ETHICS STATEMENT
This article does not contain any studies with human or animal subjects performed by any of the authors. The authors are in compliance with the ethical standards of this journal.
CONFLICT OF INTEREST
There is no conflict of interest of all authors. Medical images used in this publication were granted by the patient after informed consent.
To the Editor,
On March 11, 2020, the World Health Organization declared COVID‐19 a global pandemic. COVID‐19 is a novel coronavirus with high infectivity and severe morbidity and mortality, especially for those over 70 years old and also for those with comorbid medical conditions such as lung disease and obesity. 1 The entire world has been gripped with fear and practiced social distancing, masking, and quarantine of exposed or infected individuals in an effort to curtail the spread of the disease. 2 , 3 The new virus is characterized primarily by respiratory‐related disease, but also may involve multiple organ systems; COVID‐19 has caught the medical community by surprise due to its myriad of clinical manifestations. Additionally, COVID‐19 has been associated with various cutaneous manifestations; associated cutaneous skin eruptions from natural COVID‐19 infection are described as diffuse erythematous eruptions, widespread urticaria, and chickenpox‐like vesicles. 4 , 5 Two vaccines were approved for use by the FDA on December 11th (Pfizer) and December 18th (Moderna) of 2020 to prevent COVID‐19 infection and change the tide against this deadly pandemic. 6 , 7
At the time of our observation, two vaccines were authorized in the United States to prevent COVID‐19: Moderna (94.5% effective) and Pfizer (95% effective). 8 , 9 Two doses of the aforementioned vaccines are required to reach optimal effectiveness. Side effects may emerge after the first and/or second dose, with common side effects including injection site pain and flu‐like symptoms. 10 Recently, a third vaccine by J&J has been approved for use, requiring only one dose; to our knowledge, there are no reported cases of shingles reaction to the J&J COVID‐19 approved vaccine. 11 In clinical trials, it was noted that the Moderna vaccine was associated with swelling in the area of facial filler injections in some patients. 7 A suggested pathophysiologic mechanism involving angiotensin‐signaling pathways has been posited as the etiology of filler‐associated swelling; in fact, these patients have been successfully treated with angiotensin inhibitors. 12 The initial Pfizer vaccine application mentioned no reported adverse facial filler reactions with vaccination. 6
On 2/5/2021, the 1st case of Post‐COVID‐19 vaccine‐related varicella‐zoster virus (VZV) (Herpes Zoster (HZ) aka shingles) eruptions was seen in the Las Vegas Dermatology clinic and posted on our social media. 13 Indeed on 2/16/2021, one of the authors was on local television and discussed a 2nd case of Post‐COVID‐19 vaccine‐related HZ seen in the clinic. 14 Since those initial patients, there have been a total of 5 other Post‐COVID‐19 vaccine‐related cases seen in the Las Vegas Dermatology clinic. An additional 14 cases have been reported to us and confirmed with medical history and photographs via social media with a mean time of 6.85 days to the first signs/symptoms of shingles. (Table 1 ). However, in the literature, there has been only one other reported case of Post‐COVID‐19 vaccine shingles in Turkey. 15
TABLE 1.
Post‐COVID‐19 vaccine HZ cases seen at the Las Vegas Dermatology clinic and cases reported via social media.
| Age at time of initial vaccination (years old) | Sex | Presentation | Vaccine Type | Days until symptom onset post‐vaccination (PV) | Days until unilateral dermatomal herpetiform skin eruption post‐vaccination (PV) & Location | Comorbid conditions | Comorbid dermatologic conditions | Treatment | Confirmatory Tests |
|---|---|---|---|---|---|---|---|---|---|
| 77 | M | Evaluated in clinic | Moderna, 1st dose | 2 days PV: "odd feeling" and pain | 4 days PV: mid‐ABD, R flank, R mid‐back | Crohn's Disease, h/o treated Aspergilloma | Psoriasis | Valacyclovir, Gabapentin, LMX | Clinically diagnosed |
| 56 | M | Evaluated in clinic | Moderna, 1st dose | None | 4 days PV: R chest | None | Atopic Eczema | Valacyclovir, Gabapentin, LMX | VZV IgG (+), IgM (‐) |
| 54 | F | Evaluated in clinic | Moderna, 1st dose | 4 days PV: itchiness and deep pain | 5 days PV: L back, L shoulder, L triceps | Thyroidectomy, Hysterectomy, Cholecystectomy | Melanoma x3: Tx Wide Local Excision | Valacyclovir, Gabapentin, LMX | VZV PCR (+) |
| 65 | M | Evaluated in clinic | Pfizer, 2nd dose | 14 days PV: itchiness, 17 days post‐vaccine: pain | 38 days PV: L axilla, L shoulder, L triceps | HTN | History of Shingles, no previous shingles vaccination | Valacyclovir, Gabapentin, LMX | VZV PCR (+) |
| 43 | F | Social media | Pfizer, 2nd dose | 1 day PV: severe pain at injection site and body aches | 4 days PV: "bump" on R neck; 8 days PV: R collarbone & R lower jaw | HTN | None | Valacyclovir & Terrasil Shingles cream | Clinically diagnosed |
| 69 | F | Social media | Moderna, 1st dose | 4 days PV: severe pain in upper L back | 4–7 days PV: worsening blistering of L axilla and L upper breast | Polycythemia Vera treated with Jakfi, HTN, Dyslipidemia, Osteopenia | Psoriasis, Actinic keratosis, BCC, SCC, previously vaccinated with Zostavax and Shingrix, | Antiviral | Clinically diagnosed |
| 42 | M | Social media | Moderna, 1st dose | 14 days PV: tingling, mild “skin irritation sensation” radiating from R lower chest to mid‐ABD. 21 days: burning and itchiness | 21 days PV: scattered blistering | None | Childhood chickenpox, No history of shingles | Clinically diagnosed | |
| 47 | F | Social media | Moderna, 1st dose | 12 days PV: burning pain to R axilla and R chest | 15 days PV: T1 dermatome | "Vascular issues to my extremities" | No history of Shingles | Valacyclovir, Gabapentin, Prednisone | Clinically diagnosed |
| 39 | F | Social media | Moderna, 1st dose | 2 days PV: itchiness | 5 days PV: R back and R flank | DM, HTN | None | Valacyclovir | Clinically diagnosed |
| 74 | M | Evaluated in clinic | Pfizer, 2nd dose | None | 3 days PV: Mid‐chest, R arm | Kidney failure, Anemia, HTN, Gout, Heart failure, A‐Fib. | None | Valacyclovir & Mupirocin | Clinically diagnosed |
| 48 | M | Evaluated in clinic | Pfizer, 1st dose | Arm Soreness from Vaccine | 12 days PV: Unilateral Dermatomal L Flank and altered skin sensation | None | Chickenpox at 8 y/o | Valacyclovir, Gabapentin, LMX | VZV PCR (+) |
| 68 | F | Social media | Moderna, dose, 1st dose |
L shoulder injection site pain; 8–9 days PV: CP & tachycardia |
12 days PV: "red blotches erupted" L axilla, L triceps, L scapula |
Hypothyroidism, HTN, adrenal fatigue |
Chickenpox in 2nd grade, "hives from stress", no previous shingles vaccination, childhood rubella, measles at 47 y/o |
Valacyclovir, Tramadol | Clinically diagnosed |
| 46 | M | Social media | Pfizer, 1st dose | Within 7 days PV: Pain at site of future skin eruption | 9 days PV: Unilateral Dermatomal R Flank | None | None | Valacyclovir, Gabapentin, Hydrocodone/acetaminophen | Clinically diagnosed |
| 60 | F | Social media | Moderna, 2nd dose | 24–26 days PV: itching and burning at site of future skin eruption | 26–28 days PV: R forehead along hairline | Treated thyroid cancer, Gout | None | Valacyclovir | Clinically diagnosed |
| 43 | M | Social media | Moderna, 1st dose | 2 days PV: hotness and altered skin sensation at site of future skin eruption | 3 days PV: Unilateral Dermatomal R Flank and back | None | Chickenpox 4–5 y/o, no previous shingles vaccination | Valacyclovir and Gabapentin | Clinically diagnosed |
| 65 | F | Social media | Moderna, 1st shot | 2 days PV: injection site pain | 11 days PV: tingling, itching, burning, and eruption around and under right eye | None | Shingles 10 years prior to current episode, vaccinated for shingles in 2015 and 2019 | Valacyclovir | Clinically diagnosed |
| 44 | M | Social media | Pfizer, 1st dose | 3 days PV: itchiness | 5 days PV: L eyebrow | None | None | Acyclovir, Desonide cream | Clinically diagnosed |
| 37 | F | Social media | Moderna, 1st dose | 2 days PV: nerve pain | 4–5 days PV: L back and L ABD | None | None | Valacyclovir | Clinically diagnosed |
| 69 | M | Social media | Moderna, 1st dose | None | 14 days PV: Unilateral R back and R arm | Hypercholesterolemia | "Susceptible to poison ivy," vaccinated for shingles in July 2020 | Valacyclovir | Clinically diagnosed |
| 72 | F | Social media | Moderna, 2nd dose | 3–4 days PV: 2 in raised, red spot at injection site with accompanying pain; 4–5 days PV: itching and burning at site of future skin eruption | 5–6 days PV: L arm, L upper back, L breast | HTN, treated R breast cancer | "no, but am often itchy" | Valacyclovir | Clinically diagnosed |
Vaccine Used→Shingles: 14 Moderna & 6 Pfizer‐; Mean 6.85 Days Post‐Vaccine→Shingles; Median 4 Days Post‐Vaccine→Shingles; Mode 2 Days Post‐Vaccine→Shingles; Mean 10.2 days→Eruption.
Unknown information.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
1. DEMONSTRATIVE CASES
1.1. Case 1
A‐77‐year‐old man with a history of Psoriasis treated with Methotrexate, Crohn's Disease, and treated Aspergilloma presented to the clinic for evaluation of a severely painful, unilateral dermatomal herpetiform eruption in a T11 distribution from the right mid‐abdomen, right flank, and right mid‐back. (Figure 1 ) two days post his 1st dose of the Moderna COVID‐19 vaccine—the patient initially developed pain in the area of what would become his shingles eruption. Per the natural course of his disease, the initial pain worsened to become severely painful 4 days post‐vaccine with a classic HZ eruption seen in accompanying photographs. The patient was clinically diagnosed with Herpes Zoster and treated with a course of Valacyclovir for the virus; oral Gabapentin, Oxycodone, and topical Lidocaine 4% for the pain. Despite the shingles outbreak post‐initial vaccination, the patient was still recommended to receive his second dose of the Moderna COVID‐19 vaccine.
FIGURE 1.
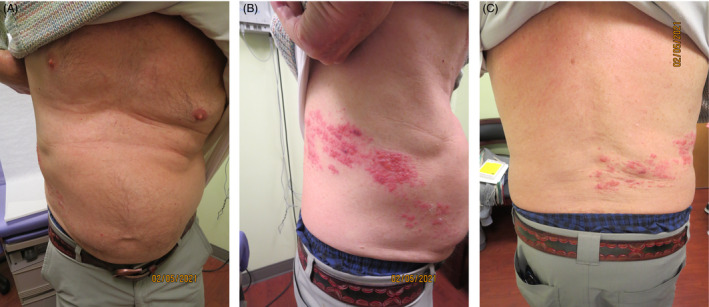
First patient to present with Post‐COVID‐19 vaccine shingles (Moderna 1st dose). Dermatological examination demonstrates unilateral dermatomal herpetiform eruption in a T11 distribution from the (a) mid‐abdomen, (b) right flank, and (c) right mid‐back.
1.2. Case 2
A 65–year‐old man with a history of shingles 35 years prior to presentation and long‐standing hypertension was consulted for painful, erythematous, and clustered skin eruptions. Symptoms first began after his 2nd dose of the Pfizer COVID‐19 vaccine, with pruritus 14 days post‐vaccination, followed by increasing chest and back pain 3 days post‐pruritus. The patient was diagnosed with HZ, later confirmed via VZV PCR, and was treated with a course of Valacyclovir for the virus, oral Gabapentin, and topical Lidocaine 4% for pain. He was the 4th patient seen in our clinic for HZ related to COVID‐19 vaccination. Several other individuals contacted us via social media to share their experience, contributing to our list of Post‐COVID‐19 vaccine HZ eruptions (Table 1 ).
HZ is attributed to reactivation of VZV. Primary infection with VZV results in chickenpox; once resolved, VZV establishes lifelong dormancy in multiple sensory ganglia and may re‐emerge due to risk factors including increasing age, immunocompromised condition, and/or stress. 16 Cases of shingles have also been seen in patients vaccinated to VZV. 17 VZV and herpes viruses in general are distinguished by their ability to form latent infections. Kaposi's varicelliform eruption (KVE), also known as eczema herpetiformis, is a rare and potentially fatal reactivation of herpes virus in individuals with a history of atopic dermatitis; outbreaks of KVE have historically been seen when atopic patients were vaccinated for smallpox. 18 Underlying skin conditions such as atopic dermatitis and psoriasis have also been associated with Kaposi's varicelliform eruption. 18 , 19 Two of our post‐COVID‐19 vaccinated patients have a chronic history of psoriasis and presented with earlier onset of shingles eruption compared to most other reported cases (Table 1 ).
There have been a number of natural COVID‐19 reported infections with HZ coinfection, including cases with ophthalmic involvement. 20 , 21 Several cases of vesicular eruptions in middle‐aged patients have also been reported earlier in the course of COVID‐19 infection. 4 COVID‐19 is associated with decreased number of lymphocytes throughout the course of infection including a decreased number of monocytes, eosinophils, and CD4/CD8 T cells. 22 VZV has been shown to reduce the number of T cells, B cells, natural killer (NK) cells, and monocytes. 23 It is still early in the course of vaccine distribution; besides personal experiences, delayed reactions of COVID‐19 vaccination are incompletely understood. It is hypothesized that immunomodulation related to COVID‐19 vaccination is associated with VZV reactivation seen in our patients. Recently, the University of Birmingham announced they will be investigating vaccine effectiveness in individuals with significant underlying diseases, transplant patients, people with liver or kidney disease, cancer, and inflammatory arthritis. 24 Because of the associated morbidity and mortality seen in patients with COVID‐19, and the high infectivity rate, society must be weary of misinformation by professional anti‐vaccine activists that would increase vaccine hesitancy. 1 Pre‐clinical studies demonstrate mRNA vaccines elicit strong CD8/CD4 T‐cell responses, with favorable safety profile in animals, and are rapid to design for emerging infectious diseases. 25 Mass vaccination is the paramount goal, and the United States is anticipated to have enough vaccines available for all adults by the end of May 2021. 26 It is likely that emerging epidemiological studies are underway. With a promising end to the pandemic, perhaps then we can better understand the relationship of COVID‐19 vaccination and VZV reactivation. COVID‐19 vaccination is the highest priority. Although there are no current recommendations from the CDC or other regulatory bodies, it is the authors’ recommendation that at‐risk patients (ie, patients over 50 years of age) obtain the shingles vaccine prior to COVID‐19 vaccination.
REFERENCES
- 1. Centers for Disease and Control Prevention . Weekly updates by select demographic and geographic characteristics. https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm. Published 2021. Accessed March 1, 2021.
- 2. Executive Office of the President . Suspension of Entry as Immigrants and Nonimmigrants of Persons Who Pose a Risk of Transmitting 2019 Novel Coronavirus and Other Appropriate Measures To Address This Risk. Federal Register: The Daily Journal of the United States Government. https://www.federalregister.gov/documents/2020/02/05/2020‐02424/suspension‐of‐entry‐as‐immigrants‐and‐nonimmigrants‐of‐persons‐who‐pose‐a‐risk‐of‐transmitting‐2019. Published 2020. Accessed March 1, 2020.
- 3. Executive Office of the President . Declaring a National Emergency Concerning the Novel Coronavirus Disease (COVID‐19) Outbreak. Federal Register: The Daily Journal of the United States Government. https://www.federalregister.gov/documents/2020/03/18/2020‐05794/declaring‐a‐national‐emergency‐concerning‐the‐novel‐coronavirus‐disease‐covid‐19‐outbreak. Published 2020. Accessed March 1, 2021.
- 4. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020. 10.1111/bjd.19163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatology Venereol. 2020. 10.1111/jdv.16387 [DOI] [PubMed] [Google Scholar]
- 6. U.S. Food and Drug Administration . Pfizer‐BioNTech COVID‐19 Vaccine. Food and Drug Administration. https://www.fda.gov/emergency‐preparedness‐and‐response/coronavirus‐disease‐2019‐covid‐19/pfizer‐biontech‐covid‐19‐vaccine. Published 2021. Accessed March 5, 2021.
- 7. U.S. Food and Drug Administration . Moderna COVID‐19 Vaccine. Food and Drug Administration. https://www.fda.gov/emergency‐preparedness‐and‐response/coronavirus‐disease‐2019‐covid‐19/moderna‐covid‐19‐vaccine#additional. Published 2021. Accessed February 23, 2021.
- 8. Mahase E. Covid‐19: Moderna vaccine is nearly 95% effective, trial involving high risk and elderly people shows. BMJ. 2020. 10.1136/bmj.m4471 [DOI] [Google Scholar]
- 9. Mahase E. Covid‐19: Pfizer vaccine efficacy was 52% after first dose and 95% after second dose, paper shows. BMJ. 2020. 10.1136/bmj.m4826 [DOI] [PubMed] [Google Scholar]
- 10. Wadman M. Public needs to prep for vaccine side effects. Science. 2020;370(6520):1022. 10.1126/science.370.6520.1022 [DOI] [PubMed] [Google Scholar]
- 11. U.S. Food and Drug Administration . Janssen COVID‐19 Vaccine. Food and Drug Administration. https://www.fda.gov/emergency‐preparedness‐and‐response/coronavirus‐disease‐2019‐covid‐19/janssen‐covid‐19‐vaccine. Published 2021. Accessed March 5, 2021.
- 12. Munavalli GG, Guthridge R, Knutsen‐Larson S, Brodsky A, Matthew E, Landau M. COVID‐19/SARS‐CoV‐2 virus spike protein‐related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment. Arch Dermatol Res. 2021;15:1–15. 10.1111/jocd.14035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenberg HL. Shingles 1 day post Covid19 Vaccination. https://www.instagram.com/tv/CK9gebWjilh/. Published 2021. Accessed February 6, 2021.
- 14. Kim K. Las Vegas doctor wants more vaccine research after treating two shingles patients. https://news3lv.com/news/local/las‐vegas‐doctor‐wants‐more‐vaccine‐research‐after‐treating‐two‐shingles‐patients. Published 2021. Accessed February 16, 2021.
- 15. Bostan E, Yalici‐Armagan B. Herpes zoster following inactivated COVID‐19 vaccine: A coexistence or coincidence? J Cosmet Dermatol. 2021;5:1566–1567. 10.1111/jocd.14035 [DOI] [PubMed] [Google Scholar]
- 16. Cohen JI. Clinical practice: Herpes zoster. N Engl J Med. 2013;369(3):255‐263. 10.1056/NEJMcp1302674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moodley A, Swanson J, Grose C, Bonthius DJ. Severe herpes zoster following varicella vaccination in immunocompetent young children. J Child Neurol. 2019;34(4):184‐188. 10.1177/0883073818821498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferrari B, Taliercio V, Luna P, Abad M, Larralde M. Kaposi′s varicelliform eruption: A case series. Indian Dermatol Online J. 2015;6(6):399‐ 10.4103/2229-5178.169714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olson J, Robles DT, Kirby P, Colven R. Kaposi varicelliform eruption (eczema herpeticum). Dermatol Online J. 2008;14(2):18–18. [PubMed] [Google Scholar]
- 20. Elsaie ML, Nada HA. Herpes zoster (shingles) complicating the course of COVID19 infection. J Dermatolog Treat. 2020;1‐3. 10.1080/09546634.2020.1782823 [DOI] [PubMed] [Google Scholar]
- 21. Ferreira ACAdF, Romão TT, Macedo YS, Pupe C, Nascimento OJM. COVID‐19 and herpes zoster co‐infection presenting with trigeminal neuropathy. Eur J Neurol. 2020;27(9):1748‐1750. 10.1111/ene.14361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med (CCLM). 2020;58(7):1021‐1028. 10.1515/cclm-2020-0369 [DOI] [PubMed] [Google Scholar]
- 23. Gerada C, Campbell TM, Kennedy JJ, et al. Manipulation of the innate immune response by varicella zoster virus. Front Immunol. 2020;11:1–17. 10.3389/fimmu.2020.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. University of Birmingham . New study launches into COVID‐19 vaccine responses in patients with impaired immune systems. University of Birmingham.
- 25. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines‐a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261‐279. 10.1038/nrd.2017.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sheryl Gay S, Sharon LaFraniere KT, M. D. S. Biden vows enough vaccine ‘for Every Adult in America’ by End of May. The New York Times. https://www.nytimes.com/2021/03/02/us/politics/merck‐johnson‐johnson‐vaccine.html. Published 2021. Accessed March 3, 2021.


