Abstract
Objective
Early reports have suggested that coronavirus disease 2019 (COVID‐19) can present with significant urinary frequency and nocturia, and that these symptoms correlate with markers of inflammation in the urine. We evaluated surrogate markers of chronic urinary symptoms to determine if they were more frequent after COVID‐19 infection.
Methods
Routinely collected data from the province of Ontario was used to conduct a matched, retrospective cohort study. We identified patients 66 years of age or older who had a positive COVID‐19 test between February and May 2020 and survived at least 2 months after their diagnosis. We matched them to two similar patients who did not have a positive COVID‐19 test during the same time period. We measured the frequency of urology consultation, cystoscopy, and new prescriptions for overactive bladder medications during a subsequent 3‐month period. Proportional hazard models were adjusted for any baseline differences between the groups.
Results
We matched 5617 patients with COVID‐19 to 11,225 people who did not have COVID‐19. The groups were similar, aside from a higher proportion of patients having hypertension and diabetes in the CoVID‐19 cohort. There was no significantly increased hazard of new receipt of overactive bladder medication (hazards ratio [HR]: 1.04, p = 0.88), urology consultation (HR: 1.40, p = 0.10), or cystoscopy (HR: 1.14, p = 0.50) among patients who had COVID‐19, compared to the matched cohort.
Conclusion
Surrogate markers of potential bladder dysfunction were not significantly increased in the 2–5 months after COVID‐19 infection.
Keywords: COVID‐19, lower urinary tract symptoms, overactive, SARS‐CoV‐2, urinary bladder
1. INTRODUCTION
The ongoing global coronavirus disease 2019 (COVID‐19) pandemic has had an unprecedented impact on humanity. Over 100 million people have had confirmed infections so far, and it is likely that there have been millions of additional cases that have gone undiagnosed. The coronavirus is a family of RNA viruses that commonly causes disease in other mammals; in the case of COVID‐19, the causative coronavirus is severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 Urologic manifestations of COVID‐19 have been limited, with the coronavirus family in general having the potential to cause kidney and testicular dysfunction, 2 and having only a low likelihood being detected in the urine. 3
However, recent reports have suggested that COVID‐19 may lead to urinary symptoms (“COVID‐19 associated cystitis”). 4 The SARS‐CoV‐2 virus may be able to bind angiotensin converting enzyme 2 (ACE2) receptors in the urinary system and cause local inflammation. 5 Small case series have reported urinary storage symptoms associated with COVID‐19, and correlated these findings with elevated urinary proinflammatory cytokines. 6 This has led to the hypothesis that, just as COVID‐19 can lead to long‐term cognitive and medical symptoms and pulmonary, cardiac, and vascular fibrosis, 7 a chronic COVID‐19 associated cystitis condition with urinary storage symptoms similar to overactive bladder may develop after the acute infection has resolved. 6 Our objective was to determine if patients who had a COVID‐19 diagnosis were more likely to seek urologic care or undergo medical treatment for urinary storage symptoms.
2. METHODS
We conducted a retrospective cohort study with the use of routinely collected data from the province of Ontario, Canada. All data was linked across databases deterministically using encoded patient identifiers and analyzed at ICES Western. Ontario has a universal healthcare system that provides healthcare for all 15 million residents. The use of the data in this study was authorized under Ontario's Personal Health Information Protection Act, which does not require approval by a Research Ethics Board, or patient consent.
2.1. Data sources
We used the following data sources for this study:
-
(1)
Ontario Laboratories Information System (OLIS): The OLIS database contains laboratory information collected from eHealth Ontario, submitted from all Public Health Ontario laboratories. COVID‐19 swab status was determined from OLIS. 8
-
(2)
Ontario Health Insurance Plan (OHIP): The OHIP claims database contains information for all services provided to Ontario residents through the province's publicly funded health insurance system. It is necessary for physicians to submit billing codes to this database to be compensated for services provided. In general, OHIP fee codes have a high accuracy. 9
-
(3)
Discharge Abstract Database (DAD)/Same Day Surgery (SDS)/National Ambulatory Care Reporting System (NACRS): The DAD/SDS/NACRS is compiled by the Canadian Institute for Health Information and tracks administrative, clinical (diagnoses and procedures/interventions), demographic and administrative information for all patients in the province of Ontario who have a same day surgery, hospitals admissions or emergency room visits. Reabstraction studies from CIHI‐DAD/SDS have shown a high agreement with procedure codes (91% CCI code exact match, 97% CCI code group match) and diagnoses (89% exact match, 95% ICD10 code group match). 10
-
(4)
Registered Persons Database (RPDB): The RPDB provides basic demographic information (such as age and sex) for all Ontario citizens.
-
(5)
Ontario Drug Database (ODB): The ODB contains records of prescriptions for all people in Ontario who are 65 years or older; it has greater than 99% accuracy. 11
2.2. Patient population
We identified all patients who had a positive nasopharyngeal swab for SARS‐CoV‐2 between February 1 and May 31, 2020. The date of the positive nasopharyngeal swab was considered the index date of the infection. We excluded patients who were: less than 66 years of age (to ensure all patients had universal drug coverage, and to allow a look‐back period to help define new medication use), those that had filled a prescription for an overactive bladder medication of interest in the 6 months before the COVID‐19 diagnosis, and those that died within 2 months of the COVID‐19 diagnosis.
We then identified a group of people in the general population who were also over 66 years of age, alive on February 1 2020, and did not have a positive COVID‐19 test result during the February–May 2020 period. We assigned each of these patients a random index date between February 1 and May 31, 2020. We applied the same three exclusion criteria that were described for the COVID‐19 population to identify a cohort of similar non‐COVID‐19 patients from the general population.
We matched (without replacement) one COVID‐19 patient to two people from the general population cohort based on the index date (±1 week), age (±1 year), sex, and whether they were a resident of a long‐term care home (because residents of long‐term care homes were disproportionally represented in the COVID‐19 cohort). We used two years of previous routinely collected data and three validated datasets (to identify patients with chronic obstructive pulmonary disease, hypertension, and diabetes) to measure several baseline characteristics (further defined in Appendix 1).
2.3. Study outcomes
We measured study outcomes that occurred in the time period from 60 to 150 days after the index date. This time period was chosen as we felt it would be unlikely that people would seek care for urinary symptoms during their acute illness, and patients may be admitted to hospital for a period of a few weeks during their acute COVID‐19 disease. 12 We identified the following a priori outcomes of interest: whether patients underwent cystoscopy (OHIP fee code Z606, Z607), consulted with a urologist (OHIP fee code A355), or filled a prescription for an overactive bladder medication (solifenacin, oxybutynin, mirabegron, fesoterodine, tolterodine, or darifenacin). Our hypothesis was that if COVID‐19 was associated with chronic urinary symptoms, these should be more frequent among COVID‐19 patients.
2.4. Statistical analysis
Baseline characteristics were compared using standardized differences; when it is greater than 10% it is considered a potentially meaningful difference. 13 Analysis of our outcomes was done using PROC PHREG (SAS 9.4; SAS institute) to create Cox proportional hazards models with robust variance estimation to account for the correlation introduced with matching. If applicable, patients were censored on the date of their death, and additionally in the non‐COVID‐19 population, the patient was censored if they were diagnosed with COVID‐19. Models were adjusted for any differences in the baseline characteristics which had a standardized difference greater than 10%. Results are reported as hazard ratios and 95% confidence intervals, and we considered two‐tailed p < 0.05 statistically significant.
3. RESULTS
We were able to match 99.9% of the eligible COVID‐19 patients (n = 5617) 1:2 to patients who did not have COVID‐19 (Figure 1). Over 90% of the COVID‐19 patients were diagnosed in the month of April or May 2020. In general, the groups had comparable proportions of comorbidities after matching, aside from a higher proportion of hypertension and diabetes in the COVID‐19 cohort (Table 1).
Figure 1.
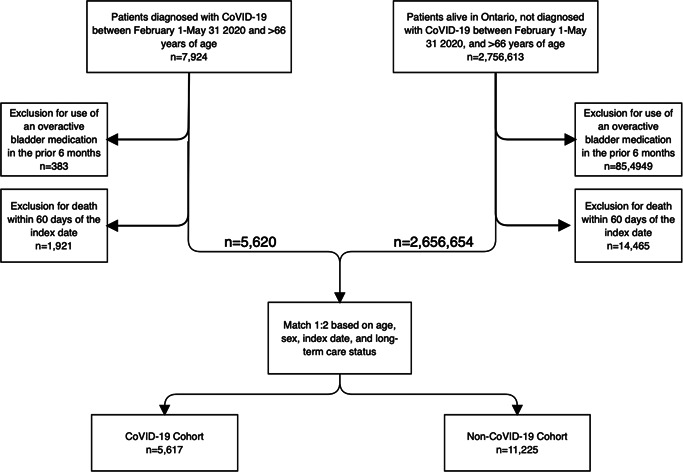
Creation of the final matched cohorts. COVID‐19, coroanvirus disease 2019
Table 1.
Demographics of the unmatched and matched cohorts of people with and without COVID‐19
| Unmatched cohorts | Matched cohorts | |||||
|---|---|---|---|---|---|---|
| COVID‐19 Cohort | Non‐COVID‐19 cohort | Standardized difference | COVID‐19 Cohort | Non‐COVID‐19 cohort | Standardized difference a | |
| n = 5620 | n = 2,656,654 | n = 5617 | n = 11,225 | |||
| Demographics | ||||||
| Age (median, IQR) | 81 (73–88) | 74 (70–81) | 0.52 | 81 (73–88) | 81 (72–88) | 0b |
| Female, N (%) | 2134 (38.0%) | 1,417,611 (53.4%) | 0.31 | 2134 (38.0%) | 4268 (38.0%) | 0b |
| Highest income quintile | 1586 (28.2%) | 543,536 (20.5%) | 0.18 | 1585 (28.2%) | 2827 (25.2%) | 7% |
| Lowest income quintile | 914 (16.3%) | 536,212 (20.2%) | 0.1 | 914 (16.3%) | 1951 (17.4%) | 3% |
| Residing in long term care, N (%) | 2851 (50.7%) | 60,142 (2.3%) | 1.31 | 2848 (50.7%) | 5687 (50.7%) | 0b |
| Comorbidities in the previous 2 years, N (%) | ||||||
| COPD | 459 (8.2%) | 144,742 (5.4%) | 0.11 | 459 (8.2%) | 923 (8.2%) | 0 |
| Diabetes | 1563 (27.8%) | 523,953 (19.7%) | 0.19 | 1562 (27.8%) | 2420 (21.6%) | 15% |
| Congestive heart failure | 655 (11.7%) | 129,219 (4.9%) | 0.25 | 654 (11.6%) | 982 (8.7%) | 9% |
| Hypertension | 2275 (40.5%) | 759,355 (28.6%) | 0.25 | 2274 (40.5%) | 3170 (28.2%) | 26% |
| Heart disease | 311 (5.5%) | 96,015 (3.6%) | 0.09 | 310 (5.5%) | 501 (4.5%) | 5% |
| Stroke/TIA | 584 (10.4%) | 72,057 (2.7%) | 0.31 | 584 (10.4%) | 880 (7.8%) | 9% |
| Obesity | 138 (2.5%) | 53,402 (2.0%) | 0.03 | 138 (2.5%) | 149 (1.3%) | 8% |
| Chronic kidney disease | 366 (6.5%) | 108,554 (4.1%) | 0.11 | 365 (6.5%) | 537 (4.8%) | 7% |
| Cancer | 409 (7.3%) | 187,737 (7.1%) | 0.01 | 409 (7.3%) | 709 (6.3%) | 4% |
| Prior urologist visit | 615 (10.9%) | 260,107 (9.8%) | 0.04 | 615 (10.9%) | 1049 (9.3%) | 5% |
Abbreviations: IQR, interquartile range; COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease 2109; TIA, transient ischemic attack.
Standardized differences are better at demonstrating potentially clinically relevant differences (>10%) between large groups.
The variables age, sex, and residing in long‐term care were used to create the matched cohorts.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The results of our analysis of the outcomes of interest are shown in Table 2. There was no significantly increased hazard of new receipt of overactive bladder medication, urology consultation or cystoscopy among patients who had COVID‐19, compared to the matched cohort. The competing risk of death during the 3 month period when we were looking for our outcomes was low and comparable between the COVID‐19 and non COVID‐19 cohort, respectively (3.0% vs. 4.1%).
Table 2.
Outcomes of interest
| Non COVID‐19 cohort (n = 11,225) | COVID‐19 cohort (n = 5617) | |
|---|---|---|
| Receipt of overactive bladder medication | ||
| N (proportion) | 45 (0.40%) | 26 (0.46%) |
| Rate per 10,000 patient days | 0.27 | 0.31 |
| Adjusteda HR (95% CI) | Reference | 1.04 (0.64–1.70) p = 0.88 |
| Cystoscopy | ||
| N (proportion) | 78 (0.69%) | 49 (0.87%) |
| Rate per 10,000 patient days | 0.47 | 0.59 |
| Adjusteda HR (95% CI) | Reference | 1.14 (0.79–1.64) p = 0.50 |
| Urology consultation | ||
| N (proportion) | 56 (0.50%) | 45 (0.80%) |
| Rate per 10,000 patient days | 0.34 | 0.54 |
| Adjusteda HR (95% CI) | Reference | 1.40 (0.94–2.07) p = 0.10 |
Abbreviations: CI, confidence interval; COVID‐19, coronavirus disease 2019; HR, hazards ratio.
HRs have been adjusted for the presence of diabetes and hypertension.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
4. DISCUSSION
Despite being an unknown disease in 2019, COVID‐19 is now the focus of significant research efforts all over the world. We evaluated whether there was evidence to support the hypothesis that COVID‐19 infection caused long‐term bladder dysfunction, 6 consistent with initial case‐series which described COVID‐19 associated cystitis with acute SARS‐CoV‐2 infection. 4 We did not find that patients who were 66 years or older with laboratory‐confirmed COVID‐19 were at increased risk of receiving overactive bladder medication or having a urology consultation or cystoscopy in the 2–5 months after their diagnosis.
Given the novelty of this disease, there have only been a few publications on this urologic manifestation of COVID‐19. Mumm et al. 4 described a series of 7/57 (12%) male patients in Germany who complained of urinary frequency as part of their presenting symptoms of COVID‐19. The authors hypothesized that this symptom was consistent with viral cystitis. This is supported by the expression of the ACE2 receptor in the urothelium, 14 and a separate report of three patients from Italy who developed gross hematuria in the setting of COVID‐19 infection. 15 Supporting the possibility that urothelium may be directly infected by COVID‐19, a study from China demonstrated that microscopic hematuria is more common among COVID‐19 patients than controls. 16 Dhar et al. 17 described similar urinary symptoms (frequency, nocturia) as Mumm's paper in a group of 39 African American patients who had previously been hospitalized with COVID‐19. The same group of authors went on to show that COVID‐19 patients with urinary frequency had markedly elevated proinflammatory cytokines in their urine compared to matched controls. 6 They hypothesized that COVID‐19 related inflammation could lead to more permanent bladder dysfunction, with symptoms similar to overactive bladder.
Our study suggests that significant bladder dysfunction after the acute infectious period may not occur with COVID‐19. The work by Kaya et al. 18 from Turkey supports this conclusion. They reported that urinary storage symptom scores decrease after resolution of COVID‐19 infection; however, their study had a significant limitation, as patients were asked to retrospectively recall what their urinary symptoms had been at the time of acute COVID‐19, and their recollections could be inaccurate or biased.
Limitations of our research include the use of routinely collected data, which while it provides a large sample size and can be rapidly accessed for research questions, it does not contain patient reported outcome measures, or direct measures of urinary symptoms. Instead, we used surrogate markers which we hypothesized would be associated with persistent COVID‐19 associated cystitis. Consistent with research on risk factors for COVID‐19 infection, 19 and more severe disease, we found patients in our study with COVID‐19 had a higher frequency of hypertension and diabetes. We adjusted for these two variables in our analysis due to the hypothesized link between metabolic syndrome and overactive bladder, 20 however residual confounding due to other factors is still a potential limitation for this observation study. Our study only included patients over 66 years of age; this is beneficial as it restricts our study population to those who were most likely to have more severe disease (and by extension more prolonged COVID‐19 associated symptoms, 21 including possibly bladder dysfunction), however this limits the generalizability of our findings. It is possible that persistent COVID‐19 urinary symptoms are more likely in younger patients. As prescription for an overactive bladder medication in the 6 months before the COVID‐19 diagnosis was an exclusion criterion, our study could not assess whether COVID‐19 patients with pre‐existing OAB symptoms experienced worsening of their urinary storage symptoms following COVID‐19. Similarly, we choose to examine patients for indicators of COVID‐19 associated cystitis 2–5 months after their diagnosis; we felt it would be unlikely that patients would have any of the surrogate markers for urinary dysfunction during their acute infection. Finally, it is possible that patients do have chronic urinary symptoms after COVID‐19, but do not seek medical care or receive treatment for them during this time period. Similarly, it is possible that physicians are not offering treatment or referral for these symptoms.
5. CONCLUSION
After COVID‐19 infection, we did not find that patients have an increased risk of receiving overactive bladder medication, consulting with a urologist or undergoing a cystoscopy. This does not support the hypothesis that COVID‐19 leads to long‐term bladder dysfunction.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the project conception, study design, and interpretation of the data. Blayne Welk obtained funding for the project, and wrote the initial draft of the manuscript. Lucie Richard performed the statistical analysis. Lucie Richard, Emmanuel Braschi, and Marcio A Averbeck revised the work, and all authors approved the final version.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The study was supported by a grant from the St. Joseph's Health Care Foundation's Health Crisis Fund. The study was carried out by ICES Western. ICES is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). Core funding for ICES Western is provided by several partners including the Academic Medical Organization of Southwestern Ontario (AMOSO), the Schulich School of Medicine and Dentistry (SSMD), Western University, and the Lawson Health Research Institute (LHRI). The opinions, results and conclusions are those of the authors and are independent from the funding sources. No endorsement by ICES, AMOSO, SSMD, LHRI, or the MOHLTC is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by CIHI. However, the analyses, conclusions, opinions and statements expressed in the material are those of the author(s), and not necessarily those of CIHI. We thank IQVIA Solutions Canada Inc. for use of their Drug Information Database.
Welk B, Richard L, Braschi E, Averbeck MA. Is coronavirus disease 2019 associated with indicators of long‐term bladder dysfunction? Neurourology and Urodynamics. 2021;40:1200‐1206. 10.1002/nau.24682
DATA AVAILABILITY STATEMENT
The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (e.g., healthcare organizations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre‐specified criteria for confidential access, available at https://www.ices.on.ca/DAS (email: das@ices.on.ca). The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. New Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Z, Wang D, Dai Y, Zhu S, Zeng H. Urogenital system damaging manifestations of 3 human infected coronaviruses. J Urology. 2021;205(3):671‐677. [DOI] [PubMed] [Google Scholar]
- 3. Kashi AH, Rosette J, la Amini E, Abdi H, Fallah‐karkan M, Vaezjalali M. Urinary viral shedding of COVID‐19 and its clinical associations: a systematic review and meta‐analysis of observational studies. Urol J. 2020;17(5):433‐441. [DOI] [PubMed] [Google Scholar]
- 4. Mumm J‐N, Osterman A, Ruzicka M, et al. Urinary frequency as a possibly overlooked symptom in COVID‐19 patients: does SARS‐CoV‐2 cause viral cystitis? Eur Urol. 2020;78(4):624‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Z, Zhang Z, Wu S. Focus on the crosstalk between COVID‐19 and urogenital systems. J Urology. 2020;204(1):7‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lamb LE, Dhar N, Timar R, Wills M, Dhar S, Chancellor MB. COVID‐19 inflammation results in urine cytokine elevation and causes COVID‐19 associated cystitis (CAC). Med Hypotheses. 2020;145:110375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oronsky B, Larson C, Hammond TC, et al. A review of persistent post‐COVID syndrome (PPCS). Clin Rev Allerg Immu. 2021:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung H, Fung K, Ferreira‐Legere LE, et al. COVID‐19 laboratory testing in Ontario: patterns of testing and characteristics of individuals tested, as of April 30, 2020. 2020. IC/ES.
- 9. Raina P, Torrance‐Rynard V, Wong M, Woodward C. Agreement between self‐reported and routinely collected health‐care utilization data among seniors. Health Serv Res. 2002;37(3):751‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. CIHI/ICIS . CIHI data quality study of the 2009–2010 discharge abstract database. 2012;1‐139. CIHI/ICIS.
- 11. Levy AR, O'Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario drug benefit database. Canad J Clin Pharmacol. 2003;10(2):67‐71. [PubMed] [Google Scholar]
- 12. Welk B, Myers JB, Kennelly M, McKibbon M, Watson J, Gervais K. A qualitative assessment of psychosocial aspects that play a role in bladder management after spinal cord injury. Spinal Cord. 2020:1‐9. [DOI] [PubMed] [Google Scholar]
- 13. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Statistics Simul Comput. 2009;38(6):1228‐34. [Google Scholar]
- 14. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med‐prc. 2020;14(2):185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luciani LG, Gallo F, Malossini G, et al. Severe involvement of the urinary tract during COVID‐19 infection. Eur Urol. 2020;78(3):e129‐e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu R, Ma Q, Han H, et al. The value of urine biochemical parameters in the prediction of the severity of coronavirus disease 2019. Clin Chem Laboratory Medicine Cclm. 2020;58(7):1121‐1124. [DOI] [PubMed] [Google Scholar]
- 17. Dhar N, Dhar S, Timar R, Lucas S, Lamb LE, Chancellor MB. De novo urinary symptoms associated with COVID‐19: COVID‐19‐associated cystitis. J Clin Medicine Res. 2020;12(10):681‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaya Y, Kaya C, Kartal T, Tahta T, Tokgöz VY. Could LUTS be early symptoms of COVID‐19. Int J Clin Pract. 2021;75(3):e13850. [DOI] [PubMed] [Google Scholar]
- 19. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bunn F, Kirby M, Pinkney E, et al. Is there a link between overactive bladder and the metabolic syndrome in women? A systematic review of observational studies. Int J Clin Pract. 2015;69(2):199‐217. [DOI] [PubMed] [Google Scholar]
- 21. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long‐COVID: analysis of COVID cases and their symptoms collected by the COVID symptoms study app. medRxiv. 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (e.g., healthcare organizations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre‐specified criteria for confidential access, available at https://www.ices.on.ca/DAS (email: das@ices.on.ca). The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.


