Abstract
Background/Objectives
The effectiveness of the BNT162b2 vaccine on preventing the spread of COVID‐19 and deaths in nursing homes (NH) is unknown.
Design
We used zero‐inflated negative binomial mixed effects regressions to model the associations of time since the vaccine clinic ending the week of December 27, 2020 (cohort 1), January 3, 2021 (cohort 2), or January 10, 2021 (cohort 3) controlling for county rate of COVID‐19, bed size, urban location, racial and ethnic census, and level of registered nurses with resident cases and deaths of COVID‐19 and staff cases of COVID‐19.
Setting and Participants
All 2501 NHs who held a vaccine clinic from the first 17 states to initiate clinics as part of the Pharmacy Partnership for Long‐Term Care Program.
Main Outcome(s) and Measure(s)
Adjusted Incidence Rate Ratio (IRR) for time in 3, 4, 5, and 6 weeks after the first vaccine clinic for resident cases and deaths of COVID‐19 and staff cases of COVID‐19.
Results
Resident and staff cases trended downward in all three cohorts following the vaccine clinics. Time following the first clinic at 5 and 6 weeks was consistently associated with fewer resident cases (IRR: 0.68 [95% CI: 0.54–0.84], IRR: 0.64 [95% CI: 0.48–0.86], respectively); resident deaths (IRR: 0.59 [95% CI: 0.45–0.77], IRR: 0.45 [95% CI: 0.31–0.65], respectively); and staff cases (IRR: 0.64 [95% CI: 0.56–0.73], IRR: 0.51 [95% CI: 0.42–0.62], respectively). Other factors associated with fewer resident and staff cases included facilities with less than 50 certified beds and high nurse staffing per resident day (>0.987). Contrary to prior research, higher Hispanic non‐white resident census was associated with fewer resident cases (IRR: 0.42, 95% CI: 0.31–0.56) and deaths (IRR: 0.18, 95% CI: 0.12–0.27).
Conclusions
The BNT162b2 vaccine is associated with decreased spread of SARS‐CoV‐2 in both residents and staff as well as decreased deaths among residents.
Keywords: BNT162b2, COVID‐19, health care worker, mortality, nursing home, SARS‐CoV‐2, vaccination
Short abstract
See related editorial by Ouslander et al and related articles by Mor et al, Moore et al, and Rudolph et al. in this issue.
Key Points
Following the first COVID‐19 vaccine clinic in nursing homes, new resident cases and deaths from SARS‐CoV‐2 and cases among staff were associated with delayed vaccine effect when controlling for other factors.
The decrease corresponded to the expected drop if the vaccine is effective compared with facilities holding clinics later.
Other factors were associated with decline in cases and deaths, including size of the facility, ethnic and racial census in the facility, and level of registered nurses.
Why Does this Paper Matter?
This is the first study to show the potential effectiveness of federal Long‐Term Care Pharmacy Partnership administration of COVID‐19 vaccines on the development of new SARS‐CoV‐2 cases and deaths, as all prior publications have only looked at impact on developing COVID‐19 in the general population. This effect was seen after the first vaccine clinic in nursing homes. If confirmed, these data support changing guidance on visitations, dining, and activities in long‐term care settings as well as on testing of health care workers based on vaccination status.
INTRODUCTION
The coronavirus disease 2019, COVID‐19, has disproportionately impacted older adults and individuals with complex medical needs, particularly those receiving care in long‐term care (LTC) settings in the United States and internationally. 1 , 2 Some estimate that over 150,000 LTC residents have died due to COVID‐19, accounting for up to a third (34%) of all COVID‐19 deaths in the United States. 3 Evidence from the first half of 2020 found that outbreaks in LTC facilities were associated with the rates of COVID‐19 in the community. 4 , 5 This association has persisted during the most recent surge in cases in the fall of 2020. 6 Therefore, a vaccine, coupled with reductions in community spread and standard infection prevention precautions, provides the most promising approach to preventing the deleterious impact of COVID‐19 in LTC residents and staff.
The U.S. Food & Drug Administration (FDA) granted Emergency Use Authorization (EUA) for two mRNA vaccines in December of 2020, BNT162b2 and mRNA‐1273, manufactured by Pfizer‐BioNTech and Moderna, respectively. 7 , 8 Although two doses are required to achieve nearly 95% effectiveness, the vaccines were found to be effective in preventing symptomatic disease after the first dose, albeit at lower levels (52% effectiveness for both Pfizer and Moderna). 7 , 8 , 9 The trials did not evaluate the impact of the vaccines on infection spread, however, emerging data from Israel and a recent study among health care workers in the United States suggest that the vaccines may prevent spread in the general population. 10 , 11
Due to high risk for COVID‐19 morbidity and mortality for LTC residents and staff, on December 1, 2020, the Advisory Committee on Immunization Practices (ACIP) recommended prioritizing long‐term care residents and staff to receive the vaccine as part of Phase 1a. 12 The Centers for Disease Control and Prevention (CDC) launched the Pharmacy Partnership for Long‐Term Care Program (Pharmacy Partnership), a public–private partnership among CDC, CVS Pharmacy, Managed Health Care Associates, Inc., and Walgreens, to administer vaccines to residents and staff in nursing homes across the country. 13
Each state selected the start date for distribution under the Pharmacy Partnership and selected the vaccine for use in the program; all providers within the state received the vaccine from the same manufacturer. Scheduling of clinics was done by the Pharmacy Partnership with a goal of scheduling over 90% of all nursing homes in the nation within 4 weeks of the program's start date. Seventeen states started clinics on December 18, 2020, all using BNT162b2 because mRNA‐1273 and Ad26.COV2.S were not yet available for use in this program. Clinics for the first dose of the vaccine were then held over the next 3–4 weeks in these 17 states.
Because antibody levels do not rise to protective levels until approximately 7–14 days after exposure to the SARS‐CoV‐2 virus, 14 any protective effect of the vaccine would not be achieved within 1–2 weeks after the first dose of the vaccine. 7 , 8 , 9 Given the median COVID‐19 incubation period of 4–5 days, we would not expect to observe the effectiveness of the vaccine on reducing new cases until approximately 2–3 weeks after the first vaccine clinic. 15 Therefore, if the vaccine impacted spread after the first dose, we would expect to observe fewer incident COVID‐19 cases in facilities starting 3 weeks after first vaccine clinic. The sequential scheduling of vaccine clinics in nursing homes in the same county over a 3‐week period provided a unique opportunity to evaluate if there was a decline in new COVID‐19 cases among nursing homes that started to vaccinate residents and staff before other nursing homes.
To describe if the COVID‐19 vaccine was associated with decreased spread, we created three cohorts among facilities in the same county based on having their initial vaccination clinic in the first, second, or third week of the Pharmacy Partnership. We then controlled for time and facility characteristics to examine the association between the resident and staff incident COVID‐19 case and resident death rates and the time‐dependent, delayed vaccination effect at 3, 4, 5, and 6 weeks after the initial vaccination clinic took place.
METHODS
Data Sources
We utilized the CMS National Health Safety Network (NHSN) Public File data released on February 25, 2021, containing staff and resident COVID‐19 data up to the week ending on February 7, 2021. In accordance with CMS regulations, nursing homes in the same county are required to test all their staff and residents at the same frequency based on the county COVID‐19 rate using either PCR or point of care antigen tests. 16 The NHSN Public File contains weekly counts of incident staff and resident COVID‐19 cases and deaths reported by LTC facilities to the CDC NHSN system. 17 , 18 We joined NHSN data for each facility with the CMS Certification and Survey Provider Enhanced Reports (CASPER) January 2021 release, the February 2021 release of Care Compare for nursing homes, the 2020Q3 Payroll Based Journal (PBJ) Daily Staffing, and the Provider of Service December 2020 release to obtain facility characteristic data. 19 , 20 , 21 Using the national distribution, we characterized the 2020Q3 adjusted registered nurse (RN) Hours per Resident Day (HPRD) in three categories with the second category containing the two middle quartiles of the distribution (Table 2). To obtain information on nursing homes that have contracts with Veteran's Affairs, we joined these data to a U.S. Department of Veterans Affairs provider list. 22 We then joined these data with the Hopkins COVID‐19 Dashboard data in order to obtain the county incident 7‐day average COVID‐19 rate per 100,000 people for each week. 23
We linked these data with the latest CMS LTC Minimum Data Set (MDS) 3.0 from 2020Q1 to calculate the proportion of residents identifying as a racial minority or Hispanic non‐white ethnicity. 24 The MDS instrument is a standardized, comprehensive assessment that must be completed for all persons who receive care in a Medicare and/or Medicaid‐certified nursing facility, capturing information about residents' demographic and clinical status. 25 , 26 We define racial minority as follows: Asian, African American, American Indian or Alaskan Native, Native Hawaiian or Pacific Islander alone, or more than one race. We characterized facilities as having low, medium, medium‐high, or high proportion of residents based on quartiles from the national distribution for racial minority and for Hispanic non‐white. 27
Primary outcomes
Because the CMS NHSN Public file provides data for each facility for each week, the primary outcomes for the study were as follows: new COVID‐19 resident cases per resident‐week, new COVID‐19 resident mortality per resident‐week, and new COVID‐19 staff cases per FTE clinical staff‐week. For each week starting from December 20, 2020, until February 7, 2021, we constructed the resident‐at‐risk denominator by taking the total resident census and subtracting the sum of the COVID‐19 resident incident cases from the previous 13 weeks. This was done to account for COVID‐19 immunity lasting three or more months after infection. 28 Similarly, we created the weekly FTE clinical staff‐at‐risk denominator by subtracting the sum of the staff COVID‐19 incident cases from the previous 13 weeks from the Care Compare reported FTE clinical staffing variable.
Study population
We used vaccine clinic schedule data from the CDC Tiberius system for LTC facilities participating in the Pharmacy Partnership for Long‐Term Care Program containing information on 12,454 nursing homes. 13 Two facilities had missing COVID‐19 counts, 28 had missing occupied beds, and 77 had missing FTE staffing information. We assigned nursing homes to three cohorts based on the date of their first vaccination clinic (Table 1). We included 18 facilities that held clinics between December 18 and 20, 2020 in the Cohort 1 sample. Facilities had to have at least 4 weeks of follow‐up NHSN data after their first vaccination clinics.
TABLE 1.
Three nursing home cohorts depicting the time periods relative to their first vaccine clinic
| Cohort name | # of NHs | Week of | ||||||
|---|---|---|---|---|---|---|---|---|
| Dec | Dec–Jan | Jan | Jan | Jan | Jan | Feb | ||
| 18–27 | 28–3 | 4–10 | 11–17 | 18–24 | 25–31 | 1–7 | ||
| 1 | 840 |
T + 0 (Vaccine) |
T + 1 | T + 2 | T + 3 | T + 4 | T + 5 | T + 6 |
| 2 | 830 |
T + 0 (Vaccine) |
T + 1 | T + 2 | T + 3 | T + 4 | T + 5 | |
| 3 | 831 |
T + 0 (Vaccine) |
T + 1 | T + 2 | T + 3 | T + 4 | ||
Note: T0 is the week of the facility's first vaccination clinic. Facilities in Cohorts 2 and 3 are in the same counties as facilities in Cohort 1. More than 7 days in the first week to include 18 facilities with clinics from Dec 18 to 20.
Statistical analyses
First, we compared differences in facility characteristics between cohorts using chi‐squared (χ 2) tests. Second, we plotted the average number of resident cases and deaths, as well as staff cases, for each cohort from the week ending on December 20 through the week ending on February 7, 2021. Third, we sought to examine if there was a relationship between having a vaccination clinic and resident COVID‐19 cases and deaths, as well as staff COVID‐19 cases. The main independent indicators were the four time‐dependent, delayed vaccination effects at 3, 4, 5, and 6 weeks respectively after the initial vaccination clinic took place. 29 We adjusted for calendar week; county 7‐day average rate of COVID‐19; urban location; certified beds divided into low (≤50 beds), medium 51–120 beds (referent group), and large (>120 beds); racial minority census by quartile with the lowest group serving as the referent group; Hispanic non‐white census by quartile with the lowest group serving as the referent group; and the level of registered nurses divided into low (<0.499 HPRD), medium (0.499–0.987 HPRD, referent group), and high (>0.987 HPRD), all covariates previously shown to be associated with COVID‐19 cases.
Approximately 69% of the observation‐weeks in the data had zero resident COVID‐19 case counts, 84% of the observation‐weeks had zero resident COVID‐19 mortality counts, and 49% of the observation‐weeks had zero staff COVID‐19 case counts. Given the study window of 8 weeks, all facilities had 4 weeks of follow‐up data, 1670 facilities had follow‐up data for T + 5 after vaccination, and 840 facilities had follow‐up data for T + 6 after vaccination.
To address the highly skewed, longitudinal count measurements with a large proportion of zeros, we adopted zero‐inflated negative binomial mixed effect regressions to model the associations of the covariates with our primary outcomes. 30 The negative binomial model addresses the issue of overdispersion by including a dispersion parameter that relaxes the assumption of equal mean and variance of the Poisson model. The zero‐inflated negative binomial (ZINB) model assumes a dual‐state process involving a zero‐COVID state and a non‐zero COVID state that is governed by the negative binomial distribution. 31 This approach also allows us to present the negative binomial incidence rate ratios (IRR) and 95 percent confidence intervals (CI).
To manage the hierarchical nature of the data and account for the correlation between measurements of the same facilities at different occasions, we extended the ZINB model by including a random intercept for each facility‐cluster in the negative binomial state. The relationships between the main outcomes and intervention effects controlling for covariates were assessed via the NLMIXED procedure with starting parameter values obtained from GLIMMIX and GENMOD. 32 Statistical significance was defined at alpha = 0.05. All analyses were done using SAS 9.4.
RESULTS
There were 840 facilities in Cohort 1, 830 facilities in Cohort 2, and 831 facilities in Cohort 3. We did not find any differences in the size, facility type, Continuing Care Retirement Community (CCRC) status, Veteran's Affairs contracting, or ownership of facilities holding their vaccine clinics in the first week of the program compared with facilities in the same counties who held vaccine clinics in weeks 2 and 3 of the program (Table 2). However, we observed that a higher proportion of facilities holding initial vaccination clinics in the first week of the program were rural than those who had their initial vaccination clinic at later periods (20.8% vs. 13.3% vs. 11.2%, respectively, P < 0.01). Similarly, a higher proportion of facilities in Cohort 1 had higher RN staffing HPRD than those in cohorts 2 and 3 (54.8% vs. 51.1% vs. 54.4%, P = 0.05). Furthermore, a higher proportion of facilities in Cohort 2 and Cohort 3 cared for, on average, a high proportion of racial minorities as compared with facilities in Cohort 1 (26.5% vs. 26.8% vs. 21.5%, P < 0.01). We observed a similar trend for facilities caring for a higher proportion of Hispanic non‐white residents, but the findings were not statistically significant.
TABLE 2.
Characteristics of facilities holding first clinics in Week 1, 2, and 3 of the pharmacy partnership program for long‐term care
| Facility characteristic | First vaccine clinic | P value | |||||
|---|---|---|---|---|---|---|---|
| Cohort 1 | Cohort 2 | Cohort 3 | |||||
| N | % | N | % | N | % | ||
| Total | 840 | 33.6 | 830 | 33.2 | 831 | 33.2 | |
| Bed size | |||||||
| ≤50 beds | 95 | 11.3 | 66 | 8.0 | 85 | 10.2 | 0.20 |
| 50–120 beds | 458 | 54.5 | 475 | 57.2 | 471 | 56.7 | |
| >120 beds | 287 | 34.2 | 289 | 34.8 | 275 | 33.1 | |
| Government or not‐for‐profit | |||||||
| No | 624 | 74.3 | 632 | 76.1 | 622 | 74.8 | 0.67 |
| Yes | 216 | 25.7 | 198 | 23.9 | 209 | 25.2 | |
| Urban | |||||||
| No | 175 | 20.8 | 110 | 13.3 | 93 | 11.2 | <0.0001 |
| Yes | 665 | 79.2 | 716 | 86.3 | 737 | 88.7 | |
| Multi‐facility ownership | |||||||
| No | 361 | 43.0 | 378 | 45.5 | 386 | 46.5 | 0.33 |
| Yes | 479 | 57.0 | 452 | 54.5 | 445 | 53.5 | |
| Hospital‐based | |||||||
| No | 825 | 98.2 | 815 | 98.2 | 814 | 98.0 | 0.91 |
| Yes | 15 | 1.8 | 15 | 1.8 | 17 | 2.0 | |
| CCRC | |||||||
| No | 755 | 89.9 | 754 | 90.8 | 742 | 89.3 | 0.57 |
| Yes | 85 | 10.1 | 76 | 9.2 | 89 | 10.7 | |
| Veterans affairs SNF | |||||||
| No | 739 | 88.0 | 724 | 87.2 | 740 | 89.0 | 0.61 |
| Yes | 101 | 12.0 | 102 | 12.3 | 90 | 10.8 | |
| Racial minority | |||||||
| Low | 187 | 22.3 | 136 | 16.4 | 141 | 17.0 | 0.01 |
| Medium | 231 | 27.5 | 226 | 27.2 | 213 | 25.6 | |
| Medium–high | 240 | 28.6 | 240 | 28.9 | 252 | 30.3 | |
| High | 181 | 21.5 | 220 | 26.5 | 223 | 26.8 | |
| Hispanic non‐White | |||||||
| Low | 246 | 29.3 | 211 | 25.4 | 219 | 26.4 | 0.06 |
| Medium | 155 | 18.5 | 133 | 16.0 | 143 | 17.2 | |
| Medium–high | 248 | 29.5 | 241 | 29.0 | 229 | 27.6 | |
| High | 190 | 22.6 | 237 | 28.6 | 238 | 28.6 | |
| RN staffing HPRD | |||||||
| ≤0.449 | 196 | 23.3 | 246 | 29.6 | 212 | 25.5 | 0.05 |
| 0.449–0.987 | 460 | 54.8 | 424 | 51.1 | 452 | 54.4 | |
| >0.987 | 184 | 54.8 | 160 | 51.1 | 167 | 54.4 | |
Note: Cohort 1 facilities had their first vaccination clinic between December 18 and 27, 2020. Cohort 2 facilities had their first vaccination clinic between December 28, 2020, and January 3, 2021. Cohort 3 facilities had their vaccination clinic between January 3 and 10, 2021.
The average rate of resident and staff cases was relatively stable for all three cohorts up until the week ending January 17, 2021 when cases in all three cohorts declined (Figure 1(A), (C)). Resident deaths were essentially unchanged up until the week of January 24, when deaths declined in two of the cohorts and the following week for the third cohort (Figure 1(B)).
FIGURE 1.
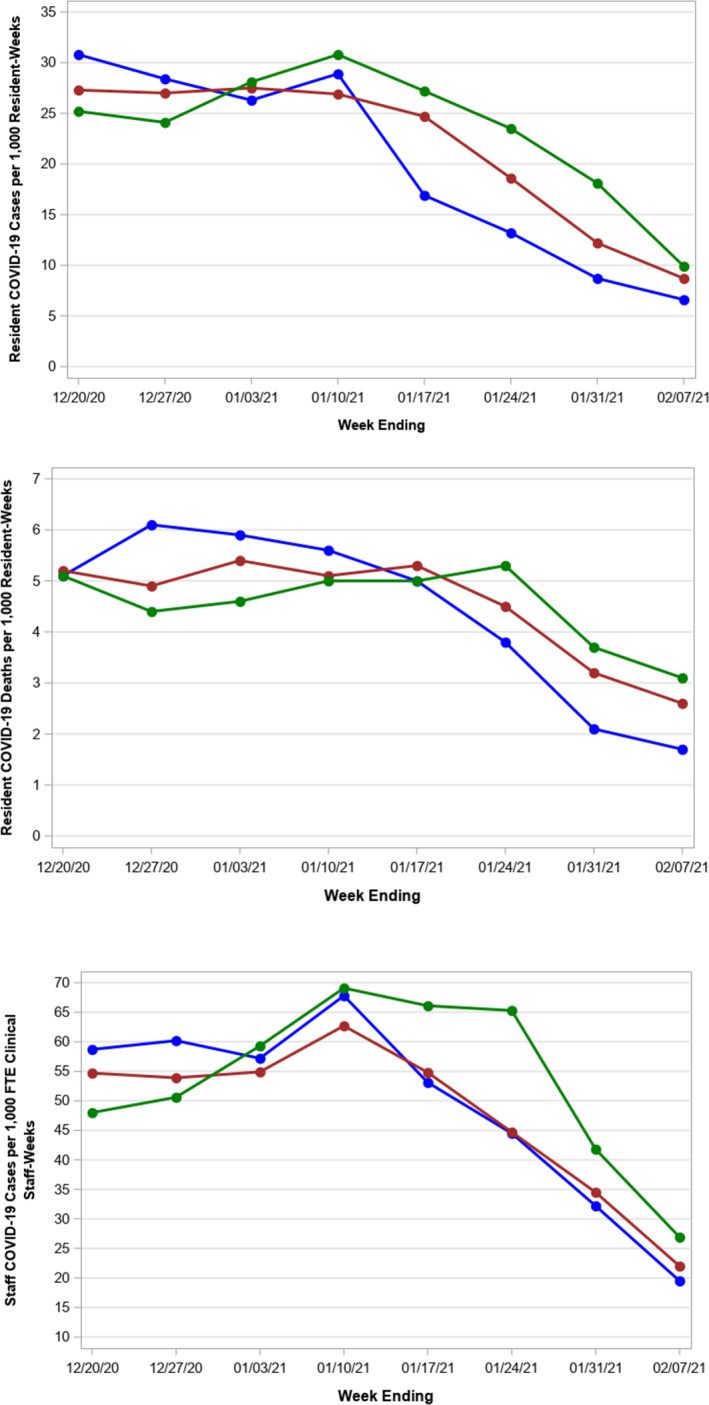
Unadjusted trends in resident and staff COVID‐19 cases and resident deaths from December 20, 2020, to February 7, 2021. Panel A, Resident COVID‐19 Cases per 1000 Residents‐Weeks. Panel B, Resident COVID‐19 Death per 1000 Residents‐Weeks. Panel C, Staff COVID‐19 Cases per 1000 FTE Clinical Staff‐Weeks. We divided SNFs into three cohorts based on the week in which they held their first vaccine clinic: Blue Line: Cohort 1 (vaccine clinics ending 12/27/2020, n = 840); Red Line: Cohort 2 (vaccine clinics ending 1/3/2021, n = 830); Green Line: Cohort 3 (vaccine clinics ending 1/10/2021, n = 831). Panel A, The unadjusted rate of COVID cases was stable in all three cohorts up until the week ending January 17th, 2021 when resident case of COVID‐19 began to decrease each week for all three cohorts. Panel B, The unadjusted resident COVID‐19 death was relatively stable and began to decline in all three cohorts starting the week ending January 24, 2021. Panel C, Depicts the trend in unadjusted staff Cases of COVID‐19 that were increasing through the end of January 10 but then began to decrease for SNFs in the blue and red cohorts, with the decline in cases lagging for the green cohort
Resident cases
In the fully adjusted multivariable ZINB model (Table S1), the delayed‐vaccination effect at 5 and 6 weeks after the vaccine clinic was associated with lower expected resident COVID‐19 counts per resident‐week by a factor of 0.68 (95% CI: 0.54–0.84) and 0.64 times (95% CI: 0.48–0.86) respectively (Figure 2). At 4 and 4 weeks after their initial vaccination clinic, facilities trended toward significance (IRR = 0.88, 95% CI: 0.77–1.10 and IRR = 0.84, 95% CI: 0.71–1.00, respectively). There was a significant independent association between time and resident COVID‐19 rates. In the 8 weeks of observation time, going from one calendar week to the next was associated with a decrease in expected resident counts by a factor of 0.93 (95% CI: 0.90–0.97), adjusting for all variables including vaccination status. Similarly, facilities with fewer than 50 certified beds and those with a high census of Hispanic non‐white residents were associated with fewer resident cases (IRR 0.66 [95% CI: 0.45–0.99] and 0.42 [95% CI: 0.31–0.56], respectively).
FIGURE 2.
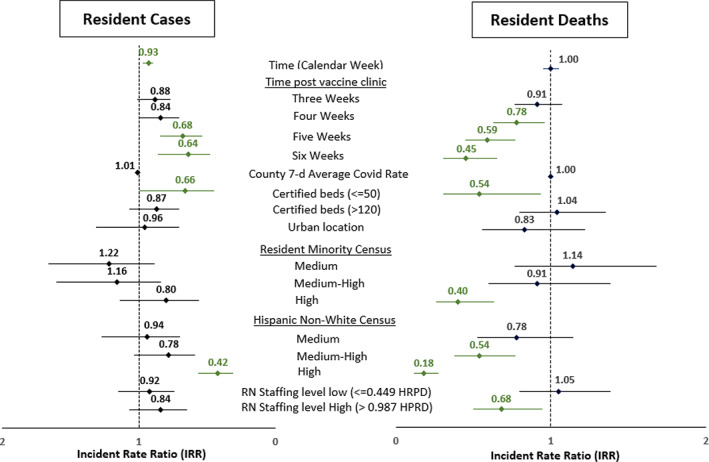
Resident COVID‐19 Cases and Deaths Incidence Rate Ratio from the Zero Inflated Negative Binomial Model. The incident rate ratio (IRR) with 95 confidence intervals in green depicts IRR whose 95% CI are less than 1.0 indicating an association with having fewer resident COVID‐19 cases or deaths. Low resident minority and low Hispanic, non‐white census, medium RN staffing level, and medium bed size were the referent groups. When controlling for all these variables that might explain trends in resident cases or deaths, time from the clinic was associated with fewer cases and deaths and had a stronger association as more time elapsed from the first clinic. In addition, time in calendar weeks was associated with fewer resident cases but not deaths corresponding to the general decline in cases seen nationally. Small facilities (less than 50 certified beds) and those with high Hispanic non‐white census were less likely to have resident cases and deaths. For resident deaths, higher nursing hours were also associated with less deaths
Resident deaths
In the fully adjusted multivariable models (available in supplemental Table S2) and depicted in Figure 2, the vaccination status following 4, 5, and 6 weeks were all associated with lower expected COVID resident mortality rates but not for 3 weeks after vaccine clinic. When controlling for all other variables, there was an independent association of fewer resident deaths and facilities with fewer than 50 certified beds (IRR: 0.54, 95% CI: 0.31–0.93), high census of racial and ethnic minorities (IRR: 0.40 [95% CI: 0.26–0.63] and 0.18 [95% CI: 0.12–0.27], respectively), and high RN staffing (>0.987 HPRD) (IRR: 0.68, 95% CI: 0.50–0.94). The other characteristics were not associated with resident deaths.
Staff cases
Figure 3 shows the significant associations of staff COVID‐19 cases with nursing homes for all time periods—3–6 weeks after clinic (see supplemental Table S3). For instance, facilities that were 3 weeks after vaccination clinic had 0.85 times the expected staff COVID rate of those that were not 3 weeks after clinic (95% CI: 0.78–0.93). The magnitude of the association increased with each vaccine status starting from 3 weeks after clinic to 6 weeks after vaccine clinic (IRRs: 0.85, 0.75, 0.64, and 0.51, for 3, 4, 5, and 6 weeks after clinic, respectively). Other factors independently associated with fewer staff COVID‐19 cases included larger facilities (IRR: 0.83, 95% CI: 0.74–0.94), high non‐white Hispanic resident census (IRR: 0.48, 95% CI: 0.40–0.57), and high level of registered nurses (IRR: 0.83, 95% CI: 0.72–0.96). Medium racial minority census, compared with low minority census, was associated with higher staff cases when controlling for all other factors (IRR: 1.20, 95% CI: 1.01–1.44).
FIGURE 3.
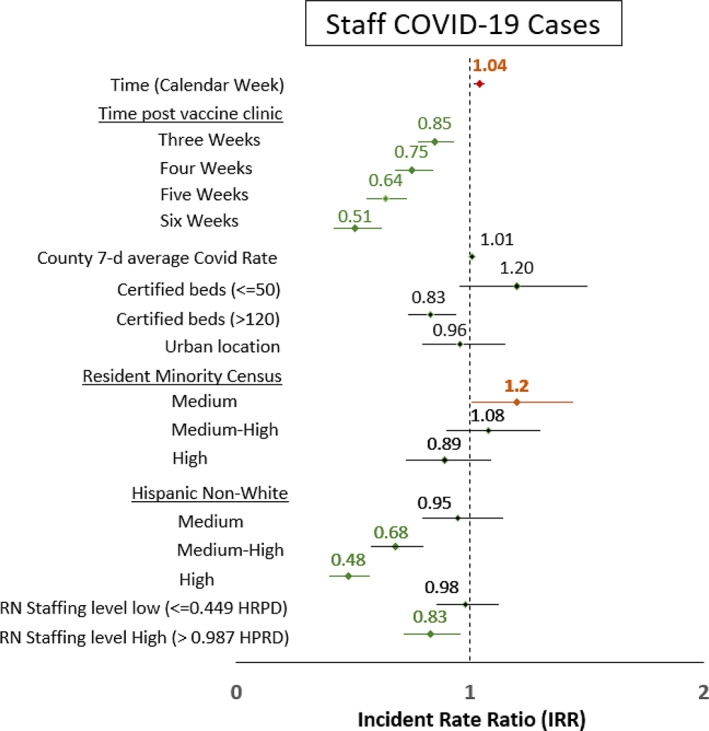
Staff COVID‐19 Cases Incidence Rate Ratio from the Zero Inflated Negative Binomial Model. The incident rate ratio (IRR) with 95 confidence intervals in green depicts IRR that is less than 1.0 indicating an association with having fewer resident COVID‐19 cases or deaths and IRRs in red indicate where 95% CI was greater than 1.0. Low resident minority and low Hispanic, non‐white census, medium RN staffing level, and medium bed size were the referent groups. When controlling for all these variables that might explain trends in resident cases of deaths, time from the clinic was associated with fewer staff cases and had a stronger association as more time elapsed from the first clinic. In addition, time in weeks was associated with more cases as was facilities with medium census of racial minorities compared with low census. However, facilities with higher Hispanic non‐white census were associated with fewer staff cases as were facilities with high nursing staffing levels
DISCUSSION
This analysis is the first look at the relationship between the COVID‐19 vaccine and spread in nursing homes in the first states to initiate the LTC Pharmacy Partnership. We found that across all three adjusted outcomes, time following the vaccine clinic was independently associated with fewer cases and deaths. The largest associations were observed at 5 and 6 weeks after the first clinic. Given that Cohort 1 and 2 facilities had clinics at earlier time periods, allowing for greater follow‐up at T + 5 and T + 6, it is possible that this association was strengthened by both more time for antibody production and for facilities to have an additional vaccination clinic. Unfortunately, second clinic data were not available to allow for disentangling this association. Nonetheless, controlling for time and other characteristics, time from the first clinic was associated with fewer cases and deaths. This association was consistent with recent findings that one dose of the COVID‐19 vaccine among health care workers had an 80% effectiveness for preventing COVID‐19 infections (e.g., testing positive for COVID‐19) 11 and similar to findings from Israel looking at the vaccine effectiveness in the general population. 10
Because COVID‐19 resident infection rates were declining in the community before and during the Pharmacy Partnership vaccinations, we introduced the effect of time, or calendar weeks, in the model. Time in calendar weeks was associated with lower resident COVID‐19 case counts and higher staff COVID‐19 case counts, adjusting for other factors including vaccination status. This supports that some of the decline in cases and deaths was due to the temporal changes in cases independent of vaccine clinics.
Other facility characteristics were associated with the number of resident cases and deaths as seen in prior research. 5 For example, we found that smaller facility size, higher proportion of residents identifying as racial and ethnic minority, and higher number of registered nurses were independently associated with lower resident COVID‐19 case and death rates, after adjusting for all other factors including vaccination status. The association of racial and ethnic minority census is contrary to findings from earlier in the pandemic where facilities with higher proportion of ethnic minorities experienced higher rates of COVID‐19 infections. 27 However, our results are consistent with recent published literature using data from the end of 2020, corresponding to our time period, showing that there were higher deaths in facilities with higher proportion of residents identifying as white. 33 As we found, smaller bed size has been shown to be associated with fewer COVID‐19 outbreaks, most likely due to the fewer number of people interacting with each other to spread the virus. 5 Also, as seen by others, higher nursing staffing levels were associated with fewer number of COVID‐19 deaths. 34 , 35
The rate of infection in the county has been previously shown to be one of the strongest predictors of resident and staff cases of COVID‐19. 4 , 6 However, we did not find such an association. When creating our analytic cohorts, we included only facilities in the same counties as the 840 facilities that held initial clinics in the first week of the Pharmacy Partnership. This may explain why the county 7‐day average rate of COVID‐19 was not associated with cases in our model.
Our ecologic analysis has limitations. We could only compare counts at the facility level without knowing the vaccination status of individuals or the facility's overall vaccination rates as that data are not available. The vaccination rates among staff have been previously shown to be lower than among residents. 13 However, there is wide variation in vaccination rates for both staff and residents, and these data were not available for this analysis. Ideally, evaluations should compare the effect of vaccination status for individual‐level residents or staff on the rate of COVID‐19 infections over time. Our findings would be bolstered if there is a stronger association in COVID‐19 infections and facilities with higher vaccination rates of staff and residents than those with lower vaccination rates.
Differences in COVID‐19 testing may also contribute to the differences, if following vaccination clinics, facilities tested staff and residents less frequently. Although we could not control for the frequency of testing for COVID‐19 among residents and staff, a benefit of including only LTC facilities in the same counties in our sample is that the testing frequency for COVID‐19 should be the same in all the facilities. CMS requires all nursing homes to test residents and staff at the same frequency dictated by the rate of COVID‐19 in the community not in the facility. 16 , 18 This CMS guidance on testing frequency issued in August 2020 has not been updated and remains in effect throughout the follow‐up period in our study. As such, the rate of testing should be similar in facilities with vaccine clinics and those without vaccine clinics that are in the same counties.
It is possible that staff work at multiple facilities in the same community or received their vaccine from other locations because as healthcare workers they were prioritized for vaccination in all states. Thus, facilities may have benefitted from staff being vaccinated earlier in a different facility, so that staff in facilities in cohorts 2 and 3 may have been vaccinated before their facility's initial vaccine clinic. 36 However, because individual‐level staff vaccination information was not available, we were not able to further disentangle this relationship.
Evaluation on vaccine effectiveness in the LTC setting needs to be a priority. Without evidence on the vaccine effectiveness in long‐term care on spread, morbidity, and mortality, policies intended to prevent spread of COVID‐19 such as limitations on visitors, communal dinning, group activities, and community outings may continue. 37 , 38 The unintended effect these policies have had on the residents in LTC facilities support the need to rapidly determine when these policies can be safely lifted. 39 , 40
Our finding may signal that the mRNA vaccines are associated with lower COVID‐19 counts, but further study of individual vaccination rates are necessary to determine if the vaccine prevents spread. Data and funding to complete more in‐depth analyses need to be made available to the research community to determine if these ecological findings showing an association with decreased COVID‐19 counts hold after the second and third vaccine clinics across the country.
CONFLICT OF INTEREST
David Gifford reports no financial conflicts of interest. He reports the following potential personal conflicts of interests: his spouse serves in state government as acting Commissioner of Public Health and Commissioner of Social Services in the state of Connecticut where she is responsible for the COVID‐19 vaccine program, nursing home licensure, and Medicaid. The other authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Study Design: Domi, Gifford, Leitson, and Sreenivas. Methods: Domi and Nicolaou. Datasets: Leitson and Domi. Analysis: Domi. Figures: Leitson and Gifford. Interpretation: All authors. Manuscript Writing: Domi and Gifford.
SPONSOR'S ROLE
AHCA/NCAL did not have any role in the design, methods, analysis, or preparation of the paper. The findings, conclusions, and recommendations do not necessarily represent the position of AHCA/NCAL.
Supporting information
Table S1: Adjusted Resident COVID‐19 Log‐Linear Estimates of the Zero Inflated Negative Binomial Model. Log‐linear estimates modeling the adjusted effect of the independent indicators on the resident COVID‐19 case counts per resident‐weeks with a random intercept for each cluster‐facility in the negative binomial state.
Table S2: Adjusted Resident COVID‐19 Mortality Log‐Linear Estimates of the Zero Inflated Negative Binomial Model. Log‐linear estimates modeling the adjusted effect of the independent indicators on the resident COVID‐19 mortality counts per resident‐weeks with a random intercept for each cluster‐facility in the negative binomial state.
Table S3: Adjusted Staff COVID‐19 Log‐Linear Estimates of the Zero Inflated Negative Binomial Model. Log‐linear estimates modeling the adjusted effect of the independent indicators on the staff COVID‐19 case counts per FTE staff‐weeks with a random intercept for each cluster‐facility in the negative binomial state.
ACKNOWLEDGMENTS
The authors would like to thank the entire staff in the CDC Division of Healthcare Quality Promotion team who provided help understanding the vaccination schedules and NHSN data and Anita Patel, Ruth Link‐Gelles, Angela Guo, and Radhika Gharpure who designed and oversaw the Pharmacy Partnership for Long‐Term Care Program that made the immunization clinics possible. The authors would like to thank Dr. Roee Gutman for his advice on the statistical approach and Terry Hawk for assistance in creating the figures. The authors also would like to thank all the CVS, Walgreens, and Managed Health Care Associates, Inc. pharmacists, and other personnel who conducted the immunization clinics.
Domi M, Leitson M, Gifford D, Nicolaou A, Sreenivas K, Bishnoi C. The BNT162b2 vaccine is associated with lower new COVID‐19 cases in nursing home residents and staff. J Am Geriatr Soc. 2021;69(8):2079–2089. 10.1111/jgs.17224
See related editorial by Ouslander et al and related articles by Mor et al, Moore et al, and Rudolph et al. in this issue.
Preliminary results were posted on February 4, 2021 as a report on AHCA's website: https://www.ahcancal.org/Data-and-Research/Center-for-HPE/Documents/CHPE-Report-Vaccine-Effectiveness-Feb2021.pdf.
REFERENCES
- 1. NYT . More than one‐third of U.S. coronavirus deaths are linked to nursing homes. New York Times. February 2, 2021. https://www.nytimes.com/interactive/2020/us/coronavirus-nursing-homes.html.
- 2. Comas‐Herrera A, Zalakaín J, Lemmon E, et al. Mortality associated with COVID‐19 outbreaks in care homes: early international evidence. International Long‐Term Care Policy Network. 2020. October 14, 2020. https://ltccovid.org/2020/04/12/mortality-associated-with-covid-19-outbreaks-in-care-homes-early-international-evidence/.
- 3. Chidambaram P, Garfield R, Neuman T. COVID‐19 has claimed the lives of 100,000 Long‐term care residents and staff. Kaiser Family Foundation. November 25, 2020. https://www.kff.org/policy‐watch/covid‐19‐has‐claimed‐the‐lives‐of‐100000‐long‐term‐care‐residents‐and‐staff/.
- 4. Chidambaram P, Garfield R. Patterns in COVID‐19 cases and deaths in long‐term care facilities in 2020. Kaiser Family Foundation. January 14, 2021. https://www.kff.org/coronavirus‐covid‐19/issue‐brief/patterns‐in‐covid‐19‐cases‐and‐deaths‐in‐long‐term‐care‐facilities‐in‐2020/.
- 5. Abrams H, Loomer L, Gandhi A, Grabowski D. Characteristics of U.S. nursing homes with COVID‐19 cases. J Am Geriatr Soc. 2020;68(8):1653‐1556. 10.1111/jgs.16661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bagchi S, Mak J, Li Q, et al. Rates of COVID‐19 Among Residents and Staff Members in Nursing Homes ‐ United States, May 25 ‐ November 22, 2020. Morbid Mortal Wkly Rep: Centers Dis Control Prev. 2021;70(2):52‐55. https://www.cdc.gov/mmwr/volumes/70/wr/mm7002e2.htm?s_cid=mm7002e2_w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polack F, Thomas S, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA‐1273 SARS‐CoV‐2 Vaccine. N Engl J Med. 2021;384(5):403‐416. 10.1056/nejmoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inc P. FDA Decisional Memorandum. Emergency Use Authorization (EUA) for an Unapproved Product Review Memorandum; 2020. Accessed Feb 1, 2021. https://www.fda.gov/media/144416/download.
- 10. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid‐19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412‐1423. 10.1056/nejmoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thompson M, Burgess J, Naleway A, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA‐1273 COVID‐19 vaccines in preventing SARS‐CoV‐2 infection among health care personnel, first responders, and other essential and frontline workers—eight U.S. locations, December 2020–march 2021. Morbid Mortal Wkly Rep (MMWR). 2021;70(13):495‐500. 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on immunization practices' Interim recommendation for allocating initial supplies of COVID‐19 vaccine—United States, 2020. Morbid Mortal Wkly Rep: Centers Dis Control Prev. 2020;69(49):1857‐1859. https://www.cdc.gov/mmwr/volumes/69/wr/mm6949e1.htm?s_cid=mm6949e1_w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gharpure R, Guo A, Bishnoi CK, et al. Early COVID‐19 first‐dose vaccination coverage among residents and staff members of skilled nursing facilities participating in the pharmacy partnership for long‐term care program — United States, December 2020–January 2021. Morbid Mortal Wkly Rep: Centers Dis Control Prev. 2021;70(5):178‐182. https://www.cdc.gov/mmwr/volumes/70/wr/mm7005e2.htm?s_cid=mm7005e2_w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Self WH, Tenforde MW, Stubblefield WB, et al. Decline in SARS‐CoV‐2 antibodies after mild infection among frontline health care personnel in a multistate hospital network—12 states, April–August 2020. Morbid Mortal Wkly Rep; Centers Dis Control Prev. 2020;69(47):1762‐1766. https://www.cdc.gov/mmwr/volumes/69/wr/mm6947a2.htm?s_cid=mm6947a2_w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. CDC . Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID‐19). Centers Dis Control Prev. 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. [Google Scholar]
- 16. Wright DR. Interim Final Rule (IFC), CMS‐3401‐IFC, Additional Policy and Regulatory Revisions in Response to the COVID‐19 Public Health Emergency related to Long‐Term Care (LTC) Facility Testing Requirements and Revised COVID‐19 Focused Survey Tool Center for Clinical Standards and Quality/Survey & Certification Group. QSO‐20‐38‐NH. CMS; 2020. https://www.cms.gov/files/document/qso-20-38-nh.pdf.
- 17. CMS . Data from: COVID‐19 Nursing Home Data; 2021. https://data.cms.gov/stories/s/COVID-19-Nursing-Home-Data/bkwz-xpvg. Deposited Feb 25 2021.
- 18. Wright DR. Upcoming Requirements for Notification of Confirmed COVID‐19 (or COVID19 Persons under Investigation) Among Residents and Staff in Nursing Homes. Center for Clinical Standards and Quality/Quality, Safety & Oversight Group. QSO‐20‐26‐NH. CMS; 2020. April 19, 2020. https://www.cms.gov/files/document/qso-20-26-nh.pdf.
- 19. CMS . Data from: Nursing Homes Including Rehab Services. Care Compare. 2021. https://data.cms.gov/provider-data/topics/nursing-homes. Deposited Jan 27, 2021.
- 20. CMS . Data from: PBJ Daily Nurse Staffing CY 2020Q3. Payroll‐Based J. 2021. https://data.cms.gov/Special-Programs-Initiatives-Long-Term-Care-Facili/PBJ-Daily-Nurse-Staffing-CY-2020Q3/2qky-49qq. Deposited Jan 5 2021.
- 21. CMS . Provider of Services Current Files. https://www.cms.gov/Research-Statistics-Data-and-Systems/Downloadable-Public-Use-Files/Provider-of-Services.
- 22. Affairs USDoVs . Data from: Skilled Nursing Facilities with Contracts with the U.S. Dept of Veteran's Affairs; 2018.
- 23. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20(5):533‐534. 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. ResDAC . Data from: Long Term Care Minimum Data Set (MDS) 3.0. 2021. https://www.resdac.org/cms-data/files/mds-3.0/data-documentation.
- 25. Saliba D, Buchanan J. Making the investment count: revision of the minimum data set for nursing homes, MDS 3.0. J Am Med Direct Assoc. 2012;13(7):602‐610. 10.1016/j.jamda.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 26. Saliba D, Jones M, Streim J, Ouslander J, Berlowitz D, Buchanan J. Overview of significant changes in the minimum data set for nursing homes version 3.0. J Am Med Direct Assoc. 2012;13(7):595‐601. 10.1016/j.jamda.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 27. Li Y, Cen X, Cai X, Temkin‐Greener H. Racial and ethnic disparities in COVID‐19 infections and deaths across U.S. nursing homes. J Am Geriatr Soc. 2020;68(11):2454‐2461. 10.1111/jgs.16847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS‐CoV‐2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hughes JP, Granston TS, Heagerty PJ. Current issues in the design and analysis of stepped wedge trials. Contemp Clin Trials. 2015;45:55‐60. 10.1016/j.cct.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu H, Luo S, DeSantis SM. Zero‐inflated count models for longitudinal measurements with heterogeneous random effects. Stat Methods Med Res. 2017;26(4):1774‐1786. 10.1177/0962280215588224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu M‐C, Pavlicova M, Nunes EV. Zero‐inflated and hurdle models of count data with extra zeros: examples from an HIV‐risk reduction intervention trial. Am J Drug Alcohol Abuse. 2011;37(5):367‐375. 10.3109/00952990.2011.597280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kurada RR. Fitting Multilevel Hierarchical Mixed Models Using PROC NLMIXED. SAS Institute, Inc. 2016 (Paper SAS4720‐2016). https://support.sas.com/resources/papers/proceedings16/SAS4720‐2016.pdf.
- 33. Kumar A, Roy I, Karmarkar AM, et al. Shifting US patterns of COVID‐19 mortality by race and ethnicity from June–December 2020. J Am Med Direct Assoc. 2021. 10.1016/j.jamda.2021.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Y, Temkin‐Greener H, Shan G, Cai X. COVID‐19 infections and deaths among Connecticut nursing home residents: facility correlates. J Am Geriatr Soc. 2020;68(9):1899‐1906. 10.1111/jgs.16689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gorges RJ, Konetzka RT. Staffing levels and COVID ‐19 cases and outbreaks in U.S. nursing homes. J Am Geriatr Soc. 2020;68(11):2462‐2466. 10.1111/jgs.16787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen MK, Chevalier JA, Long EF. Nursing home staff networks and COVID‐19. Proc Natl Acad Sci. 2021;118(1):e2015455118. 10.1073/pnas.2015455118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wright DR. Nursing Home Visitation—COVID‐19. Center for Clinical Standards and Quality/Quality, Safety & OversightGroup. QSO‐20‐39‐NH. CMS; 2020. https://www.cms.gov/files/document/qso-20-39-nh.pdf.
- 38. CDC . Preparing for COVID‐19 in Nursing Homes; 2020. Accessed March 7 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/long-term-care.html.
- 39. O'Caoimh R, O'Donovan MR, Monahan MP, et al. Psychosocial impact of COVID‐19 nursing home restrictions on visitors of residents with cognitive impairment: a cross‐sectional study as part of the engaging remotely in care (ERiC) project. Front Psychiatry. 2020;11:1115. 10.3389/fpsyt.2020.585373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Verbeek H, Gerritsen DL, Backhaus R, de Boer BS, Koopmans RTCM, Hamers JPH. Allowing visitors Back in the nursing home during the COVID‐19 crisis: a Dutch National Study into First Experiences and impact on well‐being. J Am Med Dir Assoc. 2020;21(7):P900‐P904. 10.1016/j.jamda.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Adjusted Resident COVID‐19 Log‐Linear Estimates of the Zero Inflated Negative Binomial Model. Log‐linear estimates modeling the adjusted effect of the independent indicators on the resident COVID‐19 case counts per resident‐weeks with a random intercept for each cluster‐facility in the negative binomial state.
Table S2: Adjusted Resident COVID‐19 Mortality Log‐Linear Estimates of the Zero Inflated Negative Binomial Model. Log‐linear estimates modeling the adjusted effect of the independent indicators on the resident COVID‐19 mortality counts per resident‐weeks with a random intercept for each cluster‐facility in the negative binomial state.
Table S3: Adjusted Staff COVID‐19 Log‐Linear Estimates of the Zero Inflated Negative Binomial Model. Log‐linear estimates modeling the adjusted effect of the independent indicators on the staff COVID‐19 case counts per FTE staff‐weeks with a random intercept for each cluster‐facility in the negative binomial state.


