Abstract
This study describes the baseline characteristics and treatment patterns of US patients hospitalized with a diagnosis of coronavirus disease 2019 (COVID‐19) and pulmonary involvement. Patients hospitalized with pulmonary involvement due to COVID‐19 (first hospitalization) were identified in the IBM Explorys® electronic health records database. Demographics, baseline clinical characteristics, and in‐hospital medications were assessed. For evaluation of in‐hospital medications, results were stratified by race, geographic region, age, and month of admission. Of 6564 hospitalized patients with COVID‐19‐related pulmonary involvement, 50.4% were male, and mean (SD) age was 62.6 (16.4) years; 75.2% and 23.6% of patients were from the South and Midwest, respectively, and 50.2% of patients were African American. Compared with African American patients, a numerically higher proportion of White patients received dexamethasone (19.7% vs. 31.8%, respectively), nonsteroidal anti‐inflammatory drugs (NSAIDs; 27.1% vs. 34.9%), bronchodilators (19.8% vs. 29.5%), and remdesivir (9.3% vs. 21.0%). Numerically higher proportions of White patients than African American patients received select medications in the South but not in the Midwest. Compared with patients in the South, a numerically higher proportion of patients in the Midwest received dexamethasone (20.1% vs. 34.5%, respectively), NSAIDs (19.6% vs. 55.7%), bronchodilators (15.9% vs. 41.3%), and remdesivir (10.6% vs. 23.1%). Inpatient use of hydroxychloroquine decreased over time, whereas the use of dexamethasone and remdesivir increased over time. Among US patients predominantly from the South and Midwest hospitalized with COVID‐19 and pulmonary involvement, differences were seen in medication use between different races, geographic regions, and months of hospitalization.
Keywords: antiviral agents, cytokine/chemokine, disease control, immune responses, immunodulators, inflammation, respiratory tract, SARS coronavirus
Research Highlights
In this retrospective, electronic health records study, including US patients hospitalized with COVID‐19 and pulmonary involvement, a numerically higher proportion of patients in the Midwest received dexamethasone, nonsteroidal anti‐inflammatory drugs, bronchodilators, and remdesivir than patients in the South.
Analysis of select medication use by the US region showed that, in the South, numerically higher proportions of White patients than African American patients received multiple select medications, including dexamethasone and remdesivir; however, in the Midwest, select medication use was generally comparable among White and African American patients.
These study results demonstrating differences in rates of medication use between different geographic regions, in addition to differences in the race within some regions and among different age groups, warrant further study.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) was first reported in China in December 2019 and quickly spread globally, 1 with the first known case in the United States (US) confirmed in January 2020. 2 After initial outbreaks in US states in the Northeast and West, outbreaks in the South followed, with spread to areas throughout the country over the course of the pandemic. 3 As of March 22, 2021, the United States had the most confirmed cases of COVID‐19 and COVID‐19‐related deaths of any country in the world, with over 29 000 000 confirmed cases and over 540 000 deaths. 4
Patients with COVID‐19 may present with mild to critical, life‐threatening illnesses. Severe COVID‐19 cases often progress to acute respiratory distress syndrome (ARDS)‐related respiratory failure, septic shock, and/or multiple organ dysfunction and failure, which are associated with high mortality rates.5, 6 Patients with severe and critical COVID‐19 illness have been shown to have elevated levels of multiple pro‐inflammatory cytokines, including interleukin‐1 (IL‐1), interleukin‐6 (IL‐6), and tumor necrosis factor α (TNF‐α), 7 which are thought to play a role in the pathogenesis of the disease.
The risk of severe infection increases with age and is higher in those with underlying conditions, such as obesity, diabetes, and hypertension.8, 9, 10, 11 In addition, racial and ethnic minority populations have been shown to have an increased risk of hospitalization and death due to COVID‐19.10, 12, 13, 14 It is important to consider the underlying reasons related to these differences, which are not well understood, but may include health and socioeconomic inequalities.12, 15
As of December 2020, only one medication, remdesivir, is approved for the treatment of COVID‐19; however, treatment of severe COVID‐19 currently involves several therapies, including corticosteroids, antiviral drugs, immunomodulatory drugs, and others, to help manage symptoms and to prevent respiratory failure. Results from studies of therapies in patients with COVID‐19 pneumonia are published daily, and government and professional organization treatment guidelines and recommendations have changed regularly as new evidence and data are published.
Awareness of the demographics, baseline clinical characteristics, and patterns of treatments of patients with COVID‐19 is important to gain a better understanding of evolving standard of care in hospitalized patients with COVID‐19 pneumonia and of potential differences by region or patient demographics. This study used the IBM Explorys® database, which includes a racially diverse group of patients primarily from the Midwest and South, to evaluate demographics, baseline characteristics, and medication use of patients hospitalized with COVID‐19 and pulmonary involvement.
2. METHODS
2.1. Study design and patient population
This retrospective analysis identified patients hospitalized with COVID‐19‐related pulmonary involvement using data from the IBM Explorys database from December 1, 2019, through August 27, 2020. The Explorys database, a convenience sample of health systems containing data on approximately 70 million patients (since 2000), comprises data from approximately 400 hospitals and 400 000 providers in 39 large US health systems, including electronic health record (EHR) data, outgoing billing, and adjudicated claims from commercial and public payers. Most patients in the database are from the Midwest (39%) and South (33%), followed by the West (18%) and Northeast (5%). Institutional Review Board (IRB) approval was not required because study data were deidentified and fully compliant with the Health Insurance Portability and Accountability Act of 1996.
Variables were measured using International Classification of Diseases, Clinical Modification (ICD‐9‐CM and/or ICD‐10‐CM) diagnosis codes, and additional EHR terminology and drug coding systems, including Systemized Nomenclature of Medicine, Healthcare Common Procedure Coding System, and National Drug Codes. Hospitalized patients with a diagnosis code for COVID‐19 or other coronavirus and a diagnosis code for at least 1 of 6 conditions representing pulmonary involvement, including pneumonia (J12.89), acute bronchitis (J20.8), bronchitis not otherwise specified (J40), lower respiratory infection (J22), associated with a respiratory infection not otherwise specified (J98.8), and acute respiratory distress syndrome (J80), based on the Centers for Disease Control and Prevention coding guidelines 16 for patients with COVID‐19, were included (Table S1). If a patient had multiple hospitalizations for COVID‐19 with pulmonary involvement, only the first hospitalization was analyzed.
2.2. Outcomes
Patient demographics, including age, sex, self‐reported race/ethnicity, and geographic region; comorbidities, including Charlson Comorbidity Index (CCI) 17 conditions and additional selected conditions; and selected prescribed medications from the baseline period of up to 12 months through 14 days before hospital admission were assessed. Body mass index (BMI), self‐reported smoking status, the month of first hospital admission, and select inpatient medications during hospitalization were captured; medications were selected based on the published literature of emerging therapeutic options. For inpatient medications, results were stratified by race, geographic region, age (<60 or ≥60 years), and month of hospitalization (patients hospitalized from December 2019 through March 2020 were grouped together). Results are descriptive, and all comparisons were qualitative in nature.
This was a retrospective, observational study, and all study data were deidentified and fully compliant with Health Insurance Portability and Accountability Act regulations (45 CFR 164.514e); therefore, approval from an IRB was not required, and informed consent was not obtained.
3. RESULTS
3.1. Baseline demographics and clinical characteristics
Of 6564 hospitalized patients who had a diagnosis of COVID‐19 and pulmonary involvement, 50.4% were male, and mean (SD) age was 62.6 (16.4) years; most patients were from the South (75.2%) or Midwest (23.6%), and 50.2% of patients self‐identified as African American and 34.8% as White (Table 1). The months of admission with the highest proportion of patients were April 2020 (30.5%), July 2020 (21.3%), and March 2020 (17.3%). The mean (SD) Deyo CCI was 0.91 (1.92), and the most common select comorbidities were hypertension (29.8%), hyperlipidemia (18.6%), and diabetes (17.8%; Table 2). Mean (SD) BMI was 32.25 (8.69), and 26.2% had a history of smoking or tobacco use. Private insurance (20.1%) and Medicare (18.9%) were the most common insurance plan types; however, for almost half of patients, insurance data were unknown.
Table 1.
Baseline demographics among hospitalized patients in the United States with COVID‐19 and pulmonary involvement
| Total patients (N = 6564) | |
|---|---|
| Age, mean (SD), years | 62.6 (16.4) |
| Median, years | 64.0 |
| Age group, n (%), years | |
| <18 | 14 (0.2) |
| 18–29 | 177 (2.7) |
| 30–39 | 427 (6.5) |
| 40–49 | 757 (11.5) |
| 50–59 | 1303 (19.9) |
| 60–69 | 1532 (23.3) |
| 70–79 | 1264 (19.3) |
| 80–89 | 774 (11.8) |
| ≥90 | 311 (4.7) |
| Missing | 5 (0.1) |
| Sex, n (%) | |
| Male | 3306 (50.4) |
| Female | 3258 (49.6) |
| Region, n (%) | |
| Northeast | 48 (0.7) |
| Midwest | 1549 (23.6) |
| South | 4936 (75.2) |
| West | 22 (0.3) |
| Unknown/missing | 9 (0.1) |
| Race, n (%) | |
| Hispanic/Latino | 6 (0.1) |
| African American | 3297 (50.2) |
| White | 2281 (34.8) |
| Asian | 73 (1.1) |
| Other | 592 (9.0) |
| Unknown/refused | 315 (4.8) |
| Insurance plan type, n (%) | |
| Private | 1321 (20.1) |
| Medicare | 1240 (18.9) |
| Medicaid | 318 (4.8) |
| Self‐pay | 421 (6.4) |
| Other | 92 (1.4) |
| Unknown | 3172 (48.3) |
| Month of hospital admission, n (%) | |
| December 2019 | 3 (<0.05) |
| January 2020 | 6 (0.1) |
| February 2020 | 5 (0.1) |
| March 2020 | 1134 (17.3) |
| April 2020 | 2000 (30.5) |
| May 2020 | 1061 (16.2) |
| June 2020 | 608 (9.3) |
| July 2020 | 1396 (21.3) |
| August 2020 | 351 (5.3) |
Table 2.
Twelve‐month baseline clinical characteristics before hospitalizationa
| Total patients (N = 6564) | |
|---|---|
| Deyo CCI | |
| Mean (SD) | 0.91 (1.92) |
| Median | 0 |
| Deyo CCI comorbidities, n (%) | |
| Mild to moderate diabetes | 763 (11.6) |
| Renal disease | 673 (10.3) |
| Chronic pulmonary disease | 570 (8.7) |
| Diabetes with chronic complications | 512 (7.8) |
| Congestive heart failure | 442 (6.7) |
| Primary cancer | 247 (3.8) |
| Cerebrovascular disease | 200 (3.0) |
| Peripheral vascular disease | 164 (2.5) |
| Dementia | 145 (2.2) |
| Myocardial infarction | 125 (1.9) |
| Rheumatologic disease | 84 (1.3) |
| Metastatic solid tumor | 59 (0.9) |
| Mild liver disease | 33 (0.5) |
| Hemiplegia and paraplegia | 30 (0.5) |
| Peptic ulcer disease | 27 (0.4) |
| HIV infection | 17 (0.3) |
| Moderate to severe liver disease | 13 (0.2) |
| Select comorbidities, n (%) | |
| Hypertension | 1958 (29.8) |
| Hyperlipidemia | 1222 (18.6) |
| Diabetes | 1169 (17.8) |
| Obesity | 761 (11.6) |
| Coronary artery disease | 505 (7.7) |
| Respiratory conditions | |
| COPD | 468 (7.1) |
| Sleep apnea | 397 (6.0) |
| Asthma | 265 (4.0) |
| Pulmonary fibrosis | 104 (1.6) |
| Organ transplant | 107 (1.6) |
| Influenza | 87 (1.3) |
| Pregnancy | 43 (0.7) |
| History of smoking/tobacco use, n (%) | 1721 (26.2) |
| Has BMI measurement, n (%) | 4629 (70.5) |
| BMI, mean (SD) | 32.25 (8.69) |
| BMI, median | 31.00 |
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; COPD, chronic obstructive pulmonary disease.
Diagnoses and prescriptions using available data from up to 12 months through 14 days before hospital admission.
3.2. Inpatient medications
Overall, the most common inpatient medications were corticosteroids (including dexamethasone and methylprednisolone; 31.3%), nonsteroidal anti‐inflammatory drugs (NSAIDs; 28.2%), bronchodilators (22.1%), hydroxychloroquine (19.6%), and remdesivir (13.5%; Table 3). Compared with African American patients, a numerically higher proportion of White patients received dexamethasone (19.7% vs. 31.8%, respectively), NSAIDs (27.1% vs. 34.9%, respectively), bronchodilators (19.8% vs. 29.5%), and remdesivir (9.3% vs. 21.0%). A comparable proportion of African American and White patients received hydroxychloroquine (21.9% vs. 20.4%, respectively; Table 3).
Table 3.
Inpatient medications used during first hospitalization stratified by race or ethnicity and US regiona
| Race or ethnicity | US region | ||||||
|---|---|---|---|---|---|---|---|
| Select medications, n (%) | All, N = 6564 | African American, n = 3297 | White, n = 2281 | Hispanic/Latino/Asian/Other/Unknown, n = 986 | South, n = 4936 | Midwest, n = 1549 | Northeast/West/Unknown, n = 79 |
| Corticosteroids | 2055 (31.3) | 898 (27.2) | 951 (41.7) | 206 (20.9) | 1289 (26.1) | 748 (48.3) | 18 (22.8) |
| Dexamethasone | 1532 (23.3) | 650 (19.7) | 726 (31.8) | 156 (15.8) | 991 (20.1) | 535 (34.5) | 6 (7.6) |
| Methylprednisolone | 407 (6.2) | 195 (5.9) | 179 (7.8) | 33 (3.3) | 285 (5.8) | 117 (7.6) | 5 (6.3) |
| NSAIDs | 1848 (28.2) | 894 (27.1) | 796 (34.9) | 158 (16.0) | 969 (19.6) | 863 (55.7) | 16 (20.3) |
| Bronchodilators | 1450 (22.1) | 654 (19.8) | 673 (29.5) | 123 (12.5) | 787 (15.9) | 640 (41.3) | 23 (29.1) |
| Hydroxychloroquine | 1289 (19.6) | 723 (21.9) | 466 (20.4) | 100 (10.1) | 988 (20.0) | 296 (19.1) | 5 (6.3) |
| Remdesivir | 883 (13.5) | 307 (9.3) | 479 (21.0) | 97 (9.8) | 522 (10.6) | 358 (23.1) | 3 (3.8) |
| ACE inhibitors | 395 (6.0) | 159 (4.8) | 191 (8.4) | 45 (4.6) | 145 (2.9) | 245 (15.8) | 5 (6.3) |
| Angiotensin II receptor blockers | 341 (5.2) | 145 (4.4) | 156 (6.8) | 40 (4.1) | 153 (3.1) | 184 (11.9) | 4 (5.1) |
| IL‐6 inhibitors | 206 (3.1) | 53 (1.6) | 123 (5.4) | 30 (3.0) | 78 (1.6) | 125 (8.1) | 3 (3.8) |
| Tocilizumab | 204 (3.1) | 53 (1.6) | 123 (5.4) | 28 (2.8) | 78 (1.6) | 125 (8.1) | 1 (1.3) |
| Sarilumab | 2 (0.0) | 0 | 0 | 2 (0.2) | 0 | 0 | 2 (2.5) |
| Alteplase | 279 (4.3) | 167 (5.1) | 94 (4.1) | 18 (1.8) | 201 (4.1) | 77 (5.0) | 1 (1.3) |
| Lopinavir/ritonavir | 11 (0.2) | 4 (0.1) | 7 (0.3) | 0 | 3 (0.1) | 7 (0.5) | 1 (1.3) |
| Dornase alfa | 3 (0.0) | 3 (0.1) | 0 | 0 | 2 (0.0) | 1 (0.1) | 0 |
| Intravenous immunoglobulin | 2 (0.0) | 1 (0.0) | 0 | 1 (0.1) | 1 (0.0) | 1 (0.1) | 0 |
| TNF inhibitors | 1 (0.0) | 0 | 0 | 0 | 0 | 1 (0.1) | 0 |
| Infliximab | 1 (0.0) | 1 (0.0) | 0 | 0 | 0 | 1 (0.1) | 0 |
Abbreviations: ACE, angiotensin‐converting enzyme; IL‐6, interleukin‐6; NSAID, nonsteroidal anti‐inflammatory drug; TNF, tumor necrosis factor.
No patient in this study received the following drugs: siltuximab, antirejection medications, chloroquine, tofacitinib, filgotinib, baricitinib, etanercept, certolizumab, golimumab, adalimumab, or baloxavir marboxil.
Compared with patients in the South, a higher proportion of patients in the Midwest received dexamethasone (20.1% vs. 34.5%, respectively), NSAIDs (19.6% vs. 55.7%), bronchodilators (15.9% vs. 41.3%), and remdesivir (10.6% vs. 23.1%; Table 3). The proportion of patients who received hydroxychloroquine was comparable in the South and Midwest.
After analysis of select medication use by US region, numerically higher proportions of White patients than African American patients in the South received multiple select medications, including dexamethasone (30.0% vs. 16.0%) and remdesivir (20.6% vs. 5.7%), but select medication use was generally comparable among White and African American patients in the Midwest (Table 4). When patients were stratified by age (<60 or ≥60 years), numerically higher proportions of White patients than African American patients in both age groups received multiple select medications, including dexamethasone (<60 years: 35.6% vs. 23.0%; ≥60 years, 30.1% vs. 17.3%) and remdesivir (<60 years: 24.7% vs. 11.2%; ≥60 years, 19.3% vs. 7.9%; Table 4).
Table 4.
Proportion of African American and White patients treated with select medications by US region and age group
| US Region | Age group | |||||||
|---|---|---|---|---|---|---|---|---|
| South | Midwest | <60 years | ≥60 years | |||||
| Select medications, n (%) | African American, n = 2706 | White, n = 1495 | African American, n = 580 | White, n = 749 | African American, n = 1429 | White, n = 721 | African American, n = 1865 | White, n = 1558 |
| Corticosteroids | 602 (22.2) | 556 (37.2) | 294 (50.7) | 382 (51.0) | 441 (30.9) | 322 (44.7) | 457 (24.5) | 629 (40.4) |
| Dexamethasone | 432 (16.0) | 448 (30.0) | 217 (37.4) | 275 (36.7) | 328 (23.0) | 257 (35.6) | 322 (17.3) | 469 (30.1) |
| Methylprednisolone | 151 (5.6) | 117 (7.8) | 44 (7.6) | 58 (7.7) | 101 (7.1) | 43 (6.0) | 94 (5.0) | 136 (8.7) |
| NSAIDs | 523 (19.3) | 378 (25.3) | 369 (63.6) | 407 (54.3) | 447 (31.3) | 278 (38.6) | 446 (23.9) | 517 (33.2) |
| Bronchodilators | 402 (14.9) | 318 (21.3) | 249 (42.9) | 337 (45.0) | 287 (20.1) | 183 (25.4) | 367 (19.7) | 490 (31.5) |
| Hydroxychloroquine | 629 (23.2) | 292 (19.5) | 94 (16.2) | 169 (22.6) | 320 (22.4) | 128 (17.8) | 403 (21.6) | 338 (21.7) |
| Remdesivir | 154 (5.7) | 308 (20.6) | 153 (26.4) | 170 (22.7) | 160 (11.2) | 178 (24.7) | 147 (7.9) | 301 (19.3) |
Abbreviation: NSAID, nonsteroidal anti‐inflammatory drug.
Inpatient use of hydroxychloroquine decreased over time from a peak of 54.0% in December 2019 through March 2020 to 2.0% in August 2020, whereas the use of dexamethasone and remdesivir increased over the same period, from 11.9% to 59.8% and 0.8% to 36.5%, respectively. Methylprednisone use remained relatively constant (Table 5; Figure 1). The proportion of patients who received NSAIDs and bronchodilators also remained generally comparable over time.
Table 5.
Inpatient medications used during first hospitalization stratified by month of admission between December 2019 and August 2020a
| Select medications, n (%) | December to March, n = 1148 | April, n = 2000 | May, n = 1061 | June, n = 608 | July, n = 1396 | August, n = 351 |
|---|---|---|---|---|---|---|
| Corticosteroids | 286 (24.9) | 342 (17.1) | 134 (12.6) | 201 (33.1) | 869 (62.2) | 223 (63.5) |
| Dexamethasone | 137 (11.9) | 147 (7.4) | 57 (5.4) | 168 (27.6) | 813 (58.2) | 210 (59.8) |
| Methylprednisolone | 98 (8.5) | 108 (5.4) | 36 (3.4) | 39 (6.4) | 103 (7.4) | 23 (6.6) |
| NSAIDs | 372 (32.4) | 450 (22.5) | 221 (20.8) | 198 (32.6) | 500 (35.8) | 107 (30.5) |
| Bronchodilators | 301 (26.2) | 348 (17.4) | 174 (16.4) | 158 (26.0) | 392 (28.1) | 77 (21.9) |
| Hydroxychloroquine | 620 (54.0) | 589 (29.5) | 42 (4.0) | 14 (2.3) | 17 (1.2) | 7 (2.0) |
| Remdesivir | 9 (0.8) | 5 (0.3) | 86 (8.1) | 184 (30.3) | 471 (33.7) | 128 (36.5) |
| ACE inhibitors | 41 (3.6) | 98 (4.9) | 58 (5.5) | 40 (6.6) | 125 (9.0) | 33 (9.4) |
| Angiotensin II receptor blockers | 47 (4.1) | 69 (3.5) | 40 (3.8) | 43 (7.1) | 122 (8.7) | 20 (5.7) |
| IL‐6 inhibitors | 37 (3.2) | 50 (2.5) | 30 (2.8) | 30 (4.9) | 49 (3.5) | 10 (2.8) |
| Tocilizumab | 37 (3.2) | 50 (2.5) | 30 (2.8) | 28 (4.6) | 49 (3.5) | 10 (2.8) |
| Sarilumab | 0 | 0 | 0 | 2 (0.3) | 0 | 0 |
| Alteplase | 111 (9.7) | 87 (4.4) | 25 (2.4) | 19 (3.1) | 33 (2.4) | 4 (1.1) |
| Lopinavir/ritonavir | 9 (0.8) | 2 (0.1) | 0 | 0 | 0 | 0 |
| Dornase alfa | 3 (0.3) | 0 | 0 | 0 | 0 | 0 |
| Intravenous immunoglobulin | 0 | 0 | 0 | 0 | 2 (0.1) | 0 |
| TNF inhibitors | 0 | 0 | 1 (0.1) | 0 | 0 | 0 |
| Infliximab | 0 | 0 | 1 (0.1) | 0 | 0 | 0 |
Abbreviations: ACE, angiotensin‐converting enzyme; IL‐6, interleukin‐6; NSAID, nonsteroidal anti‐inflammatory drug; TNF, tumor necrosis factor.
No patient in this study received the following drugs: siltuximab, antirejection medications, chloroquine, tofacitinib, filgotinib, baricitinib, etanercept, certolizumab, golimumab, adalimumab, or baloxavir marboxil.
Figure 1.
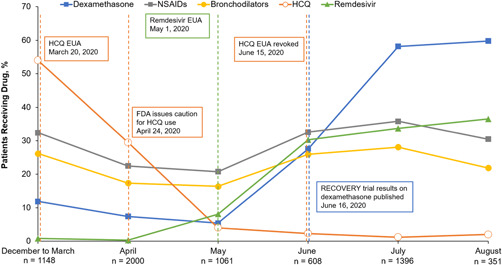
Inpatient medications used during first hospitalization by month of admission between December 2019 and August 2020. EUA, emergency use authorization; FDA, US Food and Drug Administration; HCQ, hydroxychloroquine; NSAID, nonsteroidal anti‐inflammatory drug
4. DISCUSSION
This study evaluated the baseline characteristics, clinical characteristics, and treatment patterns of US patients hospitalized with COVID‐19 and pulmonary involvement. Of 6564 hospitalized patients, the proportions of males and females were comparable, mean age was >60 years, and most patients were from the South or Midwest. The most common select comorbidities were hypertension, diabetes, and hyperlipidemia. The months of admission with the highest proportion of patients (April and July) likely reflect the peaks of hospitalization in the Midwest and South, where the majority of patients in the Explorys database are located.
Overall, 50.2% of patients in this study self‐identified as African American. This percentage is not due to an overrepresentation in the Explorys database in which approximately 10% of patients in all regions and 16% of patients in the South identify as African American. The Explorys database is reflective of the US population, which is 13% Black or African American and 60% non‐Hispanic White based on the latest US Census data. 18 Higher rates of COVID‐19 infection and pulmonary involvement in African American patients in this study may be due to a multitude of factors, including increased rates of infection in some areas of the country, as well as the increased numbers of African American or Black people who work in essential jobs that cannot be performed remotely and who have living situations that increase their potential exposure to COVID‐19. The complex effect of race in COVID‐19 disease also involves socioeconomic, health, and healthcare disparities, which are often the result of systemic inequities.12, 15
Our study showed that across all regions, for most of the select medications evaluated, a numerically smaller proportion of African American patients received the medication than White patients, with a >10% numerical difference for dexamethasone and remdesivir. Notably, when analyzed by region, the difference in medication use by race was seen in the South, but not in the Midwest. The comparisons are unadjusted for disease severity, type of hospital (academic vs. community), insurance status, and other factors, such as concomitant medications or underlying conditions that may affect the differences noted by race. Racial differences in medication use by region may reflect a multitude of factors, including access to healthcare, access to insurance coverage, and structural inequities. However, insurance coverage is unlikely to account for differences in use of a low‐cost therapy, such as dexamethasone. Also, inpatient care (including medications) is reimbursed based on diagnosis‐related group codes, so costs may not be a major factor in deciding whether patients receive a particular therapy. Although exploring all the possible reasons for regional differences in medication use by race is beyond the scope of this manuscript, these results raise important questions and warrant further study.
Within this population of patients predominantly from the Midwest and South, we found regional differences in the use of medications. Differences in the patterns of prescriptions may reflect varying treatment guidelines and implementation of evidence‐based medicine and differences in practice between hospitals and healthcare systems across regions, and variable access to medications and healthcare resources due to regional surges in COVID‐19‐related hospitalizations. Further study is warranted with regard to regional differences and potential resulting health outcome disparities.
This study demonstrated notable changes in medication use in patients with COVID‐19 and pulmonary involvement over a relatively short period of time. Hydroxychloroquine and chloroquine are approved to treat or prevent malaria, and hydroxychloroquine is approved to treat certain autoimmune conditions, including rheumatoid arthritis and systemic lupus erythematosus, due to their immunomodulatory effects. 19 The US Food and Drug Administration (FDA) granted emergency use authorization to chloroquine and hydroxychloroquine in March 2020 based on in vitro and observational studies in patients with COVID‐19. However, this authorization was revoked in June 2020 based on updated scientific evidence, indicating a lack of benefit. Results of our study show a decrease in in‐hospital hydroxychloroquine use over time; the highest proportion of patients receiving hydroxychloroquine did so in December 2019 through March 2020 (54.0%) and April (29.5%); in May, only 4.0% of patients received hydroxychloroquine, and the proportion of patients was <2.5% thereafter.
In the Adaptive COVID‐19 Treatment Trial (ACTT‐1), remdesivir was shown to shorten the time to recovery compared with placebo in patients with COVID‐19 pneumonia. 20 In May 2020, remdesivir received emergency use authorization for patients with severe COVID‐19 that was expanded in August for all hospitalized adult and pediatric patients; remdesivir received FDA approval in October. This timeline is reflected in the results of our study, which showed a notable increase in the proportion of patients receiving remdesivir, from 8.1% in May to 30.3% in June, 33.7% in July, and 36.5% in August. Dexamethasone has been shown to decrease the mortality rate in patients with COVID‐19 who were receiving either invasive mechanical ventilation or oxygen support. 21 Results of the RECOVERY trial were released in June and are reflected in our study, showing 5.4% of patients receiving dexamethasone in May, 27.6% in June, and >58% in July and August.
Research into potential COVID‐19 therapies is ongoing with hundreds of COVID‐19 clinical trials, including trials examining the safety and efficacy of immunomodulatory therapies, including IL‐1, IL‐6, and Janus kinase inhibitors, antithrombotic therapies, and interferons, among other medications, as well as large observational studies. Medication use in patients with severe COVID‐19 is primarily driven by clinical status (e.g., dexamethasone for patients who require mechanical ventilation or supplemental oxygen). As more study results are published, recommendations based on available scientific evidence and expert opinion from government and national professional societies will continue to be revised and updated to inform healthcare professionals on optimal patient care. In this unprecedented pandemic environment, understanding treatment patterns in light of the frequent and rapid release of scientific information and evaluating medication use in different racial and ethnic, and geographic populations are important to identify potential barriers to access to care.
Limitations of this study include those inherent to retrospective EHR data analysis, including potential bias and missing data (e.g., care and medications provided outside of Explorys). For almost half of patients, insurance data were missing; EHR data are not used for administrative and/or billing purposes, so insurance status is more likely to be missing than in claims data. This study included only first hospital admission; however, it is unlikely that including the small number of readmissions would have changed observed treatment patterns. Another potential limitation is that patients were included based on a COVID‐19 diagnosis, rather than a positive test. Thus, a few patients may have been presumed to have COVID‐19 and given a diagnosis of COVID‐19, particularly early in the pandemic when testing was not readily available. Furthermore, the absence of correlation between disease severity/clinical condition and medication use is an important limitation of this study because we cannot demonstrate that patients in all regions and races had equal disease severity when evaluating treatments received.
Most patients in this study were from the South and Midwest, and our results may not be generalizable to the overall US population. However, because the selection criteria included nationally used ICD codes, the selection of these populations was unintentional and likely reflects the geographic distribution of the Explorys database and the pandemic outbreak itself. As the treatment of patients with COVID‐19 is evolving in this rapidly changing and unprecedented pandemic environment, it is possible that treatment patterns have changed over time by region and race, and these potential trends were not evaluated. In part due to this ever‐changing treatment environment, this study reported on only select medications (based on published literature of emerging therapies), and data on the use of other COVID‐19 therapies, including convalescent plasma or anticoagulants, were not evaluated. Despite these limitations, this study using a large database, including a racially diverse group of patients, reveals important information about differences in medication use in patients with COVID‐19 and pulmonary involvement. Identification and awareness of disparities in medication use are the first steps, and additional studies are warranted to further evaluate and understand these study findings.
5. CONCLUSION
This retrospective EHR study included US patients hospitalized with COVID‐19 and pulmonary involvement predominantly from the South and Midwest. These study results, which demonstrate different rates of medication use between geographic regions, in addition to differences in medication use among African American and White patients within some regions and age groups, warrant further study.
CONFLICT OF INTEREST
Jennie H. Best, Shalini V. Mohan, and James L. Zazzali are employees and shareholders of Genentech, Inc. Amanda M. Kong was an employee of IBM Watson Health at the time of this study and manuscript preparation. Krutika Jariwala‐Parikh is an employee of IBM Watson Health. Emma Kaplan‐Lewis is a principal investigator for a Genentech‐sponsored clinical trial at Elmhurst Hospital Center, with no direct financial support received, and has served on medical advisory boards for ViiV Pharmaceuticals. Otis W. Brawley reports no conflicts of interest. Rachel Baden is a principal investigator for a Genentech‐sponsored clinical trial at Highland Hospital, with no direct financial support received. Karen S. Miller is a principal investigator for a Genentech‐sponsored clinical trial at St Luke′s Health System, with no direct financial support received. James Loveless has served as an investigator for Genentech‐sponsored clinical trials, and as an advisor and on a speaker′s bureau for Genentech, all outside of the submitted work.
AUTHOR CONTRIBUTIONS
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All authors had access to the data, provided critical feedback, and contributed to the writing of the manuscript. Jennie H. Best and Shalini V. Mohan conceived and designed the study. Amanda M. Kong provided subject matter expertise, and Krutika Jariwala‐Parikh conducted programming and data analysis support. Jennie H. Best, Amanda M. Kong, Emma Kaplan‐Lewis, Otis W. Brawley, Rachel Baden, James L. Zazzali, Karen S. Miller, James Loveless, and Shalini V. Mohan contributed to the interpretation of the results.
ETHICS STATEMENT
This was a retrospective, observational study, and all study data were deidentified and fully compliant with Health Insurance Portability and Accountability Act regulations (45 CFR 164.514e); therefore, approval from an Institutional Review Board was not required, and informed consent was not obtained.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors would like to thank Boris Ivanov of IBM Watson Health for programming assistance on this study. This study was funded by Genentech, Inc., South San Francisco, CA, USA. Genentech, Inc., was involved in the design of the study; analysis and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Best JH, Kong AM, Kaplan‐Lewis E, et al. Treatment patterns in US patients hospitalized with COVID‐19 and pulmonary involvement. J Med Virol. 2021;93:5367‐5375. 10.1002/jmv.27049
REFERENCES
- 1. Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. J Am Med Assoc. 2020;323(8):709‐710. [DOI] [PubMed] [Google Scholar]
- 2. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oster AM, Kang GJ, Cha AE, Beresovsky V, Rose CE, Rainisch G. Trends in number and distribution of Covid‐19 hotspot counties – United States, March 8–July 15, 2020. Morb Mortal Wkly Rep. 2020;69(33):1127‐1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Coronavirus COVID‐19 global cases. Accessed March 22, 2021. https://coronavirus.jhu.edu/map.html
- 5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. J Am Med Assoc. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 6. Pascarella G, Strumia A, Piliego C, et al. COVID‐19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Costela‐Ruiz VJ, Illescas‐Montes R, Puerta‐Puerta JM, Ruiz C, Melguizo‐Rodríguez L. SARS‐CoV‐2 infection: the role of cytokines in COVID‐19 disease. Cytokine Growth Factor Rev. 2020;54:62‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coronavirus Disease 2019 Case Surveillance – United States, January 22–May 30, 2020. Morb Mortal Wkly Rep. Centers for Disease Control and Prevention. Accessed September 8, 2020. https://www.cdc.gov/mmwr/volumes/69/wr/mm6924e2.htm
- 9. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory‐confirmed coronavirus disease 2019 ‐ COVID‐NET, 14 states, March 1‐30, 2020. Morb Mortal Wkly Rep. 2020;69(15):458‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suleyman G, Fadel RA, Malette KM, et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw Open. 2020;3(6):e2012270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pan D, Sze S, Minhas J, et al. The impact of ethnicity on clinical outcomes in COVID‐19: a systematic review. EClinicalMedicine. 2020:100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Price‐Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid‐19. N Engl J Med. 2020;382:2534‐2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karaca‐Mandic P, Georgiou A, Sen S. Assessment of COVID‐19 hospitalizations by race/ethnicity in 12 states. JAMA Intern Med. 2020;382:2534‐2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El‐Khatib Z, Jacobs G, Ikomey G, Neogi U. The disproportionate effect of COVID‐19 mortality on ethnic minorities: genetics or health inequalities? EClinicalMedicine. 2020;23:100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. ICD‐10‐CM official coding guidelines‐supplement coding encounters related to COVID‐19 coronavirus outbreak effective: February 20, 2020. https://www.cdc.gov/nchs/data/icd/ICD-10-CM-Official-Coding-Gudance-Interim-Advice-coronavirus-feb-20-2020.pdf
- 17. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613‐619. [DOI] [PubMed] [Google Scholar]
- 18.United States Census Bureau. Quick Facts, United States Population Estimates v2019. Accessed September 25, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 19. Ben‐Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42(2):145‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid‐19 – final report. N Engl J Med. 2020;383:1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horby P, Lim WS, Emberson J, et al. Dexamethasone in hospitalized patients with Covid‐19 – preliminary report. N Engl J Med. 2020:NEJMoa2021436. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


