Abstract
The newborn coronaivus disease 2019 (COVID‐19) pandemic has become the foremost concern of health system worldwide. Interferon typeI (IFN‐I) are among the well‐known antiviruses. Hence IFN‐α have gained much attention as a treatment for COVID‐19 recently. To sum up the efficiency of IFN‐α against COVID‐19, we searched PubMed, SCOPUS, and EMBASE, from the date of genesis to the 1st of October 2020. Discharge from hospital and virus clearance considered as primary and secondary outcomes, respectively. We compared the aforementioned outcomes of patients treated with standard care protocol and the patients treated with IFN‐α in addition to standard care protocol. Out of 356 identified records, 14 studies were subjected for full‐text screening. Finally, a systematic review was performed with inclusion of five studies. Majority of the participants were males (ranged from 43.50% to 90.0%). We found that time of viral clearance and polymerase chain reaction negative (days) in most studies were decreased in the INF‐α + standard care group. The mean days of virus's clearance in INF‐α group and standard group reported 27.3 and 32.43. Likewise, the average days of hospitalization was found also lower in INF‐α group (18.55 vs. 24.36). This study provides a stand to conclude that early administration of INF‐α may be accounted as a promising treatment of COVID‐19.
Keywords: COVID‐19, discharge, interferon, systematic review
1. INTRODUCTION
Latest pandemic well known as coronavirus disease 2019 (COVID‐19) has caused the foremost health and economic challenges all over the world. 1 Several research works have been conducted worldwide addressing this newborn concern but to date no efficient antiviral intervention have been approved. Clues arise from investigating molecular patho‐mechanism of severe acute respiratory syndrome coronavirus 2 (SARS‐COV‐2) (virus causing COVID‐19). Interferons (IFNs) are well known for their action of interfering with virus replication and resulted in antiviral functions. 2 Moreover, type I IFNs support immune system in viral clearance via several mechanisms. But regretfully production and function of type I IFNs attenuated notably in COVID‐19 patients since circulating levels of IFNs type I presented to define the stage of the condition. Deterioration of patients' clinical symptoms occurs following a decrease in plasma levels of IFNs type I. Therefore, type I IFNs insufficiency could be a sign of severe stages of COVID‐19 and may help identify a high‐risk population. Among type I IFNs, IFN‐α is the first‐line therapy of viral diseases, including hepatitis B and C infections. IFN‐α revealed beneficial effects on resolution of lung abnormalities in hospitalized patients with SARS during 2003 SARS‐CoV outbreak in Canada and China.3, 4
In our previous conducted systematic review and meta‐analysis study of clinical trials we presented that early administration of IFN‐β with antiviral drugs is a promising treatment against COVID‐19. 5 Moreover, it was reported that nasal drops of IFN‐α had protective function against COVID‐19 among healthcare workers. 16 IFN‐α can be efficient as preventive and therapeutic medicine for COVID‐19, not only for its antiviral activity but also owing to its role in regulating inflammatory factors. Thereafter IFN‐α seems a promising candidate to manage several stages of COVID‐19.
In such a situation where finding a way to control and treatment of COVID‐19 is urgent, thorough review and analysis of the studies and performed clinical trials may offer a new way of deducing the results besides introducing efficient treatments. Hence, we developed a systematic review to evaluate the findings of IFN‐α treatment in patients with COVID‐19.
IFN‐α treatment powerfully inhibits severe in vitro cytopathology induced by Middle Eastern respiratory syndrome coronavirus (MERS‐C0V) replication. Additionally, MERS‐CoV was found to be 50–100 times more sensitive to IFN‐α treatment than SARS‐CoV in vitro. These findings highlight relevant differences between different CoVs in terms of their interaction with and evasion from the cellular innate immune response
2. METHODS
2.1. Search strategy and data extraction
MEDLINE, SCOPUS, EMBASE, Google Scholar databases, and websites (WHO and CDC) systematically searched for identifying relevant articles to find out the effects of IFN‐α in severe COVID‐19 patients up to the October 1st, 2020. The references list of all found records was checked to select more related studies. We searched English language records. The search keywords were 2019‐nCoV, 2019 novel coronavirus, COVID‐19, coronavirus disease 2019, INF‐α and ‐β.
Inclusion criteria was: (1) hospitalized COVID‐19 patients receiving IFN‐α, (2) be any of randomized controlled trial, cohort, or retrospective studies (all observational and interventional studies).
Records independently selected by two authors (HA, AN) and appropriate information gathered. Disagreements and doubted records were solved by consent of two more authors. All information about included records, such as article character, therapeutic impact of IFN, sign and symptoms release, hospital discharge, mortality, other relevant information and expected outcomes, as well as intervention, were gathered in the predesigned EXCELL form with brief details.
2.2. Comparison
We compared standard care protocol of laboratory‐confirmed and hospitalized patients with COVID‐19, with standard care combination with IFN‐α. We considered patients who received standard antiviral treatment (lopinavir/ritonavir/arbidol) as control group and (antiviral treatment + IFN‐α/IFN‐β) as intervention group.
2.3. Outcomes
The primary and secondary outcomes were considered as virus clearance (mean days) and hospitalization days (length of stay), respectively.
The time (days) of treatment initiation (with IFN) from symptom onsets was evaluated in the studies if reported, to identify the association between the golden time of IFN‐α therapy after symptoms beginning.
Inflammatory factors as well as symptoms release, including fever resolve, adverse reactions, blood cells count, and disease level were studied and assessed in all included studies.
3. RESULTS
We identified 356 records by searching a total of 4 literature databases, which were evaluated for duplications and inclusion criteria by title and abstracts review. Out of these, one randomized controlled trial (RCT) and four observational studies were included for full‐text screening and finally, five records were included in the systematic review (Figure 1).
Figure 1.
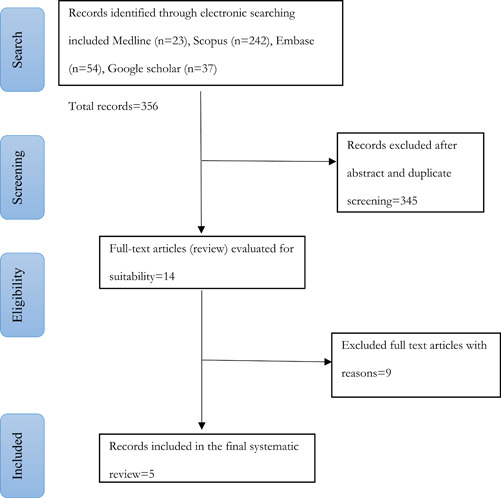
Search flow diagram
The total included patients were 541. The PRISMA flow diagram (Figure 1) indicated the included studies. Out of the 14 records, the following were excluded after full‐text screening: beta IFN = 7, letter to the editor = 1, case reports = 2, poor information = 4.
The details of the included studies are demonstrated in Table 1. Majority of the patients were males in most of the articles. The percentage of male patients ranged from 43.50% to 60.0%. The mean age of the patients in most studies was more than 41. All of included records were performed in China.
Table 1.
Important features of included studies
| Author | Country | Mean age ± SD | Sex (male %) | Type of study | No. of patients | Study groups | Treatment onset from symptom onset | Stage of disease | |
|---|---|---|---|---|---|---|---|---|---|
| Intervention | control | ||||||||
| Bo Wang 12 | China | 56.2 ± 9.7 | 44% | Single‐center, retrospective cohort study | 41 | LPV/r 200 mg/50 mg/pill), 400 mg/time, twice a day in combination with IFN α ‐2b | LPV/r | Intervention: 12 ± 7.3 Control: 11 ± 6.8 | Hospitalized |
| Yin‐Qiu Huang 10 | China | 42.5 | 46% | RCT | 101 | (A) RBV and IFN‐α, (B) LPV/r and IFN‐α, (C) RBV and LPV/r and IFN‐α at a 1:1:1 ratio | Not defined | Mild to moderate | |
| Qiong Zhou 7 | China | 41.3 | IFN: 0.0% IFN + ARB:4 3.5% ARB: 45.8% | Cohort uncontrolled, exploratory study | Total :77 IFN‐α2b: 7ARB: 24 IFN‐α2b + ARB: 46 | IFN‐ α2b (5 mU b.i.d) 1 ml in 2 ml of sterile water | ARB (200 mg t.i.d.) or IFN‐α2b and ARB | IFN:8 (5.5, 15.5) IFN + ARB: 6.5 (3, 10) ARB: 10 (4.5, 19.9) | Moderate |
| Yan Zuo 8 | China | 44.3 ± 13.3 | 55.2% | Retrospective study | 181 | LPV/r and IFN‐α | Not defined | <5 days from onset versus ≥5 days | Severe (34%) versus non severe |
| Ping Xu 13 | China | 52.4 | 60% | Retrospective multicenter cohort | 141 | IFN‐α2b | ARB (200 mg, oral, three times per day, for 7–10 days)/with IFN‐α2b combination | Not defined | Not defined |
Abbreviations: ARB, Arbido; INF, interferon; LPV/r, lopinavir/ritonavir; RBV, ribavirin; RCT, randomized controlled trial.
3.1. Clinical symptoms
Table 1 shows characteristics of studies included. Most of the included participants in all of the investigations had fever at admission. The fever of the majority of participants in the intervention group had disappeared by the end of the study. White blood cell and lymphocyte count were decreased in the majority of articles in the intervention group. Qiong Zhou et al. reported that fever was resolved in all patients during (median) 4 (2.0, 7.0) days. Time‐to‐viral clearance (duration from the symptoms onset to the time of the first two repeated negative polymerase chain reaction [PCR] tests) reported 13.0 (9.0, 21.5) days among patients who were treated with INF‐α.6, 7
In all studies, hospitalized patients with mild to severe COVID‐19 had been investigated. The time of treatment onset from symptom onset were between 5 and 10 days. The shortest time (<5 days) to IFN administration after initiation of symptoms was reported in the Yan Zuo study. 8 Longest time, 8 (5.5, 15.5), from symptom onset to treatment with IFN was related to the China study by Qiong Zhou. 7
3.2. Outcomes
Table 2 indicated the outcome variables and clinical features among patients treated with IFN‐α. We found that time of viral clearance and PCR negative (days) in most studies were decreased in the intervention group (IFN‐α + standard care). The mean days of virus clearance in IFN‐α group and standard group reported 27.3 and 32.43. The virus's clearance was markedly faster in patients who started IFN‐α earlier than control group (5.13 days). Wang et al. found that the average length of hospitalization (16±9.7 vs. 23±10.5 days) in the combination group with IFN α‐2b was shorter than control group (standard). Moreover, in the all included studies the average days of hospitalization was found lower in IFN‐α group (18.55 vs. 24.36). Likewise, in Bo Wang study, hospitalization duration in early intervention group declined from 25±8.5 days to 10±2.9 days compared with delayed intervention group. 9 However, some studies did not reported death and hospitalization days. 7 The average duration of viral clearance ranged from 5 to 21 days. The shortest duration of virus clearance (5 days) was reported by Yan Zuo study for patients who started antiviral treatment. 8
Table 2.
Clinical features among patients treated with interferon α (INF‐α)
| Author | IFN‐α‐ dose | INF type | INF administration | WBC count | Lymphocyte count | Primary outcome; the time to viral clearance(int. vs. cont.) | IL‐6 levels | Hospitalization days (int. vs. cont.) |
|---|---|---|---|---|---|---|---|---|
| Bo Wang | 3 million IU/dose) | IFN α‐2b | Subcutaneous injection | Decreased WBC count: Not significant | Not defined | Faster viral clearance in IFN α‐2b group: (37 v 42 days) | Not reported | 16 ± 9.7/23 ± 10.5 |
| Yin‐Qiu Huang | 5 million U or 50 mg per dose twice a day for 14 d | IFN‐α | Atomizing inhalation | 4.8 (4.0, 6.0) | 1.5 (1.1, 1.9) Neutrophil (109/L): 2.9 (2.0, 3.9) | 13.0 (9.0, 21.5) | Not reported | 17 (12, 24) |
| Qiong Zhou | 5 mU b.i.d 10 mIU/day | IFN‐α2b | Aerosol inhalation | Varied around the normal range with no obvious or consistent difference noticeable among antiviral treatment groups. | 21.1 days for IFN 27.9 for ARB 20.3 days for IFN + ARBtreatment speeded viral clearance by ~7 days. | IFN treatment markedly decreased circulating IL‐6 levels in IFN treated group | Not defined | |
| Yan Zuo | Not defined | IFN‐α | Not defined | Lymphocytopenia recorded in 41 (22.7%) patients. | Accelerated in patients who started antiviral treatment <5 days after symptoms onset | The median IL‐6 level of 17.5 pg/ml (IQR, 5.9‐43.2) in the prolonged group was higher than the median level of 9.0 pg/ml (IQR, 4.0‐27.5) in the short‐term group | 17/23 | |
| Ping Xu | 5 × 10 (5) IU, twice per day, inhale, for 10–14days | IFN‐α2b | Aerosol inhalation | No differences between the two groups | Lymphocytopenia recorded in 36.4% patients | 23.8/27.4 | Not defind | 24.2/27.1 |
Abbreviations: IL, interleukin; IFN, interferon; IQR, interquartile range; WBC, white blood cell.
In the RCT study from China (Yin‐Qiu Huang), no death was reported among study groups. This study did not have any control group (without IFN‐α) but the results indicated that after 4 days fever of all patients had subsided and the median days of hospitalization reported were 17 days (Table 2). 10
One other open‐label single‐arm clinical trial, reported only one death after 45 days of hospitalization. 11 A noncontrolled study reported that fever disappeared in all patients within the first seven days. 6 In the majority of included studies there was no important difference in the adverse effect compared between two groups.9, 10
3.3. Intervention condition (IFN‐α‐ dose)
In most of the studies, 5 micrograms/ml (12 million IU/ml) of IFN‐α were administered twice per day for two consecutive weeks or until discharge. IFN‐α administrated subcutaneously in Bo Wang study whereas other studies administered IFN‐α by aerosol inhalation (Table 2).
4. DISCUSSION
The newborn idiopathic coronavirus named as COVID‐19 and growing number of infected patients has engulfed the whole world since December 2019. Since then, drugs with antiviral and immunomodulation properties are assessed to inhibit the coronavirus and several trials have been conducted. But to date no official antiviral drugs with confirmed efficiency for the treatment of COVID‐19 have been established. Thereafter it seems of crucial importance to collect and summarize several evidence to achieve an efficient treatment for COVID‐19. In the current systematic review, we indicated that IFN‐α in combination with antiviral drugs proficiently suppress the SARS‐CoV‐2 in terms of lessening hospitalization duration and virus clearance duration.
Among several antivirals, type I IFNs(α/β) target and inhibit the replication and development of a various viral pathogens besides supporting an immune response to clear virus infection.14, 15 Type I IFNs are accepted medications for hepatitis B and C, autoimmune disorders, and certain cancers treatment.16, 17
IFN‐ α demonstrated promising properties in faster remission of lung abnormalities during the 2003 SARS‐CoV outbreak in Toronto, Canada among hospitalized SARS patients. 18 Also several evidence supported beneficial effects of IFNs‐α/β on severe MERS‐CoV‐infected patients19, 20. Different recombinant IFNs (rIFN‐α2a, rIFN‐α2b, rIFN‐β1a, and rIFN‐β1b) have shown promising effects against MERS‐CoV in vitro. 21 Regarding similar properties of SARS‐CoV‐2 with MERS‐CoV and SARS‐CoV, the abovementioned evidence could be very helpful in selecting promising managements against SARS‐CoV‐2. Furthermore, SARS‐CoV‐2 has shown a far greater sensitivity to type I IFNs than SARS‐CoV in vitro, which suggests more efficiency in treatment using type I IFNs.
The risk of viral transmission is very high in the viral replication stage. Also, it is well documented that extended virus shedding has been directly related to mortality or disease severity. 8 Hence shortening the duration of virus shedding is considered as the most effective strategy to control the epidemic and increase the recovery percentage. Therefore, the duration of viral clearance and hospital stay counted as the outcomes of the study.
Although a little time has passed from the beginning of the epidemic but it is well documented that the uncontrolled inflammation induced by SARS‐CoV‐2 is the main reason of disease severity and death. High levels of inflammatory markers, including C‐reactive protein (CRP), ferritin and d ‐dimer, high neutrophil‐to‐lymphocyte ratio, and elevated levels of inflammatory cytokines and chemokine have been recorded in patients with severe diseases. So far, interleukin‐6 (IL‐6) and TNF‐α serum levels are introduced as independent and major prognosticators of disease severity and death since the increase of these factors are correlated with most organ failures, such as heart failure. 22 Therefore, attenuation of inflammatory markers, including IL‐6 and CRP, could be a significant achievement in treatment of COVID‐19 patients.
Combination therapy of IFN‐α with antiviral drugs is suggested as treatment of COVID‐19 in the guidelines of National Health Commission of the People's Republic of China. 23 Several clinical trials and retrospective studies have been evaluated the efficacy of IFN‐α monotherapy or in combination with other antiviral drugs against COVID‐19.
Wang et al. assessed the effectiveness of subcutaneously injected IFN‐α‐2b combined with lopinavir/ritonavir (LPV/r, 200 mg/50 mg/pill), 400 mg/time, twice a day in the treatment of COVID‐19. 9 The study demonstrated that subcutaneous injection of IFN‐α‐2b and LPV/r reduced the duration of hospitalization and enhanced viral clearance in COVID‐19 patients. It is noteworthy that early administration of the drug also has a notable impact on the result of the study. Moreover, they highlighted that subcutaneous and intravenous administration of IFN had several advantages over inhalation
Zhou et al. reported the clinical development of disease in hospitalized confirmed cases of COVID‐19 who were treated with IFN‐α2b, Arbidol (ARB) (200 mg t.i.d), or IFN‐α2b and ARB combination. 7 They demonstrated promising impact of IFN‐α in shortening the duration of viral shedding as well as decreasing inflammation markers, including CRP and IL‐6. Thereafter they have introduced IFN‐α2b as a conceivable and promising therapeutic for COVID‐19. Also, they suggested that inhalation of IFN‐α2b make the viral clearance from the respiratory tract faster and accelerated the resolution of systemic inflammatory process. This study also suggests the administration of IFN‐α2b for mild cases of COVID‐19 as reducing the duration of viral shedding also leads to attenuate the spread of the virus among the populations.
Zuo et al. performed a retrospective, multicenter study of hospitalized patients with laboratory‐positive SARS‐CoV‐2 infection. 8 They investigated the impact of initial antiviral therapy (lopinavir/ritonavir, arbidol, IFN‐α) (monotherapy/combination therapy) on viral shedding. They reported significant beneficial effects of early beginning of lopinavir/ritonavir + IFN‐α combination therapy on duration of SARS‐CoV‐2 shedding. Whereas lopinavir/ritonavir monotherapy may had no effect on virus clearance.
Xu et al. 13 conducted a multicenter retrospective cohort study on hospitalized patients with laboratory‐positive COVID‐19 infection pneumonia. They evaluated the impacts of combination therapy of ARB (200 mg, oral, three times per day, for 7–10 days) and IFN‐α and monotherapy of IFN‐α in decreasing hospitalization days and shortening time of virus RNA clearance. They revealed that IFN‐α2b monotherapy was more efficient than ARB/IFN‐α2b combination therapy in accelerating discharge and COVID‐19 RNA clearance. Moreover, they suggested that combination therapy of ARB and IFN‐α2b have probable advantages on inhibiting lung inflammation more than monotherapy of IFN‐α2b in mild cases.
Huang et al, compared the effectiveness of ribavirin (RBV) + IFN‐α, lopinavir/ritonavir (LPV/r) + IFN‐ α, and RBV + LPV/r plus IFN‐α in patients with mild to moderate COVID‐19. 10 RBV was administered by intravenous injection (2 g), and followed by oral doses of 400–600 mg every 8 h depending on patients' body weight, for 14 days, LPV/r was administered orally (400 mg/100 mg per dose) twice per day for 14 days. They observed no major difference in terms of duration of SARS‐CoV‐2 nucleic acid negativity by nasopharyngeal swab (from treatment onset) among the three groups, and not between any two of the three groups. Moreover, no significant difference in the percentage of patients with SARS‐CoV‐2 nucleic acid negativity among the three groups at Day 14 and at Day 28 was not observed. Consequently, they concluded that antiviral efficacy of the aforementioned three antiviral regimens in mild to moderate COVID‐19 was not different remarkably. Moreover, they interpreted that the three regimens do not have any promising antiviral clinical efficiency, consequently no difference in effectiveness was reported in the analyzed data. However, further studies are required in this regard since they did not have blank‐controlled or placebo‐controlled treatment group in their study.
It should be taken into consideration that auto‐antibodies (auto‐Abs) against type I IFNs have been adverted in some patients. Whereas the severity of COVID‐19 was recognized due to neutralizing auto‐Abs against type I IFNs among 10% of patients. In such cases, treatment with IFN‐ɑ is less effective and it should be redirected to IFN‐β therapy. 24 Even though it was documented that SARS‐CoV‐2 suppress type I IFN signaling at the first stages of infection, some studies reported upregulation of IFN‐I responses and increased ISGs expression in severe COVID‐19. Inconsistency in interpretations about IFN‐I responses in patients with COVID‐19 may be attributed to different characteristics in determining moderate, severe, and critical forms of COVID‐19, between studies. Accordingly, further investigations on efficacy of IFN‐I in mild to severe forms of COVID‐19 are crucial. 25
Taken all studies together, IFN‐α therapy seems to have a significant performance against COVID‐19 in terms of decreasing hospitalization days as well as virus clearance duration. In addition, no significant adverse drug reactions or IFN‐α side effects were reported. Indeed, hints could arise from INF‐β therapy for emerging effective therapeutic strategy against COVID‐19. It seems that the earlier administration of IFN‐α lead to more efficiency. Possibly the combination of IFN‐α with two/more antiviral drug in the first days of virus shedding may lead to a rapid suppression of high initial viral load and strengthen the antiviral response and lead to more favorable effects. Thereafter timely administration of the drug is highly suggested. Most of the studies have administered the drug through inhalation; however, one of them subcutaneously injected the IFN‐α. Due to controversy between the results and some limitations further studies with focus on the administration route is suggested.
5. LIMITATIONS
Some studies lacked control and were performed with a small sample size. Some of the studies were retrospective studies or the groups were not adjusted properly. However, controlled trial studies were also performed and confirmed the therapeutic impacts of IFN‐α on COVID‐19. It should be noted that COVID‐19 is a recent phenomena and there are no ample studies. On the other hand, in some studies, the participants were not proportionate and were mostly male; however, it could be unavoidable since being male accounted as a risk factor for COVID‐19 infection and the patient was selected by random selection. 26
Owing to the mysterious nature of COVID‐19 and broad range of symptoms and consequences, engaging various and different interventions in patients is inevitable; therefore, having a uniform intervention in all studies is impossible. However, the data were adjusted to different interventions and attempts have been made to present reliable results.
Some studies did not balance standard characteristics of the two groups, which can lead to overestimation of the IFN efficiency. Additionally, the effects of comorbidities have been ignored in studies while highly suggested to be considered in the further investigations. On the other hand, mechanism of SARS‐CoV‐2 pathogenesis and interactions with IFN‐α may change as several documents indicated changes in the function of virus ORF proteins. 26
The current data demonstrated IFN‐α as the potent and efficient therapeutic for COVID‐19 disease. Though, the interpreting the results must be performed thoughtfully by regarding the limitations of the study.
6. CONCLUSION
Conclusively, the reported data brought us to the conclusion that IFN‐α may promise innovative therapeutic options in terms of decreasing viral shedding and hospitalization duration in COVID‐19 patients. However, owing to small amount of studies, further investigations are deeply suggested. Several factors seem to affect IFN‐α efficacy, including disease severity and treatment onset time. Early administration of IFN‐α combined with antiviral drugs demonstrated notable progress toward overcoming COVID‐19.
AUTHOR CONTRIBUTIONS
Ailar Nakhlband gave the initial idea, contributed in developing protocol and interpreting, and data collection as well as drafted all sections of the manuscript and language editing. Hosein Azizi participated in the all sections of the manuscript development, data analysis, technical comment, electronic searching, data collecting and extracting, and interpretation. Ali Fakhari participated in the all sections of the manuscript development, technical comment, confirmation of extracted information, and interpretation.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.27072
ETHICS STATEMENT
This study was approved by the central ethics committee of Tabriz University of Medical Sciences to number TBZMED. REC.1399.015 on August 2020.
ACKNOWLEDGMENTS
The authors would like to acknowledge high support of their colleagues in Research Center of Psychiatry and Behavioral Sciences. As well, they would like to appreciate the support of Development Research Unit in Tabriz University of Medical Sciences.
Nakhlband A, Fakhari A, Azizi H. Interferon‐alpha position in combating with COVID‐19: A systematic review. J Med Virol. 2021;93:5277‐5284. 10.1002/jmv.27072
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Azizi H, Esmaeili ED. Challenges and potential solutions in the development of COVID‐19 pandemic control measures. New Microbes New Infect. 2021;40:100852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fukuda Y, Homma T, Inoue H, et al. Downregulation of type III interferons in patients with severe COVID‐19. J Med Virol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ströher U, DiCaro A, Li Y, et al. Severe acute respiratory syndrome‐related coronavirus is inhibited by interferon‐alpha. J Infect Dis. 2004;189:1164‐1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zuo Y, Liu Y, Zhong Q, Zhang K, Xu Y, Wang Z. Lopinavir/ritonavir and interferon combination therapy may help shorten the duration of viral shedding in patients with COVID‐19: a retrospective study in two designated hospitals in Anhui, China. J Med Virol. 2020;92:2666‐2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakhlband A, Fakhari A, Azizi H. Interferon‐beta offers promising avenues to COVID‐19 treatment: a systematic review and meta‐analysis of clinical trial studies. Naunyn‐Schmiedeberg's Arch Pharmacol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dastan F, Nadji SA, Saffaei A, et al. Subcutaneous administration of Interferon beta‐1a for COVID‐19: A non‐controlled prospective trial. Int Immunopharmacol. 2020:106688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou Q, Wei X‐S, Xiang X, et al. Interferon‐a2b treatment for COVID‐19. MedRxiv. 2020. [Google Scholar]
- 8. Zuo Y, Liu Y, Zhong Q, Zhang K, Xu Y, Wang Z. Lopinavir/ritonavir and interferon combination therapy may help shorten the duration of viral shedding in patients with COVID‐19: a retrospective study in two designated hospitals in Anhui, China. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang B, Li D, Liu T, Wang H, Luo F, Liu Y. Subcutaneous injection of IFN alpha‐2b for COVID‐19: an observational study. BMC Infect Dis. 2020;20:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang Y‐Q, Tang S‐Q, Xu X‐L, et al. No statistically apparent difference in antiviral effectiveness observed among ribavirin plus interferon‐alpha, lopinavir/ritonavir plus interferon‐alpha, and ribavirin plus lopinavir/ritonavir plus interferon‐alpha in patients with mild to moderate coronavirus disease 2019: Results of a randomized, open‐labeled prospective study. Front Pharmacol. 2020;11:1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Payandemehr P, Azhdarzadeh M, Bahrami‐Motlagh H, et al. In terferon beta‐1a as a candidate for COVID‐19 treatment; an open‐label single‐arm clinical trial. Adv J Emergency Med. 2020;4:e51. [Google Scholar]
- 12. Wang B, Li D, Liu T, Wang H, Luo F, Liu Y. Subcutaneous injection of IFN alpha‐2b for COVID‐19: an observational study. Book Subcutaneous Injection of IFN alpha‐2b for COVID‐19: An Observational Study. Research Square; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu P, Huang J, Fan Z, et al. Arbidol/IFN‐α2b therapy for patients with corona virus disease 2019: a retrospective multicenter cohort study. Microb Infect. 2020;22:200‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pourseif MM, Moghaddam G, Naghili B, et al. A novel in silico minigene vaccine based on CD4(+) T‐helper and B‐cell epitopes of EG95 isolates for vaccination against cystic echinococcosis. Comput Biol Chem. 2018;72:150‐163. [DOI] [PubMed] [Google Scholar]
- 15. Lokugamage KG, Hage A, Vries M, et al. SARS‐CoV‐2 is sensitive to type I interferon pretreatment. bioRxiv. 2020. [Google Scholar]
- 16. Rezaiemanesh A, Majidi J, Baradaran B, et al. Impacts of anti‐EGFR monoclonal antibody in prostate cancer PC3 cells. Hum Antibodies. 2010;19:63‐70. [DOI] [PubMed] [Google Scholar]
- 17. Nakhlband A, Barar J. Impacts of nanomedicines in ocular pharmacotherapy. BioImpacts. 2011;1:7‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang J, Xie B, Hashimoto K. Current status of potential therapeutic candidates for the COVID‐19 crisis. Brain Behav Immun. 2020;87:59‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al Ghamdi M, Alghamdi KM, Ghandoora Y, et al. Treatment outcomes for patients with Middle Eastern respiratory syndrome coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infect Dis. 2016;16:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shalhoub S, Farahat F, Al‐Jiffri A, et al. IFN‐α2a or IFN‐β1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129‐2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lokugamage KG, Schindewolf C, Menachery VD. SARS‐CoV‐2 sensitive to type I interferon pretreatment. bioRxiv. 2020. [Google Scholar]
- 22. Rameshrad M, Maleki‐Dizaji N, Vaez H, Soraya H, Nakhlband A, Garjani A. Lipopolysaccharide induced activation of toll like receptor 4 in isolated rat heart suggests a local immune response in myocardium. Iranian J Immunol. 2015;12:104‐116. [PubMed] [Google Scholar]
- 23. Qiu T, Liang S, Dabbous M, Wang Y, Han R, Toumi M. Chinese guidelines related to novel coronavirus pneumonia. J Mark Access Health Policy. 2020;8:1818446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life‐threatening COVID‐19. Science. 2020:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee JS, Shin E‐C. The type I interferon response in COVID‐19: implications for treatment. Nat Rev Immunol. 2020;20:585‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Azizi H, Esmaeili ED, Fakhari A. Challenges and accurate estimates of mortality and case‐fatality rates due to COVID‐19. New Microbes New Infect. 2020:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


