Abstract
Over the last year, the novel coronavirus disease 2019 (COVID‐19) has continued to spread across the globe, causing significant morbidity and mortality among transplantation candidates and recipients. Patients with end‐stage liver disease awaiting liver transplantation and patients with a history of liver transplantation represent vulnerable populations, especially given the high rates of associated medical comorbidities in these groups and their immunosuppressed status. In addition, concerns surrounding COVID‐19 risk in this patient population have affected rates of transplantation and general transplantation practices. Here, we explore what we have learned about the impact of COVID‐19 on liver transplantation candidates and recipients as well as the many key knowledge gaps that remain.
Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- ACLF
acute‐on‐chronic liver failure
- AIH
autoimmune hepatitis
- ALD
alcohol‐related liver disease
- ALI
acute liver injury
- CI
confidence interval
- CLD
chronic liver disease
- CLIF-C
European Foundation for the study of chronic liver failure
- CLIF-OF
European Foundation for the study of chronic liver failure ‐ Organ Failure
- COLD
COVID‐19 in Chronic Liver Disease
- COVID‐19
coronavirus disease 2019
- CTP
Child‐Turcotte‐Pugh
- DDLT
deceased donor liver transplantation
- EUA
emergency use authorization
- HCC
hepatocellular carcinoma
- ICU
intensive care unit
- IL
interleukin
- IV
intravenous
- MELD
Model for End‐Stage Liver Disease
- mRNA
messenger ribonucleic acid
- N/A
not applicable
- OPTN
Organ Procurement and Transplantation Network
- po, per os (orally); RT‐PCR
reverse transcription polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SOT
solid organ transplantation
- UNOS
United Network for Organ Sharing
- WHO
World Health Organization
The novel coronavirus disease 2019 (COVID‐19), the clinical syndrome caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has had a profound impact on all aspects of transplantation in the United States and worldwide. The infection continues to spread at an alarming rate around the world, having reached over 3.3 million deaths globally and over 580,000 deaths in the United States alone as of May 2021.( 1 ) Although mortality from COVID‐19 is primarily related to pulmonary complications, there are numerous extrapulmonary manifestations of the disease, including gastrointestinal and hepatic.( 2 , 3 , 4 , 5 ) Patients with preexisting medical comorbidities including chronic liver disease (CLD) and cirrhosis are likely to be at high risk of severe disease and death when compared with healthy patients and those without liver disease.( 6 , 7 ) There has been significant interest in the outcomes of patients with COVID‐19 among liver transplantation candidates and recipients, as well as the impact the pandemic has had on the rates and logistics of the transplantation itself. Here, we review what we have learned in the first year of this pandemic about the impact of COVID‐19 in liver transplantation and identify key knowledge gaps to guide research in this area.
The Impact of the COVID‐19 Pandemic on the Practice of Liver Transplantation
Many aspects of health care have changed dramatically during the pandemic, with practices in liver transplantation being no exception. Most centers rapidly converted to telehealth for the majority of their patient visits.( 8 ) In the first quarter of 2020, there was a significant impact of the pandemic on the number of liver transplantations performed (Fig. 1), number of additions to the liver transplantation waiting list, and number of liver transplantation waitlist inactivations as the pandemic evolved throughout the United States.( 9 ) Adding additional complexity, these data must be interpreted in the context of the virtually simultaneous change in the liver allocation system in February 2020.( 10 ) This initial decline in transplantation activities was likely fueled by concerns about donor and recipient safety, as well as the safety of the transplantation teams and unknowns about the resources available in transplantation centers as the virus spread geographically and access to viral testing was very limited. In addition, there was a notable increase in number of waitlist inactivations because of COVID‐19 precautions at this same time. Between March 15, 2020 and April 30, 2020, states with the highest incidence of COVID‐19 cases saw 33% fewer new listings for liver transplantation than expected, with 34% fewer deceased donor liver transplantations (DDLTs). Over this period, transplantation centers in these states had 59% more waitlist deaths than expected.( 11 )
FIG. 1.
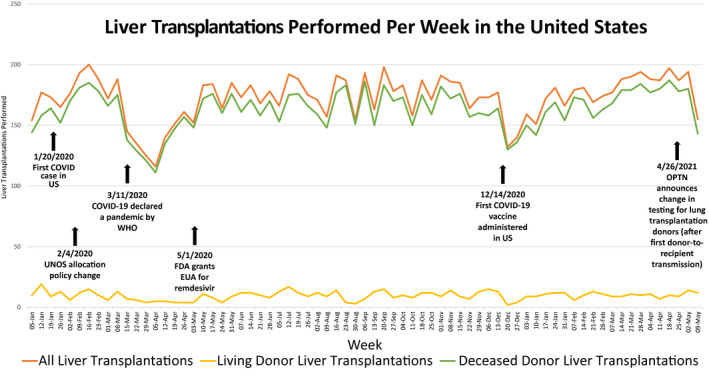
Impact of the COVID‐19 pandemic on liver transplantation volume in the United States.( 9 ) Abbreviations: EUA, emergency use authorization; OPTN, Organ Procurement and Transplantation Network; WHO, World Health Organization.
Interestingly, even within geographic areas with similar COVID‐19 rates, there were significant variations in transplantation practices between individual centers. When changes in adult DDLT in the month preceding and following the onset of widespread COVID‐19 infection in the United States were evaluated, there was an 11.0% decrease in deceased donors from February 2020 to March 2020 overall, with a resultant decrease in adult DDLTs nationally of 24.7%. When region and center were considered, there was much greater variability seen in 2020 compared with 2019 when looking at transplantation practices within the same region, despite similar rates of COVID‐19 at these centers. This was observed among regions with high and low rates of COVID‐19, although notably with some of the highest within‐region variability seen in that with the highest burden of COVID‐19 cases (such as region 9).( 12 ) It is not clear what accounts for these differences, though it is possible that center‐specific resources, COVID‐19 transmission among transplantation team members, and center leadership philosophy regarding risk may have played a role.
Despite the risks and unknowns, these early effects of the pandemic on transplantation and waitlist rates are largely seen only in the first 2 months of the pandemic. Subsequently, even as COVID‐19 cases spread between states, waitlist registrations, rates of transplantation, and waitlist mortality were very near that expected in new high‐incidence areas.( 11 ) Some of this restoration of transplantation volume may be attributed to the rapid implementation of guidance surrounding COVID‐19 from the United Network for Organ Sharing (UNOS) as well as medical societies.( 13 , 14 ) With the adoption of COVID‐19 testing in donors and recipients based on society guidelines, increased access to personal protective equipment, and especially now with health care workers having been offered vaccination against COVID‐19, concerns about risk of transmission to the transplantation team in the perioperative period are diminished. Figure 1 shows the number of liver transplantations performed in the United States from January 2020 to May 2021, highlighting key events related to COVID‐19.( 15 )
There was a particularly sharp initial decline in living donor liver transplantation early in the pandemic, which has also subsequently recovered. As reported also in countries where living donor liver transplantation is the main source of liver allografts,( 16 , 17 , 18 ) with improved safety protocols and testing, these operations are likely safe if the local hospital resources are not overwhelmed. Overall, we believe living donor liver transplantation evaluation and programming should continue, especially in the context of widespread vaccination, with additional precautions as described here for donor testing to ensure safety of the potential donor, recipient, and transplantation team.
CLD, Cirrhosis, and Liver Transplantation Candidates
Since the early reports of the clinical characteristics of COVID‐19, it has been clear that liver injury as measured by both elevations in liver enzymes and post‐mortem pathology has been common, perhaps a particular threat to patients with decompensated liver disease.( 19 , 20 , 21 , 22 , 23 ) Liver enzyme elevations have been reported in up to 63.0% of patients hospitalized with COVID‐19 infection, with a more severe disease course including higher rates of intensive care unit (ICU) admission, intubation, renal replacement therapy, and mortality seen in patients with marked elevations.( 2 , 5 , 24 , 25 , 26 ) In addition, there have been reports of less common but more severe forms of liver disease in the context of COVID‐19 that could create additional indications for liver transplantation, including acute liver failure( 27 ) as well as progressive cholangiopathy.( 28 )
Patients with CLD and cirrhosis have also been greatly affected by COVID‐19 (Table 1). While patients with CLD do not appear to be overrepresented in the largest epidemiological cohorts,( 29 ) it does appear that patients with CLD overall are at increased risk of severe disease and death.( 6 , 7 ) This has been demonstrated in large data set analyses, where CLD is associated with increased rates of hospitalization and a significantly higher risk of mortality when compared with patients without liver disease, with the greatest risk among patients with cirrhosis.( 6 , 29 ) From subsequent studies with more granular patient data, it is likely that both the etiology of CLD and the disease severity impact this risk, and it is possible that cirrhosis is underdiagnosed in these larger data sets.( 30 , 31 , 32 )
TABLE 1.
Outcomes and Predictors of COVID‐19 in Patients with CLD and Cirrhosis
| Study | Cohort | Outcomes in CLD | Outcomes in Cirrhosis | Outcomes in Comparator Group |
|---|---|---|---|---|
| Iavarone et al.( 33 ) |
|
N/A |
|
|
| June 2020 | ||||
| 9 centers in Italy | ||||
| Sarin et al.( 34 ) |
|
|
|
CLIF‐C [Chronic Liver Failure Consortium] CLIF‐OF [Chronic Liver Failure Organ Failure] |
| July 2020 | ||||
| 13 countries in Asia | ||||
| Qi et al.( 35 ) |
|
N/A |
|
N/A |
| May 2020 | ||||
| 16 centers in China | ||||
| Bajaj et al.( 36 ) |
|
N/A |
|
|
| July 2020 | ||||
| 7 centers in the United States | ||||
| Hashemi et al.( 32 ) |
|
|
|
|
| July 2020 | ||||
| 9 centers in the United States | ||||
| Singh and Khan( 6 ) |
|
|
|
|
| May 2020 | ||||
| 34 centers in the United States | ||||
| Kim et al.( 30 ) |
|
|
|
N/A |
| September 2020 | ||||
| 21 centers in the United States | ||||
| Marjot et al.( 31 ) |
|
|
|
|
| October 2020 | ||||
| International registry (29 countries) |
The earliest multicenter cohorts to explore outcomes among patients with cirrhosis, though relatively small, were from China and Italy, the first epicenters of the pandemic.( 33 , 34 , 35 ) Following these initial reports, larger studies from the United States and multinational cohorts have further refined our understanding of the relationship between CLD, cirrhosis, and COVID‐19 outcomes. The first of these is the COVID‐19 in Chronic Liver Disease (COLD) consortium, which included 867 patients across 21 US centers with CLD (71.5% with cirrhosis, 10.7% with decompensated cirrhosis) and confirmed SARS‐CoV‐2 infection. In this cohort, decompensated cirrhosis was associated with an increased risk of severe COVID‐19 infection and higher mortality. Higher mortality was also seen in alcohol‐related liver disease (ALD), patients with hepatocellular carcinoma (HCC), and several medical comorbidities including older age, diabetes mellitus, hypertension, chronic obstructive pulmonary disease, and current smoking.( 30 ) Similar findings were seen in the SECURE‐Liver Registry (formerly SECURE‐Cirrhosis) and the COVID‐Hep registry, which included 745 patients with CLD from 29 countries. Within this cohort, patients with cirrhosis (n = 386) had a mortality rate of 32.0% compared with only 8.0% in patients with CLD but no cirrhosis. Among patients with cirrhosis, there was a stepwise increase in mortality with increasing Child‐Turcotte‐Pugh (CTP) class. Multivariable analysis showed older age, cirrhosis (with any CTP class), and ALD to be associated with increased risk for mortality.( 31 )
However, it is also clear that this literature must be interpreted in the context of carefully selected control groups to understand what is driving these outcomes. In a US‐based multicenter study comparing patients with cirrhosis and COVID‐19 with 2 age‐ and sex‐matched comparator groups, patients with cirrhosis and COVID‐19 had higher mortality than those without cirrhosis with COVID‐19, but similar mortality rates when compared with patients with cirrhosis without COVID‐19, perhaps indicating that cirrhosis itself is driving the high inpatient mortality rates.( 36 ) In a recent international study comparing outcomes among patients with autoimmune hepatitis (AIH) and COVID‐19 with a propensity score–matched cohort of patients with other etiologies of CLD and COVID‐19, cirrhosis was again identified as the strongest predictor for severe COVID‐19 in patients with AIH.( 37 )
Treatment of COVID‐19 in Transplantation Candidates
Data for the treatment of COVID‐19 specifically among patients with cirrhosis and decompensated cirrhosis are limited. There remain concerns about the potential hepatotoxicity of several SARS‐CoV‐2 therapies, particularly remdesivir and interleukin 6 (IL6) inhibitors such as tocilizumab. Remdesivir has been associated with elevation in aspartate aminotransferase and/or alanine aminotransferase in 10.0%‐11.0% of patients treated for SARS‐CoV‐2 in the trials to date, though grade 3‐4 elevations in liver enzymes were rare and not greater than those in the control populations in the phase 3 trials.( 38 , 39 , 40 , 41 , 42 , 43 , 44 ) However, remdesivir is currently recommended to be discontinued among patients with liver enzyme elevation over 10 times the upper limit of normal or if symptomatic liver disease is diagnosed.( 45 ) The use of remdesivir in patients with CLD and cirrhosis has not been reported outside of 1 case report, where a patient with decompensated cirrhosis received the medication without adverse effects.( 46 )
There are also numerous studies regarding tocilizumab‐related hepatotoxicity in the rheumatology literature, including reports of up to 71.0% of patients with liver injury in this context.( 47 , 48 , 49 ) In the trials currently published of tocilizumab for the treatment of COVID‐19, liver enzyme elevations were reported in over 15.0% of patients, although these reflect only significant enzyme elevations.( 50 , 51 , 52 ) In other series, for example, liver enzyme elevations were seen in over 80.0% of patients who received tocilizumab during their hospitalization.( 53 ) However, it is challenging to understand whether there is a direct tocilizumab‐related liver injury in many cases given the many competing etiologies of acute liver injury (ALI) in this population, especially among hospitalized patients.
Despite these concerns, there have been no reports to suggest that patients with CLD and cirrhosis should not receive these COVID‐19‐directed therapies. Given the significant mortality associated with severe COVID‐19, the increased risk of severe disease in the context of liver enzyme elevation, and the limited therapeutic options available, it will be important to report outcomes among these patients who receive these agents.
SARS‐CoV‐2 Testing of Donors and Recipients at Transplantation
Screening of donors (both deceased and living) and recipients at the time of transplantation is now widely performed, although not yet standardized outside of society consensus statements.( 13 , 14 , 54 , 55 ) All patients who present for transplantation should be tested for SARS‐CoV‐2 with a nasopharyngeal polymerase chain reaction (PCR) test prior to undergoing the operation. Current recommendations advise against transplantation in recipients with active COVID‐19 and recommend proceeding to transplantation ideally 2‐3 weeks after symptoms have resolved and only if at least 1 nasopharyngeal PCR test is negative, with some physicians recommending 2 negative tests.
All donors should also undergo a nasopharyngeal PCR test prior to acceptance of the organ offer. In lung transplantation donors a lower respiratory specimen is also required,( 56 ) with this policy change being implemented after a case of SARS‐CoV‐2 transmission from a lung transplantation donor to recipient was reported.( 57 ) Potential living donors with prior SARS‐CoV‐2 infection should have at least 1 negative SARS‐CoV‐2 RNA test; if repeat testing remains positive, proceeding to donation should be considered only if they are asymptomatic and the infection was between 21 and 90 days prior to donor evaluation, as this is felt to reflect viral shedding and not new or active infection.( 58 )
One unique challenge in testing is the prolonged viral shedding that has been observed in patients with end‐stage liver disease, especially among those with nonalcoholic fatty liver disease, where PCR testing can remain positive beyond the period expected in the general population.( 59 , 60 ) In addition, for patients who cannot wait, including those with acute liver failure or high Model for End‐Stage Liver Disease (MELD) scores, it has been debated whether it is safe to proceed without negative testing, especially in the absence of respiratory symptoms. There has been 1 published report describing transplantation of a patient with COVID‐19 in the setting of acute liver failure thought to be due to Wilsonian crisis. The patient had symptomatic COVID‐19, including respiratory manifestations, and immediately after transplantation received remdesivir. At 3‐month follow‐up, the patient had recovered from COVID‐19 and had good graft function.( 61 ) In carefully selected patients with risks of transplantation deferral outweighing those of decompensation from COVID‐19, liver transplantation may be considered. However, further studies are needed to better understand the risks and benefits of proceeding to transplantation to establish clear guidelines and recommendations.
Safety concerns related to SARS‐CoV‐2 infection in donors and recipients include both ongoing manifestations of COVID‐19, which is increasingly recognized to have a prolonged effect, and concerns about immunologic events including rejection in the early postoperative period. Early in the pandemic, there were small numbers of case reports describing patients who successfully underwent DDLT after recovery from SARS‐CoV‐2 infection.( 62 , 63 , 64 ) In these reports, all patients recovered with negative PCR tests prior to transplantation, with the earliest transplantation occurring 36 days after onset of symptoms. In 1 case where the patient’s induction immunosuppression regimen consisted of only steroids and a calcineurin inhibitor, the patient experienced acute rejection requiring thymoglobulin and high‐dose steroids, without subsequent adverse events.( 64 ) There have since been larger case series describing both living donors and recipients with positive SARS‐CoV‐2 RT‐PCR as recently as 2 weeks before transplant, without COVID‐19–related complications.( 65 , 66 )
Data surrounding safety in donor SARS‐CoV‐2 positivity are more limited. There has been 1 case report of a patient with neutralizing antibodies to SARS‐CoV‐2 who received a liver allograft from a patient who tested positive for SARS‐CoV‐2 at the time of procurement, with good graft function at 2‐month follow‐up.( 67 ) However, there has also been a fatal case of transmission of SARS‐CoV‐2 in a lung transplantation recipient, highlighting the need for ongoing vigilance in this area. While it continues to be debated whether the SARS‐CoV‐2 virus is actively replicating in the liver in humans, the presence of the angiotensin‐converting enzyme 2 (ACE2) receptor on hepatocytes and cholangiocytes( 68 ) as well as the growing evidence that the virus can infect these cells and cause virally mediated liver injury( 23 , 69 , 70 ) does raise the possibility of the liver allograft as a potential reservoir of virus that could be transmitted.( 71 )
Outcomes of COVID‐19 in Liver Transplantation Recipients
Numerous studies have reported patient outcomes in liver transplantation recipients with SARS‐CoV‐2 infection. Major findings are summarized in Table 2. Early studies from the United States and Europe described the clinical course of solid organ transplantation (SOT) recipients with COVID‐19, with mortality rates approaching 20% in this patient population.( 72 , 73 , 74 )
TABLE 2.
Outcomes and Predictors of COVID‐19 in Liver Transplantation Recipients
| Study | Cohort | Outcomes in SOT and Liver Transplantation Recipients | Outcomes in Comparator Group |
|---|---|---|---|
| Pereira et al.( 72 ) |
|
|
N/A |
| May 2020 | |||
| 2 centers in New York City | |||
| Belli et al.( 73 ) |
|
|
N/A |
| June 2020 | |||
| Multicenter European cohort | |||
| Colmenero et al.( 74 ) |
|
|
|
| August 2020 | |||
| 25 centers in Spain | |||
| Webb et al.( 76 ) |
|
|
|
| August 2020 | |||
| International registry study | |||
| Rabiee et al.( 75 ) |
|
|
|
| September 2020 | |||
| 15 centers in the United States | |||
| Becchetti et al.( 92 ) |
|
|
N/A |
| June 2020 | |||
| 12 centers in Europe | |||
| Mansoor et al.( 77 ) |
|
|
|
| September 2020 | |||
| Health research network database |
As rates of COVID‐19 have continued to climb, there have been additional studies from multicenter consortia and large data sets. In the multicenter cohort from the COLD consortium including 15 medical centers in the United States, the mortality rate was 22.3%, similar to the early studies described. In this cohort, 72.3% of patients required hospitalization and 26.8% required ICU‐level care.( 75 ) Risk factors for death in this group included the presence of diabetes mellitus as well as ALI in the graft at the time of presentation. In a subsequent multicenter cohort from 2 international registries, increased age, serum creatinine, and nonliver cancer were associated with increased mortality rate in liver transplantation recipients with COVID‐19. In propensity score–matched analysis (comparing liver transplantation recipients with COVID‐19 to patients with COVID‐19 without a history of liver transplantation), liver transplantation itself was not associated with death.( 76 ) There was similarly no difference in the observed risk of mortality, thrombosis events, and ICU‐level care in a recent large, multicenter research network study comparing outcomes in liver transplantation recipients with COVID‐19 to those with COVID‐19 without a history of liver transplantation.( 77 )
Thus, despite initial concerns that the virus would be much more severe among our immunosuppressed transplantation recipients, it appears that when matched for other risk factors, transplantation status does not seem to be predictive of outcomes. There have been similar findings in the SOT literature in general,( 78 ) raising important questions about the role of a hyperimmune response in the pathogenesis of severe COVID‐19 and whether there are specific types or degrees of immunosuppression that may be helpful.
Management of COVID‐19 in Liver Transplantation Recipients
The approach to management of liver transplantation recipients with COVID‐19 is shown in Fig. 2. Overall, treatment of COVID‐19 is largely the same as in nonliver transplantation recipients( 79 ); however, large‐scale safety data of COVID‐19 treatments in liver transplantation recipients are lacking. In some of the studies discussed earlier where COVID‐19‐targeted therapy was investigated, medications including remdesivir and anti‐IL6‐receptor monoclonal antibodies were administered to liver transplantation recipients without clear adverse events.( 72 , 76 , 80 , 81 , 82 ) Larger patient cohorts are needed to further study the safety and efficacy of these therapies in this patient population.
FIG. 2.
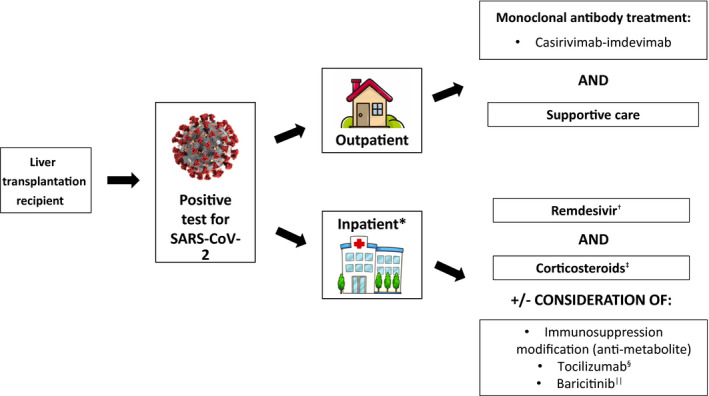
Algorithm for treatment of COVID‐19 infection among liver transplantation recipients.( 79 ) *Requiring supplemental oxygen. †Not recommended for routine use if the patient is on mechanical ventilation (200 mg IV followed by 100 mg IV daily × 4 days or until discharge, may extend to 10 days if no improvement). ‡Dexamethasone 6 mg (IV or po) once daily × 10 days or until discharge (can use alternative steroid formulations at equivalent doses if dexamethasone is not available). §Within 3 days of hospital admission (if requiring oxygen delivery through high‐flow nasal cannula or noninvasive ventilation) or within 24 hours of ICU admission; 8 mg/kg of actual body weight (up to 800 mg) IV once. ||If unable to tolerate corticosteroids (4 mg po once daily × 14 days or until discharge).
As in the general population, treatment recommendations are currently based on the severity of illness, including whether the patient is hospitalized and the level of supplemental oxygen that is required (Fig. 2). For outpatients with asymptomatic or mild disease, an important treatment consideration is the use of monoclonal antibodies. Bamlanivimab‐etesevimab is the most recent monoclonal antibody combination, with emergency‐use authorization granted by the Food and Drug Administration as of February 2021 for nonhospitalized patients with mild to moderate COVID‐19 and high risk of disease progression however this is no longer recommended by the FDA due to decreased efficacy against specific variants and is no longer available. Immunocompromised patients including transplantation recipients are eligible for this treatment,( 83 , 84 ) though again specific studies in this population have not been performed. We currently recommend the use of monoclonal therapies for outpatients when available, though treatment in the outpatient setting can be logistically challenging and we encourage enrollment of transplantation recipients in trials of these agents when available.
For patients who are admitted to the hospital and/or have moderate or severe disease as defined by their oxygen requirement, current treatment recommendations include the use of remdesivir and corticosteroids,( 79 ) including in transplantation recipients. Additional therapies that are currently available include the use of baricitinib when steroids are not tolerated, though this is less frequently used and there are no reports to date of the use of this agent among transplantation recipients for COVID‐19. In addition, successful and safe use of convalescent plasma has been described in SOT recipients in small case series, mostly in those thought to be at risk of progressing to severe infection.( 85 , 86 , 87 , 88 , 89 ) While no safety concerns have been reported with the use of convalescent plasma in transplantation recipients, there are no data that convalescent plasma is of particular benefit, either in the general population or in transplantation recipients.( 79 )
An additional area of interest in the management of SARS‐CoV‐2 infection in liver transplantation recipients is the approach to immunosuppression. In one of the first studies to detail immunosuppression in liver transplantation recipients, baseline immunosuppression using mycophenolate was associated with severe COVID‐19, especially at doses higher than 1 g per day( 74 ); one proposed mechanism for severe disease with mycophenolate is the possibility of a synergistic effect of mycophenolate and COVID‐19 depleting T lymphocytes.( 74 , 90 ) This association was not seen with calcineurin inhibitors or everolimus, and in fact one other study showed tacrolimus to be associated with a positive effect on patient survival.( 91 ) However, this has not been seen in subsequent studies investigating immunosuppression in transplantation recipients with COVID‐19, including a large multicenter cohort of SOT recipients where various measures of immunosuppression intensity did not show an association with mortality risk.( 80 ) In cohorts of liver transplantation recipients alone, rates of immunosuppression modification ranged from 44% to 49.4%, with more severe disease associated with a greater likelihood of change in immunosuppression.( 75 , 92 ) In these studies, only 1 patient had acute cellular rejection, and changes in immunosuppression were not associated with the risk of ALI or risk of mortality.( 75 ) Patient outcomes were not impacted by immunosuppression reduction or discontinuation.
It has also been postulated COVID‐19 may impact immunosuppression levels, with a multicenter study of SOT recipients reporting increased serum tacrolimus concentrations at the time of presentation with COVID‐19 compared with baseline( 93 ); however, this area needs further exploration, as does the significance of prolonged viral shedding and high viral burden among this patient population.( 94 )
Finally, an important consideration in the management of liver transplantation recipients with COVID‐19 is the approach to abnormalities in liver tests and the presence of ALI. In data from the multicenter COLD consortium including 112 adult liver transplantation recipients, the incidence of ALI was lower in liver transplantation recipients when compared with age‐ and sex‐matched controls with CLD and COVID‐19. However, among liver transplantation recipients, rates of ICU admission and mortality were higher in patients with ALI.( 75 ) ALI and abnormalities in liver tests present a unique challenge in liver transplantation recipients, where COVID‐19 and rejection can coexist. Reports of concomitant acute cellular rejection and acute hepatitis secondary to COVID‐19 have been described in a liver allograft recipient,( 95 ) highlighting the importance of liver biopsy in select circumstances, given that the laboratory abnormalities for rejection and COVID‐19 may be indistinguishable from one another.
COVID‐19 Vaccination in Liver Transplantation Candidates and Recipients
The safety and efficacy of the currently available SARS‐CoV‐2 vaccines among liver transplantation candidates and recipients. Given the high rates of morbidity and mortality in this population associated with infection, there have been broad recommendations in support of vaccination in both of these populations.( 96 , 97 ) Both the American Society of Transplantation and American Association for the Study of Liver Diseases have released guidelines on COVID‐19 vaccination in liver transplantation candidates and recipients.( 97 , 98 )
It is recommended that all transplantation candidates and their household contacts receive the COVID‐19 vaccine. The vaccine series should be completed at least 2 weeks prior to transplantation, if possible. SOT recipients are known to have a lowered response to vaccination in general( 99 , 100 , 101 ) therefore, prioritization of vaccination in the pretransplantation population is especially important. While there are not yet robust data regarding antibody and cellular immune responses to these vaccines among patients with cirrhosis, some clinical efficacy data are emerging in real world datasets.
A large cohort of patients with cirrhosis from the Veterans Administration was recently studied who received at least 1 dose of the vaccine (n = 20,037) compared with 20,037 propensity‐matched controls to assess the association between vaccination and new COVID‐19 infection and COVID‐19 hospitalization and death.( 102 ) By 28 days after the initial dose, receipt of 1 dose of an mRNA vaccine was associated with a 64.8% reduction in COVID‐19 infections and 100% protection against hospitalization or death due to COVID‐19 infection. Receipt of a second dose was associated with a 78.6% reduction in COVID‐19 infections and 100% reduction in COVID‐19–related hospitalization or death after 7 days.
In transplantation recipients, COVID‐19 vaccination is also recommended, although concerns regarding the efficacy of the vaccine in this population have been raised. SOT recipients have a lower antibody‐related response to natural infection,( 103 ) with factors such as time from transplantation and number of immunosuppressive agents being predictive of antibody nonresponse. Similar data have now been reported with antibody response to vaccination as well. In a study of 658 transplantation recipients, only 14.9% of patients mounted a detectable antispike antibody response after dose 1, and 54.3% after dose 2.( 104 , 105 ) Risk factors for lack of antibody response included antimetabolite immunosuppression. Reduced cellular immune response to vaccination has also been described in renal transplantation recipients compared with healthy controls,( 106 ) though some patients without documented antibody responses may have significant cellular responses.( 107 ) As a result, several groups have now studied administration of a third dose of mRNA vaccines in an attempt to improve antibody responses in transplant recipients. In France, a third dose of mRNA vaccine for immunocompromised patients including transplant recipients has been recommended since April 2021, resulting in documentation of higher antibody response rates after the third dose in this group. In an initial cohort of 101 solid organ transplant recipients, 40% had detectable antibodies before the third dose, which increased to 68% 4 weeks after the third dose.( 108 ) Subsequently, a prospective randomized controlled trial from Canada comparing 2‐ and 3‐dose vaccine regimens also confirmed higher antibody response rates in the 3‐dose arm, including increased titers of neutralizing antibodies.( 109 ) As a result of these and other studies, the FDA recently authorized a third dose of mRNA vaccination in immunocompromised patients, including transplant recipients, and this is now also recommended by the CDC.( 110 ).
The ideal timing for vaccination in liver transplantation recipients who receive standard induction immunosuppression is unknown. Risk of acquiring COVID‐19 must be balanced with the potential benefits of waiting longer to vaccinate after transplantation in an effort to optimize vaccine response. In particular, for patients who require B or T cell ablative therapies after transplantation, it may be reasonable to wait until 3 months after these treatments to begin vaccination.
Concerns have been raised about safety of vaccination, including low rates of allergic reactions, thrombotic events, myocarditis, and others. While there have been no reports to date of adverse effects of vaccination, thrombosis or otherwise, that are specifically common in liver transplantation candidates or recipients, additional safety and efficacy data in this population will be important with a focus on both thrombotic and immune‐mediated events.
What We Have Learned and Key Remaining Knowledge Gaps
COVID‐19 has had a significant impact on liver transplantation candidates and recipients, as well as the practices surrounding liver transplantation. Our knowledge has improved greatly since the first US case was described over a year ago, in March 2020. Despite an initial decline in liver transplantations performed, transplantation metrics have improved throughout the year and perhaps exceeded projections. The transplantation community made rapid adjustments including shifting to telehealth, implementing measures to protect clinical teams from exposures, and facilitating donor and recipient testing such that after a whole year, there have been no reported cases of viral transmission with COVID‐19 in the recipient of a liver allograft. In addition, the community has worked together to describe risk factors for severe COVID‐19 and outcomes among liver transplantation candidates and recipients with SARS‐CoV‐2 infection, and have successfully treated these patients for COVID‐19, balancing the risks and benefits of immunosuppression modification specifically in our liver transplantation recipients. Perhaps most remarkably, we are now seeing our transplantation candidates and recipients undergo vaccination, offering hope of a preventative strategy, even if the response is not as robust as in the general population.
Despite these tremendous accomplishments, there remain several key gaps in knowledge which should guide future studies and research. In particular, more data are needed in the following high‐priority areas:
Safety and efficacy of COVID‐19 treatment strategies in patients with cirrhosis and in liver transplantation recipients.
Safety and efficacy of prevention of COVID‐19 with vaccination in patients with cirrhosis and in liver transplantation recipients, including characterization of cellular immune responses and rates of infection following the vaccine, understanding of the risk of rejection and/or hepatitis flare with vaccination, and risk of SARS‐CoV‐2 infection.
Impact of the specific immunosuppression regimens and intensities on COVID‐19 outcomes and vaccine responses.
Risk assessment of donors and recipients with recent or active SARS‐CoV‐2 infection in the peritransplantation period.
The long‐term outcomes of liver transplantation candidates and recipients with COVID‐19, including the proportion with prolonged symptoms.
Potential conflict of interest: Elizabeth C. Verna receives grant support from Salix
References
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis 2020;20:533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, et al. Acute liver injury in COVID‐19: prevalence and association with clinical outcomes in a large US cohort. Hepatology 2020;72:807‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nobel YR, Phipps M, Zucker J, Lebwohl B, Wang TC, Sobieszczyk ME, Freedberg DE. Gastrointestinal symptoms and coronavirus disease 2019: a case‐control study from the United States. Gastroenterology 2020;159:373‐375.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID‐19. Nat Med 2020;26:1017‐1032. [DOI] [PubMed] [Google Scholar]
- 5. Mao R, Qiu Y, He J‐S, Tan J‐Y, Li X‐H, Liang J, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID‐19: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol 2020;5:667‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh S, Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology 2020;159:768‐771.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID‐19 in patients with liver and kidney diseases: an early systematic review and meta‐analysis. Tropical Med Infect Dis 2020;5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sherman CB, Said A, Kriss M, Potluri V, Levitsky J, Reese PP, et al. In‐person outreach and telemedicine in liver and intestinal transplant: a survey of national practices, impact of COVID‐19 and areas of opportunity. Liver Transpl 2020;26:1354‐1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Data ‐ OPTN. https://optn.transplant.hrsa.gov/data/. Accessed January 6, 2021. [Google Scholar]
- 10. Leventhal TM, Florek E, Chinnakotla S. Changes in liver allocation in United States. Curr Opin. Organ Transplant 2020;25:52‐58. [DOI] [PubMed] [Google Scholar]
- 11. Strauss AT, Boyarsky BJ, Garonzik‐Wang JM, Werbel W, Durand CM, Avery RK, et al. Liver transplantation in the United States during the COVID‐19 pandemic: national and center‐level responses. Am J Transplant 2021;21:1838‐1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agopian V, Verna E, Goldberg D. Changes in liver transplant center practice in response to coronavirus disease 2019: unmasking dramatic center‐level variability. Liver Transpl 2020;26:1052‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clinical and Best Practice Advice for Hepatology and Liver Transplant Providers during the COVID‐19 Pandemic: AASLD Expert Panel Conensus Statement. https://www.aasld.org/sites/default/files/2021‐03/AASLD‐COVID19‐ExpertPanelConsensusStatement‐March92021.pdf. Accessed March 20, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Summary of current evidence and information—donor SARS‐CoV‐2 testing and organ recovery from donors with a history of COVID‐19. OPTN. https://optn.transplant.hrsa.gov/media/4424/sars‐cov‐2‐summary‐ofevidence.pdf. Accessed March 20, 2021. [Google Scholar]
- 15. Current state of organ donation and transplantation. COVID‐19 and solid organ transplant. https://unos.org/covid/. Accessed May 17, 2021. [Google Scholar]
- 16. Singhal A, Sahota GS, Srivastava P, Makki K, Agarwal A, Ali Khan A, et al. Living donor liver transplantation during the COVID‐19 pandemic: “elective” but “necessary.” Transplantation 2020;104:e351‐e353. [DOI] [PubMed] [Google Scholar]
- 17. Menon J, Hakeem AR, Rammohan A, Sundaramoorthy S, Kanagavelu RG, Reddy MS, Rela M. Living donor liver transplantation during the COVID‐19 pandemic: a serendipitous silver lining! Transplantation 2021;105:e20‐e21. [DOI] [PubMed] [Google Scholar]
- 18. Verma S, Agarwal S, Chikkala BR, Dey R, Singh S, Varma S, et al. Living donor liver transplants for sick recipients during COVID‐19 pandemic—an experience from a tertiary center in India. Am J Transplant 2020;20:3257‐3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen T, Wu DI, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical features of COVID‐19—related liver functional abnormality. Clin Gastroenterol Hepatol 2020;18:1561‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, et al. COVID‐19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy 2020;75:1742‐1752. [DOI] [PubMed] [Google Scholar]
- 22. Cao W. Clinical features and laboratory inspection of novel coronavirus pneumonia (COVID‐19) in Xiangyang, Hubei. medRxiv 2020. 10.1101/2020.02.23.20026963. [DOI] [Google Scholar]
- 23. Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, et al. Hepatic pathology in patients dying of COVID‐19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol 2020;33:2147‐2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA 2020;323:2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid‐19 in New York City. N Engl J Med 2020;382:2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, et al. Systematic review with meta‐analysis: liver manifestations and outcomes in COVID‐19. Aliment Pharmacol Ther 2020;52:584‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Melquist S, Estepp K, Aleksandrovich Y, Lee A, Beiseker A, Hamedani FS, Bassett J. COVID‐19 presenting as fulminant hepatic failure: a case report. Medicine 2020;99:e22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roth NC, Kim A, Vitkovski T, Xia J, Ramirez G, Bernstein D, Crawford JM. Post‐COVID‐19 cholangiopathy: a novel entity. Am J Gastroenterol 2021;116:1077‐1082. [DOI] [PubMed] [Google Scholar]
- 29. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature 2020;584:430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, et al. Predictors of outcomes of COVID‐19 in patients with chronic liver disease: US multi‐center study. Clin Gastroenterol Hepatol 2021;19:1469‐1479.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marjot T, Moon AM, Cook JA, Abd‐Elsalam S, Aloman C, Armstrong MJ, et al. Outcomes following SARS‐CoV‐2 infection in patients with chronic liver disease: an international registry study. J Hepatol 2021;74:567‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, et al. Impact of chronic liver disease on outcomes of hospitalized patients with COVID‐19: a multicentre United States experience. Liver Int 2020;40:2515‐2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, et al. High rates of 30‐day mortality in patients with cirrhosis and COVID‐19. J Hepatol 2020;73:1063‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sarin SK, Choudhury A, Lau GK, Zheng M‐H, Ji D, Abd‐Elsalam S, et al. Pre‐existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; the APCOLIS study (APASL COVID‐19 Liver Injury Spectrum Study). Hepatol Int 2020;14:690‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qi X, Liu Y, Wang J, Fallowfield JA, Wang J, Li X, et al. Clinical course and risk factors for mortality of COVID‐19 patients with pre‐existing cirrhosis: a multicentre cohort study. Gut 2021;70:433‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bajaj JS, Garcia‐Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, et al. Comparison of mortality risk in patients with cirrhosis and COVID‐19 compared with patients with cirrhosis alone and COVID‐19 alone: multicentre matched cohort. Gut 2021;70:531‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Efe C, Dhanasekaran R, Lammert C, Ebi B, Higuera‐de la Tijera F, Aloman C, et al. Outcome of COVID‐19 in patients with autoimmune hepatitis: an international multi‐centre study. Hepatology 2021;73:2099‐2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid‐19—preliminary report. N Engl J Med 2020;383:1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 days in patients with severe Covid‐19. N Engl J Med 2020;383:1827‐1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Montastruc F, Thuriot S, Durrieu G. Hepatic disorders with the use of remdesivir for coronavirus 2019. Clin Gastroenterol Hepatol 2020;18:2835‐2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet 2020;395:1569‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee C, Ahn MY, Byeon K, Choi J‐P, Hahm C, Kim H, et al. Clinical experience with use of remdesivir in the treatment of severe acute respiratory syndrome coronavirus 2: a case series. Infect Chemother 2020;52:369‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Antinori S, Cossu MV, Ridolfo AL, Rech R, Bonazzetti C, Pagani G, et al. Compassionate remdesivir treatment of severe Covid‐19 pneumonia in intensive care unit (ICU) and non‐ICU patients: clinical outcome and differences in post‐treatment hospitalisation status. Pharmacol Res 2020;158:104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID‐19: a randomized clinical trial. JAMA 2020;324:1048‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Center for Drug Evaluation and Research . Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/214787Orig1s000Sumr.pdf. Accessed March 20, 2021. [Google Scholar]
- 46. Umemura T, Nishikawa K, Mutoh Y, Sasano H, Kozaki K, Yamada T, Ichihara T. Usage experience of remdesivir for SARS‐CoV‐2 infection in a patient with chronic cirrhosis of Child‐Pugh class C. J Antimicrob Chemother 2021;76:1947‐1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Genovese MC, Kremer JM, van Vollenhoven RF, Alten R, Scali JJ, Kelman A, et al. Transaminase levels and hepatic events during tocilizumab treatment: pooled analysis of long‐term clinical trial safety data in rheumatoid arthritis. Arthritis Rheumatol 2017;69:1751‐1761. [DOI] [PubMed] [Google Scholar]
- 48. Schiff MH, Kremer JM, Jahreis A, Vernon E, Isaacs JD, van Vollenhoven RF. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther 2011;13:R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Feist E, Burmester G‐R. Is tocilizumab in combination with traditional DMARDs safe and effective for patients with active RA? Nat Clin Pract Rheumatol 2009;5:128‐129. [DOI] [PubMed] [Google Scholar]
- 50. Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID‐19 pneumonia: a randomized clinical trial. JAMA Intern Med 2021;181:24‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID‐19. JAMA Intern Med 2021;181:41‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stone JH, Frigault MJ, Serling‐Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with Covid‐19. N Engl J Med 2020;383:2333‐2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Piano S, Dalbeni A, Vettore E, Benfaremo D, Mattioli M, Gambino CG, et al. Abnormal liver function tests predict transfer to intensive care unit and death in COVID‐19. Liver Int 2020;40:2394‐2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. SARS‐CoV‐2 (Coronavirus, 2019‐nCoV): Recommendations and Guidance for Organ Donor Testing. AST 2020. https://www.myast.org/sites/default/files/Donor%20Testing_100520_revised_ReadyToPostUpdated10‐12.pdf. Accessed March 22, 2021. [Google Scholar]
- 55. COVID‐19 Information | American Society of Transplantation . https://www.myast.org/covid‐19‐information. Accessed January 6, 2021.
- 56. Lower Respiratory SARS‐CoV‐2 Testing forLung Donors. 2021. https://optn.transplant.hrsa.gov/media/4576/policy_notice_lunglowerrespiratorytesting_20210426.pdf. Accessed May 17, 2021. [Google Scholar]
- 57. Kaul DR, Valesano AL, Petrie JG, Sagana R, Lyu D, Lin J, et al. Donor to recipient transmission of SARS‐CoV‐2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant 2021;21:2885‐2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Interim Guidance on Duration of Isolation and Precautions for Adults with COVID‐19. 2021. https://www.cdc.gov/coronavirus/2019‐ncov/hcp/duration‐isolation.html. Accessed May 19, 2021. [Google Scholar]
- 59. Kushner T, Cafardi J. Chronic liver disease and COVID‐19: alcohol use disorder/alcohol‐associated liver disease, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, autoimmune liver disease, and compensated cirrhosis. Clin Liver Dis 2020;15:195‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, et al. Non‐alcoholic fatty liver diseases in patients with COVID‐19: a retrospective study. J Hepatol 2020;73:451‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yohanathan L, Campioli CC, Mousa OY, Watt K, Friedman DZP, Shah V, et al. Liver transplantation for acute liver failure in a SARS‐CoV‐2PCR‐positive patient. Am J Transplant 2021;21:2890‐2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tabrizian P, Pourmand K, Florman S. Liver transplantation in a patient with human immunodeficiency virus and COVID‐19. Liver Transpl 2020. Nov 18. 10.1002/lt.25947. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Niess H, Börner N, Muenchhoff M, Khatamzas E, Stangl M, Graf A, et al. Liver transplantation in a patient after COVID‐19—rapid loss of antibodies and prolonged viral RNA shedding. Am J Transplant 2021;21:1629‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dhand A, Bodin R, Wolf DC, Schluger A, Nabors C, Nog R, et al. Successful liver transplantation in a patient recovered from COVID‐19. Transpl Infect Dis 2021;23:e13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kulkarni AV, Parthasarathy K, Kumar P, Sharma M, Reddy R, Chaitanya Akkaraju Venkata K, et al. Early liver transplantation after COVID‐19 infection: the first report. Am J Transplant 2021;21:2279‐2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Malleeswaran S, Mohanka R, Yalakanti RB, Patcha R, Rao PS, Appusamy E, et al. Living donor hepatectomy after proven SARS‐CoV‐2 infection: first report of 9 cases from 3 centers. Transplantation 2021;105:e70‐e71. [DOI] [PubMed] [Google Scholar]
- 67. Manzia TM, Gazia C, Lenci I, Angelico R, Toti L, Monaco A, et al. Liver transplantation performed in a SARS‐CoV‐2 positive hospitalized recipient using a SARS‐CoV‐2 infected donor. Am J Transplant 2021;21:2600‐2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schaefer EAK, Arvind A, Bloom PP, Chung RT. Interrelationship between coronavirus infection and liver disease. Clin Liver Dis 2020;15:175‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, et al. SARS‐CoV‐2 infection of the liver directly contributes to hepatic impairment in patients with COVID‐19. J Hepatol 2020;73:807‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhao B, Ni C, Gao R, Wang Y, Yang LI, Wei J, et al. Recapitulation of SARS‐CoV‐2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell 2020;11:771‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Marjot T, Webb GJ, Barritt AS, Moon AM, Stamataki Z, Wong VW, Barnes E. COVID‐19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol 2021;18:348‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, et al. COVID‐19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant 2020;20:1800‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Belli LS, Duvoux C, Karam V, Adam R, Cuervas‐Mons V, Pasulo L, et al. COVID‐19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry. Lancet Gastroenterol Hepatol 2020;5:724‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Colmenero J, Rodríguez‐Perálvarez M, Salcedo M, Arias‐Milla A, Muñoz‐Serrano A, Graus J, et al. Epidemiological pattern, incidence and outcomes of COVID‐19 in liver transplant patients. J Hepatol 2021;74:148‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rabiee A, Sadowski B, Adeniji N, Perumalswami PV, Nguyen V, Moghe A, et al. (COVID‐19): US multicenter experience. Hepatology 2020;72:1900‐1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, et al. Outcomes following SARS‐CoV‐2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol 2020;5:1008‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mansoor E, Perez A, Abou‐Saleh M, Sclair SN, Cohen S, Cooper GS, et al. Characteristics, hospitalization, and mortality rates of coronavirus disease 2019 among liver transplant patients in the United States: a multicenter research network study. Gastroenterology 2021;160:459‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pereira MR, Aversa MM, Farr MA, Miko BA, Aaron JG, Mohan S, et al. Tocilizumab for severe COVID‐19 in solid organ transplant recipients: a matched case‐control study. Am J Transplant 2020;20:3198‐3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Therapeutic Management of Adults with COVID‐19 . COVID‐19 Treatment Guidelines 2021. https://www.covid19treatmentguidelines.nih.gov/therapeutic‐management/. Accessed May 17, 2021. [Google Scholar]
- 80. Kates OS, Haydel BM, Florman SS, Rana MM, Chaudhry ZS, Ramesh MS, et al. COVID‐19 in solid organ transplant: a multi‐center cohort study. Clin Infect Dis 2020. Aug 7. 10.1093/cid/ciaa1097. [Epub ahead of print] [DOI] [Google Scholar]
- 81. Pereira MR, Aversa MM, Farr MA, Miko BA, Aaron JG, Mohan S, et al. Tocilizumab for severe COVID‐19 in solid organ transplant recipients: a matched case‐control study. Am J Transplant 2020;20:3198‐3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Laracy JC, Verna EC, Pereira MR. Antivirals for COVID‐19 in solid organ transplant recipients. Curr Transplant Rep 2020;7:355‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Frequently asked questions onthe Emergency Use Authorization for bamlanivimab and etesevimab. Food and Drug Administration. 2021. https://www.fda.gov/media/145808/download. Accessed May 12, 2021. [Google Scholar]
- 84. Anti‐SARS‐CoV‐2 Monoclonal Antibodies | COVID‐19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/statement‐on‐bamlanivimab‐plus‐etesevimab‐eua/. Accessed May 12, 2021. [Google Scholar]
- 85. Jiang J, Miao Y, Zhao Y, Lu X, Zhou P, Zhou X, et al. Convalescent plasma therapy: helpful treatment of COVID‐19 in a kidney transplant recipient presenting with serve clinical manifestation and complex complications. Clin Transplant 2020;34:e14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rahman F, Liu STH, Taimur S, Jacobs S, Sullivan T, Dunn D, et al. Treatment with convalescent plasma in solid organ transplant recipients with COVID‐19: experience at large transplant center in New York City. Clin Transplant 2020;34:e14089. [DOI] [PubMed] [Google Scholar]
- 87. Fung M, Nambiar A, Pandey S, Aldrich JM, Teraoka J, Freise C, et al. Treatment of immunocompromised COVID‐19 patients with convalescent plasma. Transpl Infect Dis 2020;23:e13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Naeem S, Gohh R, Bayliss G, Cosgrove C, Farmakiotis D, Merhi B, et al. Successful recovery from COVID‐19 in three kidney transplant recipients who received convalescent plasma therapy. Transpl Infect Dis 2020;23:e13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lima B, Gibson GT, Vullaganti S, Malhame K, Maybaum S, Hussain ST, et al. COVID‐19 in recent heart transplant recipients: clinicopathologic features and early outcomes. Transpl Infect Dis 2020;22:e13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L, et al. Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J Infect Dis 2020;221:1762‐1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Belli LS, Fondevila C, Cortesi PA, Conti S, Karam V, Adam R, et al. Protective role of tacrolimus, deleterious role of age and comorbidities in liver transplant recipients with Covid‐19: results from the ELITA/ELTR Multi‐center European Study. Gastroenterology 2021;160:1151‐1163.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Becchetti C, Zambelli MF, Pasulo L, Donato MF, Invernizzi F, Detry O, et al. COVID‐19 in an international European liver transplant recipient cohort. Gut 2020;69:1832‐1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Salerno DM, Kovac D, Corbo H, Jennings DL, Lee J, Choe J, et al. SARS‐CoV‐2 infection increases tacrolimus concentrations in solid‐organ transplant recipients. Clin Transplant 2020;35:e14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Theodore DA, Greendyke WG, Miko B, Whittier S, Green DA, Shoucri S, et al. Cycle thresholds among solid organ transplant recipients testing positive for SARS‐CoV‐2. Transplantation 2021;105:1445‐1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lagana SM, De Michele S, Lee MJ, Emond JC, Griesemer AD, Tulin‐Silver SA, et al. COVID‐19 associated hepatitis complicating recent living donor liver transplantation. Arch Pathol Lab Med 2020;144:929‐932. [DOI] [PubMed] [Google Scholar]
- 96. Fix OK, Blumberg EA, Chang K, Chu J, Chung RT, Goacher EK, et al. AASLD expert panel consensus statement: vaccines to prevent COVID‐19 in patients with liver disease. 2021. https://www.aasld.org/sites/default/files/2021‐03/AASLD‐COVID19‐VaccineDocument‐March162021‐FINAL.pdf. Accessed March 20, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fix OK, Blumberg EA, Chang K‐M, Chu J, Chung RT, Goacher EK, et al. AASLD Expert Panel Consensus Statement: vaccines to prevent COVID‐19 infection in patients with liver disease. Hepatology 2021;74:1049‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. COVID‐19 Vaccine FAQ Sheet . AST 2021. https://www.myast.org/sites/default/files/2021%2003%2018%20COVID19%20VACCINE%20FAQS_update.pdf. Accessed March 20, 2021. [Google Scholar]
- 99. Chow J, Golan Y. Vaccination of solid‐organ transplantation candidates. Clin Infect Dis 2009;49:1550‐1556. [DOI] [PubMed] [Google Scholar]
- 100. Sester M, Gärtner BC, Girndt M, Sester U. Vaccination of the solid organ transplant recipient. Transplant Rev 2008;22:274‐284. [DOI] [PubMed] [Google Scholar]
- 101. Danziger‐Isakov L, Kumar D, AST ID Community of Practice . Vaccination of solid organ transplant candidates and recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant 2019;33:e13563. [DOI] [PubMed] [Google Scholar]
- 102. John BV, Deng Y, Scheinberg A, Mahmud N, Taddei TH, Kaplan D, et al. Association of BNT162b2 mRNA and mRNA‐1273 vaccines with COVID‐19 infection and hospitalization among patients with cirrhosis. JAMA Intern Med 2021. 10.1001/jamainternmed.2021.4325. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Burack D, Pereira MR, Tsapepas DS, Harren P, Farr MA, Arcasoy S, et al. Prevalence and predictors of SARS‐CoV‐2 antibodies among solid organ transplant recipients with confirmed infection. Am J Transplant 2021;21:2254‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, Garonzik‐Wang JM. Immunogenicity of a single dose of SARS‐CoV‐2 messenger RNA vaccine in solid organ transplant recipients. JAMA 2021;325:1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, Garonzik‐Wang JM. Antibody response to 2‐dose SARS‐CoV‐2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021;325:2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sattler A, Schrezenmeier E, Weber U, Potekhin A, Bachmann F, Straub‐Hehenbleicher H. Impaired humoral and cellular immunity after SARSߚCoV2 BNT162b2 (Tozinameran) primeߚboost vaccination in kidney transplant recipients. J Clin Invest 2021;131:e150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cucchiari D, Egri N, Bodro M, Herrera S, Del Risco‐Zevallos J, Casals‐Urquiza J, et al. Cellular and humoral response after MRNA‐1273 SARS‐CoV‐2 vaccine in kidney transplant recipients. Am J Transplant 2021;21:2727–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid‐19 vaccine in solid‐organ transplant recipients. N Engl J Med 2021;385:661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hall VG, Ferreira VH, Ku T, Ierullo, Majchrzak‐Kita B, Chaparro C. Randomized trial of a third dose of mRNA‐1273 vaccine in transplant recipients. N Engl J Med 2021. 10.1056/NEJMc2111462. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html. Accessed August 2021.


