Abstract
Objectives
Occurrence of invasive fungal respiratory superinfections in patients with COVID‐19 has gained increasing attention in the latest studies. Yet, description of acute invasive fungal sinusitis with its management in those patients is still scarce. This study aims to describe this recently increasing clinical entity in relation to COVID‐19 patients.
Study Design
Longitudinal prospective study.
Methods
Prospective longitudinal study included patients diagnosed with acute invasive fungal rhinosinusitis after a recent COVID‐19 infection. Antifungal agents given included amphotericin B, voriconazole, and/or posaconazole. Surgical treatment was restricted to patients with PCR negative results for COVID‐19. Endoscopic, open, and combined approaches were utilized to eradicate infection. Follow‐up for survived patients was maintained regularly for the first postoperative month.
Results
A total of 36 patients with a mean age of 52.92 ± 11.30 years old were included. Most common associated disease was diabetes mellitus (27.8%). Mycological analysis revealed infection with Mucor and Aspergillus species in 77.8% and 30.6% of patients, respectively. Sino‐nasal, orbital, cerebral, and palatine involvement was found in 100%, 80.6%, 27.8%, and 33.3% of patients, respectively. The most common reported symptoms and signs are facial pain (75%), facial numbness (66.7%), ophthalmoplegia, and visual loss (63.9%). All patients were treated simultaneously by surgical debridement with antifungal medications except for two patients with PCR‐positive swab for COVID‐19. These two patients received antifungal therapy alone. Overall survival rate was 63.89% (23/36).
Conclusion
Clinical suspicion of acute invasive fungal sinusitis among COVID‐19 patients and early management with antifungal therapy and surgical debridement is essential for better outcomes and higher survival.
Level of Evidence
4 Laryngoscope, 131:2652–2658, 2021
Keywords: Invasive fungal infection, COVID‐19, SARS‐CoV‐2, sinusitis
INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is the leading cause of an emergency global pandemic. 1 , 2 Beside the Acute Respiratory Distress Syndromes (ARDS) caused by it, COVID‐19 patients were found to have immune‐suppression attributed to a decrease in CD4+T and CD8+T cells. 3 This results in a diverse range of bacterial and fungal infections that may co‐exist with possible association with a preexisting morbidity (diabetes mellitus, lung disease) or may develop as a hospital‐acquired infection.
Acute invasive fungal rhino‐sinusitis (AIFR) is a potentially fatal infection that is notably found in immune‐compromised patients and considered the most aggressive subtype of fungal sinusitis with subsequent serious morbidity and mortality. The reported most frequently affected individuals with invasive fungal infections include those with malignancy, uncontrolled diabetes, AIDS, immunosuppressive, and chemotherapeutic drugs and recently, COVID‐19. 4 , 5
Literature review regarding association of AIFR with COVID‐19 revealed only isolated case reports. 6 , 7 Herein, the primary objective of this study is to demonstrate this new clinical entity in relation to COVID‐19 pandemic and its aspects and management in our institution.
PATIENTS AND METHODS
Study Design
Longitudinal prospective study.
Setting
Tertiary referral center.
Participants
Patients diagnosed with AIFR, associated with a recent COVID‐19 infection, and treated at Department of Otorhinolaryngology, Mansoura University from August 2020 to December 2020 were analyzed. All patients had a definite diagnosis of COVID‐19 by polymerase chain reaction (PCR) and computed tomography (CT) studies with post‐COVID invasive fungal sinusitis. Diagnosis of invasive fungal sinusitis was made according to recent guidelines. 8 It included an acute course (less than 4 weeks) with histological documented fungal invasion within sinus mucosa, submucosa, or bone. Patients with incomplete data or lost follow‐up were excluded from the study.
After institutional review board approval and obtaining written consent from studied population, patient demographics, presenting symptoms and signs, imaging findings, pathological data, medical and surgical treatment approaches and clinical outcomes were tabulated and analyzed.
Investigations
Computed tomography scans were obtained for all studied patients for both chest and nose and paranasal sinuses. Magnetic resonance imaging (MRI) studies were performed when orbital or intracranial involvement was suspected. Also, magnetic resonance venography (MRV) was obtained to assess sinus thrombosis.
Patients were biopsied for histopathological confirmation of AIFR and determination of the causative organism. The histopathological diagnosis was determined from the morphology using hematoxylin and eosin (H&E), periodic acid‐Schiff, and Gomori's methenamine silver staining. Mucor and Aspergillus species were histologically differentiated based on their microscopic appearance.
Interventions
Both antifungal medications and surgical intervention together with control of any associated medical condition were performed. Antifungal therapy was the main line of treatment for PCR‐positive patients for COVID‐19 in this study. Choice of antifungal agents typically consisted of amphotericin B, voriconazole, and/or posaconazole and was managed by infectious disease specialists depending on the organism detected and patient's condition with therapeutic drug monitoring. Surgical intervention was not performed except after obtaining two successive negative swab results for COVID‐19.
Surgical procedure was tailored according to each patient's findings and by extension of the infection. Endoscopic, open, and combined approaches were utilized with serial debridement to eradicate infection. Management of intracranial sinus thrombosis by anticoagulants was carried out in coordination with neurology consultants. Operated patients were considered cleared from fungal infection after two endoscopic negative histopathologic evaluations. Survived patients were maintained on periodic ambulatory endoscopic evaluation regularly during the follow up period to ensure eradication of the disease.
Statistical Analysis
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 18.0 (SPSS, Chicago, IL, USA). Continuous variables are presented as means ± standard deviation (SD) while discrete variables are presented as number and percent. P‐value < .05 is considered statistically significant.
RESULTS
Thirty‐six patients were diagnosed with AIFR in relation to COVID‐19 infection. All patients were diagnosed in the recent post COVID‐19 infection period, with mean duration of 17.82 ± 2.97 days after being PCR negative, except for two patients who developed AIFR with active COVID‐19 infection. The mean age was 52.92 ± 11.30 years (range 22–73). Patients' demographic, clinical data, and comorbidities are listed in Table I. The most common associated disease was diabetes mellitus (DM) (27.8%) and hypertension (16.67%). Among 10 patients diagnosed with diabetes, 3 of them had a recent onset diagnosis caused by corticosteroid therapy given during COVID‐19 treatment. Associated malignancy, uncontrolled leukemia, and pancreatic cancer, was found in two patients (5.56%). Histopathological evaluation of mucosal biopsies confirmed that the main causative fungi were Mucor species in 77.8% and Aspergillus fumigatus in 30.6% while three patients (8.3%) were infected with both (Table I).
TABLE I.
Demographic Data, Associated Comorbidities, Causative Organisms, Medical and Surgical Management Outlines, and Outcomes for the Studied AIFR Patients After COVID‐19 Infection.
| Variable | Number | Frequency (%) |
|---|---|---|
| Total cases | 36 | 100 |
| Age (years) (mean ± SD) | 52.92 ± 11.30 | |
| Sex (male/female) | 19/17 | 52.8/47.22 |
| Comorbidities | ||
| DM | 10 | 27.8 |
| Malignancy | 2 | 5.56 |
| HTN | 6 | 16.67 |
| CKD | 3 | 8.33 |
| Asthma | 3 | 8.33 |
| Cardiac | 1 | 2.78 |
| Hypothyroidism | 1 | 2.78 |
| SLE | 2 | 5.56 |
| Days passed since COVID –ve (mean ± SD) | 17.82 ± 2.97 | |
| Organism | ||
| Mucor | 28 | 77.8 |
| Aspergillus | 11 | 30.6 |
| Antifungal therapy | ||
| Amphotericin B | 28 | 77.8 |
| Voriconazole | 10 | 27.8 |
| Posaconazole | 3 | 8.33 |
| Surgical therapy | ||
| No. of interventions | 2 ± 1 | |
| Purely endoscopic approach | 24 | 66.7 |
| Outcomes | ||
| Survival | 23 | 63.89 |
| Died | 13 | 36.11 |
AIFR = acute invasive fungal rhinsinusitis; CKD = chronic kidney disease; DM = diabetes mellitus; HTN = hypertension; SD = standard deviation; SLE = systemic lupus erythematosus.
A summary of presenting symptoms and signs and disease extent is presented in Table II. Endoscopic findings and CT scan showed various stages of sinonasal (100%), orbital (80.6%), cerebral (27.8%), and palatine (33.3%) involvement. Headache and facial pain (75%), facial numbness (66.7%), ophthalmoplegia, and visual loss (63.9%) were the most common symptoms and signs. Proptosis was documented in 19 patients while 6 patients complained of diplopia. Palatal affection had variable patterns which involved small palatal ulcers with necrosis (Fig. 1A) (11.1%), unilateral large palatal necrosis (Fig. 1B) (19.4%), and central palatal necrosis (Fig. 1C) (2.7%). Skin in the present study was involved with fungal infection in two cases, one with necrosis of cheek area (Fig. 2A) and the other with necrosis of cheek, eye, and lateral nasal wall area (Fig. 2B).
TABLE II.
Clinical Extension With Presenting Symptoms and Signs for the Studied AIFR Patients After COVID‐19 Infection
| Variable | Number (n = 36) | Frequency (100%) |
|---|---|---|
| Clinical extension | ||
| Nasal | ||
| Maxillary sinus | 17 | 47.22 |
| Ethmoid sinus | 26 | 72.22 |
| Sphenoid sinus | 20 | 55.6 |
| Frontal sinus | 9 | 16.7 |
| Lateral nasal wall | 31 | 86.1 |
| Septum | 25 | 69.4 |
| Orbit | ||
| Subperiosteal/orbital abscess | 2 | 5.6 |
| Orbital invasion | 7 | 19.4 |
| Orbital apex syndrome | 20 | 55.6 |
| Intracranial | 10 | 27.8 |
| Palate | 12 | 33.3 |
| Skin | 2 | 5.6 |
| Symptoms and signs | ||
| Nasal | ||
| Headache | 27 | 75 |
| Facial numbness | 24 | 66.7 |
| Nasal discharge | 17 | 47.2 |
| Orbital | ||
| Ophthalmoplegia | 23 | 63.9 |
| Proptosis | 19 | 52.8 |
| Visual loss | 23 | 63.9 |
| Diplopia | 6 | 16.7 |
| Central/cranial nerve affection | ||
| Altered mental state | 6 | 16.7 |
| Facial nerve palsy | 7 | 19.4 |
| Bulbar palsy | 3 | 8.3 |
| Palatal necrosis and ulceration | 12 | 33.3 |
AIFR = acute invasive fungal rhinsinusitis.
Fig 1.
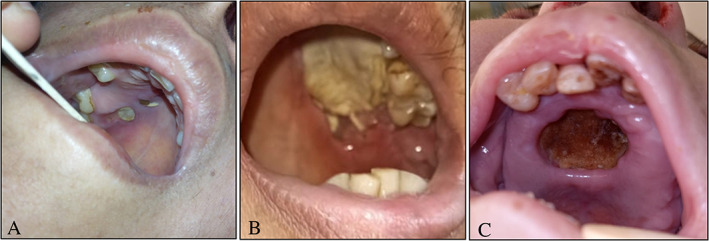
Different patterns of palatal involvement with invasive fungal infection; (A) small palatal ulcers. (B) Unilateral large palatal necrosis. (C) Central palatal ulceration with necrosis. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Fig 2.
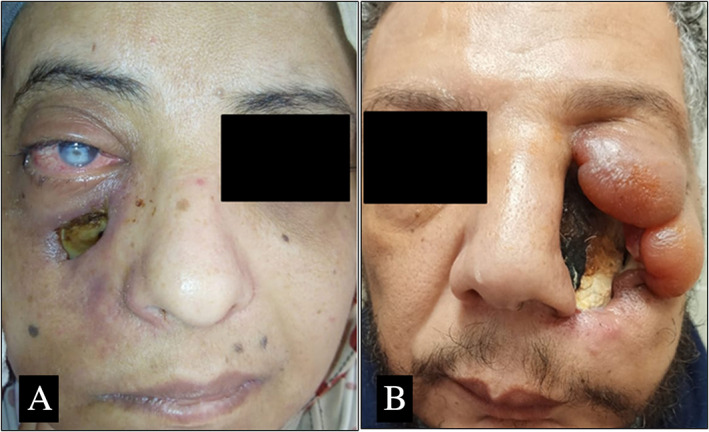
Skin necrosis and ulceration as a complication of post COVID‐19 AIFR. (A) Necrosis involves cheek area. (B) Large skin necrotic area over cheek, lateral nasal wall and eye with edematous inflammatory changes. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
CT scans of the chest revealed highly positive findings of COVID‐19 (CORAD 5) in 61.1% of patients, CORAD 4 in 19.4%, CORAD 3 in 5.5%, and no abnormal findings in 13.8% of patients. Radiological studies of nose and paranasal sinuses showed variable patterns. Those patterns range from mucosal thickening of the involved sinus mucosa to osteomyelitis of the related bone. The most commonly involved sinonasal site is lateral nasal wall (86.1%) with ethmoid (72.2%) and sphenoid (55.6%) sinuses being the most commonly affected sinuses. Orbital involvement showed evidence of either subperiosteal abscess (Fig. 3A) (5.6%), extension of inflammation giving symptoms of orbital apex syndrome (55.6%) or orbital invasion with fungal infection compressing orbital contents (Fig. 3B) (19.4%). Among 10 patients with intracranial extension, 5 patients had cavernous sinus thrombosis, one case had transverse and sigmoid sinus thrombosis (Fig. 3C), one case had temporal lobe abscess (Fig. 3D), and the remaining 2 shows suggested encephalitis. Also, central retinal artery thrombosis was found in two patients.
Fig 3.

Important radiological findings and variations found in post COVID‐19 AIFR cases. (A) Axial CT showing right subperiosteal abcess. (B) T1‐weighted axial MRI showing right orbital invasion with proptosis. (C) MRV of the same patient demonstrated in (B) showing left side transverse and sigmoid sinus thrombosis (white arrows). (D) T2‐weighted axial MRI shows right orbital invasion with right temporal lobe abscess (yellow arrow) with occlusion of the right internal carotid artery.
Interestingly, a kind of paradoxical clinical and CT findings was noticed in two patients. One patient was presented with right orbital proptosis, ophthalmoplegia, and visual loss with isolated left sphenoidal opacity. Another patient had right orbital invasion with left transverse and sigmoid sinuses thrombosis (Fig. 3B,C).
During active COVID‐19 infection, 11 patients were classified as mild, 13 as moderate, and 12 as severe, out of them 3 patients only needed invasive mechanical ventilation. All moderate and severe cases were admitted, while only one case of mild disease required hospitalization due to associated chronic renal failure. Treatment protocol for hospitalized patients generally consisted of antiviral, anticoagulant, and vitamins ± steroids. Steroid treatment was reserved for some selected moderate (1 mg/kg/day) and severe (1–2 mg/kg/day) cases. Classification and treatment of patients have been implemented according to the national management protocol guidelines provided by Ministry of Health and Population in Egypt. 9
Regarding AIFS, all patients in the present study were treated with a combined antifungal therapy and surgery except for two patients who were maintained on antifungal therapy only with no surgical debridement. These two patients were admitted in ICU with PCR‐positive test for COVID‐19 with bad chest and general condition raising the opinion against surgical intervention. Liposomal amphotericin B was given to 26 patients, voriconazole to 8 patients, and a combination of both in two patients. In three patients, voriconazole was replaced by posaconazole.
Surgical approach was purely endoscopic in 24 (66.7%) patients; while combined endoscopic and open approaches were utilized in the remaining 10 patients. Endoscopic debridement included resection of the middle turbinate, wide middle meatal antrostomy, ethmoidectomy, sphenoidotomy, and draf III in some cases according to the involved sinuses. Orbital involvement in this case series was managed by a wide variety of surgical approaches including endoscopic evacuation of subperiosteal abscess, orbitotomy, orbital decompression, and orbital exenteration in case of orbital invasion with blindness. Only three patients, diagnosed as orbital apex syndrome, recovered their vision and showed improvement of ophthalmoplegia. Neurosurgery was consulted for operative intervention in the case complicated by temporal lobe abscess. Palatal necrosis was managed either by infrastructure maxillectomy or palatectomy according to extension of necrosis with post‐debridement obturator insertion. Skin necrosis due to invasive fungal infection was managed by debridement only with planned future skin grafting after eradication of infection was established.
Overall survival was 63.89% (23/36) at the conclusion of the study. Thirteen patients (36.11%) died, eight from extensive intracranial extension, two from uncontrolled renal failure, the two COVID‐19 PCR + ve cases with continuous severe respiratory failure, and a single case of uncontrolled leukemia. Early cases (seven patients) with limited sinonasal involvement showed easier treatment, single surgical debridement procedure, and better results unlike late cases with intracranial extension and venous sinus thrombosis.
DISCUSSION
Studies on SARS‐CoV and SARS‐CoV‐2 have shown that both viruses belong to the same species and have similar biological and clinical characteristics. 10 Fungal infections were observed in SARS patients in earlier studies and were considered the leading cause of death in 25% to 73.7% of patients. 11 , 12 , 13 Based on this experience, it is critically important to pay attention to the probability of fungal infections accompanying COVID‐19.
In COVID‐19 patients, co‐infections of fungi were found. In China, Chen et al. found five cases of pulmonary fungal infection in 99 COVID‐19 patients. 14 Yang et al. found three (3/52, 5.8%) patients with pulmonary fungal co‐infection in 52 critically ill patients. 15 Other studies have found a higher percentage of secondary pulmonary infections (8%–15%) in COVID‐19 patients, but it is not clear whether it is bacterial or fungal infection. 16 , 17 A German study found COVID‐19‐associated invasive pulmonary aspergillosis in 26.3% of critically ill patients with moderate to severe ARDS. 18 In another study from Netherlands, out of 31 ICU patients, there were six patients (19.4%) presumed invasive pulmonary aspergillosis. 19
AIFR is a time‐sensitive condition that must be recognized and treated promptly to avoid life‐threatening complications. In Egypt, a 10‐year study in a pediatric oncology center, reported 45 cases of proven invasive fungal sinusitis which is comparable to the relative incidence present in other countries. 20 , 21 In our institution, nearly 5 to 7 invasive fungal sinusitis cases are diagnosed per year. Unusually, a notably rising number of this infection has been recorded recently in COVID‐19 patients. The incidence of invasive fungal sinusitis in COVID‐19 patients with critical illness is still unknown. Only isolated case reports were published demonstrating this evolving clinical entity. 6 , 7 The present study, to the best of our knowledge, is the first to present a relatively larger number of patients, their management, and outcomes.
Standardized definition of AIFR includes presence of tissue invasion by fungal elements over an acute clinical course of less than 4 weeks. 8 Progression occurs over a number of days, but no longer than a few weeks with possible vascular invasion and thrombosis. 22 Usually, it presents with acute onset of facial pain, fever, and nasal congestion with frequent extension into adjacent structures, including the paranasal soft tissues, orbit, and cranial vault. Orbital involvement can result in attenuation of vision, while sinus or intracranial extension can be associated with proptosis or neurological impairments, respectively. 22 , 23
Previous study carried out by Kursun et al., on non‐COVID AIFR showed that the most common co‐morbidity was DM, and to a lesser extent, hematological malignancies and chronic kidney disease. 24 In other pre‐COVID pandemic studies, the most common concomitant disease was also DM. 25 , 26 , 27 , 28 , 29 , 30 Turner et al. at 2013 reported presence of diabetes, hematologic malignancies, and corticosteroid use in 47.8%, 39.0% and 27.6% of their patients, respectively. 31 As Egypt has a high prevalence rate of type 2 diabetes mellitus (15.6% of all adults aged 20–79), it is also a well‐known risk factor for developing fungal infections. 32 This is in accordance with results in our study as the most common associated disorder was diabetes mellitus (27.8%) followed by hypertension (16.67%).
Many fungal species, Rhizopus, Mucor, Rhizomucor, and Aspergillus, were reported to cause AIFR. 33 Histopathologically confirmed fungal organisms in the present study were Mucor species (77.8%) and Aspergillus fumigatus (30.6%). Most studies reported mainly the Mucorales species. 24 , 25 , 27 , 29
The most common presenting symptoms of patients in the current study were headache and facial pain (75%), facial numbness (66.7%), and ophthalmoplegia and visual loss (63.9%). Previous publications discussing non‐COVID AIFR showed concomitant results. Abu El‐Naaj et al., mentioned symptoms as pain resembling sinusitis, facial swelling, and fever in his case series. 34 Kursun et al. listed fever (79%), periorbital cellulitis (75%), and periorbital oedema (70%) as the most common manifestations among their cases 24 while Ketenci et al. 27 reported fever, facial edema, facial pain, and nasal obstruction as the most frequent symptoms. In the same study, nine (64%) patients had skin and/or palatal involvement in comparison to 14 cases in the present study. Also, Ketenci et al., had five cases (35%) of ophthalmoplegia and blindness in comparison to 23 (63.9%) cases in the current study. 27
This disease and its aggressive orbital and intracranial extension should be given close scrutiny. Single most important element for successful attenuation of this infection is early diagnosis followed by aggressive medical care, surgical debridement, and control of associated diseases. In AIFR cases occurring with COVID‐19, management is not an easy task both for the patient and the provider. The morbidity of isolated medical treatment, patient's general and chest condition, hazards of general anesthesia for both patient and surgical team must be balanced with benefits of aggressive surgical debridement together with an understanding of the overall prognosis of the patient.
Regarding antifungal medications, some previous studies have demonstrated a link between invasive Mucor sinusitis and worse outcomes which needs amphotericin B as antifungal therapy. 35 Voriconazole was suggested to have superiority over amphotericin B as a primary treatment for invasive Aspergillus infections with improved disease clearance. 33 , 36 The majority of patients in the current study, 26 patients, received intravenous liposomal amphotericin B (80.56%). Voriconazole was used in eight patients with Aspergillus fumigatus infection or patients with high creatinine levels limiting amphotericin B use. Both drugs were used in two patients with advanced disease. In the three severe cases of renal impairment that occurred in the current study, voriconazole was replaced by posaconazole.
As high numbers of health care workers were infected during the first phase of the COVID‐19 pandemic, many research studies were carried out to assess risk of surgical procedures and how to minimize it. Latest recommendations considered endoscopic sinus surgery as a very high risk procedure that should be delayed as it carries great risk of infection to all attendants of the operating and recovery rooms together with risk of delayed patient recovery. 37 Accordingly, in the current study, only PCR‐negative patients for COVID‐19 were surgically treated. In our experience, 66.7% of cases underwent an exclusive endoscopic debridement while Turner et al. performed endoscopic approach in 46.4% of their patients. 31 Orbital exenteration was performed in 20% of cases, which is close to our results (16.7%). 31
AIFR has high mortality and morbidity rates (18%–80%) despite improvements in medical and surgical management protocols. 22 , 38 , 39 Orbital and intracranial extension is associated with an increased risk of death. Mortality has been reported to range from 20% to 68% in previous studies. 39 , 40 , 41 , 42 , 43 Using WHO data on the cumulative number of deaths caused by COVID‐19, mortality rates would be 15.2% (12.5–17.9) outside of China. 44
It has been observed that patients, whose diagnosis was early with limited disease extension, have the best outcome with minimal mortality and morbidity. The survival rate reported in studies for AIFS ranges from 20% to 80%. 24 , 27 , 38 , 45 , 46 Studies have suggested that recovery of immune system, which is potentially compromised in COVID‐19 patients, is a critical factor to clear this infection. 41 The overall survival rate of non‐COVID AIFR cases reviewed by Turner et al., was 49.7% in comparison to 63.89% in the present study. This relatively better survival rate may be due to early diagnosis while close observation during the follow‐up period after COVID‐19 recovery, aggressive surgical debridement, and early use of antifungal medications.
Limitations of this study include relatively limited patient number, single tertiary referral center experience, and short‐term follow‐up. Future research studies are planned to update this experience with long‐term follow‐up and to target larger group of patients on a comparative basis with evaluation of risk factor association between AIFR and COVID‐19 infection.
CONCLUSION
Clinical suspicion and early diagnosis of AIFR in COVID‐19 patients are essential for better treatment outcomes. Rapid initiation of antifungal therapy and immediate management with surgical intervention could affect the prognosis of the patients and improve survival rates.
AUTHOR CONTRIBUTIONS
Noha Ahmed El‐Kholy: Conception of the study, collection, analysis, interpretation of data, drafting and revising the manuscript. Ahmed Musaad Abd El‐Fattah: Conception and design of the study, analysis and interpretation of data, performing the surgical intervention, revising and final approval of manuscript. Yasser W. Khafagy: Conception and design of the study, performing the surgical intervention, analysis of data, revising and final approval of manuscript.
Editor's Note: This Manuscript was accepted for publication on May 10, 2021.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses , Gorbalenya AE, Baker SC, et al. The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol 2020;5:536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID‐19): a multi‐center study in Wenzhou city, Zhejiang, China. J Infect 2020;80:388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fekkar A, Lampros A, Mayaux J, et al. Occurrence of invasive pulmonary fungal infections in patients with severe COVID‐19 admitted to the ICU. Am J Respir Crit Care Med 2021;203:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim DW, Heo ST, Jeon S‐Y, et al. Invasive paranasal mucormycosis with peripheral eosinophilia in an immunocompetent patient. Med Mycol 2010;48:406–409. [DOI] [PubMed] [Google Scholar]
- 6. Werthman‐Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID‐19. Am J Emerg Med 2020;42:264.e5–264.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehta S, Pandey A. Rhino‐orbital mucormycosis associated with COVID‐19. Cureus 2020;12:e10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chakrabarti A, Denning DW, Ferguson BJ, et al. Fungal rhinosinusitis: a categorization and definitional schema addressing current controversies. Laryngoscope 2009;119:1809–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Masoud H EG, Zaky S, Baki A, et al. Management Protocol for COVID‐19 Patients Version 1.4, Ministry of Health and Population (MOHP), Egypt. May 13, 2020.
- 10. Peeri NC, Shrestha N, Rahman MS, et al. The SARS, MERS and novel coronavirus (COVID‐19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol 2020;49:717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yin C, Wang C, Tang Z, Zhang S, Wang B. Clinical analysis of 146 patients with critical severe acute respiratory syndrome in Beijing areas. Clin J Emerg Med 2004;1:12–14. [Google Scholar]
- 12. Zhang Y, Li W, Huang K, Cao Z, Hao J. Hospital acquired pneumonia occurring after acute stage of the serious SARS and its treating strategies. Chin J Nosocomiol 2003;11:1081–1087. [Google Scholar]
- 13. Li C, Pan S. Analysis and causation discussion of 185 severe acute respiratory syndrome dead cases. Zhongguo Weizhongbing Jijiuyixue 2003;15:582–584. [PubMed] [Google Scholar]
- 14. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med 2020;8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koehler P, Cornely OA, Böttiger BW, et al. COVID‐19 associated pulmonary aspergillosis. Mycoses 2020;63:528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Arkel AL, Rijpstra TA, Belderbos HN, Van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID‐19–associated pulmonary aspergillosis. Am J Respir Crit Care Med 2020;202:132–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi 2019;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madney Y, Khedr R, Ahmed N, et al. Overview and outcome of mucormycosis among children with cancer: report from the Children's cancer hospital Egypt. Mycoses 2019;62:984–989. [DOI] [PubMed] [Google Scholar]
- 22. Aribandi M, McCoy VA, Bazan C III. Imaging features of invasive and noninvasive fungal sinusitis: a review. Radiographics 2007;27:1283–1296. [DOI] [PubMed] [Google Scholar]
- 23. Momeni AK, Roberts CC, Chew FS. Imaging of chronic and exotic sinonasal disease. Am J Roentgenol 2007;189:S35–S45. [DOI] [PubMed] [Google Scholar]
- 24. Kursun E, Turunc T, Demiroglu YZ, Alışkan HE, Arslan AH. Evaluation of 28 cases of mucormycosis. Mycoses 2015;58:82–87. [DOI] [PubMed] [Google Scholar]
- 25. Bellazreg F, Hattab Z, Meksi S, et al. Outcome of mucormycosis after treatment: report of five cases. New Microbes New Infect 2015;6:49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kermani W, Bouttay R, Belcadhi M, Zaghouani H, Ali MB, Abdelkéfi M. ENT mucormycosis. Report of 4 cases. Eur Ann Otorhinolaryngol Head Neck Dis 2016;133:83–86. [DOI] [PubMed] [Google Scholar]
- 27. Ketenci I, Ünlü Y, Kaya H, et al. Rhinocerebral mucormycosis: experience in 14 patients. J Laryngol Otol 2011;125:e3. [DOI] [PubMed] [Google Scholar]
- 28. Mohammadi R, Meidani M, Mostafavizadeh K, et al. Case series of rhinocerebral mucormycosis occurring in diabetic patients. Caspian J Intern Med 2015;6:243. [PMC free article] [PubMed] [Google Scholar]
- 29. Saedi B, Sadeghi M, Seilani P. Endoscopic management of rhinocerebral mucormycosis with topical and intravenous amphotericin B. J Laryngol Otol 2011;125:807–810. [DOI] [PubMed] [Google Scholar]
- 30. Vaezi A, Moazeni M, Rahimi MT, de Hoog S, Badali H. Mucormycosis in Iran: a systematic review. Mycoses 2016;59:402–415. [DOI] [PubMed] [Google Scholar]
- 31. Turner JH, Soudry E, Nayak JV, Hwang PH. Survival outcomes in acute invasive fungal sinusitis: a systematic review and quantitative synthesis of published evidence. Laryngoscope 2013;123:1112–1118. [DOI] [PubMed] [Google Scholar]
- 32. Hegazi R, El‐Gamal M, Abdel‐Hady N, Hamdy O. Epidemiology of and risk factors for type 2 diabetes in Egypt. Ann Glob Health 2015;81:814–820. [DOI] [PubMed] [Google Scholar]
- 33. Bakhshaee M, Bojdi A, Allahyari A, et al. Acute invasive fungal rhinosinusitis: our experience with 18 cases. Eur Arch Otorhinolaryngol 2016;273:4281–4287. [DOI] [PubMed] [Google Scholar]
- 34. El‐Naaj IA, Leiser Y, Wolff A, Peled M. The surgical management of rhinocerebral mucormycosis. J Craniomaxillofac Surg 2013;41:291–295. [DOI] [PubMed] [Google Scholar]
- 35. Ingley AP, Parikh SL, DelGaudio JM. Orbital and cranial nerve presentations and sequelae are hallmarks of invasive fungal sinusitis caused by Mucor in contrast to Aspergillus. Am J Rhinol 2008;22:155–158. [DOI] [PubMed] [Google Scholar]
- 36. Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 2002;347:408–415. [DOI] [PubMed] [Google Scholar]
- 37. Givi B, Schiff BA, Chinn SB, et al. Safety recommendations for evaluation and surgery of the head and neck during the COVID‐19 pandemic. JAMA Otolaryngol Head Neck Surg 2020;146:579–584. [DOI] [PubMed] [Google Scholar]
- 38. Gillespie MB, O'Malley BW, Francis HW. An approach to fulminant invasive fungal rhinosinusitis in the immunocompromised host. Arch Otolaryngol Head Neck Surg 1998;124:520–526. [DOI] [PubMed] [Google Scholar]
- 39. Parikh SL, Venkatraman G, DelGaudio JM. Invasive fungal sinusitis: a 15‐year review from a single institution. Am J Rhinol 2004;18:75–81. [PubMed] [Google Scholar]
- 40. Iwen PC, Rupp ME, Hinrichs SH. Invasive mold sinusitis: 17 cases in immunocompromised patients and review of the literature. Clin Infect Dis 1997;24:1178–1184. [DOI] [PubMed] [Google Scholar]
- 41. Kennedy CA, Adams GL, Neglia JR, Giebink GS. Impact of surgical treatment on paranasal fungal infections in bone marrow transplant patients. Otolaryngol Head Neck Surg 1997;116:610–616. [DOI] [PubMed] [Google Scholar]
- 42. Süslü AE, Öğretmenoğlu O, Süslü N, Yücel ÖT, Önerci TM. Acute invasive fungal rhinosinusitis: our experience with 19 patients. Eur Arch Otorhinolaryngol 2009;266:77. [DOI] [PubMed] [Google Scholar]
- 43. Talbot GH, Huang A, Provencher M. Invasive aspergillus rhinosinusitis in patients with acute leukemia. Rev Infect Dis 1991;13:219–232. [DOI] [PubMed] [Google Scholar]
- 44. Baud D, Qi X, Nielsen‐Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID‐19 infection. Lancet Infect Dis 2020;20:773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen C‐Y, Sheng W‐H, Cheng A, et al. Invasive fungal sinusitis in patients with hematological malignancy: 15 years experience in a single university hospital in Taiwan. BMC Infect Dis 2011;11:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mohindra S, Mohindra S, Gupta R, Bakshi J, Gupta SK. Rhinocerebral mucormycosis: the disease spectrum in 27 patients. Mycoses 2007;50:290–296. [DOI] [PubMed] [Google Scholar]


