Dear Editor,
The novel Coronavirus SARS‐CoV‐2 is suspected of acting as a trigger for autoimmune diseases and the production of autoantibodies. Retinoic acid‐inducible gene (RIG)‐I‐like receptors (RLR), including melanoma differentiation‐associated protein 5 (MDA5) and RIG‐I, recognize the double‐strand (ds) virus RNA and induce the production of Type I interferon (Type I IFN) as well as pro‐inflammatory cytokines like Interleukin (IL)‐6 1 (Fig. 1). High IL‐6 levels are associated with the induction of pro‐inflammatory cytokines (“cytokine storm”) and development of respiratory failure. 2 On the other hand, chronically high levels of Type I IFN are related to several autoimmune diseases such as systemic Lupus erythematodes (SLE), Sjögren Syndrom, systemic sclerosis, inflammatory myopathies and rheumatoid arthritis (RA). In terms of clinical presentation and biomarkers, many similarities can be found between Covid‐19 and anti‐MDA5 positive dermatomyositis. 2 Moreover, allelic variations within IFN‐pathway‐genes can be found in those autoimmune diseases. 3 The first cases of new autoimmune phenomena related to Covid‐19 were found with some delay after the outbreak of the pandemic. With a new awareness for possible induction of autoimmune‐mediated phenomena associated with SARS‐CoV‐2 infection, the topic gained attention. In recent years, the potential role of viruses in the pathogenesis of autoimmune diseases, e.g. Epstein‐Barr‐Virus, has been published. 4 There have also been reports of post‐vaccination onset of autoimmune diseases, most recently following SARS‐CoV2 vaccination. 5 Therefore, it stands to reason to consider SARS‐CoV‐2 as a trigger for autoimmune phenomena. We performed a meta‐analysis of recently published articles on autoimmune phenomena associated with concomitant SARS‐CoV‐2 infection. 6 , 7 , 8 , 9 , 10 Table 1 shows reported autoantibodies, increased levels of IL‐6 as well as frequently reported clinical symptoms.
Figure 1.
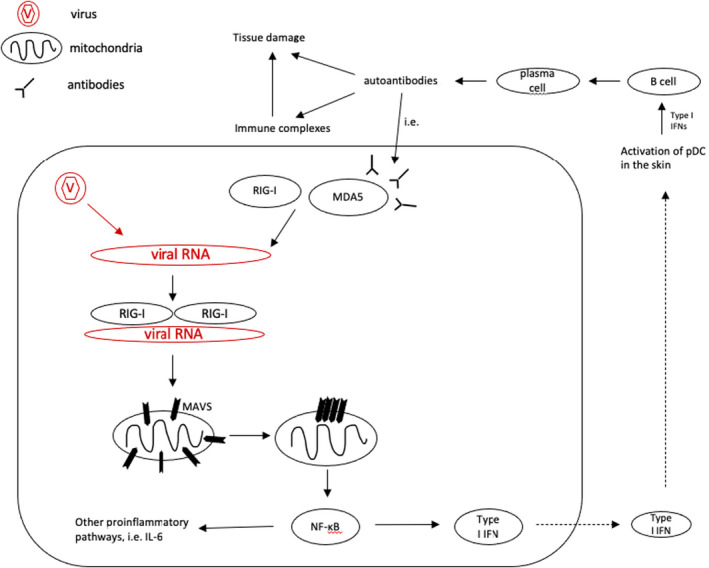
Retinoic acid‐inducible gene (RIG)‐I‐like receptors (RLR), including melanoma differentiation‐associated protein 5 (MDA5) and RIG‐I, recognize the double‐strand (ds) virus RNA and induce the production of Type I interferon (Type I IFN) and pro‐inflammatory cytokines, 1 which are associated with autoimmune diseases, such as systemic Lupus erythematodes (SLE) and Dermatomyositis. After binding to the viral dsRNA, N‐terminal caspase activation and recruitment domains (CARDs) of RLR interact with mitochondrial antiviral‐signalling protein (MAVS) and eventually, prion‐like aggregates are formed. These aggregates activate transcription factor NF‐ĸB, which in turn stimulates the production of Type I IFN, interleukin‐6 (IL‐6) and further pro‐inflammatory cytokines. 15 Activation of plasmacytoid dendritic cells (pDC) follows Type I IFN‐mediated activation of B cells which can lead to autoantibody production, e.g, anti‐MDA5 antibodies.
Table 1.
| Number of patients | |
|---|---|
| Autoantibodies | 108 |
| Lupus anticoagulant | 75 |
| Anti‐nuclear antibodies | 10 |
| Anti‐erythrocyte antibodies | 7 |
| anti‐60 kDa SSA/Ro antibodies | 5 |
| anti‐52 kDa SSA/Ro antibodies | 4 |
| Anti‐cardiolipin IgA + anti‐β2‐glycoprotein I IgA und IgG | 4 |
| anti‐GD1b‐IgG | 2 |
| Anti‐ADAMTS‐13 antibodies | 1 |
| Other laboratory findings | 51 |
| IL6 ↑ | 51 |
| Clinical symptoms | 143 |
| Chilblain‐like lesions | 43 |
| Pulmonary embolism | 25 |
| Stroke | 11 |
| Exanthema | 4 |
| Thrombosis of the extremities | 3 |
| Coagulopathy | 3 |
| Chickenpox‐like vesicles | 2 |
| Eruptive cherry angioma | 1 |
Several authors reported an increased frequency of at least nine autoantibodies in patients with Covid‐19, with Lupus Anticoagulant (LA) being the most common (75 out of 107 patients).
LA is associated with prolonged activated partial thromboplastin time (aPTT), arterial or venous thrombosis, and in consequence cardiovascular events. Besides LA, anticardiolipin‐ and anti‐β2‐glycoprotein‐I antibodies are numbered among the group of antiphospholipid antibodies and were found in three more cases. Congruent to these findings, Covid‐19 patients often showed clinical signs of coagulopathies such as hypercoagulation and thromboembolic events including pulmonary embolism and stroke. 11 , 12 Microangiopathic changes were represented by chilblain‐like skin lesions and eruptive cherry‐angioma. 13 Kolivars et al. hypothesized that chilblain‐like lesions and microangiopathic changes are due to immunologic reactions to the viral infection. In this case, the Type I IFN response most likely happens to be early and strong in young patients resulting in microangiopathy and chilblains, overall with a short and indolent course of the infection, whereas older patients react late and inadequately to Type I IFN, which results in hypercytokinemia, hypercoagulation, and thus with an increased morbidity and mortality. 14
A potential reason for the significantly lower rate of six out of nine mentioned autoantibodies could be their delayed presence compared to LA and anticardiolipin IgA antibodies. Furthermore, severe and acute coagulopathies need rapid investigation, due to their ability to evoke an acute life‐threatening situation. Therefore, most hospitals have implemented diagnostic algorithms. In contrast, autoantibody‐screenings are not part of these routine work‐ups. They are time consuming and are usually done posthoc. Additionally, in most cases patients’ basal autoantibody levels are not available, making it difficult to give a clear statement regarding the coherence of autoimmune phenomena and antibodies with a SARS‐CoV‐2 infection. In our opinion, a correlation between a SARS‐CoV‐2 infection and autoimmune phenomena is likely, and we propose to consider autoantibody screenings more often in diagnostic procedures, keeping autoimmune phenomena as a differential diagnosis in mind. Further studies are needed for a more founded statement on/better understanding of the coherence of the appearance of autoantibodies following SARS‐CoV‐2 infection.
Conflicts of interest
JB declares to have no conflict of interest. SV declares to have no conflict of interest.
Funding source
No funding sources to declare.
References
- 1. Theofilopoulos AN, Kono DH, Baccala R. The multiple pathways to autoimmunity. Nat Immunol 2017; 18: 716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Lorenzis E, Natalello G, Gigante L, Verardi L, Bosello SL, Gremese E. What can we learn from rapidly progressive interstitial lung disease related to anti‐MDA5 dermatomyositis in the management of COVID‐19? Autoimmun Rev 2020; 19: 102666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol 2014; 14: 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steed AL, Stappenbeck TS. Role of viruses and bacteria‐virus interactions in autoimmunity. Curr Opin Immunol 2014; 31: 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gambichler T, Scholl L, Dickel H, Ocker L, Stranzenbach R. Prompt onset of Rowell's syndrome following the first BNT162b2 SARS‐CoV‐2 vaccination. J Eur Acad Dermatol Venereol 2021; 35: e415–e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ehrenfeld M, Tincani A, Andreoli L et al. Covid‐19 and autoimmunity. Autoimmun Rev 2020; 19: 102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lazarian G, Quinquenel A, Bellal M et al. Autoimmune haemolytic anaemia associated with COVID‐19 infection. Br J Haematol 2020; 190: 29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albiol N, Awol R, Martino R. Autoimmune thrombotic thrombocytopenic purpura (TTP) associated with COVID‐19. Ann Hematol 2020; 99: 1673–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou Y, Han T, Chen J et al. Clinical and autoimmune characteristics of severe and critical cases of COVID‐19. Clin Transl Sci 2020; 13: 1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dalakas MC. Guillain‐Barré syndrome: The first documented COVID‐19–triggered autoimmune neurologic disease. More to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm 2020; 7: e781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klok FA, Kruip M, van der Meer NJM et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res 2020; 191: 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oxley TJ, Mocco J, Majidi S et al. Large‐vessel stroke as a presenting feature of Covid‐19 in the young. N Engl J Med 2020; 382: e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bouaziz J, Duong T, Jachiet M et al. Vascular skin symptoms in COVID‐19: a french observational study. J Eur Acad Dermatol Venereol 2020; 34: e451–e452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kolivras A, Dehavay F, Delplace D et al. Coronavirus (COVID‐19) infection‐induced chilblains: A case report with histopathologic findings. JAAD Case Rep 2020; 6: 489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hou F, Sun L, Zheng H, Skaug B, Jiang Q‐X, Chen ZJ. MAVS forms functional prion‐like aggregates to activate and propagate antiviral innate immune response. Cell 2011; 146: 448–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen N, Zhou M, Dong X et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]


