Abstract
Kawasaki‐like disease (KLD) and multisystem inflammatory syndrome in children (MIS‐C) are considered as challenges for pediatric patients under the age of 18 infected with coronavirus disease 2019 (COVID‐19). A systematic search was performed on July 2, 2020, and updated on December 1, 2020, to identify studies on KLD/MIS‐C associated with COVID‐19. The databases of Scopus, PubMed, Web of Science, Embase, and Scholar were searched. The hospitalized children with a presentation of Kawasaki disease (KD), KLD, MIS‐C, or inflammatory shock syndromes were included. A total number of 133 children in 45 studies were reviewed. A total of 74 (55.6%) cases had been admitted to pediatric intensive care units (PICUs). Also, 49 (36.8%) patients had required respiratory support, of whom 31 (23.3%) cases had required mechanical ventilation/intubation, 18 (13.5%) cases had required other oxygen therapies. In total, 79 (59.4%) cases had been discharged from hospitals, 3 (2.2%) had been readmitted, 9 (6.7%) had been hospitalized at the time of the study, and 9 (6.7%) patients had expired due to the severe heart failure, shock, brain infarction. Similar outcomes had not been reported in other patients. Approximately two‐thirds of the children with KLD associated with COVID‐19 had been admitted to PICUs, around one‐fourth of them had required mechanical ventilation/intubation, and even some of them had been required readmissions. Therefore, physicians are strongly recommended to monitor children that present with the characteristics of KD during the pandemic as they can be the dominant manifestations in children with COVID‐19.
Keywords: children, COVID‐19, Kawasaki Disease, multisystem inflammatory syndrome, pediatrics
Abbreviations
- AHF
acute hearth failure
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- CSS
cytokine storm syndrome
- CT
computed tomography
- ECMO
extra corporeal membrane oxygenation
- ECO
echocardiography
- G6PD
glucose‐6‐phosphate dehydrogenase
- GI
gastrointestinal
- HFNC
high flow nasal cannula
- IL‐6
interleukin 6
- KD
Kawasaki disease
- KLD
Kawasaki‐like disease
- LVSD
left ventricular systolic dysfunction
- MERS
Middle East respiratory syndrome
- MIS‐C
multisystem inflammatory syndrome
- PICU
pediatric intensive care unit
- PIMS‐TS
pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2
- RT‐PCR
reverse transcription polymerase chain reaction
- SARS
severe acute respiratory syndrome
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- VSD
ventricular septal defect
1. INTRODUCTION
Since December 2019, the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) from the family Coronaviridae causing coronavirus disease 2019 (COVID‐19) emerged in Wuhan, China, and then became a global health challenge. This was the third epidemic of the large family Coronaviridae, which induced SARS and the Middle East respiratory syndrome at the beginning of the present century.1, 2 The authors' knowledge of COVID‐19 was thus based on two previous experiments in their early phases, that hyperinflammation caused by macrophage‐activating syndrome and cytokine storm release (CSR) was involved in the pathogenesis of COVID‐19.3, 4, 5, 6
Initially, COVID‐19 patients were identified with symptoms such as dry cough, fever, dyspnea, headache, weakness, and lethargy, which later appeared with gastrointestinal (GI), neurological, and cutaneous manifestations.7, 8 Early on, it seemed that children were not the target groups and they were less likely to be affected, so there were a small number of reports on childhood illnesses.9, 10 After a while, the surge of comparable reports of children attending medical centers increased with identical clinical characteristics of this disease in different countries during the pandemic. Signs and symptoms in these patients had something in common with Kawasaki disease (KD), KD shock syndrome, toxic shock syndrome (TSS), fever, shock, and skin rash. Also, conjunctivitis, extremity edema, and GI manifestations were observed based on the positive nasopharyngeal reverse transcription‐polymerase chain reaction (RT‐PCR) or antibody (viz. serological) testing for SARS‐CoV‐2 within 4 weeks of the onset of the symptoms.11, 12, 13 With reference to the growing number of reports in the first half of May, the Royal College of Pediatrics and Child Health of the United Kingdom and the Centers for Disease Control and Prevention of the United States, respectively, declared an alert on this condition under the label “Pediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS‐CoV‐2 (PIMS‐TS)” and “Multisystem Inflammatory Syndrome in Children (MIS‐C).” 14
In this respect, MIS‐C refers to a hyperinflammatory systemic condition that shares numerous similar features of KD such as lymphadenopathy, diarrhea, elevated inflammatory biomarkers, prolonged fever, skin rash alongside some separate specifications like older onset, the predominance of abdominal symptoms, cases with left ventricular systolic dysfunction and acute heart failure (AHF).11, 15 The present study was designed and implemented to demonstrate the relationship between severely ill cases with KD/MIS‐C and COVID‐19.
2. METHODS
2.1. Search databases and search strategies
This study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) reporting guidelines 16 on July 2, 2020, and updated on December 1, 2020, to identify the studies on KD/MIS‐C associated with COVID‐19. To this end, the relevant studies were searched using the databases of Scopus, PubMed (i.e., MEDLINE), Web of Science, Embase (Elsevier), and Scholar. The following search keywords were also used: “coronavirus,” “COVID‐19,” “coronavirus and infection,” “SARS‐CoV‐19,” “2019 novel and coronavirus,” “Kawasaki,” “hyperinflammatory,” “inflammatory syndrome,” “Kawasaki‐like,” and “MIS‐C.” The keywords list utilized in the search is provided as a Supporting Information appendix. The PRISMA flow diagram of the study selection process is also illustrated in Figure 1.
Figure 1.
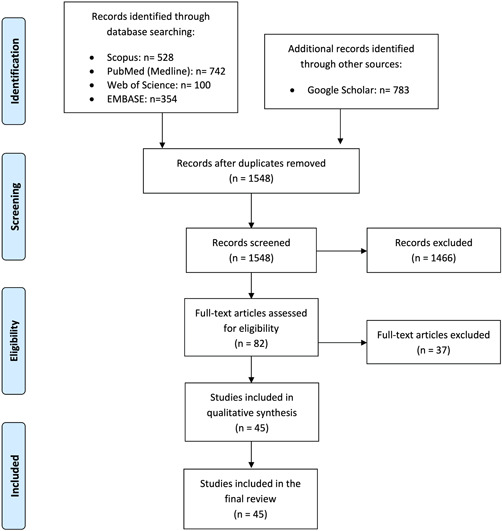
Flow diagram of the study selection process. Preferred reporting items for systematic reviews and meta‐analyses (PRISMA). Adapted from Moher et al. 16 (doi.org/10.1371/journal.pmed.1000097) ©2009, under terms of Creative Commons Attribution 4.0 International License (creativecommons.org/licenses/by/4.0/legalcode)
2.2. Eligibility criteria
The pediatric patients (0–≤18 years of age) with more than 1 day of subjective or measured fever (≥100.4°F/38°C) and hospital stay, that presented with at least KD, Kawasaki‐like disease (KLD), MIS‐C, or inflammatory shock syndromes, with the evidence of COVID‐19, and with confirmed SARS‐CoV‐2 infection using nasopharyngeal RT‐PCR or antibody (viz. serological) testing were included in this study. Moreover, irrelevant studies, conference abstracts, and duplicates were excluded.
2.3. Data extraction and quality assessment
Two independent and blinded reviewers extracted the data and then performed crosschecking. A third reviewer also resolved the disagreements via consensus. Accordingly, the data were extracted: first author's name, study setting, type of study, patient's age, gender, initial presentations at the time of hospital admission, type of hospital admission ward (pediatric ward and pediatric intensive care unit [PICU]), type of COVID‐19 confirmation tests (RT‐PCR and antibody [viz. serological] testing), mechanical ventilation and intubation, pulmonary and extra‐pulmonary findings, electrocardiography reports, echocardiography reports, methods of treatment, complications, and outcomes. Additionally, a series of images from two studies included were presented 17, 18 (Figures 2 and 3) through formal permissions obtained from their publishers. Regarding the quality assessment, two independent reviewers evaluated the risk of bias of the included cohort studies using the modified version of the Newcastle–Ottawa Scale, 19 and the National Institutes of Health Quality Assessment tool for case series/reports 20 (Tables S1 and S2).
Figure 2.
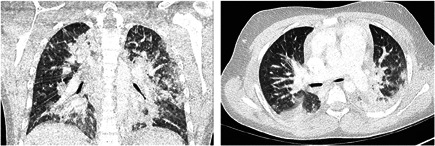
Lung window axial and coronal CT images of patient 3 that show diffuse bilateral consolidations predominantly located in the posterior aspects of the upper and inferior lobes. Images obtained from Dallan et al. 17 The Lancet Child & Adolescent Health, Vol 4(7), E21‐23 July 1, 2020, and permission to use granted by Elsevier License Terms and Conditions. CT, computed tomography
Figure 3.
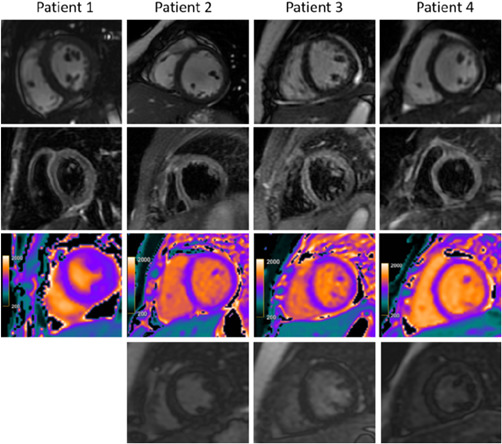
Cardiac MRI for four children with a clinical diagnosis of acute myocarditis in the setting of COVID‐19‐related Kawasaki‐like symptoms. The top panel demonstrates minimal pericardial effusion on cine images. The second panel demonstrates increased T2‐STIR signal intensity with average ratios between myocardium and muscle more than 2 in patient 2 (12‐year‐old male), patient 3 (11‐year‐old female), and patient 4 (6‐year‐old female). The third panel demonstrates abnormal native‐T1 mapping, which was more than 1100 ms in patients 2, 3, and 4 and normal in patient 1 (8‐year‐old female). The bottom panel demonstrates absence of late gadolinium enhancement (LGE) in patients 2 and 3. Myocardial null times were recognized as too short in patient 4 but could not be repeated due to lack of further patient cooperation; however, a review of Look‐Locker images and additional sequences revealed no LGE. Images obtained from Blondiaux et al. 18 Radiology, June 9, 2020, and permission to use granted by Ashley E. Daly, Senior Manager, Journal Rights & Communications Publications, Radiological Society of North America (RSNA). COVID‐19, coronavirus disease 2019; MRI, magnetic resonance imaging
3. RESULTS
A total number of 69 studies were recognized in the initial search. After removing the duplicates and the irrelevant articles, 45 eligible studies including 6 cohort studies, 7 case series, 24 case reports, and 8 correspondences or letters to editors related to KLD/MIS‐C associated with COVID‐19 were included. As a whole, 133 children with the mean age of 9 ± 4.2 (age range: 4 months to 17 years old, interquartile range: 5.5–13 years old) with 82 (61.6%) male cases, 50 (37.5%) females, and one unknown gender were reviewed (Table 1).
Table 1.
Characteristics of children (n = 133) with Kawasaki‐like multisystem inflammatory syndrome and COVID‐19 infection
| Patient no./Sex/Age(y) | First author | Country | Type of disease (at hospital admission) | Significant findings (imaging, echocardiography) | Outcome |
|---|---|---|---|---|---|
| P1/F/10 | Saeed et al. 21 | Iran | atypical KD | Patchy infiltration in chest CT, Lt. ventricle function and dilated IVC | Hospitalized |
| P2/F/13 | Saeed et al. 21 | Iran | MIS‐C | Bilateral patchy GGOs in chest CT, poor Lt. ventricle systolic function and borderline Rt. ventricle systolic function with dilated IVC | Expired |
| P3/F/15 | Fraser et al. 22 | Canada | atypical KD | Normal echocardiography | Discharged |
| P4/M/16 | Schnapp et al. 23 | Israel | PIMS‐TS | Lt. ventricular dilatation | Hospitalized |
| P5/M/5 | Rauf et al. 23 | India | atypical KD | Cardiomegaly with Lt. ventricular dilatation in chest X‐ray, global Lt. ventricular hypokinesia with systolic dysfunction | Discharged |
| P6/M/5 | Schupper et al. 24 | USA | Cardiogenic shock | Rt. MCA infarction, cerebral edema, diffuse contralateral subarachnoid, bilateral MCA and PCA territory infarctions, bilateral hemispheric transformation, bilateral subdural collections in brain CT | Brain death, Hospitalized |
| P7/M/2 ma | Schupper et al. 24 | USA | Refractory respiratory failure | Bilateral MCA and PCA territory infarctions with the hemorrhagic transformation. evolving hemorrhagic infarctions in bilateral occipitoparietal lobes, Lt. temporal and frontal lobes in brain MRI | Hospitalized |
| P8/F/11 | Greene et al. 25 | USA | Incomplete KD | LV systolic function mildly decreased based on decreased shortening fraction | Discharged & readmitted |
| P9/M/9 | Giannattasio et al. 26 | Italy | MIS‐C | Two small bilateral areas of atelectasis associated to minimal pleural effusion in chest CT, Normal echocardiography | Discharged |
| P10/M/4 ma | Acharyya et al. 27 | India | atypical KD | Normal Lt. ventricular function, perivascular brightness and diffuse coronary arteries ectasia | Hospitalized |
| P11/F/3 | Yozgat et al. 28 | Turkey | PIMS‐TS | Significant increase in echogenicity of coroner vessels | Discharged |
| P12/F/8 | Bloniaux et al. 18 | France | PIMS‐TS | hypokinesis, mitral regurgitation | Discharged |
| P13/M/12 | Bloniaux et al. 18 | France | PIMS‐TS | Diffuse echo‐bright appearance in myocardium, septal dyskinesia, pericardial effusion | Discharged |
| P14/F/11 | Bloniaux et al. 18 | France | PIMS‐TS | Peripheral, posterior, multilobar and bilateral distribution of a combination of GGOs and consolidations in chest CT, hypokinesis, mitral regurgitation, pericardial effusion | Discharged |
| P15/F/6 | Bloniaux et al. 18 | France | PIMS‐TS | Pericardial effusion, transient systolic dysfunction | Discharged |
| P16/M/5 | Rivera‐Figueroa et al. 29 | USA | Incomplete KD, KDSS | Enlarged cardiac silhouette in chest x‐ray, a small pericardial effusion | Discharged |
| P17/M/16 | Rosenzweig et al. 30 | USA | Acute ITP | NR | Discharged |
| P18/F/14 | Rosenzweig et al. 30 | USA | Mixed‐type AIHA | NR | Discharged |
| P19/M10 | Chiu et al. 31 | USA | atypical KD | NR, severely diminished Lt. ventricular systolic function with trace pericardial effusion | Hospitalized |
| P20/F/14 | Chiotos et al. 11 | USA | Incomplete KD | Bilateral pulmonary infiltrates in chest CT, Rt. coronary artery dilation (Boston Z score, 3.15) | Discharged |
| P21/M/12 | Chiotos et al. 11 | USA | MIS‐C | Diffuse bilateral infiltrates in chest CT, mild LV dysfunction | Discharged |
| P22/F/9 | Chiotos et al. 11 | USA | MIS‐C | Cardiomegaly and pulmonary edema in chest X‐ray, Normal echocardiography | Discharged |
| P23/F/5 | Chiotos et al. 11 | USA | MIS‐C | Peribronchial thickening with Rt. patchy infiltrates in chest X‐ray, moderately diminished LV systolic function | Discharged |
| P24/F/5 | Chiotos et al. 11 | USA | MIS‐C | Significant cardiac silhouette and mild central vascular congestion in chest X‐ray, LV dilation, mildly diminished LV function | Discharged |
| P25/F/6 | Chiotos et al. 11 | USA | MIS‐C | Dense bilateral airspace opacities and heart appears prominent in chest X‐ray, moderate LV dilation with mildly diminished systolic shortening, developed intermittent premature ventricular contractions, bigeminy and trigeminy | Hospitalized |
| P26/F/16 | Foong Ng et al. 32 | UK | PIMS‐TS | Bilateral basal and peripheral airspace shadowing in chest x‐ray, mildly impaired Lt. ventricular function, small pericardial effusion | Discharged |
| P27/M/17 | Foong Ng et al. 32 | UK | PIMS‐TS | Cardiomegaly, retrocardiac and Lt. lobe airspace opacification, Lt. pleural effusion in chest x‐ray, coronary artery dilatation, RCA 4.9 mm ectasia (Z‐score +3) | Discharged |
| P28/M/13 | Foong Ng et al. 32 | UK | PIMS‐TS | Collapse‐consolidation in Rt. lobe in chest x‐ray, mild mitral regurgitation. coronary artery dilatation, dilated RCA 4.6 mm (Z score +2.2) and LCA 4.9–5.7 mm (Z score +2.2–3.7) | Discharged |
| P29/M/13 | Joshi et al. 33 | USA | Cardiac dysfunction & shock | Lt. basal opacity in chest X‐ray, Normal echocardiography | Discharged |
| P30/M/6 | Joshi et al. 33 | USA | Cardiac dysfunction & shock | Mild mitral regurgitation | Discharged |
| P31/F/13 | Joshi et al. 33 | USA | Cardiac dysfunction & shock | Moderately decreased Lt. ventricular systolic function with mild mitral regurgitation, and a small pericardial effusion | Discharged |
| P32/M/12 | Licciardi et al. 34 | Italy | PIMS‐TS | Decreased systolic function, pleural effusion | NR |
| P33/M/7 | Licciardi et al. 34 | Italy | PFAPA syndrome | Cardiomegaly and pleural effusion in chest CT, reduced systolic function | NR |
| P34/M/8 | Verdoni et al. 35 | Italy | Incomplete KD | Regurgitation of mitral valve, pericardial effusion, aneurysm more than 4 mm | NR |
| P35/M/7 | Verdoni et al. 35 | Italy | Incomplete KD | Normal echocardiography | NR |
| P36/F/3 | Verdoni et al. 35 | Italy | Classic KD | Normal echocardiography | NR |
| P37/F/7 | Verdoni et al. 35 | Italy | Incomplete KD | Mitral valve regurgitation, pericardial effusion | NR |
| P38/F/7 | Verdoni et al. 35 | Italy | Incomplete KD | Mitral valve regurgitation, pericardial effusion | NR |
| P39/M/16 | Verdoni et al. 35 | Italy | Classic KD | Pericardial effusion | NR |
| P40/M/5 | Verdoni et al. 35 | Italy | Classic KD | Normal echocardiography | NR |
| P41/M/9 | Verdoni et al. 35 | Italy | Incomplete KD | Pneumonia in chest X‐ray, mitral valve regurgitation, aneurysm more than 4 mm | NR |
| P42/M/5 | Verdoni et al. 35 | Italy | Classic KD | Normal echocardiography | NR |
| P43/M/5 | Verdoni et al. 35 | Italy | Classic KD | Pneumonia in chest X‐ray, Normal echocardiography | NR |
| P44/F/12 | Riollano‐Cruz et al. 36 | USA | PIMS‐TS | Progressive lower lobe GGOs in chest X‐ray, Normal echocardiography | Discharged |
| P45/M/14 | Riollano‐Cruz et al. 36 | USA | PIMS‐TS | Mild regurgitation in both the tricuspid and mitral valves, Normal Rt. Ventricular systolic function. Mildly dilated Lt. ventricle. | Discharged |
| Normal Lt. ventricular systolic function. | |||||
| P46/F/14 | Riollano‐Cruz et al. 36 | USA | PIMS‐TS | Normal, Mildly dilated Lt. ventricle | Discharged |
| P47/M/5 | Riollano‐Cruz et al. 36 | USA | PIMS‐TS | Progressive lung GGOs in chest x‐ray, approximately total Rt. MCA infarction involving cortex, subcortical white matter and deep gray matter, Lt. frontal subarachnoid hemorrhage in brain CT, Severely depressed biventricular systolic function, Trivial posterior pericardial effusion | Expired |
| P48/M/6 | Riollano‐Cruz et al. 36 | USA | PIMS‐TS | Normal echocardiography | Hospitalized |
| P49/F/11 | Riollano‐Cruz et al. 36 | USA | PIMS‐TS | Mild peri‐bronchial thickening throughout the lungs in chest X‐ray, Normal echocardiography | Discharged |
| P50/M/17 | Riollano‐Cruz et al. 36 | USA | PIMS‐TS | Lt. ventricle systolic function mildly depressed | Discharged |
| P51/F/3 | Riollano‐Cruz et al. 36 | USA | PIMS‐TS | Normal echocardiography | Discharged |
| P52/M/10 | Riollano‐Cruz et al. 36 | USA | PIMS‐TS | Small bilateral pleural effusions, ill‐defined airway opacities in X‐ray, Normal echocardiography | Discharged |
| P53/M/12 | Riollano‐Cruz et al. 36 | USA | PIMS‐TS | Reactive airway disease, Mild proximal LMCA ectasia | Discharged |
| P54/M/13 | Riollano‐Cruz et al. 36 | USA | PIMS‐TS | Progressive lung opacities in chest X‐ray, mildly diffusely ecstatic Lt. main and LAD, Lt. prominent circumflex | Discharged |
| P55/M/5 | Riollano‐Cruz et al. 36 | USA | PIMS‐TS | Reactive airway disease, Mildly dilated Lt. main and proximal Lt. anterior descending coronary arteries | Discharged |
| P56/F/6 | Leon et al. 37 | USA | Incomplete KD | Prominent cardiac silhouette with clear lung fields, diffuse patchy GGOs in chest X‐ray, mildly decreased LV function, mild mitral valve insufficiency | Discharged & readmitted |
| P57/NR/14 | Pain et al. 38 | USA | PIMS‐TS | Typical findings of COVID‐19 pneumonia in chest CT, aortic regurgitation, and progressive Lt. coronary dilatation | Discharged |
| P58/M/8 | Oberweis et al. 39 | Luxembourg | Myocarditis with heart failure | Bilateral pneumopathies and bilateral pleural effusions in chest CT, impaired LV function, and trace mitral insufficiency as well as a small pericardial effusion | Discharged |
| P59/M/6 | Labé et al. 40 | France | COVID‐19‐associated with Erythema multiforme | NR | Discharged |
| P60/M/3 | Labé et al. 40 | France | PIMS‐TS | GGOs and consolidation in the Rt. poster basal area in chest CT | NR |
| P61/M/4 | DeBiasi et al. 41 | USA | atypical KD | NR | NR |
| P62/M/8 | Balasubramanian et al. 42 | India | MIS‐C | Rt. lobe infiltrates in chest x‐ray, Normal echocardiography | Discharged |
| P63/M/14 | Riphagen et al. 43 | UK | hyperinflammatory shock | Bilateral basal lung consolidations and diffuse nodules in chest X‐ray, Rt. ventricular dysfunction, elevated Rt. ventricular systolic pressure | Expired |
| P64/M/8 | Riphagen et al. 43 | UK | hyperinflammatory shock | Pleural effusions in chest X‐ray, mild biventricular dysfunction, | Discharged |
| severely dilated coronaries | |||||
| P65/M/4 | Riphagen et al. 43 | UK | hyperinflammatory shock | Pleural effusions in chest X‐ray | Discharged |
| P66/F/13 | Riphagen et al. 43 | UK | hyperinflammatory shock | Moderate to severe LV dysfunction | Discharged |
| P67/M/6 | Riphagen et al. 43 | UK | hyperinflammatory shock | Dilated LV, AVR, peri coronary hyper echogenicity | Discharged |
| P68/F/6 | Riphagen et al. 43 | UK | hyperinflammatory shock | Mild LV systolic impairment | Discharged |
| P69/M/12 | Riphagen et al. 43 | UK | hyperinflammatory shock | Pleural effusions in chest X‐ray, severe biventricular impairment | Discharged |
| P70/F/8 | Riphagen et al. 43 | UK | hyperinflammatory shock | Moderate LV dysfunction | Discharged |
| P71/M/13 | Waltuch et al. 44 | USA | atypical KD, CSS, TSS | Hazy bilateral opacities in chest X‐ray, coronary artery dilatation and moderately depressed LV systolic function | NR |
| P72/M/10 | Waltuch et al. 44 | USA | atypical KD, TSS | Peribronchial thickening with ill‐defined airspace opacities in the Rt. lung in chest X‐ray, mild regurgitation in both the tricuspid and mitral valves | NR |
| P73/M/5 | Waltuch et al. 44 | USA | atypical KD, TSS | Mildly dilated proximal Lt. anterior descending coronary artery | NR |
| P74/F/12 | Waltuch et al. 44 | USA | NR | Normal imaging | NR |
| P75/M/13 | Bapst et al. 45 | Switzerland | MIS‐C | Normal imaging | Discharged |
| P76/F/6 ma | Jones et al. 46 | USA | Classic KD | A faint opacity in the Lt. lung in chest X‐ray, Normal echocardiography | Discharged |
| P77/F/5 | Bahrami et al. 47 | Iran | MIS‐C | Normal echocardiography | Discharged |
| P78/F/8 | Dasgupta et al. 48 | USA | PIMS‐TS | Bibasilar reticulonodular opacities, enlarged cardiac silhouette with pulmonary edema and small bilateral pleural effusions, systolic and diastolic dysfunction, valvular regurgitation | NR |
| P79/M/12 | Dallan et al. 17 | Switzerland | Septic shock | Normal imaging | Discharged |
| P80/M/10 | Dallan et al. 17 | Switzerland | Hypotensive septic shock associated with MODS | Rt. lobe consolidation with bilateral pleural effusions | Discharged |
| P81/M/10 | Dallan et al. 17 | Switzerland | NR | Diffuse bilateral consolidations in chest CT, Lt. anterior descending artery and Rt. coronary aneurysms, with Z scores of 4.53 and 3.30, respectively | Hospitalized |
| P82/M/7 | Akca et al. 49 | Turkey | Incomplete KD | Bilateral diffuse GGOs, diffuse enlargement in the Lt. coronary artery (Z score of 2.0) | Expired |
| P83/F/10 | Akca et al. 49 | Turkey | KD | Pleural effusion and GGOs | Discharged |
| P84/F/2 | Akca et al. 49 | Turkey | Incomplete KD | Increased perivascular echogenicity in the Rt. coronary artery | NR |
| P85/F/2 | Akca et al. 49 | Turkey | Incomplete KD | An aneurysm in the Lt. coronary artery | NR |
| P86/F/6 | Burger et al. 50 | USA | MIS‐C | Mildly decreased Lt. LV function, septal hypokinesis, and mild mitral valve insufficiency | Discharged |
| P87/F/13 | Al Ameer et al. 51 | Saudi Arabia | atypical KD | Mild mitral regurgitation, mild pericardial effusion, and moderate depression in Lt. ventricle function | Expired |
| P88/M/8 | Khan et al. 52 | Pakistan | atypical KD | Parenchymal opacification and pleural effusion in the Lt. lobe | Discharged & Readmitted |
| P89/F/9 | Jackson et al. 53 | USA | MIS‐C | NR | Discharged |
| P90/M/5 | Falah et al. 54 | Pakistan | Incomplete KD | Cardiomegaly, Pericardial effusion | NR |
| P91/M/3 | Falah et al. 54 | Pakistan | KD | GGOs and consolidation in the Rt. lung | NR |
| P92/M/10 | Falah et al. 54 | Pakistan | Incomplete KD | Pericardial effusion | NR |
| P93/F/11 | Falah et al. 54 | Pakistan | Incomplete KD | NR | NR |
| P94/F/6 ma | Falah et al. 54 | Pakistan | KD | Faint opacity in Lt. lobe of lung | NR |
| P95/M/8 | Falah et al. 54 | Pakistan | KD | Rt. lobe infiltrates | NR |
| P96/M/4 ma | Falah et al. 54 | Pakistan | KD | NR | NR |
| P97/M/5 | Falah et al. 54 | Pakistan | Incomplete KD | Cardiomegaly | NR |
| P98/M/11 | Falah et al. 54 | Pakistan | Incomplete KD | Cardiomegaly, Pericardial effusion | NR |
| P99/M/6 | Falah et al. 54 | Pakistan | KD | Bilateral pulmonary infiltrates in the Rt. base of lung | NR |
| P100/M/7 | Almoosa et al. 55 | Saudi Arabia | NR | Acute respiratory distress syndrome (ARDS), pericardial effusion | Expired |
| P101/F/7 | Almoosa et al. 55 | Saudi Arabia | NR | NR | Discharged |
| P102/M/11 | Almoosa et al. 55 | Saudi Arabia | MIS‐C | NR | Discharged |
| P103/F/3 | Almoosa et al. 55 | Saudi Arabia | MIS‐C | NR | Discharged |
| P104/M/1 | Almoosa et al. 55 | Saudi Arabia | MIS‐C | NR | Discharged |
| P105/F/12 | Almoosa et al. 55 | Saudi Arabia | MIS‐C | NR | NR |
| P106/F/6 | Almoosa et al. 55 | Saudi Arabia | MIS‐C | Rt. sided pleural effusion | Discharged |
| P107/M/5 | Almoosa et al. 55 | Saudi Arabia | MIS‐C | Lt. ventricular dysfunction | Discharged |
| P108/M/11 | Almoosa et al. 55 | Saudi Arabia | MIS‐C | NR | Discharged |
| P109/M/5 ma | Raut et al. 56 | India | Incomplete KD | Mild GGOs in Rt. lung, Dilated Lt. main coronary artery (3.0 mm, Z score of 4.30) and Lt. anterior descending artery (2.37 mm, score = 3.76) | Discharged |
| P110/M/11 | Kim et al. 57 | South Korea | MIS‐C | Cardiomegaly, pleural effusion with lung parenchymal consolidation, Lt. main coronary artery dilation. Rt. coronary artery dilatation and aneurysmal changes with mild pericardial effusion | Discharged |
| P111/F/7 ma | De Farias et al. 58 | Brazil | TSS | NR | Expired |
| P112/M/4 | De Farias et al. 58 | Brazil | TSS | NR | Expired |
| P113/M/11 | De Farias et al. 58 | Brazil | KDSS | NR | Discharged |
| P114/M/4 | De Farias et al. 58 | Brazil | KD | NR | Discharged |
| P115/M/7 | De Farias et al. 58 | Brazil | KD | NR | Discharged |
| P116/F/2 | De Farias et al. 58 | Brazil | atypical KD | NR | Discharged |
| P117/M/9 | De Farias et al. 58 | Brazil | KDSS | NR | Discharged |
| P118/M/6 | De Farias et al. 58 | Brazil | KDSS | NR | Discharged |
| P119/M/4 | De Farias et al. 58 | Brazil | KDSS | NR | Discharged |
| P120/M/4 | De Farias et al. 58 | Brazil | KD | NR | Discharged |
| P121/M/10 | De Farias et al. 58 | Brazil | KD | NR | Discharged |
| P122/M/12 | Shahbaznejad et al. 59 | Iran | NR | Patchy GGOs and interlobar septal thickening, mild regurgitation in both the tricuspid and mitral valves, mild diastolic dysfunction | Expired |
| P123/F/5 | Shahbaznejad et al. 59 | Iran | NR | Bilateral plural effusion and patchy infiltration, GGOs, mild regurgitation in tricuspid valves | Discharged |
| P124/M/1 | Shahbaznejad et al. 59 | Iran | NR | Bilateral plural effusion, basilar patchy infiltration and reverse halo | Discharged |
| sign, mild regurgitation in both the tricuspid and mitral valves | |||||
| P125/F/10 | Shahbaznejad et al. 59 | Iran | NR | Mild bilateral plural effusion, mild regurgitation in both the tricuspid and mitral valves | Discharged |
| P126/M/1 | Shahbaznejad et al. 59 | Iran | NR | Pleural effusion, mild mitral valves regurgitation | Discharged |
| P127/M/6 | Shahbaznejad et al. 59 | Iran | NR | Bilateral GGOs, mild regurgitation in both the tricuspid and mitral valves | Discharged |
| P128/F/7 | Shahbaznejad et al.(75) | Iran | NR | Bilateral GGOs, moderate regurgitation in both the tricuspid and mitral valves, Dilated Rt. and Lt. ventricle, myocarditis | Discharged |
| P129/M/1 | Shahbaznejad et al. 59 | Iran | NR | Bilateral GGOs, LAD, moderate regurgitation in mitral valve, diastolic dysfunction | Discharged |
| P130/M/7 | Shahbaznejad et al. 59 | Iran | NR | Bilateral nodular like lesions in in lungs, Sub plural atelectasis, | Discharged |
| mild bilateral pleural effusion | |||||
| P131/F/1 | Shahbaznejad et al. 59 | Iran | NR | Bilateral nonspecific opacities in inferior lobes | Discharged |
| P132/M/11 | Cirks et al. 60 | USA | MIS‐C | Bilateral patchy infiltrates, bilateral pleural effusions, prominent peri bronchial cuffing, retro cardiac atelectasis, Lt. anterior descending coronary artery aneurysm (4 mm, Boston Z score +3.3) | NR |
| P133/F/15 | Nelson et al. 61 | USA | MIS‐C | Mild dilatation of the Lt. main coronary artery | Discharged |
Abbreviations: AIHA, autoimmune hemolytic anemia; CSS, cytokine storm syndrome; EF, ejection fraction; FS, fractional shortening; GGOs, Ground‐glass opacities; ITP, Immune thrombocytopenic purpura; IVC: Inferior vena cava; KD, Kawasaki disease; KDSS, Kawasaki disease shock syndrome; Lt, left; LV: Left ventricular; MCA: middle cerebral artery; MIS‐C, multisystem inflammatory syndrome in children; MODS, multiple organ dysfunction syndrome; NR, not reported; PCA, posterior cerebral artery; PFAPA, Periodic fever, aphthous stomatitis, pharyngitis, adenitis; PIMS‐TS, pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2; TSS, toxic shock syndrome.
These patients age based on months.
Moreover, the type of disease mentioned in the studies was reported, and was then classified as KD in 46 (34.6%) patients (including subtypes of atypical KD in 12 [9%] patients, incomplete KD in 18 [13.5%], classic KD in 6 [4.5%], and KD in 10 [7.5%]), MIS‐C in 22 (16.5%), PIMS‐TS in 25 (18.8%), and hyperinflammatory shock in 8 (6%) patients. Other diseases such as refractory/respiratory failure, cardiac dysfunction, and shock, hypotensive septic shock, myocarditis with heart failure, Kawasaki disease shock syndrome, and TSS were detected in 18 (13.5%) patients. Additionally, the type of disease had not been reported in 14 (10.5%) cases.
With regard to the symptoms of patients, skin rash (n = 74, 55.6%) was the most common one followed by conjunctivitis (n = 65, 48.8%), and then lip and oral cavity changes in 43 (32.3%) patients, as summarized in Table 2. Other KD clinical features such as GI symptoms (n = 97, 72.9%), hypotension (less than 90/50, n = 45, 33.8%), and pulmonary abnormalities (n = 35, 26.3%) were also present in these patients (Table 2). Comorbidities had been further reported in 10 (7.5%) patients, of whom one patient (No. 67) suffered from attention‐deficit/hyperactivity disorder and autism, one (No. 31) had a mid‐muscular ventricular septal defect, one patient (No. 69) had been affected with alopecia areata (viz. spot baldness) and hay fever. Moreover, one case (No. 71) had hypothyroidism, two patients (Nos. 30, 72, and no. 79) had mild asthma, two (Nos. 80 and 81) were suffering from obesity, one had glucose‐6‐phosphate dehydrogenase (G6PD) (No. 87) and one patient born with congenital adrenal hyperplasia (CAH) (No. 85).
Table 2.
Clinical characteristics of children (n = 133) included in the study
| KD principal clinical criteria | Total N (%) |
|---|---|
| Complete presentation (fever for at least 4 days and ≥4 principal criteria) (23) | 46 (34.5) |
| Cervical lymphadenopathy | 19 (14.3) |
| Rash | 74 (55.6) |
| Lips and oral cavity changes | 43 (32.3) |
| Changes to extremities | 25 (18.8) |
| Conjunctival symptoms | 65 (48.8) |
| KD associated clinical features | |
| Gastrointestinal symptoms | 97 (72.9) |
| Pulmonary symptoms or abnormalities | 35 (26.3) |
| Malaise, fatigue, lethargy | 24 (18) |
| Myalgia, chest and thoracic pain | 12 (9) |
| Hypotension | 45 (33.8) |
| Edema (facial, eyelid, periorbital) | 16 (12) |
| Other neurological features | 28 (21) |
Note: Values and numbers (percentages) unless stated otherwise.
Abbreviation: KD, Kawasaki disease.
Moreover, 122 (91.7%) patients had been confirmed to have COVID‐19 infection with reference to nasopharyngeal RT‐PCR or antibody (viz. serological) testing for SARS‐CoV‐2, of whom 14 (10.5%) cases were positive for both of them. In addition, among all patients admitted to hospitals, 74 (55.6%) cases had been admitted to PICUs. Also, 49 (36.8%) patients had required some levels of respiratory support, of whom 31 (23.3%) cases had required mechanical intubation/ventilation, 18 (13.5%) had required oxygen therapy (such as venous arterial extracorporeal membrane oxygenation [ECMO] and cases that required high flow nasal cannula [HFNC]). With regard to the outcomes, 79 (59.4%) cases had been discharged from hospitals, 3 (2.2%) had been readmitted, 9 (6.7%) had been hospitalized at the time of the study, and 9 (6.7%) patients had expired. Likewise, the outcomes had not been reported in the rest of the patients (n = 33).
In the initial analysis, the measured pooled mean (SE) for inflammatory cytokines such as C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR), ferritin, troponin, and interleukin 6 (IL‐6) concentrations in patients were 226.5 ± 12.4, 67.4 ± 4.1, 1036.6 ± 108.3, 525 ± 117.7, and 412.2 ± 82.5, respectively, in those cases that have been reported.
Coronary artery dilation and aneurysm were reported in seven patients (Nos. 20, 27, 28, 81, 82, 109, and 132); of these, only one died due to severe hypoxia and disseminated intravascular coagulation, and the rest were discharged on medication.
Accordingly, patient No. 6, a healthy 5‐year‐old boy presented with numerous days of fever, cough, and abdominal pain. He proceeded to cardiogenic shock and tested positive for COVID‐19 antibodies, and had high IL‐6 levels. He developed cardiopulmonary failure requiring ECMO. After 5 days of ECMO, pupils became fixed and dilated, and head computed tomography (CT) revealed a middle cerebral artery (MCA) infarction, cerebral edema, and diffuse contralateral subarachnoid hemorrhage. His examination disclosed blank brainstem reflexes and movement. After 3 days, brain death proved following normalization of his electrolytes.
3.1. Patients with a death outcome
In this systematic review, a total of 9 (6.7%) patients, mostly male dominance (66.6%) expired due to a wide variety of reasons. Severe heart failure and cardiac arrest played a predominant role in the mortality of these patients (n = 6). As patients Nos. 2, 87, 100, 111, 112, and 122 expired because of refractory hypotension and cardiac arrest despite all adjuvant and complementary therapies. Patients Nos. 47 and 63 showed evidence of MCA ischemic infarction in CT of the head that might be due to heart failure and shock, which are not directly mentioned in the literature. Although the SARS‐CoV‐2 is known as a respiratory infection that causes respiratory symptoms, only one patient (No. 82) passed away due to severe hypoxia even with venovenous ECMO.
All dead cases came with an initial stable presentation that their condition deteriorated days (ranged 3–180 days) after hospitalization. Greater severity of the condition and a higher risk of mortality may be triggered by poor nutrition, comorbidities (that may facilitate the expansion of marked hyperinflammatory syndrome), and also cardiac dysfunction evidenced by lower cardiac output and ejection fraction. Patients Nos. 111 and 112 suffered from poor nutrition; patient No. 122 had a history of chronic renal failure, and patient No. 87 was with G6PD deficiency. All the mentioned patients were hospitalized in the PICU and required supplemental oxygenation, of which six patients had tracheal ventilation, and the rest of them were ventilated by veno‐arterial ECMO (Nos. 2, 47, and 82). Although RT‐PCR tests for COVID‐19 were positive for all these patients, immunoglobulin G antibody against SARS‐CoV‐2 detected just in two of them.
Coronary artery dilation in dead patients was reported in one patient (No. 82) with a Z score of 2, and a coronary artery aneurysm was not revealed in each of them. Lung parenchyma condition was reported in six patients of the death cases, of which most appeared as diffuse patch ground‐glass opacification/opacity in both lungs (Nos. 2, 47, 63, 82, and 122), and one of them (No. 100) progressed to acute respiratory distress syndrome.
4. DISCUSSION
As with the COVID‐19 pandemic and its progress, the incidence of patients admitted with KD and other related diseases and symptoms such as KLD and MIS‐C has increased, so there have been several reports from different countries.21, 22 In this study, available evidence published updated globally until December 1st were systematically reviewed, and hyperinflammatory condition in children following COVID‐19, threatening their lives, were reported. We found that more than half of children with KLD associated with COVID‐19 had been admitted to PICUs, a quarter had required mechanical ventilation/intubation and even some of them had been required readmissions. Moreover, a 6.7% mortality rate was observed in children with KLD associated with COVID‐19 that mostly due to severe heart failure, cardiac arrest, and refractory hypotension. Additionally, the male gender could be recognized as a poor prognostic factor with a fatality rate of two times higher than the female.
Despite more than half a century since the initial reports of KD, no clear cause has been thus far identified, and no specific pathology has been recognized. 23 Considering the higher number of cases with KD after the outbreak of viral respiratory diseases, the most accepted hypothesis is the strange response of the immune system to one or more unknown pathogens in genetically predisposed patients.24, 25 The presumed causative pathogens include influenza, chlamydia pneumonia, retroviruses, the Epstein‐Barr virus, and primate erythroparvovirus 1, generally referred to as parvovirus B19. 26 There are also conflicting reports about coronaviruses, while there are pieces of evidence of KD, following a novel human coronavirus (HCoV), called new haven coronavirus, alphacoronaviruses, and respiratory diseases, which have been ruled out in some studies.27, 28, 29
The aforementioned systematic review of the cohort studies, case series/reports, and correspondences or letters to editors also unveiled various clinical hallmarks of patients admitted to hospitals with the manifestations of KD during the COVID‐19 pandemic. In 133 cases examined, COVID‐19 had been confirmed in 91.7% of the cases based on the nasopharyngeal RT‐PCR and antibody (viz. serological) testing, albeit the RT‐PCR had been less sensitive compared with serology, which was consistent with the findings by Akca et al.62, 65 Despite a wide range, all cases had been under the age of 18, and the average age had been higher than patients with KD. In the studies reviewed, not all patients had typical KD characteristics, and even with possessing similar symptoms, several names had been mentioned for diagnoses, among which most patients that had been admitted with a diagnosis of PIMS‐TS and MIS‐C had been placed next in this line. Other diagnoses, including various types of KD alongside hyperinflammatory shock, hypotension, and AHF, had lower proportions.
With an overview of the gender distribution of the patients developing KD in association with COVID‐19, male dominance had been apparent, which had the same order with KD alone. 30 Among the death reports in the reviewed cases, the ratio of male to female had been 2:1, so that the male gender could be recognized as a poor prognostic factor, which was consistent with mortality in adults with COVID‐19, with the fatality rate of male two to three times higher than female. In adults, this effect had been hypothesized to be dependent on males with higher angiotensin‐converting enzyme 2 expression, X chromosome immunological consequences, and hormonal differences31, 32; So, ignoring the latter, the two other causes could be generalized to children.
The study results revealed that GI manifestations were also prevalent in patients exhibiting KD associated with COVID‐19, with a higher incidence rate than those in both non‐COVID‐19 KD and children with COVID‐19 alone. Other subsequent symptoms including skin rash, conjunctivitis, hypotension, lip, and oral cavity changes, and pulmonary abnormalities were not consistent with both KD patients with or without SARS‐CoV‐2 infection.23, 32 The particular reason for this was obscured by a probable scenario of the profile of the complex inflammatory factors along with the preceding two diseases and its discrete unfamiliar immunopathology.
More than half of the hospitalized patients had been admitted to PICUs, a quarter of them undergoing mechanical ventilation, and others receiving HFNC and ECMO. This level of dependence on respiratory support was higher than the values reported in COVID‐19 patients with pulmonary involvement, both in children and adults, 33 probably due to various reasons including different types of immunopathology and hyperinflammatory state of this condition 34 indicating the necessity of conducting a multidisciplinary study on pediatric patients during the COVID‐19 pandemic.
Elevated cytokine production is a prominent characteristic of severe COVID‐19. Like most severe COVID‐19 cases, which present an extreme increase in inflammatory cytokines, including IL‐6, CRP, ESR, and ferritin,3, 4, 5, 6 the literature review revealed the abnormal quantity of these values. On the other hand, a high level of troponin in patients indicates cardiac tissue damage, which was one of the leading causes of death in this study.
Also, this study revealed a higher mortality rate among patients with KD associated with COVID‐19, compared to each of them alone, given that nine children had died and one had proceeded to brain death.35, 36 Hyperinflammation state and augmented cytokine production in KD amidst the CSR associated with COVID‐19, leading to several increased cytokines was further presumed causative in the progress of this condition that could make it more common in COVID‐19; however, its inflammatory profile was different from that of COVID‐19. The increased CRP and ferritin observed in the reviewed cases were supportive here. Nevertheless, the role of high troponin levels and hypotension in patients should not be missed, which could show serious cardiovascular diseases and their effects on higher mortality rates, insofar as inotropes had particular importance in the treatment of these patients following intravenous immunoglobulin and antibiotics (Table 3).
Table 3.
Treatment of children (n = 133) included in this study
| Characteristics, (%) otherwise stated | Total (N = 133) | |
|---|---|---|
| Treatment | ||
| Intravenous immunoglobulin (2 g/kg) infusion | 91 (68.4) | |
| Intravenous immunoglobulin (2 g/kg) retreatment | 5 (3.7) | |
| Steroids (2–10 mg/kg/day) | 47 (35.3) | |
| Aspirin | 45 (33.8) | |
| Broad‐spectrum antibiotics | 77 (57.9) | |
| Inotropes | 55 (41.3) | |
| Hydroxychloroquine | 14 (10.5) | |
| Anakinra | 8 (6) | |
| Tocilizumab | 23 (17.3) | |
| Remdesivir | 2 (1.5) | |
| Diphenhydramine | 1 (0.7) | |
| Favipiravir | 9 (6.7) | |
| Ritonavir | 1 (0.7) | |
| Mesenchymal stem cell treatment | 1 (0.7) | |
The majority of the cases examined in this study were from the US and Europe, although 27 patients were from Iran and India, and Saudi Arabia. The geographical dispersion could have diverse reasons, which were in line with the study by Akca et al. 62 At the first glance, it was not evident whether this trend was due to different coronavirus variants, genetic predisposition, or more accurate registration and care systems in those countries or not. It did not necessarily mean the absence or scarcity of cases in other parts of the world. The vast majority of demised patients in this review had circulation problems, and they suffered from heart failure and refractory hypotension despite aggressive therapy that didn′t respond to efforts. In most of them, the cytokine profile is different from whatever is generally seen in COVID‐19 patients. Echocardiography of these patients mostly has various degrees of cardiac dysfunction, and in some cases, the arterial aneurysm was observed. It seems that their condition gets more critical when the cytokine storm begins to rise; while, in most of the above patients (death patients), the number of cytokines at the time of death is much higher than from the first examination. An equivocal point that needs further investigation is cytokine profile correlation with prognosis in these patients. However, we encounter a CSS in most patients who die; not all patients with high levels of inflammatory cytokines had complicated conditions. Although differences in the approach and treatment of patients can be one of the causes of this problem, due to the similarity of drug treatment and their focus on suppressing the immune system, other factors, including genetic variations, might be remarkable in this regard. One of the expired patients (No. 87) had a history of G6PD deficiency. The G6PD deficiency was introduced as a predisposing factor in the increasing incidence of COVID‐19 because it plays a pivotal role in coronavirus viral gene expression and viral particle production. So, the action of this deficiency in the increase of mortality rate should also be investigated. 37
Among the limitations of this review study, at first, incomplete datasets for numerous properties, which made it impossible to present appropriate comparisons and conclusions. Second, the duration of follow‐ups was inadequate, leading to the omission of their long‐term sequel. Thirdly, selection bias in the studies concerned in some countries was undeniable, as they might have been written in different languages rather than English. Considering the studies were slightly heterogeneous and their information was not comparable in some characteristics, multidisciplinary research in this field is recommended to shed light on the obscure dimensions of this condition.
5. CONCLUSION
This systematic review established that approximately two‐thirds of children with KLD associated with COVID‐19 had been admitted to PICUs. Moreover, around one‐fourth of them had required mechanical ventilation/intubation and even some of them had required readmissions. Therefore, pediatricians and physicians are strongly recommended to monitor children that present with fever, GI symptoms, and other characteristics of KD during the pandemic as they can be the dominant manifestations in children with COVID‐19. Accordingly, irreversible complications can be prevented through early diagnosis and treatment.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Pedram Keshavarz: investigation, writing—original draft, writing—review and editing, data curation. Fereshteh Yazdanpanah: investigation, writing—original draft, writing—review and editing, data curation. Sara Azhdari: writing—review and editing, data curation. Hadiseh Kavandi: data curation, writing—review and editing. Parisa Nikeghbal: investigation, data curation, writing—review and editing. Amir Bazyar: Investigation, data curation. Faranak Rafiee: investigation, data curation. Seyed Faraz Nejati: investigation, data curation. Faranak Ebrahimian Sadabad: investigation, data curation, writing—review and editing. Nima Rezaei: writing—review and editing, conceptualization, investigation.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Cecilia Dallan and Dr. Eleonore Blondiaux for providing the images and figures for this study and also, thank publisher holder of Radiology (RSNA) and Elsevier for granting permission to use the images.
Keshavarz P, Yazdanpanah F, Azhdari S, et al. Coronavirus disease 2019 (COVID‐19): A systematic review of 133 Children that presented with Kawasaki‐like multisystem inflammatory syndrome. J Med Virol. 2021;93:5458‐5473. 10.1002/jmv.27067
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ashraf MA, Keshavarz P, Hosseinpour P, et al. Coronavirus disease 2019 (COVID‐19): a systematic review of pregnancy and the possibility of vertical transmission. J Reprod Infertil. 2020;21(3):157‐168. [PMC free article] [PubMed] [Google Scholar]
- 3. Yazdanpanah F, Hamblin MR, Rezaei N. The immune system and COVID‐19: friend or foe? Life Sci. 2020;256:117900. 10.1016/j.lfs.2020.117900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akbari H, Tabrizi R, Lankarani KB, et al. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. Life Sci. 2020;258:118167. 10.1016/j.lfs.2020.118167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mansourabadi AH, Sadeghalvad M, Mohammadi‐Motlagh H‐R, Rezaei N. The immune system as a target for therapy of SARS‐CoV‐2: a systematic review of the current immunotherapies for COVID‐19. Life Sci. 2020;258:118185. 10.1016/j.lfs.2020.118185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rafiee F, Keshavarz P, Katal S, et al. Coronavirus disease 2019 (COVID‐19) in molecular imaging: a systematic review of incidental detection of SARS‐CoV‐2 pneumonia on PET studies. Semin Nucl Med. 2020;51:28‐191. 10.1053/j.semnuclmed.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective [published online ahead of print March 26, 2020]. J Eur Acad Dermatol Venereol. 2020;34:e212. 10.1111/jdv.16387 [DOI] [PubMed] [Google Scholar]
- 8. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang E, Brar K. COVID‐19 in children: an epidemiology study from China. J Allergy Clin Immunol Pract. 2020;8(6):2118‐2120. 10.1016/j.jaip.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoang A, Chorath K, Moreira A, et al. COVID‐19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. 2020;24:100433. 10.1016/j.eclinm.2020.100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiotos K, Bassiri H, Behrens EM, et al. Multisystem inflammatory syndrome in children during the COVID‐19 pandemic: a case series. J Pediatric Infect Dis Soci. 2020;9(3):393‐398. 10.1093/jpids/piaa069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaushik S, Aydin SI, Derespina KR, et al. Multisystem inflammatory syndrome in children (MIS‐C) associated with SARS‐CoV‐2 infection: a multi‐institutional study from New York City. J Pediatr. 2020;224:24‐29. 10.1016/j.jpeds.2020.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keshavarz P, Rafiee F, Kavandi H, Goudarzi S, Heidari F, Gholamrezanezhad A. Ischemic gastrointestinal complications of COVID‐19: a systematic review on imaging presentation. Clin Imaging. 2020;73:85‐96. 10.1016/j.clinimag.2020.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mustafa NM, Selim LA. Characterisation of COVID‐19 pandemic in paediatric age group: a systematic review. J Clin Virol. 2020;128:104395. 10.1016/j.jcv.2020.104395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daskalakis D. health alert# 13: pediatric multi‐system inflammatory syndrome potentially associated with COVID‐19. NYC Health [Internet]. 2020. [DOI] [PMC free article] [PubMed]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dallan C, Romano F, Siebert J, Politi S, Lacroix L, Sahyoun C. Septic shock presentation in adolescents with COVID‐19. Lancet Child Adolesc Health. 2020;4(7):E21‐E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blondiaux E, Parisot P, Redheuil A, et al. Cardiac MRI of children with multisystem inflammatory syndrome (MIS‐C) associated with COVID‐19: case series. Radiology. 2020;297:202288. 10.1148/radiol.2020202288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Margulis AV, Pladevall M, Riera‐Guardia N, et al. Quality assessment of observational studies in a drug‐safety systematic review, comparison of two tools: the Newcastle–Ottawa scale and the RTI item bank. Clin Epidemiol. 2014;6:359‐368. 10.2147/CLEP.S66677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. aBIwNH L. Study quality assessment tools. http://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.Accessed
- 21. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771‐1778. 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones VG, Mills M, Suarez D, et al. COVID‐19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10(6):537‐540. 10.1542/hpeds.2020-0123 [DOI] [PubMed] [Google Scholar]
- 23. McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long‐term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927‐e999. 10.1161/CIR.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 24. Turnier JL, Anderson MS, Heizer HR, Jone P‐N, Glodé MP, Dominguez SR. Concurrent respiratory viruses and Kawasaki disease. Pediatrics. 2015;136(3):e609‐e614. 10.1542/peds.2015-0950 [DOI] [PubMed] [Google Scholar]
- 25. Shulman ST, Rowley AH. Kawasaki disease: insights into pathogenesis and approaches to treatment. Nat Rev Rheumatol. 2015;11(8):475‐482. [DOI] [PubMed] [Google Scholar]
- 26. Chang L‐Y, Lu C‐Y, Shao P‐L, et al. Viral infections associated with Kawasaki disease. J Formos Med Assoc. 2014;113(3):148‐154. 10.1016/j.jfma.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shimizu C, Shike H, Baker SC, et al. Human coronavirus NL63 is not detected in the respiratory tracts of children with acute Kawasaki disease. J Infect Dis. 2005;192(10):1767‐1771. 10.1086/497170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang L‐Y, Chiang B‐L, Kao C‐L, et al. Lack of association between infection with a novel human coronavirus (HCoV), HCoV‐NH, and Kawasaki disease in Taiwan. J Infect Dis. 2006;193(2):283‐286. 10.1086/498875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191(4):499‐502. 10.1086/428291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakamura Y. Kawasaki disease: epidemiology and the lessons from it. Int J Rheum Dis. 2018;21(1):16‐19. 10.1111/1756-185X.13211 [DOI] [PubMed] [Google Scholar]
- 31. Chanana N, Palmo T, Sharma K, Kumar R, Graham BB, Pasha Q. Sex‐derived attributes contributing to SARS‐CoV‐2 mortality. Am J Physiol Endocrinol Metab. 2020;319:E562‐E567. 10.1152/ajpendo.00295.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID‐19 infection: origin, transmission, and characteristics of human coronaviruses. J Advanced Res. 2020;24:91‐98. 10.1016/j.jare.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID‐19: a systematic review and meta‐analysis. Aging. 2020;12(13):12493‐12503. 10.18632/aging.103579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Consiglio CR, Cotugno N, Sardh F, et al. The immunology of multisystem inflammatory syndrome in children with COVID‐19. Cell. 2020;183(4):968‐981. 10.1016/j.cell.2020.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakamura Y, Yanagawa H, Kato H, Kawasaki T. Mortality rates for patients with a history of Kawasaki disease in Japan. J Pediatrics. 1996;128(1):75‐81. 10.1016/S0022-3476(96)70430-4 [DOI] [PubMed] [Google Scholar]
- 36. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model‐based analysis. Lancet Infect Dis. 2020;20(6):669‐677. 10.1016/S1473-3099(20)30243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu Y‐H, Tseng C‐P, Cheng M‐L, Ho H‐Y, Shih S‐R, Chiu DT‐Y. Glucose‐6‐phosphate dehydrogenase deficiency enhances human coronavirus 229E infection. J Infect Dis. 2008;197(6):812‐816. 10.1086/528377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saeed A Case report: Pediatric patients with COVID‐19 presented as multi‐system inflammatory syndrome. 2020.
- 39. Fraser D, Patterson E, Daley M, Cepinskas G Inflammation and Endothelial Injury Profiling in COVID‐19 Pediatric Multi‐system Inflammatory Syndrome. 2020. [DOI] [PMC free article] [PubMed]
- 40. Schnapp A, Abulhija H, Maly A, et al. Introductory histopathologic findings may shed light on COVID19 pediatric hyperinflammatory shock syndrome. J Eur Acad Dermatol Venereol. 2020;34:e665. 10.1111/jdv.16749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rauf A, Vijayan A, John ST, Krishnan R, Latheef A. Multisystem inflammatory syndrome with features of atypical Kawasaki disease during COVID‐19 pandemic. Indian J Pediatr. 2020;89(9):745‐747. 10.1007/s12098-020-03357-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schupper AJ, Yaeger KA, Morgenstern PF. Neurological manifestations of pediatric multi‐system inflammatory syndrome potentially associated with COVID‐19. Child's Nervous Sys. 2020;36:1579‐1580. 10.1007/s00381-020-04755-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Greene AG, Saleh M, Roseman E, Sinert R. Toxic shock‐like syndrome and COVID‐19: a case report of multisystem inflammatory syndrome in children (MIS‐C). Am J Emerg Med. 2020;38(11):2492.e5‐2492.e6. 10.1016/j.ajem.2020.05.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giannattasio A, Maglione M, Zenzeri L, et al. A child with a severe multi‐system inflammatory syndrome following an asymptomatic COVID‐19 infection: a novel management for a new disease? J Med Virol. 2020;93:1‐3. 10.1002/jmv.26189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Acharyya BC, Acharyya S, Das D Is Novel Corona Virus Truly a Trigger for Kawasaki Disease in Infants?–Reporting the First Asian Case. 2020. 10.21203/rs.3.rs-29420/v1. [DOI]
- 46. Yozgat CY, Uzuner S, Bursal Duramaz B, et al. Dermatological manifestation of pediatrics multisystem inflammatory syndrome associated with COVID‐19 in a 3‐year‐old girl. Dermatol Ther. 2020;33:13770. https://hdl.handle.net/20.500.12645/18109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rivera‐Figueroa EI, Santos R, Simpson S, Garg P. Incomplete Kawasaki disease in a child with Covid‐19. Indian Pediatr. 2020;57(7):680‐681. 10.1007/s13312-020-1900-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosenzweig JD, McThenia SS, Kaicker S. SARS‐CoV‐2 infection in two pediatric patients with immune cytopenias: a single institution experience during the pandemic. Pediatr Blood Cancer. 2020;67:e28503. 10.1002/pbc.28503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chiu JS, Lahoud‐Rahme M, Schaffer D, Cohen A, Samuels‐Kalow M. Kawasaki disease features and myocarditis in a patient with COVID‐19. Pediatr Cardiol. 2020;41:1526‐1528. 10.1007/s00246-020-02393-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ng KF, Kothari T, Bandi S, et al. COVID‐19 multisystem inflammatory syndrome in three teenagers with confirmed SARS‐CoV‐2 infection. J Med Virol. 2020;92:2880‐2886. 10.1002/jmv.26206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Joshi K, Kaplan D, Bakar A, et al. Cardiac dysfunction and shock in pediatric patients with COVID‐19. J Am Coll Cardiol Case Rep. 2020;2(9):1267‐1270. 10.1016/j.jaccas.2020.05.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Licciardi F, Pruccoli G, Denina M, et al. SARS‐CoV‐2‐induced Kawasaki‐like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. 2020;146:e20201711. [DOI] [PubMed] [Google Scholar]
- 53. Riollano‐Cruz M, Akkoyun E, Briceno‐Brito E, et al. Multisystem inflammatory syndrome in children (MIS‐C) related to COVID‐19: a New York City experience. J Med Virol. 2020;93:1‐10. 10.1002/jmv.26224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Deza Leon MP, Redzepi A, McGrath E, et al. COVID‐19–associated pediatric multisystem inflammatory syndrome. J Pediatr Infect Dis Soc. 2020;9(3):407‐408. 10.1093/jpids/piaa061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pain CE, Felsenstein S, Cleary G, et al. Novel paediatric presentation of COVID‐19 with ARDS and cytokine storm syndrome without respiratory symptoms. Lancet Rheumatol. 2020;2(7):E376‐E379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oberweis M‐L, Codreanu A, Boehm W, et al. Pediatric life‐threatening coronavirus disease 2019 with myocarditis. J Pediatr Infect Dis. 2020;39(7):e147‐e149. 10.1097/INF.0000000000002744 [DOI] [PubMed] [Google Scholar]
- 57. Labé P, Ly A, Sin C, et al. Erythema multiforme and Kawasaki disease associated with COVID‐19 infection in children. J Eur Acad Dermatol Venereol. 2020;34:e539. 10.1111/jdv.16666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. DeBiasi RL, Song X, Delaney M, et al. Severe COVID‐19 in children and young adults in the Washington, DC metropolitan region. J Pediatr. 2020;223:199‐203.E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Balasubramanian S, Nagendran T, Ramachandran B, Ramanan A. Hyper‐inflammatory syndrome in a child with COVID‐19 treated successfully with intravenous immunoglobulin and tocilizumab. Indian Pediatr. 2020;57(7):681‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Riphagen S, Gomez X, Gonzalez‐Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID‐19 pandemic. Lancet. 2020;395(10237):1607‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Waltuch T, Gill P, Zinns LE, et al. Features of COVID‐19 post‐infectious cytokine release syndrome in children presenting to the emergency department. Am J Emerg Med. 2020;38(10):2246.e3‐2246.e6. 10.1016/j.ajem.2020.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Akca UK, Kesici S, Ozsurekci Y, et al. Kawasaki‐like disease in children with COVID‐19. Rheumatol Int. 2020;40:2105‐2115. 10.1007/s00296-020-04701-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bahrami A, Vafapour M, Moazzami B, Rezaei N. Hyperinflammatory shock related to COVID‐19 in a patient presenting with multisystem inflammatory syndrome in children: first case from Iran. J Paediatr Child Health. 2020. 10.1111/jpc.15048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dasgupta K, Finch SE. A case of pediatric multisystem inflammatory syndrome temporally associated with COVID‐19 in South Dakota. SD Med. 2020;73(6):246‐251. [PubMed] [Google Scholar]
- 65. Bapst T, Romano F, Müller M, Rohr M. Special dermatological presentation of paediatric multisystem inflammatory syndrome related to COVID‐19: erythema multiforme. BMJ Case Rep CP. 2020;13(6):e236986. 10.1136/bcr-2020-236986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Burger MW, Moore MA, Wilburn JM. Case report: pediatric patient with COVID‐19 and multisystem inflammatory syndrome in children. Clin Pract Cases Emerg Med. 2020;4(4):513‐516. 10.5811/cpcem.2020.8.48376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Al Ameer HH, AlKadhem SM, Busaleh F, AlKhwaitm S, Llaguno MBB. Multisystem inflammatory syndrome in children temporally related to COVID‐19: a case report From Saudi Arabia. Cureus. 2020;12(9):e10589. 10.7759/cureus.10589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Khan I, Sarwar A, Ahmed Z. Atypical case of COVID‐19 associated Kawasaki Disease in an eight‐year‐old Pakistani Boy. Cureus. 2020;12(9):e10670. 10.7759/cureus.10670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jackson RJ, Chavarria HD, Hacking SM. A case of multisystem inflammatory syndrome in children mimicking acute appendicitis in a COVID‐19 pandemic area. Cureus. 2020;12(9):e10722. 10.7759/cureus.10722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Falah NU, Hashmi S, Ahmed Z, et al. Kawasaki Disease‐like features in 10 pediatric COVID‐19 cases: a retrospective study. Cureus. 2020;12(10):e11035. 10.7759/cureus.11035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Almoosa ZA, Al Ameer HH, AlKadhem SM, Busaleh F, AlMuhanna FA, Kattih O. Multisystem inflammatory syndrome in children, the real disease of COVID‐19 in pediatrics‐a multicenter case series From Al‐Ahsa, Saudi Arabia. Cureus. 2020;12(10):e11064. 10.7759/cureus.11064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Raut S, Roychowdhoury S, Bhakta S, Sarkar M, Nandi M. Incomplete Kawasaki disease as presentation of COVID‐19 infection in an infant: a case report. J Trop Pediatr. 2020:1‐4. 10.1093/tropej/fmaa047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kim H, Shim JY, Ko J‐H, et al. Multisystem inflammatory syndrome in children related to COVID‐19: the first case in Korea. J Korean Med Sci. 2020;35(43):e291. 10.3346/jkms.2020.35.e391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. de Farias E, Pedro Piva J, de Mello M, et al. Multisystem inflammatory syndrome associated with coronavirus disease in children: a multi‐centered study in Belém, Pará, Brazil. Pediatr Infect Dis J. 2020;39(11):e374‐e376. 10.1097/INF.0000000000002865 [DOI] [PubMed] [Google Scholar]
- 75. Shahbaznejad L, Navaeifar MR, Abbaskhanian A, Hosseinzadeh F, Rahimzadeh G, Rezai MS. Clinical characteristics of 10 children with a pediatric inflammatory multisystem syndrome associated with COVID‐19 in Iran. BMC Pediatr. 2020;20(513):1‐12. 10.1186/s12887-020-02415-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cirks BT, Geracht JC, Jones OY, et al. Multisystem inflammatory syndrome in children during the COVID‐19 pandemic: a case report on managing the hyperinflammation. Mil Med. 2020;186(1‐2):e270‐e276. 10.1093/milmed/usaa508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nelson C, Ishimine P, Hayden SR, Correia M, Wardi G. Multisystem inflammatory syndrome in children (MIS‐C) in an Adolescent that developed coronary aneurysms: a case report and review of the literature. J Emerg Med. 2020;59(5):699‐704. 10.1016/j.jemermed.2020.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


