Abstract
Background
COVID‐19 convalescent plasma (CCP) is plasma collected from individuals who have recovered from SARS‐CoV‐2 infection. The FDA Emergency Use Authorization restricts use of CCP to high‐titer units only. The purpose of this study was to determine if donor ABO blood group was associated with SARS‐CoV‐2 antibody response, and subsequent qualification as high‐titer CCP.
Methods
All CCP donations collected from April 21, 2020 to September 1, 2020 were included. The Abbott ARCHITECT semi‐quantitative chemiluminescent microparticle immunoassay was used to assess IgG antibodies to the nucleocapsid protein of SARS‐CoV‐2. Units with a S/C value ≥4.5 were considered high titer.
Results
A total of 232 CCP donations were evaluated. There were no significant differences in the distribution of sex, age, and interval from symptom resolution to donation by ABO blood group. The mean SARS‐CoV‐2 IgG antibody S/C value was significantly lower in blood group O donations (3.6), compared to blood group A (5.0) donations (p < .001). There was no difference in antibody response between the other blood group pairings. Blood group O donations resulted in a lower percentage of high‐titer units (35%), compared to blood group A (60%), B (58%), and AB (65%) donations.
Conclusion
Blood group O donations were found to have significantly lower levels of SARS‐CoV‐2 IgG nucleocapsid antibodies compared to blood group A donations and were less likely to produce CCP units that qualified as high titer. These findings may aid donor recruitment to promote availability of high‐titer CCP to meet patient needs.
Keywords: ABO blood group, antibody titer, Covid‐19 convalescent plasma, SARS‐CoV‐2
Abbreviations
- ANOVA
analysis of variance
- CCP
COVID‐19 convalescent plasma
- EUA
emergency use authorization
- FDA
food and drug administration
- IgG
immunoglobulin G
- RBD
receptor binding domain
- S/C
signal to cutoff ratio
- SD
standard deviation
1. INTRODUCTION
COVID‐19 convalescent plasma (CCP) is plasma collected from individuals who have recovered from SARS‐CoV‐2 infection. In August 2020, the United States Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the use of CCP for the treatment of hospitalized patients with COVID‐19. In February 2021, the EUA was revised to authorize the use of high‐titer CCP only, and included a list of acceptable tests to be used for the purpose of qualifying CCP as high titer. 1 Identifying donor characteristics predictive of antibody response to COVID‐19 may aid in the selection of high‐titer CCP donors.
The purpose of this study was to determine if donor ABO blood group was associated with SARS‐CoV‐2 IgG antibody response and subsequent qualification as high‐titer CCP.
2. MATERIALS AND METHODS
The study was approved by the Institutional Review Board. All CCP donations collected from April 21, 2020 to September 1, 2020 were included in the study. Donors were at least 18 years of age, met routine blood donor eligibility requirements, and had a history of symptomatic COVID‐19 with complete resolution of symptoms at least 28 days before donation. A negative HLA antibody test was required for female donors with a history of pregnancy. Data elements collected at the time of donation included donor age, sex, interval from COVID‐19 symptom resolution to donation, and documentation of a laboratory result confirming prior SARS‐CoV‐2 infection. Donor screening did not include questions regarding donor ethnicity. Following donation, donors were deferred for 2 weeks and permitted to donate CCP a maximum of four times.
A retained sample collected at the time of donation was used to assess IgG antibodies to the nucleocapsid protein of SARS‐CoV‐2 using the Abbott ARCHITECT semi‐quantitative chemiluminescent microparticle immunoassay (Abbott Laboratories, Abbott Park, IL). 2 This test was deemed acceptable by the FDA for use in the manufacture of high‐titer CCP using a signal to cutoff ratio (S/C) ≥ 4.5. 1 Donor ABO typing was performed at Creative Testing Solutions (Phoenix, AZ) using the BECKMAN COULTER PK7200, PK7300, and/or PK7400 Automated Microplate Systems.
A one‐way analysis of variance (ANOVA) was used to compare continuous data stratified by ABO blood group, including donor age, interval from symptom resolution to donation, and antibody S/C value. Post hoc comparisons were performed using the Tukey multiple comparisons test. A chi‐square test was used to compare categorical data stratified by ABO blood group, including donor sex, number of high‐titer donations, and number of repeat donations. Data analysis was performed using Microsoft Excel (2008), GraphPad Prism (8.3.1), and Social Science Statistics (https://www.socscistatistics.com/).
3. RESULTS
A total of 232 CCP donations from 161 donors were evaluated. Donation frequency included a single donation (50%), 2 donations (24%), 3 donations (14%), and 4 donations (12%) within the study period. Overall, 133 (57%) donations were from male donors and mean donor age was 45.1 years (standard deviation [SD] = 14 years). The mean interval from symptom resolution to donation was 73.3 days (SD = 38.2 days). The study population included 88 (38%) blood group O, 103 (44.4%) blood group A, 24 (10.3%) blood group B, and 17 (7.3%) blood group AB donations. The frequency of blood group O in the CCP donor population was lower than what is typically observed in the standard blood donor population (49.9%). Donation characteristics stratified by ABO blood group are presented in Table 1. There were no significant differences in the distribution of donor sex (p = .166), donor age (p = .342), and interval from symptom resolution to donation (p = .770) by ABO blood group. The frequency of repeat donations within the study period did not significantly differ by ABO blood group (p = .543).
TABLE 1.
Donation characteristics stratified by ABO blood group
| O (n = 88) | A (n = 103) | B (n = 24) | AB (n = 17) | |
|---|---|---|---|---|
| Sex | ||||
| Male (%) | 44 (50) | 65 (63) | 12 (50) | 12 (71) |
| Female (%) | 44 (50) | 38 (37) | 12 (50) | 5 (29) |
| Age (mean ± SD) | 45.8 ± 13.2 | 45.7 ± 14.7 | 40.2 ± 14 | 44.9 ± 13.4 |
| Repeat donations (%) | 45 (51) | 53 (51) | 9 (38) | 10 (59) |
| Days from symptom resolution to donation (mean ± SD) | 76.1 ± 44.1 | 70.9 ± 33.9 | 76.1 ± 34.8 | 69.6 ± 37 |
| SARS‐CoV‐2 IgG status | ||||
| High titer a (%) | 31 (35) b | 62 (60) | 14 (58) | 11 (65) |
| Low titer (%) | 57 (65) b | 41 (40) | 10 (42) | 6 (35) |
| Abbott architect S/C (mean ± SD) | 3.6 ± 2.3 b | 5.0 ± 2.4 | 4.9 ± 2.7 | 5.0 ± 2.2 |
Abbreviations: IgG, immunoglobulin G; S/C, signal/cutoff ratio; SD, standard deviation.
High titer = S/C ≥ 4.5.
Significant p ≤ .05.
There was a significant difference between groups when comparing antibody response and ABO blood type as determined by one‐way ANOVA (F[3228] = 6.118, p < .001). Post hoc testing revealed that the mean SARS‐CoV‐2 IgG antibody S/C value was significantly lower in blood group O (3.6) donations compared to blood group A (5.0) donations (p < .001; Figure 1). Although not statistically significant, the mean S/C value for blood group O was also lower than the mean S/C value for blood groups B (4.9, p = .084) and AB (5.0, p = .113). The mean SARS‐CoV‐2 IgG antibody S/C value did not significantly differ between the remaining blood groups (A vs B p = .999, A vs AB p > .999, B vs AB p = .999).
FIGURE 1.
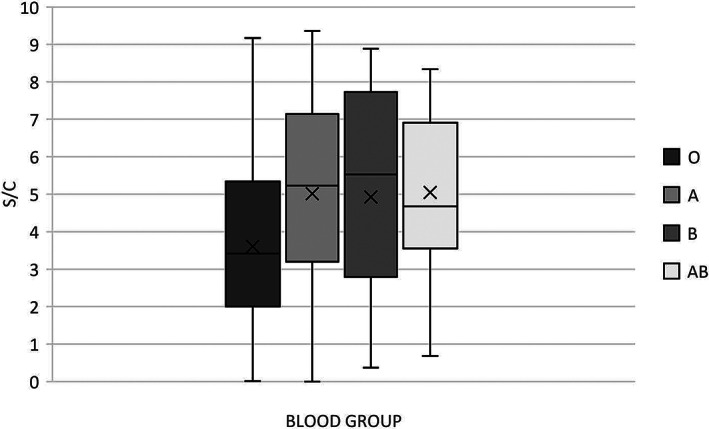
Distribution of SARS‐CoV‐2 IgG S/C value by ABO blood group in CCP donations. Box‐and‐whisker plot depicts the median (horizontal line), mean (X), interquartile ranges, and minimum and maximum values. p‐values using a one‐way ANOVA with a post hoc Tukey multiple comparison test are as follows: O vs A < 0.001, O vs B = 0.084, O vs AB = 0.113, A vs B = 0.999, A vs AB > 0.999, and B vs AB = 0.999
There was a significant relationship between ABO blood group and number of high‐titer donations (p = .003), with blood group O donations having fewer high SARS‐CoV‐2 IgG titer units. The prevalence of high‐titer SARS‐CoV‐2 IgG antibodies (S/C ≥ 4.5) was 35% (31/88) in blood group O donations, 60% (62/103) in blood group A donations, 58% (14/24) in blood group B donations, and 65% (11/17) in blood group AB donations (Figure 2).
FIGURE 2.
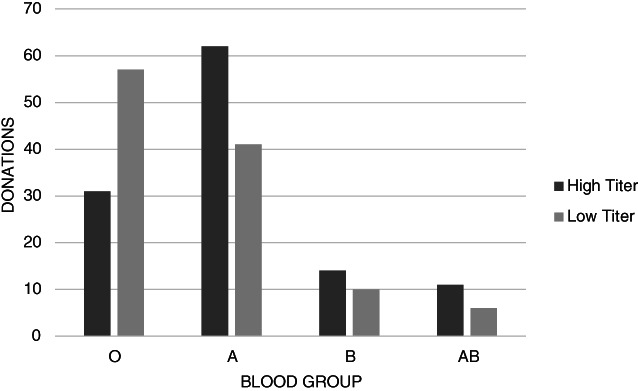
Number of high‐titer (S/C ≥ 4.5) and low‐titer donations by ABO blood group
4. DISCUSSION
The purpose of this study was to determine if ABO blood group was associated with SARS‐CoV‐2 IgG antibody response in CCP donors. Blood group O donations were found to have significantly lower levels of SARS‐CoV‐2 IgG nucleocapsid antibodies compared to blood group A donations, and were less likely to produce CCP units that qualified as high titer using the EUA‐defined ARCHITECT threshold of S/C ≥ 4.5.
An association between ABO blood group and COVID‐19 disease susceptibility has previously been described, with blood group O individuals reported to have a lower risk of acquiring COVID‐19 than non‐O individuals. 3 , 4 , 5 , 6 , 7 , 8 , 9 However, it is unclear how this leads to a diminished antibody response in blood group O individuals following SARS‐CoV‐2 infection. Similar to the current findings, prior studies also report a lower antibody titer in blood group O CCP donors, compared to non‐O blood groups. Bloch et al. found blood group O CCP donors have relatively lower neutralizing antibody titers compared to blood group B donors, but did not identify a difference in anti‐spike IgA or anti‐spike IgG titers by ABO blood group. 10 Madariaga et al. reported lower anti‐receptor binding domain (RBD) and anti‐spike antibodies in blood group O CCP donors compared to blood group AB donors. 11 Additionally, de Freitas Dutra et al. describe lower titers of neutralizing antibodies and lower levels of anti‐nucleocapsid antibodies in blood groups O and B COVID‐19 individuals compared to blood groups A and AB COVID‐19 individuals. 12 However, additional studies found no correlation between ABO blood type and anti‐nucleocapsid level, anti‐spike‐RBD level, 13 or maintenance of neutralizing antibody titer, 14 in convalescent individuals. Possible explanations for these conflicting findings include a difference in the donor demographic being studied and differences in antibody testing methodologies.
Limitations of the current study include a small sample size, which resulted in a low number of some blood groups, such as blood group B and blood group AB. Future studies with a larger sample size are needed to examine differences in antibody responses between blood group O and blood groups B and AB. There was also relative underrepresentation of blood group O in the CCP donor population compared to the standard blood donor population. This may reflect recruitment efforts focused on non‐blood group O CCP donations to improve donor/recipient compatibility or differences in the frequency of COVID‐19 disease amongst blood groups. 8 The study was also limited to a population from a single geographic region and donor screening did not include questions regarding ethnicity, so it is unclear if the donors included in this study are representative of the general population. Additionally, screening did not include questions regarding disease severity or requirement for hospitalization; however, blood donors who felt healthy at the time of donation likely represent a group that had a milder course of illness. It is not clear if the association between ABO blood group and antibody response would persist if sampling was expanded to include those who experienced a more severe disease course. Moreover, the mean interval from symptom resolution to donation in the studied CCP donor population was rather long at 73 days. Significant waning of anti‐SARS‐CoV‐2 antibody levels within 4 months of symptom onset has been reported, 15 and it is possible that the relative late presentation of the studied population impacted the relationship between ABO blood group and antibody response.
In conclusion, blood group O CCP donations have significantly lower levels of SARS‐CoV‐2 IgG nucleocapsid antibodies compared to blood group A donations and are less likely to produce CCP units that qualify as high titer. The EUA restricts use of CCP to high‐titer units only and identifying donor characteristics associated with an elevated antibody response to COVID‐19, such as non‐O blood groups, may aid in donor recruitment to promote availability of high‐titer CCP to meet patient needs. If future studies confirm the blood group‐associated differences in quantitative and qualitative SARS‐CoV‐2 antibodies, elucidation of the responsible pathophysiology could contribute to a better understanding of human COVID‐19 biology and lead to additional treatment options.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Hayes C, Rubenstein W, Gibb D, Klapper E, Tanaka J, Pepkowitz S. Blood group O convalescent plasma donations have significantly lower levels of SARS‐CoV‐2 IgG antibodies compared to blood group A donations. Transfusion. 2021;61:2245–2249. 10.1111/trf.16524
REFERENCES
- 1. Hinton D. Emergency use authorization for COVID‐19 convalescent plasma: letter of authorization [monograph on the internet]. U.S. Food and Drug Administration. 2021. https://www.fda.gov/media/141477/download. Accessed March 8, 2021.
- 2. ARCHITECT SARS‐CoV‐2 IgG Package Insert [monograph on the internet]. Abbott Laboratories. 2020. https://www.fda.gov/media/137383/download. Accessed June 17, 2020.
- 3. Zhao J, Yang Y, Huang H, Li D, Gu D, Lu X, et al. Relationship between the ABO blood group and the COVID‐19 susceptibility. Clin Infect Dis. 2020. 10.1093/cid/ciaa1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu Y, Feng Z, Li P, Yu Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID‐19. Clin Chim Acta. 2020;509:220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muñiz‐Diaz E, Llopis J, Parra R, Roig I, Ferrer G, Grifols J, et al. Relationship between the ABO blood group and COVID‐19 susceptibility, severity and mortality in two cohorts of patients. Blood Transfus. 2021;19:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gallian P, Pastorino B, Morel P, Chiaroni J, Ninove L, de Lamballerie X . Lower prevalence of antibodies neutralizing SARS‐CoV‐2 in group O French blood donors. Antiviral Res. 2020;181:104880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Severe Covid‐19 GWAS Group , Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, et al. Genomewide association study of severe Covid‐19 with respiratory failure. N Engl J Med. 2020;383:1522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goel R, Bloch EM, Pirenne F, al‐Riyami AZ, Crowe E, Dau L, et al. ABO blood group and COVID‐19: a review on behalf of the ISBT COVID‐19 working group. Vox Sang. 2021. 10.1111/vox.13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li J, Wang X, Chen J, Cai Y, Deng A, Yang M. Association between ABO blood groups and risk of SARS‐CoV‐2 pneumonia. Br J Haematol. 2020;190:24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bloch EM, Patel EU, Marshall C, Littlefield K, Goel R, Grossman BJ, et al. ABO blood group and SARS‐CoV‐2 antibody response in a convalescent donor population. Vox Sang. 2021. 10.1111/vox.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Madariaga MLL, Guthmiller JJ, Schrantz S, Jansen MO, Christensen C, Kumar M, et al. Clinical predictors of donor antibody titre and correlation with recipient antibody response in a COVID‐19 convalescent plasma clinical trial. J Intern Med. 2020;289(4):559–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Freitas Dutra V, Bonet‐Bub C, Yokoyama APH, Achkar R, Machado RRG, Assunção M, et al. Anti‐A and SARS‐CoV‐2: an intriguing association. Vox Sang. 2021;116(5):557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li L, Tong X, Chen H, He R, Lv Q, Yang R, et al. Characteristics and serological patterns of COVID‐19 convalescent plasma donors: optimal donors and timing of donation. Transfusion. 2020;60:1765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wendel S, Fontão‐Wendel R, Fachini R, Candelaria G, Scuracchio P, Achkar R, et al. A longitudinal study of convalescent plasma (CCP) donors and correlation of ABO group, initial neutralizing antibodies (nAb), and body mass index (BMI) with nAb and anti‐nucleocapsid (NP) SARS‐CoV‐2 antibody kinetics: proposals for better quality of CCP collections. Transfusion. 2021;61(5):1447–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perreault J, Tremblay T, Fournier MJ, Drouin M, Beaudoin‐Bussières G, Prévost J, et al. Waning of SARS‐CoV‐2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset. Blood. 2020;136:2588–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


