Abstract
During the COVID‐19 pandemic, management of SARS‐CoV‐2 infection in children with underlying chronic lung disease has been challenging. There are limited studies in children with respiratory comorbidities, apart from asthma, presumably due to low morbidity of SARS‐CoV‐2 infection in the general pediatric population along with the low incidence of certain pulmonary conditions. Compassionate use of remdesivir has been shown to reduce time to clinical improvement in adults and has been retrospectively studied in small pediatric cohorts with promising results. Whether children with underlying respiratory conditions may benefit from antiviral treatment in the context of different pathophysiologic backgrounds and unknown drug safety and efficacy needs to be further evaluated. We present a case of COVID‐19 infection in a 3‐year old toddler with severe postinfectious bronchiolitis obliterans, who received compassionate treatment with 5‐day‐course of remdesivir, and recovered with favourable outcome.
Keywords: antiviral agents, bronchiolitis obliterans, corticosteroids, COVID‐19, treatment
1. INTRODUCTION
During the COVID‐19 pandemic, management of SARS‐CoV‐2 infection in children with chronic lung disease has been challenging. There are limited studies in children with respiratory comorbidities, apart from asthma, presumably due to low morbidity of SARS‐CoV‐2 infection in the general paediatric population along with the low incidence of certain pulmonary conditions.1, 2, 3
Remdesivir, a broad spectrum antiviral agent against several RNA viruses, has been shown to reduce time to clinical improvement in adults and has been retrospectively studied in small pediatric cohorts with promising results. In anticipation of randomized controlled trials for the pediatric population, an expert consensus suggests the use of antivirals in children on a case‐by‐case basis.2, 3
We present a case of COVID‐19 infection in a 3‐year old toddler with severe postinfectious bronchiolitis obliterans (PIBO), who received compassionate treatment with 5‐day‐course of remdesivir, and recovered with favourable outcome.
2. CASE REPORT
The patient is a 3‐year‐old toddler, without significant perinatal complications, who was diagnosed with PIBO at the age of 13 months, following an RSV and adenovirus coinfection. He was hospitalized for approximately 12 months due to difficulty in weaning off invasive and noninvasive respiratory support. He received multiple courses of pulse methylprednisolone treatment (10–30 mg/kg) and was placed on long term treatment with inhaled fluticasone and salbutamol, low dose oral azithromycin and oral montelukast (FAM). His HRCT chest demonstrates bronchial thickening, ground glass opacities, severe air trapping bilaterally and bronchiectasis (Figure 1A,B).
Figure 1.
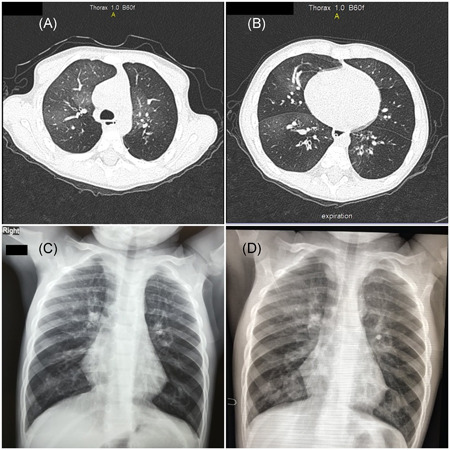
(A, B) Patient's baseline chest CT findings. (C) Chest X‐ray at presentation. (D) Three‐month follow‐up chest X‐ray post COVID‐19 admission [Color figure can be viewed at wileyonlinelibrary.com]
Since his discharge, at the age of 22 months, he remains on the FAM treatment and receives supplemental oxygen during the day (1–2 L/min) and nocturnal noninvasive ventilation (BLPAP). He is steroid‐responsive and requires a pulse dose of methylprednisolone (10 mg/kg) every 4–6 weeks in line with his respiratory status.
To date, he has established mixed hypercapnic and hypoxemic respiratory failure and a profound exercise intolerance. At baseline, he has tachypnea, subcostal and suprasternal retractions, diminished respiratory sounds, fine crackles on lung auscultation and hyperresonance on percussion.
This patient presented at the Emergency Room due to fever 24 h before his admission, tired‐appearing, with worsening tachypnea and mildly increased oxygen needs to 3 L/min. COVID‐19 infection was confirmed by molecular testing in nasopharyngeal swab (cycle threshold 21) and chest X‐ray showed peribronchial thickening and severe hyperinflation (Figure 1C). Laboratory evaluation revealed normal inflammatory biomarkers (Table 1). The patient was transferred to the COVID‐19 Department for further management.
Table 1.
Patient's results
| Reference range | Units | At presentationa | Day 4 | Day 6 | Day 8 | Day 9 | Day 11b | |
|---|---|---|---|---|---|---|---|---|
| WBC | 5–15.5 | ×103/ml | 6.65 | 5.87 | 2.11 | 5.48 | 3.36 | |
| Neutrophil | % | 58 | 58.9 | 37.9 | 22.7 | 70 | ||
| Lymphocytes | % | 24 | 26.4 | 48.7 | 73 | 19.5 (↓) | ||
| Eos | % | 1.5 | 0.0 | 0.9 | 0.6 | |||
| Hgb | g/dl | 11.5 | 10.3 | 11.3 | 10.6 | 10.9 | ||
| Platelets | 150–350 | ×103/ml | 205 | 174 | ||||
| CRP | 1–10 | mg/L | 0.13 | 1.41 | <1 | 2.37 | 1.09 | |
| Ferritin | 10–150 | ng/ml | 155 | 34 | ||||
| IL‐6 | <7 | pg/ml | 6.7 | |||||
| D‐dimmers | <0.5 | μg/ml | 0.6 | |||||
| Creatinine | 0.20–1.00 | mg/dl | 0.41 | 0.27 | 0.34 | 0.27 | 0.28 | 0.28 |
| Urea | 10–35 | mg/dl | 26 | 21 | 19 | 16 | 16 | 20 |
| ALT | 5–45 | U/L | 11 | 11 | 13 | 14 | 22 | 64 (↑) |
| AST | 10–60 | U/L | 32 | 26 | 32 | 25 | 30 | 44 |
| ALP | 60–240 | U/L | 161 | 120 | 131 | 130 | ||
| Albumin | 3.7–5.5 | g/dl | 4.7 | 4.5 | 4.9 | 4.6 | 4.6 | 4.7 |
| Bilirubin | <1 | mg/dl | 0.30 | 0.15 | 0.08 | |||
| Na | 135–150 | mmol/l | 137 | 134 | 135 | 139 | 141 | 139 |
| K | 3.5–5.5 | mmol/l | 4.2 | 4.2 | 5.1 | 4.4 | 5.1 | 4.9 |
Note: Remdesivir treatment was prescribed on Day 4 of disease.
Day 2 of disease.
Hospital discharge.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Upon arrival, the patient was initiated on broad spectrum antibiotics and intravenous dexamethasone at a dose of 0.15 mg/kg for 5 days and weaning thereafter, for another 5 days. Following parental informed consent, a compassionate access application for Emergency Use Authorisation of remdesivir was requested and granted by the National Organisation for Health Care Provision. On day 4 from disease onset, an intravenous loading dose of remdesivir (5 mg/kg) was administered, followed by 2.5 mg/kg maintenance dose for 5 days with continuous hepatic and renal function monitoring. No associated drug toxicity was observed. Fever regressed and the patient returned to baseline oxygen requirements (2 L/min) within 24 h from remdesivir administration. He was discharged after 11 days of hospitalization, on baseline respiratory support. Follow‐up examinations did not show signs of COVID‐19 sequelae, his chest radiograph three months post COVID‐19 course did not show significant changes and his baseline oxygen support remains unaltered (Figure 1D).
3. DISCUSSION
We describe the case of a toddler with underlying severe PIBO and COVID‐19 infection that was treated early in his course and improved with 5 days of remdesivir. He also received intravenous dexamethasone acutely, not only to likely inhibit the inflammatory cascade of his COVID‐19 infection, but also in the setting of his bronchiolitis obliterans.
Little evidence exists, more than one year after the onset of SARS‐CoV‐2 pandemic, whether pediatric patients with chronic lung disease may benefit from antiviral or immunomodulatory treatment. According to Chiotos et al. 4 there is insufficient evidence demonstrating that such patients are at higher risk for severe COVID‐19. However, the panel suggests the use of antivirals with a preference for remdesivir in patients with significant pulmonary disease, as they may be more likely to experience severe illness.
In a European multicentre cohort study from the PTBNET, remdesivir was used in 3% of 582 patients and 4% received systemic corticosteroids; the majority had underlying medical conditions including chronic pulmonary disease. Nevertheless, the low numbers of treated patients prevented investigators from drawing firm conclusions regarding effectiveness. 2
A retrospective multicentre study from Spain describes eight patients treated with remdesivir (four previously healthy, four with complex medical conditions) with 87.5% survival rate and nonsignificant adverse events. Interestingly, they report a 15 year‐old patient diagnosed with bronchiolitis obliterans post HSCT for CTLA‐4 haplo‐insufficiency on corticosteroids treatment and intermittent home oxygen support. Their COVID‐19 admission was uneventful; they did not require invasive mechanical ventilation, received remdesivir along with hydroxychloroquine and were discharged home after 11 days of hospitalization.
In our case, we treated our patient with systemic steroids, mostly due to his underlying refractory lung disease rather than for severe COVID‐19 with striking inflammation. Chrousos and Meduri have suggested the occurence of CIRCI (Critical Illness‐related Corticosteroid Insufficiency) phenomenon which translates to the relative inability of endogenous cortisol to control the profound inflammation and the significance of early onset of therapy with glucocorticoids before the homeostatic mechanisms reach irreversible exhaustion. 5
Similarly, there is no pediatric‐specific evidence that remdesivir mitigates disease severity.
As the great majority of children suffer minimal symptoms, anyone could question if the potential benefits of antivirals are also limited and, therefore, the threshold of using it should be much higher, given the unknown side effects. In our case, remdesivir was well tolerated, although, it is impossible to determine its contribution to our patient's uneventful recovery.
In conclusion, this unprecedented pandemic has put many clinicians under intense pressure to provide equivocal therapies based on compelling preclinical data. We feel that remdesivir may have contributed to our patient's relatively quick recovery, halted him from ICU admission and prolonged hospital stay. Notwithstanding, there is a long way before we really know what is actually beneficial to this challenging subset of patients.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Patra Koletsi conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. Marita Antoniadi participated in the clinical care of the patient, collected the data and drafted the initial manuscript. Despina Mermiri, Georgia Koltsida, Vana Spoulou, and Athanasios Michos participated in the clinical care of the patient, reviewed and revised the manuscript. Dimitra Koukou and Maria Noni collected the data. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
Thank you to Dr Stavroula Kostaridou and Dr Marina Letsiou for their contribution in the patient's care.
REFERENCES
- 1. Moeller A, Thanikkel L, Duijts L, et al. COVID‐19 in children with underlying chronic respiratory diseases: survey results from 174 centres. ERJ Open Res. 2020;6(4):409‐2020. 10.1183/23120541.00409-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Götzinger F, Santiago‐García B, Noguera‐Julián A, et al. ptbnet COVID‐19 Study Group. COVID‐19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4(9):653‐661. 10.1016/S2352-4642(20)30177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Méndez‐Echevarría A, Pérez‐Martínez A, Gonzalez Del Valle L, et al. Compassionate use of remdesivir in children with COVID‐19. Eur J Pediatr. 2021;180(4):1317‐1322. 10.1007/s00431-020-03876-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chiotos K, Hayes M, Kimberlin DW, et al. Multicenter initial guidance on use of antivirals for children with coronavirus disease 2019/severe acute respiratory syndrome coronavirus 2. J Pediatric Infect Dis Soc. 2020;9(6):701‐715. 10.1093/jpids/piaa045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chrousos GP, Meduri GU. Critical COVID‐19 disease, homeostasis, and the “surprise” of effective glucocorticoid therapy. Clin Immunol. 2020;219:108550. 10.1016/j.clim.2020.108550 [DOI] [PMC free article] [PubMed] [Google Scholar]


