Abstract
Background and Purpose
Blood pressure (BP) control is strongly recommended, but BP control rate has not been well studied in patients with stroke. We evaluated the BP control rate with fimasartan-based antihypertensive therapy initiated in patients with recent cerebral ischemia.
Methods
This multicenter, prospective, single-arm trial involved 27 centers in South Korea. Key inclusion criteria were recent cerebral ischemia within 90 days and high BP [systolic blood pressure (SBP) >140 mm Hg or diastolic blood pressure (DBP) >90 mm Hg]. BP lowering was initiated with fimasartan. BP management during the follow-up was at the discretion of the responsible investigators. The primary endpoint was the target BP goal achievement rate (<140/90 mm Hg) at 24 weeks. Key secondary endpoints included achieved BP and BP changes at each visit, and clinical events (ClinicalTrials.gov Identifier: NCT03231293).
Results
Of 1,035 patients enrolled, 1,026 were included in the safety analysis, and 951 in the efficacy analysis. Their mean age was 64.1 years, 33% were female, the median time interval from onset to enrollment was 10 days, and the baseline SBP and DBP were 162.3±16.0 and 92.2±12.4 mm Hg (mean±SD). During the study period, 55.5% of patients were maintained on fimasartan monotherapy, and 44.5% received antihypertensive therapies other than fimasartan monotherapy at at least one visit. The target BP goal achievement rate at 24-week was 67.3% (48.6% at 4-week and 61.4% at 12-week). The mean BP was 139.0/81.8±18.3/11.7, 133.8/79.2±16.4/11.0, and 132.8/78.5±15.6/10.9 mm Hg at 4-, 12-, and 24-week. The treatment-emergent adverse event rate was 5.4%, including one serious adverse event.
Conclusions
Fimasartan-based BP lowering achieved the target BP in two-thirds of patients at 24 weeks, and was generally well tolerated.
Keywords: blood pressure, prevention and control, stroke, fimasartan
INTRODUCTION
More than two-thirds of patients with stroke have hypertension. 1,2,3,4 Well-designed randomized controlled trials (RCTs) and meta-analyses have demonstrated that blood pressure (BP) lowering reduces the risk of recurrent stroke as well as subsequent major vascular events.5,6,7,8 Stroke guidelines strongly recommend BP control after stroke or transient ischemic attack (TIA).
It is unclear whether BP is well controlled in patients who have experienced stroke or TIA. In patients enrolled in RCTs, which are generally required to ensure adequate control of risk factors, the BP control rate was less than optimal. A posthoc analysis of the Vitamin Intervention for Stroke Prevention (VISP) trial showed that only 30% of participants had their BP values controlled for ≥75% of the follow-up period.9 About 40% of the patients enrolled in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial had a mean systolic blood pressure (SBP) of ≥140 mm Hg during the study period.10 Even in RCTs that have primarily focused on target BP levels, 25–30% of patients in the less-intensive BP-lowering arms and 33–68% of those in the intensive BP-lowering arms did not achieve the target BP goals.11,12 Furthermore, the BP control rate after stroke or TIA in realworld practice has been less well studied.
Fimasartan is a new nonpeptide angiotensin II receptor blocker with a selective AT1-receptor blocking effect, and was approved by the Korea Food and Drug Administration in 2010 for the treatment of hypertension.13 A comparative trial found fimasartan to be as safe as and more effective for BP reduction than losartan in patients with mild-to-moderate hypertension. 14 The fimsasartan-based blood pressure control after acute cerebral ischemia (FABULOUS) Study aimed to determine the BP control rate with fimasartan-based antihypertensive therapy in patients with recent ischemic stroke or TIA.
METHODS
Study design and participants
The FABULOUS Study was a multicenter, prospective, single-arm trial involving 27 academic medical centers in South Korea. This study was initiated and sponsored by Boryung Pharmaceutical Co., Ltd. (Seoul, Korea). Representatives of the sponsor participated in the design and conduct of the study, but the academic investigators were allowed to fully access the obtained data after confidentiality agreements, were responsible for the completeness, accuracy, and analysis of the data, drafted the manuscript, and decided to submit the manuscript after receiving agreement with the sponsor. The study was approved by the institutional ethics committee of each participating institution, and all patients provided written informed consent. The study was registered at ClinicalTrials.gov (NCT03231293), and was conducted and reported in accordance with the study protocol.
Inclusion criteria were 1) ischemic stroke or TIA at 5–90 days before study entry, 2) high SBP (>140 mm Hg) or diastolic blood pressure (DBP) (>90 mm Hg), 3) being suitable for fimasartan treatment, 4) age ≥19 years, and 5) life expectancy >6 months. Exclusion criteria included 1) currently taking an antihypertensive agent, 2) hemorrhagic stroke, 3) endstage renal disease, 4) moderate-to-severe hepatic insufficiency or biliary tract obstruction, 5) galactose intolerance, Lapp lactase deficiency, or glucose-galactose malabsorption, 6) contraindication to fimasartan use, 7) pregnancy or lactation, 8) planned intervention or surgery requiring interruption of antihypertensive agent before the study end, 9) inability or unwillingness to comply with the study protocol, or 10) participation in another clinical trial. However, we allowed the enrollment of patients 1) who had taken beta blockers (BBs), calcium-channel blockers (CCBs), diuretics, or alpha blockers for purposes other than BP control in atrial fibrillation, coronary heart disease, congestive heart failure, or benign prostatic hypertrophy, and 2) whose BP was >140/90 mm Hg at study entry.
Procedures
At baseline, we compiled data on demographics, characteristics of the index ischemic stroke or TIA, functional disability, vascular risk factors, BP, heart rate, height, weight, body mass index, concomitant medications, and laboratory test results (complete blood count, blood urea nitrogen, creatinine, glucose, HbA1c, aspartate transaminase, alanine transaminase, high-sensitivity C-reactive protein, lipid profile, and prothrombin time).
At baseline, after resting for 5 minutes in a sitting position, BP was measured three times in each arm and then averaged using an automated measurement system (Model HEM-7080 IC; Omron Healthcare, Kyoto, Japan). Of BP values measured in both arms, the higher value was used as the baseline BP value, and subsequent BP values during the follow-up were measured in the arm with a higher BP value.
BP lowering was initiated in eligible patients with fimasartan after obtaining informed consent. Since this study aimed to determine the BP control rate in routine clinical practice, the dose of fimasartan (range, 30–120 mg) and BP management during the study period were determined at the discretion of the responsible physicians. However, concurrent use of angiotensin-converting-enzyme inhibitors or direct renin inhibitors was not allowed. Patients were followed up at 4, 12, and 24 weeks after enrollment.
Efficacy endpoints
The primary efficacy endpoint was the rate of achieving the target BP at 24 weeks, defined as the proportion of patients who had SBP <140 mm Hg and DBP <90 mm Hg. Secondary efficacy endpoints included 1) the rates of achieving the target BP at 4 and 12 weeks, 2) the BP and BP changes at 4, 12, and 24 weeks, and 3) clinical events of recurrent stroke, other vascular events, vascular death, and all-cause death at 24 weeks.
Safety endpoints included any treatment-emergent adverse event (TEAE), which was related to antihypertensive agents or for which a causal relationship with antihypertensive agents could not be excluded. Serious TEAEs included those that 1) resulted in death or were life-threatening, 2) required or prolonged hospitalization, 3) resulted in persistent or significant disability/incapacity, 4) caused a congenital anomaly/ birth defect, or 5) resulted in the development of drug dependency/abuse or significant medical events.
Sample-size calculation and statistical analysis
A previous study that enrolled 14,151 patients with hypertension found that the rate of achieving the target BP (SBP/DBP <140/90 mm Hg) with fimasartan at 60 mg or 120 mg per day for more than 2 months was 75.6%.15 Given that patients with ischemic stroke or TIA would be older, have more comorbidities, and be less responsive to antihypertensive agents than those enrolled in that previous study, we assumed the target BP goal achievement rate of 70% at 24 weeks. We then calculated that a sample size of 928 was required to ensure a two-sided 95% confidence interval (CI) of 67–73% for the expected rate of achieving the target BP. Assuming a 10% dropout rate, we planned to enroll 1,032 patients. The efficacy analysis included patients who took at least one dose of fimasartan, had at least one set of follow-up BP data, met the eligibility criteria, and did not withdraw their informed consent. The safety analysis included patients who took at least one dose of fimasartan, did not withdraw their informed consent, and undertook at least one follow-up visit.
Categorical variables are summarized as frequencies and percentages, while continuous variables are presented as mean±SD or median and interquartile range (IQR) values. The exact Clopper-Pearson method was used to calculate the 95% CI of the rate of achieving the BP target at each follow-up visit. The BP values were compared between the baseline visit and each follow-up visit using the paired t-test or Wilcoxon signed-rank test, depending on the distribution normality of data. The primary analysis of BP only included patients with BP data available for each visit without considering missing BP data. As an additional analysis, we used the last-observation-carried-forward (LOCF) method to impute the missing BP data, with any missing BP value at a follow-up visit replaced by the last observed BP value. We also analyzed BP data separately for patients who received fimasartan monotherapy and those who received antihypertensive therapies other than fimasartan monotherapy (fimasartan plus other antihypertensive agents, other antihypertensive agents without fimasartan, or no antihypertensive agents) at at least one visit during the follow-up. We conducted multivariable logistic regression analysis to explore factors associated with achieving the target BP. Variables with p<0.1 in the univariable analysis or biological relevance were included in the multivariable model. All statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC, USA).
RESULTS
Characteristics of the patients
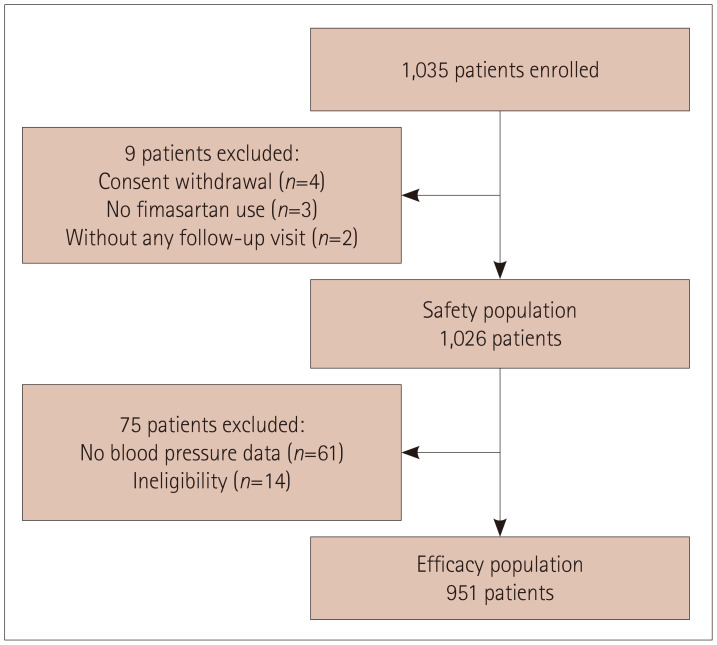
This study enrolled 1,035 patients between July 2016 and November 2018. Of these patients, 1,026 were included in the safety analysis after excluding 9 for the following reasons: 4 withdrew their informed consent, 3 did not take any dose of fimasartan, and 2 did not participate in any follow-up visit. The efficacy analysis included 951 patients, after further excluding 75 patients: 61 for whom there were no follow-up BP data and 14 who did not meet the eligibility criteria (Fig. 1).
Fig. 1. Enrollment, withdrawal, safety population, and efficacy population.
The baseline characteristics of the patients in the efficacy population are presented in Table 1. They were aged 64.1± 12.1 years, 32.7% were female, the qualifying event was ischemic stroke in 882 (92.7%) and TIA in 69 (7.3%), and the median baseline score on the NIHSS (National Institutes of Health Stroke Scale) was 2 (IQR, 1–4). The median interval from the qualifying event to initiating antihypertensive therapy was 10 days (IQR, 7–18 days), and 298 (31.3%) patients were enrolled between 5 and 7 days after the index event. The mean BP value at baseline obtained from 951 patients was 162.3±16.0 mm Hg for SBP and 92.2±12.4 mm Hg for DBP. During the follow-up, BP data were obtained from 934 patients at 4 weeks, 875 at 12 weeks, and 829 at 24 weeks.
Table 1. Baseline characteristics of the patients in the efficacy population.
| Characteristic | Value (n=951) | ||
|---|---|---|---|
| Age, years | 64.1±12.1 | ||
| Sex, female | 311 (32.7) | ||
| Race, Korean | 951 (100.0) | ||
| Baseline BP, mm Hg | |||
| SBP | 162.3±16.0 | ||
| DBP | 92.2±12.4 | ||
| Height, cm | 164.1±8.8 | ||
| Body weight, kg | 66.7±11.9 | ||
| BMI, kg/m2 | 24.7±3.4 | ||
| Qualifying event | |||
| Transient ischemic attack | 69 (7.3) | ||
| Ischemic stroke | 882 (92.7) | ||
| Small-vessel occlusion | 382 (43.3) | ||
| Large-artery atherosclerosis | 340 (38.5) | ||
| Cardiac embolism | 55 (6.2) | ||
| Other determined | 24 (2.7) | ||
| Undetermined | 81 (9.2) | ||
| NIHSS score at baseline | 2 (1–4) | ||
| Onset to BP-lowering therapy, days | |||
| Mean | 14.8±13.0 | ||
| Median | 10 (7–18) | ||
| Risk factors | |||
| Prior stroke or transient ischemic attack | 109 (11.5) | ||
| Current smoker | 335 (35.2) | ||
| Diabetes | 255 (26.8) | ||
| Dyslipidemia | 431 (45.3) | ||
| Atrial fibrillation | 62 (6.5) | ||
| Coronary artery disease | 34 (3.6) | ||
Data are mean±SD, n (%), or median (IQR) values.
BMI: body mass index, BP: blood pressure, DBP: diastolic blood pressure, IQR: interquartile range, NIHSS: National Institutes of Health Stroke Scale, SBP: systolic blood pressure.
Use of antihypertensive agents
In the efficacy population (n=951), fimasartan (as monotherapy or combined with other antihypertensive agents) was administered to 940 patients at baseline, 938 at 4 weeks, 848 at 12 weeks, and 778 at 24 weeks. The median (IQR) doses of fimasartan administered per day were 30 (30–60) mg at baseline, 60 (30–60) mg at 4 weeks, 60 (30–60) mg at 12 weeks, and 60 (30–60) mg at 24 weeks. During the study period, 528 (55.5%) patients were maintained on fimasartan monotherapy, while 423 (44.5%) received antihypertensive therapies other than fimasartan monotherapy at at least one visit. Compared with those on antihypertensive therapies other than fimasartan monotherapy, patients on fimasartan monotherapy were more likely to be younger, to have lower SBP and DBP values, and to start receiving BP-lowering therapy later, and were less likely to have atrial fibrillation and dyslipidemia (Supplementary Table 1 in the online-only Data Supplement). The proportions of patients who received fimasartan monotherapy, fimasartan plus other antihypertensive agents, other antihypertensive agents without fimasartan, and no antihypertensive agent at each visit are provided in Supplementary Table 2 (in the online-only Data Supplement). Among the antihypertensive agents other than fimasartan, CCBs were administered to 327 (34.4%) patients, diuretics to 108 (11.4%), BBs to 37 (3.9%), and other agents to 77 (8.1%) at at least one visit. During the follow-up, 29 (3.0%) patients stopped taking BP-lowering medications. Among patients who did not achieve the target BP at each visit, intensification of BP lowering (adding other antihypertensive agents or increasing the fimasartan dose) was provided to 55.0% at 4 weeks, 38.2% at 12 weeks, and 11.2% at 24 weeks.
Efficacy outcomes
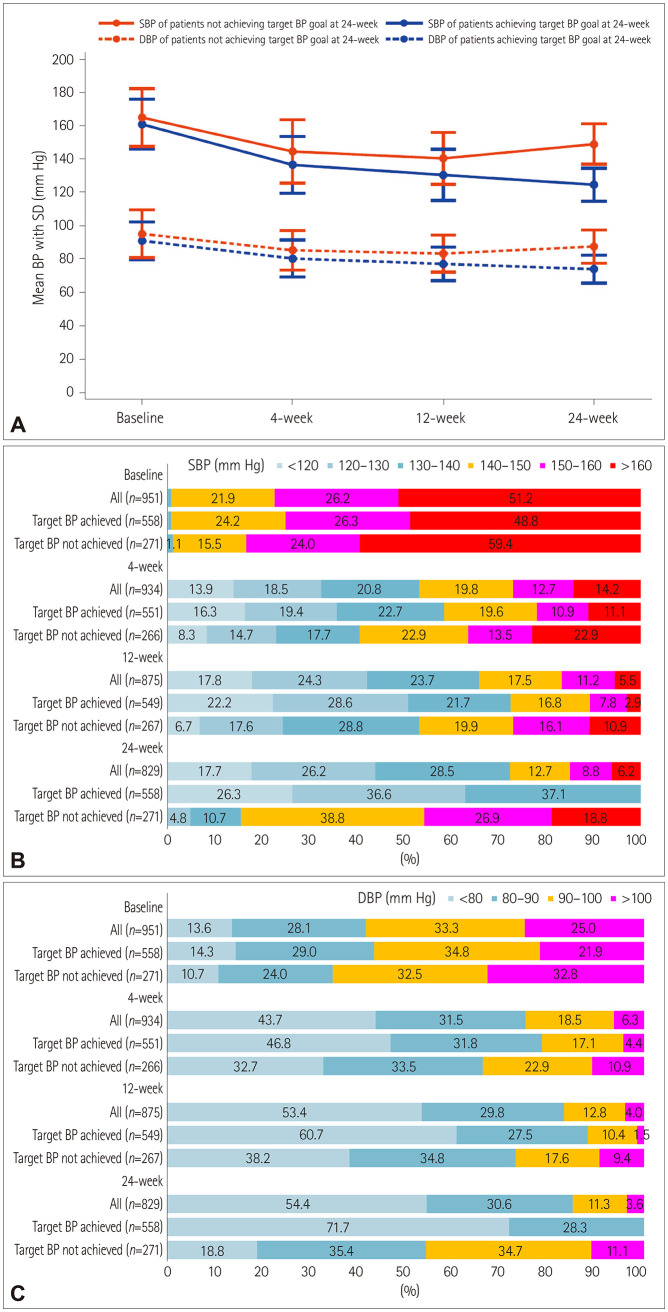
The rate of achieving the target BP (SBP <140 mm Hg and DBP <90 mm Hg) at 24 weeks was 67.3% (95% CI, 64.0–70.5). It was 48.6% (95% CI, 45.4–51.9) at 4 weeks and 61.4% (95% CI, 58.1–64.6) at 12 weeks (Table 2). LOCF analysis showed a slightly lower but similar rate of achieving the target BP at each visit (Table 2). The proportion of patients who achieved SBP <140 mm Hg was 53.2% at 4 weeks, 65.8% at 12 weeks, and 72.4% at 24 weeks; the corresponding proportions for achieving DBP <90 mm Hg were 75.2%, 83.2%, and 85.0%, respectively. Fig. 2 shows the proportions of SBP and DBP categories at each visit.
Table 2. Rate of achieving the target BP.
| Visit | Rate | n/total n | ||
|---|---|---|---|---|
| Overall | ||||
| At 24 weeks | ||||
| Observed (n=829) | 67.3 (64.0–70.5) | 558/829 | ||
| LOCF (n=951) | 65.9 (62.8–68.9) | 627/951 | ||
| At 4 weeks | ||||
| Observed (n=934) | 48.6 (45.4–51.9) | 454/934 | ||
| LOCF (n=934) | 48.6 (45.4–51.9) | 454/934 | ||
| At 12 weeks | ||||
| Observed (n=875) | 61.4 (58.1–64.6) | 537/875 | ||
| LOCF (n=949) | 61.0 (57.8–64.1) | 579/949 | ||
Rate values with 95% confidence intervals were calculated using the exact Clopper-Pearson method. Target BP was defined as SBP <140 mm Hg and DBP <90 mm Hg.
BP: blood pressure, DBP: diastolic blood pressure, LOCF: last-observation-carried-forward, SBP: systolic blood pressure.
Fig. 2. Comparison of BP parameters at each visit between patients who did and those who did not achieve the target BP at 24 weeks: mean SBP and DBP (A), SBP category (B), and DBP category (C). BP: blood pressure, DBP: diastolic blood pressure, SBP: systolic blood pressure.
Compared with the baseline SBP of 162.3±16.0 mm Hg, SBP significantly decreased to 139.0±18.3 mm Hg at 4 weeks, 133.8±16.4 mm Hg at 12 weeks, and 132.8±15.6 mm Hg at 24 weeks (all p<0.0001). DBP also decreased significantly, from 92.2±12.4 mm Hg at baseline to 81.8±11.7 mm Hg at 4 weeks, 79.2±11.0 mm Hg at 12 weeks, and 78.5±10.9 mm Hg at 24 weeks (all p<0.0001). Therefore, the magnitude of the SBP/DBP reduction was 23.4±19.9/10.6±11.8 mm Hg at 4 weeks, 28.6±20.4/13.2±12.5 mm Hg at 12 weeks, and 29.7±20.1/14.0±12.4 mm Hg at 24 weeks. LOCF analysis produced similar results (Table 3).
Table 3. BP values and decrease in BP at each visit.
| Visit | SBP/DBP, mm Hg | BP decrease, mm Hg | ||
|---|---|---|---|---|
| Overall | ||||
| Baseline (n=951) | 162.3±16.0/92.2±12.4 | |||
| At 4 weeks | ||||
| Observed (n=934) | 139.0±18.3/81.8±11.7 | 23.4±19.9*/10.6±11.8* | ||
| LOCF (n=934) | 139.0±18.3/81.8±11.7 | 23.4±19.9*/10.6±11.8* | ||
| At 12 weeks | ||||
| Observed (n=875) | 133.8±16.4/79.2±11.0 | 28.6±20.4*/13.2±12.5* | ||
| LOCF (n=949) | 133.9±16.6/79.1±10.9 | 28.4±20.2*/13.1±12.4* | ||
| At 24 weeks | ||||
| Observed (n=829) | 132.8±15.6/78.5±10.9 | 29.7±20.1*/14.0±12.4* | ||
| LOCF (n=951) | 132.9±16.2/78.5±11.0 | 29.5±20.0*/13.7±12.4* | ||
Data are mean±SD values.
*p<0.0001 for BP decrease compared with baseline value (p value in paired t-test).
BP: blood pressure, DBP: diastolic blood pressure, LOCF: last-observation-carried-forward, SBP: systolic blood pressure.
We compared BP data at each visit between the 558 patients who achieved the target BP at 24 weeks and the 271 who did not (Fig. 2). Baseline characteristics of patients who did and those who did not achieve the target BP are presented in Supplementary Table 3 (in the online-only Data Supplement). Patients who achieved the target BP compared to those who did not had lower SBP and DBP values at baseline (SBP, 161.1±15.0 vs. 165.2±17.4 mm Hg, p=0.001; DBP, 91.1± 11.4 vs. 95.3±14.3 mm Hg, p<0.001), 4 weeks (SBP, 136.7± 17.0 vs. 144.7±19.1 mm Hg, p<0.001; DBP, 80.5±11.1 vs. 85.4±11.9 mm Hg, p<0.001), 12 weeks (SBP, 130.7±15.3 vs. 140.6±15.6 mm Hg, p<0.001; DBP, 77.2±10.1 vs. 83.4±11.2 mm Hg, p<0.001), and 24 weeks (SBP, 124.8±9.9 vs. 149.2± 12.1 mm Hg, p<0.001; DBP, 74.1±8.3 vs. 87.7±10.0 mm Hg, p<0.001). The difference in the SBP and DBP values between patients who did and did not achieve the target BP increased gradually over time. In addition, logistic regression analysis using the generalized estimating equation method indicated that there were increases over time in the differences between the two groups in the proportion of patients with SBP <140 mm Hg (58.4% vs. 40.6% at 4 weeks, 72.5% vs. 53.2% at 12 weeks, and 100% vs. 15.5% at 24 weeks) and those with DBP <90 mm Hg (78.6% vs. 66.2%, 88.2% vs. 73.0%, and 100% vs. 54.2%, respectively) (Fig. 2).
In addition to the baseline BP values, patients who achieved the target BP compared with those who did not were more likely to be older, to have lower height and weight, and to have atrial fibrillation, and less likely to be current smokers (Supplementary Table 3 in the online-only Data Supplement). In multivariable analysis adjusted for age, sex, baseline SBP and DBP, height, weight, interval from onset to BP-lowering therapy, stroke severity, qualifying event, and risk factors, independent factors associated with target BP achievement were baseline DBP [for 10-mm Hg increase in DBP: odds ratio (95% CI), 0.84 (0.71–0.99), p=0.040] and current smoker at the time of the qualifying event [0.69 (0.48–1.00), p=0.049]. Baseline SBP values showed a trend of achieving the target BP, but did not reach a statistical significance [for 10-mm Hg SBP increase: 0.90 (0.81–1.00), p=0.059].
At 24 weeks, compared with patients on antihypertensive therapies other than fimasartan monotherapy, those on fimasartan monotherapy had trends of the rate of achieving the target BP being higher (70.3% vs. 63.9%, p=0.051) and the SBP level being lower (131.5±15.0 vs. 134.2±16.3 mm Hg, p=0.0128) (Supplementary Table 4 in the online-only Data Supplement). However, patients on fimasartan monotherapy compared with those on antihypertensive therapies other than fimasartan monotherapy had lower baseline SBP (SBP, 158.1±14.1 vs. 167.7±16.7 mm Hg, p<0.001) and DBP (91.3±11.2 vs. 93.±13.8 mm Hg, p=0.016) and smaller reductions in SBP (26.4±18.5 vs. 33.5±21.3 mm Hg, p<0.001) and DBP (12.8±11.8 vs. 15.4±12.8 mm Hg, p=0.003) at 24 weeks.
We additionally explored the changes in BP as the fimasartan dose increased. To exclude the effect of other antihypertensive agents on BP changes, this analysis only included patients who were on fimasartan monotherapy. When the fimasartan dose increased from 30 to 60 mg (n=64), SBP/DBP decreased significantly from 147.5±13.4/85.1±10.2 mm Hg to 139.8±16.3/81.9±12.4 mm Hg (SBP/DBP decrease, 7.7±15.6/3.2±10.6 mm Hg; p=0.0002 for SBP change and p=0.0191 for DBP change). When the fimasartan dose increased from 60 to 120 mg (n=33), SBP/DBP decreased significantly from 152.7±16.3/87.5±12.9 mm Hg to 142.2±20.0/82.3±14.2 mm Hg (SBP/DBP decrease, 10.3±9.2/5.±8.8 mm Hg; p=0.0048 for SBP change and p=0.0013 for DBP change). Therefore, the rate of achieving the target BP increased from 18.8% to 42.2% when the fimasartan monotherapy dose was increased from 30 to 60 mg, and from 15.2% to 56.3% with the dose increase from 60 to 120 mg.
Clinical events
During the study period, 28 (2.7%) patients in the safety population experienced recurrent strokes and 9 (0.9%) experienced other vascular events. There were six deaths: three vascular deaths (two cardiac deaths and one death due to hemorrhagic stroke), two deaths related to pneumonia, and one death of undetermined cause.
Safety
TEAEs occurred in 55 (5.4%) patients: 36 (3.5%) were related to fimasartan and 19 (1.9%) were related to other antihypertensive agents (Table 4). The most common TEAE was dizziness (n=23, 2.2%), followed by headache (n=9, 0.9%), syncope (n=4, 0.4%), cough (n=3, 0.3%), and dyspepsia (n=2, 0.2%). One patient had the serious TEAE of ischemic stroke, which was judged as being related to BP lowering with fimasartan.
Table 4. TEAEs based on safety population.
| TEAE | Value | |
|---|---|---|
| All TEAEs | 55 (5.36) | |
| Dizziness | 23 (2.24) | |
| Headache | 9 (0.88) | |
| Syncope | 4 (0.39) | |
| Cough | 3 (0.29) | |
| Dyspepsia | 2 (0.19) | |
| Fimasartan-related TEAE | 36 (3.51) | |
| Serious TEAE* | 1 (0.10) | |
| Fimasartan-related serious TEAE* | 1 (0.10) | |
Data are n (%) values. TEAEs were defined as any adverse events related to antihypertensive agents or for which a causal relationship with antihypertensive agents could not be excluded.
*One serious TEAE was recurrent ischemic stroke.
TEAE: treatment-emergent adverse event.
DISCUSSION
In this study, fimasartan-based antihypertensive therapies achieved the target BP at 24 weeks in about two-thirds of patients with recent cerebral ischemia and high BP. The target BP was achieved in nearly 50% of patients at 4 weeks and about 60% at 12 weeks. The rate of TEAEs was low at 5.4%, and serious TEAEs were very rare. This is the first study to have prospectively and systematically evaluated the BP control rate in Korean patients with recent cerebral ischemia, and showed that fimasartan-based BP lowering initiated in the subacute period was effective and safe.
It would be informative to compare the rate of achieving the target BP in our study with the rates found in secondary stroke prevention RCTs testing target BP levels. In the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, 75% of patients in the less-intensive BP-lowering group (SBP 130–149 mm Hg) and 67% in the intensive BP-lowering group (SBP <130 mm Hg) achieved their target BP levels.11 In the Recurrent Stroke Prevention Clinical Outcome (RESPECT) study, the rate of achieving the target BP was 61.7% in the standard BP-lowering group (SBP/DBP <140/90 mm Hg or <130/80 mm Hg for patients with diabetes, chronic kidney disease, or prior myocardial infarction) and 32.0% in the intensive BP-lowering group (SBP/DBP <120/80 mm Hg).12 Therefore, the rate of achieving the target BP (SBP/DBP <140/90 mm Hg) in our study is comparable with those obtained in patients randomized to less-intensive BP lowering in the SPS3 and RESPECT trials.
The magnitude of BP reduction with fimasartan-based antihypertensive therapies was substantial: almost 30 mm Hg in SBP and 14 mm Hg in DBP at 24 weeks (from 162.3/92.2 mm Hg at baseline to 132.8/78.5 mm Hg at 24 weeks). These BP reductions were achieved relatively early after initiating antihypertensive therapies; at 4 weeks, the mean SBP/DBP was 139.0/81.8 mm Hg, corresponding to a reduction of about 23 mm Hg in SBP and almost 11 mm Hg in DBP. These reductions appear to be greater than those observed in the SPS3 and RESPECT trials: the magnitude of the SBP reduction at 1 year was 6 mm Hg in the less-intensive group and 15 mm Hg in the intensive group in the SPS3 trial, and it was 13 mm Hg in the less-intensive group and 22 mm Hg in the intensive group in the RESPECT trial.11,12 A previous study of fimasartan-based BP lowering in Korean hypertensive patients found that the magnitude of SBP/DBP reduction in patients who newly started antihypertensive therapies was 26.4/13.9 mm Hg, which is comparable to our findings.15 In another study, the reduction in BP among patients with mild-to-moderate hypertension was greater with fimasartan than with losartan. 14 These findings support the substantial BP-lowering effect of fimasartan-based antihypertensive therapy.
Our study was neither a randomized comparative trial nor a clinical endpoint trial, and thereby we were not able to assess the clinical benefit of the observed BP reduction. However, this can be indirectly inferred from previous studies. Based on meta-regression curves from a previous meta-analysis of secondary stroke prevention trials, the calculated relative risk reduction (RRR) when lowering SBP from 162 mm Hg to 132 mm Hg, as observed in this study, was 45% for recurrent stroke and 67% for cardiovascular death. The findings of post-hoc analyses of individual large RCTs conform to this meta-analysis. In Perindopril Protection Against Recurrent Stroke Study (PROGRESS), the annual risk of stroke recurrence was 2.81% in patients who achieved SBP values of 120–139 mm Hg and 5.54% among those who achieved SBP values of >160 mm Hg, indicating an RRR of 49%.16 In PRo-FESS, the estimated RRR with SBP levels of 130–139 mm Hg versus ≥150 mm Hg was 52% for recurrent stroke; 48% for the composite of stroke, myocardial infarction, or vascular death; and 60% for fatal stroke.10 The SPS3 trial found that achieving SBP levels of around 130 versus >150 mm Hg was associated with an RRR of about 35% for recurrent stroke.17 Therefore, the expected clinical benefit of BP reduction observed in the present study is likely to be significant.
In this study, patients who did not achieve the target BP at 24 weeks compared with those who did had higher SBP and DBP values and greater proportions of SBP >140 mm Hg and DBP >90 mm Hg at baseline, and these differences increased over time during the follow-up (Fig. 2). Intensification of BP lowering in patients who failed to achieve the target BP level at each visit were provided to 55% of them at the 4-week visit and 38% at the 12-week visit. The insufficient BP-lowering intensification during the study period is likely to partially account for the failure to achieve the target BP at 24 weeks. In addition to baseline DBP, current smoking at the time of the qualifying event was associated with not achieving the target BP. Patients who had smoked were likely to have more advanced atherosclerosis, and some of them might smoke again, which could impair BP control. Therefore, stricter BP control and smoking cessation interventions should be provided to these patients.
While this study was not designed to evaluate the efficacy of fimasartan monotherapy, 55.5% of the patients included in the efficacy population were maintained on fimasartan monotherapy during the study period. Their mean SBP/DBP was 158.1/91.3 mm Hg at baseline, and the magnitude of SBP/DBP reduction was 24.5/11.6 mm Hg at 4 weeks and 26.4/12.8 mm Hg at 24 weeks. Therefore, 59.5% of these patients achieved the target BP at 4 weeks and 70.3% at 24 weeks. The findings are largely consistent with those of another Korean study, where the magnitude of the SBP/DBP reduction was 13.6/8.2 mm Hg at 4 weeks and 17.6/11.2 mm Hg at 12 weeks, and the response rate (defined as DBP <90 mm Hg or a DBP decrease of >10 mm Hg) was 55.1% at 4 weeks and 71.7% at 12 weeks with fimasartan monotherapy (initiated at 60 mg once daily and increased to 120 mg once daily as needed) in patients with mild-to-moderate hypertension.14 We found that increasing the dose of fimasartan monotherapy from 30 to 60 mg resulted in an SBP/DBP decrease of 7.7/3.2 mm Hg and a 23.4% increase in the rate of achieving the target BP, while increasing it from 60 to 120 mg led to an SBP/DBP decrease of 10.3/5.5 mm Hg and a 41.1% increase in the rate of achieving the target BP. Therefore, fimasartan monotherapy could be considered as an effective initial BP-lowering therapy in stroke/TIA patients with nonsevere hypertension. Compared with those on other antihypertensive therapies (mostly using combination therapy), patients on fimasartan monotherapy had a lower SBP and a nonsignificantly higher rate of achieving the target BP at 24 weeks. However, it should be noted that patients treated with other antihypertensive therapies had a higher baseline BP and a greater BP reduction at 24 weeks (Supplementary Table 4 in the online-only Data Supplement).
The study had several limitations. Most of the included patients had experienced very mild strokes and were younger than general Korean stroke patients, which limits the generalizability of our findings. BP management was at the discretion of individual physicians rather than standardized, and we did not record the reason for changing or maintaining antihypertensive therapies at each visit. In addition to the BP value at each visit, BP management in real-world practice is probably influenced by the attitudes of physicians and patients, which are difficult to reliably measure. Therefore, the analysis exploring factors associated with achieving the target BP had a high risk of bias by unmeasured confounders. About 30% of the patients in this study were enrolled between 5 to 7 days after the qualifying event when physiologic responses to acute stroke potentially increase BP. Therefore, spontaneous BP decreases after the acute period could have affected our results. We found that fimasartan-based BP lowering was generally safe, as indicated by the low incidence of any TEAEs (5.4%) and only one serious TEAE. The current safety profiles were similar to those in previous large observational studies conducted in Korea.15,18 However, our study as well as previous ones did not routinely evaluate laboratory results during the follow-up, and accordingly TEAEs relied on patient self-reporting and/or clinical judgment, which is likely to underestimate the incidence of TEAEs. Finally, we used office BP data, and thereby the target BP goal achievement rate might be underestimated due to patients with white-coat hypertension.
In conclusion, fimasartan-based antihypertensive therapies significantly reduced BP and achieved the target BP at 24 weeks in two-thirds of patients in whom BP-lowering therapy was initiated in the subacute stage of cerebral ischemia.
Acknowledgements
This study was sponsored by Boryung Pharmaceutical Co., Ltd. (Seoul, Korea).
Footnotes
- Conceptualization: Sun Uck Kwon, Keun-Sik Hong, Oh Young Bang.
- Data curation: all authors.
- Formal analysis: Sun Uck Kwon, Keun-Sik Hong.
- Investigation: all authors.
- Methodology: Sun Uck Kwon, Keun-Sik Hong, Oh Young Bang.
- Writing—original draft: Keun-Sik Hong.
- Writing—review & editing: all authors.
Conflicts of Interest: All the investigators received research grant based on the study contracts for the number of subjects enrolled and/or for the conduct of the study from Boryung Pharmaceutical Co., Ltd., Seoul, Korea.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2021.17.3.344.
Characteristics of patients on fimasartan monotherapy and those on antihypertensive therapies other than fimasartan monotherapy at least one visit
Antihypertensive therapies at each visit
Characteristics of patients who did and did not achieve the target BP
Comparison of BP parameters between patients on fimasartan monotherapy and those on antihypertensive therapies other than fimasartan monotherapy
References
- 1.Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke. 2001;32:2559–2566. doi: 10.1161/hs1101.098524. [DOI] [PubMed] [Google Scholar]
- 2.Kimura K, Kazui S, Minematsu K, Yamaguchi T. Analysis of 16,922 patients with acute ischemic stroke and transient ischemic attack in Japan. Cerebrovasc Dis. 2004;18:47–56. doi: 10.1159/000078749. [DOI] [PubMed] [Google Scholar]
- 3.Fonarow GC, Reeves MJ, Smith EE, Saver JL, Zhao X, Olson DW, et al. Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in get with the guidelines-stroke. Circ Cardiovasc Qual Outcomes. 2010;3:291–302. doi: 10.1161/CIRCOUTCOMES.109.921858. [DOI] [PubMed] [Google Scholar]
- 4.Kim BJ, Park JM, Kang K, Lee SJ, Ko Y, Kim JG, et al. Case characteristics, hyperacute treatment, and outcome information from the Clinical Research Center for stroke-fifth division registry in South Korea. J Stroke. 2015;17:38–53. doi: 10.5853/jos.2015.17.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PROGRESS Collaborative Group. Randomised trial of a perindoprilbased blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Wang Z, Gong L, Zhang Y, Thijs L, Staessen JA, et al. Blood pressure reduction for the secondary prevention of stroke: a Chinese trial and a systematic review of the literature. Hypertens Res. 2009;32:1032–1040. doi: 10.1038/hr.2009.139. [DOI] [PubMed] [Google Scholar]
- 7.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741–2748. doi: 10.1161/01.STR.0000092488.40085.15. [DOI] [PubMed] [Google Scholar]
- 8.Katsanos AH, Filippatou A, Manios E, Deftereos S, Parissis J, Frogoudaki A, et al. Blood pressure reduction and secondary stroke prevention: a systematic review and metaregression analysis of randomized clinical trials. Hypertension. 2017;69:171–179. doi: 10.1161/HYPERTENSIONAHA.116.08485. [DOI] [PubMed] [Google Scholar]
- 9.Towfighi A, Markovic D, Ovbiagele B. Consistency of blood pressure control after ischemic stroke: prevalence and prognosis. Stroke. 2014;45:1313–1317. doi: 10.1161/STROKEAHA.113.001900. [DOI] [PubMed] [Google Scholar]
- 10.Ovbiagele B, Diener HC, Yusuf S, Martin RH, Cotton D, Vinisko R, et al. Level of systolic blood pressure within the normal range and risk of recurrent stroke. JAMA. 2011;306:2137–2144. doi: 10.1001/jama.2011.1650. [DOI] [PubMed] [Google Scholar]
- 11.SPS3 Study Group. Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–515. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitagawa K, Yamamoto Y, Arima H, Maeda T, Sunami N, Kanzawa T, et al. Effect of standard vs intensive blood pressure control on the risk of recurrent stroke: a randomized clinical trial and meta-analysis. JAMA Neurol. 2019;76:1309–1318. doi: 10.1001/jamaneurol.2019.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Lee JH, Paik SH, Kim JH, Chi YH. Fimasartan, a novel angiotensin II receptor antagonist. Arch Pharm Res. 2012;35:1123–1126. doi: 10.1007/s12272-012-0700-z. [DOI] [PubMed] [Google Scholar]
- 14.Lee SE, Kim YJ, Lee HY, Yang HM, Park CG, Kim JJ, et al. Efficacy and tolerability of fimasartan, a new angiotensin receptor blocker, compared with losartan (50/100 mg): a 12-week, phase III, multicenter, prospective, randomized, double-blind, parallel-group, dose escalation clinical trial with an optional 12-week extension phase in adult Korean patients with mild-to-moderate hypertension. Clin Ther. 2012;34:552–568. doi: 10.1016/j.clinthera.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 15.Park JB, Sung KC, Kang SM, Cho EJ. Safety and efficacy of fimasartan in patients with arterial hypertension (Safe-KanArb study): an openlabel observational study. Am J Cardiovasc Drugs. 2013;13:47–56. doi: 10.1007/s40256-013-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arima H, Chalmers J, Woodward M, Anderson C, Rodgers A, Davis S, et al. Lower target blood pressures are safe and effective for the prevention of recurrent stroke: the PROGRESS trial. J Hypertens. 2006;24:1201–1208. doi: 10.1097/01.hjh.0000226212.34055.86. [DOI] [PubMed] [Google Scholar]
- 17.Odden MC, McClure LA, Sawaya BP, White CL, Peralta CA, Field TS, et al. Achieved blood pressure and outcomes in the Secondary Prevention of Small Subcortical Strokes trial. Hypertension. 2016;67:63–69. doi: 10.1161/HYPERTENSIONAHA.115.06480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho EJ, Sung KC, Kang SM, Shin MS, Joo SJ, Park JB. Fimasartan reduces clinic and home pulse pressure in elderly hypertensive patients: a K-MetS study. PLoS One. 2019;14:e0214293. doi: 10.1371/journal.pone.0214293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of patients on fimasartan monotherapy and those on antihypertensive therapies other than fimasartan monotherapy at least one visit
Antihypertensive therapies at each visit
Characteristics of patients who did and did not achieve the target BP
Comparison of BP parameters between patients on fimasartan monotherapy and those on antihypertensive therapies other than fimasartan monotherapy