Abstract
The most consequential challenge raised by coinfection is perhaps the inappropriate generation of recombinant viruses through the exchange of genetic material among different strains. These genetically similar viruses can interfere with the replication process of each other and even compete for the metabolites required for the maintenance of the replication cycle. Due to the similarity in clinical symptoms of most viral respiratory tract infections, and their coincidence with COVID‐19, caused by SARS‐CoV‐2, it is recommended to develop a comprehensive diagnostic panel for detection of respiratory and nonrespiratory viruses through the evaluation of patient samples. Given the resulting changes in blood markers, such as coagulation factors and white blood cell count following virus infection, these markers can be of diagnostic value in the detection of mixed infection in individuals already diagnosed with a certain viral illness. In this review, we seek to investigate the coinfection of SARS‐CoV‐2 with other respiratory and nonrespiratory viruses to provide novel insights into the development of highly sensitive diagnostics and effective treatment modalities.
Keywords: COVID‐19, diagnosis, mixed infection, SARS‐CoV‐2, viral coinfection, viral respiratory tract infection, virus infection
1. INTRODUCTION
In December 2019, the first cases of Coronavirus Disease 2019 (COVID‐19) were reported in Wuhan, China. Soon after, the rapid spread of COVID‐19 in the world prompted nations to recognized it as a global threat. The causative virus, Severe Acute Respiratory Coronavirus 2 (SARS‐CoV‐2), had infected 109,594,835 people as of May 7, 2020, resulting in 2,424,060 deaths.1, 2, 3 The substantial disease burden associated with COVID‐19 necessitated the development of novel techniques for detection of SARS‐CoV‐2 and repurposing of drugs with potential therapeutic value, including lopinavir, ritonavir, chloroquine/hydroxychloroquine, oseltamivir, darunavir, zanamivir, peramivir, and remdesivir.1, 4, 5, 6
Transmitted primarily through respiratory droplets released into the air upon coughing or sneezing, SARS‐CoV‐2 can frequently lead to pneumonia in individuals exposed to a sufficient amount of viral particles.7, 8 The virus is believed to target the upper respiratory tract, in particular, with a natural history characterized by high‐grade fever, cough, and shortness of breath. Following the acute phase of COVID‐19, some patients may experience clinical symptoms, such as nausea, exhaustion, headache, and low‐grade fever.9, 10 Beside SARS‐CoV‐2, infection with other viruses, including the influenza viruses, rhinoviruses, adenoviruses, human metapneumoviruses, and other coronaviruses (CoVs), namely, 229E, OC43, NL63, and HKU1, can also manifest as regular symptoms of the common cold. 11 Other notable CoVs, including the SARS‐CoV and the Middle East Respiratory Syndrome Coronavirus (MERS‐CoV), which tend to target the lower respiratory tract, can also result in pneumonia and death.12, 13 With a 79% genetic sequence identity, SARS‐CoV and SARS‐CoV‐2 are remarkably similar strains.12, 14 Furthermore, SARS‐CoV‐2 infection may occur in parallel to SARS‐CoV, leading to the activation of inflammatory responses that result in lung damage. The severity of the disease is distinguished by the host immune response to the virus infection.15, 16, 17
To date, several instances of SARS‐CoV and MERS‐CoV mixed infection have been reported; however, in the case of COVID‐19, coinfection is less frequent. 18 The prevalence of coinfection in different individuals with COVID‐19 is thought to vary to a significant extent as only 50% of deaths associated with COVID‐19 are suspected to stem from mixed infection. 18 Coinfection can result in the inhibition of host immune response, resistance to antibacterial drugs, and an overall poor prognosis of the disease. 19 One study discovered that 94.2% of people with COVID‐19 were also coinfected with several other microorganisms, such as viruses, bacteria, and fungi. 20 Important viral copathogens include the influenza A and B viruses, rhinovirus/enterovirus, parainfluenza virus, metapneumovirus, respiratory syncytial virus, human immunodeficiency virus (HIV), dengue virus (DENV), hepatitis B virus (HBV), cytomegalovirus (CMV), Epstein Barr virus (EBV), and other CoVs, among which the influenza A virus and rhinovirus/enterovirus are the most common copathogens.18, 21 Nonetheless, due to the remarkable advances made in the diagnosis and treatment, influenza is significantly less associated with mortality. 22
Interestingly, the SARS‐CoV‐2 epidemic in December 2019 coincided with the seasonal outbreak of the influenza virus. 22 An investigation by Xia et al. 23 found high levels of serum procalcitonin in pediatric patients with COVID‐19 and other common respiratory infections, suggesting that coinfection with other pathogens might have been responsible for the enhanced inflammatory response. A significant increase in the serum levels of D‐dimer (>1 mcg/ml), LDH (lactate dehydrogenase; >350 U/L), and ferritin and troponin (>1000 ng/ml), along with a decline in the lymphocyte count to less than 800, stand among notable laboratory findings in patients with COVID‐19, which could be helpful in determining the prognosis of the disease24, 25 (Table 1). Nonetheless, there is no definite knowledge regarding the exact coincidence of other diseases with COVID‐19. 26 With an emphasis on potential coinfections with SARS‐CoV‐2, the present review seeks to provide a more clear perspective to facilitate the development of strategies for diagnosis and containment of the disease.18, 27
Table 1.
Similar blood markers in viral disease
| Serum biomarker for COVID‐19 | Prognostic value | Viral coinfection | Correlation with coinfections |
|---|---|---|---|
| C‐reactive protein | Increased levels, especially in the early stages of the disease indicate an acute risk of pulmonary lesions and infection.23, 28 | ||
| IL‐6 and TNF‐α | Increased levels predict the progression of the disease to severe forms. 29 | DENV 30 CMV 31 | Increased in coinfections |
| Lymphocyte count | Evaluation of the severity of the disease and the indication of hospitalization in the intensive care unit. It is less than 5% of the normal value in patients with a critical illness. 32 | HIV, 33 BV, 34 DENV | Decreased counts determine lymphopenia 35 |
| LDH | Increased levels are reported in mild and severe cases of COVID‐19, especially in coinfection with adenovirus.36, 37, 38 | Adenovirus | Increased levels along with D‐dimer and ferritin in coinfections |
| D‐dimer | Increased levels are reported in vasculopathies and coinfection with adenovirus.36, 39 | Adenovirus 36 | |
| Procalcitonin | Prompts immune response against other viruses. 23 | Respiratory infection 23 | Increased in coinfection |
| Creatine kinase‐MB | Increased levels are reported in COVID‐19 patients with kidney failure40, 41 | CMV 41 | Elevated along with ALT and IL‐6, IL‐10 and TNF‐α in coinfections. |
| Platelet count | Predicts development of thrombocytopenia in rare cases and subsequent myocardial injury. 42 | DENV 43 | A decreased platelet count is associated with thrombocytopenia in dengue virus coinfection. |
Abbreviations: CMV, cytomegalovirus; DENV, dengue virus; EBV, Epstein Barr virus; HBV, hepatitis B virus; HIV, human immunodeficiency virus; IL, interleukin, LDH, lactate dehydrogenase; TNF‐α, tumor necrosis factor‐α.
1.1. Coinfection with respiratory viruses and SARS‐CoV‐2
Identifying coinfections of more than one respiratory virus can help with understanding the various clinical symptoms, long‐lasting effects on health, and appropriate methods of prevention. In the case of viral respiratory tract infections resulting in pneumonia, a mixed infection can lead to serious disease in individuals with a suppressed immune system. 44 There have been reports of disease exacerbation and hospitalization in children under 3 years of age, following simultaneous infection with RSV and Human metapneumovirus (hMPV). 45 Furthermore, patients with viral respiratory tract coinfections are more likely to be admitted to the hospital than individuals with a single respiratory virus infection. 46 Moreover, due to the similarity of common clinical symptoms of COVID‐19 with that of the diseases caused by other respiratory viruses, it has become quite challenging to precisely distinguish SARS‐CoV‐2 infection from other viral infections. 47 It is thought that coinfection with common respiratory viruses can still occur in individuals infected with other respiratory‐specific strains. 48 Mixed infection of SARS‐CoV‐2 with other respiratory viruses has been reported to be uncommon, with a rate of 1.4%.27, 49, 50 It is speculated that the spread of another infection through aerosol production as a result of the respiratory symptoms of different pathogens might increase the likelihood of coinfection. 48 Thus, there are discussions about the utilization of routine tests, that may greatly help to detect SARS‐CoV‐2 and other respiratory viruses in cases suspected to have been coinfected with SARS‐CoV‐2 and another pathogen of the respiratory tract.51, 52 This has led to concerns about coinfection of SARS‐CoV‐2 with other respiratory microorganisms, especially unusual pathogens.49, 53 Moreover, the severity of COVID‐19, in cases of mixed infection with other respiratory viruses, is correlated with lymphocyte count and serum levels of D‐dimer. 54 High rates of interleukin (IL)‐6, IL‐10, and tumor necrosis factor‐α (TNF‐α) were also observed in high‐risk groups; thus, the severity of COVID‐19 disease can be assessed according to the levels of IL‐10 and TNF‐α.7, 17, 55
It has been suggested that the incidence of coinfection with influenza virus in COVID‐19 patients is lower than human adenovirus (HAdV) and human rhinovirus (HRV), which is also associated with lower mortality. In spite of low mortality, coinfection with influenza virus may result in a substantial economic burden due to the adverse effects accompanied by either infection. 20 The effect of the mixed infection of EBV and herpes simplex virus in COVID‐19 still remains unclear. 20 CMV, human bocavirus, and hMPVs, the major causes of viral respiratory tract infection in children, are too different in terms of pathogenesis to be missed in COVID‐19 patients 20 (Figure 1). More importantly, coinfection of SARS‐CoV‐2 with other upper and lower respiratory tract viruses can result in overlapping of clinical symptoms, and make it difficult for clinicians to rule out other infections. 20 As a result, infection with other viruses should also be appraised during the diagnosis and treatment of COVID‐19. In general, coinfection could be associated with a diminished immune response due to an already present SARS‐CoV‐2 virus infection. 20
Figure 1.
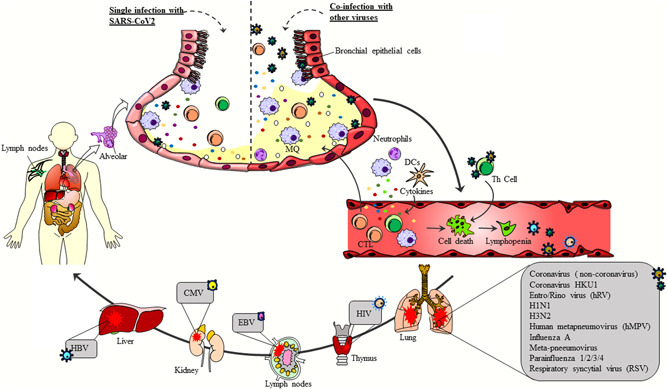
Coinfection of COVID‐19 with other viruses. Coinfection of SARS‐CoV‐2 with other respiratory viruses, such as Flu, HRV, hMPV, RSV, and parainfluenza viruses, as well as systemic viruses, such as HIV, HBV, and CMV, can aggravate the clinical symptoms of the disease, increase the migration of inflammatory cells, such as macrophages and neutrophils, to the site of infection, and ultimately elevate the secretion of proinflammatory cytokines in organs infected with specific pathogens. Therefore, these events might lead to a severe form of the disease. In contrast, SARS‐CoV‐2 infection alone may only result in mild or absent clinical symptoms, as is the case with asymptomatic patients. In this respect, identification of coinfection of SARS‐CoV‐2 with other viruses can contribute to the development of novel approaches for the treatment of COVID‐19. CMV, cytomegalovirus; HBV, hepatitis B virus; HIV, human immunodeficiency virus; HRV, human rhinovirus; hMPV, human metapneumovirus; SARS‐CoV‐2, severe acute respiratory Coronavirus 2
1.2. Coinfection with influenza virus and SARS‐CoV‐2
Epidemiological data from 2014 to 2020 were investigated by Sakamoto et al.22, 56 in a study, which suggested a lower incidence of influenza in 2020, compared to the years before, indicating different levels of seasonal influenza virus infection with SARS‐CoV‐2. SARS‐CoV‐2 and influenza virus‐mixed infection were found to be common during the COVID‐19 outbreak. Individuals coinfected with SARS‐CoV‐2 and influenza B were indicated to be affected by less severe complications compared with the coinfection of SARS‐CoV‐2 and influenza A virus, which was also found to be common. 57 Accordingly, coinfection with influenza A virus, an important respiratory tract pathogen, during the COVID‐19 outbreak, might lead to false‐negative results in individuals with SARS‐CoV‐2 infection. 18 Nevertheless, a positive result for influenza A infection in patients with underlying COVID‐19 can also bring about particular challenges in terms of diagnosis 47 asa clinical suspicion for COVID‐19 is more likely to be neglected once an individual has been tested positive for influenza A virus. 57 Therefore, studying the clinical features and effects of coinfection in COVID‐19 patients is important. A recent investigation on coinfection of SARS‐CoV‐2 with influenza viruses revealed a significantly high prevalence for these strains (Influenza A: 49.8% and Influenza B: 7.5%) during the initial phase of the outbreak of COVID‐19 in Wuhan, with slightly different frequencies of mixed infection with Influenza A (0.9%) and Influenza B (0%) in a 3‐week period (March 3–25).44, 57 Studies have also shown that clinical symptoms and transmission dynamics are highly similar in patients with SARS‐CoV‐2 infection alone, and those with influenza virus coinfection.58, 59 As a result, in patients with coinfection, rapid identification of the influenza virus might be of great diagnostic value in the case of simultaneous SARS‐CoV‐2 infection. 57 Also, in a study conducted by Hashemi et al., 60 it was reported that in patients with mixed infection of SARS‐CoV‐2 and influenza virus, the presence of underlying diseases, such as heart disease, asthma, diabetes, and chronic neurological pathologies might lead to increased mortality. Arguably, patients with SARS‐CoV‐2 and influenza B virus coinfection are more likely to develop a chronic disease compared to individuals with influenza A virus coinfection. 57 In this regard, Yue et al. 57 showed that coinfection of SARS‐CoV‐2 and influenza B virus, unlike SARS‐CoV‐2 and influenza A virus, might accentuate the symptoms and result in chronic illness. Along the similarities of respiratory symptoms caused by both SARS‐CoV‐2 and influenza viruses, such as high‐grade fever, cough, headache, and pneumonia, the pathogenicity of SARS‐CoV‐2 overlaps with that of the influenza virus in winter, leading to a rise in the number of individuals with undetected coinfection.27, 47, 61, 62, 63 Nevertheless, SARS‐CoV‐2 and influenza virus infection still differ in terms of epidemiology and clinical manifestations, due chiefly to immunity, as infection with the latter usually results in asymptomatic to moderate illness. COVID 19 patients, on the contrary, experience complications for 5 to 7 days, and develop a serious disease.64, 65, 66 Furthermore, acute respiratory distress syndrome (ARDS) is uncommon in influenza outbreaks, with a mortality rate of less than 1%. In COVID 19, on the contrary, ARDS is more prevalent, with a mortality rate of 3%–4%. Besides, the span of virus shedding in influenza‐related illnesses is 5–10 days, whereas it is 2–5 weeks in SARS‐CoV‐2 infection.67, 68
Moreover, hyperinflammation, as well as disorders, such as shock, ARDS, myocarditis, acute kidney injury, and dysfunction of other organs are found in COVID‐19 patients with influenza virus coinfection, owing to the stronger and more frequent activation of the cytokine cascade caused by the flu virus infection.25, 69, 70, 71
As early clinical symptoms of COVID‐19 disease and the flu are so similar, false negatives in the diagnosis of COVID‐19 might increase, resulting in increased, albeit overlooked, viral shedding. Furthermore, proinflammatory cytokine levels in the blood have been shown to rise considerably in these patients,35, 71 leading to serious pulmonary sequelae, as well as increased neutrophil and macrophage penetration into the infected region. 71 Furthermore, individuals with ARDS have a higher number of neutrophils in their blood and lungs.72, 73 Increased neutrophil penetration into the pulmonary tissue, on the contrary, has been linked to lung injury and exacerbation of clinical symptoms in influenza patients. 74 In a study by Ma et al., 69 neutrophil levels and proinflammatory cytokines were shown to be slightly higher in the deceased than in the recovered cases. They also discovered that the amount of d‐dimer, which is a risk factor for mortality, was considerably higher in deceased patients than in recovered individuals. 69 Furthermore, d‐dimer levels were slightly higher in flu patients than non‐flu patients, indicating that these patients had died as the result of local vascular damage, ischemia, and thrombosis caused by a cytokine cascade triggered by virus infection. 75 Besides, according to a report by Wang et al., 76 the frequency of coinfection with other CoVs and influenza A virus was 2.88% and 1%, respectively, suggesting that SARS‐CoV‐2 coinfection with influenza A, influenza B, and other CoVs might rather be uncommon. Nevertheless, coinfection with influenza A and B viruses might enhance the incidence of COVID‐19.76, 77 ARDS is one of the primary respiratory sequelae of SARS‐CoV‐2, influenza A (H1N1 and H3N2) and influenza B infection, which have resulted in major pandemics through the last century.77, 78, 79 Due to the similarities between the clinical manifestations of SARS‐CoV‐2 and the influenza virus, coinfection of these two viruses may potentially disrupt the patient response to therapeutic agents for either infection, thus a simultaneous diagnosis of these viruses is necessary to provide effective care. 78
1.3. Coinfection with adenovirus and novel coronavirus (SARS‐CoV‐2)
Although rare, mixed infection of SARS‐CoV‐2 and adenoviruses, another family of respiratory viruses, in individuals with diabetes mellitus may be associated with findings, such as elevated d‐dimer, LDH, and ferritin, along with life‐threatening conditions like hypoxia and ARDS, which could necessitate hospitalization in the intensive care unit (ICU). 36 SARS‐CoV‐2 and adenovirus coinfection can instigate a cascade of detrimental sequelae, for example, lymphopenia, thrombocytopenia, and septic shock, rendering the patient dependent on mechanical ventilation. To date, coinfection of adenovirus and SARS‐CoV‐2 has been reported in a limited number of patients over 15 years of age and adults.20, 50, 51 A case report of adenovirus coinfection with SARS‐CoV‐2 in a 4‐month‐old infant negated the misconception regarding the unlikelihood of adenovirus coinfection in infants, indicating that complete screening for COVID‐19 in addition to other virus infections, in this particular case, significantly reduced the risk of being an asymptotic carrier. 80
1.4. Coinfection with human CoVs and SARS‐CoV‐2
CoVs are highly recombinant due to the large size of their RNA genome. Several investigations have reported a recombination rate of 25% throughout the entire viral genome, which is relatively high compared to other single‐stranded RNA viruses, resulting in enhanced susceptibility to mutations. 81 Of the seven human CoVs, four are endemic (HCoV‐229E, HCoV‐OC43, HCoV‐NL63, and HCoV‐HKU1) and cause symptoms similar to the common cold and lower respiratory tract infections. 81 Coinfection or super‐infection with endemic or epidemic HCoVs (SARS‐CoV‐1, MERS‐CoV, and SARS‐CoV‐2) can accentuate the patients' symptoms.82, 83, 84 In a study conducted by Grieven et al., 85 on a population of Kenyan children residing in coastal endemic areas, with a seasonal rotation of about 4%, three cases of HCoV (HCoV‐NL63, OC43, and 229E) were identified to be vulnerable to coinfection with other respiratory viruses, without any seasonal pattern. Considering the spread of SARS‐CoV‐2, the relatively high prevalence of seasonal MERS‐CoV in the Middle East, for example, the Kingdom of Saudi Arabia, particularly in the spring, warrants careful screening for both viruses in these regions as MERS‐CoV infection is significantly more fatal (34% mortality rate) that COVID‐19.86, 87 As a result, the possibility of coinfection with SARS‐CoV‐2 and other HCoVs, particularly in children who are susceptible to seasonal coronavirus infections, should not be overlooked.
2. COINFECTION WITH SYSTEMIC DISEASE‐CAUSING VIRUSES AND SARS‐COV‐2
In addition to the respiratory viruses mentioned earlier, many systemic disease‐causing viruses, including HIV, HBV, CMV, EBV, and dengue fever virus, can be superimposed on SARS‐CoV‐2 infection amid the widespread outbreak of this virus.88, 89 However, the severity of infection remains unknown. Coinfection with SARS‐CoV‐2 and HIV has also been demonstrated to be more severe because of immune deficiencies, severe infection, and slower development of antibodies. 76 It has also been documented that the risk of re‐infection with SARS‐CoV‐2 is increased in HIV‐infected patients due to immunosuppression, as well as deficiency or failure of the humoral immune response. Thus, it is imperative to avoid any exposure to SARS‐CoV‐2 in these patients. 90 Studies suggest that the HBV virus is either reactivated or suppressed following coinfection with another viral strain. 91 For example, the rate of coinfection with HIV and HBV is high due to the similarity of their transmission. In this respect, studies have indicated that, after the suppression of the immune system during HIV infection, the amount of antibodies associated with hepatitis B as a result of its reactivation declines, ultimately leading to chronic hepatitis B. 92 The innate immune response associated with hepatitis C virus (HCV) infection can also suppress the replication of HBV if a coinfection with HCV is present. 93 In addition, Liu et al.94, 95, 96 discovered that secreted cytokines, including IL‐6 and TNF‐α, counteract HBV replication and pathogenicity during COVID‐19 disease.
2.1. Coinfection with HIV and SARS‐CoV‐2
Despite the expansion of information regarding the SARS‐CoV‐2 infection, little is known about the potential impact of HIV infection on COVID‐19. 33 As common diseases including lymphoma and opportunistic infections, such as pneumocystis pneumonia, have been observed in people with HIV/acquired immunodeficiency syndrome (AIDS); the compromise in the immune system caused by HIV often contributes to the enhanced occurrence of SARS‐CoV‐2 infection.97, 98, 99, 100, 101 An investigation on HIV‐infected patients reported a significantly lower number of CD4+ T cells before coinfection with SARS‐CoV‐2. Considering that tissue damage in the case of SARS‐CoV‐2 infection is triggered by cytokine‐induced cascades, 102 deaths from mixed infection of HIV and SARS‐CoV‐2 are rare due to the inadequacy of CD4+ T cells, preventing upregulation of immune cytokines. Lymphopenia has been documented in extreme cases with HIV‐negative COVID‐19; however, the distribution of immune cells in patients coinfected with HIV and SARS‐CoV‐2, as well as in patients with COVID‐19 alone, is slightly different.103, 104 Nonetheless, the precise impact of lymphocyte deficiency in peripheral blood on the clinical outcome of HIV and SARS‐CoV‐2 coinfection is yet to be elucidated. 100 Patients with AIDS, especially those with comorbidities, and a high load of HIV RNA in their general circulation are more vulnerable to SARS‐CoV‐2 infection. However, the clinical symptoms of simultaneous infection with HIV and SARS‐CoV‐2 are not clearly defined.33, 105, 106 Interestingly, a lower prevalence of comorbidities, such as high blood pressure, high body mass index, diabetes, chronic kidney disease, and chronic liver disease has been documented in patients with SARS‐CoV‐2 or HIV infection alone compared to those with coinfection.33, 107, 108
In the case of HIV infection, factors determining disease severity, including lymphocyte or platelet loss, as well as increased IL‐6 levels are associated with reduced CD4+ T‐lymphocyte counts. Accordingly, the count of CD4+ T lymphocytes in individuals with severe coinfection with HIV and SARS‐CoV‐2 appears to be much lower than in patients with moderate symptoms.33, 35, 109 Despite the lack of any particular association between the CD4+ lymphocytes and COVID‐19, inhibition of the immune system might still contribute to the severity of disease, resulting in adverse consequences and persistence of viral infection. 33
Delayed antibody response could be another complication associated with such coinfection, as indicated by a case report regarding a patient with mixed infection of SARS‐CoV‐2, HIV‐1, and HCV. 110 This, in turn, denotes the impact of HIV‐1 on the function of the immune system, in relation to SARS‐CoV‐2. 110 However, the grade of infection with SARS‐CoV‐2 due to the compromised immune state of the host is controversial. A major decrease in the count of B and T lymphocytes, as well as natural killer (NK) cells, was shown in patients with severe COVID‐19, in contrast to those with mild symptoms. According to a report by Qin et al., the function of the immune system after SARS‐CoV‐2 infection is also impaired in the chronic phase of HIV infection, leading to a decline in the count of T lymphocytes. 111 In addition, an analysis by Suwanwongse & Shabarek. 112 on clinical symptoms in individuals with SARS‐CoV‐2/HIV coinfection, found that CD4+ T‐cell dysfunction was inversely correlated with the clinical outcome in COVID‐19 patients with HIV infection. 112
It was documented that individuals with AIDS were neither immune to SARS‐CoV‐2 nor developed a less serious COVID‐19. Though, patients with reduced T lymphocyte counts had poorer outcomes. 113 Owing to the regular administration of immunosuppressive drugs, or antiviral agents, such as protease inhibitors, nucleoside reverse transduction inhibitors, or non‐nucleoside reverse transduction inhibitors (NNRTIs), the risk of SARS‐CoV‐2 infection and the severity of its clinical symptoms are markedly mitigated.102, 114, 115
2.2. Coinfection with hepatitis B virus (HBV) and SARS‐CoV‐2
In individuals with SARS‐CoV‐2 infection, impaired liver function is identified in addition to common symptoms.116, 117, 118 This inordinate hepatic activity, in turn, contributes to the development of severe disease, and ultimately, death from SARS‐CoV‐2 infection. However, the exact cause of liver injury in COVID‐19 patients has not been explained.117, 118, 119 Due to the globally high prevalence of hepatitis B, and the high serum levels of HBsAg in these patients, hepatitis B is considered to be a major public health concern. 120 Furthermore, according to an investigation by Huang et al., 21 people with SARS‐CoV‐2 infection are more likely to develop severe hepatitis and hepatic failure if they become infected with HBV.However, there is no reliable information on the clinical outcome of SARS‐CoV‐2/HBV coinfection. 121
Angiotensin‐converting enzyme 2 (ACE2) has been recognized by numerous studies as an important receptor for SARS‐CoV‐2, which can be found on the surface of the liver cells in the bile ducts.55, 122 Accordingly, due to the binding of SARS‐CoV‐2 to ACE2‐expressing hepatocytes, normal liver function is impaired and the illness is aggravated. Still, increased serum level of alkaline phosphatase (ALP), a bile duct injury marker, was not reported in COVID‐19 patients. 39 It has also been shown that elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels are related to impaired liver function in COVID‐19 patients. There is a possibility for liver damage in COVID‐19 patients with moderate symptoms. 118 As a result, the liver is a target organ for SARS‐CoV‐2. 21
SARS‐CoV‐2 and HBV coinfection can result in the depletion of pre‐albumin deposits in the liver. 21 Interestingly, the extent of impairment in liver function is not substantially different in patients with HBV coinfection, and those without HBV coinfection. However, pre‐albumin levels in HBsAg‐negative individuals were higher than HBsAg+ individuals. 21 In general, SARS‐CoV‐2/HBV coinfection does not seriously affect the progression of COVID‐19, including disease severity, mortality, and hospitalization. Yet, it has recently been suggested that SARS‐CoV infection might lead to increased liver damage and induction of HBV activity in people with hepatitis B. 123 The clinical effects of COVID‐19 have been confirmed not to be associated with HBV infection, although some patients might present with elevated liver enzymes. 124 Accordingly, a few theories can be considered in such situations:
1. Long‐term use of HBV antiviral medications has been linked to the progression of COVID‐19 disease, as have nucleoside analogs, such as entecavir, tenofovir, and others. As a result, tenofovir binds to the RNA‐dependent RNA polymerase (RdRp) enzyme of SARS‐CoV‐2, halting the synthesis of its RNA.125, 126 As a consequence, during the COVID‐19 pandemic, nucleotide analogs might be considered as potential therapeutic agents. 124
2. Immune system dysfunction plays a key role in the development of COVID‐19 in individuals with chronic HBV infection. It has been shown that the persistence of viral genes can lead to a decrease in CD4+ and CD8+ T cells in these patients.127, 128 In this case, a disruption in the proinflammatory cytokine secretion, such as IL‐2 and TNF‐α, may result in repressed antiviral function. 129 Furthermore, the release of IL‐2, IL‐6, and TNF‐α is suggested to be correlated with the development of COVID‐19, as well as increased mortality. In individuals with chronic HBV infection, however, immune system dysfunction and a reduction in the number of T cells may cause moderate symptoms of COVID‐19, preventing appropriate immune response to SARS‐CoV‐2. 124
3. In the case of coinfection with SARS‐CoV‐2 and HBV, viral interference, a mechanism in which one virus suppresses the replication of another strain, can be observed. Autoantibodies and genetic mutations are also affected by defective interferon type 1 activity in acute COVID‐19.130, 131 As a result, increased IFN‐І secretion in viral coinfections can suppress the replication of the other strain. 132
HBV reactivation, on the contrary, has been confirmed in coinfection with SARS‐CoV‐2 and HBV. Immunosuppressive drugs like tosilizumab and siltoximab; IL‐6 receptor antagonists, namely tosilizumab and siltoximab; IL‐1 receptor antagonists, including anakinra and high‐dose corticosteroids, are used to regulate the aggravated cytokine cascade upon HBV reactivation when viral DNA is present in the peripheral blood.133, 134, 135 As a consequence, an important risk factor for HBV reactivation following COVID‐19 could be the disequilibrium of the immune system and virus replication. 124
Accordingly, there is a possibility of SARS‐CoV‐2/HBV coinfection in individuals with COVID‐19. 21
2.3. Coinfection with DENV and SARS‐CoV‐2
One of the most significant public health concerns in Asian countries is DENV, 116 which is more prevalent in rainy seasons, causing conditions varying from mild dengue fever (DF) to severe hemorrhagic DF and dengue shock syndrome.136, 137 High‐grade fever and flu‐like symptoms are the most important clinical prognostic manifestations of both DENV disease and COVID‐19, and the similarity of these symptoms can increase the risk of misdiagnosis. An investigation conducted by Yan et al. 138 suggested the possibility of DENV and SARS‐CoV‐2 coinfection, characterized by prolonged fever. Similarly, in Thailand, Yan et al. 138 and Joob et al. 139 reported mixed infection of DENV and SARS‐CoV‐2, characterized by petechial rash, and respiratory symptoms as a result of COVID‐19. In this respect, it was speculated that patients with fever could be coinfected with SARS‐CoV‐2 and DENV, a condition most prevalent in areas, including Singapore, Thailand, Malaysia in South East Asia and Brazil in South America. In terms of clinical manifestations, 87.9% of COVID‐19 patients presented with fever, 67.7% reported cough and 13.7% complained of headache. On the contrary, all patients with DENV infection had fever, whereas only 25.7% reported headache.61, 140
The findings above suggested that DF and COVID‐19 may present with similar clinical signs and symptoms. For example, thrombocytopenia is observed in both infections. 141 However, serious respiratory symptoms were not found in individuals with either SARS‐CoV‐2 or DENV infection or coinfection. 142 Due to the similarity among the symptoms of DF and COVID‐19, including fever, cough, myalgia, and petechiae, it is not readily possible to discriminate the two diseases from each other. 142 Skin rashes and reduced platelet counts can also be observed in COVID‐19 patients, increasing the likelihood of misdiagnosis.139, 142 Symptoms such as reduced lymphocyte and monocyte counts, elevated glucose levels, and serious respiratory disorders have been reported in cases with SARS‐CoV and DENV coinfection. 143 In the absence of any major clinical symptoms in dormant DENV infection, serious clinical manifestations and a need for hospitalization have been confirmed during the active phase of DENV infection. 143 In cases with no history of DENV‐related symptoms, coinfection with SARS‐CoV‐2 might result in the exacerbation of respiratory complications, mandating hospitalization. Although the number of lymphocytes and monocytes can decrease in coinfection with SARS‐CoV‐2 and DENV, leukopenia has only been documented in patients with classic DF. 144 The number of lymphocytes in the peripheral blood may increase in the mixed infection of DENV and SARS‐CoV‐2, hence, the reports of impaired innate and acquired immune systems in these patients. 145 It should be noted that coinfection with SARS‐CoV‐2 and DENV are associated with elevated blood glucose levels, which favor replication of SARS‐CoV‐2. 146
Therefore, it is necessary to utilize the fastest and most sensitive serological tests, such as antibodies against the nonstructural glycoprotein 1 (NS1), secreted by all flaviviruses, at the appropriate time after the onset of symptoms (5 days after onset of fever), especially in endemic areas. 147 Increased IL‐6 levels in DENV infection are associated with enhanced severity of symptoms. 30 However, serum studies of cases with acute symptoms of COVID‐19 revealed increased amounts of proinflammatory cytokines (TNF‐α, IL‐1, and IL‐6). 148 One of the markers facilitating the identification of individuals suspected to have COVID‐19 is lymphocytopenia, which may incapacitate the immune system against DENV infection.61, 149 Bleeding disorders have been identified in a few cases during COVID‐19. The most frequent sign of DF is bleeding, which might aggravate the infection. 150 Lymphopenia has been documented to contribute to bleeding in the presence of SARS‐CoV‐2 and DF infection alone; therefore, the ratio of neutrophils to lymphocytes in DENV infection can become inverse. 151
Coinfection with two viruses can either alleviate or increase the severity of the disease. 152 A common consequence in such cases is viral interference. During viral interference, one virus competes with another, interfering with the replication mechanism. 153 Accordingly, this mechanism might have a major impact on the development of DF and its natural history. 154
2.4. Coinfection with CMV and SARS‐CoV‐2
CMV can predispose individuals to severe cases of COVID‐19 through disruption of peripheral blood T‐cell differentiation, and upregulation of inflammatory cytokines, including IL‐6, especially in the elderly. 31 T‐cell dysfunction is mostly due to decreased thymic function, determined by the age of the patient. T cells are highly important in the regulation of immune responses against viral infections.155, 156 The prevalence of CMV infection in elderly patients compromises the immune response to subsequent viral infections, including SARS‐CoV‐2, by mitigating the diversity in the population of naïve T cells. The correlation between the ineffectiveness of influenza vaccine in the elderly patients and the decreased count of CD8+ T cells due to thymic dissolution, 157 as well as the increase in the count of T‐reg and CD4+ T cells in individuals with CMV reactivation, 40 explains the pivotal role of cellular immunity mediated by T cells in viral infections.
The serum level of IL‐6 is usually increased in individuals infected with CMV, 157 which is very similar to COVID‐19 patients, who exhibit increased levels of cytokines (IL‐6, IL‐10, and TNF‐α), decreased lymphocytes (CD4+ and CD8+ T cells), and diminished IFN‐γ secretion from CD4+ T cells. 41 On the contrary, the clinical use of tocilizumab as a monoclonal antibody versus IL‐6, has been demonstrated to be very effective in the treatment of COVID‐19 patients admitted to the ICU. 158 Therefore, it can be concluded that coinfection with CMV or activated CMV in COVID‐19 patients can exacerbate the underlying disease and induce cytokine storms. Thus, identification of this latent virus can be effective in adopting appropriate treatment methods for suppressing the inflammation.
ACE2 is the major receptor mediating the entry of SARS‐CoV‐2 into the host cells, which is expressed in large amounts in organs, such as the kidneys and lungs. 159 Patients with renal insufficiency due to immunosuppression stand among the most sensitive groups and are at risk of death from COVID‐19. 160 Carll et al. 161 investigated the clinical symptoms of systemic coinfection or CMV reactivation in SARS‐CoV‐2 infection with hemorrhagic enterocolitis. In this regard, the pathogenesis of CMV and serious enterocolitis in severe COVID‐19 has been the subject of several speculations, such as:
1. Serious lymphocytopenia occurs in severe SARS‐CoV‐2 infection, which leads to cellular immune system deficiencies and, as a result, CMV reinfection or reactivation. 70 Subsequently, impairment of the reticuloendothelial and hematopoietic systems, such as the bone marrow, spleen, and lymph nodes may result in the death of T cells in severe COVID‐19.162, 163 In this respect, as the population of effector and CD8+ cytotoxic T cells decline, the effects of SARS‐CoV‐2 infection become more serious, leading to innate and adaptive immune system failure and eventually predisposing individuals to viral infections.162, 164, 165
2. Immune modulators like tocilizumab suppress the biological and pathological effects of hemophagocytosis caused by cytokine secretion and, as a result, intensify COVID‐19 symptoms.35, 161 B and T lymphocytes, macrophages, dendritic cells, fibroblasts, and endothelial cells also secrete IL‐6. 161 In H1N1 flu, a decrease in the IL‐6 activity has also been associated with serious pulmonary complications. 166 As a consequence, immune modulators, according to reports, might play a key role in the development of CMV coinfection.167, 168, 169
CMV is the most common viral infection among patients with kidney failure, especially kidney transplant recipients. 170 Studies show that COVID‐19‐induced cytokines increase creatine kinase, which, in turn, elevates serum creatinine (Cr) and blood urea nitrogen (BUN), particularly in patients with kidney transplants.171, 172 Accordingly, SARS‐CoV‐2 and CMV coinfection in kidney transplant recipients may potentially aggravate the clinical status of these patients. Thus, continuous monitoring of Cr, BUN, ALT, and CRP levels, as prognostic factors, as well as the lymphocyte count is recommended to be regularly measured in these patients to mitigate the risk of death. 173
2.5. Coinfection with EBV and SARS‐CoV‐2
The causative agent of infectious mononucleosis, EBV is involved in the development of chronic active infections due to a variety of lymphoproliferative disorders, for example, Hodgkin's lymphoma, Burkitt's lymphoma, B‐cell lymphoma, plasma myeloma, NK/T‐cell lymphoma, and NK cell carcinoma, especially in individuals with immunodeficiency or patients with transplantation. 174 As patients with COVID‐19 may frequently develop lymphocytopenia, it is advised to rule out SARS‐CoV‐2 and EBV coinfection, especially in the elderly or patients with transplants, who are susceptible to both viruses. 34 This is because the decrease in the count of lymphocytes, and the inability of the cellular immune system to eliminate the infectious agent can lead to the development of dysplasia, and ultimately, malignancy. In this regard, the mortality rate can increase as a result of simultaneous infection with EBV and SARS‐CoV‐2. 34
3. DIAGNOSIS OF VIRAL COINFECTIONS
On the basis of their prevalence, the clinical signs and symptoms associated with SARS‐CoV‐2 infection include fever (more than 90%), cough (69.8%), shortness of breath (34.5%), myalgia (27.7%), sore throat (17.4%), headache (7.2%), diarrhea (6.1%), and rhinorrhea (4.0%).90, 175 COVID‐19 also causes radiological changes in the lungs, such as ground‐glass opacity, thickened bronchial wall, and pleural effusion. 176 As a result, due to changes in radiological imaging and similarity of clinical symptoms caused by SARS‐CoV‐2 with those of other viral respiratory tract infections, it is difficult to distinguish the exact type of virus and the count of viruses that might have infected the patients, which, in turn, leads to misdiagnosis of COVID‐19 and other respiratory diseases.90, 177, 178 SARS‐CoV‐2 infection is also associated with lymphopenia, prolonged prothrombin time (PT), high levels of LDH, ALT, AST, d‐dimer, C‐reactive protein (CRP), and troponin, along with neutrophilia and eosinophilia. 111 Lymphocytopenia and increased levels of CRP are believed to be the most important lab findings, which are frequently observed in other viral infections, too. 179 Despite the multiple diagnostic indicators, reverse transcription‐polymerase chain reaction (RT‐PCR) is one of the most reliable methods to identify coinfection with SARS‐CoV‐2 and other viruses.90, 180 The test is performed on the sputum or nasopharyngeal secretions of individuals suspected to be infected. The risk of bias, false‐negative reports, and the necessity to repeat the test for proper diagnosis should be considered by clinicians.90, 181
4. CONCLUSION
Coinfections, compared with single infections, may lead to changes in transmission of the pathogen, progression of clinical symptoms, and the adverse effects associated with any given infection, which ultimately determines the management of infectious diseases.182, 183, 184, 185 The inability of conventional methods to detect coinfection, in the absence of sufficient evidence, in turn, can result in underdiagnosis of coinfections. 186 Coinfections or mixed infections have been reported to negatively affect the efficiency of diagnostic methods used for detection of SARS‐CoV‐2; for instance, RT‐PCR may not be the optimal diagnostic test for diagnosis of COVID‐19 in patients with influenza A virus coinfection. 18 In contrast, nucleic acid amplification test would be a suitable method for detection of influenza virus RNA in the case of coinfection with SARS‐CoV‐2. 78 Similarly, a proper diagnosis of DENV and SARS‐CoV‐2 coinfection in countries endemic to DF can only be made through the adoption of reliable laboratory tests, such as the PCR of the nasopharyngeal swab and anal swab, NS1 DENV, immunoglobulin (Ig) M and IgG assays. 187 It is also necessary to develop new strategies to better understand the clinical signs of coinfections and discover suitable therapeutic options for them. 188 Accordingly, early detection of coinfections is important due to the discrepancies in treatment and appropriate prognosis. 189 Considering the fact that there is more than one mechanism involved in the occurrence of coinfections, one might hypothesize that the mere existence of a virus and its impact on the immune system may provide the foundation for the replication of the other virus and suppression of the molecular mechanism of normal cells. For instance, in the case of COVID‐19, the triad of lymphocytopenia, overexpression of inflammatory cytokines, and dysfunction of the acquired immune system pave the way for the development of coinfection, which may result in detrimental sequelae in an otherwise healthy individual.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Hossein B. Baghi was involved in the study design, review of the manuscript, and is the corresponding author. Parisa S. Aghbash was involved in literature search, figure design, manuscript preparation, and review of the manuscript. Nergar Eslami was involved in table design and review of the manuscript. Milad Shirvaliloo was involved in editing and reviewing of the manuscript.
ACKNOWLEDGMENT
This project was supported by the Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Aghbash PS, Eslami N, Shirvaliloo M, Baghi HB. Viral coinfections in COVID‐19. J Med Virol. 2021;93:5310–5322. 10.1002/jmv.27102
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCE
- 1. Lai C‐C, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and corona virus disease‐2019 (COVID‐19): the epidemic and the challenges. Int J Antimicro Agents. 2020;55:105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aghbash PS, Eslami N, Shamekh A, Entezari‐Maleki T, Baghi HB. SARS‐CoV‐2 infection: the role of PD‐1/PD‐L1 and CTLA‐4 axis. Life Sci. 2021;270:119124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Coronavirus Disease (COVID‐19) pandemic. 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed February 19, 2021.
- 4. Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID‐19. Int J Antimicrob Agents. 2020;55(4):105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ko W‐C, Rolain JM, Lee NY, et al. Arguments in favour of remdesivir for treating SARS‐CoV‐2 infections. Int J Antimicro Agents. 2020;55:105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oroojalian F, Haghbin A, Baradaran B, et al. Novel insights into the treatment of SARS‐CoV‐2 infection: an overview of current clinical trials. Int J Biol Macromol. 2020;165:18‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chu D, Pan Y, CheNg S, et al. Molecular diagnosis of a novel Coronavirus (2019‐nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66:549‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donyavi T, Bokharaei‐Salim F, Baghi HB, et al. Acute and post‐acute phase of Covid‐19: analyzing expression patterns of miRNA‐29a‐3p, 146a‐3p, 155‐5p, and Let‐7b‐3p in PBMC. Int Immunopharmacol. 2021;97:107641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tabacof L, Tosto‐Mancuso J, Wood J, et al. Post‐acute COVID‐19 syndrome negatively impacts health and wellbeing despite less severe acute infection. medRxiv. 2020. [Google Scholar]
- 11. Gaunt ER, Hardie A, Claas EC, Simmonds P, Templeton KE. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real‐time PCR method. J Clin Microbiol. 2010;48(8):2940‐2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gorbalenya A, Baker SC, Baric RS, et al. The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5:536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hemmat N, Derakhshani A, Bannazadeh Baghi H, Silvestris N, Baradaran B, de Summa S. Neutrophils, crucial, or harmful immune cells involved in coronavirus infection: a bioinformatics study. Front Genet. 2020;11:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hemmat N, Asadzadeh Z, Ahangar NK, et al. The roles of signaling pathways in SARS‐CoV‐2 infection; lessons learned from SARS‐CoV and MERS‐CoV. Arch Virol. 2021;166:1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136(1):95‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aghbash PS, Hemmat N, Nahand JS, et al. The role of Th17 cells in viral infections. Int Immunopharmacol. 2021;91:107331. [DOI] [PubMed] [Google Scholar]
- 18. Lai C‐C, Wang C‐Y, Hsueh P‐R. Co‐infections among patients with COVID‐19: the need for combination therapy with non‐anti‐SARS‐CoV‐2 agents? J Microbiol Immunol Infect. 2020;53:505‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li X‐X, Zhou X‐N. Co‐infection of tuberculosis and parasitic diseases in humans: a systematic review. Parasit Vectors. 2013;6(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu X, Ge Y, Wu T, et al. Co‐infection with respiratory pathogens among COVID‐2019 cases. Virus Res. 2020;285:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen L, Huang S, Yang J, et al. Clinical characteristics in patients with SARS‐CoV‐2/HBV co‐infection. J Viral Hepatitis. 2020;27(12):1504‐1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirotsu Y, Maejima M, Shibusawa M, et al. Analysis of Covid‐19 and non‐Covid‐19 viruses, including influenza viruses, to determine the influence of intensive preventive measures in Japan. J Clin Virol. 2020;129:104543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID‐19 infection: different points from adults. Pediatr Pulmonol. 2020;55(5):1169‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alfaraj SH, Al‐Tawfiq JA, Alzahrani NA, Altwaijri TA, Memish ZA. The impact of co‐infection of influenza A virus on the severity of Middle East Respiratory Syndrome Coronavirus. J Infect. 2017;74(5):521‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ding Q, Lu P, Fan Y, Xia Y, Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020;92:1549‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ling W. C‐reactive protein levels in the early stage of COVID‐19. Med Mal Infect. 2020;50:332‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feng Z, Yu Q, Yao S, et al. Early prediction of disease progression in 2019 novel coronavirus pneumonia patients outside Wuhan with CT and clinical characteristics. medRxiv. 2020. [Google Scholar]
- 30. Butthep P, Chunhakan S, Yoksan S, Tangnararatchakit K, Chuansumrit A. Alteration of cytokines and chemokines during febrile episodes associated with endothelial cell damage and plasma leakage in dengue hemorrhagic fever. Pediatr Infect Dis J. 2012;31(12):e232‐e238. [DOI] [PubMed] [Google Scholar]
- 31. Kadambari S, Klenerman P, Pollard AJ. Why the elderly appear to be more severely affected by COVID‐19: the potential role of immunosenescence and CMV. Rev Med Virol. 2020;30(5):e2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vizcarra P, Pérez‐Elías MJ, Quereda C, et al. Description of COVID‐19 in HIV‐infected individuals: a single‐centre, prospective cohort. The Lancet HIV. 2020;7:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roncati L, Lusenti B, Nasillo V, Manenti A. Fatal SARS‐CoV‐2 coinfection in course of EBV‐associated lymphoproliferative disease. Ann Hematol. 2020;99:1945‐1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England). 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Motta JC, Gómez CC. Adenovirus and novel coronavirus (SARS‐Cov2) coinfection: a case report. IDCases. 2020;22:e00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xiang J, Wen J, Yuan X, et al. Potential biochemical markers to identify severe cases among COVID‐19 patients. medRxiv. 2020. [Google Scholar]
- 38. Huang Y, Yang R, Xu Y, Gong P Clinical characteristics of 36 non‐survivors with COVID‐19 in Wuhan, China. medRxiv. 2020. [Google Scholar]
- 39. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnstone J, Parsons R, Botelho F, et al. Immune biomarkers predictive of respiratory viral infection in elderly nursing home residents. PLOS One. 2014;9(10):e108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chang CS, Lin S, Wallace CG, et al. Clinical and immunologic features in severe and moderate forms o f Coronavirus Disease. J Clin Invest. 2019;82:137244. [Google Scholar]
- 42. Liu Y, Sun W, Guo Y, et al. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets. 2020;31(4):490‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bicudo N, Bicudo E, Costa JD, Castro J, Barra GB. Co‐infection of SARS‐CoV‐2 and dengue virus: a clinical challenge. Braz J Infect Dis. 2020;24(5):452‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ozaras R, Cirpin R, Duran A, et al. Influenza and COVID‐19 coinfection: report of six cases and review of the literature. J Med Virol. 2020;92(11):2657‐2665. [DOI] [PubMed] [Google Scholar]
- 45. König B, König W, Arnold R, Werchau H, Ihorst G, Forster J. Prospective study of human metapneumovirus infection in children less than 3 years of age. J Clin Microbiol. 2004;42(10):4632‐4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Drews AL, Atmar RL, Glezen WP, Baxter BD, Piedra PA, Greenberg SB. Dual respiratory virus infections. Clin Infect Dis. 1997;25(6):1421‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee N, Chan PK, Yu IT, et al. Co‐circulation of human metapneumovirus and SARS‐associated coronavirus during a major nosocomial SARS outbreak in Hong Kong. J Clin Virol. 2007;40(4):333‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wee LE, Ko K, Ho WQ, Kwek G, Tan TT, Wijaya L. Community‐acquired viral respiratory infections amongst hospitalized inpatients during a COVID‐19 outbreak in Singapore: co‐infection and clinical outcomes. J Clin Virol. 2020;128:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lin D, Liu L, Zhang M, et al. Co‐infections of SARS‐CoV‐2 with multiple common respiratory pathogens in infected patients. Sci China Life Sci. 2020;63(4):606‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co‐infection between SARS‐CoV‐2 and other respiratory pathogens. JAMA. 2020;323:2085‐2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Khaddour K, Sikora A, Tahir N, Nepomuceno D, Huang T. Case report: the importance of novel coronavirus disease (COVID‐19) and coinfection with other respiratory pathogens in the current pandemic. Am J Trop Med Hyg. 2020;102(6):1208‐1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lum LHW, Tambyah PA. Outbreak of COVID‐19–an urgent need for good science to silence our fears? Singapore Med J. 2020;61(2):55‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lv Z, Cheng S, Le J, et al. Clinical characteristics and co‐infections of 354 hospitalized patients with COVID‐19 in Wuhan, China: a retrospective cohort study. Microb Infect. 2020;22(4–5):195‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sarkesh A, Daei Sorkhabi A, Sheykhsaran E, et al. Extrapulmonary clinical manifestations in COVID‐19 patients. Am J Tropic Med Hyg. 2020;103(5):1783‐1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sakamoto H, Ishikane M, Ueda P. Seasonal influenza activity during the SARS‐CoV‐2 outbreak in Japan. JAMA. 2020;323(19):1969‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yue H, Zhang M, Xing L, et al. The epidemiology and clinical characteristics of co‐infection of SARS‐CoV‐2 and influenza viruses in patients during COVID‐19 outbreak. J Med Virol. 2020;92(11):2870‐2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lai C‐C, Liu YH, Wang CY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARSCoV‐2): facts and myths. J Microbiol Immunol Infect. 2020;53:404‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chow EJ, Doyle JD, Uyeki TM. Influenza virus‐related critical illness: prevention, diagnosis, treatment. Crit Care. 2019;23(1):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hashemi SA, Safamanesh S, Ghasemzadeh‐Moghaddam H, Ghafouri M, Azimian A. High prevalence of SARS‐CoV‐2 and influenza A virus (H1N1) coinfection in dead patients in Northeastern Iran. J Med Virol. 2021;93(2):1008‐1012. [DOI] [PubMed] [Google Scholar]
- 61. Guan W‐j, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cao B, Li XW, Mao Y, et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;361(26):2507‐2517. [DOI] [PubMed] [Google Scholar]
- 63. Cuadrado‐Payán E, Montagud‐Marrahi E, Torres‐Elorza M, et al. SARS‐CoV‐2 and influenza virus co‐infection. Lancet (London, England). 2020;395(10236):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang N, Wang L, Deng X, et al. Recent advances in the detection of respiratory virus infection in humans. J Med Virol. 2020;92(4):408‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jiang C, Yao X, Zhao Y, et al. Comparative review of respiratory diseases caused by coronaviruses and influenza A viruses during epidemic season. Microb Infect. 2020;22(6–7):236‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Konala VM, Adapa S, Gayam V, et al. Co‐infection with Influenza A and COVID‐19. Eur J Case Rep Intern Med. 2020;7(5):001656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fang Z, Zhang Y, Hang C, Ai J, Li S, Zhang W. Comparisons of viral shedding time of SARS‐CoV‐2 of different samples in ICU and non‐ICU patients. J Infect. 2020;81(1):147‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jin CC, Zhu L, Gao C, Zhang S. Correlation between viral RNA shedding and serum antibodies in individuals with coronavirus disease 2019. Clin Microbiol Infect. 2020;26:1280‐1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ma S, Lai X, Chen Z, Tu S, Qin K. Clinical characteristics of critically ill patients co‐infected with SARS‐CoV‐2 and the influenza virus in Wuhan, China. Int J Infect Dis. 2020;96:683‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. The lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cao X. COVID‐19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nicholls JM, Poon LL, Lee KC, et al. Lung pathology of fatal severe acute respiratory syndrome. The Lancet. 2003;361(9371):1773‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang Y‐H, Lin AS, Chao TY, et al. A cluster of patients with severe acute respiratory syndrome in a chest ward in southern Taiwan. Intensive Care Med. 2004;30(6):1228‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kulkarni U, Zemans RL, Smith CA, Wood SC, Deng JC, Goldstein DR. Excessive neutrophil levels in the lung underlie the age‐associated increase in influenza mortality. Mucosal Immunol. 2019;12(2):545‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Davidson JA, Warren‐Gash C. Cardiovascular complications of acute respiratory infections: current research and future directions. Expert Rev Anti Infect Ther. 2019;17(12):939‐942. [DOI] [PubMed] [Google Scholar]
- 76. Wang M, Wu Q, Xu W, et al. Clinical diagnosis of 8274 samples with 2019‐novel coronavirus in Wuhan. medRxiv. 2020. [Google Scholar]
- 77. Na W, Nam D, Lee H, Shin S. Rapid molecular diagnosis of infectious viruses in microfluidics using DNA hydrogel formation. Biosens Bioelectron. 2018;108:9‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ji M, Xia Y, Loo JFC, et al. Automated multiplex nucleic acid tests for rapid detection of SARS‐CoV‐2, influenza A and B infection with direct reverse‐transcription quantitative PCR (dirRT‐qPCR) assay in a centrifugal microfluidic platform. RSC Adv. 2020;10(56):34088‐34098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lakdawala SS, Nair N, Hutchinson E. Educational material about influenza viruses. Viruses. 2019;11(3):231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Danley K, Kent P. 4‐month‐old boy coinfected with COVID‐19 and adenovirus. BMJ Case Reports CP. 2020;13(6):e236264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. To KK, Hung IF, Chan JF, Yuen KY. From SARS coronavirus to novel animal and human coronaviruses. J Thorac Dis. 2013;5(suppl 2):S103‐S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yu X, Lu R, Wang Z, et al. Etiology and clinical characterization of respiratory virus infections in adult patients attending an emergency department in Beijing. PLOS One. 2012;7(2):e32174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kanwar A, Selvaraju S, Esper F. Human coronavirus‐HKU1 infection among adults in Cleveland, Ohio. Open Forum Infectious Diseases. Oxford, UK: Oxford University Press; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Otieno GP, Murunga N, Agoti CN, Gallagher KE, Awori JO, Nokes DJ. Surveillance of endemic human coronaviruses (HCoV‐NL63, OC43 and 229E) associated with pneumonia in Kilifi, Kenya. Wellcome Open Res. 2020;5:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. World Health Organization . Middle East Respiratory Syndrome Coronavirus (MERS‐CoV)—the Kingdom of Saudi Arabia. Disease outbreak news: update, February 24. 2020.
- 87. World Health Organization . Middle East Respiratory Syndrome Coronavirus (MERS‐CoV)—Saudi Arabia. Disease outbreak news: update, May 5. 2020.
- 88. Blanco JL, Ambrosioni J, Garcia F, et al. COVID‐19 in patients with HIV: clinical case series. The Lancet HIV. 2020;7(5):e314‐e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kiley JL, Chung KK, Blyth DM. Viral infections in burns. Surg Infect (Larchmt). 2020;22:88‐94. [DOI] [PubMed] [Google Scholar]
- 90. Chen X, Liao B, Cheng L, et al. The microbial coinfection in COVID‐19. Appl Microbiol Biotechnol. 2020;104(18):7777‐7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Liu R, Zhao L, Cheng X, et al. Clinical characteristics of COVID‐19 patients with hepatitis B virus infection—a retrospective study. Liver Int. 2021;41(4):720‐730. [DOI] [PubMed] [Google Scholar]
- 92. Hoffmann CJ, Thio CL Clinical implications of HIV and hepatitis B co‐infection in Asia and Africa. Lancet Infect Dis. 2007;7(6):402‐409. [DOI] [PubMed] [Google Scholar]
- 93. Cheng X, Uchida T, Xia Y, et al. Diminished hepatic IFN response following HCV clearance triggers HBV reactivation in coinfection. J Clin Invest. 2020;130(6):3205‐3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhao K, Liu A, Xia Y Insights into Hepatitis B Virus DNA Integration‐55 years after virus discovery. The Innovation. 2020;1:100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID‐19 patients reveals IL‐6 and IL‐10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Xia Y, Protzer U Control of hepatitis B virus by cytokines. Viruses. 2017;9(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang J‐C, Zhang HJ, Li Y, et al. Changes in levels of T cell subpopulations to monitor the response to antiretroviral therapy among HIV‐1‐infected patients during two years of HIV‐1 replication suppression. Scand J Infect Dis. 2013;45(5):368‐377. [DOI] [PubMed] [Google Scholar]
- 98. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020; 130(5):2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kanwugu ON, Adadi P. HIV/SARS‐CoV‐2 coinfection: a global perspective. J Med Virol. 2020;93:726‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Guo W, Ming F, Feng Y, et al. Patterns of HIV and SARS‐CoV‐2 co‐infection in Wuhan, China. J Int AIDS Soc. 2020;23(7):25568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jiang H, Zhou Y, Tang W. Maintaining HIV care during the COVID‐19 pandemic. The Lancet HIV. 2020;7(5):e308‐e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Guo W, Ming F, Yu D, et al. A survey for COVID‐19 among HIV/AIDS patients in two Districts of Wuhan, China, 2020.
- 103. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID‐19. JAMA. 2020;323(14):1406‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J Infect Dis. 2020;221:1762‐1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhu F, Cao Y, Xu S, Zhou M. Reply to Comments on 'Co‐infection of SARS‐CoV‐2 and HIV in a patient in Wuhan city, China'. J Med Virol. 2020;92:1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhu F, Cao Y, Xu S, Zhou M. Co‐infection of SARS‐CoV‐2 and HIV in a patient in Wuhan city, China. J Med Virol. 2020;92:1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jiehao C, Jin X, Daojiong L, et al. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020;71:1547‐1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yan S, Song X, Lin F. Clinical characteristics of coronavirus disease 2019 in Hainan, China. medRxiv. 2020. [Google Scholar]
- 109. Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin‐6 (IL‐6) blockade for coronavirus disease 2019 (COVID‐19)‐induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhao J, Liao X, Wang H, et al. Early virus clearance and delayed antibody response in a case of COVID‐19 with a history of co‐infection with HIV‐1 and HCV. Clin Infect Dis. 2020;71:2233‐2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020;71:762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Suwanwongse K, Shabarek N. Clinical features and outcome of HIV/SARS‐CoV‐2 co‐infected patients in the Bronx, New York City. J Med Virol. 2020;92:2387‐2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Maggiolo F, Zoboli F, Arosio M, et al. SARS‐CoV‐2 infection in persons living with HIV: a single center prospective cohort. J Med Virol. 2020;93:1145‐1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Park SY, Lee JS, Son JS, et al. Post‐exposure prophylaxis for Middle East respiratory syndrome in healthcare workers. J Hosp Infect. 2019;101(1):42‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Joob B, Wiwanitkit V. SARS‐CoV‐2 and HIV. J Med Virol. 2020;92:1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lei F, Liu YM, Zhou F, et al. Longitudinal association between markers of liver injury and mortality in COVID‐19 in China. Hepatology. 2020;72:389‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fan Z, Chen L, Li J, et al. Clinical features of COVID‐19‐related liver damage. Clin Gastroenterol Hepatol. 2020;18:1561‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cai Q, Huang D, Yu H, et al. COVID‐19: abnormal liver function tests. J Hepatol. 2020;73:566‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Xu S, Zhang W, Wang Q, et al. Hepatitis B virus serological screen in a general hospital in Beijing from 2008 to 2018, and challenges to our vaccination policy. Vaccine: X. 2020;4:100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhang C, Shi L, Wang F‐S. Liver injury in COVID‐19: management and challenges. Lancet. Gastroenterol Hepatol. 2020;5(5):428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Huang Y‐h, Gao Z‐l. Study of the relationship SARS and hepatitis virus B. Chin J Clini Hepatol. 2003;6:342‐343. [Google Scholar]
- 124. Xiang T‐D, Zheng X. Interaction between hepatitis B virus and SARS‐CoV‐2 infections. World J Gastroenterol. 2021;27(9):782‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Elfiky AA. Remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS‐CoV‐2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253:117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Jockusch S, Tao C, Li X, et al. A library of nucleotide analogues terminate RNA synthesis catalyzed by polymerases of coronaviruses that cause SARS and COVID‐19. Antiviral Res. 2020;180:104857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rehermann B. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nature Med. 2013;19(7):859‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Anugwom CM, Aby ES, Debes JD. Inverse association between chronic hepatitis B infection and Coronavirus Disease 2019 (COVID‐19): immune exhaustion or coincidence? Clin Infect Dis. 2021;72(1):180‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79(16):10514‐10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID‐19 patients. Science. 2020;369(6504):718‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life‐threatening COVID‐19. Science. 2020;370(6515):eabd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Li N, Ma WT, Pang M, Fan QL, Hua JL. The commensal microbiota and viral infection: a comprehensive review. Front Immunol. 2019;10:1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sonneveld MJ, Murad SD, van der Eijk AA, de Man RA. Fulminant liver failure due to hepatitis B reactivation during treatment with tocilizumab. ACG Case Rep J. 2019;6(12):e00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chen Y‐M, Huang WN, Wu YD, et al. Reactivation of hepatitis B virus infection in patients with rheumatoid arthritis receiving tofacitinib: a real‐world study. Ann Rheum Dis. 2018;77(5):780‐782. [DOI] [PubMed] [Google Scholar]
- 135. Wong GL, Wong VW, Yuen BW, et al. Risk of hepatitis B surface antigen seroreversion after corticosteroid treatment in patients with previous hepatitis B virus exposure. J Hepatol. 2020;72(1):57‐66. [DOI] [PubMed] [Google Scholar]
- 136. Halstead SB. Dengue. The Lancet. 2007;370(9599):1644‐1652. [DOI] [PubMed] [Google Scholar]
- 137. Setiati TE, Wagenaar JFP, de Kruif M, Mairuhu A. Changing epidemiology of dengue haemorrhagic fever in Indonesia. Dengue Bull. 2006;30:1‐14. [Google Scholar]
- 138. Yan G, Lee CK, Lam L, et al. Covert COVID‐19 and false‐positive dengue serology in Singapore. Lancet Infect Dis. 2020;20(5):536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Joob B, Wiwanitkit V COVID‐19 can present with a rash and be mistaken for Dengue. J Am Acad Dermatol. 2020;82(5):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Chen D, Zhang Y, Wu X, et al. A survey of clinical and laboratory characteristics of dengue fever epidemic from 2014 to 2018 in Guangzhou, China. Ann Palliative Med. 2020;9(1):70‐81. [DOI] [PubMed] [Google Scholar]
- 141. Kembuan GJ. Dengue serology in Indonesian COVID‐19 patients: coinfection or serological overlap? IDCases. 2020;22:e00927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bicudo N, Bicudo E, Costa JD, Castro J, Barra GB. Co‐infection of SARS‐CoV‐2 and dengue virus: a clinical challenge. Braz J Infect Dis. 2020;24(5):452‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Teotônio IMSN, de Carvalho JL, Castro LC, et al. Clinical and biochemical parameters of COVID‐19 patients with prior or active dengue fever. Acta Trop. 2021;214:105782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Saddique A, Rana MS, Alam MM, et al. Emergence of co‐infection of COVID‐19 and dengue: a serious public health threat. J Infect. 2020;81:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Azeredo EL, Neves‐Souza PC, Alvarenga AR, et al. Differential regulation of toll‐like receptor‐2, toll‐like receptor‐4, CD16 and human leucocyte antigen‐DR on peripheral blood monocytes during mild and severe dengue fever. Immunology. 2010;130(2):202‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Codo AC, Davanzo GG, Monteiro LB, et al. Elevated glucose levels favor SARS‐CoV‐2 infection and monocyte response through a HIF‐1α/glycolysis‐dependent axis. Cell Metab. 2020;32(3):437‐446e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. de Paula SO, da Fonseca BAL. Dengue: a review of the laboratory tests a clinician must know to achieve a correct diagnosis. Braz J Infect Dis. 2004;8(6):390‐398. [DOI] [PubMed] [Google Scholar]
- 148. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan China. Clin Infect Dis. 2020;71(15):762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ng K‐H, Zhang SL, Tan HC, et al. Persistent dengue infection in an immunosuppressed patient reveals the roles of humoral and cellular immune responses in virus clearance. Cell Host Microbe. 2019;26(5):601‐605. [DOI] [PubMed] [Google Scholar]
- 150. Al‐Samkari H, Karp Leaf RS, Dzik WH, et al. COVID‐19 and coagulation: bleeding and thrombotic manifestations of SARS‐CoV‐2 infection. Blood. 2020;136(4):489‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Imad HA, Phumratanaprapin W, Phonrat B, et al. Cytokine expression in dengue fever and dengue hemorrhagic fever patients with bleeding and severe hepatitis. Am J Trop Med Hyg. 2020;102(5):943‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Díaz‐Muñoz SL. Viral coinfection is shaped by host ecology and virus–virus interactions across diverse microbial taxa and environments. Virus Evol. 2017;3(1):011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kumar N, Sharma S, Barua S, Tripathi BN, Rouse BT. Virological and immunological outcomes of coinfections. Clin Microbiol Rev. 2018;31(4):e001111‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Hilmy AI, Dey RK, Imad HA, Yoosuf AN, Latheef AA, Nazeem A. Coronavirus disease 2019 and dengue: two case reports. J Med Case Rep. 2021;15:171‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Gruver A, Hudson L, Sempowski G. Immunosenescence of ageing. J Pathol. 2007;211(2):144‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Boraschi D, Aguado MT, Dutel C, et al. The gracefully aging immune system. Sci Transl Med. 2013;5(185):185ps8. [DOI] [PubMed] [Google Scholar]
- 157. Trzonkowski P, Myśliwska J, Szmit E, et al. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti‐hemagglutinins during the anti‐influenza vaccination—an impact of immunosenescence. Vaccine. 2003;21(25–26):3826‐3836. [DOI] [PubMed] [Google Scholar]
- 158. Zhang W, Zhao Y, Zhang F, et al. The use of anti‐inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID‐19): the experience of clinical immunologists from China. Clin Immunol. 2020;214:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Alberici F, Delbarba E, Manenti C, et al. Management of patients on dialysis and with kidney transplant during SARS‐COV‐2 (COVID‐19) pandemic in Brescia, Italy. Kidney International Reports. 2020;5:580‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Osman NM, Sayed NM, Abdel‐Rahman SM, Hamza SA, Abd al Aziz AA. The impact of cytomegalovirus infection on mechanically ventilated patients in the respiratory and geriatric intensive care units. Egypt J Chest Dis Tuberc. 2014;63(1):239‐245. [Google Scholar]
- 161. Carll WC, Rady MY, Salomao MA, Patel B, Singh VP, Sen A. Cytomegalovirus haemorrhagic enterocolitis associated with severe infection with COVID‐19. BMJ Open Gastroenterol. 2021;8(1):e000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020;17(5):533‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID‐19: a post‐mortem study. Lancet Microbe. 2020;1(6):e245‐e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Maucourant C, Filipovic I, Ponzetta A, et al. Natural killer cell immunotypes related to COVID‐19 disease severity. Sci Immunol. 2020;5(50):eabd6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Jeannet R, Daix T, Formento R, Feuillard J, François B. Severe COVID‐19 is associated with deep and sustained multifaceted cellular immunosuppression. Intens Care Med. 2020;2020(46):1769‐1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Dienz O, Rud JG, Eaton SM, et al. Essential role of IL‐6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 2012;5(3):258‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID‐19. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Antinori S, Bonazzetti C, Gubertini G, et al. Tocilizumab for cytokine storm syndrome in COVID‐19 pneumonia: an increased risk for candidemia? Autoimmun Rev. 2020;19(7):102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kimmig LM, Wu D, Gold M, et al. Il‐6 inhibition in critically ill COVID‐19 patients is associated with increased secondary infections. Front Med. 2020;7:583897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID‐19 infection in kidney transplant recipients. Kidney Int. 2020;97:1076‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clinica Chimica Acta. 2020;506:145‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Billah M, Santeusanio A, Delaney V, Cravedi P, Farouk SS. A catabolic state in a kidney transplant recipient with COVID‐19. Transpl Int. 2020;33:1140‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Molaei H, Khedmat L, Nemati E, Rostami Z, Saadat SH. Iranian kidney transplant recipients with COVID‐19 infection: Clinical outcomes and cytomegalovirus coinfection. Tranpl Infect Dis. 2020;23:e13455. [DOI] [PubMed] [Google Scholar]
- 174. Kim H‐J, Ko YH, Kim JE, et al. Epstein‐Barr virus‐associated lymphoproliferative disorders: review and update on 2016 WHO classification. J Pathol Transl Med. 2017;51(4):352‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Singhai T. A review of the coronavirus disease‐2019. Indian J Pediatr. 2020;87:281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhou S, Wang Y, Zhu T, Xia L. CT features of coronavirus disease 2019 (COVID‐19) pneumonia in 62 patients in Wuhan, China. Am J Roentgenol. 2020;214(6):1287‐1294. [DOI] [PubMed] [Google Scholar]
- 177. Azekawa S, Namkoong H, Mitamura K, Kawaoka Y, Saito F. Co‐infection with SARS‐CoV‐2 and influenza A virus. IDCases. 2020;20:e00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Danis K, Epaulard O, Bénet T, et al. Cluster of coronavirus disease 2019 (Covid‐19) in the French Alps, 2020. Clin Infect Dis. 2020;71:825‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Li X, Wang L, Yan S, et al. Clinical characteristics of 25 death cases with COVID‐19: a retrospective review of medical records in a single medical center, Wuhan, China. medRxiv preprint. 2020;10(02):19.20025239‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Mahony JB. Detection of respiratory viruses by molecular methods. Clin Microbiol Rev. 2008;21(4):716‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Rodriguez JA, Rubio‐Gomez H, Roa AA, Miller N, Eckardt P. Co‐infection with SARS‐COV‐2 and Parainfluenza in a young adult patient with pneumonia: case report. IDCases. 2020;20:e00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Chiodini P. Chemotherapy for patients with multiple parasitic infections. Parasitology. 2001;122(S1):S83‐S89. [DOI] [PubMed] [Google Scholar]
- 183. Griffiths EC, Pedersen AB, Fenton A, Petchey OL. The nature and consequences of coinfection in humans. J Infect. 2011;63(3):200‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Ray S, Maunsell JH. Different origins of gamma rhythm and high‐gamma activity in macaque visual cortex. PLOS Biol. 2011;9(4):e1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Pullan R, Brooker S. The health impact of polyparasitism in humans: are we under‐estimating the burden of parasitic diseases? Parasitology. 2008;135(7):783‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Abouelkhair MA. Non‐SARS‐CoV‐2 genome sequences identified in clinical samples from COVID‐19 infected patients: evidence for co‐infections. PeerJ. 2020;8:10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Wu D, Lu J, Liu Q, Ma X, He W. To alert co‐infection of SARS‐COV‐2 and dengue virus in developing countries in the dengue‐endemic area. Infect Control Hosp Epidemiol. 2020;41:1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Birdsell DN, Özsürekci Y, Rawat A, et al. Coinfections identified from metagenomic analysis of cervical lymph nodes from tularemia patients. BMC Infect Dis. 2018;18(1):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. D'Abramo A, Lepore L, Palazzolo C, et al. Acute respiratory distress syndrome due to SARS‐CoV‐2 and influenza A co‐infection in an Italian patient: mini‐review of the literature. Int J Infect Dis. 2020;97:236‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


