee et al., 2020) | |||
| Postop endometrial (scheduled for initiation chemotherapy) | Inoperable endometrial cancer | |||
| Postop endometrial cancer (Wright et al., 2020) | ||||
| Adjuvant RT: up to 3 months from surgery (unless there is a residual disease, positive resection margins, or aggressive histological subtype) | Locally advanced and high-risk groups (Hypo-F RT) (Guidance for radiotherapy for gynaecological cancer and COVID-19 [Online], 2021) | |||
| Microscopic disease: 45 - 50 Gy / 25 frs | ||||
| Gross residue in postop cases (add boost: a total dose of 60 -70 Gy) | ||||
| Neoadjuvant RT: 45 - 50 Gy (Parashar et al., 2020) | ||||
| Postop stage IA, grade I-II endometrioid carcinoma with higher risk features (age > 60 yrs, LVSI) | Postop stage IA, grade III or stage IB, grade I-II, and low-risk stage II endometrioid carcinoma | Patients with extreme vaginal bleeding | ||
| Inoperable endometrioid carcinoma candidates for hormone therapy | Postop stage IB, grade III, and stage II endometrioid carcinoma | Inoperable patients with non-endometrioid histology (not candidates for systemic therapy) | ||
| Postop stage III-IV: chemotherapy alone (+/−RT after chemo) | Postop patients with grade I -histology with positive nodes (Stage IIIC) | Recurrent vaginal cuff disease (Elledge et al., 2020) | ||
| Postop stage IA-IV non-endometrioid histology | ||||
| In case of COVID + after 1-2 fr, further sessions may be postponed until 10–14 days after recovery from infection | The higher dose of 50.4 Gy instead of 45 Gy instead of a brachytherapy boost (Dewan et al., 2021) | |||
| Surgical stage III and IV a: Adjuvant RT/ In case of pelvic RT: Hypo-F | ||||
| Pelvic recurrence: Hypo-F RT (Lee et al., 2020b) | ||||
| Stages IB Gr 3, Stage II (RT 8-12 weeks post-op): 45 Gy / 25 frs (IMRT preferred) | ||||
| Stage IIIA-IIIC (RT 6–8 weeks post-op): 45 Gy / 25 frs (IMRT preferred) | ||||
| Stage IVB (palliative): 8 Gy/1 fr or 20 Gy / 5 frs (Hinduja et al., 2020) | ||||
| High priority: | ||||
| -High-risk patients: Post-op RT ± C | ||||
| -Symptomatic unresectable primary tumor (not a candidate for surgery): RT | ||||
| Medium priority: | ||||
| -Intermediate–high risk: Brachytherapy | ||||
| -Isolated vaginal relapse after surgery: RT (curative intent) | ||||
| Low priority: | ||||
| Asymptomatic vaginal/pelvic recurrence: RT (Colombo et al., 2020) | ||||
| Stage I, G2-G3 intermediate-risk or Stage II, and Stage III: no RT according to comorbidities | Postpone RT start (3 months) | |||
| Interruption for a suspected or confirmed case of COVID-19 within 15 days (Carvalho et al., 2020) | ||||
| Ovarian | Isolated locoregional relapse in patients with former surgery and chemotherapy | Bleeding or extremely painful disease in metastatic patients (not candidates for surgical or systemic therapies) (Elledge et al., 2020) | ||
| Vulvar | Curative intent RT: | |||
| - Radical RT for patients not appropriate for surgery (using IMRT for reduction of skin toxicity) | ||||
| - Adjuvant RT | ||||
| - Palliative RT: a single fraction to control symptoms until re-treatment is feasible (Guidance for radiotherapy for gynaecological cancer and COVID-19 [Online], 2021) | ||||
| Postop vulvar cancer | Locally advanced vulvar cancer-causing extreme pain (Wright et al., 2020) | |||
| Postop stage IB-II (close margins not candidates for margin re-excision or possibly with + LVSI, tumor size ≥4 cm) | Postop patients with close/positive margins or involved nodes with no gross residual disease | Bleeding or extremely painful lesions in metastatic patients | ||
| Postop patients with ≥ 1 positive lymph node | ||||
| Intact stage III/IVA disease | ||||
| Recurrent vulvar disease (not candidates for surgery and formerly not received RT) | ||||
| Intact recurrent inguinal or pelvic disease (not candidates for surgery) (Elledge et al., 2020) | ||||
| Locally advanced vaginal cancer-causing extreme pain (Wright et al., 2020) | ||||
| Neoadjuvant: CRT | ||||
| Radical: Locally advanced vulvar cancer with or without nodal involvement: CRT (SIB) (Garganese et al., 2020) | ||||
| Vaginal recurrence with bleeding: brachytherapy or RT (Lee et al., 2020b) | ||||
| Vulva, radical (CRT): 45 Gy / 25 frs + 18-20 Gy or 9-10 frs to gross disease (IMRT or VMAT only) / SIB to primary and nodes | ||||
| Vulva, adjuvant (Groin and pelvic RT in high-risk features): 45 Gy / 25 frs | ||||
| Vulva, palliative: 8 Gy/1 fr or 20 Gy / 5 frs (Hinduja et al., 2020) | ||||
| Interruption for a suspected or confirmed case of COVID-19 | No changes, No Postpone RT start | |||
| Adjuvant RT (Carvalho et al., 2020) | ||||
| Radical RT (Alkatout et al., 2020) | ||||
| Sarcoma | Adjuvant and Neoadjuvant: if alternative exist (Prioritize by age and other comorbidities) (Samiee et al., 2020) | |||
| Neoadjuvant/Adjuvant/Definitive RT | Severe pain | |||
| Uncontrolled bleeding (Wright et al., 2020) | ||||
| Neoadjuvant and adjuvant: Hypo-F RT (Starling et al., 1992) | ||||
| Delay Postop RT for: | Soft tissue sarcoma: | |||
| - Soft tissue sarcoma | - Preop RT for non-complex tumors not close to critical structures (few patients): Hypo-F RT using 25 Gy /5 frs | |||
| - Fibromatosis | - Postop RT: Hypo-F RT (40 –45 Gy / 15 - 20 frs and 36 Gy / 6 once-weekly frs not for younger patients) | |||
| - Ewing's sarcoma: postop RT for | Bone sarcoma: | |||
| - Low grade tumors such as chordoma or lower grade chondrosarcomas | Ewing's sarcoma: Surgery (1st local therapy) /definitive RT (curative treatment) | |||
| Definite RT for: | - Postop RT based on resection histology | |||
| - Non-malignant locally aggressive condition | - Definitive RT if surgery is not feasible/suitable | |||
| - Low-grade tumors, including chordoma (slow-growing indolent tumors): delay for a couple of months | Non-Ewing's bone sarcomas (osteosarcoma, chondrosarcoma, chordoma) | |||
| High-grade tumors: urgent RT | ||||
| Locally advanced high-grade tumors including osteosarcoma: definitive RT with shorter fraction schedules (Seddon and Zaidi, 2021) | ||||
| Soft-Tissue Sarcomas (NCC recommendations): | ||||
| Postop RT doses: RT (50 Gy) + RT boost (Negative margins: 10 - 16 Gy | ||||
| Microscopically positive margins: 16-18 Gy | ||||
| Gross residual disease: 20-26 Gy | ||||
| Using SBRT as a preop regimen (e.g., 35 Gy / 5 frs) for sarcomas (Parashar et al., 2020) | ||||
| Preop RT or chemotherapy: | ||||
| - High-risk surgery cases (e.g., retroperitoneal sarcoma) | ||||
| Adjuvant RT for soft tissue sarcoma: | ||||
| - operable grade II-III soft tissue sarcoma (not to defer surgery) (Vordermark, 2020a) | ||||
| Other forms of sarcoma | Soft tissue sarcoma: protracted RT regimens (25 Gy /5 frs) if the disease is not close to critical structures | |||
| Hypo-F RT:40-45 Gy / 15-20 frs and 36 Gy /6 frs once weekly (except in young patients due to increased late RT related toxicities) (Hinduja et al., 2020) | ||||
| Pediatric | All optional or unnecessary radiation cases | All cases where chemo or other interventions to delay initiation of RT | All curable cases (delay of RT is not feasible) (Wright et al., 2020) | |
| Active surveillance for grade I–II primary CNS | Chemo-sensitive tumors: e.g., rhabdomyosarcoma and Ewing sarcoma, medulloblastoma, ependymoma, and germ cell tumors presenting with metastases | Standard RT if feasible for: | ||
| Low-grade gliomas and craniopharyngiomas after primary biopsy or debulking surgery | Highly proliferative tumors: rhabdomyosarcoma, | - RT has a high effect on the outcome | ||
| Poor prognostic tumors and palliative care | Ewing sarcoma, medulloblastoma, germ cell tumors, and ATRT | Hypo-F RT, change dose/fr from1·6–1·8 Gy to above 2·0 Gy: | ||
| - Poor prognosis patients where RT shouldn't delay (neuroblastoma, rhabdomyosarcoma, Ewing sarcoma, and high-grade or diffuse midline gliomas) (Janssens et al., 2020) | ||||
| Medulloblastoma/embryonal CNS tumors, RMS, Ewing Sarcoma, chemo-sensitive NRSTS, intracranial germ cell tumors, neuroblastoma, ependymoma | Priority 1: Radical RT (any delay or interruption of RT decreases cure) | |||
| - Medulloblastoma | ||||
| - Embryonal CNS tumors/ pineoblastoma | ||||
| - RMS/ Ewings - definitive treatment/incomplete resection | ||||
| - Intracranial Germ Cell tumors | ||||
| Benign/ slowly proliferative tumors (whenever possible) | - Ependymoma G2/G3 | |||
| - Nasopharynx/ Head and neck | ||||
| - Total body irradiation | ||||
| - Retinoblastoma | ||||
| - ATRT | ||||
| Priority 2: Urgent palliative RT (save the loss of function/ life) | ||||
| - Cord compression | ||||
| - Bleeding, hemorrhage | ||||
| - Pontine/ spinal diffuse midline or high-grade glioma | ||||
| Priority 3: Adjuvant RT (aggressive tumors or with recognized residue) RMS/ Ewings- complete resection | ||||
| - Wilms' tumor | ||||
| - Neuroblastoma | ||||
| - Chordoma/ Chondrosarcoma | ||||
| - Bone tumors | ||||
| - NRSTS | ||||
| - Hodgkin Lymphoma | ||||
| - Salivary gland tumors/ Adenoid cystic carcinoma | ||||
| - Esthesioneuroblastoma | ||||
| - High grade/ diffuse midline glioma other than pontine or spinal | ||||
| - Metastatic RMS/ Ewings | ||||
| - Meningioma G3/ anaplastic | ||||
| - Pineal parenchymal tumors | ||||
| Priority 4: Palliative RT (control of symptoms to enhance the quality of life) | ||||
| -Symptomatic metastatic sites | ||||
| -Symptomatic local recurrence / re-irradiation | ||||
| Priority 5: Radical RT | ||||
| - Benign/ gradually proliferative tumors Craniopharyngioma | ||||
| - Optic pathway | ||||
| - Low-grade glioma | ||||
| - Desmoid-type fibromatosis | ||||
| - Pituitary Adenoma | ||||
| - Meningioma- G1/G2 | ||||
| - Myxopapillary Ependymoma (Mandeville, 2021) | ||||
| CNS tumors including medulloblastoma, grade II-III ependymoma, embryonal CNS tumors, intracranial germ cell tumors, atypical teratoid/ rhabdoid. | ||||
| Total body irradiation, retinoblastoma, nasopharynx, and head and neck malignancies (Hinduja et al., 2020) | ||||
| Considering Hypo-F where RT is required: | ||||
| Wilms tumor | ||||
| Low-grade glioma | ||||
| Palliative cases with urgent symptoms (Sullivan et al., 2020) | ||||
| Individualize interruption for a suspected or confirmed case of COVID-19 | Pediatric tumors: No changes and no delay of RT (Carvalho et al., 2020) | |||
|
Lymphoma and hematological malignancies |
Consolidation therapy for high-grade lymphomas | High-grade lymphomas with severe or life-threatening symptoms (Wright et al., 2020) | ||
| Low-grade lymphomas (most patient) | ||||
| HL: if RT not available | Peripheral T-Cell Lymphomas (PTCL): aggressive disease (Perini, 2020) | |||
| Early-stage HL (RT elimination costs of 6–8% disease control) (Vordermark, 2020a) | ||||
| For ≥ 60 yrs: | When there is no/little expected adverse effect: | Using alternative Hypo-F RT when RT cannot be omitted or delayed to maintain high cure/palliation rates without excessive toxicity (e.g., For patients with symptomatic sites of disease and Localized aggressive NHL, primary RT alone, and NK-/T-cell lymphoma) (Yahalom et al., 2020; Di Ciaccio et al., 2020) | ||
| - for the palliative purpose where alternative treatment is available | - for asymptomatic localized low-grade lymphomas; | |||
| - for localized low-grade lymphomas if completely excised | - for localized nodular lymphocyte-predominant HL | |||
| - for localized nodular lymphocyte-predominant HL if completely excised | - in a palliative setting for low-grade lymphomas in stable cases; | |||
| - in consolidation RT for diffuse large B-cell lymphoma/ | - for whom develop COVID-19 infection before commencing RT | |||
| aggressive NHL for those who have completed a full chemotherapy course with complete remission. | ||||
| In a minority of cases, if alternative treatment options were available | Shortening of treatment via Hypo-F RT or limitation of total dose for hemato-oncological patients: | |||
| - Painful osteolytic lesion caused by multiple myeloma in non-weight bearing bones after stabilizing surgery: 8 Gy/1 fr, 20 Gy/5frs, 24 Gy/8frs, and 30 Gy/10frs | ||||
| - Osteolytic lesion of multiple myeloma in weight-bearing bones (e.g., axial skeleton) without surgery: 20 Gy/5frs and 24–36 Gy/ 8-12 frs | ||||
| - Diffuse large B-cell lymphoma with the initial abdominal bulky disease after completion of six cycles of R-CHOP | ||||
| a) With no information on PET status: 27-30 Gy/ 9-10 frs | ||||
| b) PET-positive after treatment: 36 Gy/12frs | ||||
| - Early-stage indolent lymphoma in a noncritical location: | ||||
| 4 Gy/1 fr and 27 Gy/9 frs or 4 Gy/2 frs (Oertel et al., 2020) | ||||
| High-risk lymphomas (Carvalho et al., 2020) | ||||
| Skin | Primary and resected skin cancers (if not use short courses and limit radiation to the mucosa) | Unresected SCC/BCC: | ||
| - Primary tumors < 2 cm: 30 Gy / 5 frs over 2 weeks | ||||
| - Primary tumors > 2 cm: 45 - 55 Gy over 3 to 4 weeks, 10·2 Gy in 3 frs weekly | ||||
| Resected SCC/BCC: | ||||
| - 50 Gy / 4 weeks (2·5 Gy / fr) | ||||
| - 44 Gy / 10 frs in 4 days a week | ||||
| Melanoma | ||||
| Definitive cases: 35 Gy / 5 frs over a week for < 3 cm2 | ||||
| Postop: 30 Gy / 5 frs twice a week or every other day (Parashar et al., 2020) | ||||
| CM: | ||||
| - Patients with ≥ T2 disease for three months with negative biopsy margins | ||||
| - T0-T1 disease for three months if no macroscopic residue is detected at biopsy | ||||
| BCC: up to 3 months except for extremely symptomatic patients | ||||
| cSCC: | ||||
| - T1-T2a disease for 2-3 month except for prompt growth or symptomatic/immunosuppressed patients (prioritize patients with ≥ T2b disease) | ||||
| MCC: | ||||
| - Around one month for patients with favorable T1b disease High-risk patients: COVID-19 infection, elderly, and/or weak patients (Baumann et al., 2020) | ||||
| Non-melanoma skin tumors (Slotman et al., 2020) | ||||
| Adjuvant RT for BCC (with limited benefit) | cSCC, MCC, and rare skin pathologies incompletely excised: for 2-3 months | Definitive RT: cSCC, MCC, and rare skin pathologies (modified fractionation) (Rembielak et al., 2020) | ||
| Definitive RT including incompletely excised | ||||
| Melanoma (involved high-risk nodal basins) NMSC | Melanoma: LM, LMM, and melanoma in situ within 2-3 months | Melanoma: | ||
| BCC (definitive and postop) incomplete excised | NMSC: SCC, MCC, and rare skin pathologies incompletely excised in 2-3 months | - MM: radical RT just for unsuitable patients for surgery or inoperable mucosal melanomas (modified fractionation as definitive RT) | ||
| Adjuvant RT (benefit limited) for patients with closely excised cSCC <1 mm or with minor risk factors (lower/intermediate risk of recurrence) | - Adjuvant RT – primary site: just for postop insufficient margins and more surgery is not possible or in high-risk cases, with involved margins (<1 mm) | |||
| - Adjuvant RT – nodal basin: regional metastases from mucosal primaries | ||||
| - Patients at high risk of nodal recurrence (not candidates for systemic adjuvant therapy): 48 Gy / 20 frs, 40 Gy / 15 frs as Hypo-F RT | ||||
| Oligometastatic intracranial disease: SRS | ||||
| Standard palliative RT (excluding brain metastases): 20 Gy /4 frs instead of 20 Gy / 5frs, 30 Gy / 8 frs instead of 30 Gy/ 10 frs or single fraction of 8-10 Gy (Rembielak et al., 2021) | ||||
| Suspend all treatment forms until the pandemic is over (Hinduja et al., 2020) | ||||
| Rare indications of Melanoma (e.g., lentigomaligna, lentigo malignant melanoma, and melanoma in situ) should be deferred for 2-3 months | In palliative or case (e.g., bleeding or fungating skin nodules): Hypo-F RT (1-4 frs for 8-20 Gy) (Nahm et al., 2021) | |||
| Radical RT for advanced SCC: | Radical RT for advanced SCC: | |||
| COVID-19 positive patients: based on patient’s and lesion’s characteristics (site and size) | COVID-19 negative patients: No delay, especially for a large lesion or palliative setting or facial lesion | |||
| Adjuvant RT for advanced SCC: | COVID-19 positive patients: | |||
| COVID-19 positive patients | Hypo-F RT (based on patient’s and lesion’s characteristics | |||
| Radical RT for advanced BCC: (both COVID-19 negative and positive patients): Multidisciplinary discussion based on the lesion size and location (priority for face lesion) | Dedicated COVID-19 positive RT pathways | |||
| Adjuvant RT for advanced SCC: | ||||
| COVID-19 negative patients: Choice is based on patient’s (age, comorbidities) and lesion’s characteristics (location and size) | ||||
| Adjuvant RT for advanced BCC: COVID-19 positive patients | Radical RT for advanced BCC (both COVID-19 negative and positive patients): | |||
| Multidisciplinary discussion based on the lesion size and location (priority for face lesion): Hypo-F RT | ||||
| Adjuvant RT for advanced BCC (COVID-19 negative patients): Choice based on patient’s prognosis, age, comorbidities, and the location (priority for face lesion) (Tagliaferri et al., 2020) | ||||
| Skin : not treating (Carvalho et al., 2020) | ||||
| Palliative RT | Bone metastasis | Cord compression, superior vena cava obstruction, life-threatening bleeding: | ||
| Multiple brain metastasis | Do not defer until a reasonable alternative (Radical treatment) (Samiee et al., 2020) | |||
| Painful spine metastasis, Spinal cord compression or spine metastases with the epidural disease, Brain metastases <5 mm, Patients with stable or minimum symptomatic oligo-metastatic disease | Cord compression, Symptomatic brain metastases or brain metastases >5 mm, Malignant airways obstruction, SVCO, Severe pain from primary, Heterotopic bone (Wright et al., 2020) | |||
| Brain metastases (SRS for good PS/SRS of resection cavity for postop) (Combs et al., 2020) | ||||
| Painful metastasis, uncomplicated, other systemic options | Painful metastases without impending structural/neurologic compromise | Bone metastasis, no fracture, +/- cord compression | ||
| Oligometastatic (e.g., prostate cancer) | Bone metastasis, fracture/surgery | |||
| Postoperative radiotherapy (for pathologic fracture) | Brain metastasis | |||
| CNS metastasis from NSCLC needing WBRT | Esophageal bleeding/ dysphagia | |||
| GBM, poor KPS | ||||
| Head & Neck | ||||
| SVCO Syndrome/Lung cancer | ||||
| Lymphoma, low grade | ||||
| Pelvic/GI bleeding (reduction of fractionation) (Simcock et al., 2020) | ||||
| Prostate cancer patients, breast cancer patients, benign CNS tumor (up to 3 months from diagnosis to treatment) | Urgent treatments: | |||
| - Spinal cord compression, superior vena cava syndrome, life-threatening lower airway obstruction, digestive or respiratory hemorrhage, and life-threatening brain lesion (RT delivered within 24–48 h) | ||||
| Non-urgent treatments: | ||||
| - Painful metastatic bone lesions, lung cancer-causing chest pain or Pancoast syndrome, tumors causing nerve root and soft tissue infiltration, relief of impending airways or bowel obstruction (start of RT within seven days) | ||||
| Elective priority treatments: | ||||
| - Head and neck cancers, rectal and anal cancer, the gastroesophageal junction (Montesi et al., 2020a) | ||||
| Palliative non-emergent indications (Slotman et al., 2020) | ||||
| In cases of spinal cord compression, metastatic bone pain irresponsive to other treatments or micro-vascular bleeding: single fraction (Starling et al., 1992) | ||||
| Palliative intent in asymptomatic or oligosymptomatic patients | Spinal cord compression, SVCO, or bleeding in confirmed cases of COVID-19 (Ismael et al., 2020) | |||
| Symptomatic brain metastases: 20 Gy / 4 - 5 frs | ||||
| For COVID-19 patient: | ||||
| Palliative RT for a highly symptomatic patient (life expectancy > 3-6 months) and without any other therapeutic alternative (Amaoui et al., 2020) | ||||
| Painful bone metastasis patients (controlled by level 1 to 3 oral analgesics) | Adjuvant bone metastasis RT: | MESCC: RT without delay (if surgical treatment is contraindicated or not appropriate) (Thureau et al., 2020) | ||
| MESCC: Adjuvant RT after surgery for 4 to 12 weeks | ||||
| Bone oligometastases and other SBRT indications: for a few weeks, esp. for hormone-sensitive tumors | ||||
| Very algic bone metastases refractory to analgesics: 8 Gy / 1 fr (Amaoui et al., 2020) | ||||
| Urgent cases: pain due to bone metastases, cord compression, SVCO, and tumor bleeding: 5-8 Gy/1 fr (single fraction) | ||||
| Hypo-F RT and single fraction palliative RT (Upadhyay and Shankar, 2020) | ||||
| Treatment is limited to function- or life-threatening situations (e.g., spinal cord compression) | ||||
| The shortest possible course (e.g., single-fraction treatment for bone pain (Weisel et al., 2020) | ||||
| Palliative RT: spinal cord compression, uncontrolled bleeding from fungating tumors, and intractable pain (Ng et al., 2020a) | ||||
| RT for emergencies (spinal cord compression, symptomatic brain metastases) (Ismaili and El Majjaoui, 2020) | ||||
| Palliative RT, e.g., in painful bone metastases a single 8 Gy / fr (Mahmoodzadeh et al., 2020) | ||||
| Postop for a pathological fracture | Palliative treatment of bleeding/fungating inoperable breast mass, spinal cord compression, and symptomatic brain metastases (Elghazawy et al., 2020) | |||
| Single or two weekly fractions for palliative thoracic RT (Bakhribah et al., 2020) | ||||
| - Single-fraction for bone metastases and spinal cord compression: 8-24 Gy / 1 fr | ||||
| - Airway obstruction:17 Gy / 2 frs (Singh et al., 2020) | ||||
| No spinal compression | Bone Mets, fracture/spinal compression, SVCO: 8 Gy / 1 fr (Kochbati et al., 2020) | |||
| Stage IVB of cervical cancer: RT for cord compression/Brain metastasis (Dewan et al., 2021) | ||||
| BCLC C: Palliative RT in a single 8 Gy fr for symptomatic disease (local or metastatic) (Barry et al., 2020) | ||||
| Arteriovenous malformations: SRS or Hypo-F SRS | Brain metastases: | |||
| - For solitary/limited brain met with good DS-GPA (single fraction frameless SRS) | ||||
| - Hypo-F RT:30–35 Gy / 5–6 frs | ||||
| - For multiple brain metastases/whole-Brain: Hypo-F RT (20 Gy / 5frs) | ||||
| Spinal cord compression: Hypo-F RT (8 Gy/single fr or 20 Gy / 5 frs) (Balakrishnan et al., 2020) | ||||
| Palliative/temporary control of vulvar cancer: | ||||
| - Long course: 30 Gy/10 frs | ||||
| - Short course: 16 Gy/4 frs or 20 Gy/5 frs (symptomatic patients) (Garganese et al., 2020) | ||||
| Palliative treatment of head and neck malignancies (Short fractionation schedules): | ||||
| 25 Gy / 5 frs, 20 Gy / 5 frs, 30 Gy / 6 frs, IMRT over 2 weeks, or Single 8 Gy fr (Hinduja et al., 2020) | ||||
| Brain metastases: | ||||
| - For metastases <10 cc: single fraction treatment | ||||
| - SRS (replace neurosurgical options) | ||||
| - Postop: SRS to the cavity 5 Gy/fr for 7 frs | ||||
| - If life expectancy >3 months: 4 Gy/fr for 5 frs to the whole brain | ||||
| Spinal cord compression: 8 Gy in a single fraction | ||||
| Tumor bleeding: | ||||
| - 20 Gy / 5 frs given daily | ||||
| - Single fraction of 8 Gy | ||||
| SVCO: | ||||
| - 20 Gy / 5 daily frs | ||||
| - 8-10 Gy in a single fraction | ||||
| Painful bone metastases: 8 Gy single fraction (Hinduja et al., 2020) | ||||
| Thoracic patients with oligometastatic disease | Symptomatic metastases (pain, obstruction, or bleeding) palliative short course Hypo-F RT: 8 Gy/10 Gy or SBRT (Arrieta et al., 2020) | |||
| Cord compression or bony metastases: 20 Gy/5 frs or 8-10 Gy/1 fr or 30 Gy/10 frs for good prognosis | ||||
| Breast palliation: 6 Gy for 5 to 6 weeks | ||||
| Brain metastases: where appropriate: SRS; all others: 20 Gy/5 frs or 12 Gy/2frs (daily) (Chan et al., 2020) | ||||
| Hypo-F RT: | ||||
| -Bone metastasis: 6–8 cGy/1 fr; 15 Gy/3 frs; 20 Gy/5 frs to a small radiation field | ||||
| - Brain metastasis: 20 Gy/5frs (Elkhouly et al., 2020) | ||||
| Palliative RT for melanoma: | ||||
| COVID-19 negative patients (No delay) | ||||
| COVID-19 positive patients: In case of pain or dedicated COVID-19 positive RT pathways (Tagliaferri et al., 2020) | ||||
| Brain metastases from lung cancer (whole brain RT) | ||||
| Short course Hypo-F RT: 20 Gy/5 frs; 30 Gy/10 frs (patients with better survival outcomes); 12 Gy /2 frs (once a week) in patients with poor PS | ||||
| Hypo-F boost of 10 - 15 Gy after WBRT | ||||
| Single fraction SRS as an alternative to surgery (oligo-metastases and controlled extracranial disease) (Mummudi et al., 2020) | ||||
| In patients with short survival | Malignant spinal cord compression: 8 Gy/1 fr (Cameron, 2020) | |||
| Brain metastases: 20 Gy/5 frs | ||||
| Cord compression: 8 Gy/1 fr | ||||
| Tumor bleeding: 14.8 Gy / 4 twice daily frs; 20 Gy/5 daily frs | ||||
| SVCO: 17 Gy/2 weekly frs; 20 Gy/5 daily frs | ||||
| Bone metastases: 8 Gy/1 daily fr (Yerramilli et al., 2020a) | ||||
| The omission of whole-brain radiation: multiple brain metastases and limited life-expectancy (<3–6 months) | Single-fraction palliative RT for bone metastases/metastatic spinal cord compression (Gupta et al., 2020a) | |||
| High priority: | ||||
| Spinal cord compression with potential neurological recovery | ||||
| Moderate priority: | ||||
| Palliation of symptoms like hemoptysis in lung cancer (Talapatra et al., 2020) | ||||
| Selected palliative treatments (Carvalho et al., 2020) | ||||
| High priority: | ||||
| Spinal cord compression, brain metastases, other critical metastatic lesions | ||||
| Low priority: | ||||
| Palliative RT for asymptomatic recurrence not amenable to surgery (Colombo et al., 2020) | ||||
| Pain or bony lesion: 8 Gy / 1 fr; | ||||
| Bleeding: 10 Gy / 1 fr; 20 Gy /5frs (If single fraction not possible, Hypo-F RT) | ||||
| Multiple brain metastases: 20 Gy / 5 frs (in the favorable subgroup) | ||||
| MSCC: 8 Gy / 1 fr (Counago et al., 2020) | ||||
| Symptomatic bone metastases: 8 Gy/1 fr (Kwek et al., 2021) | ||||
| Benign Disease | Keloid, heterotopic Ossification, Actinic Keratosis | Benign Disease, Pituitary Adenoma, Fibromatosis Other: Actinic Keratosis, Recurrent/Refractory Fasciitis, other rare benign (Simcock et al., 2020) | ||
| Benign tumors (schwannomas and asymptomatic meningiomas) (Starling et al., 1992) | ||||
| Keloid, heterotopic ossification, actinic keratosis (Marcus and Mahajan, 2020) | ||||
| Non-malignant indications (Slotman et al., 2020) | ||||
| Benign tumors: RT after 3 months (Carvalho et al., 2020) | ||||
RT: radiotherapy, BT: brachytherapy, SBRT: stereotactic body radiotherapy, Hypo-F RT: hypo-fractionated RT, HypeF-RT: hyper-fractionated RT, SABR: stereotactic ablative radiotherapy. KPS: karnofsky performance status, ADT: androgen deprivation therapy, CALGB: cancer and leukemia group b; COMS: collaborative ocular melanoma study. SIB: simultaneous integrated boosts, ER: estrogen receptor, HER2: Human epidermal growth factor receptor 2, ATRT: atypical teratoid rhabdoid tumors, PCI: prophylactic cranial irradiation., CNS: central nervous system, GBM: glioblastoma multiform, PNET: primitive neuroectodermal tumor, RMS: rhabdomyosarcoma, AVM: arteriovenous malformation, OSCC: oesophageal squamous cell carcinoma, OAC:oesophageal adenocarcinoma, dCRT: definitive radiation chemotherapy, HCC: Hepatocellular Carcinoma, cCRT: concurrent radiation chemotherapy, TME: total mesorectal excision, SCRT: short-course radiotherapy, PS:performance status, GS: gleason score, ATRT : atypical teratoid rhabdoid tumors, BCC: basal cell carcinoma, SCC: squamous cell carcinoma, CM: cutaneous melanoma, cSCC: cutaneous squamous cell carcinoma, MCC: merkel cell carcinoma, MM: malignant melanoma, LM: lentigo maligna, LMM: lentigo maligna melanoma, NMSC: non-melanoma skin cancer, LVI: lymphovascular invasion, PTV: planning tumor volume, PMRT: postmastectomy radiation therapy, NAC: neoadjuvant chemotherapy, TNBC: triple negative breast cancer, BC: breast cancer, BCT: breast conserving therapy, NCCN: National Comprehensive Cancer Network, SRS: stereotactic radiosurgery, VMAT: volumetric modulated arc therapy, postop: postoperative, preop: preoperative, HNSCC: head and neck squamous cell carcinoma, ER: estrogen receptor,HER2: human epidermal growth factor receptor 2, DCIS: ductal carcinoma in situ, DCIS-RH+: hormone receptor-positive ductal carcinoma, LVI: lymphovascular invasion, PCI: prophylactic cranial irradiation, NSCLC: non-small cell lung cancer, LS-SCLC: limited stage small cell lung cancer, IMN: internal mammary nodes, SBRT: stereotactic body radiation, LAPC: locally advanced pancreatic cancer, MESCC: metastatic epidural spinal cord compression, GI: gastrointestinal, LVSI: lymphovascular space invasion, CALGB: cancer and leukemia group b; COMS :collaborative ocular melanoma study, HL: Hodgkin lymphoma, NHL: Non-Hodgkin lymphoma, NK: natural killer, BCLC: Barcelona Clinic Liver Cancer, OSCC: oral cavity squamous cell cancer, LCCRT long-course chemoradiotherapy, MRF-D distance from mesorectal fascia, R-CHOP rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin, prednisone, PET positron emission tomography, EBC: Early breast cancer, IDH: isocitrate dehydrogenase, SVCO: superior vena cava obstruction, APBI: accelerated partial breast irradiation, WBI: whole breast irradiation, DORSCON, Disease Outbreak Response System Condition, IMNC: internal mammary nodal chain.
Table 2.
Summary of international guidelines or national multi-cancer recommendation for brachytherapy prioritization during COVID-19 pandemic.
| Cancer type | Hold BT and choose another treatment option | Delay BT until the end of the pandemic | Continue BT during the pandemic | |
|---|---|---|---|---|
| CNS | Brain (For primary or metastases/adjuvant cases): | |||
| - Avoid BT until pandemic solves | ||||
| - SRS/SRT for glioma or metastatic cases (Mohindra et al., 2020) | ||||
| Head and neck | Oral tongue (pT1-T2, N0) high risk of local recurrence: | |||
| - Adjuvant BT (39 Gy /fr in 7 days, twice daily instead of 60 Gy /30 frs by EBRT) (Aghili et al., 0) | ||||
| Definitive/boost oral cavity/oropharynx, boost nasopharynx or any re-irradiation: | ||||
| - Avoid BT until pandemic solves | ||||
| - For COVID-19+ patients, continue EBRT rather than BT boost (Mohindra et al., 2020) | ||||
| SSC of lip, oral mucosa, or nasal region cases: - Continue (Cyrus et al., 2020) |
||||
| Switch interstitial BT to EBRT | If BT can be employed as a sole modality for cases such as the lip and oral mucosa (Barthwal et al., 2020) | |||
| Recurrent nasopharyngeal carcinoma: time-sparing interstitial or intracavitary brachytherapy (if feasible) | ||||
| - 198Au grains: 60 Gy | ||||
| - 125I grains: 130 Gy; 120 Gy | ||||
| - HDR intracavitary: 24 Gy / 3 frs (Svajdova et al., 2020) | ||||
| Breast | Early-stage cases: | |||
| - Use balloon- or multicatheter-based BT instead of EBRT () | ||||
| Low-risk cases: | ||||
| Postpone interstitial BT for up to 16–20 weeks for ER + invasive cases or 12 weeks for DCIS (Mohindra et al., 2020) | ||||
| Patients prescribed for definitive or adjuvant therapy: | Early-stage: | |||
| - Shorten BT fractionation schedules | - Neoadjuvant endocrine therapy due to delay of surgeries during the crisis; | |||
| - Adjuvant therapy after BCS | ||||
| - Deem BT as an equivalent option to EBRT | ||||
| - BT for APBI with a single-entry intra-cavitary or multi-catheter interstitial technique after surgery | ||||
| Invasive cases: | ||||
| - Induction of therapy for within 12 weeks after surgery, not more than 20 weeks (BT after BCS) (Williams et al., 2020) | ||||
| Accelerated partial breast irradiation (Exclusive): | Accelerated partial breast irradiation (Exclusive): | |||
| - Opt for EBRT according to local facilities (Chargari et al., 2020) | - Postpone (8–12 weeks) | |||
| Apply EBRT instead of BT (Barthwal et al., 2020) | ||||
| Very Low-, Low- and Intermediate Risk: | ||||
| -HDR-ISBT 27 Gy/2frs Monotherapy | ||||
| - 125I LDR-ISBT | ||||
| Intermediate Risk High - Very High Risk: | ||||
| - HDR-ISBT boost 15 Gy/1 fr (Murakami et al., 2020) | ||||
| Lung | For palliative and post-transplant stenosis: | |||
| - Avoid BT until the pandemic solves (Mohindra et al., 2020) | ||||
| Gastrointestinal | Esophageal | Palliation with symptoms: | ||
| - Continue BT () | ||||
| -Palliative and re-irradiation: | ||||
| - Avoid BT until the pandemic solves (Mohindra et al., 2020) | ||||
| BT with Endoscopic procedures (esophagus or bronchus): | ||||
| - Omit and consider EBRT options (Chargari et al., 2020) | ||||
| Hepato-biliary | Avoid delaying the treatment using BT () | |||
| Hilar Cholangiocarcinoma cases with COVID-19+ during RT: | ||||
| - Continue EBRT rather than BT boost | ||||
| Palliative unresectable malignant biliary obstruction or hepatocellular carcinoma cases (not for the transplant) and metastatic lesions: | ||||
| - Avert BT until pandemic solves (Mohindra et al., 2020) | ||||
| Rectal | For COVID-19+ patients: | Preoperative or definitive postpone brachytherapy until pandemic solves (Hypo-F RT) (Mohindra et al., 2020) | ||
| - Hypo-F-EBRT rather than BT boost | ||||
| After SCRT: | ||||
| - For Unresectable, Medically inoperable, or Frail elderly cases: 10-20 Gy in 2-4 frs (Siavashpour et al., 2020) | ||||
| Anal | - Switch interstitial BT to EBRT | |||
| - Switch to IORT if facilities are available (Barthwal et al., 2020) | ||||
| Genitourinary | Prostate | Continue (Chargari et al., 2020) | ||
| If BT can be employed as a sole modality for penile region cases (Barthwal et al., 2020) | ||||
| Low-risk patient: | High-risk patients: | |||
| - Delay BT up to 3–6 months | - BT as a boost, avoiding any deferent: | |||
| 13·5 Gy /2 frs of BT alone or 15 Gy/1 fr as EBRT boost () | ||||
| For COVID-19+ patients during EBRT: | High-risk cases: | High-risk cases: | ||
| - Interrupt treatment to let recovery up to 10–14 days before restarting/plan for BT | - Delay all monotherapy BT | - Defer starting EBRT and keep on hormone therapy-- Consider EBRT boost instead of BT (Mohindra et al., 2020) | ||
| For COVID-19+ patients after 1 st session of HDR, defer 2nd fraction to allow recovery up to10–14 days | ||||
| Low and intermediate-risk cases: | For anxious patients, minimize the time of treatment (definitive) | |||
| - Delay BT for at least 3–6 months | Definitive or adjuvant therapy (using endocrine): | |||
| - Shorten BT fractionation schedules (Williams et al., 2020) | ||||
| Low-risk prostate cancer (Exclusive): | Low-risk prostate cancer (Exclusive): | |||
| - Postpone (8–12 weeks) | - Opt for surveillance | |||
| Intermediate and high-risk prostate: | Intermediate and high-risk prostate: | |||
| - Postpone (8–12 weeks) | - Opt for EBRT according to local facilities (Chargari et al., 2020) | |||
| Brachytherapy should be avoided as far as possible | In centers where prostate BT is common: | |||
| – all (HDR) monotherapy cases (2 implants) should be converted to HDR boost (single implant 15 Gy in 1 fr) or switching to EBRT or starting of ADT | ||||
| – EBRT that are due for HDR boosts (15 Gy in 1 fr) can be converted to 37.5 Gy/15 fractions, | ||||
| – For experienced centers, BT can be delivered using LDR (Barthwal et al., 2020) | ||||
| Temporarily defer certain specialized procedures (HDR-BT) (Kwek et al., 2021) | ||||
| Gynecological | Cervix | locally advanced cases (excluding verified or doubtful patients with COVID-19 infection) () | ||
| Positive COVID-19 patients: | Negative COVID-19 patients: | |||
| - Postpone up to 10–14 days | - Finalize treatment within 7–8 weeks (Mohindra et al., 2020) | |||
| - Increase dose by 5 Gy / week deferent (consider OAR constraints) | ||||
| - Keep on BT boost with PPE precautions | ||||
| Chemotherapy/RT + BT ≤ 8 weeks (Williams et al., 2020) | ||||
| Boost: Continue for locally advanced case (Chargari et al., 2020) | ||||
| When that is not feasible EBRT boost should be considered. | Adding approx. 5 Gy per week for each week of BT delay | |||
| beyond seven weeks, respecting (OARs) tolerance doses (Barthwal et al., 2020) | ||||
| - Reducing the number of applications by delivering multiple fractions with each application | ||||
| - Using higher dose/fr (fewer fraction number) considering the indications (e.g., 3 × 8 Gy or 4 × 7 Gy) (Miriyala and Mahantshetty, 2020; ElMajjaoui et al., 2020; Kumar and Dey, 2020; Ismaili and Elmajjaoui, 2020) | ||||
| Adjuvant treatment: 9 Gy / 2 frs over 2 weeks, over conventional 7 Gy / 3–4 frs or 6 Gy / 5 frs (Upadhyay and Shankar, 2020) | ||||
| 9 Gy × 2 frs weekly (in patients with low volume disease post-RT and in whom inferior local control) (Kumar and Dey, 2020) | ||||
| Stages IB3, IIA2-IIIC2, and early IVA: Intracavitary HDR brachytherapy 3 frs | ||||
| Stages IA1, IA2, IB1, IB2, IIA1: Vault brachytherapy 12 Gy/2 frs (Hinduja et al., 2020) | ||||
| For centers with single brachytherapy operating: | Reduced number of fractions: 24 Gy/3 frs or 28 Gy/4 frs | |||
| postpone at least 24 days or until the infection is resolved | HDR ICBT: 7 Gy/4 frs at 1 week apart or 2 frs per day separated by a 6 h interval | |||
| For patients >70 yrs, significant comorbidities, small tumors, or responding well to RT: | ||||
| -Shortened schedule (9 Gy /2 frs at 1 week apart) | ||||
| -Brachytherapy for cervical cancer (stage IB1, IIIB) (ElMajjaoui et al., 2020) | ||||
| Advanced cervical cancer: temporarily defer interstitial brachytherapy (Kwek et al., 2021) | ||||
| Uterine | - Postpone BT but no more than 12 weeks after surgery (Williams et al., 2020) | |||
| Endometrial | - Standard treatment (preferably three frs) () | |||
| Inoperable definitive positive COVID-19 symptomatic patients: | ||||
| - Hold on RT for 10–14 days | ||||
| - Start BT after recovery (Mohindra et al., 2020) | ||||
| High-risk cases: | ||||
| - Postpone boost (8–12 weeks) | ||||
| - Opt EBRT according to local facilities (Chargari et al., 2020) | ||||
| Interstitial BT for definitive COVID-19+ cases: | ||||
| - Delay treatment up to 10–14 days after recovery | ||||
| - Increase BT dose by 5 Gy / week deferent (Mohindra et al., 2020) | ||||
| Intermediate risk endometrial cancer (Exclusive): Postpone (8–12 weeks) or opt for surveillance (Chargari et al., 2020) | ||||
| Postop vaginal cuff cases: | - Postpone BT up to 8–9 weeks after surgery | |||
| - Avert BT boost after RT if no adverse factor exists | - Postpone BT boost by 2–3 weeks after RT (Mohindra et al., 2020) | |||
| - COVID-19+ patients: postpone BT until pandemic solves | ||||
| Early-stage high risk | Early-stage intermediate risk: | |||
| - Postpone BT up to 12 weeks to 6 months based on patient comorbidities | ||||
| −7 Gy (to 0.5 cm depth) in 3 frs allowing 14 days inter-fraction interval | ||||
| Stage II: | ||||
| - Postpone by 1–2 months | ||||
| - Postpone at least 24 days for COVID-19 positive cases (ElMajjaoui et al., 2020) | ||||
| Stages IA Gr I-Gr III and IB Gr I-II: Vault brachytherapy if positive margins, suboptimal surgery | Stages IB Gr 3, stage II G1 and G2 with no high-risk features, stage IIIA-IIIC: Vault brachytherapy (Hinduja et al., 2020) | |||
| High-risk patients (received adjuvant RT): Omitting VVB | For patients with significant comorbidities: for 6 months | Patients who should start VVB: 7 Gy/3 frs (depth of 0.5 cm) with an interval spacing of 14 days between the fractions | ||
| Intermediate-risk endometrial cancer: | Stage II endometrial cancer with poor prognostic factors (if invasion > 50 % of the myometrium, G3), and for stage I high-risk endometrial cancer: Adjuvant RT and brachytherapy (ElMajjaoui et al., 2020) | |||
| Delaying VVB up to 12 weeks | ||||
| Stage II endometrial cancers: | ||||
| Adjuvant VVB (exclusively: if invasion < 50 % of the myometrium, G1 and 2 or after RT: if invasion > 50 % of the myometrium, G3): postpone brachytherapy by 1–2 months | ||||
| COVID-19 positive patient: postpone treatment (at least 24 days) | ||||
| Vaginal | Stage I: | Advanced stage (ElMajjaoui et al., 2020) | ||
| - Postpone BT up to 1–6 months for patients with significant comorbidities | ||||
| Upper and lower vagina (Hinduja et al., 2020) | ||||
| Early vaginal cancer (stage I, < 5 mm of invasion) with significant comorbidities: | For advanced stage: | |||
| postpone brachytherapy by 1–2 months | CRT followed by vaginal brachytherapy (7 Gy/3frs) | |||
| Brachytherapy without any delay (curative treatment): | ||||
| stage I, < 5 mm of invasion, locally advanced stage (ElMajjaoui et al., 2020) | ||||
| Vulvar | low priority and only be carried out when operation theatre capacity allows it (Barthwal et al., 2020) | |||
| Vulva: radical, adjuvant and palliative (Hinduja et al., 2020) | ||||
| Sarcoma | Postpone BT boost until pandemic solves. | |||
| For COVID-19+ patients during RT, continue EBRT rather than brachytherapy boost (Mohindra et al., 2020) | ||||
| Soft-tissue sarcoma: | ||||
| - BT alone (HDR instead of LDR with iridium-192 wires) rather than 60−66 Gy / 1·8−2 Gy/ fr adjuvant EBRT () | ||||
| Pediatrics | BT can be employed in specialized centers, especially for rhabdomyosarcoma (Barthwal et al., 2020) | |||
| Pediatrics indication: To be discussed on an individual basis (Chargari et al., 2020) | ||||
| Skin | Non-melanoma skin cancers: | |||
| - Use BT with fewer fractions, especially in inoperable patients () | ||||
| Definitive cases: | ||||
| - Avoid BT until the pandemic solves (Mohindra et al., 2020) | ||||
| Basal cell carcinoma (Exclusive): | Basal cell carcinoma: | |||
| - Postpone according to functional risk | - Do not postpone (Chargari et al., 2020) | |||
| Hypo-F RT can be delivered in a twice-daily frs | Until it is suitable for the institute (Barthwal et al., 2020) | |||
| - Switch interstitial BT to EBRT | ||||
| - Switch to IORT if facilities are available (Barthwal et al., 2020) | ||||
| Keloids (Exclusive) | Omit BT and consider options (Chargari et al., 2020) | |||
| Uveal Melanoma | Continue (Mohindra et al., 2020;) | |||
| Palliative | BT should be avoided and replaced by Hypo-F EBRT (Barthwal et al., 2020) | |||
RT: radiotherapy, BT: brachytherapy, EBRT: external beam radiotherapy, HDR: high-dose-rate, LDR: low-dose-rate, SCC: squamous cell carcinoma, PPE: personal protective equipment, IORT, intra-operative radiotherapy, Hypo-F RT: hypo-fractionated RT, ISBT: interstitial brachytherapy, VVB: Vaginal vault brachytherapy.
Table 3.
Summary of radiotherapy departments' consensus for suggested dose/fractionation during COVID-19 pandemic based on the cancer type.
| Cancer type | Country | Radical | Palliative | Pre-pandemic EBRT technique | Indication of EBRT during the pandemic | Suggested EBRT technique during the pandemic | ||
|---|---|---|---|---|---|---|---|---|
| CNS | Glioblastoma | USA (Noticewala et al., 2020a) | ✓ | 60 Gy / 30 frs | Not recurrent cases | a) KPS ≥ 70: 60 Gy / 30 frs | ||
| b) KPS < 70 or elderly: 40 Gy / 15 frs | ||||||||
| c) KPS < 50: 34 Gy / 10 frs or 25 Gy / 5 frs | ||||||||
| Canada (Patrick et al., 2020) | ✓ | 60 Gy / 30 frs | 40 Gy in 15 frs OR 25 Gy in 5 frs | |||||
| Head and neck | Italy (De Felice et al., 2020) | ✓ | Almost a sequential technique | dCRT should be limited to SIB techniques in the standard (5 fractions per week) or accelerated schedule (6 fractions per week) | ||||
| Canada (Huang et al., 2020) | ✓ | HNSCC | 60 Gy / 25 frs (5 weeks; 2·4 Gy / frs) | |||||
| HPV + T1-T3N0-N2c (TNM-7), HPV– T1-T2N0 HNSCCs, and select stage III HNSCCs | ||||||||
| India, USA (Gupta et al., 2020c) | ✓ | 1·8−2 Gy / fr | Hypo-F RT: 55 Gy / 20 frs | |||||
| UK (Higgins et al., 2020) | ✓ | 35 frs regimens | 20 frs regimen | |||||
| USA (Kang et al., 2020) | ✓ | Treatment guidelines for curable patients | Treatment guidelines for curable patients | |||||
| -Nasopharynx | -Nasopharynx: | |||||||
| a) T1N0 | a) RT alone (69.96 Gy/33 frs or 70 Gy/35 frs) | |||||||
| b) All other M0 patients | b) CRT (69.96 Gy/33 frs or 70 Gy/35 frs) | |||||||
| - Nasal cavity and paranasal sinuses (T1-T4) | -Nasal cavity and paranasal sinuses: | |||||||
| - Oral cavity (T1-T4) | Adjuvant RT (60−66 Gy/30–33 frs) + cC | |||||||
| - Oropharynx and unknown primary | In the absence of surgery: Definitive CRT: 70 Gy/35 frs + cC | |||||||
| a) p16-positive | -Oral cavity: | |||||||
| a1) T1N0-T2N0 | Definitive CRT: 70 Gy / 35 frs + Cc (proton therapy if feasible) | |||||||
| a2) Any T3, T4, or N+ | Adjuvant RT (60−66 Gy/30–33 frs) + cC | |||||||
| b) p16-negative | In the absence of surgery: Definitive RT (70 Gy/35 frs) | |||||||
| b1) T1N0-T2N0 | Consider proton therapy if feasible. | |||||||
| b2) Any T3, T4, or N+ | -Oropharynx and unknown primary: | |||||||
| - Larynx | a1, b1) T1N0-T2N0: Definitive RT (69.96 Gy/33 frs or | |||||||
| a) T1N0 glottic larynx | 70 Gy/35 frs) | |||||||
| b) T2N0 glottic larynx | a2, b2) Any T3, T4, or N+: Definitive CRT (70 Gy/35 frs) + Cc | |||||||
| c) T1-T2N0 supraglottic or subglottic larynx | - Larynx: | |||||||
| d) T3, T4, or N + glottic larynx; all other larynx | a) Definitive RT (63 Gy / 28 frs) | |||||||
| -Hypopharynx | b) Definitive RT (65.25 Gy/29 frs) | |||||||
| a) T1N0-T2N0 | c) Definitive RT (70 Gy/35 frs or 69.96 Gy/33 frs) | |||||||
| b) Any T3, T4, or N+ | d) Definitive CRT (70 Gy/35 frs) + cC | |||||||
| -Hypopharynx | ||||||||
| Treatment guidelines where LRC is important | a) Definitive RT (69.96 Gy/33 frs or 70 Gy/35 frs) | |||||||
| b) Definitive CRT (70 Gy/35 frs) + cC | ||||||||
| -Recurrent HNC in need of re-irradiation: | Treatment guidelines where LRC is important | |||||||
| Recurrent HNC in need of re-irradiation: | ||||||||
| a) Postop patients | a) Conventionally fractionated RT (60−66 Gy/30–33 frs) | |||||||
| b) No surgery: >2 y from RT or good KPS | b) Conventionally fractionated RT (70 Gy/35 frs) | |||||||
| c) No surgery and rapid recurrence from first course | c) Quad Shot (3.7 Gy/frs twice daily × 2 consecutive days = 1 cycle; may repeat cycle every 3–4 weeks for up to 4 total cycles) | |||||||
| Severe restrictions or limitations in radiation | Severe restrictions or limitations in radiation oncology operations | |||||||
| oncology operations | ||||||||
| -Larynx | -Larynx: | |||||||
| a) T1N0 glottic larynx | a) Definitive RT (50–52.5 Gy/16 frs) | |||||||
| b) T1-T2N0 glottic | b) Definitive RT (51 Gy/20 frs) | |||||||
| c) Larynx | c) Definitive RT (55 Gy/ 20 frs) | |||||||
| - Oropharynx | - Oropharynx: | |||||||
| a) T1-T2N0-N1 oropharynx | a) Definitive IMRT (66 Gy/ 30 frs) | |||||||
| b) p16+ T1N1-T2N2b or T3N0- | b) Definitive CRT (60 Gy/30 frs) + cC | |||||||
| T3N2b with | -Locally advanced HNC: | |||||||
| ≤10-pack-y smoking history | a) Definitive CRT (55 Gy/ 20 frs) + cC | |||||||
| -Locally advanced HNC (oral cavity, oropharynx, | b) Definitive CRT (55 Gy/20 frs) + cC | |||||||
| hypopharynx) | c) Definitive RT (51 Gy/20 frs) | |||||||
| a) T1N0-T4N3 SCC | ||||||||
| b) T1-T4N2-N3 SCC | ||||||||
| c) T3-T4N0 or any N + SCC | ||||||||
| Breast | Canada (Al-Rashdan et al., 2020) | ✓ | Hypo-F RT (42·5 Gy / 16 frs) | All refereed | - APBI (27 Gy / 5 frs) for suitable (40 % of referred) | |||
| - Hypo-F RT | ||||||||
| France (Belkacemi et al., 2020a) | ✓ | 50 Gy / 25 frs with 16 Gy / 8 frs boost | - 45 Gy / 18 frs | |||||
| - 40 Gy / 15 frs ± 10 Gy | ||||||||
| - 15 Gy / 6 frs | ||||||||
| - Boost: 12 Gy / 3 frs | ||||||||
| Canada (Koch et al., 2020) | ✓ | a) Standard fractionation (50 Gy / 25 frs) | a) Hypo-F RT (40·5 Gy / 15 frs) for breast RT, including regional node irradiation | |||||
| b) 50 Gy / 25 frs for BBI and 40 Gy / 15 frs or 42·4 Gy / 16 frs for WBI | b) UK FAST-Forward trial technique (26 Gy/ 5 frs daily for WBI or PBI) | |||||||
| c) Conventional boost | c) 10 Gy / 4 frs as boost | |||||||
| Iran (Samiee et al., 2020) | ✓ | 50 Gy / 25 frs or 40 Gy / 15 frs | 40 Gy / 15 frs | |||||
| Italy, Portugal, Belgium, Australia, Switzerland, Poland (Thureau et al., 2020) | ✓ | Standard fractionation (50 Gy / 25 frs) or moderate Hypo-F RT (40 Gy / 15 frs) | a) All breast/chest wall and nodal RT | a) 40 Gy / 15 frs | ||||
| b) All patients requiring RT with node-negative tumors | b) 28-30 Gy / 5 frs (1 fr/ week) or 26 Gy / 5 frs daily | |||||||
| c) Accelerated partial breast RT can also be considered for selected low-risk patients | c) 30 Gy / 5 frs (over 2 weeks) | |||||||
| d) Omission RT and boost RT for the elderly or no significant risk factors for local relapse. | ||||||||
| USA (Dietz et al., 2020) | ✓ | a) High priority case (Locally advanced or inflammatory patients) | a) 42·5 Gy / 16 or 40 Gy / 15 frs | |||||
| b) Selected patients undergoing breast RT (without regional-nodal RT) | b) 28·5 Gy / 5 frs (1 fr/ week) | |||||||
| c) boost should be reserved for patients with the greatest absolute benefit (e.g., positive margins, age ≤ 40) | ||||||||
| Spain, UK (Pardoa et al., 2020) | ✓ | Hypo-F RT | Adjuvant irradiation | Adjuvant irradiation | ||||
| a) Any breast cancer (first choice) | a) Hypo-F RT (boost with Hypo-F RT or even integrated with whole-breast irradiation (complete the treatment in 15 frs)). | |||||||
| b) Eligible for ultra-short schedules | b) Ultra-short schedules (5-7 frs) | |||||||
| c) Whole breast and node irradiation | c) A 26 Gy / 5 frs (daily) and 29 Gy at the tumor bed with an integrated boost dose of 5·8 Gy | |||||||
| d) Partial breast irradiation (for eligible ones) | d) 5 frs × 6 Gy for a 30 Gy dose or 37·5 Gy in 3·75 Gy / fr (twice daily) on the tumor bed with a negative margin. (Brachytherapy can also be an alternative) | |||||||
| Neoadjuvant irradiation | Neoadjuvant irradiation | |||||||
| a) All the case with delayed surgery | a) 40·5 Gy / 15 frs in the breast with 54 Gy concomitant boost delivered 3·6 Gy daily. | |||||||
| b) Selected cases | b) 26 Gy / 2·6 Gy/ fr and concomitant 29-30 Gy boost in 5·7-5·8 Gy / frs at the tumor bed. | |||||||
| Elderly cases | Elderly cases | |||||||
| Hypo-F RT: | ||||||||
| -weekly 6·5 Gy dose delivered for five weeks for a total of 32·5 Gy | ||||||||
| -A boost of two 6·5 Gy / fr can be | ||||||||
| −5·5 Gy / fr will be delivered up to a total dose of 27·5 Gy if axillary nodes are to be included. | ||||||||
| UK, Netherland, Italy, Australia, Israel, Spain, Denmark, France, Norway, Brazil (Coles et al., 2020) | ✓ | a) Patients that require RT with node negative tumors (not require a boost) | a) 28-30 Gy / 5 frs (1 fr / week) or 26 Gy / 5 daily fr | |||||
| b) Patients that require RT breast/chest wall and nodal | b) Moderate Hypo-F RT: 40 Gy / 15 frs | |||||||
| UK (Higgins et al., 2020) | ✓ | Hypo-F RT: 26 Gy / 5 frs | ||||||
| France (Beddok et al., 2020) | ✓ | Hypo-F RT | ||||||
| Slovenia (Orazem and Ratosa, 2020) | ✓ | Normo-fractionation and Hypo-F RT | Increase of Hypo-F RT n (from 65% to over 80%) | |||||
| Switzerland (Achard et al., 2020) | ✓ | Normo-fractionation or moderate Hypo-F RT | - Moderate Hypo-F RT (42·5 Gy / 16 frs or 40 Gy / 15 fr) for majority of stages | |||||
| - Hypo-F RT (26 Gy / f frs daily or 28·5 Gy / 5 frs once-weekly) | ||||||||
| Zambia, USA (Lombe et al., 2020) | ✓ | 50 Gy / 25 frs | a) Breast Chest wall | a) 28·5 Gy/5 frs for 5 weeks | ||||
| b) Breast supraclavicular + chest wall | b) 40 Gy/ 10 frs | |||||||
| Belgium (Machiels et al., 2020) | ✓ | 40 Gy / 15 frs | All eligible patients adopting the Fast-Forward | Ultra-Hypo-F RT: 26 Gy / 5 frs | ||||
| regimen | + A single boost dose of 6 Gy was delivered using an IMRT technique for deeply seated tumors and a single electron field for superficial tumors | |||||||
| Canada (Patrick et al., 2020) | ✓ | 40 Gy / 15 frs | Hypo-F RT: 26 Gy / 5 frs | |||||
| Egypt, Morocco, Saudi Arabia, USA, Jordan (Elghazawy et al., 2020) | ✓ | 50 Gy / 25 frs | a) Partial breast irradiation (EBRT) | a) 30 Gy/5 frs, daily | ||||
| 28.5 Gy/5 frs, daily | ||||||||
| 38 Gy/10 frs, twice a day | ||||||||
| b) Partial breast irradiation (IORT) | b) 20 Gy once | |||||||
| c) WBRT +/- regional lymph nodes | c) -Hypo-F RT: 40.05 Gy/15 frs, daily, 3DCRT | |||||||
| - Extreme Hypo-F RT (node-negative, without boost): | ||||||||
| 28.5 Gy/5 frs, weekly or 26 Gy/5 frs, daily | ||||||||
| d) Chest wall +/- regional lymph nodes | d) 40.05 Gy/15 frs, daily, 3DCRT | |||||||
| 43.5 Gy/15 frs, daily, 3DCRT | ||||||||
| 37.5 Gy/15 frs, daily, 3DCRT | ||||||||
| USA (Ling et al., 2020) | ✓ | 40 Gy / 15 frs | a) Partial breast | a) 30 Gy / 5 frs | ||||
| b) Whole breast | b) 26 Gy / 5 frs | |||||||
| Poland (Łacko et al., 2020) | ✓ | 50 Gy / 25 frs | a) APBI: | a) 30 Gy/ 5 frs every 2nd day or IMRT technique | ||||
| -Age > 50 yrs; tumor ≤2 cm T1, negative margin width min. 2 mm without LVI, ER+, BRCA negative. | - FAST Forward: 26 Gy/ 5 frs within a week | |||||||
| -DCIS of low and medium differentiation level, detected using screening MMG, size ≤ 2 cm with negative margins ≥3 mm, located mainly on the left side. | ||||||||
| b) WBI: | b) UK FAST: 28.5 Gy /5 frs each once a week | |||||||
| -Resignation from BOOST: patients T 1-2 N0 (≤50 yrs) with negative margins ≥2 mm, without unfavorable prognostic factors (G3, DCIS component) | - FAST Forward: 26 Gy / 5 frs within a week | |||||||
| -Resignation from the radiation of patients T1, ER+, HER–, G 1-2, lymph nodes: Post-menopausal SLND up to 2 lymph nodes affected. | ||||||||
| c) WBI + BOOST ± RNI | c) SIB: 40 Gy/15 frs per breast (2.66 Gy) + 3.2 Gy per boost (total dose of 48 Gy) | |||||||
| - SIB: 42.56 Gy/16 frs per breast + 3 Gy per boost (total dose of 48 Gy) | ||||||||
| d) WBI + RNI | d) 40 Gy / 15 frs | |||||||
| e) Patients after mastectomy with breast reconstruction | e) 40 Gy 15 frs or 45 Gy / 20 frs | |||||||
| Lung | USA (Wu et al., 2020) | ✓ | ✓ | a) NSCLS | a) NSCLS | a) NSCLS | ||
| 1,2,3) 18 Gy/ 3frs, 12 Gy/ 4frs, or 10 Gy/5frs | 1) Peripheral T 1-2 N0 | 1) 34 Gy/1 fr | ||||||
| 2) Central T 1-2 N0 | 2) 50 Gy/5 frs | |||||||
| 3) Ultra-central T 1-2 N0 | 3) 60 Gy/8 frs | |||||||
| 4) 60-70 Gy/ 30-35 frs | 4) Locally advanced NSCLC | 4) 55 Gy/20 frs or 45-60 Gy/15 frs | ||||||
| 5) 54-60 Gy/ 27-30frs for margin-positiveor 50-54 Gy/ 25-30 frs for margin negative | 5) Postoperative radiation for NSCLC | 5) 50 Gy/25 frs | ||||||
| b) SCLC: | b) SCLC: | b) SCLC: | ||||||
| 1) 45 Gy in twice-daily 1·5Gy or 66-70 Gy/ 33-35frs | 1) Limited-stage SCLC (thoracic RT) | 1) 45 Gy/30 twice-daily frs | ||||||
| 2) 25 Gy/ 10frs 3,4,5) 20 Gy/5frs | 2) Limited-stage SCLC (prophylactic cranial RT) | 2) 25 Gy/10 frs vs. MRI surveillance | ||||||
| - consolidative thoracic RT: 30 Gy/10 frs | 3) Extensive-stage SCLC (thoracic RT) | 3) 30 Gy/10 frs vs. observation | ||||||
| 4) Extensive-stage SCLC (prophylactic cranial RT) | 4) MRI surveillance | |||||||
| 5) Palliative lung RT | 5) 20 Gy/5 frs, 17 Gy/2frs or 10 Gy/1 fr | |||||||
| Canada (Rathod et al., 2020) | ✓ | a) NSCLC: | a) NSCLC: | a) NSCLC: | ||||
| 60 Gy / 30 frs or 66 Gy / 33 frs | 1) peripheral | 1) SBRT: 54 Gy / 3 frs | ||||||
| 2) central | 2) SBRT: 50 Gy / 5 frs | |||||||
| 3) concurrent CTRT | 3) 60 Gy / 30 frs | |||||||
| 4) sequential CTRT | 4) 40 Gy / 15 frs or 50 Gy / 20 frs | |||||||
| b) SCLC: | b) SCLC: | b) SCLC: | ||||||
| 45 Gy / 30 frs or 66 Gy / 33 frs | 1) Limited stage: Radical | 1) 40 Gy / 15 frs | ||||||
| 2) Limited stage: PCI | 2) 25 Gy / 10 frs | |||||||
| 3) Extensive stage: Consolidation RT | 3) 25 Gy / 5frs | |||||||
| PCI: 25 Gy / 10 frs | 4) Extensive stage: PCI | 4) 25 Gy / 10 frs | ||||||
| USA (Kumar et al., 2020) | ✓ | LA-NSCLS: Hypo-F RT or standard schedules | When concurrent chemotherapy is not necessary | Hypo-F IMRT (with SIB were needed): | ||||
| a) 60 Gy/ 15 frs | ||||||||
| b) 60 Gy / 20 frs | ||||||||
| c) 55 Gy / 20 frs | ||||||||
| USA, France, China, Spain, the UK (Liao et al., 2020) | ✓ | a) NSCLC | a) NSCLC: | |||||
| 1) SABR IN 1-3 frs for stages I-II | ||||||||
| 2) 30-34 Gy / 1 fr for tumors < 2 cm and ≥ 1 cm from the chest wall | ||||||||
| 3) 48-54 Gy / 3 frs for peripheral lesions | ||||||||
| 4) 45 – 60 Gy / 4-8 frs for central and ultra-central lesions | ||||||||
| 5) 55 Gy / 20 frs for stage II-III | ||||||||
| 6) 45 Gy / 15 frs for poor performance patients | ||||||||
| b) SCLC | b) SCLC: | |||||||
| Early-stage: For the limited stage standard of care is concurrent chemoradiation with 45 Gy / 30 frs twice daily | 1) SABR in 3-5 frs, 60 Gy / 3 frs, 48 Gy / 4 frs or 50 Gy / 5 frs for stage I-II of peripheral lesions | |||||||
| 2) Early stage: 40-42 Gy / 15 frs daily or 50-55 / 20-25 frs daily | ||||||||
| Extensive stage | 3) Extensive stage: 30 Gy / 10 frs | |||||||
| c) PCI | c) PCI | |||||||
| - 25 Gy / 10 frs | 1) Can be performed during radio(chemo)therapy | |||||||
| 2) Can be omitted for p-stage I | ||||||||
| Canada (Kidane et al., 2020) | ✓ | SABR: | ||||||
| a) Early-stage (T1-T2N0M0) NSCLC (non-central tumors) | a) 30–34 Gy / 1 fr; 45–55 Gy /3–5 frs (e.g., 54/3,48/4, and 55/5); 60 Gy / 8 frs | |||||||
| b) Pulmonary oligometastases (central tumors) | b) bronchial tree (central or ultra-central tumors: 60 Gy /8 frs or 50 Gy / 5 frs) | |||||||
| USA (Ng et al., 2020b) | ✓ | Peripheral early-stage NSCLC | Single-fraction SBRT: 30 - 34 Gy | |||||
| Gastrointestinal | Esophageal | UK (Jones et al., 2020a) | ✓ | ✓ | dCRT: 2 Gy / fr | - dCRT as the most appropriate curative option for both OSCC and OAC | Definitive treatment: | |
| - dCRT (2 Gy / fr) | ||||||||
| - High-risk patients for readmission, such as those with high-grade dysphagia, may not be appropriate for dCRT | Where dCRT is unavailable or inappropriate: | |||||||
| - Hypo-F RT: | ||||||||
| - Where dCRT is unavailable or inappropriate, consider Hypo-F-dRT | 50 Gy / 16 frs tumors of up to 5 cm in length 55 Gy / 10 frs for tumors up to 10 cm in lengthNeoadjuvant: Hypo-F dCRT with 40 Gy/15 frs |
|||||||
| Brazil (Riechelmann et al., 2020) | ✓ | Early-stage | ||||||
| 1) cT2-T4 and/or clinically lymph-node positive (cN+) SCC cases | 1) Neoadjuvant chemoradiation with reduced dose (41·4 Gy) | |||||||
| 2) Patients with obstructive symptoms or hemorrhage | 2) Ultra- Hypo-F RT | |||||||
| India (Talapatra et al., 2020) | ✓ | ✓ | a) Operable patients | a) 41.4 Gy/23 frs or 40 Gy/15 frs (cCRT) | ||||
| b) Inoperable patient | b) Moderate Hypo-F RT (definitive CRT): 50 Gy/25 frs | |||||||
| c) Palliation of symptoms such as bleeding and dysphagia | c)20 Gy/5 frs or single fraction schedule (avoid protracted fractionation) | |||||||
| Pancreatic | Italy (Barcellini et al., 2020) | ✓ | Conventional RT or SBRT | Essential | CIRT | |||
| USA (Ng et al., 2020b) | ✓ | Locally advanced pancreatic cancer | Single-fraction SBRT: 25 Gy | |||||
| UK (Jones et al., 2020b) | ✓ | Conventional- or Hypo-F RT | Where surgery is unlikely to be available for the resectable and borderline disease | Hypo-F RT: | ||||
| 25–35 Gy/5 frs (RT alone) or 36 Gy/15 frs CRT with concurrent capecitabine | ||||||||
| Liver | UK (Aitken et al., 2020) | ✓ | ✓ | Standard techniques | SABR: 24 – 60 Gy /1-5 frs | |||
| Brazil (Riechelmann et al., 2020) | ✓ | Localized BCLC stage A | radiofrequency ablation or stereotactic RT | |||||
| India (Talapatra et al., 2020) | ✓ | ✓ | SBRT: | |||||
| a) Hepatocellular carcinoma | a) 48–60 Gy/3–5 frs | |||||||
| b) Oligometastases in liver | b) 16–45 Gy/1–5 frs | |||||||
| Rectal | Italy (De Felice and Petrucciani, 2020b) | ✓ | SCRT: 25 Gy / 5 frs | Locally advanced | SCRT | |||
| LCCRT: 50·4-54 Gy / 28-30 frs | ||||||||
| USA (Romesser et al., 2020) | ✓ | LCCRT (25-28 frs) | Locally advanced | SCRT | ||||
| UK (Higgins et al., 2020) | ✓ | SCRT: 25 Gy / 5 frs | ||||||
| France (Beddok et al., 2020) | ✓ | SCRT: 25 Gy / 5 frs | ||||||
| Switzerland (Achard et al., 2020) | ✓ | SCRT (neoadjuvant) | ||||||
| USA (Skowron et al., 2020) | ✓ | a) Stage I: high-risk feature patients | a) Chemoradiation as an alternative to TME | |||||
| b) Stage II or III | b) Neoadjuvant SCRT: 25 Gy / 5 frs | |||||||
| Brazil (Riechelmann et al., 2020) | ✓ | a) For cT3b/c or cN+ (middle or low rectum) with clear circumferential margins cases | a) SCRT | |||||
| b) If a major response is needed for sphincter preservation | b) LCCRT | |||||||
| c) For cT4, or threatened/involved CRM, or lateral pelvic lymph nodes, or suspected cN2/bulky LN involvement | c) neoadjuvant therapy with long-course chemoradiation or short-course radiotherapy followed by four to six cycles of chemotherapy | |||||||
| USA (Ling et al., 2020) | ✓ | SCRT: 25 Gy / 5 frs | All localized rectal cancers | SCRT: 25 Gy / 5 frs | ||||
| LCCRT: 45-50.4 Gy / 25-28 frs | ||||||||
| Genitourinary | Prostate | Italy (Barra et al., 2020) | ✓ | standard fractionation (i.e., 74–81 Gy in 37–45 frs) or Hypo-F RT (dose per fraction 2·75-3 in 20–28 frs) | Early prostate cancer | SBRT (ultra- Hypo-F RT): | ||
| 36·25 Gy in 5 frs (twice a week) | ||||||||
| The USA, UK (Zaorsky et al., 2020) | a) Localized, oligometastatic, and low volume M1 | a) Ultra- Hypo-F RT (1-6 frs) | ||||||
| b) Post-prostatectomy and clinical node positive disease. | b) Moderate Hypo-F RT (5-20 frs) | |||||||
| c) Adjuvant radiation | c) Salvage (20 frs) | |||||||
| Iran (Aghili et al., 2020) | ✓ | Standard techniques | Radiation of the whole pelvis is not intended | - SBRT | ||||
| - Abbreviated radiotherapy | ||||||||
| - A single 19 Gy /1 fr HDR brachytherapy | ||||||||
| Singapore (Tan et al., 2020) | ✓ | Standard techniques | localized prostate cancer (pT1b–T3aN0M0) | CHHiP: 60 Gy / 20 frs over four weeks or 57 Gy / 19 frs over 3·8 weeks (Dearnaley et al., 2016) | ||||
| Canada (Kokorovic et al., 2020) | ✓ | -UIR, HR, and VHR prostate cancer patients for whom RT should begin NADT | Hypo-F RT | |||||
| - High-risk features post-RP (early salvage RT) | ||||||||
| - Node-positive without evidence of further metastases | ||||||||
| - Oligometastatic HSPC | ||||||||
| Zambia, USA (Lombe et al., 2020) | ✓ | 74 Gy / 37 frs | High risk | 60 Gy/ 20 frs | ||||
| Canada (Patrick et al., 2020) | ✓ | 60 Gy / 30 frs | 36.25 Gy in 5 frs | |||||
| USA (Ling et al., 2020) | ✓ | All risk groups of localized prostate cancer | -SBRT with Ultra Hypo-F RT in 5-7 frs | |||||
| USA (Ng et al., 2020b) | ✓ | Localized prostate cancer | Single-fraction SBRT: 24 Gy | |||||
| Gynecological | Morocco (Ismaili, 2020a) | ✓ | The same as before | Not changed | ||||
| Zambia, USA (Lombe et al., 2020) | ✓ | EBRT: 50 Gy/ 25 frs | a) Cervix stage III bulky | a) 41·25 Gy / 15 frs | ||||
| Brachytherapy: 7 Gy /4 frs | b) Cervix | b) 8 Gy / 3 frs | ||||||
| 9 Gy / 2 frs one week apart; | ||||||||
| 9·4 Gy / 2 frs one week apart | ||||||||
| UK, Canada (Mendez et al., 2020) | ✓ | Standard dose/fr | Cervix | HEROICC-trial | ||||
| All but for the patients that may need elective radiotherapy to the paraaortic drainage, or if significant downstaging is necessary, like for the cases with FIGO stage IIIA–IVA. | -PTVLD = 40 Gy / 15 frs | |||||||
| -PTVHD = 48 Gy / 15 frs (SIB) | ||||||||
| -Brachytherapy as a boost to the CTVHR in early cancers | ||||||||
| Sarcoma | France (Belkacemi et al., 2020a) | ✓ | 50 Gy / 25 frs | TB: 50 Gy / 20 frs, 4 frs/week | ||||
| + boost: 10 Gy / 5 frs | +boost: 10 Gy/4 frs | |||||||
| Poland (Spalek and Rutkowski, 2020) | ✓ | Preoperative Soft tissue sarcoma: 50 Gy / 25 frs | Hypo-F RT (e.g., 28 Gy / 8 frs or 25 Gy / 5 frs) | |||||
| Canada (Patrick et al., 2020) | ✓ | Preoperative Soft tissue sarcoma: 50 Gy / 25 frs | Hypo-F RT (35 Gy / 5 frs) | |||||
| Lymphoma | France (Belkacemi et al., 2020a) | ✓ | High-grade: 40 Gy / 20 frs | 36 Gy / 12 frs, 4 frs / week | ||||
| Skin | UK (Rembielak et al., 2020) | ✓ | cSCC, MCC, and rare skin pathologies for which definitive RT should be considered | Hypo-F RT: | ||||
| a) 35 Gy / 5 frs | a) 32·5 Gy / 4 frs | |||||||
| b) 45 Gy / 10 frs | b) 40 Gy / 8 frs | |||||||
| c) 55 Gy / 20 frs | c) 50 Gy / 15 frs | |||||||
| France (Belkacemi et al., 2020a) | ✓ | 45 Gy / 15 frs, 3 frs/week | 30 Gy / 5 frs, 1 fr/week | |||||
| Australia (Veness, 2020) | ✓ | Non-Melanoma (NMSC): | 1)BCC | <70 years ECOG 0/1: | ≥80 years or ECOG 2/3 | |||
| 50–55 Gy (2-2·5 Gy / fr) | 1a) Definitive | 1a) 30–45 Gy / 5–15 frs | 1a) 15–28 Gy / 1–4 frs | |||||
| 1b) Adjuvant | 1b) 30–45 Gy / 5–15 frs | 1b) 15–28 Gy /1–4 frs | ||||||
| 1c) Adjuvant high-risk site (perioral/orbital) | 1c) 45–50 Gy /15–20 frs | 1c) 30–36 Gy / 5–6 frs | ||||||
| 2) SCC | ||||||||
| 2a) Definitive | 2a) 30–45 Gy /5–15 frs | 2a) 15–28 Gy /1–4 frs | ||||||
| 2b) Definitive high-risk site (perioral/orbital) | 2b) 45–50 Gy /15–20 frs | 2b) 15–28 Gy /1–4 frs | ||||||
| 2c) Adjuvant | 2c) 30–40 Gy /5–10 frs | 2c) 15–28 Gy /1–4 frs | ||||||
| 2d) Adjuvant high-risk site (perioral/orbital) | 2d) 45–50 Gy /15–20 frs | 2d) 30–36 Gy / 5–6 frs | ||||||
| 70–80 years ECOG 0/1: | ECOG 3/4 | |||||||
| 1a) 30–40 Gy / 5–10 frs | 1a) 15–18 Gy single frs | |||||||
| 1b) 30–40 Gy /5–10 frs | 1b) no RT | |||||||
| 1c) 40–45 Gy /10–15 frs | 1c) no RT | |||||||
| 2a) 30–40 Gy /5–10 frs | 2a) 15–18 Gy / single frs | |||||||
| 2b) 40–45 Gy /10–15 frs | 2b) 15–18 Gy / single frs | |||||||
| 2c) 30–40 Gy /5–10 frs | 2c) no RT | |||||||
| 2d) 40–45 Gy /5–10 frs | 2d) no RT | |||||||
| Palliative | Italy (van der Linden et al., 2020) | ✓ | SFRT or MFRT | If Unavoidable | SFRT: bone metastasis | |||
| France, Switzerland, Belgium (GEMO) (Thureau et al., 2020) | ✓ | SFRT or MFRT | If Unavoidable | - SFRT: almost all | ||||
| - MFRT: adjuvant case or highly suspicious for fracture | ||||||||
| USA (Yerramilli et al., 2020b) | ✓ | a) 30 Gy /10frs | a) Brain met. For patients with urgent indications¥ | a) Brain: 20 Gy / 5 frs | ||||
| b) 8 Gy / 1 fr | b) Spinal cord compression and bone met. | b) Spinal cord and bone met.: 8 Gy/ 1 fr | ||||||
| c) 10 Gy /1 fr or 3·7 Gy / 4 frs twice daily | c) Tumor bleeding | c) 3·7 Gy / 4 twice daily fractions or 4 Gy / 5 daily fractions | ||||||
| d) 8·5 Gy / 2 weekly fractions or 4 Gy / 5 daily fractions | d) SVCO or airway obstruction | d) 8·5 Gy / 2 weekly fractions or 4 Gy / 5 daily fractions | ||||||
| Canada (Hahn et al., 2020) | ✓ | a) Tumor bleeding | a) 8 Gy / 1 fr | |||||
| b) Other Palliative RT regimen | b) 8 Gy in 0-7-21 (3 days) regimen (ensuring the final fraction is off-cord and brainstem) | |||||||
| Iran (Aghili et al., 2020) | ✓ | - 8 Gy/ 1 fr | ||||||
| - 20 Gy/ 4 frs | ||||||||
| France (Belkacemi et al., 2020a) | ✓ | 20 Gy / 5 frs | 20 Gy / 4 frs | |||||
| Canada (Rathod et al., 2020) | ✓ | 20 Gy / 5 frs | a) Stage IV NSCLC | a) 8-10 Gy / 1 fr | ||||
| 30 Gy / 10 frs | b) Extensive stage (III-IV) SCLC | b) 8 Gy / 1 fr | ||||||
| Singapore (Tan et al., 2020) | ✓ | 20 Gy / 5 frs | 8 Gy / 1 fr | |||||
| 30 Gy / 10 frs | ||||||||
| USA (Chaves et al., 2020) | ✓ | Locally advanced HNSCC | - 24 Gy / 3 frs (D0-D70D21) | |||||
| - 25 Gy / 5 frs | ||||||||
| - QUAD SHOT technique: 3·7 Gy bid given over two consecutive days, a total dose of 14·8 Gy per cycle, each cycle every four weeks | ||||||||
| Italy, Switzerland (Banna et al., 2020) | ✓ | Lung | - 8-10 Gy / 1 fr | |||||
| - 17 Gy / 2 frs | ||||||||
| USA, France, China, Spain, the UK (Liao et al., 2020) | ✓ | a) Brain | a) Brain | |||||
| - SRS: 1-3 frs | ||||||||
| - WBI: 20 Gy / 5 frs | ||||||||
| b) Lung (stage IV) | b) Lung: 8 Gy / 1 fr | |||||||
| Argentina (Ismael et al., 2020) | ✓ | - patients with spinal cord compression, | 8 Gy or 18 Gy in 3 frs | |||||
| - superior vena cava syndrome | ||||||||
| - bleeding identified by a specialist | ||||||||
| Zambia, USA (Lombe et al., 2020) | ✓ | 20 Gy/5 frs | a) Breast | a) 8 Gy/1 fr | ||||
| 41·25/15 frs | b) Cervix EBRT Stage IVA (VVF, RVF) | b) 10 Gy / 2 frs four weeks apart | ||||||
| 30 Gy/ 10 frs | c) Head and Neck | c) 20 Gy/ 5 frs | ||||||
| 20 Gy/ 5 frs or 30 Gy/ 5 frs | d) Spinal Cord Compression | d) 8 Gy/ 1 fr | ||||||
| Brazil (Riechelmann et al., 2020) | ✓ | Metastatic esophagus | single fraction or Hypo-F RT | |||||
| UK (Jones et al., 2020a) | High risk esophageal cases | - 8 Gy / 1 fr | ||||||
| Egypt, Morocco, Saudi Arabia, USA, Jordan (Elghazawy et al., 2020) | ✓ | a) Brain metastasis | a) SRS: 15 Gy/1 fr for 1–3 metastases, good KPS, no extracranial disease. | |||||
| −3D whole-brain RT:20 Gy/5 frs | ||||||||
| b) Bone metastasis | b) With or without cord compression: 8 Gy/1 fr | |||||||
| Pathological fracture: 20 Gy/5frs | ||||||||
| USA (Kang et al., 2020) | ✓ | 30 Gy/10 frs | Treatment guidelines where LRC is important: | |||||
| 20 Gy/5 frs | - Metastatic HNC in need of local therapy | - Metastatic HNC in need of local therapy: | ||||||
| a) Prior RT | a) Quad Shot (3.7 Gy/frs twice daily × 2 consecutive days = 1cycle; may repeat cycle every 3-4 weeks for up to 4 total cycles) | |||||||
| b) No prior RT | b) Quad Shot (3.7 Gy/frs twice daily × 2 consecutive days = 1 cycle; may repeat cycle every 3-4 weeks for up to 4 total cycles) | |||||||
| - Other primary cancer metastatic to H&N | - Other primary cancer metastatic to H&N: | |||||||
| Quad Shot (3.7 Gy/frs twice daily × 2 consecutive days = 1 cycle; may repeat cycle every 3-4 wk for up to 4 total cycles) | ||||||||
| Other palliative regimens: 30 Gy/10 frs, 20 Gy/5 frs, 8 Gy/1 frs | ||||||||
| USA (Ng et al., 2020b) | ✓ | Oligometastatic disease: | - Single-fraction SBRT: | |||||
| a) Lung metastasis | a) 30 Gy | |||||||
| b) Bone, lymph node, or both | b) 20 Gy | |||||||
| c) Liver metastasis | c) 18-30 Gy; 35-40 Gy | |||||||
| d) Adrenal metastasis | d) 14-18 Gy | |||||||
frs: fractions, fr: fraction, Bone-Met: bone metastases, Hypo-F RT: hypo-fractionated RT, SFRT: single fraction radiotherapy, MFRT: multiple fraction radiotherapy, GEMO: European study group of bone metastases, KPS: karnofsky performance status, dCRT :definitive chemoradiotherapy, OSCC: oesophageal squamous cell carcinoma, OAC: oesophageal adenocarcinoma, SIB: simultaneous integrated boost, SABR: stereotactic ablative radiotherapy, SVCO: superior vena cava syndrome, CIRT: carbon ion radiotherapy, SBRT: Stereotactic body radiotherapy, SCRT: Short-course radiotherapy, LCCRT: long course chemoradiotherapy, cSCC: cutaneous squamous cell carcinoma, MCC: Merkel cell carcinoma, HNSCC: head and neck squamous cell carcinoma, HPV: human papillomavirus–positive, WBI: whole breast irradiation, PBI: partial breast irradiation, LA-NSCLS: locally-advanced non-small cell lung cancer, SRS: stereotactic radiosurgery, UIR: unfavorable-intermediate-risk, HR: high-risk, VHR: very high-risk, NATD: neoadjuvant androgen-deprivation therapy, HSPC: hormone-sensitive prostate cancer, BCLC: Barcelona Clinic Liver Cancer, PTVLD: Low risk PTV, PTVHR: High risk PTV. NSCLC: non-small cell lung cancer, SCLC: small cell lung cancer, PCI: prophylactic cranial irradiation, VVF: vesicovaginal fistula; RVF: rectovaginal fistula. ¥: progressive neurologic symptom from multiple brain metastases or leptomeningeal disease, LRC: Locoregional control.
Table 4.
Summary of national consensus for applying different patient’s preparation strategies of radiotherapy departments during COVID-19 pandemic.
| Cancer | Country | Routine EBRT/BT Technique | EBRT/BT Technique during the pandemic |
|---|---|---|---|
| External beam radiotherapy | |||
| Head and neck | USA (Yanagihara et al., 2020) | Thermoplastic mask with/without an intraoral device | - Mask-on policy by fitting the thermoplastic mask to the patient after wearing a personal protective mask and cutting the end of a tongue depressor |
| - to use an open-faced thermoplastic mask and place a nonstick barrier between it and a surgical mask | |||
| Italy (Alterio et al., 2020) | Mouthpiece-assisted head and shoulder thermoplastics masks during all positioning and setup process |
The patient was asked to wear one surgical mask (or a second mask if the patient has tracheostomy) during the positioning steps. The thermoplastic mask was used after the setup confirmation. - All treatment was done by VMAT technique and image-guidance. |
|
| USA (Pannullo et al., 2020) | SRS with frame-based immobilization | SRS with mask-based treatment | |
| Breast | USA (Song et al., 2020) | ABC (DIBH) | - Voluntary DIBH |
| - Prone positioning | |||
| - Supine position with further plan optimization | |||
| Canada (Barnett et al., 2020) | ABC (DIBH) | A visually monitored voluntary breath-hold technique | |
| USA (Wright et al., 2020) | ABC | - ABC with a new single-use mouthpiece and filter kit must be used per treatment per patient. (in a case with cardiac mean dose >4 Gy or lung V20 > 40 %) | |
| - IMRT/VMAT to meet dose objectives | |||
| Italy (Youssef et al., 2020) | CBCT | CBCT with a prompt review of the lung windows is recommended | |
| France (Beddok et al., 2020) | Supine positioning, DIBH, isocentric lateral decubitus irradiation | - 4D scanner imaging and daily CBCT-based positioning | |
| - Prone position using free-breathing VMAT technique (for COVID + patients) | |||
| Slovenia (Orazem and Ratosa, 2020) | ABC | - Prone positioning | |
| - Voluntary deep inspiration breath-hold | |||
| Egypt, Morocco, Saudi Arabia, USA, Jordan (Elghazawy et al., 2020) | DIBH | -Avoid active breathing control due to the risk of aerosol contamination | |
| -Voluntary breath-holding techniques | |||
| Uveal melanoma | PTCOG (Mishra et al., 2020) | Bite block | Using chin rest or chin strap |
| Gastrointestinal | USA (Wright et al., 2020) | ABC | - Free-breathing or abdominal compression |
| Lymphoma | - 4DCT | ||
| Thoracic | - ABC with a new single-use mouthpiece and filter kit must be used per treatment per patient | ||
| Sarcoma | - IMRT/VMAT to meet dose objectives | ||
| Pediatric | |||
| Lung | USA (Kumar et al., 2020) | CBCT | - Daily image guidance using CBCT to help assess the development of infiltrates in asymptomatic patients |
| USA, France, China, Spain, the UK (Liao et al., 2020) | CBCT | IGRT (CT on rail, or CBCT) before the first fraction of the treatment | |
| France (Beddok et al., 2020) | Spirometry for respiratory gating | - 4D scanner imaging and daily CBCT-based positioning | |
| All cases with EBRT indication | Italy (Sepulcri et al., 2020) | CBCT | - Daily image guidance using CBCT |
| USA (Parashar et al., 2020) | Daily CBCT imaging | - Weekly CBCT imaging or orthogonal films, especially when motion is minimal (brain lesions). | |
| Brachytherapy | |||
| Breast, prostate, gynecologic | USA (Williams et al., 2020) | Brachytherapy: | |
| a) General anesthesia for implantations | a) Procedural sedation and analgesia (PSA): | ||
| - neuraxial analgesia (epidural, spinal, or combined spinal-epidural anesthesia; CSE) | |||
| - pudendal nerve block | |||
| - moderate sedation (midazolam and fentanyl) | |||
| - local analgesia (with topical/mucosal lidocaine and/or tissue infiltration) | |||
| b) MRI guidance for IGBT of gynecologic malignancies | b) Confined MR-based planning: | ||
| - Just CT-based planning for local cervical cancer patients with limited vaginal involvement (T1b-2a stages) | |||
| - MRI-based planning for the extra-cervical spread of malignancies (T2b-T4a stages) and choose one of these strategies: | |||
| 1) Inpatient strategy: MRI-based BT with the applicator in situ for two treatment fractions | |||
| 2) Outpatient strategy: have a pre-BT MRI and incorporated it with CT performed at implantation time | |||
| 3) Using a smit sleeve placed at first implant time for CT-based planning with subsequent MR fusion | |||
| 4) Using ‘cognitive fusion’ and contouring on a CT with the applicator in place referring to a pre- BT MRI | |||
| Breast, prostate, gynecologic, head and neck, skin | Iran () | General anesthesia for implantations | - Give priority to local or spinal anesthesia for applicator insertion |
| - Balloon or catheter-based APBI is preferred to be inserted intraoperatively | |||
| All cases with BT indication | USA (Mohindra et al., 2020) | - General anesthesia for implantations | - Consider using MRI for just the first GYN BT fraction (especially if 1 st MRI shows a minimal residual tumor) |
| India (Barthwal et al., 2020; Kumar and Dey, 2020) | - Vaginal cuff gold seeds placement for postoperative vaginal cuff BT | - Consider spinal/epidural anesthesia, oral analgesia, or intravenous conscious sedation | |
| - Avoid placement of gold seeds and consider CT for confirming vaginal applicator placement | |||
ABC (DIBH): active breathing control/coordinator (deep inspiration breath-hold), PTCOG: particle therapy co-operative group, IMRT: intensity-modulated radiotherapy, VMAT: volumetric modulated arc therapy, CBCT: cone-beam computed tomography, LA-NSCLC: locally advanced non-small cell lung cancer, IGBT: image-guided brachytherapy, APBI: accelerated partial breast irradiation, BT: brachytherapy, GYN: gynecological, SRS: stereotactic radiosurgery.
Fig. 2, Fig. 3 illustrate the distribution of selected papers versus the cancer type and the distribution of included documents concerning the countries that presented them, respectively. As shown, the number of guidelines and consensus is almost related to the frequency of cancer type with radiotherapy indication as one of the treatment strategies. For instance, breast, gynecological, and prostate cancer include more than 32 % of all diagnosed cancer type. About 23 % of all cancer patients who need to receive radiotherapy also have one of these three malignancies around the world (Joiner et al., 2019).
Fig. 2.
Distribution of papers concerning studied cancer type.
Fig. 3.
Distribution of papers concerning the origin of presented consensus or guideline.
4. Discussion
Numerous recommendations were consistently published to guide radiation oncologists in the era of the COVID-19 crisis. In the beginning, the radiotherapy of some cases was postponed; however, the pandemic has been taking an unexpectedly long time. Therefore, patient selection and prioritization protocols proposed alternative treatments and modification of delivery techniques (Chakraborty and Pandey, 2020). Making proper treatment comments require weighing the risk of infection exposure and the benefit of treatment in a careful manner. A comprehensive review was done to extract the essential recommendations and consensus for radiotherapy during the current pandemic. Fig. 1 summarized the results of the review based on the PRISMA protocol.
Fig. 2 indicates the distribution of papers versus the considered disease site in the coronavirus outbreak. As illustrated, the published recommendations' rate matches the frequency of the most common cancer type worldwide. As presented in this figure, about 24 % of the recommendations were related to the radiotherapy of breast and prostate malignancies. However, based on a recent meta-analysis, most death rates between COVID-19 infected cancer patients were associated with hematological malignancies followed by lung. The higher degree of immunosuppression utilized in treating patients with hematological malignancies was known as the reason for this significant death rate (Venkatesulu et al., 2020). Previous studies did not indicate any apparent connection between any anticancer treatment modality and the chance of COVID-19 mortality, while the higher intubation and fatality rate of cancer patients was reported (Garassino et al., 2020; Venkatesulu et al., 2020).
Fig. 3 shows the distribution of papers versus countries where released guidelines and determines treatment priorities for cancer patients during the coronavirus era. The countries extracted based on the publication's author affiliation or the propounded departments. About 29 % of these included articles came from the USA and UK based on this figure. Lots of the proposed radiotherapy guidelines are dependent on the existence of advanced radiotherapy facilities and techniques. Despite worldwide improvements in financial safety and service coverage, some significant gaps remain, particularly for the most vulnerable countries and nations such as the Asian and African countries. Many centers, even in developed countries, do not have MV/MeV radiotherapy facilities, based on the IAEA Directory of Radiotherapy Centers (DIRAC) database (I. A. E. A. (IAEA), 2021). Therefore, many centers cannot technically apply some of these recommendations, such as hypo-fractionated and short-course radiotherapy techniques.
According to Fig. 3, developing countries published less guidance to face this scope. They rarely addressed their consensus, which may be due to fewer radiotherapy centers/high-tech equipment comparing to the developed ones. Eventually, some of these prescribed consensuses or even international recommendations do not fit the facilities, equipment, and staff knowledge across the whole RT centers. Considering the availability of dedicated high-tech equipment and human resources and tailoring COVID-19 pandemic management strategies to the regional context was not only recommended but also seemed mandatory (Kochbati et al., 2020).
4.1. General prioritization of radiotherapy during COVID-19 pandemic
Table 1, Table 2 summarizes the prioritization strategies of common cancer types to mitigate the demand of EBRT and brachytherapy during this crisis retrospectively. Some authors categorized the priority scale in three levels of omission, delayed, and continuing the irradiation. Using the short-course irradiation or hypo-fractionated radiotherapy (Hypo-F RT) over normal fractionation is the most frequent and preferred standard of care for radiotherapy during the pandemic.
Recommendations support the utility of active surveillance in low-risk tumors, which permitted to defer the treatment based on the disease biology and pathology, for several months or until an expected fall or management of COVID-19 pandemic. Taking into account that deferred therapy should not lead to detrimental impacts on treatment consequences. Moreover, it suggested avoiding radiotherapy for patients with poor prognostic tumors in early-stages (e.g., Hodgkin's Lymphoma) and low-risk (e.g., postoperative radiotherapy for thymoma) disease. It was recommended to omit the treatment of palliative setting as long as the patient symptom can be under control by adopting alternative approaches, elderly patients with severe health circumstances, benign disease (e.g., keloids), and boost whenever possible (Wright et al., 2020; Simcock et al., 2020; Wallis et al., 2020). These approaches have been summarized in Table 1.
Based on the suggested prioritizations (Table 1), radiation treatment should maintain and continue according to the pre-pandemic schedule for patients underway of therapy unless the COVID-19 virus infects them. The treatment should sustain for urgent issues, where there is no alternative modality to radiation therapy and those with symptomatic metastatic disease with a life expectancy of at least 3–6 months. Preoperative RT has also been reported to buy some time for postponing surgery (Zhao et al., 2020). Deferral treatment commencement for patients with a potentially promptly growing tumor and curative intention can jeopardize outcomes and should be classified as a high priority level (Janssens et al., 2020).
Based on the published references (Table 1, Table 2), some cases including high- or intermediate-grade cancer, frail patients who are not eligible for surgery, hormone-sensitive cancers (e.g., breast and prostate cases), malignancies in locally advanced stages (e.g., breast, lung, cervix locally advanced cancers) should treat as the standard of care. Emergency cases (known as the urgent category) such as superior vena cava syndrome (SVCO), uncontrolled pain or bleeding, occlusion, and spinal cord compression are recommended for radiotherapy continuation with high priority (Ismaili, 2020b; Cruz et al., 2020).
Table 3 summarized the department's consensus for radiotherapy candidates during pandemic and indications of the feasible Hypo-F RT and short-course treatment regimens. Extending the use of an evidence-based Hypo-F RT schedule or simultaneous integrated boost (SIB) (e.g., for prostate, breast, and head and neck cases) and short-course radiation therapy (e.g., for rectal cancer) were recommended frequently. There are also other classifications based on (1) the urgent/critical and non-urgent/non-critical treatment indication, (2) high-risk/ high-grade pathological malignancy stages, (3) degree of cancer cell proliferation, (4) the feasibility of treatment options during the pandemic, and (5) patient's performance status (Wright et al., 2020; Combs et al., 2020; Simcock et al., 2020; Montesi et al., 2020b).
4.2. Comprehensive cancer-based radiotherapy guidelines during the COVID-19 pandemic
Presented consensuses and recommendations of Table 1, Table 2, Table 3 can be summarized based on the cancer type as follow:
4.2.1. Central nervous system (CNS)
In glioblastoma multiform (GBM) cases, age and karnofsky performance status (KPS) of patients introduced as the determining factors in choosing radiotherapy schedule and fractionation (e.g. KPS ≥ 70: 60 Gy / 30 frs, KPS < 70 or elderly: 40 Gy / 15 frs, KPS < 50: 34 Gy / 10 frs or 25 Gy / 5 frs) (Noticewala et al., 2020a). Continuing treatment was generally recommended for high-grade glioma cases with not poor KPS. For example, Hypo-F RT can be considered where there is not any probability of compromising outcome (e.g., for patients with brain metastases or O6- methylguanine DNA methyltransferase (MGMT) promoter- unmethylated glioblastoma) based on the ESMO recommendation to reduce hospital visits (Weller and Preusser, 2020). Stereotactic radiosurgery (SRS) was suggested for solitary or limited brain metastases (up to four lesions with less than 4 cm maximum size) with good KPS patients. SRS with 15−24 Gy can be prescribed based on the maximum lesion size. Whole-brain radiotherapy (WBRT) is still introduced as the standard of care for more or/and larger brain metastatic lesions (Table 1, Table 2, Table 3) (Di Franco et al., 2020).
4.2.2. Head and neck
For head and neck cancer patients, all indications for continuing the combined chemo-RT must be preserved following the acceptable delay time between diagnosis and RT (i.e., ≤ four weeks) or between surgery and RT (i.e., 6–8 weeks) (Belkacemi et al., 2020b). Radiotherapy omission was allowed just for benign or low-risk slow-growing lesions (Table 1). Delaying radiotherapy is also permitted not more than 4–6 weeks for COVID-19 positive cases or in cases such as melanomas, as indicated in Table 1 in detail. RT fractionation must be optimized using Hypo-F RT, simultaneous integrated boost (SIB), accelerated RT scheduling (6 frs / week), or SBRT techniques. Strong agreement was reported following ASTRO-ESTRO consensus to shift from the standard approach (2–2.4 Gy / fr) to the Hypo-F regimen (2.21–3.2 Gy / fr) or Ultra-Hypo-F RT (e.g., 8 Gy / 1 fr or 20 Gy / 4 fr) for palliative cases, during the breakdowns and shortage of RT capacities. However, in these cases, concomitant chemotherapy was restricted to the RT regimen with a prescribed dose of less than 2.4 Gy / fr (Thomson et al., 2020b). It is recommended to continue brachytherapy of oral tongue cases with high local recurrence probability and SCC of the lip, oral mucosa, and nasal region. Switching to EBRT is preferred for COVID-19 positive cases, also patients and caregivers with a higher risk of infection (Table 2).
4.2.3. Breast
Based on a previous review, the RT of breast cancer in cases with locally advanced and inflammatory, residual positive lymph node (N2), recurrent, triple-negative node-positive, and extensive lymph vascular invasion categorized with high priority indication (Zaniboni et al., 2020). The most frequent thresholds for age and maximum tumor size were 65 years old and 2.5−3 cm, respectively. Standard Hypo-F RT (i.e., 40 Gy / 15 frs), the routine schedule for breast irradiation, is the most highlighted proposed technique during the pandemic. However, using FAST (26 Gy in 5 fractions once weekly) and FAST-Forward (26 Gy in 5 fractions daily) regimens were also emphasized for a patient requiring breast or partial breast EBRT (Table 1, Table 3) and a center that dedicated with IGRT. The omission of radiotherapy was proposed for low-grade elderly patients (or post-menopausal cases) with negative lymph node involvement, ER-positive and HER2-negative case for whom adjuvant endocrine therapy was planned (Table 1). Switching to EBRT instead of BT is highly recommended due to the additional demand for resources and hospitals. However, when BT is feasible, applying HDR accelerated partial breast irradiation (APBI) or LDR interstitial brachytherapy (LDR) technique using a single applicator or needle entry was proposed for early-stage disease. It allowed a maximum delay of 12 weeks for patients' RT of ductal carcinoma in situ (DCIS) cases with high RT indication (e.g., ER-negative with positive surgical margin).
4.2.4. Lung
Almost all related kinds of literature recommended continuing RT for non-small cell lung cancer (NSCLC), limited-stage of small cell lung cancer (LS-SCLC), or palliative setting (Table 1) during the pandemic. However, they proposed to hold off RT for the extensive-stage (ES-SCLC). Delaying the prophylactic cranial irradiation (PCI) of SCLC with both limited and extensive disease was highly recommended in the COVID-19 pandemic setting (Madan et al., 2020). The stereotactic body radiotherapy (SBRT) technique with a limited fraction number is the ideal RT option during the pandemic era. For instance, the fractionation suggested for the peripheral and central tumors of NSCLC was 54 Gy / 3 frs and 50 Gy / 5 frs, respectively. Besides, for limited-stage and extensive SCLC stage, 40 Gy / 15 frs and 25 Gy / 5 frs for radical and consolidation radiotherapy, and 25 Gy / 10 frs for PCI, respectively (Rathod et al., 2020).
4.2.5. Gastrointestinal
Continuing CRT or neoadjuvant RT for esophageal cancer treatment using the Hypo-F RT regimen was frequently recommended (e.g., 50 Gy / 16 frs for tumors up to 5 cm, 55 Gy / 10 frs for tumors up to 10 cm in length, and 40 Gy/15 frs for neoadjuvant Hypo-F dCRT) (Jones et al., 2020a). Surgery can be postponed up to 3 months for these cases (Belkacemi et al., 2020b). Tumor length was defined as a restricting factor for dose per radiotherapy fraction (Table 1, Table 3).
SBRT (e.g., 24–60 Gy /1–5 frs), proton therapy, or systematic RT was suggested for the liver malignancies based on the cancer stage (Aitken et al., 2020). For locally advanced pancreatic cancer continuing with Hypo-F RT with/without SBRT technique is recommended for both unresected (single fraction SBRT (8−10 Gy) for palliation) and resected cases (SBRT: 30−33 Gy / 5 frs and without SBRT: 25 Gy / 5 frs, or 30 Gy /10 frs) (Tchelebi et al., 2020). For operable cholangiocarcinoma, surgery can be the option of cancer management. Avoiding BT was suggested for patients with esophageal- and cholangial-carcinoma until the pandemic and the risk of virus transmission reduces.
For locally advanced rectal cancer (LARC), delaying radiotherapy is not recommended to decrease the recurrence rate and increase anal sphincter preservation probability (Siavashpour et al., 2020). However, neoadjuvant short-course radiation therapy (SCRT) (i.e., 25 Gy in 5 frs) with postponed surgery (up to three months) for the intermediate- to high-risk patients can be an optimum choice based on the recommendations of the pandemic setting to decrease the frequency and duration of the patients' exposure. However, distance from the mesorectal fascia (MRF-D) is considered a restricting factor for SCRT selection. Long-course chemoradiotherapy (LCCRT) (i.e., 45−54 Gy in 25–30 frs) was suggested for patients with MRF-D ≤ 2 mm to safely delay the surgery and improve the chance of clinical response. Adjuvant RT can be omitted or postponed for early-stage and low-risk cases (Madan et al., 2020). Delaying or omitting rectal BT is recommended for all patients except for unresectable lesions, frail elderly, or medically inoperable ones (Siavashpour et al., 2020; Mohindra et al., 2020). It was suggested to continue the anal cancer radiotherapy by Hypo-F RT regimen (e.g., 30 Gy / 10−15 frs) or following the standard treatment. However, switching from BT to EBRT or IORT was suggested in these cases (Table 1, Table 2, Table 3).
4.2.6. Genitourinary
Delaying or omitting surgery for muscle-invasive bladder cancer (MIBC) patients and choosing treatment options like RT and chemotherapy may be suboptimal. However, in the COVID-19 pandemic, this delay has been avoidable due to operating room closure and saturation of ICU beds (Sarkis et al., 2020). Therefore, some recommendations were proposed for treating these patients using RT even by curative or palliative indication. Hypo-F RT was the dominant suggested regimen by, for example, 55 Gy / 20 frs and 21 Gy / 3 frs for curative and palliative purposes, respectively (Table 1). SIB technique can also be applied for the unresected cases. It's better to continue RT, but with a Hypo-F regimen (e.g., 24 Gy / 1−4 frs) for unresectable or medically inoperable renal cell carcinoma (RCC) cases.
In prostate cancer, EBRT omission and active surveillance (AS) were recommended for very low-, low-, and intermediate-to-low-risk cases during the pandemic. 3–6 months delaying radiotherapy and using AS, ADT, or hormonal deprivation can be chosen for low risk, intermediate-to-high, high-risk, or localized prostate cancer in a post-operation setting. It is recommended to continue radiotherapy for high-risk and advanced cases with curative intent. The Hypo-F RT regimen is highly preferred (Table 1, Table 3). This irradiation regimen (e.g., 36 Gy / 6 frs) is also suggested for oligometastatic disease (Belkacemi et al., 2020b). Radiotherapy omission of low-stage seminoma was also preferred. Shortening the BT fractionation of intermediate- and high-risk prostate cancer (e.g., 15 in one fraction) or ultimately shifting to the EBRT to reduce the risk of patient exposure to the infection is proposed during the pandemic.
4.2.7. Gynecological
In gynecological cancer, adjuvant treatment after surgery with curative intent has a high-priority for radiotherapy (Uwins et al., 2020). For example, not postponing EBRT or BT was highly suggested for locally advanced cervical cancer (Table 1, Table 2). In invasive uterine cervix carcinoma, it was proven to have lower tumor control and higher recurrence risk when the overall treatment time (OTT) exceed more than seven weeks, especially for squamous cell carcinoma (SCC) (Mohammadi, 2019; Siavashpour, 2016). Tanderup et al. suggested an additional 5 Gy dose to the high-risk CTV (CTVHR) to compensate for the local control loss if the OTT increases from one week to more than seven weeks (Tanderup et al., 2016). Therefore, the proposed consensus tried to align these principles and keep the OTT less as possible, even by hypo-fractionated brachytherapy (Table 3). Continuing EBRT in advanced stages or palliative situation of endometrial, ovarian, and vulvar cancer was also recommended during this crisis. Postponing BT for intermediate-risk gynecological malignancies except for cervical cancer or COVID-19 positive patients is also proposed (Table 2).
4.2.8. Sarcoma
Preoperative RT of soft tissue sarcoma (STS) is not generally accepted due to the higher risk of wound complications after radiotherapy. However, there are also some benefits for this neoadjuvant RT, such as the lower risk of tumor cell seeding during operation, lesser organs at risk exposure during radiotherapy. In the pandemic, two more benefits of decreasing the OTT and the risk of exposure to virus infection were added to this neoadjuvant treatment, especially for large border-line resectable sarcomas using the Hypo-F regimen (e.g., 28 Gy / 8 frs or 25 Gy / 5 frs) (Spalek and Rutkowski, 2020). SBRT is a good treatment option for these patients with unresectable or lung metastases from sarcoma. Preoperative RT for Ewing's sarcoma cases can be an option where surgery is not feasible or suitable (Gulia et al., 2020). In specialized and dedicated centers, HDR-BT can be employed for soft tissue cases such as rhabdomyosarcoma.
4.2.9. Pediatric
The oncologists recommend following the standard treatment for pediatrics as long as the radiotherapy has the most efficient clinical consequence (Janssens et al., 2020). Radiotherapy omission was just recommended for low-grade cases or where the palliative care is intended based on the pediatric part of Table 1, Table 2. Five priority levels were defined for continuing radiotherapy of pediatrics, dedicating higher RT priority to the medulloblastoma, high-grade ependymoma, retinoblastoma cases, and lower priority to the low-grade glioma and meningioma cases (Table 1). Continuing the brachytherapy of pediatric patients has also been emphasized in the pandemic period (Table 2).
4.2.10. Lymphoma
For aggressive disease, T-Cell and high-grade lymphomas, or for symptomatic patients continuing radiotherapy should be selected. However, RT was recommended for even early-stage Hodgkin lymphoma (HL) (Vordermark, 2020b). Radiotherapy can be ignored in old patients with low-grade lymphomas or when good results were obtained after surgery or chemotherapy (Table 1).
4.2.11. Skin
Definitive RT of melanoma, unresectable SCC and basal cell carcinoma (BCC), and rare cases of Merkel cell carcinoma (MCC) were suggested during the pandemic (Table 1). However, adjuvant RT's omission can be chosen for BCC, melanoma, and SCC with low relapse risk and when the limited benefit is expected. Delaying radiotherapy up to 3 months was proposed for non-prompt growing disease or rare skin pathologies, which were incompletely excised.
4.2.12. Palliative
Radiotherapy omission and switching to the supportive care accomplished with medical therapies were proposed for patients with short life expectancy (days to few weeks) during the coronavirus pandemic setting. These patients are usually in critical conditions that need supportive immobilization or even getting help from palliative sedation to reach stable positioning during treatment, which requires a higher number of caregivers with a higher risk of infection (Hinduja et al., 2020). For the other cases, prioritization was performed to ease patient selection for palliative RT. As mentioned, patients with neurological or airway compromise or tumor bleeding belong to the highest priority (Table 1, Table 2). Using Hypo-F RT with a short number of fractions reaches desirable outcomes for patients requiring palliation for oncologic emergencies without compromising care. For example, 20 Gy / 5 frs for brain metastasis (urgent indications), 8 Gy/ 1 fr for spinal cord compression and bone metastasis, 14.8 Gy / 4 frs twice daily or 20 Gy / 5 frs tumor bleeding, and 17 Gy / 2 weekly fractions of 20 Gy / 5 daily fractions for SVC or airway obstruction (Table 3) (Yerramilli et al., 2020a). Using SBRT or frameless SRS was also suggested for these patients where these radiotherapy techniques are feasible. Avoiding palliative BT was proposed to minimize coronavirus infection risk (Barthwal et al., 2020).
4.2.13. Benign
For the benign disease, delay of radiotherapy was proposed. BT has reasonable local control for keloid cases. However, during the pandemic setting, the risk-benefit analysis leads to BT omission and switching to EBRT, such as treatment with the electron beam.
4.3. Patient's preparation guidelines for radiotherapy during COVID-19 pandemic
Selecting the best techniques to reduce the organs at risk (OARs) doses of each patient relies highly on the center's available equipment, staff's experience, patient's anatomy, and disease site. However, another aspect added to the previous criteria by selecting the best technique for patient positioning and monitoring the simulation and RT delivery during the pandemic. By considering all these aspects and patient benefits, the radiotherapy can be performed by some delivery techniques for better patient management and positioning. Table 4 summarized some of these techniques recently addressed by radiotherapy professionals in the pre/post-pandemic era.
For instance, in breast cancer, RT delivery techniques such as deep inspiration breath-hold (DIBH) can be performed voluntarily, with moderate or active breathing control/coordinator (ABC) equipment. ABC's utility is clinically necessary to control the dose of lung and heart (for left breast cases). It is also applied for gastrointestinal, thoracic, or pediatric patients if using abdominal compression or free-breathing leads to severe and unacceptable toxicity without reaching the normal tissue safety objectives (Wright et al., 2020). CBCT or prone positioning can also be used, mostly in case of reducing the delivered dose of lung and heart, and suggested as an alternative to reduce the infection risk during the pandemic situation by the majority of departments based on Table 4 (Desai et al., 2019; Joseph et al., 2017). However, daily CBCT can prolong treatment time and increase staff and exposure risk in other points of view. Therefore, it is also recommended to pay attention to this note-getting weekly CBCT or even the use of orthogonal films (Parashar et al., 2020).
It can be more useful to apply BBD (Belly Board device) for pelvic malignancies whenever the small bowel dose could be a restrictive factor for target dose escalation in clinical routine (Estabrook et al., 2016). For lung cancer, the supine position is superior to prone orientation by mitigating the target margins (Guy et al., 2020). Nevertheless, using spirometry analysis for respiratory gating of lung cancers was also abandoned and replaced by 4D scanners usage to high-risk components management; it avoids the risk of contamination spread from breathing filters and droplet precautions (Table 4) (Beddok et al., 2020).
It was also suggested to apply a chin rest for a slit lamp exam or chin strap, rather than the bite block during the proton therapy of uveal melanoma by the Particle Therapy Co-Operative Group (PTCOG). It can decrease the salivary fluid and maintain the positioning and reproducibility accuracy in parallel to care about the cleaning condition (Mishra et al., 2020). However, it is more time consuming to use prone positioning than routines supine or acquire daily CBCT rather than using ABC for each case. However, getting daily CBCT of patients can help detect COVID-19 cases caused lung infection in asymptomatic or mildly symptomatic (Table 4) (Sepulcri et al., 2020). It is essential to distinguish between radiation-related pneumonitis and ground-glass opacity from pulmonary symptoms of COVID-19 on chest CT images of patients undergoing chest radiotherapy (Shaverdian et al., 2020).
Eventually, besides choosing the best alternative procedures, shortening treatment time is dramatically crucial to alleviate droplet transmission risk among patients during the pandemic.
Upper airway procedures should be performed using personal protective equipment (PPE) such as wearing an N95 facemask, eye shield, and gloves based on the American Academy of Otolaryngology recommendation. On the other hand, all head and neck cancer cases need a thermoplastic mask during the simulation and treatment steps. Some of these cases also require a tongue blade, individualized mouth prosthesis, or bite blocks. Using these additional setup helpers caused controversy by having PPEs during the RT steps. Therefore, the centers suggested their novel approaches for making and forming the masks and tongue depressors facing this challenge during the pandemic (Yanagihara et al., 2020; Portaluri et al., 2020).
4.4. General consideration in radiotherapy during COVID-19 pandemic
For patients with an indication of definitive CRT (dCRT), robust processes should be obeyed to ensure that their radiotherapy can uninterruptedly continue their treatment even with approved COVID-19 infection (Table 1) (Clinical guide for the management of cancerpatientsduring the coronavirus pandemic [Online], 2021). Patients with spinal cord compression, bleeding, or SVCO syndrome are such cases to follow the routines.
It was suggested to dedicate a treatment machine to these cases or treating them at the end of the day by obeying post-treatment cleaning protocols (Jones et al., 2020a). It was suggested to postpone RT for head and neck, lung, gynecological cancer cases for a few weeks until resolving symptoms and subsiding inflammation. Using prone positioning instead of the supine one with the DIBH technique was also a reported consensus for COVID-19 positive breast cancer cases (Beddok et al., 2020). Switching to EBRT (with standard or hypo-fractionated regimen) was proposed as an alternative for continuing the treatment of COVD-19 positive cancer patients with BT indication such as GYN or rectal cases (Mohindra et al., 2020).
Hypofractionation is the most reported consensus of RT departments during the COVID-19 pandemic to minimize the risk of cancer patients' contagion without reducing their treatments' effectiveness (Table 1, Table 3) (Larrea et al., 2020). However, there are some doubts about the long-term results and toxicity of the proposed treatment schedule during this pandemic crisis due to the absence of long-term randomized trials in some suggested regimes. Using SCRT for rectal cancer can be named an example, especially for those who suffered from low rectal tumors and bulky ones with a close or positive circumferential residual margin (Romesser et al., 2020). Definitive Hypo-F RT of inoperable esophageal cancer patients is another example of debate due to the increasing probability of late toxicities (Tchelebi et al., 2020; Jones et al., 2019). However, the centers accept these risks and mandate Hypo-F short-course radiotherapy to reduce patient infection likelihood with the coronavirus in the pandemic setting (Romesser et al., 2020). However, in some cases, de-escalation of treatment intensity, such as advanced head and neck cancers, is not as curable as standard care. Consequently, these patients should be discussed and informed about the risk and benefit of choosing Hypo-F and standard fractionated regimens, their frequency of hospital visits, the potential of immunosuppression, and the risk of exposure to coronavirus infection (Iqbal et al., 2020).
Furthermore, based on Table 1 and the previous published papers data, there has been a significant omission or reduction and less intensive prescribing of RT strategies for elderly patients during the pandemic (Koch et al., 2020; Zaniboni et al., 2020). Reducing hospital admission frequency and following the isolation procedures was highly recommended for fragile and low-performance patients. Based on the recent adaptive recommendations for the older cancer patients, some similar protocols such as breast cancer Hypo-F RT or IORT and avoiding boost for the early stages, rectal cancer SCRT, single-fraction RT for palliative purposes, SRS technique for early non-small cell lung cancer (NSCLC), or central nervous system (CNS) metastases (Battisti et al., 2020). However, RT omission can be justified for frail or older patients due to the reported comorbidity and poor outcome of age and COVID-19 infection (Meattini et al., 2020).
The relationship between previous suggested OARs dose constraints and the risk of mortality and morbidity was also addressed during the COVID-19 emergency of cancer patients (Kabarriti et al., 2020). These researchers performed a retrospective analysis to determine if the extent of prior lung irradiation can be a risk factor for death due to COVID-19 infection. They concluded that a mean lung dose of 7 Gy and 15 Gy yields a predicted COVID mortality rate of approximately 50 % and 75 %, respectively (Kabarriti et al., 2020). This result can be included in the previous radiobiological consideration in defining the organ at risk dose constraints and revising them, especially during pandemic conditions.
Multiple authors highlighted some issues in anticipating the pandemic's termination in their busy RT departments to incorporate new treatment techniques and management using the crisis experiences. These techniques include Hypo-F RT scheduling, real-time data monitoring by new visualization tools, telemedicine utilization, and remote working (Beddok et al., 2020; Orazem and Ratosa, 2020).
As mentioned previously, choosing shorter fractionation schedules for palliation and cure is critical to adapt to the regional health system. This technique necessarily needs the use of advanced RT skills and high-tech equipment for imaging, planning, immobilization, and treatment delivery to avoid the increasing of normal tissue toxicity; it also mandates to maintain the equivalent benefit as in conventionally fractionated radiotherapy (Kochbati et al., 2020)
Therefore, there is still a long way to reach optimum cancer treatment all over the world. It is necessary to renew some emergency national/international protocols parallel to different aspects of RT developments. Overall survival and disease-free survival of various cancer stages have been updating through the newly published references influenced by the improvements in screening culture, follow-ups, and the mentioned treatment progresses. Hence, it should be frequently renewed the patient prioritizing to receive RT according to anticipated outcomes.
The COVID-19 pandemic challenged healthcare resources by creating an extraordinary struggle. The oncology community has been suddenly required to protect a group of cancer patients. They are assumed to be susceptible to a potentially fatal infection without threatening cancer treatments. Risk-to-benefit ratios should be considered dealing with quarantine laws, shortages, lockdowns situations, and cancer treatment priorities (Poortmans et al., 2020). At the early of the pandemic, every cancerous patient was assumed to be at higher risk of mortality from COVID-19. This assumption originated from the rapid primary publications, which caused abandonment or delay of some anticancer treatments, particularly for those who were the candidate to receive systemic treatments (Poortmans et al., 2020). Some multi-central studies find no meaningful associations between the COVID-19 mortality with any cancer type and anticancer therapies such as their current radiotherapy, cytotoxic chemotherapy, hormone therapy, or targeted therapy. On the other hand, some recommended treatment protocols or RT fractionation for which the phase III trials were not done or ongoing (Simcock et al., 2020).
In conclusion, it should be acknowledged that a recent meta-analysis shows cancer patients have higher mortality, although some studies did not show a strong link (Garassino et al., 2020; Zhang et al., 2020). Therefore, it is imperative to reconsider and rethink suggested cancer care protocols during the COVID-19 outbreak. It should be discussed to consider the oncological care, individualized risk factor assessment to choose the pre-pandemic standard approach and avoiding definitive and effective treatment strategies or switching to the new therapeutic options based on the pandemic situations.
Role of the funding source
There was no funding source for this study.
Data statement
The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
CRediT authorship contribution statement
Zahra Siavashpour: Conceptualization, Data curation, Writing - original draft, Supervision. Neda Goharpey: Data curation, Writing - review & editing. Mosayyeb Mobasheri: Data curation, Resources.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Acknowledgments
The authors would like to thank physicians, medical physicists, radiotherapy technicians, and all the nursing team of Shohada-e Tajrish educational hospital, for staying on the front line during this crisis. The authors would like to acknowledge and express their gratitude to numerous radio-oncologist colleagues for their valuable contributions to distribute this survey. We are also grateful to all physicians and caregivers worldwide who are doing their best to treat cancer patients during the COVID-19 pandemic.
Biographies
Zahra Siavashpour: Ph.D. of Medical Radiation Engineering, Shahid Beheshti University, Tehran, Iran. Assistant professor of Shahid Beheshti University of Medical Sciences, Tehran, Iran, since 2018-present. Ten years of experience as a medical physicist in radiotherapy and brachytherapy departments and five years of health physicist officer experience. Three years of experience in eye plaque brachytherapy. Have been elected and member of the National Elite Foundation of Iran, 2018-Present. ORCID ID: https://orcid.org/0000-000 3-4 061-099X
Neda Goharpey: Master of Science, medical physics, Tarbiat Modares University, Tehran, Iran. The medical physicist of the radiation oncology department of Shohada-e Tajrish hospital, from September 2016 until the present. Responsible for developing and maintaining quality assurance (QA) and quality control (QC) programs of radiation therapy modalities; also preparing and validating computerized treatment plans (both 3D CRT and advanced techniques) and calculations of patient dose.
Mosayyeb Mobasheri: Ph.D. of medical physics, Tarbiat Modares University, Tehran, Iran.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.critrevonc.2021.103402.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Achard V., Aebersold D.M., Said Allal A., Andratschke N., Baumert B.G., Thomas Beer K., et al. A national survey onradiation oncology patterns of practice in Switzerland during the COVID-19 pandemic: present changes and future perspectives. Radiother. Oncol. 2020;(June) doi: 10.1016/j.radonc.2020.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghili M., Ghalehtaki R., Mousavi Darzikolaee N., Jafari F., Moshtaghian M. Radiotherapy and COVID-19: practical recommendations from iran. Radiother. Oncol. 2020;149(May):70–71. doi: 10.1016/j.radonc.2020.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghili M., Jafari F., Vand Rajabpoor M. Brachytherapy during the COVID-19- lessons from Iran. Brachytherapy. 2020;(May) doi: 10.1016/j.brachy.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken K., Good J., Hawkins M., Grose D., Mukherjee S., Harrison M., et al. Liver stereotactic ablative radiotherapy: an effective and feasible alternative to surgery during the COVID-19 pandemic. Clin. Oncol. (R Coll Radiol) 2020;32(July):p. 477. doi: 10.1016/j.clon.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkatout I., Karimi-Zarchi M., Allahqoli L. Gynecological cancers and the global COVID-19 pandemic. J. Turk. Ger. Gynecol. Assoc. 2020;21(December):272–278. doi: 10.4274/jtgga.galenos.2020.2020.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzi R., Antoniou G., Anyamene N., Hoskin P., Hughes R., Ostler P., et al. 2021. Guidance for Treatment of Uro-oncology Patients During COVID-19 Pandemic [Online]https://www.rcr.ac.uk/sites/default/files/prostate-cancer-treatment-covid19.pdf Available: [Google Scholar]
- Al-Rashdan A., Roumeliotis M., Quirk S., Grendarova P., Phan T., Cao J., et al. Adapting radiation therapy treatments for patients with breast cancer during the COVID-19 pandemic: hypo-fractionation and accelerated partial breast irradiation to address world health organization recommendations. Adv. Radiat. Oncol. 2020;(April) doi: 10.1016/j.adro.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alterio D., Volpe S., Marvaso G., Turturici I., Ferrari A., Leonardi M.C., et al. Head and neck cancer radiotherapy amid COVID-19 pandemic: report from Milan, Italy. Head Neck. 2020;(June) doi: 10.1002/hed.26319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaoui B., Semghouli S., Benjaafar N. Organization of a radiotherapy service during the COVID-19 epidemic: experience of regional center of oncology of agadir, Morocco. Radiography (Lond) 2020;(June) doi: 10.1016/j.radi.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta O., Cardona A.F., Lara-Mejia L., Heredia D., Barron F., Zatarain-Barron Z.L., et al. Recommendations for detection, prioritization, and treatment of thoracic oncology patients during the COVID-19 pandemic: the THOCOoP cooperative group. Crit. Rev. Oncol. Hematol. 2020;153(September):p. 103033. doi: 10.1016/j.critrevonc.2020.103033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhribah H., Zeitouni M., Daghistani R.A., Almaghraby H.Q., Khankan A.A., Alkattan K.M., et al. Implications of COVID-19 pandemic on lung cancer management: a multidisciplinary perspective. Crit. Rev. Oncol. Hematol. 2020;156(December):p. 103120. doi: 10.1016/j.critrevonc.2020.103120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan R., Sebastian P., Rajkrishna B., Venkatasai J.P., Backianathan S. Radiotherapeutic management of brain tumours during the COVID-19 pandemic. J. Radiother. Pract. 2020:1–4. [Google Scholar]
- Banna G., Curioni-Fontecedro A., Friedlaender A., Addeo A. How we treat patients with lung cancer during the SARS-CoV-2 pandemic: primum non nocere. ESMO Open. 2020;5(April) doi: 10.1136/esmoopen-2020-000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellini A., Vitolo V., Cobianchi L., Valvo F., Vischioni B., Bonora M., et al. Pancreatic cancer: does a short course of carbon ion radiotherapy worth during COVID-19 outbreak? Pancreatology. 2020;(May) doi: 10.1016/j.pan.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett E., Comsa D., Zhang B., Pestill T., Bradley C., Proctor L., et al. A rapid transition to voluntary breath hold from device-assisted moderate deep inspiration breath hold for patients receiving breast radiotherapy during the COVID-19 pandemic. Adv. Radiat. Oncol. 2020;(April) doi: 10.1016/j.adro.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra S., Guarnieri A., di Monale E.B.M.B., Marcenaro M., Tornari E., Belgioia L., et al. Short fractionation radiotherapy for early prostate cancer in the time of COVID-19: long-term excellent outcomes from a multicenter Italian trial suggest a larger adoption in clinical practice. Radiol. Med. 2020;(May) doi: 10.1007/s11547-020-01216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A., Apisarnthanarax S., O’Kane G.M., Sapisochin G., Beecroft R., Salem R., et al. Management of primary hepatic malignancies during the COVID-19 pandemic: recommendations for risk mitigation from a multidisciplinary perspective. Lancet Gastroenterol. Hepatol. 2020;5(August):765–775. doi: 10.1016/S2468-1253(20)30182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthwal M., Pareek V., Mallick S., Sharma D.N. Brachytherapy practice during the COVID-19 pandemic: a review on the practice changes. J. Contemp. Brachytherapy. 2020;12(August):393–396. doi: 10.5114/jcb.2020.97643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti N.M.L., Mislang A.R., Cooper L., O’Donovan A., Audisio R.A., Cheung K.L., et al. Adapting care for older cancer patients during the COVID-19 pandemic: recommendations from the International Society of Geriatric Oncology (SIOG) COVID-19 Working Group. J. Geriatr. Oncol. 2020;11(November):1190–1198. doi: 10.1016/j.jgo.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann B.C., MacArthur K.M., Brewer J.D., Mendenhall W.M., Barker C.A., Etzkorn J.R., et al. Management of primary skin cancer during a pandemic: multidisciplinary recommendations. Cancer. 2020;(June) doi: 10.1002/cncr.32969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddok A., Calugaru V., Minsat M., Dendale R., De Oliveira A., Costa E., et al. Post-lockdown management of oncological priorities and postponed radiation therapy following the COVID-19 pandemic: experience of the institut curie. Radiother. Oncol. 2020;(June) doi: 10.1016/j.radonc.2020.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkacemi Y., Krichenane G.L., Grellier N., Fonteneau G., Zaoui G., Coraggio G., et al. Radiotherapy department reorganization during the COVD-19 outbreak: keys to securing staff and patients during the first weeks of the crisis and impact on radiotherapy practice from a single institution experience. Adv. Radiat. Oncol. 2020 doi: 10.1016/j.adro.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkacemi Y., Grellier N., Ghith S., Debbi K., Coraggio G., Bounedjar A., et al. A review of the international early recommendations for departments organization and cancer management priorities during the global COVID-19 pandemic: applicability in low- and middle-income countries. Eur. J. Cancer. 2020;135(August):130–146. doi: 10.1016/j.ejca.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birtle A.J., Varughese M., James N., Huddart R., Hoskin P., Choudhury A. 2021. Guidance for Management of Urothelial Cancer during COVID-19 Pandemic [Online]https://www.rcr.ac.uk/sites/default/files/urothelial-cancer-covid19.pdf Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2021. Breast Cancer in the COVID-19 Era [Online]https://www.esmo.org/guidelines/breast-cancer/breast-cancer-in-the-covid-19-era Available: [Google Scholar]
- Brunt M., Chakraborty S., Chatterjee S., Cleator S., Coles C., Kirby A., et al. 2021. Emergency Guidelines for Pre-operative Breast Radiotherapy During the COVID-19 Pandemic [Online]https://www.rcr.ac.uk/sites/default/files/pre-operative-breast-radiotherapy-covid-19.pdf Available: [Google Scholar]
- Caicedo-Martínez M., González-Motta A., Gil-Quiñones S.R., Galvis J.C. Prostate cancer management challenges due to COVID-19 in countries with low-to-middle-income economies: a radiation oncology perspective. Rev. Mex. Urol. 2020;80:1–14. [Google Scholar]
- Cameron J. Malignant spinal cord compression (MSCC) presentation before and during Covid-19. J. Radiother. Pract. 2020:1–3. [Google Scholar]
- Carvalho H.A., Vasconcelos K., Gomes H.C., Salvajoli J.V. Impact of COVID-19 pandemic on a daily-based outpatient treatment routine: experience of a radiotherapy department of a tertiary public/university hospital in Brazil. Clinics (Sao Paulo) 2020;75:e2298. doi: 10.6061/clinics/2020/e2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty M., Pandey M. Caring for cancer patients in the Covid pandemic: choosing between the devil and deep sea. World J. Surg. Oncol. 2020;18(August):220. doi: 10.1186/s12957-020-02002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.J., Sim Y., Ow S.G.W., Lim J.S.J., Kusumawidjaja G., Zhuang Q., et al. The impact of COVID-19 on and recommendations for breast cancer care: the Singapore experience. Endocr. Relat. Cancer. 2020;27(September):R307–R327. doi: 10.1530/ERC-20-0157. [DOI] [PubMed] [Google Scholar]
- Chaves A.L.F., Castro A.F., Marta G.N., Junior G.C., Ferris R.L., Giglio R.E., et al. Emergency changes in international guidelines on treatment for head and neck cancer patients during the COVID-19 pandemic. Oral Oncol. 2020;107(April):p. 104734. doi: 10.1016/j.oraloncology.2020.104734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2021. Clinical Guide for the Management of Cancerpatientsduring the Coronavirus Pandemic [Online]https://www.uhb.nhs.uk/coronavirus-staff/downloads/pdf/CoronavirusCancerManagement.pdf Available: [Google Scholar]
- Coles C.E., Aristei C., Bliss J., Boersma L., Brunt A.M., Chatterjee S., et al. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin. Oncol. (R. Coll. Radiol.) 2020;32(May):279–281. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo I., Zaccarelli E., Del Grande M., Tomao F., Multinu F., Betella I., et al. ESMO management and treatment adapted recommendations in the COVID-19 era: gynaecological malignancies. ESMO Open. 2020;5(July) doi: 10.1136/esmoopen-2020-000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs S.E., Belka C., Niyazi M., Corradini S., Pigorsch S., Wilkens J., et al. First statement on preparation for the COVID-19 pandemic in large German Speaking University-based radiation oncology departments. Radiat. Oncol. 2020;15(April):p. 74. doi: 10.1186/s13014-020-01527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counago F., Navarro-Martin A., Luna J., Rodriguez de Dios N., Rodriguez A., Casas F., et al. GOECP/SEOR clinical recommendations for lung cancer radiotherapy during the COVID-19 pandemic. World J. Clin. Oncol. 2020;11(August):510–527. doi: 10.5306/wjco.v11.i8.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz M.C., Flores J.A.S., Paule J.R., Macalalad J.M., Lacuesta A.V.A., Francisco M.C., et al. Adapting to the contemporary normal in cancer management and workflow during COVID-19 situation in the Philippines: multi-cancer center collaborative approach. Radiother. Oncol. 2020;150(September):70–72. doi: 10.1016/j.radonc.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chargari Cyrus, Chopra Supriya, Viswanathan Akila N., Deutsch Eric. Brachytherapy issues and priorities in the context of COVID-19 outbreak. Adv. Radiat. Oncol. 2020 doi: 10.1016/j.adro.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice F., Petrucciani N. Treatment approach in locally advanced rectal cancer during coronavirus (COVID-19) pandemic: long course or short course? Colorectal Dis. 2020;22(June):642–643. doi: 10.1111/codi.15058. [DOI] [PubMed] [Google Scholar]
- De Felice F., Petrucciani N. Treatment approach in locally advanced rectal cancer during coronavirus (COVID-19) pandemic: long course or short course? Colorectal Dis. 2020;(April) doi: 10.1111/codi.15058. [DOI] [PubMed] [Google Scholar]
- De Felice F., Polimeni A., Tombolini V. The impact of Coronavirus (COVID-19) on head and neck cancer patients’ care. Radiother. Oncol. 2020;147(Mar ch):84–85. doi: 10.1016/j.radonc.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearnaley D., Syndikus I., Mossop H., Khoo V., Birtle A., Bloomfield D., et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(August):1047–1060. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N., Currey A., Kelly T., Bergom C. Nationwide trends in heart-sparing techniques utilized in radiation therapy for breast cancer. Adv. Radiat. Oncol. 2019;4(Apr-Jun):246–252. doi: 10.1016/j.adro.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan A., Mitra S., Aggarwal S., Barik S., Kaur I., Umesh P., et al. Management of cervical cancer during the corona virus disease-19 (COVID-19) era. Br. J. Radiol. 2021;94(January):p.20200686. doi: 10.1259/bjr.20200686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciaccio P., McCaughan G., Trotman J., Ho P.J., Cheah C.Y., Gangatharan S., et al. Australian and New Zealand consensus statement on the management of lymphoma, chronic lymphocytic leukaemia and myeloma during the COVID-19 pandemic. Intern. Med. J. 2020;50(June):667–679. doi: 10.1111/imj.14859. [DOI] [PubMed] [Google Scholar]
- Di Franco R., Borzillo V., D’Ippolito E., Scipilliti E., Petito A., Facchini G., et al. COVID-19 and radiotherapy: potential new strategies for patients management with hypofractionation and telemedicine. Eur. Rev. Med. Pharmacol. Sci. 2020;24(December):12480–12489. doi: 10.26355/eurrev_202012_24044. [DOI] [PubMed] [Google Scholar]
- Dietz J.R., Moran M.S., Isakoff S.J., Kurtzman S.H., Willey S.C., Burstein H.J., et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. The COVID-19 pandemic breast cancer consortium. Breast Cancer Res. Treat. 2020;181(June):487–497. doi: 10.1007/s10549-020-05644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans A.C., Soo R.A., Jazieh A.R., Rice S.J., Kim Y.T., Teo L.L.S., et al. Treatment guidance for patients with lung cancer during the coronavirus 2019 pandemic. J. Thorac. Oncol. 2020;15(July):1119–1136. doi: 10.1016/j.jtho.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S., Luo C., Hu X., Zhang J., Cai Q., Qian Y., et al. Expert consensus for treating cancer patients during the pandemic of SARS-CoV-2. Front. Oncol. 2020;10:p. 1555. doi: 10.3389/fonc.2020.01555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elghazawy H., Bakkach J., Zaghloul M.S., Abusanad A., Hussein M.M., Alorabi M., et al. Implementation of breast cancer continuum of care in low- and middle-income countries during the COVID-19 pandemic. Future Oncol. 2020;16(November):2551–2567. doi: 10.2217/fon-2020-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkhouly E.A., Salem R.H., Haggag M. Should cancer treatment be continued during the COVID-19 pandemic? A single Egyptian institution experience. Ecancermedicalscience. 2020;14:1077. doi: 10.3332/ecancer.2020.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge Christen R., Beriwal Sushil, Chargari Cyrus, Chopra Supriya, Erickson Beth A., Gaffney David K., Jhingran Anuja, Klopp Ann H., Small William, Jr, Yashar Catheryn M., Viswanathan Akila N., Christen S.B., Elledge R., Chargari Cyrus, Choprad Supriya, Erickson e Beth A., Gaffney David K. Radiation therapy for gynecologic malignancies during the COVID-19 pandemic: international expert consensus recommendations. Gynecol. Oncol. 2020 doi: 10.1016/j.ygyno.2020.06.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElMajjaoui S., Ismaili N., Benjaafar N. COVID-19, brachytherapy, and gynecologic cancers: a moroccan experience. SN Compr. Clin. Med. 2020;(July):1–4. doi: 10.1007/s42399-020-00402-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrook N.C., Bartlett G.K., Compton J.J., Cardenes H.R., Das I.J. Role of belly board device in the age of intensity modulated radiotherapy for pelvic irradiation. Med. Dosim. 2016;41:300–304. doi: 10.1016/j.meddos.2016.07.002. Winter. [DOI] [PubMed] [Google Scholar]
- Faivre-Finn C., Fenwick J.D., Franks K.N., Harrow S., Hatton M.Q.F., Hiley C., et al. Reduced fractionation in lung Cancer patients treated with curative-intent radiotherapy during the COVID-19 pandemic. Clin. Oncol. (R. Coll. Radiol.) 2020;(May) doi: 10.1016/j.clon.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garassino M.C., Whisenant J.G., Huang L.C., Trama A., Torri V., Agustoni F., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(July):914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garganese G., Tagliaferri L., Fragomeni S.M., Lancellotta V., Colloca G., Corrado G., et al. Personalizing vulvar cancer workflow in COVID-19 era: a proposal from Vul.CAn MDT. J. Cancer Res. Clin. Oncol. 2020;146(October):2535–2545. doi: 10.1007/s00432-020-03312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths W., Frew J.A., Chandler R., Jiang X.Y., Pedley I.D., Pearson R.A. Prostate ultrahypofractionation - rising to challenges presents opportunities in the COVID-19 era. Clin. Oncol. (R Coll Radiol) 2021;33(January):p. e90. doi: 10.1016/j.clon.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2021. Guidance for Radiotherapy for Gynaecological Cancer and COVID-19 [Online]https://www.rcr.ac.uk/sites/default/files/gynae-cancer-treatment-covid19.pdf Available: [Google Scholar]
- Gulia A., Arora R.S., Panda P.K., Raja A., Tiwari A., Bakhshi S., et al. Adapting management of sarcomas in COVID-19: an evidence-based review. Indian J. Orthop. 2020;(May):1–13. doi: 10.1007/s43465-020-00143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundavda M.K., Gundavda K.K. Cancer or COVID-19? A review of guidelines for safe cancer care in the wake of the pandemic. SN Compr. Clin. Med. 2020;(November):1–11. doi: 10.1007/s42399-020-00632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta T., Singh V.P., Balasubramian A., Menon H., Kurkure P.A., Kumar S., et al. ISNO position statement on treatment guidance in neuro-oncology during pandemics. Neurol. India. 2020;68(July-August):769–773. doi: 10.4103/0028-3886.293460. [DOI] [PubMed] [Google Scholar]
- Gupta T., Ghosh-Laskar S., Agarwal J.P. Resource-sparing curative-intent hypofractionated-accelerated radiotherapy in head and neck cancer: more relevant than ever before in the COVID era. Oral Oncol. 2020;111(December):105045. doi: 10.1016/j.oraloncology.2020.105045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta T., Agarwal J.P., Bentzen S.M. Comment on "Practice recommendations for risk-adapted head and neck cancer radiotherapy during the COVID-19 pandemic: an ASTRO-ESTRO consensus statement. Int. J. Radiat. Oncol. Biol. Phys. 2020;(May) doi: 10.1016/j.ijrobp.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy Christopher L., Weiss Elisabeth, Rosu-Bubulac Mihaela. Characterization of respiration-induced motion in prone versus supine patient positioning for thoracic radiation therapy. Adv. Radiat. Oncol. 2020;5:466–472. doi: 10.1016/j.adro.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn E., Livergant J., Millar B.A., Ringash J., Wong R., Dawson L.A., et al. Comments on the publication by Yerramilli et al. titled "Palliative Radiotherapy for Oncologic emergencies in the setting of COVID-19: approaches to Balancing Risks and Benefits. Adv. Radiat. Oncol. 2020;(May) doi: 10.1016/j.adro.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins E., Walters S., Powell E., Staffurth J. The impact of the acute phase of COVID-19 on radiotherapy demand in South East Wales. Clin. Oncol. (R. Coll. Radiol.) 2020;(May) doi: 10.1016/j.clon.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinduja R.H., George K., Barthwal M., Pareek V. Radiation oncology in times of COVID-2019: a review article for those in the eye of the storm - an Indian perspective. Semin. Oncol. 2020;47(October):315–327. doi: 10.1053/j.seminoncol.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosni A., Chiu K., Huang S.H., Xu W., Huang J., Bayley A., et al. Non-operative management for oral cavity carcinoma: definitive radiation therapy as a potential alternative treatment approach. Radiother. Oncol. 2020;154(August):70–75. doi: 10.1016/j.radonc.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseiny M., Kooraki S., Gholamrezanezhad A., Reddy S., Myers L. Radiology perspective of coronavirus disease 2019 (COVID-19): lessons from severe acute respiratory syndrome and middle east respiratory syndrome. AJR Am. J. Roentgenol. 2020;214(May):1078–1082. doi: 10.2214/AJR.20.22969. [DOI] [PubMed] [Google Scholar]
- Huang S.H., O’Sullivan B., Su J., Ringash J., Bratman S.V., Kim J., et al. Hypofractionated radiotherapy alone with 2.4 Gy per fraction for head and neck cancer during the COVID-19 pandemic: the Princess Margaret experience and proposal. Cancer. 2020;(June) doi: 10.1002/cncr.32968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang E.S., Balch C.M., Balch G.C., Feldman S.M., Golshan M., Grobmyer S.R., et al. Surgical oncologists and the COVID-19 pandemic: guiding cancer patients effectively through turbulence and change. Ann. Surg. Oncol. 2020;27(August):2600–2613. doi: 10.1245/s10434-020-08673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- I. A. E. A. (IAEA) 2021. DIrectory of RAdiotherapy Centres (DIRAC) [Online]https://dirac.iaea.org/Data/CountriesLight Available: [Google Scholar]
- Iqbal M.S., Warner L., Paleri V., Kovarik J., Kelly C. De-intensification of treatment in human papilloma virus related oropharyngeal carcinoma: patient choice still matters for de-escalation and for the COVID era. Oral Oncol. 2020;105(June):104768. doi: 10.1016/j.oraloncology.2020.104768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismael J., Losco F., Quildrian S., Sanchez P., Pincemin I., Lastiri J., et al. Multidisciplinary approach to COVID-19 and cancer: consensus from scientific societies in Argentina. Ecancermedicalscience. 2020;14:1044. doi: 10.3332/ecancer.2020.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismaili N. COVID-19 and gynecological cancers: a Moroccan point-of-view. Radiother. Oncol. 2020;148(April):227–228. doi: 10.1016/j.radonc.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismaili N. COVID-19 recommendations for patients with cancer: the post-COVID-19 era. SN Compr Clin Med. 2020;(August):1–6. doi: 10.1007/s42399-020-00425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismaili N., El Majjaoui S. Management of breast cancer during COVID-19 pandemic in Morocco. Breast J. 2020;26(August):1618–1619. doi: 10.1111/tbj.13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismaili N., Elmajjaoui S. COVID-19 and gynecological cancers: a summary of international recommendations. SN Compr Clin Med. 2020;(August):1–8. doi: 10.1007/s42399-020-00475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalali R., Goda J.S., Patil V. Coronavirus disease 2019 pandemic and its implications on triaging patients with brain tumors for surgery, radiotherapy, and chemotherapy. Cancer Res. Statistics Treat. 2020;3:49–53. [Google Scholar]
- Janssens G.O., Mandeville H.C., Timmermann B., Maduro J.H., Alapetite C., Padovani L., et al. A rapid review of evidence and recommendations from the SIOPE radiation oncology working group to help mitigate for reduced paediatric radiotherapy capacity during the COVID-19 pandemic or other crises. Radiother. Oncol. 2020;148(April):216–222. doi: 10.1016/j.radonc.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner Michael C., van der Kogel Albert J., Gordon Steel G. In: Basic Clinical Radiobiology. 5th ed. Joiner M.C., Kogel A.V.D., editors. Taylor & Francis Group, LLC; 2019. Introduction: the significance of radiobiology and radiotherapy for cancer treatment. [Google Scholar]
- Jones C., Crosby T. 2021. Considerations for Treatment of Oesophagogastric Cancers Within the United Kingdom During the COVID-19 Pandemic [Online]https://www.rcr.ac.uk/sites/default/files/upper-gi-cancer-treatment-covid19.pdf Available: [Google Scholar]
- Jones C.M., Spencer K., Hitchen C., Pelly T., Wood B., Hatfield P., et al. Hypofractionated radiotherapy in oesophageal cancer for patients unfit for systemic therapy: a retrospective single-centre analysis. Clin. Oncol. (R. Coll. Radiol.) 2019;31(June):356–364. doi: 10.1016/j.clon.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Jones C.M., Hawkins M., Mukherjee S., Radhakrishna G., Crosby T. Considerations for the treatment of oesophageal cancer with radiotherapy during the COVID-19 pandemic. Clin. Oncol. (R. Coll. Radiol.) 2020;32(June):354–357. doi: 10.1016/j.clon.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.M., Radhakrishna G., Aitken K., Bridgewater J., Corrie P., Eatock M., et al. Considerations for the treatment of pancreatic cancer during the COVID-19 pandemic: the UK consensus position. Br. J. Cancer. 2020;123(September):709–713. doi: 10.1038/s41416-020-0980-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph K., Warkentin H., Ghosh S., Polkosnik L.A., Powell K., Brennan M., et al. Cardiac-sparing radiation therapy using positioning breast shell for patients with left-sided breast cancer who are ineligible for breath-hold techniques. Adv. Radiat. Oncol. 2017;2(October-December):532–539. doi: 10.1016/j.adro.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabarriti R., Brodin N.P., Maron M.I., Tomé W.A., Halmos B., Guha C., et al. Extent of prior lung irradiation and mortality in COVID-19 patients with a cancer history. Adv. Radiat. Oncol. 2020 doi: 10.1016/j.adro.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.J., Wong R.J., Sherman E.J., Rybkin A., McBride S.M., Riaz N., et al. The 3 Bs of cancer care amid the COVID-19 pandemic crisis: "Be safe, be smart, be kind"-A multidisciplinary approach increasing the use of radiation and embracing telemedicine for head and neck cancer. Cancer. 2020;126(September):4092–4104. doi: 10.1002/cncr.33031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidane B., Spicer J., Kim J.O., Fiset P.O., Abdulkarim B., Malthaner R., et al. SABR-BRIDGE: stereotactic ablative radiotherapy before resection to avoid delay for early-stage lung cancer or oligomets during the COVID-19 pandemic. Front. Oncol. 2020;10:p. 580189. doi: 10.3389/fonc.2020.580189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C.A., Lee C., Liu Z.A., Liu F.F., Fyles A., Han K., et al. Rapid adaptation of breast radiotherapy utilization during the COVID-19 pandemic at a large academic Cancer Centre in Canada. Adv. Radiat. Oncol. 2020 doi: 10.1016/j.adro.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochbati L., Vanderpuye V., Moujahed R., Rejeb M.B., Naimi Z., Olasinde T. Cancer care and COVID-19: tailoring recommendations for the African radiation oncology context. Ecancermedicalscience. 2020;14:1144. doi: 10.3332/ecancer.2020.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokorovic A., So A.I., Hotte S.J., Black P.C., Danielson B., Emmenegger U., et al. A Canadian framework for managing prostate cancer during the COVID-19 pandemic: recommendations from the Canadian Urologic Oncology Group and the Canadian Urological Association. Can. Urol. Assoc. J. 2020;14(June):163–168. doi: 10.5489/cuaj.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;(May) doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D., Dey T. Recapitulating intracavitary brachytherapy in cervical cancer patients during the COVID-19 pandemic: a viewpoint. Future Oncol. 2020;16(October):2143–2146. doi: 10.2217/fon-2020-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Chmura S., Robinson C., Lin S.H., Gadgeel S.M., Donington J., et al. Alternative multidisciplinary management options for locally advanced NSCLC during the coronavirus disease 2019 global pandemic. J. Thorac. Oncol. 2020;28(April) doi: 10.1016/j.jtho.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwek J.W., Chan J.J., Kanesvaran R., Wang M.L.C., Neo P.S.H., Chia C.S., et al. Early outcomes of a national Cancer center’s strategy against COVID-19 executed through a disease outbreak response taskforce. JCO oncology practice. 2021;(January) doi: 10.1200/OP.20.00535. p. OP2000535. [DOI] [PubMed] [Google Scholar]
- Łacko A., Maciejczyk A., Kasprzak P., Dupla D., Senkus E., Wysocki W., et al. Proposals for the modification of diagnostics and combination treatment of breast cancer during the COVID-19 pandemic. Nowotw. J. Oncol. 2020;70:77–84. [Google Scholar]
- Lancia A., Bonzano E., Bottero M., Camici M., Catellani F., Ingrosso G. Radiotherapy in the era of COVID-19. Expert Rev. Anticancer Ther. 2020;(June) doi: 10.1080/14737140.2020.1785290. [DOI] [PubMed] [Google Scholar]
- Larrea L., Lopez E., Antonini P., Gonzalez V., Berenguer M.A., Banos M.C., et al. COVID-19: hypofractionation in the Radiation Oncology Department during the’ state of alarm’: first 100 patients in a private hospital in Spain. Ecancermedicalscience. 2020;14:p. 1052. doi: 10.3332/ecancer.2020.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L.Y.W., Cazier J.B., Starkey T., Turnbull C.D., U.K.C.C.M.P. Team, Kerr R., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;(May) doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., Kim T., Kim M., Suh D.H., Park J.Y., Lim M.C., et al. Recommendations for gynecologic cancer care during the COVID-19 pandemic. J. Gynecol. Oncol. 2020;31(July):p. e69. doi: 10.3802/jgo.2020.31.e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S., Talapatra K. Radiotherapy management of rectal cancer in the backdrop of the COVID pandemic. Cancer Rep (Hoboken) 2020;(December):p. e1320. doi: 10.1002/cnr2.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z., Rivin del Campo E., Salem A., Pang Q., Liu H., Guerra J.Ls.L. Optimizing lung cancer radiation treatment worldwide in COVID-19 outbreak. Lung Cancer. 2020 doi: 10.1016/j.lungcan.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling D.C., Vargo J.A., Beriwal S. Breast, Prostate, and Rectal Cancer: Should 5-5-5 Be a New Standard of Care? Int. J. Radiat. Oncol. Biol. Phys. 2020;108(October):390–393. doi: 10.1016/j.ijrobp.2020.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombe D.C., Mwaba C.K., Msadabwe S.C., Banda L., Mwale M., Pupwe G., et al. Zambia’s National Cancer centre response to the COVID-19 pandemic-an opportunity for improved care. Ecancermedicalscience. 2020;14:p. 1051. doi: 10.3332/ecancer.2020.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther A., Agrawal A. A practical approach to the management of breast cancer in the COVID-19 era and beyond. Ecancermedicalscience. 2020;14:1059. doi: 10.3332/ecancer.2020.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiels M., Weytjens R., Bauwens W., Vingerhoed W., Billiet C., Huget P., et al. Accelerated adaptation of ultrahypofractionated radiation therapy for breast Cancer at the time of the COVID-19 pandemic. Clin. Oncol. (R. Coll. Radiol.) 2020;(December) doi: 10.1016/j.clon.2020.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan A., Siglin J., Khan A. Comprehensive review of implications of COVID-19 on clinical outcomes of cancer patients and management of solid tumors during the pandemic. Cancer Med. 2020;9(December):9205–9218. doi: 10.1002/cam4.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoodzadeh H., Hadjilooei F., Omranipour R., Esmati E., Alipour S., Shahi F. Management policies of breast cancer surgery, chemotherapy and radiotherapy during COVID-19 outbreak in Iran. WCRJ. 2020;7:e1623. [Google Scholar]
- Mandeville H.C. 2021. Prioritisation of Paediatric and TYA Patients (with Paediatric-type Tumours) for Radiotherapy During the COVID-19 Pandemic [Online]https://www.rcr.ac.uk/sites/default/files/paediatric-tya-patients-radiotherapy-covid19.pdf Available: [Google Scholar]
- Manjandavida F.P., Honavar S.G., Kim U., Singh U., Menon V., Das S., et al. Ocular oncology practice guidelines during COVID-19 pandemic-An expert consensus. Indian J. Ophthalmol. 2020;68(July):1281–1291. doi: 10.4103/ijo.IJO_1669_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoj Gowda S., Kabeer K.K., Jafferbhoy S., Marla S., Soumian S., Misra V., et al. Breast cancer management guidelines during COVID-19 pandemic. Indian J. Surg. 2020;(July):1–8. doi: 10.1007/s12262-020-02466-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus S., Mahajan R. The impact of COVID-19 on radiation oncology and cancer care: a perspective from the Cancer belt region of India. Asian J. Oncol. 2020 [Google Scholar]
- Marshall J.L., Yarden R.I., Weinberg B.A. Colorectal cancer care in the age of coronavirus: strategies to reduce risk and maintain benefit. Colorectal Cancer. 2020 [Google Scholar]
- Martell K., McGeachy P., Quon H., Quirk S., Roumeliotis M., Husain S., et al. Rapid implementation of extreme hypofractionation protocols in prostate cancer using RapidPlan(R) in response to COVID-19. Radiother. Oncol. 2020;151(October):296–297. doi: 10.1016/j.radonc.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M., Guerrero-Zotano A., Montero A., Jara C., Filipovich E., Rojo F., et al. GEICAM guidelines for the management of patients with breast cancer during the COVID-19 pandemic in Spain. Oncologist. 2020;25(September):e1339–e1345. doi: 10.1634/theoncologist.2020-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meattini I., Franco P., Belgioia L., Boldrini L., Botticella A., De Santis M.C., et al. Radiation therapy during the coronavirus disease 2019 (covid-19) pandemic in Italy: a view of the nation's young oncologists. ESMO Open. 2020;5(April) doi: 10.1136/esmoopen-2020-000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez L.C., Raziee H., Davidson M., Velker V., D’Souza D., Barnes E., et al. Should we embrace hypofractionated radiotherapy for cervical cancer? A technical note on management during the COVID-19 pandemic. Radiother. Oncol. 2020;148(May):270–273. doi: 10.1016/j.radonc.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miriyala R., Mahantshetty U. Brachytherapy in cervical cancer radiotherapy during COVID-19 pandemic crisis: problems and prospects. J. Contemp. Brachytherapy. 2020;12(June):290–293. doi: 10.5114/jcb.2020.96873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra K.K., Afshar A., Thariat J., Shih H.A., Scholey J.E., Daftari I.K., et al. Practice considerations for proton beam radiotherapy of uveal melanoma during the COVID-19 pandemic: PTCOG Ocular experience. Adv. Radiat. Oncol. 2020;(April) doi: 10.1016/j.adro.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi Reza, et al. Evaluation of deformable image registration algorithm for determination of accumulated dose for brachytherapy of cervical cancer patients. J. Contemp. Brachytherapy. 2019 doi: 10.5114/jcb.2019.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., P. Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 2009;62(October):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4(January):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohindra P., Beriwal S., Kamrava M. Proposed brachytherapy recommendations (practical implementation, indications, and dose fractionation) during COVID-19 pandemic. Brachytherapy. 2020;(May) doi: 10.1016/j.brachy.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesi Giampaolo, et al. Radiotherapy during COVID‑19 pandemic. How to create a No fly zone: a Northern Italy experience. Radiol. Med. 2020;125:600–603. doi: 10.1007/s11547-020-01217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesi G., Di Biase S., Chierchini S., Pavanato G., Virdis G.E., Contato E., et al. Radiotherapy during COVID-19 pandemic. How to create a No fly zone: a Northern Italy experience. Radiol. Med. 2020;125(June):600–603. doi: 10.1007/s11547-020-01217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muirhead R., Jacobs C., Weaver A., Mukerjee S., Owens R. 2021. Lower GI Response to the COVID-19 Outbreak [Online]https://www.rcr.ac.uk/sites/default/files/lower-gi-cancer-treatment-covid19.pdf Available: [Google Scholar]
- Mukherjee S., Jones C. 2021. Considerations for Treatment of Pancreatic Cancer within the United Kingdom During the COVID-19 Pandemic [Online]https://www.rcr.ac.uk/sites/default/files/pancreatic-cancer-treatment-covid19.pdf Available: [Google Scholar]
- Mummudi N., Tibdewal A., Gupta T., Patil V., Prabhash K., Agarwal J.P. Tackling brain metastases from lung cancer during the COVID-19 pandemic. Cancer Rep (Hoboken) 2020;(September):p. e1276. doi: 10.1002/cnr2.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami N., Nakamura S., Kashihara T., Inaba K., Kaneda T., Takahashi K., et al. Increased number of prostate cancer patients selecting high dose-rate interstitial brachytherapy during the COVID-19 pandemic. Radiother. Oncol. 2020;154(November):274–275. doi: 10.1016/j.radonc.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahm S.H., Rembielak A., Peach H., Lorigan P.C., Contributing C. Consensus guidelines for the management of melanoma during the COVID-19 pandemic: surgery, systemic anti-cancer therapy, radiotherapy and follow-up. Clin. Oncol. (R. Coll. Radiol.) 2021;33(January):e54–e57. doi: 10.1016/j.clon.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2021. Neuro-oncology Treatment Guidance during COVID-19 Pandemic [Online]https://www.rcr.ac.uk/sites/default/files/neuro-oncology-treatment-covid-19.pdf Available: [Google Scholar]
- Ng C.W.Q., Tseng M., Lim J.S.J., Chan C.W. Maintaining breast cancer care in the face of COVID-19. Br. J. Surg. 2020;107(September):1245–1249. doi: 10.1002/bjs.11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S.S.W., Ning M.S., Lee P., McMahon R.A., Siva S., Chuong M.D. Single-fraction stereotactic body radiation therapy: a paradigm during the coronavirus disease 2019 (COVID-19) pandemic and beyond? Adv. Radiat. Oncol. 2020;5(July-August):761–7612773. doi: 10.1016/j.adro.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noticewala S.S., Ludmir E.B., Bishop A.J., Chung C., Ghia A.J., Grosshans D., et al. Radiation for glioblastoma in the era of COVID-19: patient selection and hypofractionation to maximize benefit and minimize risk. Adv. Radiat. Oncol. 2020 doi: 10.1016/j.adro.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noticewala S.S., Ludmir E.B., Bishop A.J., Chung C., Ghia A.J., Grosshans D., et al. Radiation for glioblastoma in the era of coronavirus disease 2019 (COVID-19): patient selection and hypofractionation to maximize benefit and minimize risk. Adv. Radiat. Oncol. 2020;5(July-August):743–745. doi: 10.1016/j.adro.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2021. Novel Corona Virus Update [Online]https://www.who.int/docs/default-source/maldives/novel-corona-virus-update-mav-30jan.pdf?sfvrsn=a10dfb7b_2 Available: [Google Scholar]
- O’Cathail S.M., Gilbert D.C., Sebag-Montefiore D., Muirhead R. Challenges and consequences of COVID-19 in the management of anorectal cancer: coming together through social distancing. Clin. Oncol. (R. Coll. Radiol.) 2020;32(July):413–416. doi: 10.1016/j.clon.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obek C., Doganca T., Argun O.B., Kural A.R. Management of prostate cancer patients during COVID-19 pandemic. Prostate Cancer Prostatic Dis. 2020;23(September):398–406. doi: 10.1038/s41391-020-0258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel M., Elsayad K., Engenhart-Cabillic R., Reinartz G., Baues C., Schmidberger H., et al. Radiation treatment of hemato-oncological patients in times of the COVID-19 pandemic : expert recommendations from the radiation oncology panels of the German Hodgkin Study Group and the German Lymphoma Alliance. Strahlenther. Onkol. 2020;196(December):1096–1102. doi: 10.1007/s00066-020-01705-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omeroglu Simsek G. Lung cancer management in COVID-19 pandemic. Turk. Thorac J. 2020;21(September):340–344. doi: 10.5152/TurkThoracJ.2020.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orazem M., Ratosa I. COVID-19 pandemic as an opportunity for the radiotherapy department. Clin. Oncol. (R. Coll. Radiol.) 2020;(May) doi: 10.1016/j.clon.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannullo S.C., Chidambaram S., Brandmaier A., Knisely J., Adler J.R. Clinical considerations in neurosurgical radiosurgery in the time of COVID-19. Cureus. 2020;12(April):p. e7671. doi: 10.7759/cureus.7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar B., Chen W.C., Herman J.M., Potters L. Disease site-specific guidelines for curative radiation treatment during ‘Limited Surgery’ and ‘Hospital Avoidance’: a radiation oncology perspective from the epicenter of COVID-19 pandemic. Cureus. 2020;12(May):p. e8190. doi: 10.7759/cureus.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoa R., Algarab M., Montero-Fernándezc M.A., Sanzb X., Vernetd M., Rodríguezb N., et al. Diagnosis and locoregional treatment of patients with breast cancer during the COVID-19 pandemic. Revista de Senología y Patología Mamaria. 2020;3:61–67. [Google Scholar]
- Passaro A., Addeo A., Von Garnier C., Blackhall F., Planchard D., Felip E., et al. ESMO management and treatment adapted recommendations in the COVID-19 era: lung cancer. ESMO Open. 2020;5(June) doi: 10.1136/esmoopen-2020-000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick H.M., Hijal T., Souhami L., Freeman C., Parker W., Joly L., et al. A canadian response to the coronavirus disease 2019 (COVID-19) pandemic: is there a silver lining for radiation oncology patients? Adv. Radiat. Oncol. 2020;5(July-August):774–776. doi: 10.1016/j.adro.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini Guilherme Fleury. How to manage lymphoid malignancies during novel 2019 coronavirus (CoVid-19) outbreak: a Brazilian task force recommendation. Hematol, Transfus, Cell Ther. 2020;42(2):103–110. doi: 10.1016/j.htct.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poortmans P.M., Guarneri V., Cardoso M.J. Cancer and COVID-19: what do we really know? Lancet. 2020;(May) doi: 10.1016/S0140-6736(20)31240-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaluri M.S., Bambace F., Tramacere F., Errico A., Carbone S., Portaluri T. Staff and patients’ protection in radiation oncology departments during COVID-19 pandemic. Adv. Radiat. Oncol. 2020 doi: 10.1016/j.adro.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radiation Protection in the Design of Radiotherapy Facilities . 2006. I. A. E. A. VIENNA Safety Reports Series No.47. [Google Scholar]
- Raghavan D., Tan A.R., Story E.S., Burgess E.F., Musselwhite L., Kim E.S., et al. Management changes for patients with endocrine-related cancers in the COVID-19 pandemic. Endocr. Relat. Cancer. 2020;27(September):R357–R374. doi: 10.1530/ERC-20-0229. [DOI] [PubMed] [Google Scholar]
- Ramirez P.T. COVID-19 global pandemic: options for management of gynecologic cancers. Int. J. Gynecol. Cancer. 2020:1–3. doi: 10.1136/ijgc-2020-001419. [DOI] [PubMed] [Google Scholar]
- Rathod S., Dubey A., Bashir B., Sivananthan G., Leylek A., Chowdhury A., et al. Bracing for impact with new 4R’s in the COVID-19 pandemic - A provincial thoracic radiation oncology consensus. Radiother. Oncol. 2020;149(April):124–127. doi: 10.1016/j.radonc.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembielak A., Sykes A.J., Fife K., Challapalli A., Nobes J.P. Radiotherapy and systemic treatment for non-melanoma skin cancer in the COVID-19 pandemic. Clin. Oncol. (R. Coll. Radiol.) 2020;32(July):417–419. doi: 10.1016/j.clon.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembielak A., Sykes A., Fife K., Challapalli A., Nobes J., Lawton P. 2021. Radiotherapy for Melanoma in COVID-19 Pandemic [Online]https://www.rcr.ac.uk/sites/default/files/radiotherapy-melanoma-covid19.pdf Available: [Google Scholar]
- Riechelmann R.P., Peixoto R.D., Fernandes G.D.S., Weschenfelder R.F., Prolla G., Filho D.R., et al. Evidence-based recommendations for gastrointestinal cancers during the COVID-19 pandemic by the Brazilian Gastrointestinal Tumours Group. Ecancermedicalscience. 2020;14:p. 1048. doi: 10.3332/ecancer.2020.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romesser P.B., Wu A.J., Cercek A., Smith J.J., Weiser M., Saltz L., et al. Management of locally advanced rectal cancer during the COVID-19 pandemic: a necessary paradigm change at Memorial Sloan Kettering Cancer Center. Adv. Radiat. Oncol. 2020;(April) doi: 10.1016/j.adro.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchi S., Vischioni B., Bonora M., Barcellini A., Locati L.D., Castelnuovo P., et al. Managing locally advanced adenoid cystic carcinoma of the head and neck during the COVID-19 pandemic crisis: is this the right time for particle therapy? Oral Oncol. 2020;106(July):104803. doi: 10.1016/j.oraloncology.2020.104803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roques T., Prestwich R. 2021. Head and Neck Cancer and COVID-19 [Online]https://www.rcr.ac.uk/sites/default/files/head-and-neck-cancer-treatment-covid19.pdf Available: [Google Scholar]
- Saber Soltani S., Zakeri A.M., Karimi M.R., Akhavan Rezayat S., Zomorodi Anbaji F., Tabibzadeh A.R., et al. A systematic literature review of current therapeutic approaches for COVID-19 patients. J. Pharm. Res. Int. 2020;32:13–25. [Google Scholar]
- Salari A., Jalaeefar A., Shirkhoda M. What is the best treatment option for head and neck cancers in COVID-19 pandemic? A rapid review. Am. J. Otolaryngol. 2020;41(November-December):p. 102738. doi: 10.1016/j.amjoto.2020.102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samiee S., Hadjilooei F., Alamolhoda M., Akhlaghpoor S. New policy and regulation for a Radiology-Oncology Center at the time of Covid-19 outbreak in Tehran-Iran. Adv. Radiat. Oncol. 2020;(April) doi: 10.1016/j.adro.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkis J., Samaha R., Kattan J., Sarkis P. Bladder cancer during the COVID-19 pandemic: the calm before the storm? Future Sci. OA. 2020;vol. 6(August):p. FSO615. doi: 10.2144/fsoa-2020-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon B., Zaidi S. 2021. Updated neoadjuvant/adjuvant/definitive Radiotherapy Guidelines for the Management of Newly Diagnosed Soft-tissue and Bone Sarcoma [Online]https://www.rcr.ac.uk/sites/default/files/sarcoma-treatment-covid19.pdf Available: [Google Scholar]
- Sepulcri M., Paronetto C., El Khouzai B., Novo A., Aldegheri V., Scaggion A., et al. Effectiveness of CBCT imaging during radiotherapy for the detection of initial COVID-19 lung disease. Adv. Radiat. Oncol. 2020;(May) doi: 10.1016/j.adro.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaverdian N., Shepherd A., Rimner A., Wu A.J., Simone C.B., 2nd, Gelblum D.Y., et al. Need for caution in the diagnosis of radiation pneumonitis in the COVID-19 pandemic. Adv. Radiat. Oncol. 2020;(May) doi: 10.1016/j.adro.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siavashpour Zahra, et al. A comparison of organs at risk doses in GYN intracavitary brachytherapy for different tandem lengths and bladder volumes. J. Appl. Clin. Med. Phys. 2016;17(3) doi: 10.1120/jacmp.v17i3.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siavashpour Z., Taghizadeh-Hesary F., Rakhsha A. Recommendations on management of locally advanced rectal cancer during the COVID-19 pandemic: an Iranian consensus. J. Gastrointest. Cancer. 2020;51(September):800–804. doi: 10.1007/s12029-020-00454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcock R., Thomas T.V., Estes C., Filippi A.R., Katz M.A., Pereira I.J., et al. COVID-19: global radiation oncology’s targeted response for pandemic preparedness. Clin. Transl. Radiat. Oncol. 2020;22(May):55–68. doi: 10.1016/j.ctro.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.P., Berman A.T., Marmarelis M.E., Haas A.R., Feigenberg S.J., Braun J., et al. Management of lung cancer during the COVID-19 pandemic. JCO Oncol. Pract. 2020;16(September):579–586. doi: 10.1200/OP.20.00286. [DOI] [PubMed] [Google Scholar]
- Skowron K.B., Hurst R.D., Umanskiy K., Hyman N.H., Shogan B.D. Caring for patients with rectal cancer during the COVID-19 pandemic. J. Gastrointest. Surg. 2020;(May) doi: 10.1007/s11605-020-04645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotman B.J., Lievens Y., Poortmans P., Cremades V., Eichler T., Wakefield D.V., et al. Effect of COVID-19 pandemic on practice in European radiation oncology centers. Radiother. Oncol. 2020;(June) doi: 10.1016/j.radonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A., Manukian G., Taylor A., Anne P.R., Simone N.L. Concerns for activated breathing control (ABC) with breast cancer in the era of COVID-19: maximizing infection control while minimizing heart dose. Adv. Radiat. Oncol. 2020;(April) doi: 10.1016/j.adro.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalek M.J., Rutkowski P. Coronavirus disease (COVID-19) outbreak: hypofractionated radiotherapy in Soft tissue sarcomas as a valuable option in the environment of limited medical resources and demands for increased protection of patients. Front. Oncol. 2020;10:p. 993. doi: 10.3389/fonc.2020.00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling M.T.M., Silva A., Pereira A.P.A., Ferreira Neto D.R., Restini F.C.F., Brito L.H., et al. Recommendations for radiotherapy during the novel coronavirus pandemic. Rev. Assoc. Med. Bras. 1992;66(March):359–365. doi: 10.1590/1806-9282.66.3.359. 2020. [DOI] [PubMed] [Google Scholar]
- Stepanović A., Nikitović M. Radiotherapy and COVID-19 pandemic-a review of the current recommendations. Srpski arhiv za celokupno lekarstvo. 2020;148:644–647. [Google Scholar]
- Sullivan M., Bouffet E., Rodriguez-Galindo C., Luna-Fineman S., Khan M.S., Kearns P., et al. The COVID-19 pandemic: a rapid global response for children with cancer from SIOP, COG, SIOP-E, SIOP-PODC, IPSO, PROS, CCI, and St Jude Global. Pediatr. Blood Cancer. 2020;67(July):p. e28409. doi: 10.1002/pbc.28409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svajdova M., Sicak M., Dubinsky P., Slavik M., Slampa P., Kazda T. Recurrent nasopharyngeal cancer: critical review of local treatment options including recommendations during the COVID-19 pandemic. Cancers (Basel) 2020;12(November) doi: 10.3390/cancers12123510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvia M., Ng S.W., Ning Matthew S., Lee Percy, McMahon Ryan A., Siva Shankar, Chuong Michael D. Single-Fraction Stereotactic Body Radiotherapy: A Paradigm During the COVID-19 Pandemic and Beyond? Adv. Radiat. Oncol. 2020 doi: 10.1016/j.adro.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferri L., Di Stefani A., Schinzari G., Fionda B., Rossi E., Del Regno L., et al. Skin cancer triage and management during COVID-19 pandemic. J. Eur. Acad. Dermatol. Venereol. 2020;34(June):1136–1139. doi: 10.1111/jdv.16529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talapatra K., Majumder D., Laskar S. COVID-19: an advisory for a radiation oncology department pertinent to the multidisciplinary team. Indian J. Med. Paediatr. Oncol. 2020;41(September):629–633. 2020. [Google Scholar]
- Tan B.F., Tuan J.K.L., Yap S.P., Ho S.Z., Wang M.L.C. Managing the COVID-19 pandemic as a national radiation oncology centre in Singapore. Clin. Oncol. (R. Coll. Radiol.) 2020;32(July):e155–e159. doi: 10.1016/j.clon.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanderup K., Fokdal L.U., Sturdza A., Haie-Meder C., Mazeron R., van Limbergen E., et al. Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. Radiother. Oncol. 2016;120(September):441–446. doi: 10.1016/j.radonc.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Tchelebi L.T., Haustermans K., Scorsetti M., Hosni A., Huguet F., Hawkins M.A., et al. Recommendations for the use of radiation therapy in managing patients with gastrointestinal malignancies in the era of COVID-19. Radiother. Oncol. 2020;148(April):194–200. doi: 10.1016/j.radonc.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson David J., et al. Practice recommendations for risk-adapted head and neck cancer radiotherapy during the COVID-19 pandemic: an ASTRO-ESTRO consensus statement. Int. J. Radiat. Oncol. Biol. Phys. 2020 doi: 10.1016/j.ijrobp.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson D.J., Palma D., Guckenberger M., Balermpas P., Beitler J.J., Blanchard P., et al. Practice recommendations for risk-adapted head and neck Cancer radiation therapy during the COVID-19 pandemic: an ASTRO-ESTRO consensus statement. Int. J. Radiat. Oncol. Biol. Phys. 2020;107(July):618–627. doi: 10.1016/j.ijrobp.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thureau S., Faivre J.C., Assaker R., Biver E., Confavreux C.B., Debiais F., et al. Adapting palliative radiation therapy for bone metastases during the Covid-19 pandemic: GEMO position paper. J. Bone Oncol. 2020;(April):100291. doi: 10.1016/j.jbo.2020.100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay R., Shankar A. Implementing continued radiation therapy for cancer management in low resource countries during and after the COVID-19 crisis. Expert Rev. Anticancer Ther. 2020;(December):1–5. doi: 10.1080/14737140.2021.1860761. [DOI] [PubMed] [Google Scholar]
- Uwins C., Bhandoria G.P., Shylasree T.S., Butler-Manuel S., Ellis P., Chatterjee J., et al. COVID-19 and gynecological cancer: a review of the published guidelines. Int. J. Gynecol. Cancer. 2020;30(September):1424–1433. doi: 10.1136/ijgc-2020-001634. [DOI] [PubMed] [Google Scholar]
- Uzzo R.G., Kutikov A., Geynisman D.M. 2021. Coronavirus Disease 2019 (COVID-19): Cancer care During the Pandemic [Online]https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-cancer-care-during-the-pandemic Available: [Google Scholar]
- van der Linden Y.M., Westhoff P.G., Stellato R.K., van Baardwijk A., de Vries K., Ong F., et al. Dexamethasone for the prevention of a pain flare after palliative radiotherapy for painful bone metastases: the multicenter double-blind placebo-controlled three-armed randomized Dutch DEXA study. Int. J. Radiat. Oncol. Biol. Phys. 2020;(May) doi: 10.1016/j.ijrobp.2020.05.007. [DOI] [PubMed] [Google Scholar]
- Veness M.J. Hypofractionated radiotherapy in patients with non-melanoma skin cancer in the post COVID-19 era: time to reconsider its role for most patients. J. Med. Imaging Radiat. Oncol. 2020;(May) doi: 10.1111/1754-9485.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesulu B.P., Chandrasekar V.T., Girdhar P., Advani P., Sharma A., Elumalai T., et al. A systematic review and meta-analysis of cancer patients affected by a novel coronavirus. medRxiv. 2020;(May) doi: 10.1093/jncics/pkaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vordermark D. Shift in indications for radiotherapy during the COVID-19 pandemic? A review of organ-specific cancer management recommendations from multidisciplinary and surgical expert groups. Radiat. Oncol. 2020;15:140. doi: 10.1186/s13014-020-01579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vordermark D. Shift in indications for radiotherapy during the COVID-19 pandemic? A review of organ-specific cancer management recommendations from multidisciplinary and surgical expert groups. Radiat. Oncol. 2020;15(June):p. 140. doi: 10.1186/s13014-020-01579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil M., Fong C., Sanghera P., Hartley A., Dunn J., Mehanna H. Hypofractionated chemoradiation for head and cancer: Data from the PET NECK trial. Oral Oncol. 2020;113(December):105112. doi: 10.1016/j.oraloncology.2020.105112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C.J.D., Novara G., Marandino L., Bex A., Kamat A.M., Karnes R.J., et al. Risks from deferring treatment for genitourinary cancers: a collaborative review to aid triage and management during the COVID-19 pandemic. Eur. Urol. 2020;(May) doi: 10.1016/j.eururo.2020.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang S., Wei L., Lin Z., Wang X., Wang J., et al. Recommendations on management of gynecological malignancies during the COVID-19 pandemic: perspectives from Chinese gynecological oncologists. J. Gynecol. Oncol. 2020;31(July):p. e68. doi: 10.3802/jgo.2020.31.e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Zheng D., Lei Y., Wu S., Verma V., Liu Y., et al. Radiotherapy workflow and protection procedures during the Coronavirus Disease 2019 (COVID-19) outbreak: experience of the Hubei Cancer hospital in Wuhan, China. Radiother. Oncol. 2020;148(March):203–210. doi: 10.1016/j.radonc.2020.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel K.C., Morgner-Miehlke A., Petersen C., Fiedler W., Block A., Schafhausen P., et al. Implications of SARS-CoV-2 infection and COVID-19 crisis on clinical Cancer care: report of the university cancer center hamburg. Oncol. Res. Treat. 2020;43:307–313. doi: 10.1159/000508272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller M., Preusser M. How we treat patients with brain tumour during the COVID-19 pandemic. ESMO Open. 2020;4(May) doi: 10.1136/esmoopen-2020-000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams V.M., Kahn J.M., Harkenrider M.M., Chino J., Chen J., Fang L.C., et al. COVID-19 impact on timing of brachytherapy treatment and strategies for risk mitigation. Brachytherapy. 2020;(April) doi: 10.1016/j.brachy.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J.L., Alcorn S., McNutt T., Han-Oh S., Gonzalez R., Lin L., et al. An integrated program in a pandemic: johns hopkins radiation oncology department. Adv. Radiat. Oncol. 2020;(April) doi: 10.1016/j.adro.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A.J., Rimner A., Shepherd A.F., Gelblum D.Y., Shaverdian N., Yorke E., et al. Thoracic radiation therapy during COVID-19: provisional guidelines from a comprehensive cancer center within a pandemic epicenter. Adv. Radiat. Oncol. 2020;(April) doi: 10.1016/j.adro.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahalom J., Dabaja B.S., Ricardi U., Ng A., Mikhaeel N.G., Vogelius I.R., et al. ILROG emergency guidelines for radiation therapy of hematological malignancies during the COVID-19 pandemic. Blood. 2020;135(May):1829–1832. doi: 10.1182/blood.2020006028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara T.K., Holland R.E., Chera B. Practical Challenges of mask-to-mask encounters with patients with head and neck cancers amid the coronavirus disease 2019 pandemic. Adv. Radiat. Oncol. 2020 doi: 10.1016/j.adro.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerramilli D., Xu A.J., Gillespie E.F., Shepherd A.F., Beal K., Gomez D., et al. Palliative radiation therapy for oncologic emergencies in the setting of COVID-19: approaches to balancing risks and benefits. Adv. Radiat. Oncol. 2020;5(July-August):589–594. doi: 10.1016/j.adro.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerramilli D., Xu A.J., Gillespie E.F., Shepherd A.F., Beal K., Gomez D., et al. Palliative radiotherapy for oncologic emergencies in the setting of COVID-19: approaches to balancing risks and benefits. Adv. Radiat. Oncol. 2020;(April) doi: 10.1016/j.adro.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef I., Donahue B., Flyer M., Thompson S., Huang A., Gallant F. Covert Covid-19: CBCT lung changes in an asymptomatic patient receiving radiotherapy. Adv. Radiat. Oncol. 2020 doi: 10.1016/j.adro.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaniboni A., Ghidini M., Grossi F., Indini A., Trevisan F., Iaculli A., et al. A review of clinical practice guidelines and treatment recommendations for Cancer care in the COVID-19 pandemic. Cancers (Basel) 2020;12(August) doi: 10.3390/cancers12092452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaorsky N.G., Yu J.B., McBride S.M., Dess R.T., Jackson W.C., Mahal B.A., et al. Prostate Cancer radiotherapy recommendations in response to COVID-19. Adv. Radiat. Oncol. 2020;(April) doi: 10.1016/j.adro.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Han H., He T., Labbe K.E., Hernandez A.V., Chen H., et al. Clinical characteristics and outcomes of COVID-19-Infected Cancer patients: a systematic review and meta-analysis. J. Natl. Cancer Inst. 2020;(November) doi: 10.1093/jnci/djaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Qiu H., Gong Y., Han C., Ruan S., Wang C., et al. Clinical considerations for the management of cancer patients in the mitigation stage of the COVID-19 pandemic. Am. J. Cancer Res. 2020;10:2282–2292. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.