Abstract
Myeloid‐derived suppressor cells (MDSC) are a heterogeneous population of immature myeloid cells with immunosuppressive properties. In cancer patients, the expression of lectin‐type oxidized LDL receptor 1 (LOX‐1) on granulocytic MDSC identifies a subset of MDSC that retains the most potent immunosuppressive properties. The main objective of the present work was to explore the presence of LOX‐1+ MDSC in bacterial and viral sepsis. To this end, whole blood LOX‐1+ cells were phenotypically, morphologically, and functionally characterized. They were monitored in 39 coronavirus disease‐19 (COVID‐19, viral sepsis) and 48 septic shock (bacterial sepsis) patients longitudinally sampled five times over a 3 wk period in intensive care units (ICUs). The phenotype, morphology, and immunosuppressive functions of LOX‐1+ cells demonstrated that they were polymorphonuclear MDSC. In patients, we observed the significant emergence of LOX‐1+ MDSC in both groups. The peak of LOX‐1+ MDSC was 1 wk delayed with respect to ICU admission. In COVID‐19, their elevation was more pronounced in patients with acute respiratory distress syndrome. The persistence of these cells may contribute to long lasting immunosuppression leaving the patient unable to efficiently resolve infections.
Keywords: flow cytometry, HLA‐DR, immunosuppression, sepsis
Graphical Abstract
COVID‐19 and septic shock induce emergence of LOX‐1+ MDSC
Abbreviations
- ARDS
acute respiratory distress syndrome
- COVID‐19
coronavirus disease‐19
- HV
healthy volunteers
- LOX‐1
Lectin‐type oxidized LDL receptor 1
- MDSC
Myeloid‐derived suppressor cells
- mHLA‐DR
monocyte HLA‐DR
- M‐MDSC
Monocyte myeloid‐derived suppressor
- PMN‐MDSC
Polymorphonuclear myeloid‐derived suppressor cells
- SAPS II
Simplified acute physiology score
- SOFA
Sepsis‐related organ failure assessment score
1. INTRODUCTION
Myeloid‐derived suppressor cells (MDSC) are a heterogeneous population of immature myeloid cells with immunosuppressive properties as they are potent repressors of T‐cell responses. 1 Two main subtypes of MDSC are currently described. 2 Polymorphonuclear MDSC (PMN‐MDSC), containing granulocytic progenitors, are phenotypically and morphologically similar to neutrophils. Monocytic MDSC (M‐MDSC), containing monocytic progenitors, are phenotypically and morphologically similar to monocytes. Early MDSC are also described, only in mice. They are mostly immature and do not express any lineage markers. 3 MDSC have been first recognized for their role in attenuating immune surveillance and antitumor immune response in various cancer conditions.
More recently, MDSC have been reported to be involved in other clinical contexts including acute and chronic inflammation. 4 The salient feature of MDSC in these conditions is their ability to inhibit T cell function and thus to contribute to the occurrence of subsequent immunosuppression. As an illustrative example, sepsis, defined as a life‐threatening organ dysfunction caused by a dysregulated inflammatory host response to infection, 5 is characterized by a delayed step of immunosuppression. 6 Recent studies have demonstrated the association between elevated circulating MDSC (both PMN‐ and M‐MDSC) and increased and/or higher risk of nosocomial infections during sepsis. 7 , 8 , 9 Interestingly, the most severe cases of coronavirus disease‐19 (COVID‐19, caused by SARS‐CoV‐2) are viral sepsis by virtue of international definition (i.e., infection and one organ failure that is acute respiratory failure). Once COVID‐19 patients develop acute respiratory distress syndrome (ARDS), they similarly present with features of immunosuppression: altered type‐I IFN signaling, 10 decreased monocyte HLA‐DR (mHLA‐DR), 11 lymphopenia, 12 and altered lymphocyte functions. 13 A couple of recent articles reported on the early emergence of PMN‐MDSC especially in the most severe COVID‐19 patients. 14 , 15
Although there is a growing interest in monitoring MDSC in clinical practice, the lack of a specific phenotypic marker usable in whole blood protocol renders their assessment difficult on a routine basis due to the necessity to perform cell purification before staining. This is especially true for PMN‐MDSC, which are investigated among PBMCs after isolation by density gradient centrifugation. 3 In 2016, Condamine et al. reported that the expression of lectin‐type oxidized LDL receptor 1 (LOX‐1) identified a subset of MDSC that retained the most potent immunosuppressive properties. 16 Importantly, LOX‐1 staining can be performed in whole blood. 16 Since this pioneering description, LOX‐1 expression in MDSC have been confirmed in few human cancer studies. 17 , 18
In this context, the question of the emergence of LOX‐1+ PMN‐MDSC in bacterial and viral sepsis remained unsolved. The main objective of the present work was thus to highlight the presence of these cells in two etiologies of sepsis, that is, bacterial and viral (COVID‐19) and to verify that these cells identified in whole blood possessed all the characteristics of MDSC.
2. MATERIALS AND METHODS
2.1. Study population
This clinical study was conducted on patients admitted to the intensive care unit (ICU) of Edouard Herriot Hospital (Hospices Civils de Lyon, Lyon, France). This project is part of two prospective observational studies: IMMUNOSEPSIS (on septic shock patients), and RICO (REA‐IMMUNO‐COVID on COVID‐19 ICU patients). Extended details are provided in supplemental material.
2.2. Whole blood phenotyping
Fresh peripheral blood was collected using EDTA anticoagulant tubes. A total of 100 μl of whole blood was labelled with antibodies: CD45‐KrO/CD10‐PC7/CD16‐PB (Beckman Coulter, Brea, CA, USA), CRTH2‐APC (Miltenyi, Bergisch Gladbach, Germany), CD15‐AF700, and LOX‐1‐PE or mouse IgG2a isotype control (Biolegend, San Diego, CA, USA) and incubated for 15 min at room temperature in the dark. Cells were then incubated with 1 m of lysing solution (Versalyse, Beckman Coulter) for 10 min at room temperature in the dark and washed with PBS before being analyzed by flow cytometry. Samples were run on a Navios flow cytometer (Beckman Coulter). Calibration beads (Flow Check and Flow Set, Beckman Coulter) were run daily to check for routine alignment, day‐to‐day stability, and long‐term performance validation. Gating strategy involved a first step of PMN selection on a biparametric CD45/side scatter histogram. Then, eosinophils were excluded from gated PMN according to CRTH2 expression. LOX‐1 expression was finally evaluated on the whole neutrophil population (positivity threshold was based on isotype values set up at 0.5%). Results were expressed as percentage of LOX‐1 positive cells among neutrophils. Maturity of PMN or PMN‐MDSC was also assessed based on the expression of CD10 and CD16.
2.3. Cell isolation
Fresh PBMCs were isolated from whole blood by Ficoll density gradient centrifugation. For coculture and microscopy (May‐Grünwald‐Giemsa staining performed by an SP10 automatic system, Sysmex), MDSC were sorted by flow cytometry on a FACSAria II flow cytometer (BD Bioscience, San Jose, CA, USA). The gating strategy for cell sorting experiments was to select CD45dim LOX1+ cells for PMN‐MDSC and CD45+ LOX1‐ cells for regular neutrophils among whole CD15+ neutrophil population (>90% purity). Viability of sorted cells was assessed using propidium iodide staining (>90% viability). For coculture, T cells were isolated from healthy volunteers (HV) samples by antibody‐based negative selection and Ficoll density gradient centrifugation using a human T‐cell enrichment cocktail (Rosette Sep, StemCell Technologies, Grenoble, France), as described in the manufacturer's instructions. T cell purity was assessed by flow cytometry (cells were labelled with anti‐CD3‐APC [Beckman Coulter]).
2.4. Suppression assay
Freshly purified T cells were stimulated with anti‐CD2/3/28 coated‐beads (T cell Activation/Expansion kit, 2 beads per T cell, Biolegend, Bergisch Gladbach, Germany) and cultured alone or cocultured with heterologous CD45dim LOX1+ or CD45+ LOX1‐ PMN at a 1:1 ratio for 72 h at 37°C and 5% CO2. T cell functionality was assessed by measuring IFN‐γ production in culture supernatant (ELLA, Proteinsimple, San Jose, CA, USA), according to the manufacturer's instructions.
2.5. Statistical analysis
Results regarding expression of CD45dimLOX1+ cells in patients are presented as individual values and Tukey boxplots. For clinical parameters, continuous data and biologic parameters are presented as medians and interquartile ranges (IQR) whereas categoric data are presented as numbers of cases and percentages among total population (median and IQR). Results regarding IFN‐γ production of T cells in coculture are presented as means ± sd. Nonparametric Mann‐Whitney, Fisher, and χ2 tests were used to assess differences between HV and patients or differences between viral and bacterial sepsis, and the nonparametric Wilcoxon paired test was used to assess variations between patients themselves at different time points. P‐values lower than 0.05 were considered statistically significant.
3. RESULTS AND DISCUSSION
As a preliminary step, we first developed a whole blood protocol to assess LOX‐1 expression on circulating neutrophils. As regularly performed in clinical flow cytometry, we used CD45 expression to draw a leukogate in order to exclude debris. Then, neutrophils were identified based on CD15 positivity and lack of CRTH2 (in order to exclude eosinophils). As depicted in Figure 1, we were able to identify a subset of neutrophils expressing LOX‐1. These cells presented with diminished CD45 expression (Fig. 1A) and an immature phenotype as they did not express CD16 nor CD10 (Fig. 1A). We next performed LOX‐1 staining after Ficoll purification on cells from both Ficoll ring and pellet. As expected, LOX‐1+ CD15+ neutrophils were found in the Ficoll ring with PBMCs and were absent from the pellet (Supporting Information Fig. S1). We confirmed that these LOX‐1+ cells presented with low CD10 and CD16 expressions. In addition, after staining with May‐Grünwald Giemsa, sorted CD45dim CD15+ LOX‐1+ population appeared as neutrophil precursors presenting with immature morphology (i.e., poorly segmented reniform nucleus). In contrast, CD45+ LOX‐1 negative cells (found in the pellet) appeared as mature neutrophils exhibiting a well‐segmented nucleus (Supporting Information Fig. S1). In order to further qualify LOX‐1+ neutrophils as MDSC, we next investigated their suppressive functions. 2 Due to the limited biologic resources (i.e., small amount of available blood from severely ill ICU patients), we assessed inhibition of IFN‐γ production by T cells that require fewer cells than proliferation assay. To this end, CD15+ CD45dim LOX‐1+ and CD15+ CD45+ LOX1‐ cells were sorted and cocultured with heterologous T cells from healthy donors. After 72 h of culture, IFN‐γ release was measured in culture supernatant. Although addition of regular neutrophils (CD15+ CD45+ LOX‐1‐) had no effect on IFN‐γ release by stimulated T‐cells, coculture with CD15+ CD45dim LOX‐1+ cells induced a fall in IFN‐γ production (Fig. 1B). Overall, our results identified a subpopulation of CD15+ CRTH2‐ CD10low CD16low CD45dim LOX‐1+ neutrophils, which presented with immature morphology, low‐density characteristics, and possessed immunosuppressive properties. This agreed with first report on LOX‐1 cells by Gabrilovich's group 16 and identified these cells as PMN‐MDSC. This allowed use of this phenotype in clinical studies for the whole blood phenotyping of MDSC. Thereafter, those cells will be named LOX‐1 PMN‐MDSC.
FIGURE 1.
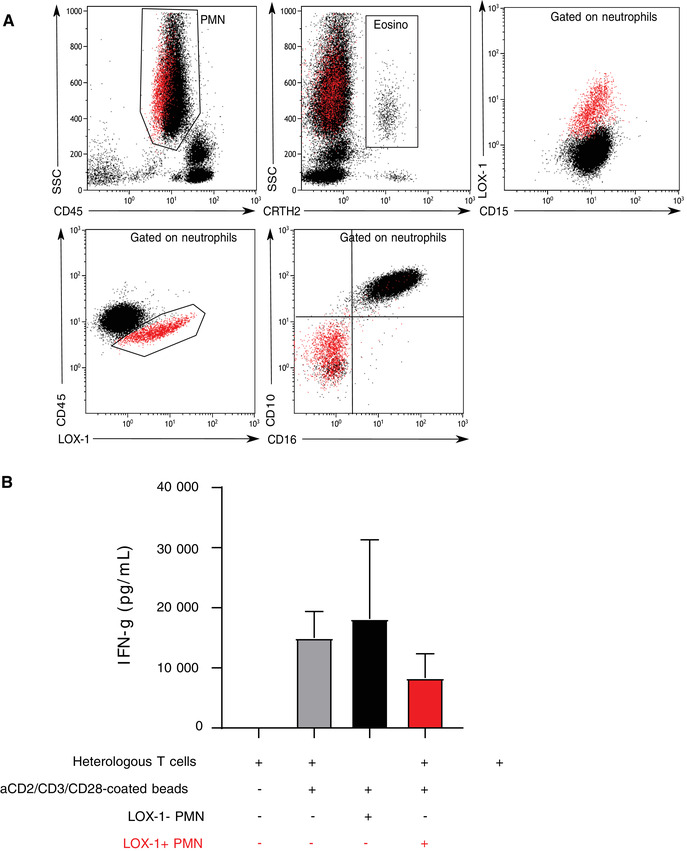
Lectin‐type oxidized LDL receptor 1 (LOX‐1) neutrophils characteristics. (A) Representative example of CD45dim LOX‐1 phenotypic characterization in septic shock patient. Polymorphonuclear cells (PMN) were first gated out from other cells on a biparametric CD45/side scatter histogram. Then, eosinophils were excluded from gated PMN on the expression of CRTH2. LOX‐1 expression was finally evaluated on whole neutrophil population (positivity threshold was defined based on isotype values set up at 0.5%). LOX‐1 expression is either represented among CD15+ cells or CD45+ cells. Red cells represent CD45dim LOX‐1+ cells gated on the biparametric CD45/LOX1 dotplot. CD10 and CD16 expressions gated on the CD45dim LOX‐1 cells is also represented to illustrate the immature nature of these cells. (B) LOX‐1+ MDSC (myeloid‐derived suppressor cells) immunosuppressive function on T cell IFNg release. IFN‐γ concentrations in the supernatants of T cells purified from healthy volunteers (HV) were measured (n = 3 independent experiments). T cells were either incubated alone, with stimulation beads, or cocultured at 1:1 ratio with CD45+ LOX‐1‐ or CD45dim LOX‐1+ MDSC purified from 3 septic shock patients. Data are presented as means ± sd
Forty‐eight septic shock patients, 39 ICU COVID‐19 patients, and 22 HV were included. Clinical and biologic characteristics are listed in Table 1. Overall, patients mostly presented with low mHLA‐DR values and lymphopenia reflecting an immunosuppression state as usually described in sepsis. 6 Upon admission, patients with bacterial septic shock did not exhibit increased percentage LOX‐1 PMN‐MDSC in comparison with HV (median = 0.15% [0.04–0.38] vs. 0.165% [0.063–0.25], respectively) (Fig. 2A). Afterward, percentage LOX‐1 PMN‐MDSC significantly increased at D3 (0.26% [0.13–0.87]; P = 0.021) with a peak at D6 (3.10% [1.02–6.58] P = 0.003). D6 values were significantly higher than those observed at D1 (P = 0.003) and D3 (P = 0.005). On patient's discharge from ICU, percentage LOX‐1 PMN‐MDSC decreased (0.43% [0.15–0.73]) without returning to completely normal values. Indeed, discharge values were still significantly higher than admission values (P = 0.004). Emergence of LOX‐1 PMN‐MDSC was also observed in COVID‐19 patients (Fig. 2B). At each time point, values were significantly higher than HV, even at patients’ ICU admission (0.73% [0.47–1.94]; P = 0.007). Values of CD45dimLOX1+ MDSC then rose up at D3 (2.83% [0.65–5.14]; P = 0.003) and reached a plateau at D6 (2.71% [1.52–5.47]; P = 0.009). The last day of their follow‐up, patients still had significantly higher LOX‐1 PMN‐MDSC values than HV (1.47% [0.745–3.450]; P = 0.007). Last, COVID‐19 patients were analyzed in regard with the development or not of ARDS within the first 3 d after ICU admission. COVID‐19 patients who developed ARDS showed a significant elevation of LOX‐1 PMN‐MDSC values between the first two samples (P = 0.002), whereas LOX‐1 PMN‐MDSC did not rise in patients without ARDS (Fig. 2C).
TABLE 1.
Demographic, clinical, and immunologic data for coronavirus disease‐19 (COVID‐19) and septic shock patients
| Parameters | COVID‐19 (n = 39) | Septic shock (n = 48) | P‐value |
|---|---|---|---|
| Age (years) | 65 [59–71] | 72 [55–78] | 0.3 |
| Gender—male, n (%) | 27 (69) | 25 (52) | 0.5 |
| McCabe score | 0.07 | ||
| 0, n (%) | 36 (92) | 34 (71) | |
| 1, n (%) | 3 (8) | 13 (27) | |
| 2, n (%) | 0 (0) | 1 (2) | |
| Delay between first symptoms (days) | 8 |5–9] | NA | |
| SAPS II score | 33 [28–40] | 54 [47–70] | <0.001 |
| SOFA score | 4 [3–5] | 9 [7–12] | <0.001 |
| Respiratory dysfunction (COVID‐19) | |||
| Mechanical ventilation, n (%) | 34 (87%) | NA | |
| PaO2/FiO2 ratio on admission (mmHg) | 108 [90–158] | NA | |
| Site of infection, n (%) | <0.001 | ||
| Pulmonary | 39 (100) | 9 (19) | |
| Abdominal | 0 (0) | 16 (33) | |
| Other | 0 (0) | 23 (48) | |
| Follow‐up | |||
| 28 d nonsurvivors, n (%) | 3 (8) | 7 (15) | 0.03 |
| Secondary nosocomial infections, n (%) | 18 (46) | 13 (27) | 0.003 |
| Immunologic parameters | |||
| Monocyte HLA‐DR (mHLA‐DR; AB/C) | 8344 [5986–8458] | 5440 [3622–8169] | 0.046 |
| Absolute CD4+ T cell count (cell/μl) | 319 [169–398] | 367 [274–570] | 0.05 |
Continuous data and biologic parameters are presented as medians and interquartile ranges [Q1–Q3]. For clinical parameters, categoric data are presented as numbers of cases and percentages among the total population in brackets. SAPS II (simplified acute physiology score II) and McCabe scores were calculated at admission. SOFA (sequential organ failure assessment) score was measured during the first 24 h after ICU admission. Absolute CD4+ T cell count was calculated on day 3 as well as mHLA‐DR, expressed as numbers of anti‐HLA‐DR antibodies bound per monocyte (AB/C). Reference values from our lab: mHLA‐DR: > 15 000 ABC, CD4+: 336–1126 cells/μl. P‐values: nonparametric Mann‐Whitney, Fisher, and χ2 tests when appropriate.
FIGURE 2.
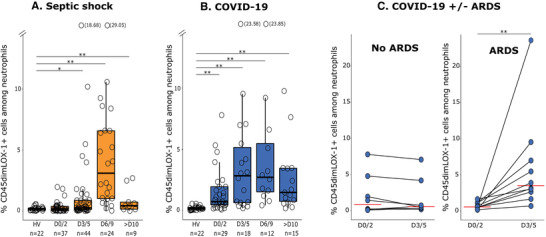
Monitoring of lectin‐type oxidized LDL receptor 1 polymorphonuclear myeloid‐derived suppressor cells (LOX‐1+ PMN‐MDSC) overtime in septic shock and coronavirus disease‐19 (COVID‐19) patients. Percentages of LOX‐1+ PMN MDSC among total neutrophils were measured in peripheral blood from 22 healthy volunteers (HV) and (A) 48 septic shock patients (day 0/2, n = 37; day 3/5, n = 44; day 6/9, n = 24; above day10, n = 9), or (B) 39 COVID‐19 ICU patients (day 0/2, n = 29; day 3/5, n = 18; day 6/9, n = 12; above day10, n = 15). Missing values corresponded to patients who died or left ICU before the end of the study and to missing samples during the weekends for which staining was not possible since lab was not operating 24/7. Data are presented as individual values and Tukey boxplots. (C) Acute respiratory distress syndrome (ARDS) in COVID‐19. For COVID‐19 ICU patients with paired samples at day 0/2 and day 3/5, kinetics of CD45dim LOX1+ percentage in patients with (n = 10) or without (n = 8) ARDS was evaluated. Nonparametric Mann‐Whitney test was used to assess differences between HV and patients. The nonparametric Wilcoxon paired test was used to assess variations between patients themselves at day 0/2 and day 3/5; *P < 0.05, **P < 0.01
Sepsis, which by international definition is defined as an organ dysfunction due to a dysregulated host response to an infection, 19 is characterized by the occurrence of delayed immunosuppression that associates with poor outcome and nosocomial infections. 6 , 20 In this condition, MDSC emergence has been described and is reported to contribute to mechanisms sustaining this state of immunosuppression. 7 , 21 Although mainly due to bacterial and fungal etiologies, viral origin is also a cause for sepsis. COVID‐19 is an illustrative example of viral sepsis because patients infected by SARS‐CoV‐2 may develop (in worst cases) ARDS (that is a major pulmonary dysfunction). Interestingly, COVID‐19 patients also present with marked immunosuppression characterized by severe lymphopenia, decreased mHLA‐DR expression, or increased IL‐10 concentrations. 11 , 22 , 23 , 24
In addition to lymphopenia, T cell altered functionality, and decreased mHLA‐DR, the current results illustrate that LOX‐1+ MDSC occurrence constitutes another similarity between sepsis and cancer. 25 Considering their potent immunosuppressive properties (T cell inhibition, arginine depletion, reactive oxygen species synthesis, production of PGE2, and anti‐inflammatory cytokines), it is reasonable to speculate on their participation in septic patients worsening due to immunosuppression. In line, their elevation is a marker of severity associated with occurrence of ARDS.
Another striking result of the present study is the similar rise of these immunosuppressive cells in bacterial and viral sepsis and the delayed maximal appearance of LOX‐1+ MDSC because they peaked approximately 1 wk after ICU admission in both etiologies of sepsis. This agrees with previous results showing that decreased mHLA‐DR, observed in septic shock and COVID‐19 patients, is associated with unfavorable outcomes in a delayed manner, that is, from day 4 after septic shock. 6 , 20 Over days persistence of low mHLA‐DR predicted nosocomial infections occurrence as well as mortality, 8 , 26 , 27 whereas inaugural values did not. Here, at ICU discharge, patients still presented elevated LOX‐1+ MDSC values. Importantly, despite constant improvements in the management of patients, the long‐term mortality in sepsis has remained dramatically high. 28 Increased mortality may last few years after sepsis. 29 The first cause of rehospitalization and death after sepsis are infectious diseases evoking a role for a long‐term persistence of immunosuppression. This phenomenon is hypothesized to be due to persistent inflammation, immunosuppression, and catabolism syndrome, and/or chronic critical illness, defined as more than 14 d spent in ICU associated with organ dysfunction and immunosuppression. In both cases, chronic low‐grade inflammation perpetuates long‐term immunosuppression that in turn favors additional infectious events. In that respect, proinflammatory compounds such as alarmins (S100A9/S100A8, HMGB‐1) are believed to induce MDSC proliferation/differentiation during late bacterial sepsis by promoting immune repressor functions in bone marrow. 9 , 30 , 31 , 32 , 33 Most importantly, S100A8 (also known as calgranulin) has been demonstrated to be an important early maker of severity in COVID‐19 patients. 34 We can thus speculate that this nonresolving inflammation observed in septic shock and COVID‐19 patients, which is also a feature of various cancers may represent a common mechanism leading to the induction of MDSC known to lower immune surveillance. 25
Nevertheless, our study has limitations. Immunosuppressive abilities of LOX‐1+ MDSC may be confirmed with additional functional testing such as lymphocyte proliferation. The relatively small sample size of the cohort (less than 50 patients in each cohort) did not allow us to deeply study the relationships with clinical outcomes. However, considering the previously described roles of these cells in cancer and their kinetics in sepsis (i.e., peak that synchronously occurs during secondary immunosuppressive step of the disease), we may hypothesize that LOX1+ MDSC participate in a deleterious immunosuppressive process. 7 , 21 , 35 , 36 , 37 Further larger studies are needed to confirm our preliminary results.
To conclude, emergence of LOX‐1+ neutrophils with proven MDSC phenotype, morphology, and function was detected for the first time in septic patients, whatever sepsis origin. In COVID‐19, their elevation was more pronounced in patients with ARDS. The peak of LOX‐1+ MDSC was delayed with respect to ICU admission. In addition, we demonstrated the feasibility of LOX‐1+ MDSC measurement in whole blood by flow cytometry, which should facilitate larger clinical research studies. Upon demonstration of a causative and deleterious role of MDSC in sepsis‐induced immunosuppression leaving the patient unable to effectively resolve infections, the repositioning of various drugs proved to be efficient as adjunctive anticancer agents by tackling MDSC could appear as a realistic therapeutic option. 38 This is all the more true because, in contrast to other putative immunoregulatory targets (regulatory T cells, inhibitory checkpoint molecules), MDSC are not present (or at very low concentration) in steady‐state conditions providing thus a unique opportunity to act on immune response while minimizing side effects on immune homeostasis. 38
AUTHORSHIP
R.C., L.W., R.P., L.J. and A.F. performed the experiments. R.C., F.V., M.G. and G.M. wrote the report and designed the experiments. M.C., T.R., H.Y., A‐C.L. and L.A. were involved with selection of the patients. All authors discussed the data, drafted or critically revised the manuscript for important intellectual content, have accepted responsibility for the entire content of this submitted manuscript, and have approved its submission.
DISCLOSURES
The authors declare no conflicts of interest.
RICO study group
Hospices Civils de Lyon, Immunology Department: Fabienne Venet, Guillaume Monneret, Morgane Gossez, Françoise Poitevin‐Later, Christophe Malcus, Remy Coudereau, Sebastien Viel, Remi Pescarmona, Lorna Garnier, Christine Lombard, Magali Perret, Marine Villard
Joint Research Unit HCL‐bioMérieux: Karen Brengel‐Pesce, Valérie Cheynet, Filippo Conti, Elisabeth Cerrato
Centre d'Investigation Clinique de Lyon (CIC 1407 Inserm): Marielle Buisson, Laetitia Itah, Inesse Boussaha, Camille Amaz
Hospices Civils de Lyon, Anesthesia and Critical Care Medicine and Medical intensive Care Departments: Martin Cour, Laurent Argaud, Marie Simon, Auguste Dargent, Pierre‐Jean Bertrand, Neven Stevic, Marion Provent, Anne‐Claire Lukaszewicz, Thomas Rimmelé, Paul Abraham, Céline Monard, Laurie Bignet, Valérie Cerro, Hodane Yonis, Jean‐Christophe Richard, Laurent Bitker, Mehdi Mezidi, Loredana Baboi, Florent Wallet, Marie‐Charlotte Delignette, Frederic Dailler
Hospices Civils de Lyon, Croix‐Rousse University Hospital, Virology laboratory: Jean‐Sebastien Casalegno
Supporting information
Supplement Material
ACKNOWLEDGMENTS
The authors thank the technical staff from the Immunology lab of Edouard Herriot Hospital (Anne Portier, Alizée Bègue, Catherine Venard, Laurence Poulat, Christelle Gonis, Mireille Bailly) for their great help in flow cytometry; the research nurses from the Anesthesia and Critical Care Medicine Department (Valérie Cerro, Laurie Bignet) and Intensive Care Department from Edouard Herriot Hospital (Marion Provent); Croix‐Rousse Hospital (Lorédana Baboi); Lyon‐sud hospital (Florence Raynal); and the “Centre d'investigation Clinique” of Lyon for their help to include patients in this study. This work was supported by funds from the Hospices Civils de Lyon, Fondation HCL, Claude Bernard Lyon 1 University and Région Auvergne Rhône‐Alpes.
Coudereau R, Waeckel L, Cour M, et al. Emergence of immunosuppressive LOX‐1+ PMN‐MDSC in septic shock and severe COVID‐19 patients with acute respiratory distress syndrome. J Leukoc Biol. 2022;111:489–496. 10.1002/JLB.4COVBCR0321-129R
REFERENCES
- 1. Gabrilovich DI. Myeloid‐derived suppressor cells. Cancer Immunol Res. 2017;5:3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bronte V, Brandau S, Chen S‐H, et al. Recommendations for myeloid‐derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Veglia F, Sanseviero E, Gabrilovich DI. Myeloid‐derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. 2021;1:1‐14. https://doi.org.10.1038/s41577-020-00490-y. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Veglia F, Perego M, Gabrilovich D. Myeloid‐derived suppressor cells coming of age. Nat Immunol. 2018;19:108‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3). JAMA. 2016;315:801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Venet F, Monneret G. Advances in the understanding and treatment of sepsis‐induced immunosuppression. Nat Rev Nephrol. 2018;14:121‐137. [DOI] [PubMed] [Google Scholar]
- 7. Uhel F, Azzaoui I, Grégoire M, et al. Early expansion of circulating granulocytic myeloid‐derived suppressor cells predicts development of nosocomial infections in patients with sepsis. Am J Respir Crit Care Med. 2017;196:315‐327. [DOI] [PubMed] [Google Scholar]
- 8. Waeckel L, Venet F, Gossez M, et al. Delayed persistence of elevated monocytic MDSC associates with deleterious outcomes in septic shock: a retrospective cohort study. Critical Care. 2020;24:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hollen MK, Stortz JA, Darden D, et al. Myeloid‐derived suppressor cell function and epigenetic expression evolves over time after surgical sepsis. Crit Care. 2019;23:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID‐19 patients. Science. 2020;369:718‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monneret G, Cour M, Viel S, et al. Coronavirus disease 2019 as a particular sepsis: a 2‐week follow‐up of standard immunological parameters in critically ill patients. Intensive Care Med. 2020:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen‐specific adaptive immunity to SARS‐CoV‐2 in acute COVID‐19 and associations with age and disease severity. Cell. 2020;183:996‐1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sacchi A, Grassi G, Bordoni V, et al. Early expansion of myeloid‐derived suppressor cells inhibits SARS‐CoV‐2 specific T‐cell response and may predict fatal COVID‐19 outcome. Cell Death Dis. 2020;11:921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reizine F, Lesouhaitier M, Gregoire M, et al. SARS‐CoV‐2‐Induced ARDS associates with MDSC expansion, lymphocyte dysfunction, and arginine shortage. J Clin Immunol. 2021;41(3):515‐525. https://doi.org.10.1007/s10875-020-00920-5. Epub 2021 Jan 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Condamine T, Dominguez GA, Youn J‐I, et al. Lectin‐type oxidized LDL receptor‐1 distinguishes population of human polymorphonuclear myeloid‐derived suppressor cells in cancer patients. Sci Immunol. 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Si Y, Merz SF, Jansen P, et al. Multidimensional imaging provides evidence for down‐regulation of T cell effector function by MDSC in human cancer tissue. Sci Immunol. 2019;4. [DOI] [PubMed] [Google Scholar]
- 18. Cassetta L, Bruderek K, Skrzeczynska‐Moncznik J, et al. Differential expansion of circulating human MDSC subsets in patients with cancer, infection and inflammation. J Immunother Cancer. 2020;8(2):e001223. 10.1136/jitc-2020-001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304‐377. [DOI] [PubMed] [Google Scholar]
- 20. Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mathias B, Delmas AL, Ozrazgat‐Baslanti T, et al. Human myeloid‐derived suppressor cells are associated with chronic immune suppression after severe sepsis/septic shock. Ann Surg. 2017;265:827‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Remy KE, Brakenridge SC, Francois B, et al. Immunotherapies for COVID‐19: lessons learned from sepsis. Lancet Respir Med. 2020;8:946‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li H, Liu L, Zhang D, et al. SARS‐CoV‐2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517‐1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benlyamani I, Venet F, Coudereau R, et al. Monocyte HLA‐DR measurement by flow cytometry in COVID‐19 patients: an interim review. Cytometry A. 2020;97:1217‐1221. [DOI] [PubMed] [Google Scholar]
- 25. Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N Engl J Med. 2014;371:380‐383. [DOI] [PubMed] [Google Scholar]
- 26. Leijte GP, Rimmelé T, Kox M, et al. Monocytic HLA‐DR expression kinetics in septic shock patients with different pathogens, sites of infection and adverse outcomes. Crit Care. 2020;24:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zorio V, Venet F, Delwarde B, et al. Assessment of sepsis‐induced immunosuppression at ICU discharge and 6 months after ICU discharge. Ann Intensive Care. 2017;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prescott HC, Osterholzer JJ, Langa KM, et al. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Delano MJ, Ward PA. The immune system's role in sepsis progression, resolution and long‐term outcome. Immunol Rev. 2016;274:330‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alkhateeb T, Kumbhare A, Bah I, et al. S100A9 maintains myeloid‐derived suppressor cells in chronic sepsis by inducing miR‐21 and miR‐181b. Mol Immunol. 2019;112:72‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dai J, Kumbhare A, Youssef D, et al. Intracellular S100A9 promotes myeloid‐derived suppressor cells during late sepsis. Front Immunol. 2017;8:1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mira JC, Brakenridge SC, Moldawer LL, et al. Persistent inflammation, immunosuppression and catabolism syndrome (PICS). Crit Care Clin. 2017;33:245‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mira JC, Gentile LF, Mathias BJ, et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation‐immunosuppression and catabolism syndrome. Crit Care Med. 2017;45:253‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Silvin A, Chapuis N, Dunsmore G, et al. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID‐19. Cell. 2020;182:1401‐1418.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tavukcuoglu E, Horzum U, Yanik H, et al. Human splenic polymorphonuclear myeloid‐derived suppressor cells (PMN‐MDSC) are strategically located immune regulatory cells in cancer. Eur J Immunol. 2020;50(12):2067‐2074. 10.1002/eji.202048666. Epub 2020 Sep 2 [DOI] [PubMed] [Google Scholar]
- 36. Chai E, Zhang L, Li C. LOX‐1+ PMN‐MDSC enhances immune suppression which promotes glioblastoma multiforme progression. Cancer Manag Res. 2019;11:7307‐7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim HR, Park S‐M, Seo S‐U, et al. The ratio of peripheral regulatory T cells to Lox‐1+ polymorphonuclear myeloid‐derived suppressor cells predicts the early response to anti‐PD‐1 therapy in patients with non‐small cell lung cancer. Am J Respir Crit Care Med. 2019;199:243‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fleming V, Hu X, Weber R, et al. Targeting myeloid‐derived suppressor cells to bypass tumor‐induced immunosuppression. Front Immunol. 2018;9:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Material


