Abstract
Since the emergence of the coronavirus disease pandemic, several effective vaccines have been introduced. These vaccines work through several different immunogenic pathways to produce effective immunity. There have been a number of reports of patients developing subacute thyroiditis and thyroid dysfunction after receiving the coronavirus (COVID-19) vaccine. This paper presents a case of a female patient who developed subacute thyroiditis soon after receiving the adenovirus-vectored COVID-19 vaccine. The patient presented with severe neck pain and her blood results demonstrated an initial thyrotoxic phase followed by a hypothyroid phase. She had no past history of thyroiditis or thyroid dysfunction. Subacute thyroiditis occurring after COVID-19 vaccination is rare but also probably underreported. We hope that this case report not only contributes to the literature but also raises awareness of subacute thyroiditis occurring after receiving the COVID-19 vaccine.
Keywords: subacute thyroiditis, coronavirus vaccine, covid 19, adenovirus-vector, overt hypothyroidism
Introduction
The emergence of severe acute respiratory distress syndrome secondary to coronavirus infection (SARS-CoV-2, COVID-19) in late 2019 posed a significant threat to the entire world. Since then several effective vaccines have been introduced. Different vaccines produce immunity through different mechanisms. Some vaccines contain adjuvants, which are mainly used to increase the response to vaccination in the population. However, adjuvants can induce other autoimmune and inflammatory reactions by stimulating immunogenic cross-reactivity in genetically susceptible individuals [1]. Several cases of subacute thyroiditis have been reported after exposure to other vaccines [2-5], but there are not many reports related to exposure to the COVID-19 vaccine. This paper presents a case of a patient who developed subacute thyroiditis soon after receiving the adenovirus-vectored COVID-19 vaccine (Oxford-AstraZeneca, United Kingdom). The objective of the article is to create awareness regarding the possible association between receiving the COVID-19 vaccine and the onset of subacute thyroid dysfunction. This incident was appropriately reported to the Medicines and Healthcare products Regulatory Agency (MHRA), United Kingdom.
Case presentation
Medical history and demographics
A 55-year-old woman presented with neck pain and swelling, which had been going on for four weeks. This was accompanied by headache, sore throat, generalized aches and palpitations. She had received her first dose of the COVID-19 vaccine (ChAdOx1 nCoV-19 vaccine, AstraZeneca) three weeks prior to the onset of these symptoms. There was no history of any viral or respiratory illness prior to the onset of symptoms. Her past medical history consisted of well-controlled asthma. She had no family history of hypothyroidism or thyroiditis. She was a non-smoker and worked as community healthcare professional. On initial examination, she had a very tender goiter with no other abnormal clinical findings.
Investigations
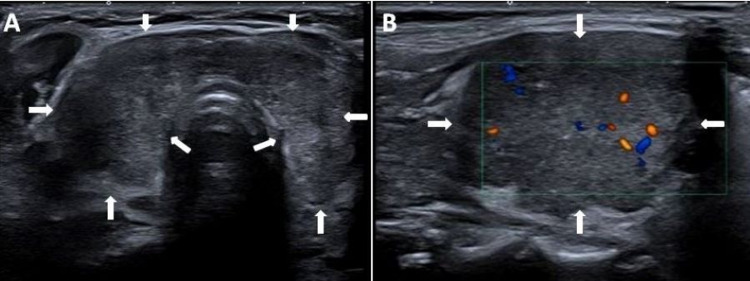
Initial blood results revealed raised inflammatory markers and thyroid hormone levels, consistent with the thyrotoxic phase of subacute thyroiditis. Her thyroid peroxidase antibody (anti-TPO) test was negative. Other routine blood results were normal (Table 1). A thyroid ultrasound scan demonstrated an enlarged thyroid gland with heterogeneous echotexture throughout (Figure 1). There were no nodules and no hyper-vascularity. Features were in keeping with thyroiditis.
Table 1. Initial blood tests and results demonstrating raised inflammatory markers and raised thyroid hormone levels consistent with subacute thyroiditis.
| Blood test | Result | Reference range |
| Sodium (mmol/L) | 139 | 132-145 |
| Potassium (mmol/L) | 4.5 | 3.4-5.1 |
| Chloride (mmol/L) | 105 | 97-110 |
| Creatinine (mmol/L) | 53 | 45-84 |
| Urea (mmol/L) | 5.0 | 2.5-7.8 |
| Thyroid-stimulating hormone (mU/L) | 0.09 | 0.3-4.2 |
| Free thyroxine (pmol/L) | 25.2 | 12.0-22.0 |
| Total protein (g/L) | 71 | 60-80 |
| Albumin (g/L) | 44 | 35-50 |
| Globulin (g/L) | 27 | 20-35 |
| C-reactive protein (mg/L) | 87 | <5 |
| Hemoglobin (g/L) | 119 | 115-165 |
| White cell count (109/L) | 8.5 | 4.0-11.0 |
| Platelet count (109/L) | 491 | 150-400 |
| Erythrocyte sedimentation rate (mm/hr) | 51 | 0-18 |
| Anti-thyroid peroxidase antibody (IU/ml) | <10 | <34 |
Figure 1. Ultrasound scan of the thyroid gland.
Enlarged thyroid gland with heterogeneous echotexture (A), and Doppler studies of right thyroid lobe showing reduced vascular flow (B).
Treatment
The patient was initially treated with a beta-blocker (propranolol) to control the palpitations. For the neck pain, she used over-the-counter ibuprofen and paracetamol.
Outcome and follow up
A repeat thyroid function test performed after six weeks demonstrated results in keeping with severe hypothyroidism (Table 2) and she was commenced on levothyroxine 50 micrograms daily. A repeat test performed six weeks later demonstrated improvement. Her thyroid receptor antibody (TRAb) was negative, but her thyroglobulin antibody test was positive. Her levothyroxine dose was increased to 75 micrograms daily and she remains well.
Table 2. Follow-up blood test results at six and 12 weeks demonstrating severe hypothyroidism at six weeks, improvement while taking levothyroxine at 12 weeks and raised thyroglobulin antibody level at 12 weeks.
| Blood test | Reference range | Results at 6 weeks | Results at 12 weeks |
| Thyroid-stimulating hormone (mU/L) | 0.3-4.2 | 20.3 | 5.35 |
| Free thyroxine (pmol/L) | 12.0-22.0 | 4.7 | 15.6 |
| Free triiodothyronine (pmol/L) | 3.1-6.8 | - | 4.2 |
| Thyroid receptor antibody (IU/L) | <2.9 | - | 1.9 |
| Thyroglobulin antibody (kU/L) | <3.0 | - | 15 |
Discussion
COVID-19 is an infectious disease caused by the novel coronavirus, SARS-CoV-2. This virus was first identified in Wuhan, a city in the Hubei Province of China [6]. Global efforts were directed towards the development of a vaccine against COVID-19. There are now several vaccines being used with positive effects on morbidity and mortality [7,8]. The common side effects of these vaccines, such as pain in the injected site, allergic skin reactions, flu-like symptoms, headache, fatigue and fever, have been well documented [9,10]. A large study revealed that the systemic and local side-effects from receiving COVID-19 vaccination occurred at frequencies lower than reported in the phase-3 trials [11].
Subacute thyroiditis is a spontaneously remitting inflammatory condition of the thyroid gland that can last for weeks to several months. Females account for 75%-80% of cases. Viral infections, immune-modulatory drugs and postpartum autoimmunity have been implicated as etiological factors. Patients characteristically present with severe pain, swelling and tenderness in the thyroid region, accompanied by malaise, fatigue, myalgia and arthralgia. There is usually an initial thyrotoxic phase with raised inflammatory markers, followed by a hypothyroid phase and then a recovery phase. Symptoms are generally self-limiting. However, the neck pain can be severe and persistent, requiring a short course of steroid (prednisone) therapy [12].
Cases of subacute thyroiditis occurring after viral vaccines have been reported [2-5]. Following the COVID-19 pandemic, there have been reports of thyroid dysfunction occurring after the COVID-19 vaccination. A recent report involved a 42-year-old female who developed subacute thyroiditis within a week of receiving the mRNA COVID-19 vaccine (SARS-CoV-2 vaccine, Pfizer-BioNTech) [13]. Another report involves three females who developed subacute thyroiditis soon after receiving the inactivated coronavirus vaccine (SARS-CoV-2 vaccine, Sinovac Biotech Ltd) [14]. A more recent report involves two females who developed Graves’ disease with thyrotoxicosis soon after receiving the mRNA COVID-19 vaccine [15]. The mechanism for post-vaccination subacute thyroiditis or thyroid dysfunction remains unknown. However, adjuvants contained in vaccines (used mainly to increase the response to vaccination in the general population) may play a role in producing diverse autoimmune and inflammatory responses [15,16]. The potential for cross-reactivity between the coronavirus spike protein target produced by the mRNA vaccine and healthy thyroid cells antigens has also been mentioned [17]. The possible interactions between genetic predisposition, a history of other autoimmune conditions, the presence of adjuvants, and cross-reactivity between spike proteins and healthy thyroid cell antigens, all require further investigation.
Subacute thyroiditis occurring after COVID-19 vaccination is rare and probably underreported. Our case may be one of the very few reports of a woman developing subacute thyroiditis after receiving the adenovirus-vectored COVID-19 vaccine. Although viral infection is a more common cause of subacute viral infection, our patient did not have any preceding viral or respiratory symptoms before developing severe neck pain and swelling. Her symptoms occurred three weeks after receiving the vaccine.
Conclusions
Subacute thyroiditis occurring after receiving the COVID-19 vaccine is a rare and probably underreported phenomenon that seems to have a predilection for the female gender. Further investigation is required to evaluate the possible predisposing factors to developing subacute thyroiditis after receiving the COVID-19 vaccine. We hope that this case report not only contributes to the literature but also raises awareness of subacute thyroiditis occurring after receiving the COVID-19 vaccine.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.ASIA syndrome and endocrine autoimmune disorders. Bragazzi NL, Hejly A, Watad A, Adawi M, Amital H, Shoenfeld Y. Best Pract Res Clin Endocrinol Metab. 2020;34:101412. doi: 10.1016/j.beem.2020.101412. [DOI] [PubMed] [Google Scholar]
- 2.Subacute thyroiditis following the H1N1 vaccine. Girgis CM, Russo RR, Benson K. J Endocrinol Invest. 2010;33:506. doi: 10.1007/BF03346633. [DOI] [PubMed] [Google Scholar]
- 3.Subacute thyroiditis following seasonal influenza vaccination. Altay FA, Güz G, Altay M. Hum Vaccin Immunother. 2016;12:1033–1034. doi: 10.1080/21645515.2015.1117716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subacute thyroiditis following influenza vaccine (Vaxigrip) in a young female. Hsiao JY, Hsin SC, Hsieh MC, Hsia PJ, Shin SJ. Kaohsiung J Med Sci. 2006;22:297–300. doi: 10.1016/s1607-551x(09)70315-8. [DOI] [PubMed] [Google Scholar]
- 5.Occurrence of subacute thyroiditis following influenza vaccination. Passah A, Arora S, Damle NA, Reddy KS, Khandelwal D, Aggarwal S. Indian J Endocrinol Metab. 2018;22:713–714. doi: 10.4103/ijem.IJEM_237_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.A novel coronavirus emerging in China - key questions for impact assessment. Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. N Engl J Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 7.COVID-19 Vaccine: A comprehensive status report. Kaur SP, Gupta V. Virus Res. 2020;288:198114. doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oxford-AstraZeneca COVID-19 vaccine efficacy. Knoll MD, Wonodi C. Lancet. 2021;397:72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efficacy and safety of COVID-19 vaccines in older people. Soiza RL, Scicluna C, Thomson EC. Age Ageing. 2021;50:279–283. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. Polack FP, Thomas SJ, Kitchin N, et al. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Menni C, Klaser K, May A, et al. Lancet Infect Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.New aspects in the pathogenesis and management of subacute thyroiditis [PREPRINT} Stasiak M, Lewiński A. Rev Endocr Metab Disord. 2021:1–13. doi: 10.1007/s11154-021-09648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subacute Thyroiditis After mRNA Vaccine for Covid-19. Franquemont S, Galvez J. J Endocr Soc. 2021;5:0–7. [Google Scholar]
- 14.Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: post-vaccination ASIA syndrome [PREPRINT] İremli BG, Şendur SN, Ünlütürk U. J Clin Endocrinol Metab. 2021:0. doi: 10.1210/clinem/dgab373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Two cases of Graves' disease following SARS-CoV-2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants [PREPRINT] Vera-Lastra O, Navarro AO, Domiguez MPC, Medina G, Valadez TIS, Jara LJ. Thyroid. 2021 doi: 10.1089/thy.2021.0142. [DOI] [PubMed] [Google Scholar]
- 16.Autoimmune/inflammatory syndrome induced by adjuvants and thyroid autoimmunity. Watad A, David P, Brown S, Shoenfeld Y. Front Endocrinol (Lausanne) 2016;7:150. doi: 10.3389/fendo.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Vojdani A, Kharrazian D. Clin Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]