Dear Editor,
We report the first registered cases of cutaneous adverse reactions in Northeast Italy after the m‐RNA COVID‐19 vaccine Comirnaty®‐BioNTech/Pfizer (BioNTech Innovative Manufacturing Services GmbH, Idar‐Oberstein, Germany).
During January 2021, in the public health jurisdiction of Trieste, a total of 19 485 individuals have been vaccinated: 13 266 (68.08%) first doses and 6219 (31.92%) completed cycles of two doses. In this population, 266 (1.36%) adverse reactions have been reported to the Pharmacovigilance Service. Notably, one or more cutaneous adverse effects were present in 44 people, accounting for 0.22% of all vaccinated individuals and 16.54% of communicated adverse effects. The reactions included both those at the injection site and more extensive manifestations (Table 1).
Table 1.
Summary of the total cutaneous adverse reactions reported to the Pharmacovigilance Service of Trieste (patients nr.1–44), including those evaluated at the Dermatology Clinic of Trieste (patients nr.38–44) and in other clinics of the Friuli Venezia‐Giulia region, in Northeast Italy (patients 45–46)
| Patient | Sex, Age | Cutaneous adverse reaction, timing of onset (if specified) | First or Second dose |
|---|---|---|---|
| 1 | F, 55 | Urticarial rash limited to the upper limbs | First |
| 2 | F, 27 | Urticarial rash limited to the neck and chest | Not reported |
| 3 | F, 64 | Itchy erythema of the neck and hands | First |
| 4 | M, 38 | Itching at the inoculation site | First |
| 5 | F, 49 | Erythema at the inoculation site | First |
| 6 | F, 23 | Urticarial rash limited to the chest | First |
| 7 | F, 49 | Itchy erythema of the palms | First |
| 8 | F, 32 | Itchy dermatitis of the face with fever | First |
| 9 | F, 34 | Generalized itching | First |
| 10 | F, 37 | Morbilliform eruption | Not reported |
| 11 | F, 43 | Painful and itchy erythematous subcutaneous nodule at the inoculation site, 3 days after the dose | First |
| 12 | F, 50 | Erythema of the chest and hands | First |
| 13 | F, 34 | Urticarial rash limited to the upper limbs | First |
| 14 | F, 53 | Painful hardening of the skin at the inoculation site with fever | Second |
| 15 | F, 65 | Swelling of the face, 18 h after the dose | First |
| 16 | F, 51 | Itchy arm with axillary lymphadenopathy, 24 h after the dose | First |
| 17 | F, 57 | Painful swelling and erythema of the right eyelid, 7 days after the dose | First |
| 18 | F, 52 | Generalized itching | First |
| 19 | F, 46 | Burning wheal at the inoculation site | First |
| 20 | F, 63 | Itchy wheal at the inoculation site | First |
| 21 | M, 28 | Itchy swelling at the inoculation site | First |
| 22 | F, 56 | Erythematous and itchy hardening of the skin at the inoculation site with fever | Second |
| 23 | F, 34 | Herpes Zoster of the scalp | First |
| 24 | F, 46 | Erythema at the inoculation site, 1 day after the dose | First |
| 25 | F, 46 | Urticarial rash limited to the chest and lower limbs | First |
| 26 | F, 37 | Cutaneous rash of the trunk | Second |
| 27 | F, 46 | Generalized itching | First |
| 28 | F, 65 | Nodule following itchy erythema at the inoculation site | First |
| 29 | F, 37 | Painful wheal at the inoculation site | First |
| 30 | F, 55 | Erythema at the inoculation site with fever | Second |
| 31 | F, 37 | Swelling of the eyelids and face with mandibular lymphadenopathy | First |
| 32 | M, 70 | Erythema at the inoculation site | First |
| 33 | F, 26 | Painful swelling at the inoculation site | First |
| 34 | F, 39 | Wheal at the inoculation site with axillary lymphadenopathy | First |
| 35 | F, 36 | Itchy erythema of the abdomen, 1 week after the dose | First |
| 36 | F, 35 | Swelling at the inoculation site with fever | Second |
| 37 | F, 48 | Herpes Zoster | First |
|
38 Fig. 1a–b |
F, 54 | Diffuse urticaria, 5 days after the dose | First |
|
39 Fig. 1e |
F, 41 | Erythematous macular rash of the hands, 8 days after the dose | First |
|
40 Fig. 1f |
F, 44 | Purplish macule on the third finger of one hand (fixed drug eruption), 10 days after the dose | Second |
|
41 Fig. 1g |
F, 42 | Pityriasis rosea‐like rash on the thighs and abdomen, 4 days after the dose | Second |
| 42 | M, 64 | Pityriasis rosea‐like rash on the neck, upper limbs, and trunk, 5 days after the dose | First |
| 43 | M, 18 | Diffuse urticaria, 60 h after the dose | First |
| 44 | F, 55 |
Malar erythema, 12 h after the first dose; erythema of the face, trunk and thighs with fever, 3 days after the second dose |
First and second |
|
45 Fig. 1c |
F, 34 | Diffuse urticaria; transient periorbital and perioral swelling, 4 days after the dose | First |
|
46 Fig. 1d |
F, 27 | Chilblain‐like rash on the first and third finger of one foot accompanied by urticarial rash, 4 days after the first dose; urticarial rash, 1 day after the second dose | First and second |
Data were collected during the vaccination campaign with Comirnaty®‐BioNTech/Pfizer (m‐RNA COVID‐19 vaccine) in January 2021.
Nine subjects were evaluated in a Dermatology Clinic of the Friuli Venezia‐Giulia region, in North‐East Italy (Table 1, patients nr. 38–46).
Three of them presented with a diffuse urticaria (Fig. 1a–c), with onset of 60 h to 5 days after the first dose. For these three subjects, advise for not applying the second dose was given. Interestingly, one more patient developed an urticarial rash limited to the chest, together with chilblain‐like manifestations on the first and third finger of one foot (Fig. 1d). The other cutaneous reactions observed in the remaining five patients were as follows: a malar erythema, an erythematous macular rash of the hands (Fig. 1e), a fixed drug eruption (FDE, Fig. 1f) and two cases resembling pityriasis rosea (Fig. 1g). Even if the first two reactions are not easy to interpret from a pathogenetic point of view, all five patients completed the two‐dose vaccine cycle.
Figure 1.
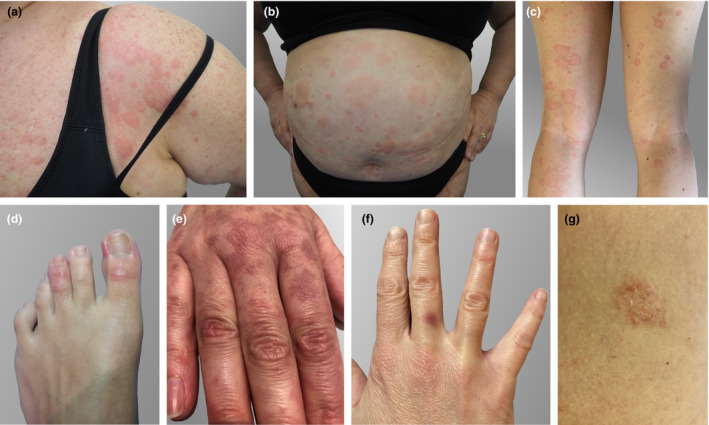
Cutaneous adverse reactions after administration of Comirnaty®‐BioNTech/Pfizer, an m‐RNA COVID‐19 vaccine. (a–c) Diffuse urticaria. (d) Chilblain‐like rash on the first and third finger of the left foot. (e) Erythematous macular rash of the hands. (f) Purplish macule on the third finger of the right hand, consistent with the diagnosis of fixed drug eruption. (g) Pityriasis rosea‐like plaque (located on abdomen).
The outlined manifestations are quite heterogeneous and occur within a time frame of 60 h to 10 days after injection. Their course was mostly mild and self‐limiting. Only one patient with urticaria (nr. 45) required intravenous steroid treatment.
With regard to the urticarial manifestations, Polyethylene glycol‐2000 (PEG‐2000), an excipient of the vaccine, may play a role. In fact, PEG contained in several drugs can produce immediate hypersensitivity reactions 1 and cases of urticaria have been reported. 2 Moreover, PEG is investigated as possible responsible for the rare cases of anaphylaxis induced by BioNTech/Pfizer vaccine. 3 , 4
The question of completing the vaccination cycle, after urticaria triggered by the first dose, remains open. We preferred to avoid exposure to the second dose, because it is currently unclear whether it should be regarded as a risk factor for anaphylaxis, although the timing of onset after exposure is not consistent with a type I Ig‐E mediated reaction. Regarding the other observed reactions, it must be admitted that FDE represents a stereotypic reaction induced by drugs, but it is rarely induced by vaccines. 5 Conversely, pityriasis rosea has been reported after vaccinations or drug use. 6 , 7 It appears remarkable that urticarial, pityriasis rosea‐like rashes or chilblain‐like changes have been frequently reported during or after COVID‐19. 8 , 9 , 10 For two patients (nr. 45–46), a swab and a serological test for SARS‐CoV‐2 resulted negative. For the remaining cases, we cannot exclude with certainty a contagion with SARS‐CoV‐2 shortly before or after the vaccine injection.
In conclusion, cutaneous adverse reactions triggered by Comirnaty®‐BioNTech/Pfizer are seldom but appear similar to those reported during SARS‐CoV‐2 infections. Limitations of this study include the use of self‐reported data. However, the reporting subjects were largely healthcare workers, and therefore, the reliability of data can be considered high. In addition, all the reports show a temporal relation with the vaccine, but this does not allow us to conclude that a true causal link exists.
The exact biological mechanisms underlying cutaneous effects after this m‐RNA COVID‐19 vaccine have still to be elucidated, and further studies based on larger cohorts are needed to better understand them.
Funding sources
None.
Conflict of interest
The authors have no conflict of interest.
Acknowledgements
The persons in this manuscript have given written informed consent to publication of their case details.
References
- 1. Cox F, Khalib K, Conlon N. PEG that reaction: a case series of allergy to polyethylene glycol. J Clin Pharmacol 2021; 61: 832–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gökay SS, Çelik T, Sari MY et al. Urticaria as a rare side effect of polyethylene glycol‐3350 in a child: case report. Acta Clin Croat 2018; 57: 187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garvey LH, Nasser S. Anaphylaxis to the first COVID‐19 vaccine: is polyethylene glycol (PEG) the culprit? Br J Anaesth 2021; 126: e106–e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blumenthal KG, Robinson LB, Camargo CA et al. Acute allergic reactions to mRNA COVID‐19 vaccines. JAMA 2021; 325: 1562–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sako EY, Rubin A, Young LC. Localized bullous fixed drug eruption following yellow fever vaccine. J Am Acad Dermatol 2014; 70: e113–e114. [DOI] [PubMed] [Google Scholar]
- 6. Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea‐like eruptions: how to distinguish them? JAAD Case Rep 2018; 4: 800–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atzori L, Pinna AL, Ferreli C, Aste N. Pityriasis rosea‐like adverse reaction: review of the literature and experience of an Italian drug‐surveillance center. Dermatol Online J 2006; 12: 1. [PubMed] [Google Scholar]
- 8. Zhao Q, Fang X, Pang Z et al. COVID‐19 and cutaneous manifestations: a systematic review. J Eur Acad Dermatol Venereol 2020; 34: 2505–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Visconti A, Bataille V, Rossi N et al. Diagnostic value of cutaneous manifestation of SARS‐CoV‐2 infection. Br J Dermatol 2021.184: 880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Masson A, Bouaziz J‐D, Sulimovic L et al. Chilblains is a common cutaneous finding during the COVID‐19 pandemic: A retrospective nationwide study from France. J Am Acad Dermatol 2020; 83: 667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]


