Abstract
Introduction/Aims
Coronavirus disease 2019 (COVID‐19), a disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, has become a global pandemic. Patients with myasthenia gravis (MG), often treated with immunosuppressants, might be at higher risk of developing COVID‐19 and of demonstrating a severe disease course. We aimed to study prevalence and describe features of COVID‐19 in MG patients.
Methods
In May 2020, we conducted telephonic interviews with MG patients followed at our referral center. We collected structured data regarding MG and COVID‐19, which was diagnosed as probable or confirmed according to the European Centre for Disease Prevention and Control case definition. We compared confirmed‐COVID‐19 prevalence calculated from the beginning of the pandemic in MG patients with that of the overall Pavia district.
Results
We interviewed 162 MG patients (median age, 66 y; interquartile range 41‐77; males 59.9%), 88 from the Pavia district. Three patients had SARS‐CoV‐2‐confirmed by polymerase chain reaction and eight had probable‐COVID‐19. In the Pavia district, the prevalence of confirmed‐COVID‐19 among MG patients (1/88, 1.14%) and overall population (4777/546 515, 0.87%) did not differ (P = .538). Higher Myasthenia Gravis Foundation of America clinicalclass and the need for recent rescue treatment, but not ongoing immunosuppressive treatments, were associated with COVID‐19 risk. Of 11 MG patients with probable/confirmed‐COVID‐19, 3 required ventilator support, and 2 elderly patients died of COVID‐19 respiratory insufficiency. Only 1 of11 patients experienced worsening MG symptoms, which improved after increasing their steroid dose.
Discussion
The risk of COVID‐19 in MG patients seems to be no higher than that of the general population, regardless of immunosuppressive therapies. In our cohort, COVID‐19 barely affected MG course.
Keywords: comorbidities, corticosteroids, COVID‐19, epidemiology, immunosuppressive treatments, myasthenia gravis
Abbreviations
- MG
myasthenia gravis
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- NMJ
neuromuscular junction
- MGFA
Myasthenia Gravis Foundation of America Clinical Classification
1. INTRODUCTION
In the past year coronavirus disease 2019 (COVID‐19), a disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, has rapidly spread to become a worldwide pandemic. 1 Therapeutic management is particularly challenging due to the lack of specific antiviral treatment and is mainly based on supportive care. Italy and in particular the Lombardy region, was one of the most affected areas in Europe during the first wave of COVID‐19 in early 2020. It is uncertain whether patients with myasthenia gravis (MG), an autoimmune disorder affecting the neuromuscular junction (NMJ), might be at higher risk of developing COVID‐19, for example, due to the immunosuppressive treatment often received. 2 Moreover, these patients might face a particularly severe COVID‐19 course since infections can trigger MG exacerbations, and some treatments administered in COVID‐19, such as hydroxychloroquine, might further worsen MG manifestations.3, 4, 5, 6 Management of MG patients during the SARS‐CoV‐2 pandemic has been guided by expert consensus, but data on COVID‐19‐MG patients are still lacking. 7 We aimed to study COVID‐19 infection risk and disease course in MG patients followed at our Institution, located in an area severely affected by the pandemic (the Pavia district in Lombardy), during the first COVID‐19 wave in Italy.
2. METHODS
We screened our electronic records for patients with a diagnosis of suspected MG with at least one outpatient visit at our Institution in the past 3 years, identifying 264 patients (Figure 1). A total of 102 patients were excluded from the study (Figure 1). MG diagnosis was confirmed by the presence of acetylcholine receptor or muscle specific tyrosine kinase autoantibodies and/or compatible neurophysiological findings of decreased compound muscle action potential after repetitive nerve stimulation and/or increased jitter on single‐fiber electromyography. In double seronegative patients, MG diagnosis required the combination of neurophysiological abnormalities, clinical improvement after cholinesterase inhibitor administration, and the exclusion of other NMJ diseases. 10
FIGURE 1.
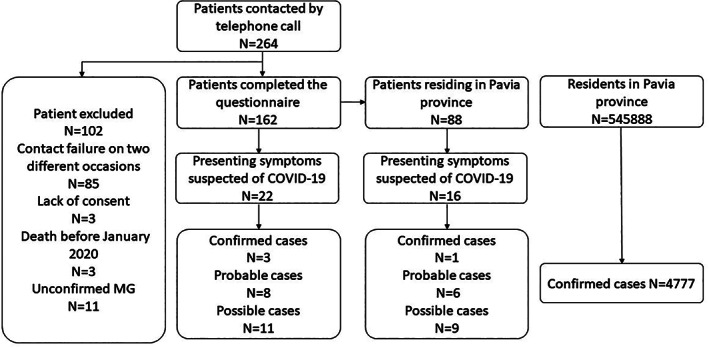
Algorithm of the study. Data regarding the Pavia province population were obtained from the national institute of statistics. 8 Data regarding the Pavia province COVID‐19 prevalence were obtained from the SARS‐CoV‐2 surveillance system 9
After receiving informed consent, we administered a telephonic interview investigating current MG condition and treatments. All the patients were contacted between April 26 and May 15, 2020, which corresponded to the final weeks of lockdown and subsequent downward trend of the SARS‐CoV‐2 infection curve. If appropriate, information on COVID‐19 occurrence was collected. If the patients were not able to respond to the interview themselves, information was collected from the caregiver. Additional information regarding the MG status at last visit was collected from our electronic records.
COVID‐19 diagnosis was assessed according to the European Centre for Disease Prevention and Control case definition as: (a) probable: at least one suggestive clinical symptom (fever, cough, shortness of breath, anosmia/ageusia/dysgeusia) with an epidemiological link and/or radiological evidence of interstitial pneumonitis; (b) confirmed: any patient with SARS‐CoV‐2 polymerase chain reaction (PCR) ‐positive nasopharyngeal swab. 11
Numerical variables are described as median and quartiles, categorical variables as percentage or row count. Categorical variables were analyzed using Fisher's exact test or Pearson's chi‐squared test, while numerical data using Mann‐Whitney test. COVID‐19 prevalence in the overall population were obtained from the SARS‐CoV‐2‐integrated‐surveillance system of the “Istituto Superiore di Sanità” (Figure 1).9, 12 COVID‐19 prevalence in MG patients was calculated from February 1 and was reported with 95% confidence intervals (CIs). 8 Our hospital is the main referral center for MG in the Pavia district; thus, we considered our cohort as representative of the MG population in this area. Prevalence estimated in MG patients was compared with that of the overall population using the exact binomial test. P values <.05 were considered significant. The study was approved by the local ethics committee.
3. RESULTS
Overall, 162 patients completed the telephonic interview. Eleven had symptoms suggestive of COVID‐19, and 6 underwent nasopharyngeal swab testing with SARS‐CoV‐2 real‐time PCR. Three patients were positive and diagnosed with “confirmed‐COVID‐19.” The remaining eight patients were classified as “probable‐COVID‐19” based on compatible symptoms and close contact with an infected subject in the weeks preceding symptoms onset. In the Pavia district, the estimate prevalence of “confirmed‐COVID‐19” was 1.14% (95% CI: 0.02%‐6.17%) in our MG patients, and 0.87% (95% CI: 0.85%‐0.90%) in the overall population, according to surveillance systems of the Italian Ministry of Health (Figure 1)8, 12 without difference in disease prevalence between the two cohorts (P = .538).
The clinical features of the 162 MG patients are summarized in Table 1. Median age was 66 y with a slight predominance of males. More than half of the patients were receiving immunosuppressive treatments at the time of the interview, most commonly oral steroids and azathioprine. Seven patients received other immunosuppressants including cyclosporine (n = 3), mycophenolate mofetil (n = 2), and rituximab (n = 2). The treatment regimen received did not correlate with the development of COVID‐19.
TABLE 1.
Clinical features in MG patients with and without COVID‐19
| MG patients, n (%) | All patients 162 (100) | No COVID‐19 151 (100) | Probable/confirmed COVID‐19 11 (100) | P Value |
|---|---|---|---|---|
| Age at the time of the interview (y), median (IQR) | 66 (54‐77) | 66 (53‐77) | 65 (55‐86) | 1.0 a |
| Age at MG onset (y), median (IQR) | 57 (43‐69) | 57 (43‐69) | 53 (50‐73) | 1.0 a |
| Males, n (%) | 84 (51.9) | 78 (51.7) | 5 (45.5) | 1.0 |
| Antibody status | ||||
| AChR | 102 (63.0) | 94 (62.3) | 8 (72.7) | .70 |
| MuSK | 6 (3.7) | 6 (4.0) | 0 (0.0) | |
| Seronegative | 54 (33.3) | 51 (33.8) | 3 (27.3) | |
| MG duration (y), median (IQR) | 5 (2‐10) | 5 (2‐10) | 6 (2‐12) | .73 a |
| Leaving the house once/week | 96 (59.3) | 88 (58.3) | 8 (72.7) | .53 |
| Comorbidities, n (%) | ||||
| Cerebrovascular | 13 (8.0) | 12 (7.9) | 1 (9.1) | 1.0 |
| Hematologic disease | 10 (6.2) | 9 (6.0) | 1 (9.1) | .51 |
| Autoimmune | 23 (14.2) | 18 (11.9) | 5 (45.5) | .01 |
| Cardiovascular | 66 (40.7) | 58 (38.4) | 8 (72.7) | .05 |
| Malignancies | 35 (21.6) | 29 (19.2) | 6 (54.5) | .01 |
| Diabetes | 37 (22.8) | 33 (21.9) | 4 (36.4) | .28 |
| Smoking, n (%) | ||||
| Active | 17 (10.5) | 17 (11.3) | 0 (0.0) | .26 |
| Previous | 45 (27.8) | 40 (26.5) | 5 (45.5) | |
| Thymectomy, n (%) | 32 (19.8) | 29 (19.2) | 3 (27.3) | .75 |
| MG symptoms, n (%) | ||||
| Ocular | 53 (32.7) | 52 (34.4) | 1 (9.1) | |
| Generalized | 109 (67.3) | 99 (65.6) | 10 (90.9) | .08 |
| Bulbar involvement | 91 (56.2) | 82 (54.3) | 9 (81.8) | .08 |
| Active treatments, n (%) | ||||
| Prednisone <10 mg/d | 31 (19.1) | 28 (18.5) | 3 (27.3) | .48 |
| Prednisone >10 mg/d | 39 (24.1) | 37 (24.5) | 2 (18.2) | .64 |
| AZA | 47 (29.0) | 42 (27.8) | 5 (45.5) | .21 |
| Other immunosuppressants | 7 (4.3) | 7 (4.6) | 0 (0) | .47 |
| No immunosuppressants | 63 (38.9) | 59 (39.1) | 4 (36.4) | .86 |
| Treatment with IvIg/PlEx in the previous year, n (%) | 26 (16.0) | 21 (13.9) | 5 (45.5) | .02 |
| MGFA >/= 3 at last visit | 22 (13.69 | 17 (11.3) | 5 (45.5) | .01 |
| MG‐ADL at last visit, median (IQR) | 1 (0‐4) | 1 (0‐4) | 4.0 (0‐10) | .43 a |
Abbreviations: AChR, acetylcholine receptor; AZA, azathioprine; IQR, interquartile range; IvIg, intravenous immunoglobulin; MG, myasthenia gravis; MG‐ADL, myasthenia gravis activity of daily living scale; MGFA, Myasthenia Gravis Foundation of America clinical classification; MuSK, muscle specific tyrosine kinase; PlEx, plasma exchange.
Mann‐Whitney test.
COVID‐19 was more frequent in MG patients with higher Myasthenia Gravis Foundation of America clinical class (MGFA), with need for rescue treatment for acute exacerbations in the previous year and with autoimmune or neoplastic comorbidities (Table 1).
Data regarding the 11 MG patients with COVID‐19 are reported in Table 2. Only 3 of 11 patients were hospitalized and required ventilatory support. Two elderly patients (93 and 86 y old) died of COVID‐19 related respiratory insufficiency, but given the rapid course of the disease, it was impossible to ascertain an MG worsening contribution to the poor outcome. In one of them oral prednisone dosage was increased, without modifying the disease course. Among the remaining patients, only one patient reported a worsening of MG symptoms during the COVID‐19 (development of double vision and swallow impairment) that promptly improved after increasing the prednisone dosage. Treatments for COVID‐19 included antibiotics (eight patients), antiviral (one patient treated with lopinavir/ritonavir), and hydroxychloroquine (one patient).
TABLE 2.
Characteristics of MG patients with COVID‐19
| Probable COVID‐19 | Confirmed COVID‐19 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient N | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| Sex | M | F | M | F | F | F | F | M | M | M | F |
| Age | 66 | 86 | 55 | 65 | 65 | 78 | 42 | 93 | 54 | 61 | 86 |
| MGFA at last visit | I | IIa | IIIa | IIIb | IIIb | IIa | IIIb | I | IIb | I | IIIb |
| MG duration (y) | 1 | 35 | 2 | 12 | 1 | 5 | 9 | 10 | 3 | 19 | 7 |
| MG signs and symptoms at last visit | Ptosis | Mild proximal weakness in UL and LL | Moderate proximal weakness in UL and LL; mild distal weakness in UL | Ptosis; diplopia; tongue weakness; neck flexors/extensors weakness | Bilateral ptosis, dysphagia; neck flexors weakness; mild proximal weakness in LL | Mild neck flexors weakness | Diplopia; facial muscles weakness; tongue weakness; moderate proximal weakness in UL and LL. | None | Ptosis; mild facial muscles weakness | None | Dysphagia, facial weakness; neck flexors weakness; weakness in UL and LL |
| Thymectomy | No | No | No | No | No | No | Yes, thymoma | No | Yes, thymic hypertrophy | Yes, thymic hypertrophy | No |
| MG antibodies | AChR | AChR | Seronegative | Seronegative | AChR | AChR | AChR | AChR | AChR | AChR | AChR |
| Nasopahryngeal swab | Not performed | Neg | Neg | Not performed | Not performed | Not performed | Not performed | Neg | Pos | Pos | Pos |
| COVID‐19 symptoms | Fever | Fever, cough | Fever, dyspnoea, myalgias | Fever, dyspnoea, cough, anosmia/ageusia, myalgias | Cough, myalgias | Fever, dyspnoea, cough, anosmia/ageusia, myalgias | Fever | Fever, cough | Dyspnoea, cough, myalgias | Fever, cough, anosmia/ageusia | Fever, dyspnoea, cough, myalgias, chest x‐ray: Interstitial pneumonia |
| Hospital admission/O2 therapy | No | No | No | No | No | No | No | Yes, unspecified O2 therapy | Yes high flow O2 therapy | No | Yes, high flow O2 therapy |
| COVID‐19 treatment | None | Piperacillin/Tazobactam | None | None | Amoxicillin/clavulanic acid | Ceftriaxone | Azithromycine, Trimetoprim/Sulfametoxazole | Ceftriaxone | Ceftriaxone, hydroxicloroquine, heparin, lopinavir/ritonavir | Azithromycine, ceftriaxone | Unspecified antibiotics |
| MG baseline treatment | Pr (12,5 mg every other day), PYR | Pr (25 mg), AZA (100 mg), PYR | PYR | Pr (25 mg), AZA (100 mg), PYR | PYR | PYR, AZA (150 mg) | PYR | PYR | Pr (5 mg), PYR | AZA (100 mg), PYR | Pr (10/5 mg every other day), AZA (50 mg), PYR |
| MG treatment modifications during COVID‐19 | None | None | None | None | None | None | Increased Pr | None | None | None | Increased Pr |
| Comorbidities | None | Autoimmune thyroiditis, metastatic colon cancer, atrial fibrillation | Coronary artery disease, type 2 diabetes | Neuromyelitis optica spectrum disorder, autoimmune thyroiditis | Breast cancer, hypertension | Abdominal aortic aneurism, dilated cardiomyopathy, type 2 diabetes | Polyglandular autoimmune syndrome, previous lung and, uterine cancer, psoriasis, diabetes | Immune trombocytopenia, atrial fibrillation, epilepsy | Hyperparathyroidism | Type 2 diabetes, hypertension | Breast cancer, COPD, polymyalgia |
| MG ADL pre‐COVID‐19 | 0 | 4 | 7 | 6 | 8 | 7 | 8 | 0 | 3 | 0 | 9 |
| MG during COVID‐19 | Stable | Stable | Stable | Stable | Stable | Stable | Worsened (dysphagia and diplopia) | NA (death due to COVID‐19) | Stable | Stable | NA (death due to COVID‐19) |
| Outcome (MG ADL post‐COVID‐19) | 0 | 3 | 11 | 5 | 10 | 8 | 10 | NA (death due to COVID‐19) | 4 | 0 | NA (death due to COVID‐19) |
Abbreviations: AChR, acetylcholine receptor; AZA, azathioprine; F, female; LL, lower limbs; M, male; MG, myasthenia gravis; MG‐ADL, myasthenia gravis activity of daily living scale; MGFA, Myasthenia Gravis Foundation of America clinical classification; Pr, prednisone; PYR, pyridostigmine; UL, upper limbs.
4. DISCUSSION
Currently, no epidemiological studies on COVID‐19 in MG patients are available, and data regarding SARS‐CoV‐2 infection risk in this population are scarce. Studies on patients with other autoimmune diseases suggest that immunosuppressive therapy does not represent a risk factor for SARS‐CoV‐2 infection and that some drugs might even be protective.13, 14 Indeed steroids, the most common medication for MG patients in our cohort, are effective in reducing the mortality in COVID‐19. 15 In our study, we found that COVID‐19 prevalence did not differ between MG patients and the general population in the Pavia district. Interestingly, among the whole cohort of MG patients, the type of treatment received did not influence the occurrence of COVID‐19, supporting the safety of MG treatments in the current pandemic.
We found that MG patients with oncologic/autoimmune comorbidity were more likely to develop COVID‐19, suggesting that SARS‐CoV‐2 infection might be more likely to manifest as a symptomatic disease in this fragile subgroup of patients. Patients with higher MGFA class/severe MG were more susceptible to COVID‐19. However, the severity of MG did not correlate with the severity of COVID‐19 infection. This might point to a higher susceptibility to COVID‐19 in patients with an active autoimmune response, as it has been suggested for other conditions, such as systemic autoimmune diseases. 13 However, it should also be considered that this particular subset of fragile patients might have been monitored and tested more carefully than the other MG patients.
In most patients, MG remains stable throughout the SARS‐CoV‐2 infection, but some of them experience worsening of MG symptoms.2, 5 In such cases, additional treatment, such as intravenous immunoglobulins or steroids, might be effective and should be considered early on to prevent severe complications. 7 In our study, the treatments received were not clearly related to the final outcome, that was overall favorable. Only two elderly patients died, possibly reflecting the high mortality rate found in COVID‐19 in this age range. 16
Our study has limitations. First of all, it is possible that some MG patients residing in the Pavia district could not be followed at our Institution. Second, the MG population we examined does not include children, who are more likely to develop an asymptomatic SARS‐CoV‐2 infection. 1 Third, no independent control for the province prevalence data was performed. Fourth, it is possible that MG patients, considering themselves as a fragile population, might have exhibited more cautious behavior and had less contact with others compared to the general population, or were more likely to undergo COVID‐19 testing. All these factors might have contributed to an imprecise prevalence estimate. Finally, the low number of MG patients with COVID‐19 does not permit us to define the full spectrum of COVID‐19 manifestations or the effect of specific medications in this population. In severely ill patients, such as those who died of respiratory failure, it was impossible to discriminate between the effects of the infection and the MG worsening.
In conclusion, in accordance with consensus guidelines, our data suggest that patients with MG should continue their immunosuppressive therapy in the current pandemic, even in cases of clinically overt COVID‐19. In most patients in our cohort, the infection did not dramatically influence the MG course, but larger studies are needed to clarify the course of COVID‐19 in MG patients.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
We thank the following Colleagues for providing patients' information: Alfredo Romani, Giovanni Piccolo, Maria Lombardi, Mauro Ceroni. This study was sponsored by the Italian Ministry of Health “Ricerca Corrente” to the IRCCS Mondino Foundation (grant code: RC20011C).
Businaro P, Vaghi G, Marchioni E, et al. COVID‐19 in patients with myasthenia gravis: Epidemiology and disease course. Muscle & Nerve. 2021;64:206–211. 10.1002/mus.27324
Pietro Businaro, Gloria Vaghi, Diego Franciotta, Matteo Gastaldi contributed equally.
Funding information Italian Ministry of Health “Ricerca Corrente” to the IRCCS Mondino Foundation, Grant/Award Number: RC20011C
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Guan W‐J, Ni Z‐Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hübers A, Lascano AM, Lalive PH. Management of patients with generalised myasthenia gravis and COVID‐19: four case reports. J Neurol Neurosurg Psychiatry. 2020;91(10):1124‐1125. 10.1136/jnnp-2020-323565. [DOI] [PubMed] [Google Scholar]
- 3. Gilhus NE, Romi F, Hong Y, Skeie GO. Myasthenia gravis and infectious disease. J Neurol. 2018;265:1251‐1258. 10.1007/s00415-018-8751-9. [DOI] [PubMed] [Google Scholar]
- 4. Anand P, Slama MCC, Kaku M, et al. COVID‐19 in patients with myasthenia gravis. Muscle Nerve. 2020;62(2):254‐258. 10.1002/mus.26918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rein N, Haham N, Orenbuch‐Harroch E, et al. Description of 3 patients with myasthenia gravis and COVID‐19. J Neurol Sci. 2020;417:117053. 10.1016/j.jns.2020.117053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koc G, Odabasi Z, Tan E. Myasthenic syndrome caused by hydroxychloroquine used for COVID‐19 prophylaxis. J Clin Neuromuscul Dis. 2020;22(1):60‐62. 10.1097/CND.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 7. Jacob S, Muppidi S, Guidon A, et al. Guidance for the management of myasthenia gravis (MG) and Lambert‐Eaton myasthenic syndrome (LEMS) during the COVID‐19 pandemic. J Neurol Sci. 2020;412:116803. 10.1016/j.jns.2020.116803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. http://dati.istat.it/Index1. Accessed May 15, 2020.
- 9. https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-integrated-surveillance.
- 10. Mantegazza R, Cavalcante P. Diagnosis and treatment of myasthenia gravis. Curr Opin Rheumatol. 2019;31(6):623‐633. 10.1097/BOR.0000000000000647. Accessed March 5, 2021. [DOI] [PubMed] [Google Scholar]
- 11. https://www.ecdc.europa.eu/en/covid‐19/surveillance/case‐definition. Accessed June 2, 2020.
- 12. http://www.salute.gov.it/. Accessed May 15, 2020.
- 13. Emmi G, Bettiol A, Mattioli I, et al. SARS‐CoV‐2 infection among patients with systemic autoimmune diseases. Autoimmun Rev. 2020;19(7):102575. 10.1016/j.autrev.2020.102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sormani MP. An Italian programme for COVID‐19 infection in multiple sclerosis. Lancet Neurol. 2020;19(6):481‐482. 10.1016/S1474-4422(20)30147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID‐19. A meta‐analysis. JAMA. 2020;324(13):1330‐1341. 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poletti P, Tirani M, Cereda D, et al. Age‐specific SARS‐CoV‐2 infection fatality ratio and associated risk factors, Italy, February to April 2020. Euro Surveill. 2020;25(31):2001383. 10.2807/1560-7917.ES.2020.25.31.2001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


