Abstract
Currently available data are consistent with increased severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) replication at temperatures encountered in the upper airways (25–33°C when breathing room temperature air, 25°C) compared to those in the lower airways (37°C). One factor that may contribute to more rapid viral growth in the upper airways is the exponential increase in SARS‐CoV‐2 stability that occurs with reductions in temperature, as measured in vitro. Because SARS‐CoV‐2 frequently initiates infection in the upper airways before spreading through the body, increased upper airway viral growth early in the disease course may result in more rapid progression of disease and potentially contribute to more severe outcomes. Similarly, higher SARS‐CoV‐2 viral titer in the upper airways likely supports more efficient transmission. Conversely, the possible significance of air temperature to upper airway viral growth suggests that prolonged delivery of heated air might represent a preventative measure and prophylactic treatment for coronavirus disease 2019.
Keywords: air temperature, COVID‐19, thermodynamics, virology
Highlights
The stability of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in vitro increases exponentially with decreases in temperature proximate to normal core body temperature (37°C).
Even room temperature air (25°C) cools upper airway surfaces to several degrees below body temperature (25–33°C), which may promote rapid viral growth.
Increased upper airway viral growth may contribute to higher transmission rates and more severe disease.
Heated, humidified air may represent a postexposure prophylactic treatment for coronavirus disease 2019.
1. INTRODUCTION
The stability of viruses is affected by changes in temperature, and severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which causes coronavirus disease 2019 (COVID‐19), is no exception.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 Heat denatures the proteins that comprise each SARS‐CoV‐2 virion, inactivating it.3, 8 Laboratory data indicate that, as temperatures increase, the rate at which virions are inactivated increases exponentially.3, 9 These in vitro data suggest that lower temperatures within the body might result in lower rates of thermal inactivation and therefore higher rates of viral growth, potentially affecting viral shedding, viral load densities, transmission rates, and patient outcomes.
SARS‐CoV‐2 replicates abundantly in the upper airways,11, 12, 13 and the temperature of the upper airways is directly affected by air temperature. Air below body temperature is warmed during inhalation through heat exchange with upper airway surfaces, reaching core body temperature (37°C) at the level of the lower airways. 14 This process cools the upper airways: Respiration of room temperature air (25°C) reduces the upper airways to an average ambient temperature of 33°C, which evolves from 25°C at the nasal vestibule to 34°C at the nasopharynx to approximately 37°C near the trachea.15, 16, 17 Lower air temperatures as well as faster and deeper respiration cause further cooling.16, 18, 19, 20 The airstream ordinarily rises to within 0.5°C of body temperature by the trachea, 14 making lower airway temperatures unique to the upper respiratory tract.
Although complicating factors preclude the trivial extension of SARS‐CoV‐2 in vitro decay rates to the in vivo environment, currently available data support increased viral replication at the cooler temperatures of the upper airways relative to the warmer temperatures of the upper airways.21, 22, 23
Abundant SARS‐CoV‐2 replication in the upper airways is a driver of its high transmissibility and is pivotal to the pathogenesis of COVID‐19.11, 12, 21, 22, 24, 25, 26, 27 If air temperature affects SARS‐CoV‐ replication rates in the upper airways, then air temperature may also contribute to transmission rates and progression of disease.
This article outlines the possible relevance of air temperature to COVID‐19, with an emphasis on how the temperatures encountered in the airways may influence the decay rate and replication dynamics of SARS‐CoV‐2. While presently available data do not permit quantification of the impact of air temperature on COVID‐19 transmission and disease progression, this article suggests that the potential for such an effect warrants further study.
2. COOL AIR MAY SUPPORT SARS‐COV‐2 REPLICATION IN THE UPPER AIRWAYS
The temperature gradient of the airways affects the replication kinetics of many respiratory viruses, including rhinoviruses, influenza viruses and coronaviruses.21, 22, 28, 29, 30, 31, 32 Airway temperatures also influence the replication kinetics of SARS‐CoV‐2,21, 22 which replicates abundantly in the human airways.11, 12, 13 A study that used an in vitro model for the human respiratory epithelium found that SARS‐CoV‐2 replicated 10‐ to 100‐fold more efficiently over 96 h at temperatures found in the upper airways than those in the lower airways, 33°C and 37°C respectively. 21 Possible differences in upper and lower airway viral replication kinetics in vivo depend upon many factors, including temperature‐dependent innate immune response that is more robust at warmer air temperatures and higher humidities,21, 33, 34, 35, 36, 37, 38 as well as cellular expression of the SARS‐CoV‐2 cell‐entry factor angiotensin‐converting enzyme 2 (ACE2) which has a gradient expression in the airways. 11 Another contributing factor may be the temperature‐dependent decay rate of SARS‐CoV‐2, which is the focus of this article.
Like many viruses, SARS‐CoV‐2 decays exponentially across a variety of mediums—including surfaces, aerosols, and solutions, such as virus transport medium, nasal mucus, and sputum.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 The rate of exponential decay increases with temperature as heat denatures the proteins that comprise the SARS‐CoV‐2 virion.1, 2, 3, 5, 6, 7, 8, 9, 10 The SARS‐CoV‐2 spike protein—which is necessary for cell entry—is temperature‐dependent, in relation to the temperature required for optimal virus replication. 22 SARS‐CoV‐2 spike proteins exhibit temperature preference to match the upper (~33°C) airways over lower (37°C) airways, with further reductions in infectivity found as temperature increases to fever levels (41°C).22, 23 Analytical models based on laboratory data for SARS‐CoV‐2 in solution and on inert surfaces at various humidity conditions indicate that virus half‐life decreases exponentially with increases in temperature (Figure 1).3, 9
Figure 1.
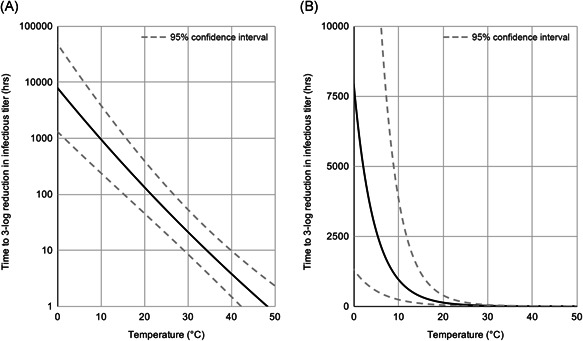
Virus lifetime as a function of temperature. (A) Predictions are shown for the duration of time required to produce a 3‐log (99.9%) reduction in infectious titer. 3 The analytical model was built on data for SARS‐CoV‐2 in viral transport medium. 1 (B) The lifetime axis is scaled linearly to emphasize the exponential relationship of virus lifetime and temperature
Complicating factors make the in vivo environment difficult to compare with the laboratory data currently available, and additional research is necessary to determine whether the rates of viral decay in vivo are similarly affected by temperature. Existing clinical data generally support the concept that viral pathogenicity decreases with elevations in temperature, although exact mechanisms for this effect remain under investigation. For example, more rapid recoveries are observed from chickenpox and rhinovirus infections with avoidance of antipyretic medication.39, 40, 41 Whether these clinical findings, including the prolongation of viral shedding with the use of antipyretics, represent an influence of body temperature on viral decay is uncertain; however, the data are at least consistent with this factor.
3. COOL AIR MAY CONTRIBUTE TO COVID‐19 DISEASE SEVERITY AND TRANSMISSION RATES
While much remains to be understood about the pathogenesis of COVID‐19, available data indicate that the rate of viral replication in the upper airways—especially during early stages of disease—may have a significant impact on disease severity. The upper airways play a pivotal role in the pathogenesis of COVID‐19. Like SARS‐CoV‐1, 42 SARS‐CoV‐2 may primarily initiate infection on nasal surfaces.11, 13 Nasal epithelial cells have high ACE2 expression, and cell cultures of nasal epithelial cells indicate high infectivity.11, 21 Clinically, high SARS‐CoV‐2 viral titers are observed in the upper respiratory tract early in infection (0–5 days).12, 13, 24 Moreover, the primary mode of COVID‐19 transmission is via aerosols,43, 44, 45 and aerosol deposition modeling suggests that inhalation of virus‐laden aerosols deposits virus in the highest concentrations on nasal surfaces.11, 46, 47, 48
After establishing infection in the nasal cavity, SARS‐CoV‐2 infection may spread from nasal surfaces through the respiratory tract, with high upper airway titers potentially enabling continual infection. Similar to other human coronaviruses, SARS‐CoV‐2 replicates productively on the nasal and tracheobronchial epithelium and sheds over time onto the luminal surface of the epithelium.21, 49, 50 The abundance of ACE2 on luminal surfaces of conducting airway epithelium may enable considerable and progressive viral growth and spread throughout the airways.11, 51, 52, 53, 54 For many lower respiratory tract infectious diseases, oral‐lung aspiration contributes to disease progression and severity.11, 55, 56, 57, 58, 59 Nasal secretions containing relatively high viral titers can accumulate in the oropharynx and are predicted to subsequently spread to lower airways via aspiration.11, 60, 61, 62, 63, 64 Chest CT and autopsy studies from COVID‐19 patients indicate patchy pulmonary infection, consistent with aspiration of virus into the lung from the upper respiratory tract.11, 65 Clinical data from COVID‐19 patients indicate early viral replication in the upper airways followed by pulmonary infection, consistent with viral spread from upper to lower airways.11, 13 Taken together, these data suggest that SARS‐CoV‐2 may establish infection in the nasal cavity and progressively replicate and spread through the airway epithelia, especially via aspiration. The data are also consistent with the concept that the upper respiratory tract serves as a viral reservoir that enables continual reinfection of proximate airway surfaces, supporting repeated cycles of viral replication and spread through the airways, which would suggest that viral growth in the upper respiratory tract might remain pathogenically significant even after infection has spread beyond the upper airways.
Rapid SARS‐CoV‐2 replication in the nasal cavity and upper airways may therefore contribute to greater and more rapid infection of the lower airways. Data on viral load are consistent with this hypothesis: SARS‐CoV‐2 upper respiratory tract viral load is strongly correlated with adverse outcomes and has been hypothesized to be a predictor of disease severity. 27 The nasopharyngeal viral load of severe COVID‐19 cases has been found to be about 60 times higher than that of mild cases. 27
As such, factors that promote more rapid viral growth in the upper airways may contribute to greater disease severity. Because cool air potentially promotes more rapid SARS‐CoV‐2 growth by reducing viral decay rate in the upper airways and suppressing innate immune response, cool air may contribute to greater disease severity. The contribution of reduced viral decay rate may be considerable, as the virion half‐life of SARS‐CoV‐2 in vitro increases exponentially with decreases in temperature. For SARS‐CoV‐2 in virus transport medium, a 3‐log (99.9%) reduction in viral titer is predicted to occur in 7.1 h at the temperature of the lower airways (37°C), 12.5 h at the average temperature of upper airways (33°C), and 52.2 h at the temperature of the nasal vestibule (25°C), where airway temperatures assume room temperature air (25°C) (95% confidence interval of 2.8–17.7, 5.1–31.0, and 19.8–138.9 h, respectively) (Figure 2). 3 Lower temperatures correspond to exponentially longer SARS‐CoV‐2 half‐life. Significantly enhanced viral growth at the lower temperatures encountered in the upper airways also likely supports the efficient transmissibility of SARS‐CoV‐2, as higher viral titer in the upper airways produces higher viral titer in expelled respiratory droplets.12, 21, 22, 24, 25, 26
Figure 2.
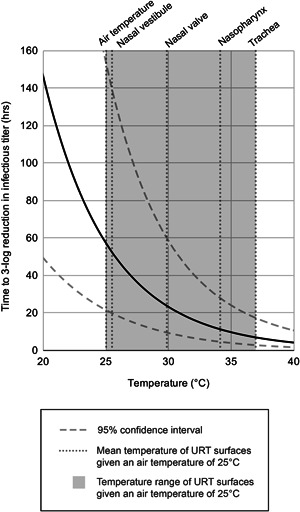
Virus lifetime at common indoor air temperatures. The mean temperatures of the upper respiratory tract when breathing 25°C (room temperature) air are transposed over the predicted in vitro virus lifetime as a function of temperature.3, 15 The upper respiratory tract spans a temperature range from air temperature to body temperature.
The seasonality of respiratory viruses is a widespread and well‐established phenomenon, affecting both transmission rates and mortality.66, 67, 68 A variety of factors have been identified as drivers of seasonality, among which outdoor air temperature features prominently. The transmission and severity of many acute respiratory viruses—including rhinoviruses, influenza viruses, and coronaviruses—are negatively correlated with outdoor air temperature.33, 35, 67, 68, 69 Seasonality has been widely hypothesized to occur for SARS‐CoV‐2, and many studies have sought to determine the relationship between the rate of COVID‐19 transmission and meteorological factors—including outdoor air temperature. The majority of currently available studies suggest that outdoor temperature is negatively correlated with COVID‐19 transmission rates, although findings are not unanimous.70, 71, 72, 73, 74, 75, 76 Despite an abundance of studies on this topic, the implications are still unclear as the data are rife with confounding variables. Some confounding variables are behavioral: For instance, during cold weather, people spend more time indoors, which supports more efficient transmission. Other confounding variables are associated with geography, such as population density,72, 77 social distancing practices, 78 quality of available health care, frequency of international travelers,72, 79 and prevalence of viral variants associated with transmissibility and virulence.80, 81, 82, 83, 84 Furthermore, other meteorological factors may affect transmission rates: Humidity results in more rapid SARS‐CoV‐2 decay in vitro,6, 7, 9, 10 improves airway immune response to infection by respiratory viruses, 33 and is negatively correlated with the transmission of many respiratory viruses66, 67; while ultraviolet radiation from sunlight rapidly inactivates SARS‐CoV‐2 viral particles, both on surfaces and in the air.85, 86, 87, 88 An additional factor to consider when attempting to use meteorological data to evaluate the effect of respired air temperature on transmissibility is that outdoor air temperature is not always equivalent to the temperature of air respired: In developed nations, people spend the majority of their time in temperature‐controlled environments,89, 90 which is where the majority of respiratory virus transmission occurs. 33 Studies on areas where people do not utilize temperature‐controlled environments may be more useful in this capacity, although such studies of course face many of the same issues that other historical meteorological studies do. In sum, despite widespread interest in the topic and an abundance of studies, it is still difficult to assess the degree to which the temperature of respired air contributes to transmission rates for SARS‐CoV‐2, although the data generally indicate decreases in transmission with increases in temperature.
Notably, although COVID‐19 patients often develop fever, fever does not heat upper airway surfaces as effectively as it does other areas of the body.91, 92 Even in a feverish patient, inhaled air begins at ambient temperature at the nostrils and converges to the patient's body temperature as it moves through the airway, cooling upper respiratory tract surfaces along the way. 15 This cooling implies diminished thermal inactivation of SARS‐CoV‐2 in the upper respiratory tract relative to other locations in the body. As such, the temperature of the upper airways would be relatively conducive to viral growth compared to other areas of the body, even during fever.
4. HEATED, HUMIDIFIED AIR MAY REPRESENT A POSTEXPOSURE PROPHYLACTIC TREATMENT FOR COVID‐19
The pathogenetic significance of the upper airways to the severity of COVID‐19 suggests that reduction of SARS‐CoV‐2 viral titer in the upper airways early in the disease may result in less severe disease and reduced transmission. Suppression of upper respiratory tract viral replication would likely have a greater impact in preventing escalation of severity if suppression occurs when SARS‐CoV‐2 is mostly contained in the upper respiratory tract, immediately postexposure or early in SARS‐CoV‐2 infection. This suggests that administration of warm air may potentially represent a therapeutic measure as a postexposure prophylaxis and an early‐stage treatment to prevent disease progression. For SARS‐CoV‐2 in solution in vitro, a 3‐log (99.9%) reduction in viral titer is predicted to occur in 12.5 h at the average temperature of upper airways (33°C), in 7.1 h at normal core body temperature (37°C), and in just 1.7 h at 45°C, an air temperature tolerable by healthy individuals for prolonged periods (95% confidence interval of 5.1–31.0, 2.8–17.7, and 0.6–4.6 h, respectively) (Figure 3).3, 93 Longer exposure to heat results in greater total reductions in viral decay. In addition to the possible effect on viral decay rates, warm air temperature and high relative humidity also facilitate a strong airway immune response, which could further contribute to the benefit of warm humidified air.21, 33, 34, 35, 36, 37, 38
Figure 3.
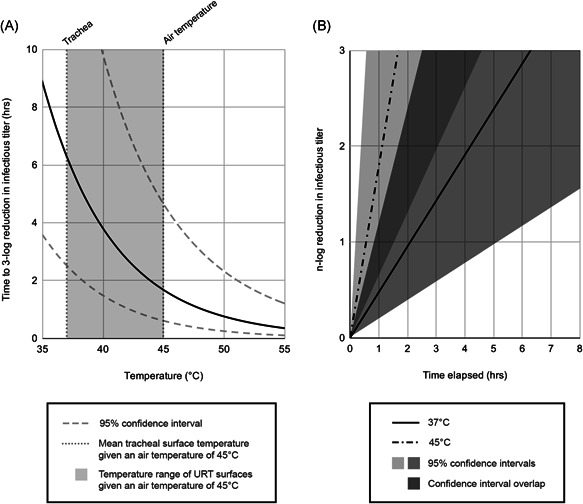
Virus lifetime at elevated air temperatures. (A) The temperature range of upper airway surfaces given an elevated temperature air (45°C) and assuming a body temperature of 37°C is transposed over the predicted in vitro virus lifetime as a function of temperature. 3 (B) The reduction in viral titer at 45°C is compared to that at body temperature (37°C).
It is important to note that air above core body temperature is cooled during inhalation, limiting the effectiveness of very warm air in reducing viral stability in lower areas of the respiratory tract. It should also be noted that warm air does not warm the entire upper respiratory tract evenly but rather elevates the temperature range in the upper airways. 14 Temperature changes caused by respiration begin immediately, with the temperature of upper airway mucosal surfaces equilibrating within 7 min at resting respiratory rate. 19
The necessary air temperature and duration of treatment depend on the exposure dose. A larger exposure dose would require greater reduction to be reduced to less than infectious levels and for that reason it would require a longer treatment duration with warm air and/or a higher air temperature (Figure 3B).
Importantly, prophylactic treatment with warm air need not reduce viral titer below infectious levels to be of potential benefit. The severity of many viral diseases is believed to be proportional to the initial viral inoculum. 94 Clinical studies conducted with influenza and coronaviruses indicate a dose‐dependent increase in disease severity.95, 96 Masks might reduce the inoculum exhaled by an infected host, and outbreaks of COVID‐19 within populations where mask‐wearing is enforced have resulted in lower rates of severe disease and fatality.94, 97 This concept is consistent with clinical data on COVID‐19 patients that indicate a strong correlation between upper respiratory tract viral load and disease severity. 27 Studies in the Syrian hamster model found that lower doses of administered SARS‐CoV‐2 virus led to less severe disease than higher doses. 98 Taken together, these data suggest that reduction of SARS‐CoV‐2 viral titer early after introduction to the upper airways may result in less severe disease. Similarly, reduced viral titer in the respiratory droplets expressed by that individual may result in less severe disease in naive hosts who contract COVID‐19 from that infected individual.
Currently available data are consistent with the hypothesis that warm air delivered early in the disease may reduce viral titer and prevent escalation of severity. An interventional study provided seven asymptomatic or pauci‐symptomatic COVID‐19 patients with 20 min of steam inhalation for 4+ consecutive days. 99 Symptomatic patients reported clinical improvement at the end of the protocol, and all patients tested negative after the first day of steam inhalation. This observation is preliminary—the study being limited in part by small sample size and the lack of a control group—and needs confirmation in a controlled trial, but the promising results suggest that further investigation of warm air as an early‐stage treatment is warranted.
Warm air delivery can be tolerated for long periods of time. Air that is humidified and heated to 37°C or 41°C and delivered via nasal high flow therapy (35 L/minute) for multiple hour‐long sessions daily is well tolerated by healthy individuals. 100 Temperatures as high as 45°C are also well tolerated for prolonged periods. 93 Risks of aerosolizing SARS‐CoV‐2 must be considered when determining method of warm air delivery for COVID‐19 patients.
5. DISCUSSION
SARS‐CoV‐2 viral stability increases exponentially with decreases in temperature, as measured for virus in solution.3, 9 The temperature‐dependent decay rate suggests that cool air temperatures might enable increased rates of SARS‐CoV‐2 growth in the upper airways.15, 16, 17 This is a proposed effect based on the review and survey of existing laboratory data, and conclusive demonstration of an effect in vivo remains to be performed. The concept is supported by currently available data: In epithelial cell cultures, SARS‐CoV‐2 replicates more efficiently at temperatures encountered in the lower airways than those in the upper airways (33°C and 37°C, respectively).21, 22, 23 Further proof of this concept will require more complex clinical studies.
More rapid SARS‐CoV‐2 replication in the upper airways has potential implications for disease severity. Clinical and laboratory data suggest that SARS‐CoV‐2 frequently initiates infection in the nasal cavity and progressively spreads through the airways, especially via aspiration.11, 13, 21 Rapid SARS‐CoV‐2 replication in the upper airways may support greater and more rapid infection of the lower airways, possibly contributing to more severe outcomes. Clinical data support this concept: SARS‐CoV‐2 upper respiratory tract viral load is strongly correlated with adverse outcomes and has been hypothesized to be a predictor of disease severity. 27 As such, respiration of cool air might be a contributing factor to COVID‐19 severity. Enhanced SARS‐CoV‐2 viral growth potentially enabled by the lower temperatures of the upper airways might also contribute to more efficient transmission, as higher viral titer in the upper airways produces higher viral titer in expelled respiratory droplets.12, 24, 25, 26 Studies based on meteorological data generally indicate negative correlation between COVID‐19 transmission and outdoor air temperature, although the strength of this correlation is obscured by a multitude of confounding variables.70, 71, 72, 73, 74, 75, 76 Further research is necessary to quantify the potential impact of respired air temperature on disease severity and transmission rates. It may be productive to examine the impact of air temperature on upper respiratory tract viral load in an animal model after exposure to air at various temperatures and for various durations.
Conversely, warmer air may reduce upper airway viral titer of SARS‐CoV‐2, which may contribute to less severe disease if upper airway viral titer is suppressed when infection is local to the nasal cavity and upper airways, immediately postexposure or very early in SARS‐CoV‐2 infection.94, 97, 98 This suggests that administration of warm air may potentially represent a therapeutic measure as a postexposure prophylaxis and an early‐stage treatment to prevent disease progression. This concept is supported by preliminary clinical data: A study that provided seven asymptomatic or pauci‐symptomatic COVID‐19 patients with 20 min of steam inhalation for 4+ consecutive days found that symptomatic patients reported clinical improvement at the end of the protocol and all patients tested negative after the first day of steam inhalation. 99 While these observations are preliminary, they suggest that further investigation of warm air as an early‐stage treatment is warranted.
Warm air delivery would need to occur for extended durations to suppress viral replication to a degree that would be beneficial, as decreases in virus infectivity are dependent on duration of exposure to elevated temperatures (Figure 3B).3, 9 Warm, humidified air is well‐tolerated over long durations.93, 100 It is important to note that air above body temperature will be cooled during inhalation, limiting the effectiveness of very warm air in reducing viral stability in lower areas of the respiratory tract. Care must be taken when administering air to COVID‐19 patients to avoid aerosolizing viral particles. Postexposure prophylaxis applications may be of particular relevance for healthcare professionals who have insufficient personal protective equipment, as well as for populations who are at risk of more severe disease.
There could also be opportunities for innovation in mask design to not only capture respiratory droplets and aerosolized viral particles, but also to warm inhaled air. Heating masks already exist and could be modified or directly applied to the aim of increasing thermal inactivation of virus in the upper respiratory tract.101, 102, 103, 104 Even simple face masks have been shown to conserve heat loss from breathing and diminish upper airway cooling, 101 suggesting that simply increasing the duration of mask usage may be beneficial. Mask design and usage could be a promising area for innovation and further study.
The impact of temperatures in the body on viral growth may help explain COVID‐19 transmission rates and disease severity. Advances made in understanding the various impacts of temperature on COVID‐19 could inform both disease prevention and treatment, with potentially significant impacts on patient outcomes and global disease burden.
CONFLICT OF INTERESTS
Erik Kulstad has equity interest in Attune Medical, a company involved in patient temperature management.
Kang D, Ellgen C, Kulstad E. Possible effects of air temperature on COVID‐19 disease severity and transmission rates. J Med Virol. 2021;93:5358–5366. 10.1002/jmv.27042
REFERENCES
- 1. Chin AWH, Chu JTS, Perera MRA, et al. Stability of SARS‐CoV‐2 in different environmental conditions. Lancet Microbe. 2020. 10.1016/S2666-5247(20)30003-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matson MJ, Yinda CK, Seifert SN, et al. Effect of environmental conditions on SARS‐CoV‐2 stability in human nasal mucus and sputum. Emerg Infect Dis. 2020;26(9):2276‐2278. 10.3201/eid2609.202267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yap TF, Liu Z, Shveda RA, Preston DJ. A predictive model of the temperature‐dependent inactivation of coronaviruses. Appl Phys Lett. 2020;117(6):060601. 10.1063/5.0020782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med. 2020;382(16):1564‐1567. 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aboubakr HA, Sharafeldin TA, Goyal SM. Stability of SARS‐CoV‐2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: a review. Transbound Emerg Dis. 2020. 10.1111/tbed.13707 [DOI] [PMC free article] [PubMed]
- 6. Biryukov J, Boydston JA, Dunning RA, et al. Increasing temperature and relative humidity accelerates Inactivation of SARS‐CoV‐2 on Surfaces. mSphere. 2020;5(4), 10.1128/mSphere.00441-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan KH, Peiris JSM, Lam SY, Poon LLM, Yuen KY, Seto WH, Kohl A. ed. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv Virol. 2011;2011:734690. 10.1155/2011/734690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sharma A, Preece B, Swann H, et al. Structural stability of SARS‐CoV‐2 degrades with temperature. bioRxiv. 2020. 10.1101/2020.10.12.336818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morris DH, Yinda KC, Gamble A, et al. Mechanistic theory predicts the effects of temperature and humidity on inactivation of SARS‐CoV‐2 and other enveloped viruses. bioRxiv. 2020. 10.1101/2020.10.16.341883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan K‐H, Sridhar S, Zhang RR, et al. Factors affecting stability and infectivity of SARS‐CoV‐2. J Hosp Infect. 2020;106(2):226‐231. 10.1016/j.jhin.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hou YJ, Okuda K, Edwards CE, et al. SARS‐CoV‐2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182(2):429‐446.e14. 10.1016/j.cell.2020.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177‐1179. 10.1056/NEJMc2001737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581(7809):465‐469. 10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- 14. Hall J. Pulmonary Ventilation. 12th edn. Guyton and Hall Textbook of Medical Physiology; 2011:474. https://archive.org/details/guytonandhalltextbookofmedicalphysiology12thed_202004/page/n493/mode/2up [Google Scholar]
- 15. Keck T, Leiacker R, Riechelmann H, Rettinger G. Temperature profile in the nasal cavity. Laryngoscope. 2000;110(4):651‐654. 10.1097/00005537-200004000-00021 [DOI] [PubMed] [Google Scholar]
- 16. McFadden ER Jr, Pichurko BM, Bowman HF, et al. Thermal mapping of the airways in humans. J Appl Physiol Bethesda Md 1985. 1985;58(2):564‐570. 10.1152/jappl.1985.58.2.564 [DOI] [PubMed] [Google Scholar]
- 17. Lindemann J, Leiacker R, Rettinger G, Keck T. Nasal mucosal temperature during respiration. Clin Otolaryngol Allied Sci. 2002;27(3):135‐139. 10.1046/j.1365-2273.2002.00544.x [DOI] [PubMed] [Google Scholar]
- 18. Gilbert IA, Fouke JM, McFadden ER. Intra‐airway thermodynamics during exercise and hyperventilation in asthmatics. J Appl Physiol. 1988;64(5):2167‐2174. 10.1152/jappl.1988.64.5.2167 [DOI] [PubMed] [Google Scholar]
- 19. Rouadi P, Baroody FM, Abbott D, Naureckas E, Solway J, Naclerio RM. A technique to measure the ability of the human nose to warm and humidify air. J Appl Physiol. 1999;87(1):400‐406. 10.1152/jappl.1999.87.1.400 [DOI] [PubMed] [Google Scholar]
- 20. Bailey RS, Casey KP, Pawar SS, Garcia GJM. Correlation of nasal mucosal temperature with subjective nasal patency in healthy individuals. JAMA Facial Plast Surg. 2017;19(1):46‐52. 10.1001/jamafacial.2016.1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. V′kovski P, Gultom M, Kelly J, et al. Disparate temperature‐dependent virus–host dynamics for SARS‐CoV‐2 and SARS‐CoV in the human respiratory epithelium. bioRxiv. 2020. 10.1101/2020.04.27.062315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laporte M, Stevaert A, Raeymaekers V, et al. The SARS‐CoV‐2 and other human coronavirus spike proteins are fine‐tuned towards temperature and proteases of the human airways. bioRxiv. 2020. 10.1101/2020.11.09.374603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou B, Thao TTN, Hoffmann D, et al. SARS‐CoV‐2 spike D614G variant confers enhanced replication and transmissibility. bioRxiv. 2020. 10.1101/2020.10.27.357558 [DOI] [Google Scholar]
- 24. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID‐19. Nat Med. 2020;26(5):672‐675. 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 25. Kim Y‐I, Kim S‐G, Kim S‐M, et al. Infection and rapid transmission of SARS‐CoV‐2 in ferrets. Cell Host Microbe. 2020;27:704‐709.e2. 10.1016/j.chom.2020.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kawasuji H, Takegoshi Y, Kaneda M, et al. Transmissibility of COVID‐19 depends on the viral load around onset in adult and symptomatic patients. PLOS One. 2020;15(12):e0243597. 10.1371/journal.pone.0243597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Y, Yan L‐M, Wan L, et al. Viral dynamics in mild and severe cases of COVID‐19. Lancet Infect Dis. 2020;0(0):656‐657. 10.1016/S1473-3099(20)30232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holwerda M, Kelly J, Laloli L, et al. Determining the replication kinetics and cellular tropism of influenza D virus on primary well‐differentiated human airway epithelial cells. Viruses. 2019;11(4), 10.3390/v11040377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Papadopoulos NG, Sanderson G, Hunter J, Johnston SL. Rhinoviruses replicate effectively at lower airway temperatures. J Med Virol. 1999;58(1):100‐104. [DOI] [PubMed] [Google Scholar]
- 30. Hoorn B, Tyrrell DAJ. A new virus cultivated only in organ cultures of human ciliated epithelium. Arch Für Gesamte Virusforsch. 1966;18(2):210‐225. 10.1007/BF01241842 [DOI] [PubMed] [Google Scholar]
- 31. Laporte M, Stevaert A, Raeymaekers V, et al. Hemagglutinin cleavability, acid stability, and temperature dependence optimize influenza B virus for replication in human airways. J Virol. 2019;94(1), 10.1128/JVI.01430-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Corman VM, Eckerle I, Memish ZA, et al. Link of a ubiquitous human coronavirus to dromedary camels. Proc Natl Acad Sci. 2016;113(35):9864‐9869. 10.1073/pnas.1604472113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moriyama M, Hugentobler WJ, Iwasaki A. Seasonality of respiratory viral infections. Annu Rev Virol. 2020;7:83‐101. 10.1146/annurev-virology-012420-022445 [DOI] [PubMed] [Google Scholar]
- 34. Foxman EF, Storer JA, Fitzgerald ME, et al. Temperature‐dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells. Proc Natl Acad Sci. 2015;112(3):827‐832. 10.1073/pnas.1411030112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mourtzoukou EG, Falagas ME. Exposure to cold and respiratory tract infections [review article]. Int J Tuberc Lung Dis. 2007;11(9):938‐943. [PubMed] [Google Scholar]
- 36. Kudo E, Song E, Yockey LJ, et al. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc Natl Acad Sci. 2019;116(22):10905‐10910. 10.1073/pnas.1902840116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williams R, Rankin N, Smith T, Galler D, Seakins P. Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit Care Med. 1996;24(11):1920‐1929. [DOI] [PubMed] [Google Scholar]
- 38. Foxman EF, Storer JA, Vanaja K, Levchenko A, Iwasaki A. Two interferon‐independent double‐stranded RNA‐induced host defense strategies suppress the common cold virus at warm temperature. Proc Natl Acad Sci. 2016;113(30):8496‐8501. 10.1073/pnas.1601942113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stanley ED, Jackson GG, Panusarn C, Rubenis M, Dirda V. Increased virus shedding with aspirin treatment of rhinovirus infection. JAMA. 1975;231(12):1248‐1251. 10.1001/jama.1975.03240240018017 [DOI] [PubMed] [Google Scholar]
- 40. Doran TF, Angelis CD, Baumgardner RA, Mellits ED. Acetaminophen: more harm than good for chickenpox? J Pediatr. 1989;114(6):1045‐1048. 10.1016/S0022-3476(89)80461-5 [DOI] [PubMed] [Google Scholar]
- 41. Graham NM, Burrell CJ, Douglas RM, Debelle P, Davies L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus‐infected volunteers. J Infect Dis. 1990;162(6):1277‐1282. 10.1093/infdis/162.6.1277 [DOI] [PubMed] [Google Scholar]
- 42. Knipe D, Howley P. Coronaviridae. Fields Virology. Vol 1, 6th ed. Lippincott Williams & Wilkins; 2013. https://archive.org/details/DavidM.KnipePeterHowleyFieldsVirologyKnipeFieldsVirology2VolumeSetLWW2013/mode/1up [Google Scholar]
- 43. World Health Organization . Coronavirus disease (COVID‐19): How is it transmitted? Accessed February 12, 2021. https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-how-is-it-transmitted
- 44. Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ. Identifying airborne transmission as the dominant route for the spread of COVID‐19. Proc Natl Acad Sci. 2020;117(26):14857‐14863. 10.1073/pnas.2009637117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS‐CoV‐2: a review of viral, host, and environmental factors. Ann Intern Med. 2020;174:69‐79. 10.7326/M20-5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Booth TF, Kournikakis B, Bastien N, et al. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J Infect Dis. 2005;191(9):1472‐1477. 10.1086/429634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Farzal Z, Basu S, Burke A, et al. Comparative study of simulated nebulized and spray particle deposition in chronic rhinosinusitis patients. Int Forum Allergy Rhinol. 2019;9(7):746‐758. 10.1002/alr.22324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Teunis PFM, Brienen N, Kretzschmar MEE. High infectivity and pathogenicity of influenza A virus via aerosol and droplet transmission. Epidemics. 2010;2(4):215‐222. 10.1016/j.epidem.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 49. Kindler E, Jónsdóttir HR, Muth D, et al. Efficient replication of the novel human betacoronavirus EMC on primary human epithelium highlights its zoonotic potential. mBio. 2013;4(1):00611‐00612. 10.1128/mBio.00611-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dijkman R, Jebbink MF, Koekkoek SM, et al. Isolation and characterization of current human coronavirus strains in primary human epithelial cell cultures reveal differences in target cell tropism. J Virol. 2013;87(11):6081‐6090. 10.1128/JVI.03368-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271‐280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Q, Zhang Y, Wu L, et al. Structural and functional basis of SARS‐CoV‐2 entry by using human ACE2. Cell. 2020. 10.1016/j.cell.2020.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sims AC, Baric RS, Yount B, Burkett SE, Collins PL, Pickles RJ. Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J Virol. 2005;79(24):15511‐15524. 10.1128/JVI.79.24.15511-15524.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007;170(4):1136‐1147. 10.2353/ajpath.2007.061088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dickson RP, Erb‐Downward JR, Martinez FJ, Huffnagle GB. The microbiome and the respiratory tract. Annu Rev Physiol. 2016;78(1):481‐504. 10.1146/annurev-physiol-021115-105238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Esther CR Jr, Muhlebach MS, Ehre C, et al. Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci Transl Med. 2019;11(486), 10.1126/scitranslmed.aav3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gaeckle NT, Pragman AA, Pendleton KM, Baldomero AK, Criner GJ. The oral‐lung axis: the impact of oral health on lung health. Respir Care. 2020;65(8):1211‐1220. 10.4187/respcare.07332 [DOI] [PubMed] [Google Scholar]
- 58. Odani K, Tachibana M, Tamashima R, Tsutsumi Y. Herpes simplex virus pneumonia: importance of aspiration etiology. Case Rep Pathol. 2019;2019:e7623576. 10.1155/2019/7623576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Phillips LK, Deane AM, Jones KL, Rayner CK, Horowitz M. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol. 2015;11(2):112‐128. 10.1038/nrendo.2014.202 [DOI] [PubMed] [Google Scholar]
- 60. Eichner H, Behbehani AA, Hochstrasser K. Diagnostic value of nasal secretions, current state: normal values. 1. Laryngol Rhinol Otol. 1983;62(12):561‐565. [PubMed] [Google Scholar]
- 61. Pandya VK, Tiwari RS. Nasal mucociliary clearance in health and disease. Indian J Otolaryngol Head Neck Surg. 2006;58(4):332‐334. 10.1007/BF03049581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gleeson K, Maxwell SL, Eggli DF. Quantitative aspiration during sleep in normal subjects. Chest. 1997;111(5):1266‐1272. 10.1378/chest.111.5.1266 [DOI] [PubMed] [Google Scholar]
- 63. Huxley EJ, Viroslav J, Gray WR, Pierce AK. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med. 1978;64(4):564‐568. 10.1016/0002-9343(78)90574-0 [DOI] [PubMed] [Google Scholar]
- 64. Quirouette C, Younis NP, Reddy MB, Beauchemin CAA. A mathematical model describing the localization and spread of influenza A virus infection within the human respiratory tract. PLOS Comput Biol. 2020;16(4):e1007705. 10.1371/journal.pcbi.1007705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xu X, Yu C, Qu J, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS‐CoV‐2. Eur J Nucl Med Mol Imaging. 2020;47(5):1275‐1280. 10.1007/s00259-020-04735-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fisman D. Seasonality of viral infections: mechanisms and unknowns. Clin Microbiol Infect. 2012;18(10):946‐954. 10.1111/j.1469-0691.2012.03968.x [DOI] [PubMed] [Google Scholar]
- 67. Du Prel J‐B, Puppe W, Gröndahl B, et al. Are meteorological parameters associated with acute respiratory tract infections? Clin Infect Dis. 2009;49(6):861‐868. 10.1086/605435 [DOI] [PubMed] [Google Scholar]
- 68. Simpson REH. Discussion on the common cold. Proc R Soc Med. 1958;51(4):267‐271. [PMC free article] [PubMed] [Google Scholar]
- 69. The Eurowinter Group . Cold exposure and winter mortality from ischaemic heart disease, cerebrovascular disease, respiratory disease, and all causes in warm and cold regions of Europe. Lancet. 1997;349(9062):1341‐1346. 10.1016/S0140-6736(96)12338-2 [DOI] [PubMed] [Google Scholar]
- 70. National Academies of Sciences, Engineering, and Medicine . Rapid Expert Consultation on SARS‐CoV‐2 survival in relation to temperature and humidity and potential for seasonality for the COVID‐19 pandemic (April 7, 2020). The National Academies Press; 2020. 10.17226/25771 [DOI]
- 71. McClymont H, Hu W. Weather variability and COVID‐19 transmission: a review of recent research. Int J Environ Res Public Health. 2021;18(2):396. 10.3390/ijerph18020396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Briz‐Redón Á, Serrano‐Aroca Á. A spatio‐temporal analysis for exploring the effect of temperature on COVID‐19 early evolution in Spain. Sci Total Environ. 2020;728:138811. 10.1016/j.scitotenv.2020.138811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Franch‐Pardo I, Napoletano BM, Rosete‐Verges F, Billa L. Spatial analysis and GIS in the study of COVID‐19. A review. Sci Total Environ. 2020;739:140033. 10.1016/j.scitotenv.2020.140033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shakil MH, Munim ZH, Tasnia M, Sarowar S. COVID‐19 and the environment: a critical review and research agenda. Sci Total Environ. 2020;745:141022. 10.1016/j.scitotenv.2020.141022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Paraskevis D, Kostaki EG, Alygizakis N, et al. A review of the impact of weather and climate variables to COVID‐19: in the absence of public health measures high temperatures cannot probably mitigate outbreaks. Sci Total Environ. 2021;768:144578. 10.1016/j.scitotenv.2020.144578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mecenas P, Bastos R, Vallinoto A, Normando D. Effects of temperature and humidity on the spread of COVID‐19: a systematic review. medRxiv. 2020. 10.1101/2020.04.14.20064923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ahmadi M, Sharifi A, Dorosti S, Jafarzadeh Ghoushchi S, Ghanbari N. Investigation of effective climatology parameters on COVID‐19 outbreak in Iran. Sci Total Environ. 2020;729:138705. 10.1016/j.scitotenv.2020.138705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Thu TPB, Ngoc PNH, Hai NM, Tuan LA. Effect of the social distancing measures on the spread of COVID‐19 in 10 highly infected countries. Sci Total Environ. 2020;742:140430. 10.1016/j.scitotenv.2020.140430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kubota Y, Shiono T, Kusumoto B, Fujinuma J. Multiple drivers of the COVID‐19 spread: the roles of climate, international mobility, and region‐specific conditions. PLOS One. 2020;15(9):e0239385. 10.1371/journal.pone.0239385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yin C. Genotyping coronavirus SARS‐CoV‐2: methods and implications. Genomics. 2020;112(5):3588‐3596. 10.1016/j.ygeno.2020.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Benvenuto D, Demir AB, Giovanetti M, et al. Evidence for mutations in SARS‐CoV‐2 Italian isolates potentially affecting virus transmission. J Med Virol. 2020;92(10):2232‐2237. 10.1002/jmv.26104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kozlovskaya L, Piniaeva A, Ignatyev G, et al. Isolation and phylogenetic analysis of SARS‐CoV‐2 variants collected in Russia during the COVID‐19 outbreak. Int J Infect Dis. 2020;99:40‐46. 10.1016/j.ijid.2020.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Korber B, Fischer WM, Gnanakaran S, et al. Spike mutation pipeline reveals the emergence of a more transmissible form of SARS‐CoV‐2. bioRxiv. 2020. 10.1101/2020.04.29.069054 [DOI] [Google Scholar]
- 84. Plante JA, Liu Y, Liu J, et al. Spike mutation D614G alters SARS‐CoV‐2 fitness. Nature. 2020:1‐6. 10.1038/s41586-020-2895-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sagripanti J‐L, Lytle CD. Estimated inactivation of coronaviruses by solar radiation with special reference to COVID‐19. Photochem Photobiol. 2020;96(4):731‐737. 10.1111/php.13293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ratnesar‐Shumate S, Williams G, Green B, et al. Simulated sunlight rapidly inactivates SARS‐CoV‐2 on surfaces. J Infect Dis. 2020;222(2):214‐222. 10.1093/infdis/jiaa274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Schuit M, Ratnesar‐Shumate S, Yolitz J, et al. Airborne SARS‐CoV‐2 is rapidly inactivated by simulated sunlight. J Infect Dis. 2020;222(4):564‐571. 10.1093/infdis/jiaa334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fischer RJ, Morris DH, Van Doremalen N, et al. Effectiveness of N95 respirator decontamination and reuse against SARS‐CoV‐2 virus. Emerg Infect Dis. 2020;26(9):2253‐2255. 10.3201/eid2609.201524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schweizer C, Edwards RD, Bayer‐Oglesby L, et al. Indoor time–microenvironment–activity patterns in seven regions of Europe. J Expo Sci Environ Epidemiol. 2007;17(2):170‐181. 10.1038/sj.jes.7500490 [DOI] [PubMed] [Google Scholar]
- 90. Klepeis NE, Nelson WC, Ott WR, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Sci Environ Epidemiol. 2001;11(3):231‐252. 10.1038/sj.jea.7500165 [DOI] [PubMed] [Google Scholar]
- 91. Sohrabi C, Alsafi Z, O'neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID‐19). Int J Surg. 2020;76:71‐76. 10.1016/j.ijsu.2020.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475‐481. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lloyd EL. Equipment for airway warming in the treatment of accidental hypothermia. J Wilderness Med. 1991;2(4):330‐350. 10.1580/0953-9859-2.4.330 [DOI] [Google Scholar]
- 94. Gandhi M, Rutherford GW. Facial masking for COVID‐19—potential for “Variolation” as we await a vaccine. N Engl J Med. 2020;383(18):e101. 10.1056/NEJMp2026913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Van Damme W, Dahake R, Van de Pas R, Vanham G, Assefa Y. COVID‐19: does the infectious inoculum dose‐response relationship contribute to understanding heterogeneity in disease severity and transmission dynamics? Med Hypotheses. 2021;146:110431. 10.1016/j.mehy.2020.110431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Callow KA, Parry HF, Sergeant M, Tyrrell D a J. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990;105(2):435‐446. 10.1017/S0950268800048019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hausdorff WP, Flores J. Low‐dose and oral exposure to SARS‐CoV‐2 may help us understand and prevent severe COVID‐19. Int J Infect Dis. 2021;103:37‐41. 10.1016/j.ijid.2020.11.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Imai M, Iwatsuki‐Horimoto K, Hatta M, et al. Syrian hamsters as a small animal model for SARS‐CoV‐2 infection and countermeasure development. Proc Natl Acad Sci. 2020;117(28):16587‐16595. 10.1073/pnas.2009799117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. la Marca G, Barp J, Frenos S, et al. Thermal inactivation of SARS COVID‐2 virus: are steam inhalations a potential treatment? Life Sci. 2021;265:118801. 10.1016/j.lfs.2020.118801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bibby S, Reddy S, Cripps T, et al. Tolerability of nasal delivery of humidified and warmed air at different temperatures: a randomised double‐blind pilot study. Pulm Med, 10.1155/2016/7951272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Eccles R. An explanation for the seasonality of acute upper respiratory tract viral infections. Acta Otolaryngol (Stockh). 2002;122(2):183‐191. 10.1080/00016480252814207 [DOI] [PubMed] [Google Scholar]
- 102. Behr RD. Heating and humidifying respiratory mask. Accessed December 15, 2020. https://patents.google.com/patent/US5570684A/en
- 103. Barghini RJ, Westberg WM, Carey JPH. Cold weather face mask. Accessed December 15, 2020. https://patents.google.com/patent/US3333585A/en
- 104. Cummins JM, Morrison G, Pierfelice RE. Respiratory heated face mask. Accessed December 15, 2020. https://patents.google.com/patent/US4793343A/en


