Abstract
Background and Purpose
To investigate the incidence and characteristics of neurological manifestations associated with coronavirus disease 2019 (COVID-19).
Methods
We reviewed the medical records of the consecutive patients with COVID-19 who were admitted to the central infectious diseases hospital designated for the treatment of COVID-19 in South Korea between March 2020 and September 2020. Newly developed neurological manifestations associated with COVID-19 were investigated. The frequency and clinical features of the neurological manifestations were analyzed according to disease severity, which was classified according to World Health Organization interim guidance.
Results
Of the 306 symptomatic patients, 186 (60.8%) developed at least one neurological manifestation during hospitalization. The most common neurological symptom was headache (n=102, 33.3%), followed by myalgia (n=96, 31.4%) and anosmia/ageusia (n=54, 17.6%). Acute stroke (all ischemic stroke) occurred in three (1.0%) patients, and new-onset seizures occurred in two (0.7%). Neurological manifestation was a presenting symptom of COVID-19 in 72 (23.5%) patients, and was the only symptom of COVID-19 in 12 (3.9%). Stroke, seizure, and impaired consciousness were significantly associated with severe to critical COVID-19, whereas headache and anosmia/ageusia were frequently found in patients with mild to moderate disease.
Conclusions
Neurological manifestations were commonly observed in patients with COVID-19. During the current pandemic, when patients present with new-onset neurological symptoms, COVID-19 may be considered as part of the differential diagnosis. Attention to severe neurological complications is needed, especially in patients with severe or critical COVID-19.
Keywords: COVID-19, neurological manifestations, Korea
INTRODUCTION
Respiratory symptoms are the most important clinical manifestations of coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 However, reports of neurological manifestations have also increased rapidly since being first described in patients in Wuhan, China.1,2 Common neurological symptoms of COVID-19 are smell and taste disturbances, myalgia, and headache.3 Rare complications include cerebrovascular disease, seizure, encephalitis, and Guillain-Barré syndrome.1,2,3
SARS-CoV-2 can directly infect structures in the peripheral nervous system and the central nervous system (CNS).4 Neurotropism may occur via an upper nasal transcribrial route (olfactory epithelium), axonal transport and transsynaptic transfer, or a hematogenous and/or lymphatic route.4,5 The SARS-CoV-2 virus can invade cells via binding of its spike protein to the cellular angiotensin-converting enzyme 2 (ACE2) receptor, which has recently been found on neurons and glial cells in several brain structures.4 In addition, neurological manifestations occur secondary to a severe systemic reaction in response to a viral infection outside the nervous system.4
As of January 13, 2021, there were more than 70,000 confirmed cases of COVID-19 in South Korea.6 Many studies have been performed on COVID-19, but there are few reports of neurological complications in Korean patients with this disease.7,8 We therefore investigated the incidence and clinical characteristics of neurological manifestations among hospitalized patients with confirmed SARS-CoV-2 infection at the central infectious diseases hospital in South Korea.
METHODS
Study population and variables
We retrospectively reviewed the electronic medical records of consecutive hospitalized patients with confirmed SARS-CoV-2 infection at the at the National Medical Center in Seoul, South Korea, between March 2020 and September 2020. The National Medical Center is an institute for infectious diseases that was designated as the headquarters for all institutions treating COVID-19 in South Korea at the start of the pandemic. All patients had a confirmed laboratory diagnosis of COVID-19 through the detection of SARS-CoV-2 RNA in a nasopharyngeal swab or sputum sample using the real-time reverse-transcription polymerase chain reaction (PCR).
The demographic data recorded for each patient included age at admission, sex, and ethnicity. Information on smoking, pregnancy, and comorbidities including hypertension, diabetes, chronic lung disease, cardiovascular and cerebrovascular diseases, chronic kidney disease, malignancies, human immunodeficiency virus (HIV) infection, neurological disease, and psychiatric disease were also collected.
Typical respiratory and other nonneurological and neurological symptoms experienced from symptom onset to discharge were extracted from the medical and nursing records of the patients. Neurological features included nonspecific symptoms (headache, dizziness, syncope, or myalgia), loss of smell (anosmia) or taste (ageusia), impaired consciousness (decreased consciousness or delirium), stroke, and seizure. The information in the medical records on symptoms or signs was generally obtained by history-taking and examinations by physicians. Neurologists were usually consulted when stroke or seizures were suspected or when there was an impaired consciousness.
COVID-19 disease severity was classified as asymptomatic (patients infected with SARS-CoV-2 who did not develop symptoms), mild (symptomatic COVID-19 without evidence of pneumonia or hypoxia), moderate [pneumonia, but no signs of severe pneumonia including a peripheral oxygen saturation level (SpO2) of ≥90% in room air], severe (severe pneumonia, with a respiration rate of >30 breaths/min; severe respiratory distress; or SpO2 <90% in room air), or critical (acute respiratory distress syndrome, sepsis, or septic shock), based on the World Health Organization (WHO) interim guidance released in May 2020.9 The patients were divided into two groups according to disease severity (asymptomatic, mild, or moderate vs. severe, critical, or fatal), and the demographic and neurological differences between these two groups were analyzed.
Statistical analyses
Continuous variables (e.g., age) were summarized using descriptive statistics, and categorical variables were summarized as absolute frequencies and percentages. Comparisons were made between patients with asymptomatic, mild, or moderate COVID-19 and those with moderate or severe COVID-19. The chi-square test (or Fisher's exact test) was used to compare categorical variables, and the Mann-Whitney test was used to compare ages between groups. All statistical analyses were performed using SPSS (version 26, IBM Corp., Armonk, NY, USA), and statistical significance was defined as p<0.05.
Ethics statement
The institutional review board of the National Medical Center reviewed and approved the study protocol (Approval No. NMC-2008-054). The requirement for patient informed consent was waived by the board because of retrospective chart review.
RESULTS
Baseline characteristics and symptoms
A total of 331 patients with confirmed COVID-19 were enrolled during the study period. During that same period, there were 16,336 patients with COVID-19 nationwide in South Korea and 5,324 in Seoul;10 thus, the patients whose data were analyzed in this study represented 2% and 6.3% of those, respectively.
The demographics and comorbidities of the patients whose data were analyzed are presented in Table 1. They ranged in age from 13 to 89 years, with a median age of 54 years; 103 of the 331 (31.1%) patients were older than 65 years. This population was predominantly male (n=213, 64.4%) and Asian (n=318, 96.1%), and 72 (21.8%) had a history of smoking. The most common comorbidities were hypertension (n=91, 27.5%), diabetes (n=63, 19.0%), and vascular disease (n=35, 10.6%). Five patients were pregnant, and four had a history of HIV infection.
Table 1. Baseline characteristics of the study patients.
| Variable | Total (n=331) | Asymptomatic, mild to moderate (n=281) | Severe to critical, or death (n=50) | p | |
|---|---|---|---|---|---|
| Age at admission, years | 54.0 [37.0–67.0] | 50.0 [36.0–64.0] | 69.0 [62.0–78.0] | <0.001† | |
| ≥65 years | 103 (31.1) | 68 (24.2) | 35 (70.0) | <0.001† | |
| Sex, male | 213 (64.4) | 180 (64.1) | 33 (66.0) | 0.792 | |
| Smoking | 72 (21.8) | 63 (22.4) | 9 (18.0) | 0.485 | |
| Comorbidity | |||||
| Hypertension | 91 (27.5) | 61 (21.7) | 30 (60.0) | <0.001† | |
| Diabetes | 63 (19.0) | 38 (13.5) | 25 (50.0) | <0.001† | |
| Chronic lung disease* | 11 (3.3) | 9 (3.2) | 2 (4.0) | 0.675 | |
| Cardio- or cerebrovascular disease | 35 (10.6) | 25 (8.9) | 10 (20.0) | 0.019† | |
| Chronic kidney disease | 3 (0.9) | 2 (0.7) | 1 (2.0) | 0.389 | |
| Malignancy | 13 (3.9) | 12 (4.3) | 1 (2.0) | 0.700 | |
| Neurological disorder other than cerebrovascular disease | 19 (5.7) | 14 (5.0) | 5 (10.0) | 0.160 | |
| Psychiatric disorder | 20 (6.0) | 17 (6) | 2 (4.0) | 0.749 | |
Data are n (%) or median [interquartile range] values.
*Chronic lung disease includes asthma and chronic obstructive lung disease, †p<0.05, statistically significant.
The clinical characteristics and severity of COVID-19 are presented in Table 2. Twenty-five (7.6%) patients were asymptomatic. Among the 306 symptomatic patients, 217 (70.9%) had experienced fever, 191 (62.4%) had experienced cough and/or sputum, and 87 (28.4%) had experienced dyspnea. According to the WHO interim guidance document on COVID-19 severity, most of the patients had mild or moderate disease (n=256, 83.7%); 34 (11.1%) had severe disease and 16 (5.2%) had critical disease. Eleven (3.3%) patients died due to COVID-19.
Table 2. Overall symptoms and severity of COVID-19.
| Variable | Total (n=331) | |
|---|---|---|
| Asymptomatic | 25 (7.6) | |
| Symptoms or signs* | 306 (92.4) | |
| Fever | 217 (70.9) | |
| Cough/sputum | 191 (62.4) | |
| Dyspnea/shortness of breath | 87 (28.4) | |
| Sore throat | 66 (21.6) | |
| Nasal congestion | 14 (4.6) | |
| Gastrointestinal symptoms (anorexia, nausea, vomiting, or diarrhea | 20 (6.5) | |
| Neurological symptoms | 186 (60.8) | |
| Disease severity*† | ||
| Mild | 80 (26.1) | |
| Moderate | 176 (57.5) | |
| Severe | 34 (11.1) | |
| Critical | 16 (5.2) | |
| Death | 11 (3.3) | |
Data are n (%) values.
*Asymptomatic patients (n=25) were excluded (symptomatic, n=306), †Classification based on the clinical management of COVID-19 in the WHO interim guidance.9
Patients with severe or critical disease and those who died due to COVID-19 were significantly older (median age 69 years in patients with severe or critical disease vs. 50 years in patients with asymtomatic, mild or moderate disease; p<0.001). In addition, hypertension, diabetes, or vascular disease were significantly more common among those with severe to critical COVID-19 (Table 1). All five pregnant patients had mild disease, and all four HIV-infected patients had asymptomatic (n=1), mild (n=2), or moderate (n=1) COVID-19.
Neurological manifestations
Of the 306 symptomatic patients excluding 25 asymptomatic patients, 186 (60.8%) developed at least one neurological symptom or disorder associated with COVID-19 during hospitalization (Table 3). The most commonly observed neurological symptom was headache (n=102, 33.3%), followed by myalgia (n=96, 31.4%), and anosmia/ageusia (n=54, 17.6%). Impaired consciousness was observed in 36 (11.8%) patients, including decreased consciousness in 16 (5.2%) and delirium or confusion in 20 (6.5%). Eighty-eight (28.8%) patients experienced more than 2 neurological manifestations.
Table 3. Neurological manifestations among symptomatic COVID-19 patients.
| Variable | Total (n=306) | |
|---|---|---|
| Any | 186 (60.8) | |
| Headache | 102 (33.3) | |
| Dizziness | 18 (5.8) | |
| Myalgia | 96 (31.4) | |
| Syncope | 4 (1.3) | |
| Loss of smell (anosmia) or loss of taste (ageusia) | 54 (17.6) | |
| Decreased consciousness (drowsiness, stupor, or coma) | 16 (5.2) | |
| Delirium or confusion | 20 (6.5) | |
| Stroke (ischemic or hemorrhagic) | 3 (1.0) | |
| Seizure | 2 (0.7) | |
| As an initial presenting manifestation of COVID-19 | ||
| Any | 72 (23.5) | |
| Headache | 7 | |
| Myalgia | 49 | |
| Syncope | 1 | |
| Anosmia or ageusia | 13 | |
| Decreased consciousness or delirium | 2 | |
| As the only manifestation of COVID-19 | ||
| Any | 12 (3.9) | |
| Headache | 3 | |
| Myalgia | 8 | |
| Anosmia or ageusia | 7 | |
Data are n or n (%) values.
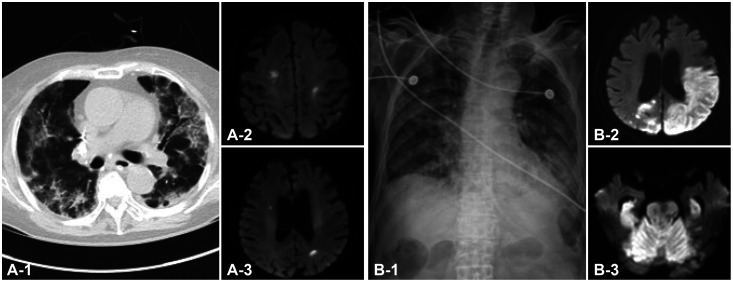
Four (1.3%) patients had syncope. Acute stroke occurred in three (1.0%) patients, and new-onset seizures occurred in two (0.7%). All three patients with stroke had ischemic strokes, which all occurred while they were being treated in the intensive care unit (Fig. 1). In one case, new-onset atrial fibrillation developed after COVID-19 infection, followed by multiple embolic infarctions. Poststroke acute symptomatic seizure subsequently occurred in one patient with acute infarction. Another patient experienced new-onset remote symptomatic seizures that might have been related to previous subarachnoid hemorrhage. Among the 16 patients with decreased consciousness, 14 had suggested underlying etiologies such as hypoxia or metabolic derangements. The remaining two patients had moderate COVID-19, but further evaluation was not performed because underlying severe dementia was thought to have contributed to their state of decreased consciousness.
Fig. 1. Chest and brain imaging in COVID-19 patients with new-onset stroke. A: Chest computed tomography shows diffuse ground-glass opacities with consolidation in both lungs of Patient 1 (A-1), and diffusion-weighted imaging (DWI) shows multiple small acute infarctions in both hemispheres (A-2 and A-3). B: Chest anteroposterior X-ray reveals diffuse hazy density in both lungs of Patient 2 (B-1), and DWI reveals massive acute infarctions in both hemispheres (B-2), brainstem and cerebellum (B-3).
A neurological manifestation was an initial presenting symptom of COVID-19 in 72 (23.5%) patients, with myalgia being the most common (n=49, 68.1%). Two patients initially presented with impaired consciousness without respiratory symptoms. Neurological manifestations were the only symptoms of COVID-19 in 12 (3.9%) patients (myalgia in 8, anosmia or ageusia in 7, and headache in 3).
Factors associated with neurological manifestations
The patients with headache were younger than those without headache [median 50.0 years, interquartile range (IQR) 37.0–61.0 years vs. median 57.0 years, IQR 38.0–72.0 years; p=0.002]. In addition, those with anosmia were younger than their counterparts without anosmia (median 38.0 years, IQR 30.0–45.0 years vs. median 59.0 years, IQR 40.0–70.0 years; p<0.001). In contrast, those who had decreased consciousness (median 78.0 years, IQR 75.0–81.0 years) or those who had delirium (median 76.0 years, IQR 69.0–80.5 years) were older than those whose consciousness was unaffected (median 52.0 years, IQR 37.0–66.0 years) (p<0.001, respectively). There was no significant difference in patient age between those with or without dizziness or myalgia, and no significant difference in the sex distribution between patients with or without individual neurological symptoms.
Neurological manifestations stratified by the COVID-19 severity are presented in Table 4. Stroke and seizure occurred only in patients with severe or critical disease, and decreased consciousness or delirium were also significantly more common in these patients. In contrast, anosmia/ageusia was found only in patients with mild or moderate disease, and headache was also more commonly found in these patients.
Table 4. Neurological manifestations according to the severity* of COVID-19.
| Variable | Mild or moderate (n=256) | Severe, critical, or death (n=50) | p |
|---|---|---|---|
| Any | 176 (68.8) | 35 (70.0) | 0.873 |
| Headache | 97 (37.9) | 5 (10.0) | <0.001 |
| Dizziness | 16 (6.3) | 2 (4.0) | 0.747 |
| Myalgia | 83 (32.4) | 13 (26.0) | 0.371 |
| Syncope | 4 (1.6) | 0 | 1.000 |
| Anosmia or ageusia | 54 (21.1) | 0 | <0.001 |
| Decreased consciousness |
2 (0.8) | 14 (28.0) | <0.001 |
| Delirium or confusion | 10 (3.9) | 10 (20.0) | <0.001 |
| Stroke | 0 | 3 (6.0) | 0.004 |
| Seizure | 0 | 2 (4.0) | 0.026 |
*Asymptomatic patients (n=25) were excluded (symptomatic, n=306).
DISCUSSION
This retrospective study of neurological features in symptomatic COVID-19 patients found that 60.8% (186/306) of patients had neurological symptoms or diagnoses. These were most commonly nonspecific manifestations (headache and myalgia) or anosmia/ageusia, which were reported primarily in relatively young patients with mild disease. Severe neurological manifestations, including altered mental status, and stroke or seizure, were significantly more common in older patients with severe to critical disease. Neurological symptoms or signs were presenting manifestations of the COVID-19 in 72 (23.5%) patients, and were the only manifestations of the disease in 12 (3.9%) patients. To the best of our knowledge, this is the first study to investigate the neurological features of COVID-19 in South Korea.
In previous studies on the neurological aspects of COVID-19, the incidence of neurological manifestations (including nonspecific symptoms) has varied widely, from 36% to 72%.2,11,12,13,14 Similar to the present findings, the most common symptoms reported previously were headache, myalgia, smell and taste impairment, and impaired consciousness. A recent systematic review found that the symptoms of headache, dizziness, smell dysfunction, and impaired consciousness occurred in 20.1, 6.8, 59.2, and 5.1% of patients, respectively.4
Several studies have focused on more specific or critical neurological manifestations of COVID-19, such as stroke, seizure, encephalopathy/encephalitis, and Guillain-Barré syndrome.15,16,17,18,19 Although the incidence of specific neurological manifestations is relatively low, they occur more commonly in patients with severe COVID-19 and are associated with a poor clinical outcome.2,4,13,15,19 We identified only three cases of stroke and two cases of seizures, and all of these patients had severe or critical COVID-19. Provisional case definitions for the association between COVID-19 with neurological disease have been proposed, which include meningitis, encephalitis, myelitis, CNS vasculitis, acute disseminated encephalomyelitis, acute neuropathies, and stroke.1 Prospective studies using the proposed new definitions are needed to ascertain the details regarding specific neurological complications associated with COVID-19.
The mechanisms by which SARS-CoV-2 invades the CNS are not fully understood; however, olfactory and hematogenous pathways have been suggested based on evidence from other coronaviruses.1,20,21,22 Direct viral entry into the brain through the olfactory bulb is a possible route.1 Several studies have indicated that olfactory dysfunction is an early and prevalent symptom of COVID-19.8,23,24,25 One case report described a COVID-19 patient who had a prefrontal lesion after experiencing anosmia, suggesting transolfactory bulb brain invasion.26 SARS-CoV-2 itself or infected leukocytes can also invade the CNS directly through hematogenous spread across the destroyed blood–brain barrier.1,4,22 Recent studies have found SARS-CoV-2 RNA in cerebrospinal fluid or brain tissues.27,28 SARS-CoV-2 can enter human host cells via binding of its spike protein to ACE2 receptors, which are found on various endothelial cells, including in the lung, blood vessels, kidney, and small intestine.21 The ACE2 receptor is also expressed in neurons, glial cells, and olfactory epithelial cells, rendering them potential targets for SARS-CoV-2 invasion.20,25
In contrast to direct invasion, indirect systemic and local inflammatory responses can cause cytokine storms and immunecell activation.22 Neurological manifestations might result from septic and hypoxic encephalopathy, coagulation dysfunction, cardiovascular-metabolic disturbance, and multiorgan failure.4,22 In addition, inflammatory conditions might be responsible for encephalitis, demyelination of the CNS, and Guillain-Barré syndrome.22
In comparison with other viral diseases, olfactory dysfunction was found to be more common in SARS-CoV-2-positive patients than in their SARS-CoV-2-negative counterparts with flu/respiratory symptoms.29 New-onset smell and taste impairments were reported more commonly in patients with COVID-19 than in a historical cohort of patients with influenza.30 Myalgia and rhabdomyolysis were more common in SARS-CoV-2 infection than in other flu/respiratory diseases.29 In another systematic review, the prevalence of myalgia among patients with COVID-19 was reported to be 20.5% [95% confidence interval (CI) 19.7–21.2]; myalgia was the fourth most common neurological symptom.3 In the case of influenza, the prevalence of myalgia was similar to that of COVID-19, with influenza A at 32% (95% CI 27–36%) and influenza B at 22.5% (95% CI 11–40%).31 It is therefore debatable whether myalgia is a more common or specific symptom of COVID-19 than of other viral infections.
Headache was the most prevalent neurological symptom of COVID-19 in our study, at 33.3%; a recent systematic review of 51 studies (16,446 patients) found that the overall prevalence of headache was 20.1%, ranging across studies from 2.0% to 66.1%.4 Headache is a common symptom of various viral infections, even in the absence of meningitis or encephalitis.32 As stated above, indirect mechanisms including hypoxia, dehydration, systemic inflammation, metabolic disturbances, and cytokine release syndrome could be involved in the development of headache in patients with COVID-19.33 A recent meta-analysis revealed a pooled prevalence of headache in COVID-19 patients of 10.1% (95% CI 8.76–11.49), which was lower than that for severe acute respiratory syndrome (20.0–61.0%), influenza (>90%), and other acute upper respiratory tract infections (>60%).34,35 Headache might be less common in SARS-CoV-2-positive patients than in patients with other respiratory-virus-associated infections.
Anosmia and ageusia were found only in patients with mild or moderate COVID-19 (n=53), which is similar to the results of a survey of patients in Daegu, South Korea.8 Although the precise underlying pathophysiology has yet to be determined, olfactory dysfunction might be related to a milder course of COVID-19 and lower mortality.13,36,37 Patients with anosmia tend to have more benign inflammatory or immune responses.37 A recent meta-analysis found that advanced age was correlated with a lower prevalence of olfactory and gustatory dysfunctions, which is consistent with the findings of the present study.38 This can be explained in part by severe COVID-19 being more prevalent in the elderly population, many of whom already have olfactory dysfunction. In addition, mild symptoms including olfactory dysfunction may be ignored or underestimated in patients with severe COVID-19.13
Stroke, seizures, and mental status changes occurred more frequently or were observed only in patients with severe to critical COVID-19. This is consistent with findings of the initial study from Wuhan and a recent Spanish registry study.2,13 Patients with severe disease are older and more likely to have stroke-associated comorbidities.4 Acute cerebrovascular disease in patients with COVID-19 may be associated with coagulopathy due to various causes, inflammatory processes, atrial fibrillation, and direct vascular endothelial injury associated with viral entry through ACE2 receptors.1,4,39 The development of major neurological manifestations, including encephalopathy, stroke, and seizure, was found to be an independent predictor of COVID-19-related death.40
It is estimated that risk of stroke is higher in COVID-19 patients than in those with influenza.41,42 Acute infection is a trigger for acute stroke, and in the UK General Practice Research Database study, acute stroke occurred within 1 month in 7% of those with acute systemic respiratory infection.43,44 Therefore, whether COVID-19 itself increases the risk of stroke is unclear.
In the present study, both patients with seizures had structural etiologies of seizures (acute ischemic stroke and prior subarachnoid hemorrhage). Nonspecific mechanisms related to COVID-19 such as fever, hypoxia, and cytokine-storm-related metabolic derangements can trigger the development of seizures in patients with underlying structural brain lesions.45 Although the occurrence of seizures in a cohort of patients with COVID-19 in Iran was reportedly low (0.08%), most patients experienced seizures as an initial manifestation.46 Several case studies have found seizures due to underlying meningoencephalitis in the setting of COVID-19.22,26
The prevalence of impaired consciousness in COVID-19 patients is reported to be 5.1% (range 1.4–69%), and has been shown to occur more commonly in those with severe to critical disease.4,47 Altered mental status has been observed in as many as 86% of patients who have died due to COVID-19.47 Most cases of impaired consciousness were associated with various causes of encephalopathy, such as hypoxia, sepsis, impaired metabolism, and toxicity/drugs. In the case of critically ill patients treated with mechanical ventilation, the diagnosis rate of impaired consciousness might have been overestimated due to the use of sedatives. In addition, as mentioned above, direct CNS invasion by SARS-CoV-2 can cause impaired consciousness and encephalitis, as observed by cerebrospinal fluid pleocytosis or a positive PCR result for cerebrospinal fluid. An extensive evaluation including a cerebrospinal fluid investigation was not performed in the present study because most of the patients with reduced consciousness had an underlying etiology related to encephalopathy. However, the possibility of direct invasion or other causes, such as nonconvulsive status epilepticus, cannot be excluded.
In the present study, 72 (23.5%) patients had a neurological manifestation as an initial presenting symptom of COVID-19, and in 12 (3.9%) patients this was the sole symptom of this disease. Most patients had mild neurological symptoms, such as myalgia, anosmia/ageusia, and headache, but two patients initially presented with altered mental status without respiratory symptoms. A study from Wuhan found that some patients with COVID-19 had neurological symptoms as their presenting manifestations,2 and one observational study found that neurological symptoms preceded respiratory symptoms in 14.5% of patients with COVID-19 and were the only symptoms of the disease in 22.7%.12 In another observational study investigating the initial symptoms of COVID-19, neurological symptoms were found in 9.7% of the patients.48 In another study, the onset of anosmia was noted early in 73% of the patients prior to the diagnosis of COVID-19.49 In addition, severe neurological manifestations, including altered mental status, stroke, and Guillain-Barré syndrome, could be the first symptom of COVID-19.47,50,51
The limitations of our study include incomplete neurological evaluations and a lack of diagnostic procedures, such as lumbar puncture and neurophysiological studies. The COVID-19 pandemic is a demanding situation during which respiratory care and contagion prevention are the critical points of focus, which makes clinicians hesitant to evaluate problems other than respiratory symptoms. In the present study, the data were collected by history-taking rather than by using structured questionnaires, and comprehensive evaluations by neurologists were performed only in selected patients. Anosmia/ageusia has received attention as a presenting symptom of COVID-19 since it was noticed in the Daegu pandemic in February 2020.8 Headache and myalgia are common symptoms of any viral illness. Therefore, we believe that the neurological symptoms were screened in most cases during the study period, although they could still be underestimated. The present findings are very similar to those of a study using structured surveys to obtain data that was performed in Tunisia.12 This study was a single-center study that included only hospitalized patients, and so the findings may not be generalizable to the overall COVID-19 patient population. Further large, prospective studies are needed to establish the details of the neurological characteristics of COVID-19.
In summary, we found that neurological manifestations were commonly observed in a population of Korean patients with COVID-19, especially headache, myalgia, and olfactory dysfunction. During the COVID-19 pandemic, when patients present with new-onset neurological symptoms, COVID-19 may be considered as part of the differential diagnosis. In addition, particular attention should be paid to severe neurological complications in patients with severe or critical COVID-19.
Acknowledgements
None
Footnotes
- Conceptualization: Hyun Kyung Kim, Seo-Young Lee.
- Data curation: Hyun Kyung Kim, Yeo Jeong Cho.
- Formal analysis: Hyun Kyung Kim, Seo-Young Lee.
- Investigation: Hyun Kyung Kim, Yeo Jeong Cho.
- Methodology: Hyun Kyung Kim, Seo-Young Lee.
- Project administration: Hyun Kyung Kim.
- Resources: Hyun Kyung Kim, Yeo Jeong Cho.
- Supervision: Seo-Young Lee.
- Validation: Hyun Kyung Kim, Seo-Young Lee.
- Visualization: Hyun Kyung Kim.
- Writing—original draft: Hyun Kyung Kim.
- Writing—review & editing: Seo-Young Lee.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Favas TT, Dev P, Chaurasia RN, Chakravarty K, Mishra R, Joshi D, et al. Neurological manifestations of COVID-19: a systematic review and meta-analysis of proportions. Neurol Sci. 2020;41:3437–3470. doi: 10.1007/s10072-020-04801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Laurent S, Onur OA, Kleineberg NN, Fink GR, Schweitzer F, et al. A systematic review of neurological symptoms and complications of COVID-19. J Neurol. 2021;268:392–402. doi: 10.1007/s00415-020-10067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 6.Ministry of Health and Welfare, Central Disease Control Headquarters. Coronavirus Disease-19, Republic of Korea: latest updates [Internet] Sejong: Ministry of Health and Welfare; 2020. [cited 2021 Jan 13]. Available from: http://ncov.mohw.go.kr/en/bdBoardList.do?brdId=16&brdGubun=161&dataGubun=&ncvContSeq=&contSeq=&board_id. [Google Scholar]
- 7.Kwon DH, Do Y, Eun MY, Lee J, Park H, Sohn SI, et al. Characteristics of acute stroke in patients with coronavirus disease 2019 and challenges in stroke management during and epidemic. J Korean Med Sci. 2020;35:e324. doi: 10.3346/jkms.2020.35.e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee Y, Min P, Lee S, Kim SW. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. 2020;35:e174. doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Heath Organization. COVID-19 clinical management: interim guidance [Internet] Geneva: World Heath Organization; 2020. [cited 2020 Oct 10]. Available from: https://www.who.int/publications/i/item/clinical-management-of-covid-19. [Google Scholar]
- 10.Statistics Korea. COVID-19 [Internet] Daejeon: Statistics Korea; 2020. [cited 2021 Feb 11]. Available from: https://kosis.kr/covid_eng/covid_index.do. [Google Scholar]
- 11.Agarwal P, Ray S, Madan A, Tyson B. Neurological manifestations in 404 COVID-19 patients in Washington State. J Neurol. 2021;268:770–772. doi: 10.1007/s00415-020-10087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kacem I, Gharbi A, Harizi C, Souissi E, Safer M, Nasri A, et al. Characteristics, onset, and evolution of neurological symptoms in patients with COVID-19. Neurol Sci. 2021;42:39–46. doi: 10.1007/s10072-020-04866-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, Sánchez-Larsen Á, Layos-Romero A, García-García J, et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95:e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campiglio L, Priori A. Neurological symptoms in acute COVID-19 infected patients: a survey among Italian physicians. PLoS One. 2020;15:e0238159. doi: 10.1371/journal.pone.0238159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frontera JA, Sabadia S, Lalchan R, Fang T, Flusty B, Millar-Vernetti P, et al. A prospective study of neurologic disorders in hospitalized patients with COVID-19 in New York city. Neurology. 2021;96:e575–e586. doi: 10.1212/WNL.0000000000010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kremer S, Lersy F, Anheim M, Merdji H, Schenck M, Oesterlé H, et al. Neurologic and neuroimaging findings in patients with COVID-19: a retrospective multicenter study. Neurology. 2020;95:e1868–e1882. doi: 10.1212/WNL.0000000000010112. [DOI] [PubMed] [Google Scholar]
- 17.Xiong W, Mu J, Guo J, Lu L, Liu D, Luo J, et al. New onset neurologic events in people with COVID-19 in 3 regions in China. Neurology. 2020;95:e1479–e1487. doi: 10.1212/WNL.0000000000010034. [DOI] [PubMed] [Google Scholar]
- 18.Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meppiel E, Peiffer-Smadja N, Maury A, Bekri I, Delorme C, Desestret V, et al. Neurologic manifestations associated with COVID-19: a multicentre registry. Clin Microbiol Infect. 2021;27:458–466. doi: 10.1016/j.cmi.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tancheva L, Petralia MC, Miteva S, Dragomanova S, Solak A, Kalfin R, et al. Emerging neurological and psychobiological aspects of COVID-19 infection. Brain Sci. 2020;10:852. doi: 10.3390/brainsci10110852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keyhanian K, Umeton RP, Mohit B, Davoudi V, Hajighasemi F, Ghasemi M. SARS-CoV-2 and nervous system: from pathogenesis to clinical manifestation. J Neuroimmunol. 2020;350:577436. doi: 10.1016/j.jneuroim.2020.577436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10:806–813. doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yachou Y, El Idrissi A, Belapasov V, Ait Benali S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol Sci. 2020;41:2657–2669. doi: 10.1007/s10072-020-04575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Guennec L, Devianne J, Jalin L, Cao A, Galanaud D, Navarro V, et al. Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia. 2020;61:e90–e94. doi: 10.1111/epi.16612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luigetti M, Iorio R, Bentivoglio AR, Tricoli L, Riso V, Marotta J, et al. Assessment of neurological manifestations in hospitalized patients with COVID-19. Eur J Neurol. 2020;27:2322–2328. doi: 10.1111/ene.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beltrán-Corbellini Á, Chico-García JL, Martínez-Poles J, Rodríguez-Jorge F, Natera-Villalba E, Gómez-Corral J, et al. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur J Neurol. 2020;27:1738–1741. doi: 10.1111/ene.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pormohammad A, Ghorbani S, Khatami A, Razizadeh MH, Alborzi E, Zarei M, et al. Comparison of influenza type A and B with COVID-19: a global systematic review and meta-analysis on clinical, laboratory and radiographic findings. Rev Med Virol. 2020:e2179. doi: 10.1002/rmv.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 33.Bobker SM, Robbins MS. COVID-19 and headache: a primer for trainees. Headache. 2020;60:1806–1811. doi: 10.1111/head.13884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Islam MA, Alam SS, Kundu S, Hossan T, Kamal MA, Cavestro C. Prevalence of headache in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of 14,275 patients. Front Neurol. 2020;11:562634. doi: 10.3389/fneur.2020.562634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–3247. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 36.Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int Forum Allergy Rhinol. 2020;10:821–831. doi: 10.1002/alr.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talavera B, García-Azorín D, Martínez-Pías E, Trigo J, Hernández-Pérez I, Valle-Peñacoba G, et al. Anosmia is associated with lower inhospital mortality in COVID-19. J Neurol Sci. 2020;419:117163. doi: 10.1016/j.jns.2020.117163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc. 2020;95:1621–1631. doi: 10.1016/j.mayocp.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salahuddin H, Afreen E, Sheikh IS, Lateef S, Dawod G, Daboul J, et al. Neurological predictors of clinical outcomes in hospitalized patients with COVID-19. Front Neurol. 2020;11:585944. doi: 10.3389/fneur.2020.585944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77:1366–1372. doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piroth L, Cottenet J, Mariet AS, Bonniaud P, Blot M, Tubert-Bitter P, et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9:251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 44.Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7:341–353. doi: 10.1016/S1474-4422(08)70061-9. [DOI] [PubMed] [Google Scholar]
- 45.Vohora D, Jain S, Tripathi M, Potschka H. COVID-19 and seizures: Is there a link. Epilepsia. 2020;61:1840–1853. doi: 10.1111/epi.16656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emami A, Fadakar N, Akbari A, Lotfi M, Farazdaghi M, Javanmardi F, et al. Seizure in patients with COVID-19. Neurol Sci. 2020;41:3057–3061. doi: 10.1007/s10072-020-04731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tyson B, Erdodi L, Ray S, Agarwal P. Comparison of influenza type A and B with COVID-19: a global systematic review and meta-analysis on clinical, laboratory and radiographic findings. Int J Neurosci. 2020. Oct 01, [Epub]. Available from: [DOI]
- 48.Nersesjan V, Amiri M, Christensen HK, Benros ME, Kondziella D. Thirty-day mortality and morbidity in COVID-19 positive vs. COVID-19 negative individuals and vs. individuals tested for influenza A/B: a population-based study. Front Med (Lausanne) 2020;7:598272. doi: 10.3389/fmed.2020.598272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaye R, Chang CWD, Kazahaya K, Brereton J, Denneny JC., 3rd COVID-19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg. 2020;163:132–134. doi: 10.1177/0194599820922992. [DOI] [PubMed] [Google Scholar]
- 50.Alberti P, Beretta S, Piatti M, Karantzoulis A, Piatti ML, Santoro P, et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7:e741. doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]