Abstract
Xiyanping (XYP) is a Chinese herbal medicine used in the clinic to treat respiratory infection and pneumonia. Recent evidence identified XYP as a potential inhibitor of severe acute respiratory syndrome coronavirus 2, implying XYP as a possible treatment for the coronavirus disease 2019 (COVID‐19). Here, we conducted a prospective, multicenter, open‐label and randomized controlled trial to evaluate the safety and effectiveness of XYP injection in patients with mild to moderate COVID‐19. We consecutively recruited 130 COVID‐19 patients with mild to moderate symptoms from five study sites, and randomized them in 1:1 ratio to receive XYP injection in combination with standard therapy or receive standard supportive therapy alone. We found that XYP injection significantly reduced the time to cough relief, fever resolution and virus clearance. Less patients receiving XYP injection experienced disease progression to the severe stage during the treatment process. No severe adverse events were reported during the study. Taken together, XYP injection is safe and effective in improving the recovery of patients with mild to moderate COVID‐19. However, further studies are warranted to evaluate the efficacy of XYP in an expanded cohort comprising COVID‐19 patients at different disease stages.
Keywords: andrographolide, coronavirus disease 2019 (COVID‐19), SARS‐CoV‐2, symptom resolution, traditional Chinese medicine, Xiyanping
1. INTRODUCTION
Since it was first detected in Wuhan City, China, the coronavirus disease 2019 (COVID‐19) has become a global health threat affecting over 200 countries and territories. As of August 19, 2020, about 22.2 million COVID‐19 cases have been documented worldwide and 782,000 patients have lost their lives. COVID‐19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which infects the respiratory tract, inflames one or both lungs and results in breathing difficulties. As there is currently no specific therapy for COVID‐19, repurposing existing drugs offers an alternative strategy to lighten the burden of the coronavirus pandemic. Several antiviral medications, such as antimalarial agents (e.g., chloroquine and hydroxychloroquine) and HIV medicine (e.g., lopinavir/ritonavir), have been tested for their effectiveness in treating coronavirus. However, due to increased toxicity, unfavorable pharmacodynamics and lack of clinical benefits, none of these drugs have been approved for COVID‐19 treatment (Boulware et al., 2020; Cao et al., 2020; Tang et al., 2020). More effort is thus needed to evaluate other potential antiviral treatments.
Xiyanping (XYP) is a marketed proprietary Chinese medicine used for the treatment of upper respiratory tract infection, viral pneumonia, influenza and bronchitis for more than three decades (Yang et al., 2019). XYP is primarily composed of 9‐dehydro‐17‐hydro‐andrographolide and sodium 9‐dehydro‐17‐hydro‐andrographolide‐19‐yl sulfate, which are derived from herbaceous plant Andrographis paniculate (Table S1) (Chong, Chen, Luo, & Jiang, 2013). The main active component of A. paniculate, andrographolide, has been characterized with broad‐spectrum antiviral properties (Gupta, Mishra, & Ganju, 2017). However, due to poor aqueous solubility, the clinical application of andrographolide has been largely limited. XYP is prepared from andrographolide through sulfonation reaction to obtain water‐soluble andrographolide sulfonate (sodium 9‐dehydro‐17‐hydro‐andrographolide‐19‐yl sulfate) (Chong et al., 2013; Zheng, Shao, Chen, & Luo, 2016), which largely improves the water solubility and bioavailability of andrographolide. In vitro study revealed that the andrographolide sulphonate in XYP shows a similar antiinflammation effect to andrographolide in inhibiting the overproduction of inflammatory cytokines, such as nitric oxide (NO) and tumor necrosis factor‐α (TNF‐α), in lipopolysaccharide (LPS)‐treated mononuclear macrophage (Yang et al., 2019). In animals, andrographolide sulphonate has been reported to promote inflammation resolution by improving the phagocytic function of peripheral blood neutrophils (Xiong et al., 2015). There is also evidence showing that XYP treatment reduces the mortality of mice infected with lethal Enterovirus 71 by inhibiting the apoptosis of T lymphocytes and suppressing the cytokines production of neutrophils (M. Li et al., 2018). Moreover, XYP has also been found to decrease the expression of proinflammatory TNF‐α, interleukin‐1β (IL‐1β), interleukin‐6 (IL‐6) and inducible nitric oxide synthase (iNOS), and attenuate liver and lung damage through inhibiting p38 mitogen‐activated protein kinase (MAPK), STAT3 and nuclear factor kappa B (NF‐κB) pathways, which dramatically improve the survival of mice with LPS‐induced sepsis (Guo et al., 2012). Of note, recent molecular docking analysis identified andrographolide with potential antiviral activity against SARS‐CoV‐2 via binding the main protease of the virus, making XYP a promising medicine for COVID‐19 treatment (Enmozhi, Raja, Sebastine, & Joseph, 2020). In the present study, we performed a multicenter, open‐labeland randomized controlled trial to further evaluate the effectiveness of XYP injection in patients with mild to moderate COVID‐19, which accounts for up to 80% of COVID‐19 patients.
2. MATERIALS AND METHODS
2.1. Trial design
This was a multicenter, prospective, open‐label and randomized controlled trial performed at five sites in Jiangxi Province, China, including the Second Affiliated Hospital of Nanchang University, the Fifth People's Hospital of Ganzhou, Ji'an Central People's Hospital, Fengcheng People's Hospital and the Ninth Hospital of Nanchang. The study was approved by the institutional review board or ethics committee at each participating site, and was conducted in accordance with the principles and standards of the Declaration of Helsinki and Good Clinical Practice guidelines of the International Conference on Harmonisation. The study is registered with ClinicalTrials.gov (identifier number: NCT04295551). Written informed consent was obtained from all the patients.
2.2. Inclusion criteria
Eligible participants were hospitalized adult patients with laboratory‐confirmed COVID‐19, who were: (1) at least 18 years of age; (2) with SARS‐CoV‐2 infection confirmed by RT‐PCR in respiratory or blood specimens; (3) with fever, respiratory symptoms or imaging features of pneumonia and (4) meeting the criteria for mild to moderate COVID‐19 according to the Diagnosis and Treatment Protocol of Novel Coronavirus Pneumonia (Table 1) (“Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7),” Wei, 2020). The participation in the study is completely voluntary. All the participants were free to discontinue the participation at any time of the study.
TABLE 1.
Clinical classification of COVID‐19
| Classification | Criteria |
|---|---|
| Mild | Mild clinical symptoms; no sign of pneumonia on imaging |
| Moderate | Fever and respiratory symptoms with radiological findings of pneumonia |
| Severe |
Cases with chest imaging that shows obvious lesion progression within 24–48 hr >50% shall be managed as severe cases. |
| Critical |
|
Abbreviations: FiO2, fractional inspired oxygen; ICU, intensive care unit; PaO2, arterial oxygen partial pressure.
2.3. Exclusion criteria
Patients with one of the following conditions were not enrolled: (1) diagnosed with severe or critical COVID‐19 pneumonia; (2) had severe primary diseases affecting survival or study outcome (e.g., uncontrolled malignant tumors, hematological diseases and human immunodeficiency virus infection); (3) had obstructive pneumonia, pulmonary interstitial fibrosis, alveolar proteinosis and allergic alveolitis caused by lung tumors; (4) were pregnant or breast‐feeding; (5) were known to be allergic to the ingredients contained in the research medication, or patients with allergies; (6) were using immunosuppressive agents or had organ transplants in the past 6 months; (7) participated in other drug clinical trials within 3 months before the screening test; and (8) were not able to complete or should not participate in the study as judged by the investigators (e.g., expected death within 48 hr, unwillingness to take active treatment).
2.4. Investigational product
The investigational product, XYP injection, is a proprietary Chinese medicine (Jiangxi Qingfeng Pharmaceutical Co. Ltd) prepared from herbaceous plant A. paniculate. The bioactive ingredient, andrographolide, was extracted from the aerial part of A. paniculate using ethanol, and was then sulfonated through sulfonation reaction to generate XYP preparation. The concentration of andrographolide sulfonation product in XYP injection is 25 mg/ml.
2.5. Sample size calculation
As little information is available regarding clinical outcomes of COVID‐19 patients at the time of study initiation, we assumed that the average time to clinical symptom resolution was 9 days for the treatment group and was 14 days for the control group, which led to an estimated sample size of 58 patients per group to provide greater than 90% power at a two‐sided type I error of 1%. We also assumed an estimated difference of 35% in the 14‐day recovery rate between the treatment group (85%) and control group (50%), and the necessary sample size was calculated to be 51 patients per group to detect such a difference with 90% power (α = 1%). By considering both the time to clinical recovery and the recovery rate, we thus proposed to enroll at least 60 patients per group allowing for a 15% dropout rate.
2.6. Randomization and blinding
Eligible patients were consequently by study investigators enrolled at each participating site until the number of participants allocated to the site was reached. Randomization was performed using block randomization with no stratification (block sizes: 2 and 4). Each patient was assigned to a serial number and was randomly assigned in 1:1 ratio to either the control group or treatment group using a computer‐generated block randomization sequence. The statistician who generated the randomization sequence was not involved in patient enrollment and patient care and was blind to the treatment allocation. The study investigators were masked to the block sizes and were not aware of treatment assignment until the enrollment of a patient was completed. Moreover, the clinicians performing vital signs, laboratory testing and outcome measurements were also blinded to treatment allocation.
2.7. Interventions
Patients in the control group received standard symptomatic treatments as necessary in accordance with the Diagnosis and Treatment Protocol of Novel Coronavirus Pneumonia (5th edition), which included supplemental oxygen therapy, antiviral medicines, antibiotic agents and immune modulators. Patients in the treatment group received XYP injection combined with standard care. XYP was given at a weight‐based dose of 10 mg/kg once per day, with a maximum daily dosage not to exceed 500 mg. The drug was diluted in 5% glucose injection or 0.9% sodium chloride injection at the concentration of 1 mg/ml and was delivered intravenously at the rate of 30–40 drops per minute. The course of treatment lasted for 7–14 consecutive days. During the treatment process, if the patients had all clinical symptoms relieved and met the discharge criteria, the treatment can be stopped after medical consultation. If the patients' clinical symptoms were improved but not fully resolved after 14 days of treatment, investigating physicians determined whether to continue the treatment plan or not. Traditional Chinese medicines (e.g., Qingkailing, Tanreqing, Xuebijing and Reduning) with the same detoxifying and circulation promoting functions as XYP were not used for both study groups. There was no co‐intervention in the present study. All the participants were followed for 28 days. Compliance with treatments was evaluated daily by study coordinators. Compliance rate was determined as the percentage of the prescribed doses of the treatment actually taken by the patients over the study period. Laboratory testing, clinical outcome, vital signs, SARS‐CoV‐2 tests and chest computed tomography imaging were assessed at baseline after randomization and on day 3, 7, 14 and 28.
2.8. Adverse events assessment
All the study participants were closely monitored for adverse events throughout the treatment process. Patients receiving XYP injection were observed by a trial staff for at least 30 minutes after the completion of each injection, as most of the adverse reactions occur within 30 minutes after administration (Wang & Xie, 2012). All the adverse events were reported regarding the manifestation, time of onset, duration and severity. The association of an adverse event with study medication was evaluated by investigating physicians. The treatment was discontinued if a severe adverse reaction occurred and was judged to be related to study medicine.
2.9. Outcome measurements
All the measurements were performed following the Diagnosis and Treatment Protocol of Novel Coronavirus Pneumonia. Fever was evaluated by measuring the underarm temperature using a medical thermometer. Fever resolution was defined as the underarm temperature lower than 37.3°C for at least 72 hr without the use of fever‐reducing medicine. The intensity and frequency of cough were self‐reported by the patients and was documented by study coordinators. Patients with no cough for over 24 hr were considered to have cough relief. SARS‐CoV‐2 infection was assessed by standard RT‐PCR. Virus clearance was defined as two consecutive negative SARS‐CoV‐2 nuclei acid tests performed more than 24 hr apart. The clinical symptoms of COVID‐19 were classified as mild, moderate, severe and critical in accordance with the Diagnosis and Treatment Protocol of Novel Coronavirus Pneumonia (Table 1). Patients with symptoms that progressed to severe or critical stage were determined to experience clinical deterioration.
2.10. Study endpoints
The primary endpoint was the time from the start of study medicine to complete symptom resolution, including fever resolution and cough recovery. Secondary endpoints comprised four measures, including the time to fever resolution, time to cough recovery, time to virus clearance, and the rate of patients with clinical deterioration.
2.11. Statistical analysis
Statistical analysis was performed using SAS software version 9.4. Continuous variables were expressed as mean ± SD and were compared between study groups using Wilcoxon rank‐sum test. Categorical variables were presented as numbers and percentages and were compared between study groups using the chi‐square test. The time to endpoints analyses was performed using Kaplan–Meier method with the log‐rank test to evaluate statistical significance. The hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated with Cox proportional hazard model. Covariate adjustments were performed by including age, trial site and baseline fever. For the analysis of time to complete fever resolution, only age and trial site were considered when doing the adjustment. Analyses were done in the intention‐to‐treat population comprising all randomized patients. The most recent assessment was used for missing values from dropout participants (Last Observation Carried Forward method). Two‐tailed p values less than .05 were considered statistically significant.
3. RESULTS
3.1. Trial patients and baseline characteristics
From January 27, 2020 to February 20, 2020, 232 patients were assessed for eligibility and 130 eligible patients with mild to moderated COVID‐19 pneumonia were recruited into the study and were randomly assigned to receive XYP injection combined with standard care (n = 65, treatment group) or standard supportive care alone (n = 65, control group). No patients dropped out of the study. All the participants completed the study with 100% compliance to their assigned treatment and were included in the final analysis (Figure 1).
FIGURE 1.
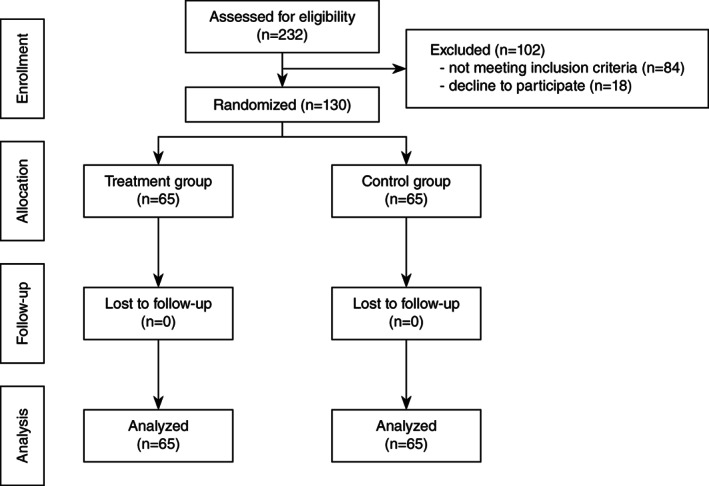
Study flow chart of patients in enrollment, allocation, follow‐up and analysis
The study population had a mean age of 46.28 years (SD, 13.93) and sex distribution was 60 men (46.2%) versus 70 women (53.8%). All the patients had positive tests for SARS‐CoV‐2. Eighty five patients (65.4%) had a recent travel to Wuhan city and 17 patients (13.1%) had close contact with confirmed COVID‐19 cases. The average time from symptom onset to randomization was 8.34 days (SD, 8.74). 88.2% of patients (n = 112) showed abnormal chest imaging findings consistent with COVID‐19 pneumonia. Cough and fever were the common symptoms occurring in 70 (53.8%) and 47 (36.2%) patients, respectively. Forty‐six patients (35.4%) had pre‐existing conditions with hypertension as the most common comorbidity reported by 21 patients (16.2%).
The baseline characteristics were generally balanced between the treatment and control groups (Table 2). No significant difference was observed for most of the demographic and clinical features, such as age, sex, exposure history, comorbidities, lung lesions on chest imaging and baseline cough, except that the treatment group showed a higher rate of baseline fever (47.7% vs. 24.6%, p = .006) and higher creatinine level (78.09 ± 21.65 μmol/L vs. 69.90 ± 15.64 μmol/L, p = .03) (Table 2).
TABLE 2.
Baseline characteristics
| Characteristics | Treatment (n = 65) | Control (n = 65) | p‐value a |
|---|---|---|---|
| Age (years) | 44.31 ± 13.45 | 48.25 ± 14.22 | .07 |
| Male sex, n (%) | 32 (49.2) | 28 (43.1) | .48 |
| Exposure | |||
| Recent travel to Wuhan city, n (%) | 46 (70.8) | 39 (60.0) | .20 |
| Close contact with confirmed cases, n (%) | 6 (9.2) | 11 (16.9) | .19 |
| Time from symptom onset to randomization (days) | 7.25 ± 7.36 | 9.43 ± 9.87 | .68 |
| Lung lesions on chest imaging, n (%) | 57 (87.7) | 55/62 (88.7) | .86 |
| Body temperature (°C) | 37.17 ± 0.62 | 36.96 ± 0.58 | .12 |
| Respiratory rate (breaths/min) | 19.98 ± 1.18 | 20.31 ± 1.58 | .18 |
| Heart rate (beats/min) | 86.02 ± 10.68 | 86.08 ± 11.86 | .53 |
| Oxygen saturation (%) | 94.87 ± 10.93 | 95.92 ± 3.02 | .54 |
| Comorbidities, n (%) | 21 (32.3) | 25 (38.5) | .46 |
| Hypertension | 10 (15.4) | 11 (16.9) | .81 |
| Diabetes | 4 (6.2) | 6 (9.2) | .74 |
| Hyperlipidemia | 1 (1.5) | 1 (1.5) | 1.00 |
| Others | 9 (13.8) | 12 (18.5) | .47 |
| Symptoms and signs | |||
| Cough, n (%) | 39 (60.0) | 31 (47.7) | .16 |
| Fever, n (%) | 31 (47.7) | 16 (24.6) | .006 |
| Anorexia, n (%) | 11 (16.9) | 7 (10.8) | .31 |
| Chest pain, n (%) | 2 (3.1) | 6 (9.2) | .27 |
| Diarrhea, n (%) | 6 (9.2) | 2 (3.1) | .27 |
| Fatigue, n (%) | 4 (6.2) | 3 (4.6) | 1.00 |
| Sore throat, n (%) | 2 (3.1) | 1 (1.5) | 1.00 |
| Joint pain, n (%) | 1 (1.5) | 1 (1.5) | 1.00 |
| Headache, n (%) | 2 (3.1) | 0 (0) | .48 |
| Vomiting, n (%) | 1 (1.5) | 0 (0) | 1.00 |
| Laboratory findings | |||
| White blood cell count (×109/L) | 5.13 ± 1.61 | 5.64 ± 1.84 | .18 |
| Lymphocytes (%) | 28.94 ± 10.10 | 26.21 ± 9.07 | .12 |
| Alanine aminotransferase (U/L) | 27.92 ± 17.72 | 31.92 ± 20.04 | .32 |
| Creatine kinase (U/L) | 84.13 ± 78.51 | 89.73 ± 86.63 | .73 |
| C‐reactive protein (mg/L) | 12.15 ± 18.09 | 15.58 ± 25.76 | .69 |
| Procalcitonin (ng/ml) | 0.05 ± 0.03 | 0.06 ± 0.03 | .61 |
| Lactate dehydrogenase (U/L) | 224.64 ± 81.31 | 209.83 ± 79.77 | .16 |
| Creatinine (μmol/L) | 78.09 ± 21.65 | 69.90 ± 15.64 | .03 |
Continuous variables were compared between study groups using the Wilcoxon rank‐sum test; categorical variables were compared between study groups using the chi‐square test; continuity correction was performed for categorical variables with at least one expected cell count less than 5.
3.2. Study endpoints
For the primary endpoint, the meantime to complete resolution of both fever and cough was 8.33 days (SD, 4.87) for the treatment group, which was significantly shorter than the 11.86 days (SD, 6.93) for the control group (HR: 1.84, 95% CI: 1.19–2.83, p = .006) (Figure 2, Table 3). Similar result was observed after adjusting for three baseline variables (age, trial site and fever at baseline) (HR: 1.93, 95% CI: 1.18–3.15, p = .008), suggesting XYP injection is effective in promoting the recovery from COVID‐19.
FIGURE 2.
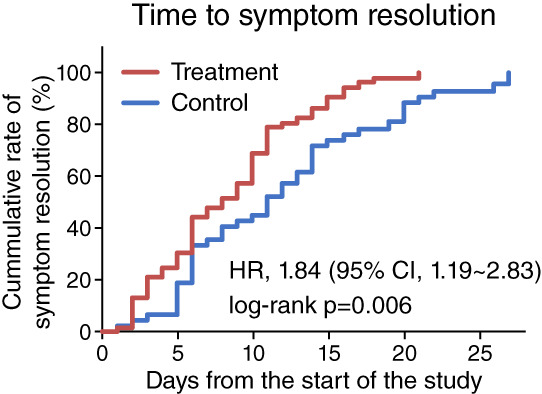
Primary study endpoint. The cumulative rate of patients with complete symptom resolution (both fever and cough resolution) in the treatment group (red line) and control group (blue line). Time to complete symptom resolution was compared between study groups using Kaplan–Meier method with the log‐rank test. Cox proportional hazard model was used to estimate hazard ratios (HR) and 95% confidence intervals (CI) [Colour figure can be viewed at wileyonlinelibrary.com]
TABLE 3.
Study endpoints
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Outcomes | Treatment group | Control group | HR (95% CI)a | p‐value | HR (95% CI) | p‐value |
| Primary endpoint | ||||||
| Time to complete symptom resolution (days) | 8.33 ± 4.87 | 11.86 ± 6.93 | 1.84 (1.19–2.83) | .006 | 1.93 (1.18–3.15) | .008 |
| Secondary endpoints | ||||||
| Time to fever resolution (days) | 3.33 ± 2.76 | 4.60 ± 3.55 | 1.61 (0.94–2.75) | .084 | 1.67 (0.95–2.92) | .075 |
| Time to cough relief (days) | 6.89 ± 4.33 | 12.25 ± 6.85 | 2.30 (1.41–3.75) | .001 | 2.56 (1.48–4.44) | .001 |
| Time to virus clearance (days) | 7.97 ± 4.08 | 12.23 ± 5.77 | 2.19 (1.51–3.16) | <.001 | 2.15 (1.46–3.17) | <.001 |
| Clinical deterioration, n (%) | 0 (0) | 6 (9.2) | 1.10 (1.02–1.19) | .014 | ||
| Severe stage | 0 (0) | 6 (9.2) | ||||
| Critical stage | 0 (0) | 0 (0) |
Relative risk (RR) and 95% CI are provided for Clinical deterioration
Secondary endpoints comprise time to cough relief, time to fever resolution, time to viral clearance and the incidence of clinical deterioration. XYP treatment significantly reduced the time to cough relief (6.89 days [SD, 4.33] vs. 12.25 days [SD, 6.85], HR: 2.30, 95% CI: 1.41–3.75, p = .001) (Figure 3a, Table 3). A better fever resolution was also observed for patients receiving XYP injection with a trend towards statistical significance (3.33 days [SD, 2.76] vs. 4.60 days [SD, 3.55], HR: 1.61, 95% CI: 0.94–2.75, p = .084) (Figure 3b, Table 3). Moreover, compared to the control group, the treatment group had a significantly shorter time to achieve negative SARS‐CoV‐2 RNA tests (7.97 days [SD, 4.08] vs. 12.23 days [SD, 5.77], HR: 2.19, 95% CI: 1.51–3.16, p < .001) (Figure 3b, Table 3). All these results remained robust after covariate adjustment (time to cough relief: HR: 2.56, 95% CI: 1.48–4.44, p = .001; time to fever resolution: HR: 1.67, 95% CI: 0.95–2.92, p = .075; time to virus clearance: HR: 2.15, 95% CI: 1.46–3.17, p < .001) (Table 3). Of note, six patients (9.2%) in the control group developed severe symptoms during the study, while no patients in the XYP treatment group showed disease progression, suggesting the potential of XYP in preventing COVID‐19 deterioration (RR: 1.10, 95% CI: 1.02–1.19, p = .014) (Figure 3d, Table 3).
FIGURE 3.
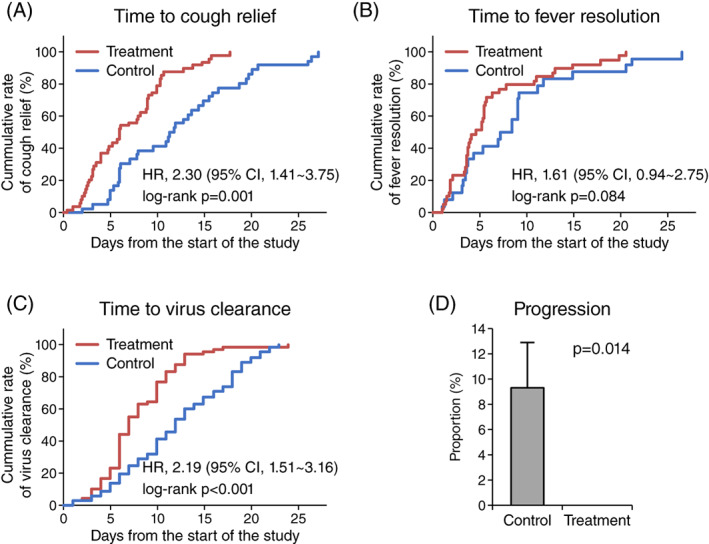
Secondary endpoints. The cumulative rate of (a) cough relief, (b) fever resolution and (c) virus clearance in the treatment group and control group (blue line). Time to endpoints was compared between study groups using Kaplan–Meier method with the log‐rank test. Cox proportional hazard model was used to estimate hazard ratios (HR) and 95% confidence intervals (CI). (d) The rate of patients with clinical deterioration in the treatment group and control group. Statistical significance was assessed using the chi‐square test with continuity correction [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. Adverse events
Adverse events were reported by 55 patients (84.6%) in the treatment group and in 53 patients (81.5%) in the control group. Most of the adverse events were abnormal laboratory findings (83 patients [63.8%]), such as lymphocytopenia (24 patients [18.5%]), neutrophilia (22 patients [16.9%]), increased C‐reactive protein level (14 patients [10.8%]) and increased alanine aminotransferase level (14 patients [10.8%]). Other common adverse events included chest pain (26 patients [20.0%]), diarrhea (25 patients [19.2%]), nausea (19 patients [14.6%]) and fatigue (10 patients [7.7%]). None of the adverse events were related to the study treatment. The incidence of adverse events showed no dramatic difference between the treatment group and the control group (Table 4). Most of the adverse events were mild and self‐limiting. No serious events were reported for both study groups and no patient died during the study. A comprehensive list of all adverse events can be found in Table S2.
TABLE 4.
Adverse events
| Adverse events | Treatment (n = 65) | Control (n = 65) | p‐value a |
|---|---|---|---|
| Laboratory findings, n (%) | 45 (69.2) | 38 (58.5) | .20 |
| Lymphocytopenia | 13 (20) | 11 (16.9) | .65 |
| Neutrophilia | 14 (21.5) | 8 (12.3) | .16 |
| Increased C‐reactive protein | 5 (7.7) | 9 (13.8) | .26 |
| Increased alanine aminotransferase | 7 (10.8) | 7 (10.8) | 1.00 |
| Hyperbilirubinaemia | 9 (13.8) | 5 (7.7) | .26 |
| Decreased blood creatine kinase | 8 (12.3) | 3 (4.6) | .21 |
| Increased lactate dehydrogenase | 5 (7.7) | 6 (9.2) | .75 |
| Decreased white blood cell count | 5 (7.7) | 5 (7.7) | 1.00 |
| Chest pain, n (%) | 15 (23.1) | 11 (16.9) | .38 |
| Diarrhea, n (%) | 10 (15.4) | 15 (23.1) | .27 |
| Nausea, n (%) | 11 (16.9) | 8 (12.3) | .46 |
| Fatigue, n (%) | 6 (9.2) | 4 (6.2) | .74 |
| Abdominal discomfort, n (%) | 3 (4.6) | 5 (7.7) | .72 |
| Shortness of breath, n (%) | 4 (6.2) | 4 (6.2) | 1.00 |
| Dizziness, n (%) | 4 (6.2) | 3 (4.6) | 1.00 |
Comparison was performed between study groups using the chi‐square test; continuity correction was performed for variables with at least one expected cell count less than 5.
4. DISCUSSION
COVID‐19 pneumonia is caused by the infection of SARS‐CoV‐2. It not only causes various respiratory symptoms but also induces a strong cytokine storm, leading to systemic inflammatory response and multiple organ failure. XYP is a Chinese herbal medicine with antiinflammatory and antiviral effects and is widely used in the clinic for respiratory infection and pneumonia. It has been reported that XYP injection improves the clinical outcome and reduces the incidence of adverse events in patients with Mycoplasma pneumoniae pneumonia (Q. Li et al., 2019). It is also superior to the conventional antibiotic and antiviral treatment for community‐acquired pneumonia (Shi et al., 2019). Notably, XYP is also generally safe treatment with a rare incidence of adverse events, of which, most can be successfully resolved if managed appropriately (Wang & Xie, 2012). All these prior studies suggested the feasibility of XYP for treating COVID‐19 pneumonia.
Here, in this multicenter randomized open‐label trial involving adult patients hospitalized with mild to moderated COVID‐19, we provided up to now the first clinical evidence that XYP injection is a safe treatment and is effective in enhancing disease recovery. Specifically, compared to standard supportive therapy, treatment with XYP was associated with accelerated symptom resolution (e.g., fever and cough) and rapid SARS‐CoV‐2 clearance. The progression of COVID‐19 pneumonia can also be delayed or prevented by XYP injection. Moreover, as no serious side effects or complications were reported, XYP showed a favorable safety profile in COVID‐19 patients. All these findings support XYP injection as a promising treatment for COVID‐19.
While western medicine is the mainstay of clinical treatment, the value of traditional Chinese medicine is growingly acknowledged. Some traditional Chinese medicines, such as acupuncture, Tai Chi, have been successfully used in western countries to treat diseases and improve health. Since the outbreak of COVID‐19, many efforts have been placed to repurposing traditional Chinese medicine, particularly those with antiviral and antiinflammatory properties, for COVID‐19 treatment alone or in combination with western medicines (Huang, Bai, He, Xie, & Zhou, 2020; Zhang et al., 2021; Zhuang et al., 2020). And some of them, such as Lianhuaqingwen capsules, have shown clinical benefits in improving COVID‐19 symptoms and promoting patient recovery (Hu et al., 2021). Here, our clinical trial provided additional evidence that XYP is also an efficacious Chinese medicine for COVID‐19 treatment, offering more therapeutic options for COVID‐19 patients.
The active component of XYP, andrographolide sulfonate, is the water‐soluble form of andrographolide, which has been characterized with antiviral property against a wide range of virus, such as influenza A virus (IAV), hepatitis C virus (HCV), herpes simplex virus (HSV), Epstein–Barr virus (EBV) and human immunodeficiency virus (HIV) (Gupta et al., 2017). It has been increasingly recognized that andrographolide exerts antiviral function by acting at each stage of the viral life cycle. Andrographolide demonstrates a high affinity to hemagglutinin and neuraminidase expressed on the surface of IAV, which thus prevents the binding of IAV to the target cell and the release of new viral particles to the extracellular region (Raja, Prabahar, Selvakumar, & Raja, 2014). Andrographolide and its derivatives can also block the attachment and penetration of HIV and HSV (Chang et al., 1991; Seubsasana, Pientong, Ekalaksananan, Thongchai, & Aromdee, 2011). In addition, there is evidence showing that andrographolide suppresses EBV genome replication by reducing the expression of two transcription factors (Rta and Zta) involved in the immediate early stage of the EBV lytic cycle (Lin, Chen, Duh, Chang, & Liu, 2008). Moreover, andrographolide can also inhibit the function of important viral proteins. It has been reported that andrographolide is able to deactivate HCV NS3/4A protease, a multi‐functional protein essential for RNA replication and signal transduction (Chandramohan, Kaphle, Chekuri, Gangarudraiah, & Bychapur Siddaiah, 2015). The expression of the HIV capsid component, p24 antigen, can also be decreased by andrographolide treatment (Reddy et al., 2005). More importantly, andrographolide has recently been characterized with high binding affinity to the main protease of SARS‐CoV‐2, a protein with a crucial role in coronaviral gene expression and replication, implying that the therapeutic role of XYP in treating COVID‐19 may involve deactivating SARS‐CoV‐2 main protease (Enmozhi et al., 2020).
In addition to directly interfering with the viral life cycle, andrographolide also defenses against viral infection through modulating the host immune response. Andrographolide is capable of activating cytotoxic T lymphocytes, augmenting natural killer cell‐mediated cytotoxicity and promoting the phagocytosis activity of macrophage, which may subsequently recognize and kill virus‐infected host cells or release antiviral cytokines to inhibit viral replication (Peng, Zhou, Ding, Li, & Yao, 2002; Sheeja & Kuttan, 2007a, 2007b). Consistent with this, XYP treatment resulted in accelerated virus clearance in COVID‐19 patients, and the related clinical symptoms were also resolved rapidly.
Apart from the antiviral function, andrographolide is also known for its strong antiinflammatory activity. Both in vitro and in vivo studies reveal that andrographolide treatment suppresses the production of pro‐inflammatory cytokines, deactivating MAPK signaling pathway and the downstream transcription factors NF‐κB (Y. Li et al., 2017). This is crucial in the setting of COVID‐19 infection because the inflammatory cytokine storm is considered as the major cause of disease progression and mortality. As the hyperinflammatory syndrome is usually observed in severely and critically ill COVID‐19 patients, our current patient cohort, which comprises mild to moderate cases only, may not have enough power to evaluate the antiinflammatory effect of XYP. However, compared to standard treatment, XYP injection prevented the progression of COVID‐19 to severe conditions. Moreover, a lower level of C‐reactive protein, a marker of acute inflammation, was observed throughout the study for XYP‐treated patients despite the lack of statistical significance (Figure S1). All these results supported an effect of XYP in neutralizing COVID‐19‐induced inflammation.
4.1. Limitations and weakness
There were several limitations in the present study. First, as this is an open‐label study, some subjective elements might be introduced. To reduce bias, statistician performing randomization was masked to treatment allocation and were not involved in patient care; the treatment assignment was not disclosed to study investigators until the enrollment process for a patient was completed; the clinicians assessing patients' symptoms were blinded to the study, and the SARS‐CoV‐2 tests were also performed by a clinician not aware of treatment allocation. Second, while many efforts were placed to reduce selection bias, we still saw an imbalance in some baseline characteristics, including baseline fever and creatinine level (Table 2). The higher baseline fever rate in the treatment group may lead to an underestimation of XYP and resulted in an unsignificant outcome regarding the fever resolution, therefore the efficacy of XYP maybe even more sound. Moreover, although the values in both study groups were within normal range, XYP treatment group also showed higher creatinine levels at baseline. As kidney damage has been seen in COVID‐19 patients who have no underlying kidney problems before SARS‐CoV‐2 infection, future studies are warranted to explore the potential influence of creatinine level or kidney function on COVID‐19 patient recovery as well as the possible interaction between creatinine and XYP treatment. Third, the sample size of the study is small, which may, for example, limit the power for detecting the difference of fever resolution between study groups. Finally, the present study comprised only patients with mild to moderate COVID‐19. The safety and effectiveness of XYP on severe and critical COVID‐19 patients require further investigation.
5. CONCLUSIONS
In summary, XYP injection is a safe and effective treatment in resolving the clinical symptoms and improving the recovery in patients with mild to moderate COVID‐19 patients. Further double‐blinded placebo‐controlled clinical trials are needed to evaluate the efficacy of XYP in more COVID‐19 patients at different disease stages.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Xiao‐Qun Ye, Xin‐Yi Zhang and Lang Lv: Designed the experiment. Xiao‐Qun Ye, Xin‐Yi Zhang, Lang Lv, Yu‐Long Zhou and Liang‐Dong Xie: Collected the data. Qin Xu, Xiao‐Fan Zou, Yan Ding, Jie Tian, Jia‐Liang Fan, Hai‐Wei Fan and Yi‐Xi Yang: Contributed to data analysis. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
ETHICS STATEMENT
The study on human participants was approved by the institutional review board or ethics committee at each participating site. Written informed consent was obtained from all the patients.
Supporting information
Figure S1. The dynamics of C‐reactive protein in treatment group (red line) and control group (blue line).
Table S1. The main ingredients of Andrographis paniculate and Xiyanping.
Table S2. All adverse events.
ACKNOWLEDGEMENTS
The authors would like to thank all the patients participating in this study. The authors also extend their gratitude to those who helped us in every way possible in this study. The study was funded by the Key Projects of Jiangxi Province (2020YBBGW0008).
Zhang X‐Y, Lv L, Zhou Y‐L, et al. Efficacy and safety of Xiyanping injection in the treatment of COVID‐19: A multicenter, prospective, open‐label and randomized controlled trial. Phytotherapy Research. 2021;35:4401–4410. 10.1002/ptr.7141
Xin‐Yi Zhang, Lang Lv, Yu‐Long Zhou, Liang‐Dong Xie, Qin Xu, Xiao‐Fan Zou and Yan Ding contributed equally to this work.
Funding information The Key Projects of Jiangxi Province, Grant/Award Number: 2020YBBGW0008
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Boulware, D. R. , Pullen, M. F. , Bangdiwala, A. S. , Pastick, K. A. , Lofgren, S. M. , Okafor, E. C. , … Hullsiek, K. H. (2020). A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid‐19. The New England Journal of Medicine, 383(6), 517–525. 10.1056/NEJMoa2016638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, B. , Wang, Y. , Wen, D. , Liu, W. , Wang, J. , Fan, G. , … Wang, C. (2020). A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. The New England Journal of Medicine, 382(19), 1787–1799. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramohan, V. , Kaphle, A. , Chekuri, M. , Gangarudraiah, S. , & Bychapur Siddaiah, G. (2015). Evaluating andrographolide as a potent inhibitor of NS3‐4A protease and its drug‐resistant mutants using in silico approaches. Advances in Virology, 2015, 972067. 10.1155/2015/972067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, R. S. , Ding, L. , Chen, G. Q. , Pan, Q. C. , Zhao, Z. L. , & Smith, K. M. (1991). Dehydroandrographolide succinic acid monoester as an inhibitor against the human immunodeficiency virus. Proceedings of the Society for Experimental Biology and Medicine, 197(1), 59–66. 10.3181/00379727-197-43225 [DOI] [PubMed] [Google Scholar]
- Chong, L. , Chen, W. , Luo, Y. , & Jiang, Z. (2013). Simultaneous determination of 9‐dehydro‐17‐hydro‐andrographolide and sodium 9‐dehydro‐17‐hydro‐andrographolide‐19‐yl sulfate in rat plasma by UHPLC‐ESI‐MS/MS after administration of xiyanping injection: Application to a pharmacokinetic study. Biomedical Chromatography, 27(7), 825–830. 10.1002/bmc.2866 [DOI] [PubMed] [Google Scholar]
- Enmozhi, S. K. , Raja, K. , Sebastine, I. , & Joseph, J. (2020). Andrographolide as a potential inhibitor of SARS‐CoV‐2 main protease: An in silico approach. Journal of Biomolecular Structure & Dynamics, 1–7. 10.1080/07391102.2020.1760136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, W. , Liu, W. , Chen, G. , Hong, S. , Qian, C. , Xie, N. , … Xu, Q. (2012). Water‐soluble andrographolide sulfonate exerts anti‐sepsis action in mice through down‐regulating p38 MAPK, STAT3 and NF‐κB pathways. International Immunopharmacology, 14(4), 613–619. 10.1016/j.intimp.2012.09.002 [DOI] [PubMed] [Google Scholar]
- Gupta, S. , Mishra, K. P. , & Ganju, L. (2017). Broad‐spectrum antiviral properties of andrographolide. Archives of Virology, 162(3), 611–623. 10.1007/s00705-016-3166-3 [DOI] [PubMed] [Google Scholar]
- Hu, K. , Guan, W. J. , Bi, Y. , Zhang, W. , Li, L. , Zhang, B. , … Zhong, N. S. (2021). Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine, 85, 153242. 10.1016/j.phymed.2020.153242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. F. , Bai, C. , He, F. , Xie, Y. , & Zhou, H. (2020). Review on the potential action mechanisms of Chinese medicines in treating Coronavirus Disease 2019 (COVID‐19). Pharmacological Research, 158, 104939. 10.1016/j.phrs.2020.104939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. , Yang, X. , Guan, C. , Wen, T. , Duan, Y. , Zhang, W. , … Liu, S. (2018). Andrographolide sulfonate reduces mortality in Enterovirus 71 infected mice by modulating immunity. International Immunopharmacology, 55, 142–150. 10.1016/j.intimp.2017.11.042 [DOI] [PubMed] [Google Scholar]
- Li, Q. , Li, Z. Y. , Zhang, J. , Guo, W. N. , Xu, X. M. , Sun, F. X. , & Xu, H. (2019). Xiyanping plus azithromycin chemotherapy in pediatric patients with mycoplasma pneumoniae pneumonia: A systematic review and meta‐analysis of efficacy and safety. Evidence‐based Complementary and Alternative Medicine, 2019, 2346583. 10.1155/2019/2346583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , He, S. , Tang, J. , Ding, N. , Chu, X. , Cheng, L. , … Wu, J. (2017). Andrographolide inhibits inflammatory cytokines secretion in LPS‐stimulated RAW264.7 cells through suppression of NF‐κB/MAPK signaling pathway. Evidence‐based Complementary and Alternative Medicine, 2017, 8248142. 10.1155/2017/8248142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, T. P. , Chen, S. Y. , Duh, P. D. , Chang, L. K. , & Liu, Y. N. (2008). Inhibition of the epstein‐barr virus lytic cycle by andrographolide. Biological & Pharmaceutical Bulletin, 31(11), 2018–2023. 10.1248/bpb.31.2018 [DOI] [PubMed] [Google Scholar]
- Peng, G. Y. , Zhou, F. , Ding, R. L. , Li, H. D. , & Yao, K. (2002). Modulation of lianbizi injection (andrographolide) on some immune functions]. Zhongguo Zhong Yao Za Zhi, 27(2), 147–150. [PubMed] [Google Scholar]
- Raja, K. , Prabahar, A. , Selvakumar, S. , & Raja, T. K. (2014). In silico analysis to compare the effectiveness of assorted drugs prescribed for swine flu in diverse medicine systems. Indian Journal of Pharmaceutical Sciences, 76(1), 10–18. [PMC free article] [PubMed] [Google Scholar]
- Reddy, V. L. , Reddy, S. M. , Ravikanth, V. , Krishnaiah, P. , Goud, T. V. , Rao, T. P. , … Venkateswarlu, Y. (2005). A new bis‐andrographolide ether from Andrographis paniculata nees and evaluation of anti‐HIV activity. Natural Product Research, 19(3), 223–230. 10.1080/14786410410001709197 [DOI] [PubMed] [Google Scholar]
- Seubsasana, S. , Pientong, C. , Ekalaksananan, T. , Thongchai, S. , & Aromdee, C. (2011). A potential andrographolide analogue against the replication of herpes simplex virus type 1 in vero cells. Medicinal Chemistry, 7(3), 237–244. 10.2174/157340611795564268 [DOI] [PubMed] [Google Scholar]
- Sheeja, K. , & Kuttan, G. (2007a). Activation of cytotoxic T lymphocyte responses and attenuation of tumor growth in vivo by Andrographis paniculata extract and andrographolide. Immunopharmacology and Immunotoxicology, 29(1), 81–93. 10.1080/08923970701282726 [DOI] [PubMed] [Google Scholar]
- Sheeja, K. , & Kuttan, G. (2007b). Modulation of natural killer cell activity, antibody‐dependent cellular cytotoxicity, and antibody‐dependent complement‐mediated cytotoxicity by andrographolide in normal and Ehrlich ascites carcinoma‐bearing mice. Integrative Cancer Therapies, 6(1), 66–73. 10.1177/1534735406298975 [DOI] [PubMed] [Google Scholar]
- Shi, H. , Guo, W. , Zhu, H. , Li, M. , Ung, C. O. L. , Hu, H. , & Han, S. (2019). Cost‐effectiveness analysis of Xiyanping injection (andrographolide sulfonate) for treatment of adult community acquired pneumonia: A retrospective, propensity score‐matched cohort study. Evidence‐Based Complementary and Alternative Medicine, 2019, 4510591. 10.1155/2019/4510591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W. , Cao, Z. , Han, M. , Wang, Z. , Chen, J. , Sun, W. , … Xie, Q. (2020). Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: Open label, randomised controlled trial. BMJ, 369, m1849. 10.1136/bmj.m1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. F. , & Xie, Y. M. (2012). Adverse event case reports for Xiyanping injection based on literature]. Zhongguo Zhong Yao Za Zhi, 37(18), 2792–2795. 10.4268/cjcmm20121830 [DOI] [PubMed] [Google Scholar]
- Wei, P.‐F. (Ed.). (2020). Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Chin Med J (Engl), 133(9), 1087–1095. 10.1097/CM9.0000000000000819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, N. , Wang, X. , Liu, X. , Chen, J. , Zheng, H. , Huo, Z. , & Jiao, J. (2015). Effect of the combination of Xiyanping and Cefazolin on the function of neutrophils in mice]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi, 32(5), 1079–1082. [PubMed] [Google Scholar]
- Yang, Q. W. , Li, Q. , Zhang, J. , Xu, Q. , Yang, X. , Li, Z. Y. , & Xu, H. (2019). Crystal structure and anti‐inflammatory and anaphylactic effects of andrographlide sulphonate E in Xiyanping, a traditional Chinese medicine injection. The Journal of Pharmacy and Pharmacology, 71(2), 251–259. 10.1111/jphp.13028 [DOI] [PubMed] [Google Scholar]
- Zhang, J. L. , Li, W. X. , Li, Y. , Wong, M. S. , Wang, Y. J. , & Zhang, Y. (2021). Therapeutic options of TCM for organ injuries associated with COVID‐19 and the underlying mechanism. Phytomedicine, 85, 153297. 10.1016/j.phymed.2020.153297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, D. , Shao, J. , Chen, W. , & Luo, Y. (2016). In vitro metabolism of sodium 9‐dehydro‐17‐hydro‐andrographolide‐19‐yl sulfate in rat liver S9 by liquid chromatography‐mass spectrometry method. Pharmacognosy Magazine, 12(Suppl 2), S102–S108. 10.4103/0973-1296.182194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang, W. , Fan, Z. , Chu, Y. , Wang, H. , Yang, Y. , Wu, L. , … Xi, S. (2020). Chinese patent medicines in the treatment of Coronavirus Disease 2019 (COVID‐19) in China. Frontiers in Pharmacology, 11, 1066. 10.3389/fphar.2020.01066 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The dynamics of C‐reactive protein in treatment group (red line) and control group (blue line).
Table S1. The main ingredients of Andrographis paniculate and Xiyanping.
Table S2. All adverse events.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


