Conflict of interest
Nothing to disclose.
Dear Editor,
According to official data published by the World Health Organization, more than 100 million cases worldwide have been confirmed to be infected with severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), causing over two million deaths. The CoronaVac vaccine, an inactivated vaccine candidate against COVID‐19 containing inactivated SARS‐CoV‐2, is a Chinese vaccine developed by Sinovac Life Sciences (Beijing, China). Randomized, double‐blind, placebo‐controlled phase 1/2 clinical trials demonstrated the safety, tolerability and immunogenicity of the CoronaVac vaccine, an inactivated COVID‐19 vaccine, in healthy adults 18 years of age and older. 1 , 2 A branch of CoronaVac vaccine phase 3 clinical trials were conducted in Turkey. Republic of Turkey Ministry of Health General Directorate for Pharmaceuticals and Pharmacy has approved the CoronaVac COVID‐19 vaccine for the emergency use of the disease on 13 January 2021. 1 , 3 Vaccination was initiated in healthcare workers in our country. The adverse effects of the inactivated CoronaVac vaccine have not been fully characterized yet. Here, we report one case of pityriasis rosea in a patient following CoronaVac COVID‐19 vaccination.
A 45‐year‐old female healthcare worker applied to our dermatology outpatient clinic for evaluation of skin rashes which developed 4 days after the first dose of CoronaVac COVID‐19 vaccine and had been present for 1 week. There was no itching, and there were no accompanying systemic symptoms. She denied any history of allergies, recent infections or drug exposure, and contact with anyone with COVID 19 infection, as well as similar skin rashes in personal or family history.
Dermatological examination revealed two oval thin plaques 2 cm in diameter with a peripheral collarette scaling consistent with the herald patch on the right scapula and the right breast. There were multiple, discrete, 1–2 cm diameter, oval to round, salmon‐coloured plaques over the trunk and proximal extremities, many of which had peripheral scales. The distribution of these plaques was along cleavage lines reminiscent of a Christmas tree pattern on the patient's body (Fig. 1). The patient had not noticed any interval between the herald patches and the diffuse rash.
Figure 1.
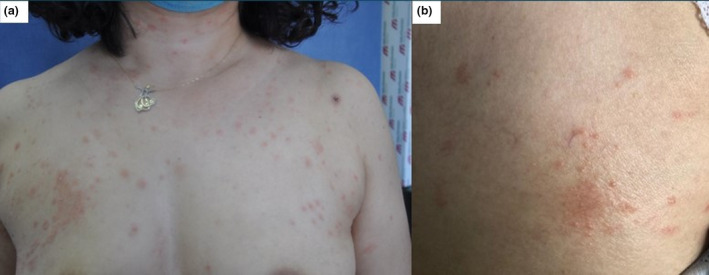
(a) Multiple oval salmon‐coloured plaques with peripheral scales over the trunk and proximal extremities and (b) the herald patch on the right breast.
Laboratory investigation did not reveal any specific abnormalities. SARS‐CoV‐2 PCR tests performed from both the nasopharyngeal swab sample and the skin lesion biopsy were negative.
Histopathological examination demonstrated focal parakeratosis in mounds with exocytosis of lymphocytes, spongiosis in the epidermis and extravasated red blood cells in the dermis (Fig. 2).
Figure 2.
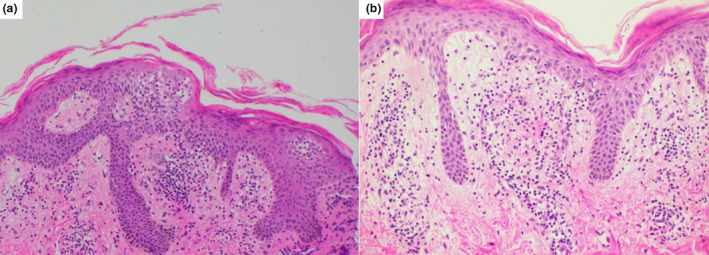
(a) Focal parakeratosis in mounds with exocytosis of lymphocytes and spongiosis in the epidermis (HE, ×100) and (b) extravasated red blood cells in the dermis (HE, ×200).
The patient was treated symptomatically with an oral antihistamine and a mid‐potency topical corticosteroid cream and the lesions faded within 3 weeks. Twenty‐eight days after the first vaccine, she received the second dose of CoronaVac COVID‐19 vaccine, and 4 days after the second dose, skin rashes were similarly reactivated at the previous lesion sites and faded within a week.
According to the criteria defined by Drago et al., 4 in our patient, the presence of discrete exanthematous lesions with the herald patch without itching, absence of eosinophilia in complete blood count and histopathological findings were consistent with typical pityriasis rosea rather than pityriasis rosea‐like eruptions.
Pityriasis rosea has been implicated with reactivation of human herpesviruses 6 and 7 triggered by other infections, psychological stress, pregnancy and drugs. 5 Pityriasis rosea has rarely been described after vaccines. The exact pathogenetic mechanism that leads to pityriasis rosea after vaccination is unknown. 6
As the worldwide vaccination campaign against the COVID‐19 pandemic continues, we emphasize that pityriasis rosea can result from new CoronaVac COVID‐19 vaccine, for both physicians and patients, and lesions may be reactivated after the second vaccination. However, pityriasis rosea is a self‐limiting benign exanthema and does not require interruption of the vaccination programme for life‐threatening SARS‐CoV‐2 infection, but close monitoring of the skin eruptions is required.
Acknowledgement
The patients in this manuscript have given written informed consent to the publication of their case details.
References
- 1. Zhang Y, Zeng G, Pan H et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18–59 years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis 2020.21: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, Hu Y, Xu M et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021. https://www.thelancet.com/action/showPdf?pii=S1473‐3099%2820%2930987‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mallapaty S. China COVID vaccine reports mixed results—what does that mean for the pandemic? Nature News 2021; 15. https://www.nature.com/articles/d41586‐021‐00094‐z. [DOI] [PubMed] [Google Scholar]
- 4. Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea‐like eruptions: how to distinguish them? JAAD Case Rep 2018; 4: 800–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drago F, Broccolo F, Rebora A. Pityriasis rosea: an update with a critical appraisal of its possible herpesviral etiology. J Am Acad Dermatol 2009; 61: 303–318. [DOI] [PubMed] [Google Scholar]
- 6. Drago F, Ciccarese G, Javor S, Parodi A. Vaccine‐induced pityriasis rosea and pityriasis rosea‐like eruptions: a review of the literature. J Eur Acad Dermatol Venereol 2016; 30: 544–545. [DOI] [PubMed] [Google Scholar]


