CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Dear Editor,
The current Sars‐CoV‐2 disease (COVID‐19) is a multisystemic disorder of global reach that has demonstrated dire medical and socioeconomic consequences. In an effort to alleviate the morbidity and mortality associated with COVID‐19 and halt viral transmission, a host of vaccines has been developed. Chief among these vaccines are the messenger RNA (mRNA vaccines that reportedly confer up to 95% protection from COVID‐19 after a two‐dose series. 1 Common vaccine‐related side effects include pain, redness, and/or swelling at the injection site, fatigue, headache, fever, and chills. 1 In what follows, we describe a unique case of varicella zoster virus (VZV) reactivation emerging after vaccination with the mRNA COVID‐19 vaccine.
A 79‐year‐old man with a history of hypertension, coronary artery disease, and antineutrophilic cytoplasmic antibody‐related glomerulonephritis presented to our dermatology department with itchy and tender lesions over the right thigh of 1 day duration. At the time of presentation, the glomerulonephritis was in remission and the patient was not on any immunosuppressive therapy for more than 3 months. On further questioning, the patient reported receiving the mRNA COVID vaccine 6 days before the skin eruption. No other symptoms, such as fever, chills, dyspnea, or cough were noted. On dermatologic examination, a confluence of vesicles, some excoriated and overlying an erythematous base were appreciated scattered over the right thigh in a dermatomal distribution (Figure 1). Based on the clinical findings, a diagnosis of herpes zoster infection was made, and systemic antiviral treatment was initiated resulting in the resolution of the condition.
Figure 1.
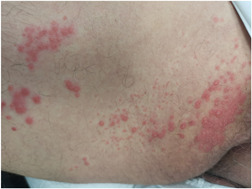
Confluence of vesicles on an erythematous base on the right thigh in a dermatomal distribution
VZV is a neurotropic virus that, on primary infection, resides and remains latent in dorsal‐root or cranial‐nerve ganglia. Reactivation of VZV leading to the clinical manifestations seen in herpes zoster may then ensue spontaneously, following activation by a trigger, such as trauma, fever, or immunosuppression. COVID‐19 infection may represent a trigger for herpes zoster reactivation as recently reported.2, 3 The rationale for the increased susceptibility to herpes zoster reactivation in COVID‐19 patients most likely centers on the tendency of COVID‐19 to produce an immunosuppressive state secondary to the functional impairment and a concomitant quantitative decrease in T lymphocytes, particularly CD4+ T cells, CD8+ T cells, and natural killer cells. 4 Actually, COVID‐19 infection has been shown to be able to reactivate several viruses, including human herpesvirus‐6, ‐7, and Epstein‐Barr virus as shown in a recent case of pityriasis rosea during COVID‐19. 5
Vaccines can also result in herpes zoster reactivation as previously described in patients receiving inactivated vaccines for hepatitis A, influenza, rabies, and Japanese encephalitis. Recently, the inactivated COVID‐19 vaccine has also been incriminated in the resurfacing of herpes zoster. 6 Furthermore, the latency between the manifestation of herpes zoster and the inactivated COVID‐19 vaccine was 5 days, akin to our patient. To our knowledge, our case represents the first instance of herpes zoster reactivation following the reception of the mRNA COVID‐19 vaccine and the second case of herpes zoster following COVID‐19 vaccination of any kind. In light of the recency of the mRNA vaccination technology, a definitive theoretical elucidation of the underlying causes for the VZV reactivation seen in our case remains elusive. However, the mass mRNA COVID‐19 vaccination campaigns conducted on a global scale will inevitably lead to the emergence of a larger number of cases with herpes zoster reactivation and will subsequently facilitate further research into the underlying pathomechanisms at play.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mMRNA COVID‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maia CMF, Marques NP, de Lucena EHG, de Rezende LF, Martelli D, Martelli‐Júnior H. Increased number of herpes zoster cases in Brazil related to the COVID‐19 pandemic. Int J Infect Dis. 2021;104:732‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tartari F, Spadotto A, Zengarini C, et al. Herpes zoster in COVID‐19‐positive patients. Int J Dermatol. 2020;59:1028‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020;17:533‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drago F, Ciccarese G, Rebora A, Parodi A. Human herpesvirus‐6, ‐7, and Epstein‐Barr virus reactivation in pityriasis rosea during COVID‐19. J Med Virol. 2021;93(4):1850‐1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bostan E, Yalici‐Armagan B. Herpes zoster following inactivated COVID‐19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021. 10.1111/jocd.14035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


