Abstract
COVID‐19 has significant case fatality. Glucocorticoids are the only treatment shown to improve survival, but only among patients requiring supplemental oxygen. WHO advises patients to seek medical care for “trouble breathing,” but hypoxemic patients frequently have no respiratory symptoms. Our cohort study of hospitalized COVID‐19 patients shows that respiratory symptoms are uncommon and not associated with mortality. By contrast, objective signs of respiratory compromise—oxygen saturation and respiratory rate—are associated with markedly elevated mortality. Our findings support expanding guidelines to include at‐home assessment of oxygen saturation and respiratory rate in order to expedite life‐saving treatments patients to high‐risk COVID‐19 patients.
Keywords: COVID‐19, death, epidemiology, hypoxemia, respiratory rate
1. SHORT REPORT
COVID‐19 is a global pandemic with significant case fatality, often due to respiratory failure. Few treatments have improved morbidity,1, 2 and the only pharmacologic intervention reported to improve survival is use of glucocorticoids in hospitalized COVID‐19 patients requiring supplemental oxygen.3 The National Institutes of Health recommends maintaining oxygen saturation levels at 92%‐96% for COVID‐19 patients.4 COVID‐19 patients often have unrecognized hypoxemia without experiencing overt respiratory symptoms5, 6 resulting in a missed opportunity to institute early, potentially life‐saving treatment. While the Centers for Disease Control (CDC) and World Health Organization (WHO) advise COVID‐19 patients to seek medical attention if they have “trouble breathing,” there are no current guidelines to assess objective signs of respiratory insufficiency at home, such as oxygen saturation or respiratory rate.7 We evaluated whether initial oxygen saturation and respiratory rate are associated with all‐cause mortality in hospitalized COVID‐19 patients.
Study cohort included consecutive COVID‐19 patients ≥18 years of age hospitalized at University of Washington, Seattle, WA, or Rush University, Chicago, IL medical centers between March 1, and June 8, 2020. Exclusion criteria included persons choosing “comfort measures only” at hospital admission and those without clinical symptoms of COVID‐19 admitted for non‐COVID‐19‐related medical issues. SARS‐CoV‐2 infection was confirmed using qRT‐PCR. Study was approved by Institutional Review Boards at both institutions.
Patient information and clinical outcomes were collected by physician chart review. Primary outcome was all‐cause in‐hospital mortality. Poisson regression models with robust standard errors were used to calculate relative risks (RR) and 95% confidence intervals (CI) for the associations of oxygen saturation and respiratory rate with the mortality. Models were adjusted for age, sex, race, health system, nursing home residence, smoking status, hypertension, diabetes, body mass index [BMI], pulmonary disease, and cardiovascular disease. In secondary analyses, linear regression models were used to examine associations of candidate risk factors with admission oxygen saturation and respiratory rate. Multiple imputation with chained equations was used to impute missing BMI values (n = 46).
During the study, 1,095 individuals were hospitalized with symptomatic COVID‐19. Patients had mean age of 58, were mostly men (62%), and commonly had comorbidities (Table 1). While patients frequently had hypoxemia (mean oxygen saturation of 91%) and tachypnea (mean respiratory rate of 23 breaths per minute) on presentation, few reported shortness of breath (10%) or cough (25%) regardless of oxygen saturation. The most common symptom at presentation was fever (73%). Patient respiratory symptoms and symptomatic fever were not associated with mortality.
TABLE 1.
Baseline characteristics
| Total (N = 1095) | |
|---|---|
| Age, years | 58 ± 17 |
| Male sex, n (%) | 684 (62%) |
| Race, n (%) | |
| White | 235 (21%) |
| Hispanic | 410 (37%) |
| Black | 384 (35%) |
| Asian | 51 (5%) |
| Unknown | 15 (1%) |
| Nursing Home Resident, n (%) | 109 (10%) |
| Body mass index,a kg/m2 | 32 ± 9 |
| Prior Medical History, n (%) | |
| Hypertension | 588 (54%) |
| Diabetes mellitus | 357 (33%) |
| Coronary disease | 129 (12%) |
| Heart Failure | 126 (12%) |
| Myocardial infarction | 35 (3%) |
| Peripheral arterial disease | 33 (3%) |
| Stroke | 98 (9%) |
| Chronic Kidney disease | 187 (17%) |
| Chronic Liver failure | 43 (4%) |
| Admission Characteristics | |
| Oxygen saturation, %b | 91 ± 9 |
| Supplemental oxygen use, n (%) | 819 (75%) |
| Heart rate, beats per minute | 93 ± 19 |
| Respiratory rate, breaths per minute | 23 ± 6 |
| Temperature, F | 99.7 ± 2 |
| Systolic blood pressure, mmHg | 128 ± 22 |
| Diastolic blood pressure, mmHg | 75 ± 15 |
| Symptoms at Presentation | |
| Fever | 792 (73%) |
| Shortness of breath | 112 (10%) |
| Cough | 282 (26%) |
| Myalgia | 237 (22%) |
| Fatigue | 275 (25%) |
| GI symptoms | 527 (48%) |
| Chest pain | 118 (11%) |
| Syncope | 20 (2%) |
| Days symptomatic prior to admission | 6 ± 4 |
Continuous variables are reported as mean ±standard deviation.
Body mass index information was missing for 46 patients; supplemental oxygen use was missing for 3 patients; temperature was missing for 3 patients; systolic and diastolic blood pressure was missing for 1 patient; symptoms at presentation were missing for 6 patients; and days symptomatic prior to admission was missing for 13 patients. Multiple imputation with chained equations was used to impute missing values of BMI (n = 46) using information on admission oxygen saturation, respiratory rate, age, sex, race, health system, and prevalent hypertension, diabetes, and cardiovascular disease, and in‐hospital mortality.
Oxygen saturation reflects measurement upon patient presentation prior to initiation of supplemental oxygen.
Overall, 197 patients died in hospital. After adjustment for risk factors, both hypoxemia and tachypnea were associated with mortality risk (Figure 1). Compared to normoxemic patients, those who were hypoxemic (oxygen saturation <92%) had a 1.8‐ to 4.0‐fold increased mortality risk, depending on initial oxygen saturation. Similarly, compared to patients with normal respiratory rate (≤20 beats per minute), those with respiratory rates >22 breaths per minute were at 1.9‐ to 3.2‐fold elevated mortality risk. Nearly, all hypoxemic (99%) and tachypneic (98%) patients required supplemental oxygen administration during hospitalization. By contrast, other clinical signs at presentation, including temperature, heart rate, and blood pressure, were not associated with mortality. Findings were similar among subgroups stratified by demographic and clinical characteristics.
FIGURE 1.
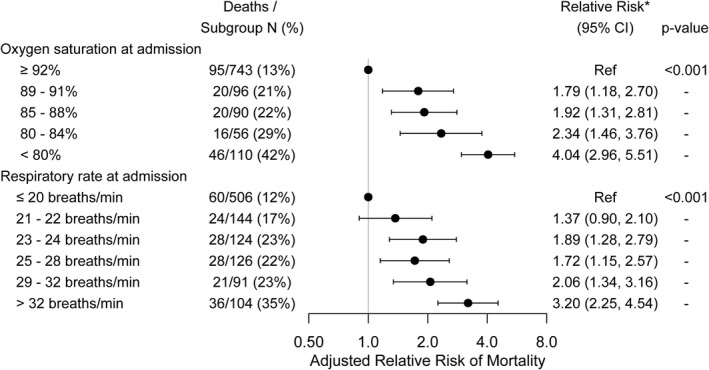
Association of oxygen saturation and respiratory rate on admission with in‐hospital mortality. *Multivariable adjustment is for age, sex, race, health system, hypertension, diabetes mellitus, body mass index, pulmonary disease, cardiovascular disease, smoking, and nursing home residence
No clinical or demographic characteristic was associated with admission oxygen saturation or respiratory rate with one notable exception. BMI was associated with both lower oxygen saturation (β‐Coefficient −0.95, [CI: −1.32, −0.57] per 5 kg/m2 higher BMI, P < 0.001) and more rapid respiratory rate (β‐Coefficient 0.36 [CI:0.14, 0.59] per 5 kg/m2 higher BMI, P = 0.001). Low admission oxygen saturation partly accounted for the higher mortality rate seen with obesity; the association between BMI and all‐cause mortality (RR 1.08 [95% CI: 1.00, 1.16] per 5 kg/m2 higher BMI, P = 0.04) was attenuated after adjustment for admission oxygen saturation (RR 1.03 [95% CI: 0.96, 1.11], P = 0.42).
Our study shows that indices of respiratory compromise at initial presentation that are readily measurable at home—oxygen saturation <92% or a respiratory rate >22 breaths per minute—were each associated with elevated mortality in hospitalized COVID‐19 patients. Current CDC and WHO COVID‐19 guidelines do not include recommendations for measuring oxygen saturation or respiratory rate at home; instead, they recommend patients seek medical attention if they have “trouble breathing.”7 In our study, only 10% of hospitalized patients reported shortness of breath, and admission respiratory symptoms were not associated with hypoxemia or mortality, underscoring that respiratory symptoms are uncommon and by themselves may not accurately identify at‐risk patients.5
Hypoxemia has significant therapeutic implications; only COVID‐19 patients who require supplemental oxygen benefit from medications that decrease mortality. We and others have shown that hypoxemia is often asymptomatic which may result in a delay in life‐saving treatment. Together, with our findings showing markedly increased mortality risk with hypoxemia, these data support expanding public health messaging to include at‐home assessment of oxygen saturation for COVID‐19 patients. In resource‐limited settings where oxygen saturation monitoring devices are not accessible, respiratory rate of >22 may serve as a surrogate marker for respiratory compromise. Consideration of inclusion of hypoxemia and tachypnea in guidelines as indication for COVID‐19 patients to seek medical consultation is warranted.
While the majority of patients in our study had one or more comorbidities, the increased mortality risk associated with hypoxemia and tachypnea was present irrespective of comorbidities.6 Obese patients have an elevated risk of silent hypoxemia and tachypnea that partly account for their elevated mortality risk and are likely to benefit most from assessing their respiratory status during acute COVID‐19.8
Study limitations include analysis restricted to hospitalized patients with comorbidities which may not generalize to non‐hospitalized patients. Since patient symptoms were retrospectively abstracted from medical records, symptom prevalence may be underestimated. We cannot exclude the possibility of residual confounding. Although pulse oximetry may underestimate the severity of hypoxemia in patients with darker skin tones,9 our study findings were robust among those of African and Hispanic ancestry. While the accuracy of at‐home pulse oximeters may be compromised at oxygen saturations less than 90%,10 ascertainment at the risk threshold identified in this study (92%) is attainable.
In summary, indices of respiratory compromise at initial presentation, that are readily measurable at home, are associated with markedly elevated risk of in‐hospital mortality in COVID‐19 patients. Public health strategies emphasizing the importance of at‐home surveillance of oxygen saturation and respiratory rate may identify at‐risk patients earlier and enable timely institution of life‐saving medical therapy.
DISCLOSURES
The authors each report no disclosures relevant to the content of the manuscript.
AUTHOR CONTRIBUTIONS
Neal Chatterjee: Conceptualization (equal); Formal analysis (lead); Investigation (equal); Methodology (equal); Project administration (equal); Writing‐original draft (lead); Writing‐review & editing (equal). Paul Jensen: Data curation (equal); Formal analysis (lead); Investigation (equal); Methodology (equal); Writing‐original draft (equal). Andrew Harris: Data curation (equal); Resources (equal). Daniel D. Nguyen: Data curation (equal); Resources (equal). Henry Huang: Data curation (equal); Writing‐review & editing (equal). Richard Cheng: Data curation (equal); Writing‐review & editing (equal). Jainy Savla: Data curation (equal). Timothy Larsen: Data curation (equal). Joanne Michelle Gomez: Data curation (equal); Writing‐review & editing (equal). Jeanne Du‐Fay‐de‐Lavallaz: Data curation (equal); Writing‐review & editing (equal). Rozenn Lemaitre: Investigation (supporting); Writing‐review & editing (equal). Barbara McKnight: Investigation (supporting); Methodology (supporting); Writing‐original draft (supporting). Sina A Gharib: Investigation (supporting); Supervision (supporting); Writing‐review & editing (equal). Nona Sotoodehnia: Conceptualization (lead); Formal analysis (equal); Investigation (equal); Project administration (lead); Supervision (lead); Writing‐original draft (equal); Writing‐review & editing (equal).
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/irv.12869.
ACKNOWLEDGEMENTS
We wish to acknowledge the frontline workers engaged in the care of patients with COVID‐19 in our communities. We also wish to acknowledge the following individuals who assisted with data abstraction at the University of Washington (Akash Kataruka, MD, Fitz Medhane, MD, Andrew Lee, MD, Michael Morcos, MD, Basil Saour, MD, Douglas Leedy, MD, Christian Ngo, MD, and Liam Hovey, BS) and Rush University Medical Center (Isabel Camara‐Planek, MD, Mary Potkonjak, MD, Vishal Birk, MD, Annas Rahman, MD, Alexander Hlepas, MD, Gianna Bosco, MD, Clay Hoster, MD, Athina Bourourkas, MD, Max Ruge, MD, Alexandra Sarau, MD, Priya P. Patel, MD, Tai Nguyen, MD, Benjamin Schwartz, MD, and Anna Zemke MD). We would also like to thank Ayushi Gupta and the University of Washington Allergy and Infectious Disease Research Collaboratory for assistance with data acquisition. Finally, we are appreciative of the many friends, family, coworkers, and patients stricken with COVID‐19 who have inspired this work.
Chatterjee NA, Jensen PN, Harris AW, et al. Admission respiratory status predicts mortality in COVID‐19. Influenza Other Respi Viruses. 2021;15:569–579. 10.1111/irv.12869
Funding information
Laughlin Family Fund (NS)
Contributor Information
Neal A. Chatterjee, Email: nchatter@uw.edu, Email: nsotoo@uw.edu.
Nona Sotoodehnia, Email: nchatter@uw.edu, Email: nsotoo@uw.edu.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in covid‐19 severe pneumonia. N Engl J Med. 2020;384(7):619‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with covid‐19 pneumonia. N Engl J Med. 2021;384(1):20‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid‐19. N Engl J Med. 2021;384(8):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. https://www.covid19treatmentguidelines.nih.gov/critical‐care/oxygenation‐and‐ventilation/
- 5.Simonson TS, Baker TL, Banzett RB, et al. Silent hypoxaemia in covid‐19 patients. J Physiol. 2021;599(4):1057‐1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with Covid‐19 in the New York City area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Covid‐19 . Accessed february 12, 2021 at https://www.Cdc.Gov/coronavirus/2019‐ncov/if‐you‐are‐sick/steps‐when‐sick.Html. World health organization coronavirus disease (covid‐19) advice for the public. Accessed february 12, 2021 at https://www.Who.Int/emergencies/diseases/novel‐coronavirus‐2019/advice‐for‐public
- 8.Hendren NS, de Lemos JA, Ayers C, et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with Covid‐19: Results from the American heart association Covid‐19 cardiovascular disease registry. Circulation. 2021;143(2):135‐144. [DOI] [PubMed] [Google Scholar]
- 9.Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial bias in pulse oximetry measurement. N Engl J Med. 2020;383(25):2477‐2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luks AM, Swenson ER. Pulse oximetry for monitoring patients with covid‐19 at home. Potential pitfalls and practical guidance. Ann Am Thorac Soc. 2020;17(9):1040‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


