CONFLICT OF INTEREST
No conflict of interest.
AUTHOR CONTRIBUTIONS
All authors have read and approved the final manuscript. P.A and K.S. wrote the paper. S.R.M provided the clinical data. P.M analyzed the data (histopathological image).
ETHICAL STATEMENT
Informed consent was taken from the patient for publication of information.
To the Editor,
While the emergent focus worldwide has shifted to vaccines for achieving herd immunity to COVID‐19, the adverse effects of these vaccines are important to recognize as it impacts their widespread acceptance.
In January 2021, the Central Drugs Standard Control Organization (CDSCO) in India granted emergency use authorization to two vaccines Covishield® (AstraZeneca's vaccine manufactured by Serum Institute of India) and Covaxin® (manufactured by Bharat Biotech Limited). Covishield or AZD‐1222 uses a weakened strain of adenovirus as vector, which contains genetic material similar to that of SARS‐COV‐2 and upon administration, stimulates the production of antibodies against the spike protein whereas Covaxin is made using an inactivated virus to mount an immune response. 1 Composition of Covaxin includes inactivated coronavirus, aluminum hydroxide gel, TLR 7/8 agonist, 2‐phenoxyethanol, and phosphate‐buffered saline. 1 Adverse effects that have been reported with Covaxin include injection site pain and swelling, fever, malaise, headache, nausea, vomiting, and skin rashes. 2 Serious allergic reactions have been rarely reported. In this article, we report a case of reactivation of varicella‐zoster virus (VZV) infection in an elderly patient who received the inactivated viral vaccine for COVID‐19.
A 60‐year‐old male patient, a known case of type II diabetes mellitus and hypertension, presented with fluid‐filled lesions over the thigh of 5‐day duration and had received inactivated vaccine for COVID‐19 (COVAXIN) 4 days prior to onset of lesions. There was no history suggestive of any other infection, emotional or physical stress, immunosuppression, radiation, or sun exposure. Patient denied any history of systemic symptoms. On examination, there were multiple grouped vesicles on an erythematous base, present over the knee, and anterior aspect of right thigh (Figure 1), and a Tzanck smear from an intact vesicle showed acantholytic cells. No multinucleated giant cells could be seen. Skin biopsy from the vesicle showed intraepidermal spongiotic vesicle containing acantholytic cells with large vesicular nuclei neutrophils and dyskeratotic cells (Figure 2A). Occasional multinucleate cell with ground‐glass chromatin and molded nuclei could be seen within the blister (Figure 2B).
FIGURE 1.
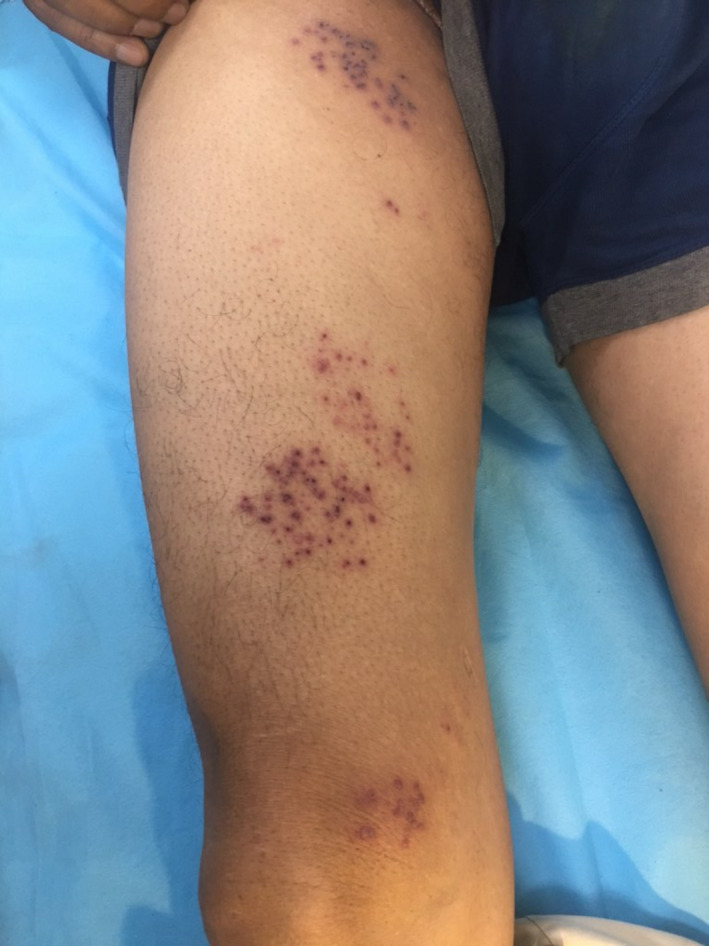
Multiple grouped vesicles on an erythematous base seen in dermatome L2‐3
FIGURE 2.
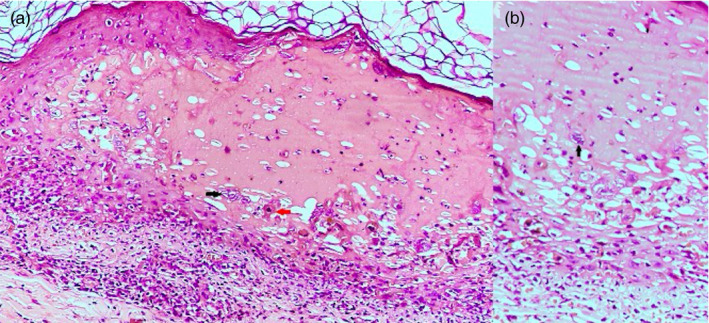
(A): Intraepidermal spongiotic vesicle containing acantholytic cells with large vesicular nuclei (black arrow), neutrophils, and dyskeratotic cells (red arrow). (H&E ×200). (B): Occasional multinucleate cell with ground‐glass chromatin and molded nuclei within the blister (H&E ×400)
A diagnosis of herpes zoster involving L2 and L3 dermatome was made and the patient was started on oral valacyclovir 1g three times a day for 7 days along with topical fusidic acid for local application twice daily. The lesions subsided in 2 weeks without any sequelae or neuralgia. The patient received second dose of the same vaccine after an interval of 4 weeks without any adverse effects.
The varicella‐zoster virus (VZV) becomes latent in neurons of dorsal root ganglion, cranial nerve ganglion, and autonomic ganglia after an episode of chicken pox or its vaccination. Certain latency‐related genes are induced during this period that cause the virus to lie dormant. However, this latency depends on endogenous and exogenous stimuli that can turn off these latency‐related genes and reactivate those that lead to viral replication. 3 Various factors that can cause reactivation of the VZV include increasing age, physical or emotional stress, immunosuppression, ultraviolet exposure, and tissue damage. Cellular immunity forms the main line of defense against VZV reactivation and a decrease in the cellular immune response due to the above factors can explain the viral reactivation.
Herpes zoster following inactivated vaccine for COVID‐19 has been reported by Bostan et al from Turkey in a 78‐year‐old male patient. 4 This was preceded by 3 cases of herpes virus reactivation after vaccination for influenza (trivalent influenza split vaccine), hepatitis A (formaldehyde inactivated), and rabies with Japanese encephalitis vaccines. 5
This highlights the fact that vaccine‐induced immunomodulation, especially T‐cell dysregulation, determines the reactivation of VZV. Increasing age (>60 years) is also one of the factors that could contribute to the development of zoster. This can occur as VZV‐CMI response progressively decreases with age (2.7%–3.9% decline each year). 6 Thus, the combination of age and vaccination could be the plausible explanation for reactivation of VZV in our patient.
While systemic adverse effects are important, cutaneous adverse effects are the immediate reason that can dissuade vaccine administration and we feel such cases should be documented to assess the overall incidence of such effects and assuage the fear that is prevalent and leads to vaccine hesitancy.
FUNDING INFORMATION
No sources of funding.
REFERENCES
- 1. COVID‐19 Vaccine FAQs. Available from https://www.mohfw.gov.in/covid_vaccination/vaccination/faqs.html#about‐the‐vaccine (Last accessed 2021 April 12)
- 2. COVID‐19:Common side effects. Available from: https://www.mohfw.gov.in/covid_vaccination/vaccination/common‐side‐effects‐aefi.html (Last accessed 2021 April 12)
- 3. Freer G, Pistello M. Varicella‐zoster virus infection: natural history, clinical manifestations, immunity and current and future vaccination strategies. New Microbiol. 2018;41:95‐105. [PubMed] [Google Scholar]
- 4. Bostan E, Yalici‐Armagan B. Herpes zoster following inactivated COVID‐19 vaccine: A coexistence or coincidence? J Cosmet Dermatol. 2021;20(6):1566‐1567. [DOI] [PubMed] [Google Scholar]
- 5. Walter R, Hartmann K, Fleisch F, Reinhart WH, Kuhn M. Reactivation of herp herpesvirus infections after vaccinations? Lancet. 1999;353(9155):810. [DOI] [PubMed] [Google Scholar]
- 6. Levin MJ, Oxman MN, Zhang JH, et al. Varicella‐zoster virus‐specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]


