Abstract
Objectives
To understand the sequelae of COVID‐19.
Methods
We followed up 1174 patients with severe coronavirus disease 2019 (COVID‐19)who were recovered and discharged for 6 months.
Results
There were 175 cases with clear IgG results 6 months after discharge, of which 82 (46.9%) were IgG (+) and 16 (9.1%) were IgG (dim+). Four hundred and forty‐one participants (55.4%) had some kind of sequelae. The most common symptoms were fatigue (25.3%), sleep disorder (23.2%) and shortness of breath (20.4%). In those who had sequelae, 262 (59.4%) had more than one symptom. Critical cases were more likely to have cough (20.5% vs 11.6%, p = 0.023) and hypomnesis (15.1% vs 8.0%, p = 0.041) than severe cases. Furthermore, univariate and multivariate logistic regression analyses revealed that women are more likely to have multiple symptoms (p = 0.002), fatigue (p = 0.009) and sleep disorder (p = 0.008), whereas critical illness was found as independent risk factor for hypomnesis (p = 0.045).
Conclusion
Our study demonstrated the duration of antibody and sequelae of COVID‐19 and compared the differences amongst different populations.
Keywords: COVID‐19, follow‐up, SARS‐CoV‐2, symptom
Introduction
The coronavirus disease 2019 (COVID‐19) has been rapidly spreading nationwide and abroad. Although the clinical characteristics, treatment and prognosis of COVID‐19 have been extensively studied [1], fewer studies focused on longitudinal observation for COVID‐19. Severe acute respiratory syndrome (SARS) and Middle Eastern respiratory syndrome (MERS) coronaviruses have been reported to be accompanied by some physical and mental symptoms during long‐term follow‐up [2, 3]. It was suggested that patients with COVID‐19 might have psychiatric sequelae based on the characteristics of SARS and MERS [4]. In this line, some studies have described the clinical sequelae of discharged patients with COVID‐19 and compared the laboratory features of COVID‐19 and other viral pneumonias in the recovery stages [5]. But unfortunately, the number of cases in these studies was less and the time of follow‐up was short. Consequently, the possible sequelae of COVID‐19 are largely unknown due to novel nature of the disease and unavailability of any existing applicable guideline for evaluating post‐COVID‐19 health‐related issues. In this study, we followed up patients who had recovered from COVID‐19 for a longer period of time and analysed the characteristics of symptoms complained by these participants.
Materials and methods
Item generation
We used four sources of information to develop the content of follow‐up: 1) the literature on health outcomes in COVID‐19 (which was scarcely available) and SARS‐CoV‐1; 2) symptom characteristics of COVID‐19 at the time of onset; 3) the expert opinions of key health professionals involved in COVID‐19 therapy and care; and 4) interviews with 30 patients who had recovered from COVID‐19. The items included respiratory system, circulatory system and digestive system symptoms such as cough, itchy throat, shortness of breath, chest pains, developing new hypertension, abdominal pain and diarrhoea; neurological symptoms such as dizziness, headache and hypomnesis; and other symptoms including muscle pain, backache, sleep disorder, fatigue, mental symptoms, hair loss and sweat.
Recruitment of patients and data collection
This cohort study included three institutions: Zhongnan Hospital of Wuhan University, No. 7 Hospital of Wuhan and Leishenshan Hospital (Wuhan, China). All patients in this study were previously confirmed COVID‐19 positive by RT‐PCR tests for SARS‐CoV‐2. The severity of COVID‐19 was defined according to the Chinese management guideline for COVID‐19 (version 7.0) [6]. The definition of severity was as follows: mild (i.e. mild clinical symptoms without imaging feature of pneumonia); moderate (i.e. clinical symptoms such as fever and cough, with imaging feature of pneumonia); severe (i.e. dyspnoea, respiratory frequency ≥ 30/min, blood oxygen saturation ≤ 93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio < 300 and/or lung infiltrates > 50% within 24 to 48 hours); and critical ill cases (i.e. respiratory failure, septic shock and/or multiple organ dysfunction or failure). In this analysis, severe cases included the severe and critical ill classification in the 7th edition. All patients were discharged between February and March, and we followed up by telephone for 6 months after they recovered and discharged in August and September. Follow‐up staff asked about their symptoms, and results of the latest SARS‐CoV‐2 nucleic acid, special IgM/IgG antibody and CT of the chest were recorded. During the treatment, whether intubation was performed and the length of hospital stay were also collected retrospectively. This study was conducted according to the principles of Helsinki and approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (No. 2020074).
Statistical analysis
Descriptive statistics for demographic information were calculated. The results were expressed as either the median or interquartile range (IQR), or the categorical data were summarized as percentage of the total group. We used the chi‐square test or Fisher’s exact test for categorical variables to compare differences between different groups using the IBM SPSS software, version 25.0. Univariate and multivariate logistic regression models were used to explore risk factors associated with multiple symptoms and neurological symptoms. All reported p values were two‐sided at significance level of 0.05.
Results
In total, 1174 severe COVID‐19 rehabilitation patients were enrolled in this cohort study. Amongst those survivors, 378 were not followed up or provided incomplete data. Finally, the data from 796 participants were available for analysis. The analysis flow chart is shown in Fig. 1a. Baseline characteristics of the participants were collected as shown in Table 1. There were 404 men (50.8%) and 392 women (49.2%), with 723 (90.8%) severe cases and 73 (9.2%) critically ill cases. The median age was 62.0 years (range: 20‐97 years; interquartile range (IQR): 51.0‐69.0 years). The median in‐hospital days were 21.0 days (IQR: 14.0‐27.0 days). There were 38 admitted to ICU and 17 experienced intubation during the stay in hospital. The follow‐up results are summarized in Table 1 and Table S1.
Fig. 1.
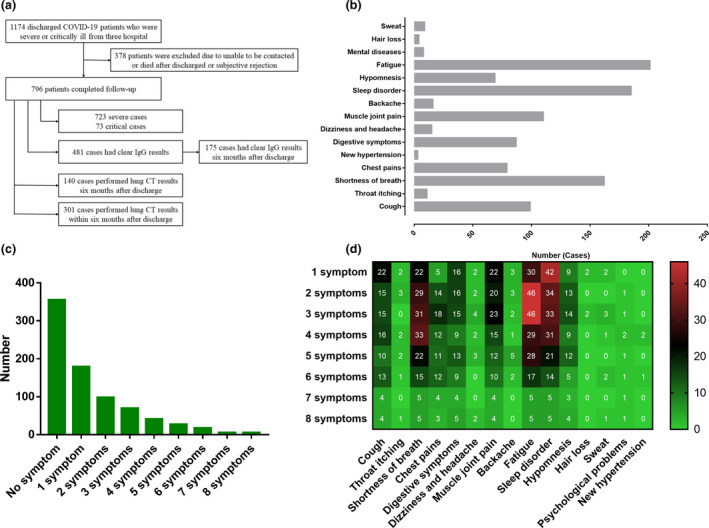
The flow chart of analysis, and the number of cases and the distribution of symptoms. The flow chart (a); the number of cases of symptoms followed up in patients recovering from severe COVID‐19 (b); the number of cases with different total symptoms (c); and the distribution of symptoms with different total symptoms (d).
Table 1.
Baseline characteristics of the participants and the follow‐up results of laboratory and CT findings
| Characteristics | Number (n = 796) | Percentage (%) |
|---|---|---|
| Basic information | ||
| Age (years) (median/IQR) | 62.0 | 51.0‐69.0 |
| Age > 65 years | 317 | 39.8 |
| Male/Female | 404/392 | 50.8/49.2 |
| Severe/Critical | 723/73 | 90.8/9.2 |
| ICU admission | 38 | 4.8 |
| Intubation | 17 | 2.1 |
| In‐hospital days (median/IQR) | 21.0 | 14.0‐27.0 |
| Follow‐up results of laboratory and CT findings | ||
| SARS‐CoV‐2 (+) in overall populations | 0 | 0 |
| IgM (+) in overall populations (n = 532) | 12 | 2.3 |
| IgG (+) in overall populations (n = 481) | 223 | 46.4 |
| IgG (+) 6 months after discharge (n = 175) | 82 | 46.9 |
| IgG (dim+)6 months after discharge (n = 175) | 16 | 9.1 |
| CT abnormal 6 months after discharge (n = 140) | 73 | 52.1 |
In the 796 participants, all were SARS‐CoV‐2 nucleic acid‐negative, 12 were IgM‐positive, and the IgG‐positive rate was 46.4% in overall populations. One hundred and seventy‐five cases had clear IgG results 6 months after discharge, of which 82 (46.9%) were IgG (+) and 16 (9.1%) were IgG (dim+) (Table 1). In addition, 140 cases had clear chest CT results 6 months after discharge, and 73 (52.1%) had abnormal CT (Table 1). The findings of abnormal chest CT included ground‐glass opacity, nodules, fibrosis and inflammation. For follow‐up symptoms, 441 participants (55.4%) had any sequelae. The most common symptoms were fatigue (25.3%), sleep disorder (23.2%) and shortness of breath (20.4%), followed by muscle joint pain (13.8%), cough (12.4%), digestive symptoms (10.9%), chest pains (9.9%) and hypomnesis (8.7%) (Fig. 1b). In those who had sequelae, 262 (59.4%) had more than one symptom. The number of cases and the distribution of symptoms with different total symptoms are shown in Fig. 1c,d.
The comparison between severe and critical illness, between men and women, and between different age groups is shown in Table 2. In all, 84 of 723 (11.6%) severe cases and 15 of 73 (20.5%) critical cases had cough (p = 0.023), whereas 8.0% of severe cases and 15.1% of critical cases had sequelae of hypomnesis (p = 0.041). Other symptoms had no statistically significant difference between severe and critical illnesses. Eighty‐six of 404 (21.3%) men and 115 of 392 (29.3%) women had fatigue sequelae (p = 0.009), and 19.3% of men and 27.3% of women suffered from sleep disorder (p = 0.008). One man and 7 women had signs of depression and anxiety (p = 0.036). Other symptoms had no statistically significant difference between men and women. No difference was found between age groups. Furthermore, univariate and multivariate logistic regression analyses revealed that women are more likely to have multiple symptoms (p = 0.002) (Table 3), fatigue (p = 0.009) and sleep disorder (p = 0.008) (Fig. 2a,b,c), whereas critical illness was found as independent risk factor for hypomnesis (p = 0.045) (Fig. 2d).
Table 2.
The comparison between severe and critical illness, between men and women, and between different age groups
| Characteristics | Severe | Critical | p value | Male | Female | p value | ≤65 years | >65 years | p value |
|---|---|---|---|---|---|---|---|---|---|
| Cough | 84 (11.6) | 15 (20.5) | 0.023 | 57 (14.1) | 42 (10.7) | 0.147 | 59 (12.3) | 40 (12.6) | 0.900 |
| Throat itching | 11 (1.5) | 0 | 0.612 | 3 (0.7) | 8 (2.0) | 0.117 | 6 (1.3) | 5 (1.6) | 0.761 |
| Shortness of breath | 143 (19.8) | 19 (26.0) | 0.206 | 72 (17.8) | 90 (23.0) | 0.072 | 101 (21.1) | 61 (19.2) | 0.527 |
| Chest pains | 70 (9.7) | 9 (12.3) | 0.471 | 36 (8.9) | 43 (11.0) | 0.331 | 55 (11.5) | 24 (7.6) | 0.071 |
| Digestive symptoms | 76 (10.5) | 11 (15.1) | 0.234 | 36 (8.9) | 51 (13.0) | 0.064 | 50 (10.4) | 37 (11.7) | 0.585 |
| Dizziness/headache | 15 (2.1) | 0 | 0.384 | 4 (1.0) | 11 (2.8) | 0.06 | 9 (1.9) | 6 (1.9) | 0.989 |
| Muscle joint pain | 95 (13.1) | 15 (20.5) | 0.080 | 47 (11.6) | 63 (16.1) | 0.07 | 74 (15.4) | 36 (11.4) | 0.101 |
| Backache | 15 (2.1) | 1 (1.4) | 0.999 | 9 (2.2) | 7 (1.8) | 0.657 | 10 (2.1) | 6 (1.9) | 0.848 |
| Fatigue | 183 (25.3) | 18 (24.7) | 0.902 | 86 (21.3) | 115 (29.3) | 0.009 | 125 (26.1) | 76 (24.0) | 0.500 |
| Sleep disorder | 172 (23.8) | 13 (17.8) | 0.249 | 78 (19.3) | 107 (27.3) | 0.008 | 111 (23.2) | 74 (23.3) | 0.956 |
| Hypomnesis | 58 (8.0) | 11 (15.1) | 0.041 | 32 (7.9) | 37 (9.4) | 0.447 | 47 (9.8) | 22 (6.9) | 0.159 |
| Hair loss | 4 (0.6) | 0 | 0.999 | 2 (0.5) | 2 (0.5) | 0.999 | 4 (0.8) | 0 | 0.155 |
| Sweat | 9 (1.2) | 0 | 0.999 | 3 (0.7) | 6 (1.5) | 0.334 | 5 (1.0) | 4 (1.3) | 0.747 |
| Psychological | 8 (1.1) | 0 | 0.999 | 1 (0.2) | 7 (1.8) | 0.036 | 7 (1.5) | 1 (0.3) | 0.155 |
| New hypertension | 3 (0.4) | 0 | 0.999 | 1 (0.2) | 2 (0.5) | 0.619 | 0 | 3 (0.9) | 0.063 |
The significant p values are in bold.
Table 3.
Comparison of clinical characteristics between different symptoms
| Characteristics | Number of symptoms | p value | Symptoms | p value | ||
|---|---|---|---|---|---|---|
| 0‐1 symptom (n = 534) Number (%) | >1 symptom (n = 262) Number (%) |
No (n = 355) Number (%) |
Yes (n = 441) Number (%) |
|||
| Age > 65 years | 220 (41.2) | 97(37.9) | 0.258 | 143 (40.3) | 174 (39.5) | 0.813 |
| Sex/female | 242 (45.3) | 150 (57.3) | 0.002 | 162 (45.6) | 230 (52.2) | 0.067 |
| Severity/critical | 52 (9.7) | 21 (8.0) | 0.429 | 32 (9.0) | 41 (9.3) | 0.891 |
| ICU admission | 26 (4.9) | 12 (4.6) | 0.858 | 16 (4.5) | 22 (5.0) | 0.751 |
| Intubation | 14 (2.7) | 3 (1.2) | 0.176 | 9 (2.5) | 8 (1.8) | 0.484 |
| In‐hospital days (median/days) | 20.0 | 22.0 | 0.141 | 21.0 | 20.0 | 0.938 |
Fig. 2.
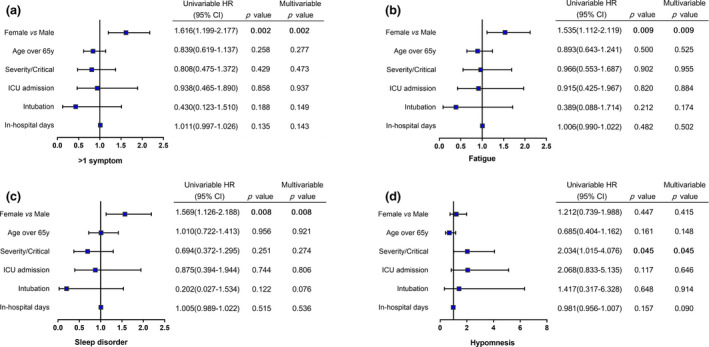
Risk factors associated with the sequelae after discharge. Risk factors associated with > 1 symptoms (a), fatigue (b), sleep disorder (c) and hypomnesis (d) after discharge. HR, hazard ratio.
Discussion
Overall, our study shows that 6 months after recovering from COVID‐19, more than half of patients had at least one common sequelae, of which nearly 60% experienced more than one symptom. The most common symptoms were fatigue, sleep disorder and shortness of breath, which was similar to other studied [7]. As we all know, respiratory symptoms were one of the most common clinical manifestations of COVID‐19 upon admission [8]. Lung damage of varying extent has been found on CT in at least 80% of patients with SARS‐CoV‐2 infection [9]. Extensive injury to alveolar epithelial cells and endothelial cells, with secondary fibroproliferation, is a signature of pulmonary SARS‐CoV‐2 infection [10]. Frija‐Masson et al reported that 1 month after SARS‐CoV‐2 infection, most patients have mild alterations of lung function [11]. Previous studies have demonstrated that recovered patients with coronavirus pneumonia can be left with damaged lungs [12]. The patients still have shortness of breath 6 months after COVID‐19 recovery, indicating that some patients may fail to achieve complete recovery of lung function leading to respiratory tract‐related problems, which could last for months or even years. Our conclusions that about half of the cases still had residual lesions of chest CT 6 months after discharge also support the long‐term existence of abnormal lung lesions.
It was reported that patients with COVID‐19 commonly have neurological manifestations, including headache, altered mental status, and other central nervous system manifestations or peripheral nervous system manifestations [13]. ACE2 was identified as the functional receptor for SARS‐CoV‐2, which is present in multiple human organs, including nervous system and skeletal muscles [14]. Autopsy results of a patient with COVID‐19 identified the SARS‐CoV‐2 in neural and capillary endothelial cells in the frontal lobe [15]. The combination of direct viral neuro‐invasion and the sequelae of systemic hyperactive immune responses could contribute to the neurological manifestations of COVID‐19 [16]. Neurological involvement is greater in severe infection, and patients with severe infection were more likely to develop neurological manifestations [13]. In this study, 44.0% patients complained they suffered hypomnesis, dizziness and headache, backache, muscle joint pain, fatigue or sleep disorder 6 months after discharge. This indicated that the sequelae of neurological symptoms, musculoskeletal disorders or general disorder may last at least several months. Compared with the severe group, the critical group had higher incidence rate of hypomnesis. More severe cytokine storm and systemic SARS‐CoV‐2 infection in critically ill patients may lead to more serious neurological damage and long‐lasting sequelae [17]. We should try our best to prevent mild or moderate status from developing severe or critical status, and prevent severe status from developing critical status of the disease.
Our study found that 23.2% of patients with COVID‐19 had a major psychiatric disorder characterized by sleep disorders. Only 8 people showed symptoms such as signs of depression and anxiety. This may be resulted from the reluctance of some people to describe their psychosocial states to us. Accurate data may require the support with questionnaires and psychometric scales. In the present analysis, women are found more prone to had multiple symptoms and are more likely to experience fatigue, sleep disorder and mental symptoms, so we should pay attention to women's neurological and mental health during and after the epidemic. Alopecia may be a unique sequela of COVID‐19 survivors. Different from the study of Xiong et al [7], who found that all hair loss occurred in female recovered from COVID‐19, we found that both men and women had hair loss symptom, and the difference was not statistically significant. Studies have shown that inflammation is associated with alopecia, but its pathogenesis, clinical course and treatment need to be further studied [18].
Several studies have shown, with a variety of tests, that 100% of severe COVID‐19 patients have seroconverted about 3 weeks after symptom onset [19]. Our previous study also showed that the prevalence of IgG was 89.1% and had a downward trend within 81 days [20]. In the present study, the prevalence of IgG was 46.9% 6 months after discharge. It was reasonable to speculate that the IgG might disappear over time. This reminds us that even people who have obtained protective antibodies should have the awareness of preventing reinfection. In addition, once the SARS‐CoV‐2 vaccine has been developed and administered to the general population, the duration of antibodies in the body should be monitored. It is worth noting that IgM was positive in some cases during follow‐up. It was unclear whether IgM continued to be positive or developed progressively from negative to positive. There was a possibility that the remaining virus in the body will be activated again when the body's immunity declines, and it is possible to reinfect. What is certain is that there were no confirmed COVID‐19 cases reported in Wuhan during the follow‐up period. False positives caused by the test kit cannot be excluded.
The main limitation of our study is its descriptive nature and that most of the symptoms were recorded as patient’s subjective descriptions. We did not obtain the specific severity of the patient's symptoms, and thus, there is no quantitative data analysis. Epidemic diseases have a psychological impact on both infected and noninfected individuals. The results of the study were only obtained from patients who have recovered from the COVID‐19 and have not been compared with non‐COVID‐19 populations in the epidemic environment. Finally, the data for follow‐ups are entirely from Wuhan, China. Besides, the population in this study is relatively small. Therefore, it may not be fully representative of the population in China or even the world.
Our study provides a preliminary database for the physical and psychological profile of patients with COVID‐19 at sixth‐month follow‐up. To our knowledge, this is the first report on detailed medium or long‐term follow‐ups of patients who recovered from severe COVID‐19. We recommend that measures should be taken to prevent mental health problems, and a comprehensive plan should be initiated to assist patients with COVID‐19 to recover from physical and psychological disorders. Further follow‐up is needed to assess whether these symptoms are persistent and to identify the duration of protective antibodies.
Conflict of interest
The authors have declared that no competing interest exists.
Supporting information
Table S1 The follow‐up symptoms.
Acknowledgements
We acknowledge all patients with COVID‐19 in Zhongnan Hospital of Wuhan University, No. 7 Hospital of Wuhan and Leishenshan Hospital (Wuhan, China).
Shang YF, Liu T, Yu JN, Xu XR, Zahid KR, Wei YC, Wang XH, Zhou FL (Zhongnan Hospital of Wuhan University, Wuhan; Shenzhen University, Shenzhen; Zhongnan Hospital of Wuhan University, Wuhan, China). Half‐year follow‐up of patients recovering from severe COVID‐19: Analysis of symptoms and their risk factors (Brief Report). J Intern Med 2021; 290: 444–450. 10.1111/joim.13284
Contributor Information
X. H. Wang, Email: wangxinghuan@whu.edu.cn.
F. L. Zhou, Email: zhoufuling@whu.edu.cn.
References
- 1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmed H, Patel K, Greenwood DC, Halpin S, Lewthwaite P, Salawu A, et al. Long‐term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta‐analysis. J Rehabil Med. 2020;52:jrm00063. [DOI] [PubMed] [Google Scholar]
- 3. Ong KC, Ng AW, Lee LS, Kaw G, Kwek SK, Leow MK, et al. 1‐year pulmonary function and health status in survivors of severe acute respiratory syndrome. Chest. 2005;128:1393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar‐Poli P, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta‐analysis with comparison to the COVID‐19 pandemic. Lancet Psychiatry. 2020;7:611–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao G, Su Y, Sun X, Cui X, Dang L, Zhao L, et al. A comparative study of the laboratory features of COVID‐19 and other viral pneumonias in the recovery stage. J Clin Lab Anal. 2020;34:e23483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Commission NH . Chinese management guideline for COVID‐19 (version 7.0). People's Republic of China: Medical Administration Hospital Authority; 2020. [Google Scholar]
- 7. Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, et al. Clinical sequelae of COVID‐19 survivors in Wuhan, China: a single‐centre longitudinal study. Clin Microbiol Infect. 2021;27:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matangila JR, Nyembu RK, Telo GM, Ngoy CD, Sakobo TM, Massolo JM, et al. Clinical characteristics of COVID‐19 patients hospitalized at Clinique Ngaliema, a public hospital in Kinshasa, in the Democratic Republic of Congo: A retrospective cohort study. PLoS One. 2020;15:e0244272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dai H, Zhang X, Xia J, Zhang T, Shang Y, Huang R, et al. High‐resolution chest CT features and clinical characteristics of patients infected with COVID‐19 in Jiangsu, China. Int J Infect Dis. 2020;95:106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Venkataraman T, Frieman MB. The role of epidermal growth factor receptor (EGFR) signaling in SARS coronavirus‐induced pulmonary fibrosis. Antiviral Res. 2017;143:142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frija‐Masson J, Debray MP, Gilbert M, Lescure FX, Travert F, Borie R, et al. Functional characteristics of patients with SARS‐CoV‐2 pneumonia at 30 days post‐infection. Eur Respir J. 2020;56:2001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, et al. Abnormal pulmonary function in COVID‐19 patients at time of hospital discharge. Eur Respir J. 2020;55:2001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China. JAMA Neurol. 2020;77:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single‐cell RNA expression profiling of ACE2, the receptor of SARS‐CoV‐2. Am J Respir Crit Care Med. 2020;202:756–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paniz‐Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). J Med Virol. 2020;92:699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID‐19 virus targeting the CNS: tissue distribution, host‐virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–8. [DOI] [PubMed] [Google Scholar]
- 17. Matschke J, Lutgehetmann M, Hagel C, Sperhake JP, Schroder AS, Edler C, et al. Neuropathology of patients with COVID‐19 in Germany: a post‐mortem case series. Lancet Neurol. 2020;19:919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saceda‐Corralo D, Pindado‐Ortega C, Moreno‐Arrones OM, Ortega‐Quijano D, Fernandez‐Nieto D, Jimenez‐Cauhe J, et al. Association of inflammation with progression of hair loss in women with frontal fibrosing Alopecia. JAMA Dermatol. 2020;156:700–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med. 2020;26:845–8. [DOI] [PubMed] [Google Scholar]
- 20. Shang Y, Liu T, Li J, Wang X, Zhou F. Factors affecting antibody response to SARS‐CoV‐2 in patients with severe COVID‐19. J Med Virol. 2021;93:612–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 The follow‐up symptoms.


