Abstract
Though it is widely believed that chronic immunosuppressive medications increase the severity of coronavirus disease 2019 (COVID‐19) illness, there is little data to support this. We performed a retrospective study of COVID‐19 positive patients diagnosed at a single academic medical center between March 10, 2020 and October 13, 2020. A total of 835 patients diagnosed with COVID‐19 by polymerase chain reaction were included (median age 64 years; 52% female). Of these, 46 (5.5%) had a prescription for an immunosuppressive therapy before diagnosis, most commonly oral steroids (20, 43%), mycophenolate (12, 26%), or tacrolimus (11, 24%). Patients on immunosuppressive therapy with COVID‐19 had increased mortality (30% vs. 17%, p = 0.036; odds ratio 2.1, 95% confidence interval 1.11–4.04), which remained significant (p = 0.040) after performing multivariate logistic regression controlling for gender, age, race, and comorbidity status. Laboratory markers of inflammation were uniformly elevated in both patients on or not on immunosuppressive therapies who died, but lymphocytes and neutrophils were decreased in both COVID‐19 patients on immunosuppressive therapies who died and who remained alive. These findings demonstrate that COVID‐19 disease is more severe in patients taking prior immunosuppressive medications. This finding emphasizes the need for aggressive monitoring and supportive care for immunosuppressed patients who are diagnosed with COVID‐19.
Keywords: COVID‐19, immunosuppression, severity, steroid
Highlights
COVID‐19 patients on immunosuppression at the time of diagnosis have increased mortality.
Laboratory markers of inflammation are elevated in severe COVID‐19 in both patients on and non on prior immunosuppression.
Age is not associated with longer hospitalization in COVID‐19 patients on prior immunosuppression.
1. INTRODUCTION
Early clinical studies 1 , 2 demonstrated that severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection can lead to a severe respiratory illness, with ensuing intensive care unit admission and high mortality. More recent analyses have raised concern that aberrant inflammation is present in a subgroup of patients with severe coronavirus disease 2019 (COVID‐19) and is a major contributor to mortality. 3 , 4 , 5 , 6 , 7 Retrospective studies describing an inflammatory cytokine profile in patients with severe COVID‐19, 8 , 9 as well as reports of a rare but detrimental multisystem inflammatory syndrome in children with COVID‐19, 10 have implicated immune dysregulation in disease severity. Characterization of the immune system in patients with severe versus mild COVID‐19, as well as the potential mechanisms by which aberrant inflammation can lead to the severe respiratory illness associated with severe COVID‐19, is necessary.
Immunosuppression is considered a risk factor for SARS‐CoV‐2 infection, but the implications of prior immunosuppressive therapies after a patient has become infected remain unclear. 4 , 11 , 12 Early case studies of patients on disease‐modifying antirheumatic drugs (DMARDs) or immunosuppressive therapy with COVID‐19 revealed that the majority of patients only develop mild disease. 13 , 14 Patients with rheumatic disease on prior DMARD therapy who contracted COVID‐19 had varying disease severity that was associated with both known risk factors and rheumatic disease severity. 15 , 16 These early observations in both adults with severe COVID‐19 and children suggest that aberrant inflammatory responses might be responsible for the high mortality observed in patients with severe COVID‐19. It has therefore been proposed that prior immunosuppressive therapies might paradoxically improve outcomes in an inpatient setting, 12 although the relative role of immunosuppressive therapy in early versus late disease and its impact on COVID‐19 outcomes remain unknown.
Clinical management of immunosuppressed patients with COVID‐19, including HIV patients, 17 solid organ transplant recipients, 18 patients undergoing irradiation or taking chemotherapy, 19 and patients with primary immunodeficiencies, 20 , 21 is currently undergoing continual reevaluation. Small observational studies 13 , 22 , 23 , 24 , 25 and recent randomized control trials 26 , 27 have provided conflicting evidence of the impact of immunomodulatory drugs, such as corticosteroids, DMARDs, and biologics like tocilizumab, on COVID‐19 outcomes. Here, we seek to clarify the role of pre‐existing immunosuppressive therapies in COVID‐19 outcomes after SARS‐CoV‐2 infection. While immunosuppression might be beneficial in later stages of the disease, we hypothesize that patients on immunosuppressive therapy before contracting SARS‐CoV‐2 develop more severe COVD‐19 due to the beneficial role of the antiviral immune response during early disease. Through a retrospective case series of 835 patients with COVID‐19, we found that patients with prior immunosuppressive therapy have worse outcomes.
2. METHODS
2.1. Study design
This study was conducted at the Beth Israel Deaconess Medical Center (BIDMC) in Boston. The BIDMC Institutional Review Board approved this retrospective cohort study (2020P000699) as minimal risk using data collected during routine clinical care and waived the requirement for informed consent. Protected health information was handled in accordance with HIPAA and BIDMC Committee on Clinical Investigation (CCI) regulations. BIDMC patients with confirmed SARS‐CoV‐2 infection by the positive result of nasopharyngeal sample polymerase chain reaction between March 10, 2020 and October 13, 2020 were included.
Data were obtained from the BIDMC COVID‐19 Observational Research Effort (CORE) Data Registry REDCap database and BIDMC InSIGHT CORE service. Length of admission was determined by the number of days between admit and discharge dates per individual patient. Length of admission values were excluded for patients still admitted at the time of data acquisition. The number of encounters was determined by the number of unique inpatient or outpatient encounters after the earliest COVID‐19 test. Encounter type was noted for each patient; for patients with multiple encounters of different types, inpatient encounters were given priority over outpatient encounters. Signs and symptoms at presentation and during the course of hospitalization were coded into categorical variables. Mean, minimum, and maximum of all recorded vital and laboratory values were calculated for each individual patient. Ventilation status, supplemental O2 requirement, length of ICU admission, and discharge disposition were obtained from inpatient records.
Patient demographics, including age, gender, race, ethnicity, language, zip code, blood type, and substance use were obtained from outpatient records. Medication lists were curated for each patient by filtering for medications with prescription dates relative to the earliest COVID‐19 test date. Medications at presentation were determined by an active prescription at the time of COVID‐19 diagnosis, and newly prescribed medications were determined by a prescription date after COVID‐19 diagnosis. Medication lists were annotated using the US FDA National Drug Code Directory (downloaded on June 18, 2020) and searching each patient's medication list for the presence of the FDA‐recognized nonproprietary drug name for selected medications. Immunosuppressive drugs were defined to include oral steroids, leflunomide, azathioprine, cyclophosphamide, cyclosporine, methotrexate, tacrolimus, and mycophenolate. Comorbidities were annotated by searching each patient's past medical history by ICD‐10‐CM code for recognized risk factors for COVID‐19, 2 , 28 including asthma (J45), chronic obstructive pulmonary disease (J44), chronic kidney disease (N18), cancer (C00‐C96), hypertension (I10‐I16), diabetes (E08‐E13), coronary artery disease (I25), and obesity (E66).
COVID‐19 severity was graded by the NIH Ordinal Severity Scale. Patients were stratified into eight groups with lower scores corresponding to greater severity: (1) death, (2) invasive mechanical ventilation, (3) noninvasive ventilation, (4) supplemental oxygen, (5) no supplemental oxygen but requiring medical care, (6) no supplemental oxygen and not requiring medical care, (7) limitation in activities, or (8) no limitation in activities.
2.2. Statistical analysis
Categorical variables are presented as frequency rates and percentages, and continuous variables are presented as median and interquartile range. Nonparametric tests of association were used throughout this study unless noted otherwise. Comparisons between binary categorical variables were calculated as odds ratios and p values were calculated by χ 2 test. Comparisons between numerical variables and binary categorical variables were performed by Mann–Whitney U test. Comparisons between continuous variables, including ordered discrete variables, were quantified by the Spearman rank correlation coefficient (rho). Hazard ratios (HRs) were calculated using a Cox proportional hazards regression model with time‐dependent incorporation by the formulation of Andersen and Gill, and p values were calculated using the Wald test statistic. To account for confounding variables, sensitivity analyses were performed using a binomial generalized linear model with iteratively reweighted least squares for multivariate regression.
All analyses were performed in R (version 3.6.1). Bar graphs and violin plots were created using ggpubr (version 0.4.0), Kaplan–Meier plots were created using survminer (version 0.4.8) and survival (version 3.2‐7), and scatter plots and forest plots were created using ggplot2 (version 3.3.0).
3. RESULTS
A total of 835 patients with PCR confirmed SARS‐CoV‐2 infection were included (Table 1). The median age was 64 years (interquartile range [IQR], 50–76 years) and 438 (52%) were female. Of these patients, 363 (43%) were white and 253 (30%) were black. Medical history was available for 549 patients and among these patients, common comorbidities included hypertension (347; 63%), diabetes (224; 41%), obesity (157; 30%), chronic kidney disease (144; 26%), and cancer (131; 24%). Most patients had an elevated temperature (median Tmax 100; IQR 99–100) and were tachypneic (median 19; IQR 18–21) but had normal heart rates (median 85; IQR 76–94). As of December 1, 2020, 656 (79%) patients required hospitalization, 336 (40%) required supplemental oxygen, and 133 (16%) required intensive care unit (ICU) stays. NIH Ordinal Scoring was available for 322 patients, and the mean ordinal score was 3.7 (SD 1.7). Overall, 149 (18%) patients died at the time of censoring.
Table 1.
Demographics, comorbidities, and outcomes of patients with COVID‐19 on or not on prior immunosuppressive therapy
| Overall | No immunosuppressive therapy | Immunosuppressive therapy | ||
|---|---|---|---|---|
| (N = 835) | (N = 789) | (N = 46) | p value | |
| Gender | 0.306 | |||
| Female | 438 (52%) | 410 (52%) | 28 (61%) | |
| Male | 397 (48%) | 379 (48%) | 18 (39%) | |
| Age | 64 (50–76) | 64 (49–76) | 65 (58–71) | 0.615 |
| Race | 0.133 | |||
| Native American | 1 (0%) | 1 (0%) | 0 (0%) | |
| Asian | 31 (4%) | 30 (4%) | 1 (2%) | |
| Black | 253 (30%) | 239 (30%) | 14 (30%) | |
| Declined | 1 (0%) | 1 (0%) | 0 (0%) | |
| Native Hawaiian | 2 (0%) | 1 (0%) | 1 (2%) | |
| Other | 90 (11%) | 86 (11%) | 4 (9%) | |
| Unknown | 94 (11%) | 92 (12%) | 2 (4%) | |
| White | 363 (43%) | 339 (43%) | 24 (52%) | |
| ABO type | 0.645 | |||
| A | 71 (9%) | 61 (8%) | 10 (22%) | |
| AB | 11 (1%) | 9 (1%) | 2 (4%) | |
| B | 40 (5%) | 37 (5%) | 3 (7%) | |
| O | 104 (12%) | 93 (12%) | 11 (24%) | |
| Missing | 609 (72.9%) | 589 (74.7%) | 20 (43.5%) | |
| BMI | 29 (25–34) | 29 (25–34) | 28 (23–33) | 0.103 |
| Comorbidities available | 549 (66%) | 511 (65%) | 38 (83%) | |
| Hypertension | 347 (63%) | 316 (62%) | 31 (82%) | 0.0238 |
| Chronic kidney disease | 144 (26%) | 127 (25%) | 17 (45%) | 0.0125 |
| Diabetes | 224 (41%) | 204 (40%) | 20 (53%) | 0.172 |
| Obesity | 167 (30%) | 151 (30%) | 16 (42%) | 0.15 |
| Rheumatologic disease | 127 (23%) | 113 (22%) | 14 (37%) | 0.0604 |
| Autoimmune disease | 49 (9%) | 38 (7%) | 11 (29%) | <0.001 |
| Cancer | 131 (24%) | 121 (24%) | 10 (26%) | 0.864 |
| COPD | 72 (13%) | 64 (13%) | 8 (21%) | 0.21 |
| Asthma | 81 (15%) | 74 (14%) | 7 (18%) | 0.672 |
| Coronary artery disease | 130 (24%) | 114 (22%) | 16 (42%) | 0.0101 |
| Cerebrovascular disease | 67 (12%) | 61 (12%) | 6 (16%) | 0.658 |
| Immunosuppressive therapy | ||||
| Oral steroid | 20 (2%) | 0 (0%) | 20 (43%) | |
| Leflunomide | 3 (0%) | 0 (0%) | 3 (7%) | |
| Azathioprine | 4 (0%) | 0 (0%) | 4 (9%) | |
| Cyclophosphamide | 1 (0%) | 0 (0%) | 1 (2%) | |
| Cyclosporine | 5 (1%) | 0 (0%) | 5 (11%) | |
| Methotrexate | 5 (1%) | 0 (0%) | 5 (11%) | |
| Tracrolimus | 11 (1%) | 0 (0%) | 11 (24%) | |
| Mycophenolate | 12 (1%) | 0 (0%) | 12 (26%) | |
| Vitals | ||||
| Respiratory rate | 19 (18–21) | 19 (18–21) | 20 (18–22) | 0.529 |
| Heart rate | 85 (76–94) | 85 (76–94) | 86 (71–96) | 0.765 |
| Tmax | 100 (99–100) | 100 (99–100) | 100 (100–100) | 0.156 |
| SBP (minimum) | 99 (91–110) | 100 (91–110) | 92 (84–100) | 0.0144 |
| DBP (minimum) | 57 (49–65) | 57 (50–65) | 50 (44–62) | 0.00688 |
| Status | 0.615 | |||
| Inpatient | 656 (79%) | 618 (78%) | 38 (83%) | |
| Outpatient | 179 (21%) | 171 (22%) | 8 (17%) | |
| ICU admission | 133 (16%) | 114 (14%) | 19 (41%) | <0.001 |
| ICU days | 8.0 (3.0–17) | 8.0 (3.0–17) | 14 (8.5–23) | 0.00349 |
| Outcomes | ||||
| Supplemental O2 | 336 (40%) | 312 (40%) | 24 (52%) | 0.123 |
| Mechanical ventilation | 196 (23%) | 179 (23%) | 17 (37%) | 0.0413 |
| Total encounters | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.881 |
| Length admission | 9.0 (5.0–18) | 9.0 (5.0–17) | 16 (10–30) | <0.001 |
| Ordinal score | 4.0 (2.0–5.0) | 4.0 (2.0–5.0) | 2.5 (1.0–4.0) | 0.0206 |
| Death | 149 (18%) | 135 (17%) | 14 (30%) | 0.0361 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease 2019; DBP, diastolic blood pressure; ICU, intensive care unit; SBP, systolic blood pressure.
Of the 835 patients with COVID‐19, 46 (5.5%) had prescriptions for immunosuppressive therapies at the time of COVID‐19 diagnosis. A spectrum of immunosuppressive medications was represented: 20 (43%) were prescribed oral steroids, 12 (26%) were prescribed mycophenolate, 11 (24%) were prescribed tacrolimus, 5 (11%) were prescribed methotrexate, 5 (11%) were prescribed cyclosporine, 4 (9%) were prescribed azathioprine, 3 (7%) were prescribed leflunomide, and 1 (2%) was prescribed cyclophosphamide. Patients on immunosuppressive therapies were more likely to have hypertension (82% vs. 65%, p = 0.024), chronic kidney disease (45% vs. 25%, p = 0.013), autoimmune disease (29% vs. 7%, p < 0.001), and coronary artery disease (42% vs. 22%, p = 0.01).
Patients with COVID‐19 having prior immunosuppressive therapy had significantly greater mortality (30% vs. 17%, p = 0.036), had longer lengths of hospitalization (median 16 days vs. 9 days, p < 0.001), had longer ICU stays (median 14 vs. 8 days, p = 0.0035), were more likely to require mechanical ventilation (37% vs. 23%, p = 0.041), and had lower ordinal scores (median 2.5 vs. 4, p = 0.021) (Table 1 and Figure 1A). Fraction of patients requiring supplemental oxygen, number of encounters, and hospitalization rate were not significantly altered in immunosuppressed patients. Maximum temperature, average respiratory rate, and average heart rates were not different in immunosuppressed patients, although minimum systolic blood pressure and diastolic blood pressure were decreased in immunosuppressed patients (Table 1). Kaplan–Meier analysis of patient survival after COVID‐19 diagnosis revealed that pre‐existing immunosuppressive therapy increased the risk of death (HR 1.8, 95% confidence interval 1.04–3.13; p = 0.033).
Figure 1.
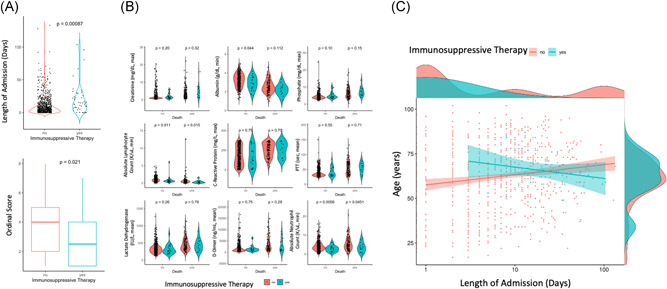
Prior immunosuppressive therapy is associated with coronavirus disease 2019 (COVID‐19) severity. (A) Violin (top) and box plot (bottom) of lengths of admission or ordinal scores of patients with COVID‐19 on (blue) or not on (red) immunosuppressive therapy. Mann–Whitney U test p value shown. (B) Violin plots of indicated laboratory values from patients with COVID‐19 grouped by immunosuppressive therapy and mortality. Mann–Whitney U test p value shown. (C) Scatter plot of patient age compared with the length of stay for patients with COVID‐19 on (blue) and not on (red) immunosuppressive therapy. Regression line and 95% confidence interval indicated on plot and patient density histograms included on sides of the plot
To account for the increased age and comorbidities of patients on immunosuppressive therapies, we performed sensitivity analysis. We built a multivariate logistic regression model to predict mortality among patients with COVID‐19 based on immunosuppressive therapy use, gender, age, race, and comorbidity status (Table 2). After sensitivity analysis, immunosuppressive therapy use remained a significant predictor of mortality in patients with COVID‐19 (p = 0.032). Gender, race, and most comorbidities were not significant predictors of mortality in our model. These results suggest that prior immunosuppressive therapy increases COVID‐19 patients’ risk of dying independent of other risk factors for COVID‐19 mortality.
Table 2.
Sensitivity analysis of the association between COVID‐19 mortality and prior immunosuppressive therapy
| Estimate | Std. Error | z value | Pr(>|z| ) | |
|---|---|---|---|---|
| Immunosuppressive therapy | 0.93995 | 0.439313 | 2.139589 | 0.032388 |
| Gender | 0.219933 | 0.267067 | 0.823511 | 0.410217 |
| Age | 0.056914 | 0.01108 | 5.136645 | 2.80E‐07 |
| Race | −0.041459 | 0.05468 | −0.758217 | 0.448321 |
| Hypertension | −0.606853 | 0.364991 | −1.662653 | 0.096382 |
| Chronic kidney disease | 0.360849 | 0.308519 | 1.169618 | 0.242155 |
| Diabetes | 0.523068 | 0.29278 | 1.786559 | 0.074009 |
| Obesity | 0.467486 | 0.307904 | 1.518285 | 0.128943 |
| Rheumatologic disease | −0.114236 | 0.302221 | −0.37799 | 0.705438 |
| Autoimmune disease | −0.724481 | 0.510117 | −1.420223 | 0.155543 |
| Cancer | 0.502552 | 0.276656 | 1.816521 | 0.06929 |
| COPD | −0.182472 | 0.372502 | −0.489855 | 0.624236 |
| Asthma | 0.56945 | 0.392756 | 1.449881 | 0.147092 |
| Coronary artery disease | 0.220466 | 0.30681 | 0.718576 | 0.472402 |
| Cerebrovascular disease | 0.744446 | 0.328264 | 2.267831 | 0.02334 |
Abbreviations: COVID‐19, coronavirus disease 2019; COPD, chronic obstructive pulmonary disease.
To further investigate the underlying clinical characteristics of patients with COVID‐19 on immunosuppressive therapies, we compared laboratory values of patients with COVID‐19 on the basis of mortality. Patients on immunosuppressive therapies had decreased albumin compared with patients not on immunosuppressive therapies who did not die. However, this difference was not present between patients on or not on immunosuppressive therapies who died (Figure 1B). Both patients on or not on immunosuppressive therapies had increased creatinine, phosphate, C‐reactive protein, partial thromboplastin time (PTT), lactate dehydrogenase, and d‐Dimer if they died. These results suggest that these markers of inflammation are altered in patients with severe COVID‐19 regardless of immunosuppressive therapy. In contrast, minimum lymphocyte count and neutrophil count were decreased in both patients on immunosuppressive therapies who died and who were still alive, suggesting that lymphocytes and neutrophils are fundamentally decreased in immunosuppressed patients regardless of COVID‐19 severity. Together, these results suggest that systemic inflammation and adaptive immunity are distinctly altered with both immunosuppressive therapy and COVID‐19 severity.
We next compared correlates of COVID‐19 severity in patients on immunosuppressive therapy to examine how immunosuppression affects risks or consequences of severe COVID‐19. We calculated the Spearman correlation of continuous clinical metrics available in our cohort and total length of hospitalization for both patients on or not on immunosuppressive therapies and compared the ratios of these coefficients to identify disparate correlations. Among the clinical metrics tested, correlation with patient age was significantly altered between immunosuppressed and nonimmunosuppressed patients: in immunosuppressed patients increasing patient age was not associated with increased length hospitalization (Spearman's rho = −0.05, p = 0.75), whereas increasing patient age was associated with increased length of hospitalization in nonimmunosuppressed patients (Spearman's rho = 0.19, p = 6.2 × 10−7) (Figure 1C). These results suggest that in patients on immunosuppressive therapies, length of hospitalization is uniquely independent of patient age and that increased COVID‐19 severity observed in patients on immunosuppressive therapies might be independent of patient age.
4. DISCUSSION
Here, we identify prior immunosuppressive therapy as a unique risk factor for severe COVID‐19. Although considered a risk factor for contracting COVID‐19, the role of immunosuppression after SARS‐CoV‐2 infection has not been extensively studied. 4 , 12 , 29 Through a retrospective cohort analysis of 835 patients with COVID‐19, we found that patients with prescriptions for immunosuppressive therapies at the time of diagnosis had an increased risk of mortality and longer lengths of hospitalization. Sensitivity analysis confirmed that this association was independent of gender, race, and comorbidities. These findings confirm that COVID‐19 disease severity is increased in patients on prior immunosuppressive therapies.
The pathogenesis of severe COVID‐19 is currently believed to be driven by both direct viral‐mediated injury and aberrant host antiviral inflammatory responses. 4 , 7 , 30 Given the contradictory role of the immune system in both of these processes, immunosuppressive therapy has the potential to affect both mechanisms of COVID‐19 pathogenesis. In contrast to the established benefit of immunosuppressive therapies, such as dexamethasone in severe COVID‐19, 31 , 32 our findings suggest that prior immunosuppressive therapy predisposes patients with COVID‐19 to worse outcomes. We propose that pre‐existing immunosuppression hinders host immune responses from preventing viral‐mediated injury, allowing COVID‐19 to progress to severe disease. Prior immunosuppression might also lead to uncontrolled viral replication at the onset of disease, leading to higher viral load at presentation, which has been associated with worse COVID‐19 prognosis. 33 , 34 Mechanisms of immunosuppression might be fundamentally different in patients with prior immunosuppressive therapy and those taking dexamethasone after disease onset, 35 , 36 and these differences in immunophenotype likely account for differences in COVID‐19 outcomes. 30 , 37 , 38 , 39 Ultimately, these results suggest that the type, mechanism, and timing of immunosuppression are critical in determining its effect on COVID‐19 pathogenesis and severity.
Mounting evidence has implicated dysregulated immune responses in severe COVID‐19. 3 , 4 , 11 , 40 Elevated LDH, CRP, d‐Dimer, creatinine, and PTT are characteristic of severe COVID‐19, 28 , 41 and we observed similar elevations of these hematologic inflammatory markers in both patients on or not on immunosuppressive therapies. These results suggest that patients with prior immunosuppressive therapy are still at risk for developing the dysregulated immune responses and hyperinflammation characteristics of severe COVID‐19. However, as the role of immune dysregulation in COVID‐19 mortality remains unclear, and without cause of death or autopsy information, our study does not answer whether the increased mortality observed in patients with COVID‐19 on immunosuppressive therapy is due to COVID‐19 itself or complications of the underlying therapy. We also observed that patients with COVID‐19 on immunosuppressive therapy who died have an even greater degree of lymphopenia, which has also been associated with severe COVID‐19. 11 , 41 Together these results suggest that immunosuppressive therapy both hinders adaptive immune responses and does not prevent hyperinflammation, both of which likely contribute to COVID‐19 severity. 3 , 4 , 11 These findings illustrate the importance of considering mechanisms when implementing immunosuppressive agents for COVID‐19 therapy.
We also observed that age was not correlated with length of hospitalization in patients with COVID‐19 on immunosuppressive therapy, in contrast to the well‐established positive correlation between age and COVID‐19 severity. 2 , 42 This observation might suggest that COVID‐19 severity is independent of age in patients on immunosuppressive therapy. Alternatively, we might have failed to observe an age‐related correlation with disease severity in patients with COVID‐19 on immunosuppressive therapy because the length of hospitalization was determined by clinical complications or decisions related to the immunosuppressive therapy itself rather than COVID‐19 severity in patients on immunosuppressive therapy. Regardless of the cause of the observed discrepancy in correlation, these results suggest that the length of hospitalization of patients with COVID‐19 on immunosuppressive therapy cannot be determined by patient age alone.
There are several limitations to this study. This retrospective study is limited to patients who sought care at our hospital and obtained a laboratory‐confirmed COVID‐19 diagnosis between March 10, 2020 and October 13, 2020 and had available medication history. We are therefore likely undersampling less severe patients with COVID‐19 and asymptomatic SARS‐CoV‐2 infections. 43 This limitation is reflected in the older age of our cohort, likely contributing to the higher mortality rate reported here. Furthermore, over the course of this study therapeutic approaches to treating COVID‐19 and the demographics of the SARS‐CoV‐2 infected population evolved, leading to notable improvements in overall mortality rates. 44 However, we expect both of these sampling biases to affect patients on and not on immunosuppressive therapy similarly, and therefore not influence the increased mortality rate observed in patients with COVID‐19 on immunosuppressive therapy. We also did not observe significant associations between prior antirheumatic therapy with mortality in patients with COVID‐19, which is consistent with other observational studies, 22 , 45 although our cohorts are small and larger studies are needed to confirm these findings.
Ultimately our study is limited to defining a cohort by active prescriptions at the time of COVID‐19 diagnosis; we do not know whether these medications were continued or discontinued following COVID‐19 diagnosis, and therefore cannot make conclusions about the use of immunosuppressive therapies during COVID‐19. We are also limited to assuming patients who had prescriptions were taking their medications, which might be confounded by nonadherence. Furthermore, prior immunosuppressive therapy use is necessarily confounded by heterogenous diseases or factors that led to that therapy, which themselves might influence COVID‐19 severity. For example, recent reports have suggested that patients with more severe rheumatologic disease might have poorer COVID‐19 outcomes. 16 , 46 , 47 We account for this in our sensitivity analysis, which revealed that immunosuppressive therapy is associated with COVID‐19 mortality independent of comorbidity status. With these limitations in mind, our findings reflect the consequences of taking immunosuppressive therapies at the time of SARS‐CoV‐2 infection.
What precautions patients on immunosuppressive therapy must take has remained unclear over the course of this pandemic. Whether immunosuppressed patients who contract COVID‐19 are likely to succumb to the disease or may avoid COVID‐19‐related immune dysregulation and inflammation have remained open questions. 4 , 12 Here, we find that patients on immunosuppressive therapy with COVID‐19 are not only at increased risk of dying but also remain susceptible to the inflammation observed in severe COVID‐19. As the number of individuals with COVID‐19 continues to rise, the number of patients on immunosuppressive therapy who contract COVID‐19 will increase as well, and these findings emphasize the importance of aggressive early monitoring and supportive care in this population. More broadly, these findings illustrate the complexities of the role of the immune system in COVID‐19 pathogenesis and illustrate the need to recognize these nuances when considering immunomodulatory therapies for COVID‐19.
CONFLICT OF INTERESTS
All the authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualization: Elliot H. Akama‐Garren and Jonathan X. Li. Methodology: Elliot H. Akama‐Garren. Software: Elliot H. Akama‐Garren. Validation: Elliot H. Akama‐Garren. Formal Analysis: Elliot H. Akama‐Garren. Investigation: Elliot H. Akama‐Garren. Data Curation: Elliot H. Akama‐Garren. Writing – Original Draft: Elliot H. Akama‐Garren and Jonathan X. Li. Visualization: Elliot H. Akama‐Garren. Supervision: Jonathan X. Li.
ACKNOWLEDGMENTS
We would like to thank S. Li and K. Pollick of the BIDMC InSIGHT CORE service for their assistance with data retrieval. This study was supported by the National Institutes of Health [T32GM007753 to E.A.G.].
Akama‐Garren EH, Li JX. Prior immunosuppressive therapy is associated with mortality in COVID‐19 patients: A retrospective study of 835 patients. J Med Virol. 2021;93:5768‐5776. 10.1002/jmv.27105
REFERENCES
- 1. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620‐2629. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. J Am Med Assoc. 2020;323(13):1239‐1242. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet Lond Engl. 2020;395(10229):1033‐1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the 'Cytokine Storm' in COVID‐19. J Infect. 2020;80(6):607‐613. 10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mangalmurti N, Hunter CA. Cytokine storms: understanding COVID‐19. Immunity. 2020;53(1):19‐25. 10.1016/j.immuni.2020.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blanco‐Melo D, Nilsson‐Payant BE, Liu W‐C, et al. Imbalanced host response to SARS‐CoV‐2 drives development of COVID‐19. Cell. 2020;181(5):1036‐1045. 10.1016/j.cell.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS‐CoV‐2 infection ‐ United Kingdom and United States, March‐August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(40):1450‐1456. 10.15585/mmwr.mm6940e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334‐346. 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Remy KE, Mazer M, Striker DA, et al. Severe immunosuppression and not a cytokine storm characterizes COVID‐19 infections. JCI Insight. 2020;5(17):e140329. 10.1172/jci.insight.140329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID‐19: a double‐edged sword? The Lancet. 2020;395(10230):1111. 10.1016/S0140-6736(20)30691-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Monti S, Balduzzi S, Delvino P, Bellis E, Quadrelli VS, Montecucco C. Clinical course of COVID‐19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. 2020;79(5):667‐668. 10.1136/annrheumdis-2020-217424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu L, Xu X, Ma K, et al. Successful recovery of COVID‐19 pneumonia in a renal transplant recipient with long‐term immunosuppression. Am J Transplant. 2020;20(7):1859‐1863. 10.1111/ajt.15869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharmeen S, Elghawy A, Zarlasht F, Yao Q. COVID‐19 in rheumatic disease patients on immunosuppressive agents. Semin Arthritis Rheum. 2020;50(4):680‐686. 10.1016/j.semarthrit.2020.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID‐19‐related death in people with rheumatic diseases: results from the COVID‐19 Global Rheumatology Alliance physician‐reported registry [published online ahead of print January 27, 2021]. Ann Rheum Dis. 2021. 10.1136/annrheumdis-2020-219498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dandachi D, Geiger G, Montgomery MW, et al. Characteristics, comorbidities, and outcomes in a multicenter registry of patients with HIV and coronavirus disease‐19 [published online ahead of print September 9, 2020]. Clin Infect Dis. 2020. 10.1093/cid/ciaa1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Avery RK, Chiang TP, Marr KA, et al. Inpatient COVID‐19 outcomes in solid organ transplant recipients compared to non‐solid organ transplant patients: a retrospective cohort [published online ahead of print December 7, 2016]. Am J Transplant. 2020. 10.1111/ajt.16431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perni S, Milligan MG, Saraf A, et al. Treating the SARS‐CoV‐2‐positive patient with cancer: a proposal for a pragmatic and transparent ethical process. Cancer. 2020;126(17):3896‐3899. 10.1002/cncr.32962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ho H‐E, Mathew S, Peluso MJ, Cunningham‐Rundles C. Clinical outcomes and features of COVID‐19 in patients with primary immunodeficiencies in New York City. J Allergy Clin Immunol Pract. 2020;9:490‐493. 10.1016/j.jaip.2020.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meyts I, Bucciol G, Quinti I, et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2020;147:520‐531. 10.1016/j.jaci.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Conticini E, Bargagli E, Bardelli M, et al. COVID‐19 pneumonia in a large cohort of patients treated with biological and targeted synthetic antirheumatic drugs. Ann Rheum Dis. 2020;80:14. 10.1136/annrheumdis-2020-217681 [DOI] [PubMed] [Google Scholar]
- 23. Damiani G, Pacifico A, Bragazzi NL, Malagoli P. Biologics increase the risk of SARS‐CoV‐2 infection and hospitalization, but not ICU admission and death: Real‐life data from a large cohort during red‐zone declaration. Dermatol Ther. 2020;33(5):e13475. 10.1111/dth.13475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Conti P, Ronconi G, Caraffa A, et al. Induction of pro‐inflammatory cytokines (IL‐1 and IL‐6) and lung inflammation by Coronavirus‐19 (COVI‐19 or SARS‐CoV‐2): anti‐inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327‐331. 10.23812/CONTI-E [DOI] [PubMed] [Google Scholar]
- 25. Hughes R, Pedotti R, Koendgen H. COVID‐19 in persons with multiple sclerosis treated with ocrelizumab: a pharmacovigilance case series. Mult Scler Relat Disord. 2020;42:102192. 10.1016/j.msard.2020.102192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with COVID‐19 pneumonia. N Engl J Med. 2020;384(15):1473‐1474. 10.1056/NEJMoa2030340 [DOI] [PubMed] [Google Scholar]
- 27. Stone JH, Frigault MJ, Serling‐Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with COVID‐19. N Engl J Med. 2020;383(24):2333‐2344. 10.1056/NEJMoa2028836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet Lond Engl. 2020;395(10229):1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thng ZX, De Smet MD, Lee CS, et al. COVID‐19 and immunosuppression: a review of current clinical experiences and implications for ophthalmology patients taking immunosuppressive drugs. Br J Ophthalmol. 2020;105:306‐310. 10.1136/bjophthalmol-2020-316586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID‐19. Nature. 2020;583(7816):437‐440. 10.1038/s41586-020-2355-0 [DOI] [PubMed] [Google Scholar]
- 31. WHO Rapid Evidence Appraisal for COVID‐19 Therapies (REACT) Working Group, Sterne JAC , Sterne J, S, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID‐19: a meta‐analysis. J Am Med Assoc. 2020;324(13):1330‐1341. 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Horby P, Lim WS, et al, RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with COVID‐19: preliminary report. N Engl J Med. 2020;384:693‐704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Y, Yan L‐M, Wan L, et al. Viral dynamics in mild and severe cases of COVID‐19. Lancet Infect Dis. 2020;20(6):656‐657. 10.1016/S1473-3099(20)30232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shi F, Wu T, Zhu X, et al. Association of viral load with serum biomakers among COVID‐19 cases. Virology. 2020;546:122‐126. 10.1016/j.virol.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giles AJ, Hutchinson M, Sonnemann HM, et al. Dexamethasone‐induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):51. 10.1186/s40425-018-0371-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coutinho AE, Chapman KE. The anti‐inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2‐13. 10.1016/j.mce.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mathew D, Giles JR, Baxter AE, et al. Deep immune profiling of COVID‐19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369(6508):eabc8511. 10.1126/science.abc8511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bost P, Giladi A, Liu Y, et al. Host‐viral infection maps reveal signatures of severe COVID‐19 patients. Cell. 2020;181(7):1475‐1488. 10.1016/j.cell.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilk AJ, Rustagi A, Zhao NQ, et al. A single‐cell atlas of the peripheral immune response in patients with severe COVID‐19. Nat Med. 2020;26(7):1070‐1076. 10.1038/s41591-020-0944-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762‐768. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liao D, Zhou F, Luo L, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID‐19: a retrospective cohort study. Lancet Haematol. 2020;7(9):e671‐e678. 10.1016/S2352-3026(20)30217-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. J Am Med Assoc. 2020;323(20):2052‐2059. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oran DP, Topol EJ. Prevalence of asymptomatic SARS‐CoV‐2 infection: a narrative review. Ann Intern Med. 2020;173(5):362‐367. 10.7326/M20-3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID‐19 risk‐adjusted mortality rates. J Hosp Med. 2020;16:90‐92. 10.12788/jhm.3552 [DOI] [PubMed] [Google Scholar]
- 45. Gianfrancesco M, Hyrich KL, Al‐Adely S, et al. Characteristics associated with hospitalisation for COVID‐19 in people with rheumatic disease: data from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis. 2020;79(7):859‐866. 10.1136/annrheumdis-2020-217871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Serling‐Boyd N, D'silva KM, Hsu TY, et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic [published online ahead of print November 30, 2020]. Ann Rheum Dis. 2020. 10.1136/annrheumdis-2020-219279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. D'silva KM, Jorge A, Cohen A, et al. COVID‐19 outcomes in patients with systemic autoimmune rheumatic diseases compared to the general population: a US multicenter, comparative cohort study [published online ahead of print December 10, 2020]. Arthritis Rheumatol. 2020. 10.1002/art.41619 [DOI] [PMC free article] [PubMed] [Google Scholar]


