Abstract
Cardiac remodeling consisted of ventricular hypertrophy and interstitial fibrosis is the pathological process of many heart diseases. Fibroblasts as one of the major cells in the myocardium regulate the balance of the generation and degeneration of collagen, and these cells transform toward myofibroblasts in pathological state, contributing to the remodeling of the heart. Peroxisome proliferator-activated receptor-γ (PPAR-γ) coactivator-1α (PGC-1α) is vital to the function of mitochondria, which contributes to the energy production and reactive oxidative species (ROS)-scavenging activity in the heart. In this study, we found that fibroblast-specific PGC-1α KO induced cardiac remodeling especially fibrosis, and Angiotensin II (AngII) aggravated cardiac fibrosis, accompanied with a high level of oxidative stress response and inflammation.
Keywords: cardiac fibroblast, fibrosis, PGC-1α, cardiac remodeling, AngII
Introduction
Cardiac remodeling is the main pathological mechanism of heart failure which is characterized by left ventricular hypertrophy and interstitial fibrosis (1, 2). Hypertension is the most common cause of cardiac remodeling, in which the renin–angiotensin system (RAS) plays an important role. AngII is a key trigger of heart remodeling, which stimulates the expression of TGF-β through the angiotensin type 1 receptor, contributing to cardiac hypertrophy and fibrosis (3). The myocardium is composed of several cell types and vascular and neuronal networks. The types of cells include cardiomyocytes, cardiac fibroblasts (CFs), and endothelial cells. Also, there is abundant and complex extracellular matrix (ECM) in the interstitium, including several types of collagen proteins. Among these, type I collagen proteins account for more than 70% (4), which is critical to maintaining the structural integrity of the heart and generates a stress-tolerant network. CFs are a key source of ECM and are responsible for the homeostasis of the ECM, contributing to tissue repair and fibrosis (5). Acute stimulation or progressive damage induces necrosis or apoptosis of cardiomyocytes, followed by phagocytosis of neighboring cells. Myofibroblasts surround the injury area and produce interstitial collagen, resulting in cardiac remodeling (6, 7), but extensive ECM accumulation will lead to the dysfunction of the myocardium and finally result in heart failure. In fact, there are no activated myofibroblasts in the healthy myocardium, and CFs transfer to myofibroblasts and migrate to the injured area upon cardiac damage (8). In addition, myofibroblasts are also derived from epithelial and endothelial cells through the process of epithelial–mesenchymal transition (EMT) and endothelial–mesenchymal transition (EndMT), respectively (9, 10). Moreover, myofibroblasts are characterized by the expression of alpha-smooth muscle actin (α-SMA), Cofilin, Palladin 4lg, and other markers (11–13). As mentioned above, in physiological conditions, the synthesis and degeneration of collagen are in dynamic equilibrium. CF has been reported to secrete the collagenase and stromelysin, which have the ability to degrade surrounding interstitial collagens (14). In an injured heart, increased synthesis of collagen that exceeds the rate of degradation can lead to cardiac fibrosis. CFs are crucial in regulating the process of cardiac remodeling, but the exact mechanism remains unclear.
Peroxisome proliferator-activated receptor gamma (PPARγ) coactivator-1 alpha (PGC-1α) is an important coactivator of several nuclear receptors and regulates mitochondrial function in various organs and tissues, including the heart, brain, and liver (15). Initially, PGC-1α was found in the brown adipose tissue and skeletal muscle of the mice exposed to cold conditions, and it plays a key role in thermogenesis (16). As the research of PGC-1α moves along, researchers found that activated PGC-1α stimulates mitochondrial oxidative metabolism. Thus, PGC-1α is highly expressed in the heart, brain, and kidney (17–19). The decreased expression of PGC-1α has been found in many diseases, which is often accompanied by a change of metabolic substrate from fatty acid to glucose (20, 21). Previous study has reported that the reduction of PGC-1α exacerbated non-alcohol fatty liver disease which eventually evolved into liver fibrosis. Moreover, this pathological process was accompanied by increased inflammation and oxidative damage (22). In terms of the heart, PGC-1α knockout mice exhibit lower treadmill running times and cardiac function decline after exercise compared to wild-type (WT) mice (23, 24). The synthesis and degeneration of collagen are energy-consuming and enzyme-dependent processes. The myocardium is mainly composed of cardiomyocytes and CFs, and both of them express PGC-1α. Although whole-body PGC-1α knockout mice have been reported to have heart dysfunction, the role of CF-specific PGC-1α in the heart is still unclear.
In the present study, we investigated the role of PGC-1α in CF. The activated fibroblasts have been considered the primary cause of cardiac fibrosis, and PGC-1α is essential to maintaining the function of CFs. Thus, we suppose that PGC-1α in CFs takes part in regulating the process of heart remodeling. Herein, we utilized the Cre-Loxp system to construct CF-specific PGC-1α knockout mouse and further examined its effects on the heart. In addition, we cultured CFs in vitro to study the function of PGC-1α.
Materials and Methods
Animal Study
PGC-1αflox (C57BL/6J background), SM22αCre/ER (C57BL/6J background) mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). PGC-1αflox/flox mice were bred with SM22αCre/ER mice to produce SM22αCre/ER;PGC-1αflox/− heterozygote mice. Then, heterozygote mice were bred with PGC-1αflox/flox mice to produce SM22αCre/ER;PGC-1αflox/flox (SP) homozygote mice. The WT mice and SP mice we used in the study are all 2- to 3-month-old male mice. The animal study was approved in accordance with institutional guidelines established by the Committee of Ethics on Animal Experiments at Shanghai Jiao Tong University School of Medicine.
Identification of Transgenic Mice
SP mice were identified by tail DNA PCR using primers for SM22α and PGC-1α (Supplementary Table 2). Mouse tail DNA was extracted by Mouse Tail Genomic DNA Kit (CWBIO). PCR was performed for 34 cycles with each cycle at 95°C for 30 s, 62°C for 30 s, and 72°C for 30 s. Finally, DNA agarose gel electrophoresis was used to identify mouse genotype (Supplementary Figure 1).
AngII-Induced Cardiac Remodeling
WT mice and SP mice were assigned to sham group and AngII group, respectively. AngII group mice were implanted subcutaneously with osmotic mini-pumps (Alzet, model: 2004, ALZET® Osmotic Pumps, Cupertino, CA, USA) to deliver AngII (1.44 mg/kg/day) for 28 days. The sham group received the same amount of saline.
Cell Culture
Primary CFs were isolated from 3 to 4-day-old male mice, as described previously (25). Cells were cultured in DMEM with 20% FBS and 1% penicillin and streptomycin. Then, CFs in the AngII group were stimulated by AngII (10−7 M) for 12 h.
Recombinant Lentiviruses
Lentiviruses carrying small hairpin RNA (shRNA) were produced and purified by GeneChem. The PGC-1α shRNA sequence was ACTATTGAGCGAACCTTAA, and the no-target control shRNA sequence was TTCTGCGAACGTGTCACGT. The viruses were used to infect CFs for 72 h (MOI = 10).
Histology and Immunostaining
Aortas fixed in formalin and embedded in paraffin were sectioned at 5 μm. Masson or wheat germ agglutinin (WGA) staining was performed using standard procedures. For immunofluorescence staining, the paraffin-embedded and frozen sections of the heart were incubated with primary antibodies for α-SMA (1:100) (19245S, CST, Danvers, MA, USA), Col1a1 (1:100) (GB11022, Servicebio, Wuhan, China), and F4/80 (1:100) (ab6640, Abcam, Cambridge, UK). Antigen retrieval of the paraffin section was obtained by heating the tissue slides in 0.01 M citrate buffer, pH 6.0, at 100°C for 5 min.
Quantitative Real-Time PCR
Total RNA was extracted from tissues and cultured cells using TRIzol (Invitrogen, Carlsbad, CA, USA) followed by chloroform extraction according to the manufacturer's protocol. Total RNA was reverse transcribed into single-stranded cDNA by incubation with reverse transcriptase (EZBioscience, Roseville, MN, USA). Real-time qRT-PCR was performed with SYBR Premix Ex Taq kits with ROX (TaKaRa) according to the manufacturer's instructions. Signals were detected on an ABI PRISM 7900 machine (Applied Biosystems, Foster City, CA, USA). β-Actin was used as a standard reference. Reactions were done at 95°C for 30 s followed by 40 cycles of 95°C for 5 s and 60°C for 30 s. Sequences of primers used in this study are provided in Supplementary Table 1.
Western Blotting
Frozen tissues were powdered and then homogenized in ice-cold RIPA buffer (50 mM Tris–HCl (pH 7.4); 10% Nonidet P-40; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM EDTA; 0.5 M NaF; 10 mM sodium pyrophosphate) supplemented with Protease Inhibitor Cocktail (BioTool Swiss, Kirchberg, Switzerland) and a phosphatase inhibitor (BioTool). Cultured cells were directly lysed in RIPA buffer. Proteins were applied into 10% SDS-PAGE gel and blotted onto a PVDF membrane (Merck Millipore, Burlington, MA, USA). Furthermore, blots were incubated with α-SMA (1:1,000) (19245S, CST), TGF-β (1:1,000) (3711S, CST), Col1a1 (1:1,000) (GB11022, Servicebio), iNOS (1:1,000) (ab178945, Abcam), and GAPDH (1:1,000) (5174S, CST) antibodies overnight at 4°C and then incubated with an HRP-conjugated antibody for 2 h at room temperature. The signal was detected by chemiluminescence.
Statistics
Differences between two independent groups were determined using Student's t-test (two-tailed). To compare more than two groups, one-way analysis of variance (ANOVA) was conducted followed by post-hoc Dunnett's testing for multiple-group comparison by GraphPad Prism (GraphPad Software, San Diego, CA, USA). Data represent mean ± SEM. The significant level was set at p < 0.05.
Results
CF-Specific PGC-1αKO Aggravates Cardiac Fibrosis
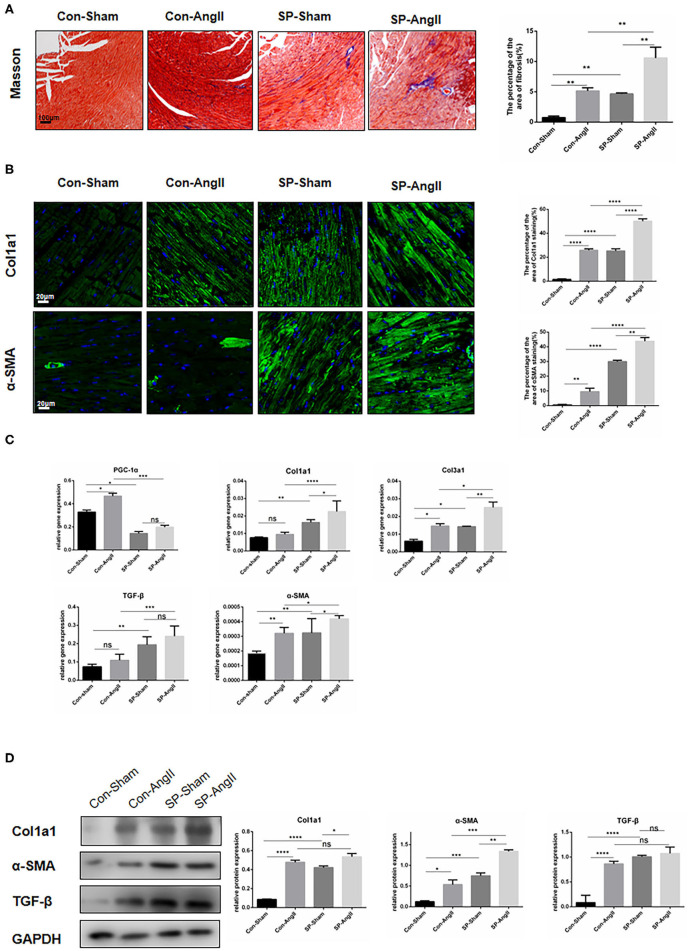
To determine whether the absence of PGC-1α in CFs could impact on heart health, WT and CF-specific PGC-1α KO mice were treated with AngII for 28 days. SM22αCre/ERT;PGC-1αflox/flox (SP) mice were utilized to study the role of CF-specific PGC-1α KO in cardiac remodeling, including interstitial fibrosis and cardiac hypertrophy. Masson's trichrome (Masson) staining was performed to analyze interstitial fibrosis. Histology analysis showed that WT mice developed significant heart fibrosis under administration of AngII for 28 days. More importantly, dramatic cardiac fibrosis was observed in both SP mice with and without the stimulation of AngII, and the SP-AngII group exhibited a higher level of fibrosis. Furthermore, SP mice developed more severe fibrosis than WT mice after AngII infusion (Figure 1A). In immunofluorescent staining of Col1a1 and α-SMA, increased expressions of Col1a1 and α-SMA were detected in WT mice after the stimulation of AngII. CF-specific PGC-1α KO induced collagen deposition in the heart, and AngII infusion aggravated it (Figure 1B). Then, qPCR was performed to explore the level of gene expression. The mRNA levels of fibrosis markers including collagen type I alpha 1 chain (Col1a1), collagen type III alpha 1 chain (Col3a1), TGF-β, and α-SMA were increased in specific PGC-1αKO mice compared to WT mice. Under the stimulation of AngII, the heart of SP-AngII mice presented upregulation of fibrosis-associated genes compared with that of control-AngII mice (Figure 1C). PGC-1αKO promoted the expressions of Col1a1, TGF-β, and α-SMA in heart (Figure 1D). These results indicated that PGC-1α plays an important role in maintaining the normal function of CFs, which can further influence heart health.
Figure 1.
Cardiac fibroblast-specific PGC-1αKO aggravates cardiac remodeling. (A) Representative cross sections of the heart stained for Masson and quantitative analysis of the area of fibrosis (Masson staining). (B) Representative immunofluorescent staining of Col1a1 and α-SMA and quantitative analysis of immunofluorescent staining of Col1a1 and α-SMA. (C) qPCR analysis of mRNA expression levels of PGC-1α and fibrotic genes (Col1a1, Col3a1, α-SMA, TGF-β). (D) Representative Western blot and analysis of the expression of fibrotic proteins (Col1a1, α-SMA, TGF-β). N.S. indicates no significant difference. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data represent mean ± SEM (n = 5 per group).
CF-Specific PGC-1αKO Aggravates Cardiac Hypertrophy
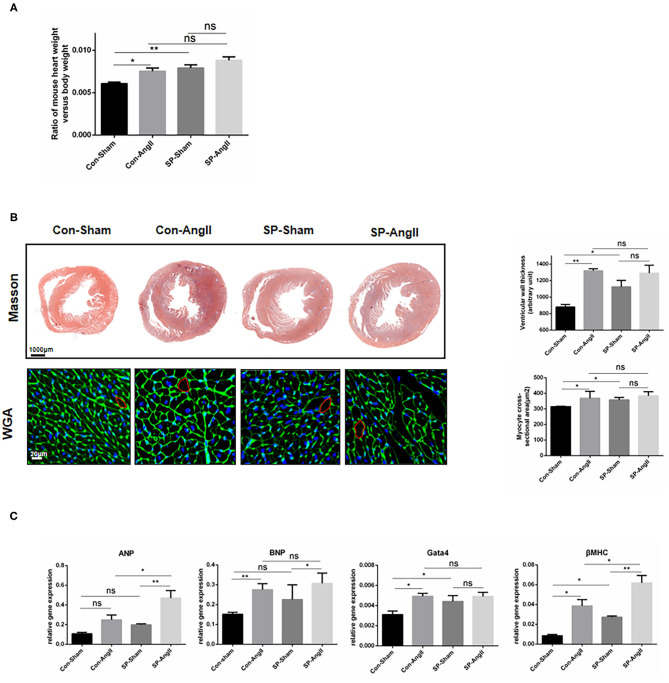
To study the possible influence of fibroblast-specific knockout of PGC-1α in cardiac hypertrophy, the following experiments were carried out. Both AngII treatment and CF-PGC1a KO induced the increase in the heart weight to body weight ratio, but there was no significant difference between the SP-sham and SP-AngII groups (Figure 2A). Masson and WGA staining results showed that ventricular wall thickness and cardiomyocyte size were increased in both WT mice and SP mice after the stimulation of AngII. Without the infusion of AngII, there were elevated ventricular wall thickness and cardiomyocyte size in SP mice compared with WT mice (Figure 2B). Besides, CF-specific PGC-1α KO resulted in elevated mRNA expression of hypertrophic relative factors, including atrial natriuretic peptide (ANP), atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), Gata4, and myosin heavy chain β (βMHC) compared with WT mice (Figure 2C). These findings suggested that CF-specific PGC-1α KO aggravated cardiac hypertrophy, which was independent of the stimulation of AngII.
Figure 2.
Cardiac fibroblast-specific PGC-1αKO aggravates cardiac hypertrophy. (A) Quantitative analysis of the ratio of the weight of mouse heart to body weight. (B) Representative cross sections of the heart stained for Masson and WGA. Quantitative analysis of the thickness of ventricular wall (Masson staining) and cardiomyocyte size (WGA staining). (C) qPCR analysis of mRNA expression levels of hypertrophic markers (ANP, BNP, Gata4, βMHC). N.S. indicates no significant difference. *p < 0.05, **p < 0.01. Data represent mean ± SEM (n = 5 per group).
CF-Specific PGC-1αKO Activates the Oxidative Stress Response in the Heart
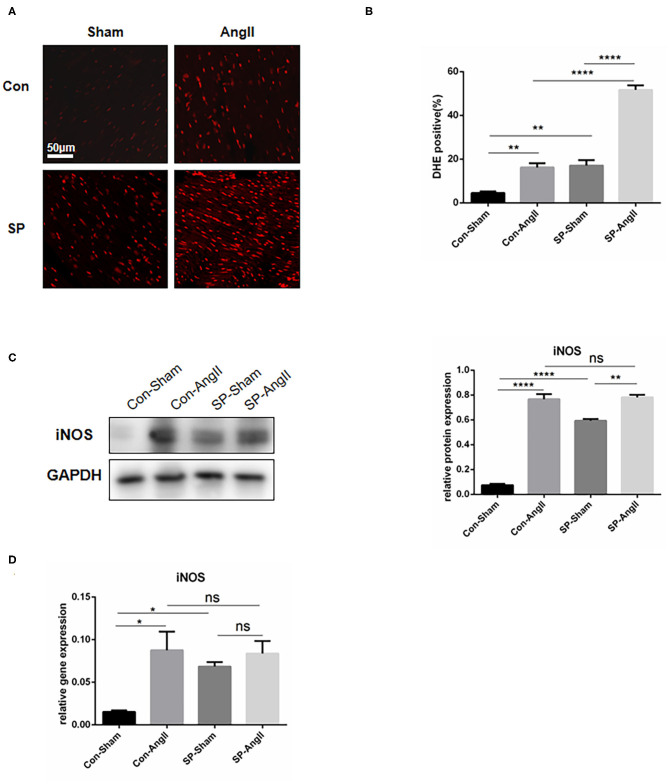
PGC-1α is considered as a suppressor in oxidative stress response (26). To explore whether the knockdown of PGC-1α in CFs could aggravate the oxidative stress in heart, the following experiments were carried out. Dihydroethidium (DHE) staining was utilized to examine the level of ROS. The results showed that the intensity and the proportion of DHE-positive cells were elevated in both WT mice and CF-PGC-1αKO mice after the stimulation of AngII. The heart of SP mice exhibited a higher level of ROS production than that of WT mice in both sham and AngII infusion (Figures 3A,B). The protein expression of inducible nitric oxide synthase (iNOS) was increased in both WT mice and SP mice after the stimulation of AngII. Moreover, the content of iNOS was at a higher level in SP mice compared to WT mice without the treatment of AngII (Figure 3C). In addition, the mRNA level of iNOS in the heart was measured. The results of analysis were identical to the expression of protein (Figure 3D). These data showed that PGC-1α knockdown in CFs aggravated the oxidative stress response in the heart.
Figure 3.
Cardiac fibroblast-specific PGC-1αKO activates the oxidative stress response in the heart. (A) Representative cross sections of the heart stained for DHE. (B) Quantitative analysis of reactive oxidative stress as assessed by DHE staining. (C) Representative Western blot and analysis of the expression of iNOS. (D) qPCR analysis of the mRNA expression levels of iNOS. N.S. indicates no significant difference. *p < 0.05, **p < 0.01. Data represent mean ± SEM (n = 3–5 per group).
CF-Specific PGC-1αKO Aggravates the Inflammatory Response in the Heart
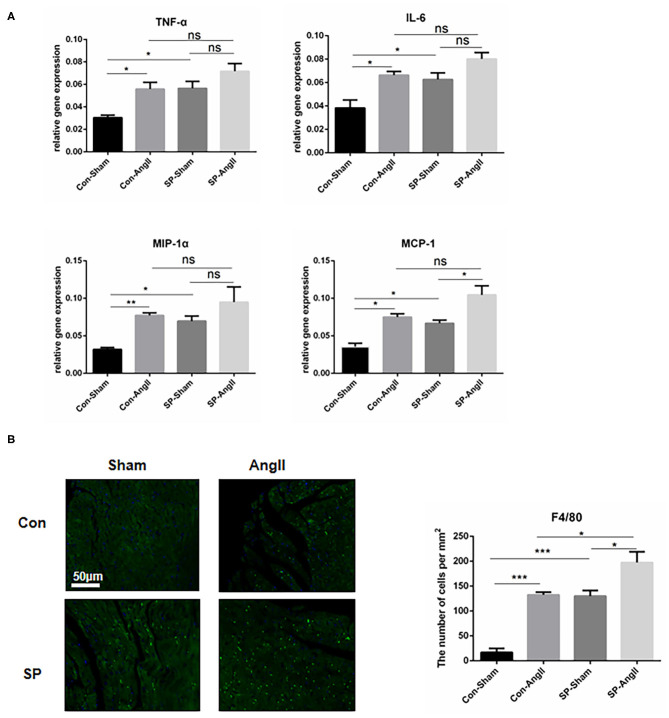
Inflammation is an important process in cardiac remodeling. The accumulation of inflammatory cells in the heart is closely associated with development of cardiac fibrosis. Furthermore, the activation of fibroblasts is responsive for elevated pro-inflammatory cytokines, which promotes the proliferation and migration of myofibroblasts and accelerates the synthesis of collagen (12, 27). To confirm the effect of PGC-1αKO in CFs on inflammation, qPCR was carried out to examine the mRNA levels of genes coding pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), macrophage inflammatory protein (MIP-1α), and monocyte chemotactic protein 1 (MCP-1). After the treatment of AngII, the levels of the above genes were elevated dramatically in WT mice. CF-PGC1a KO mice showed upregulation of TNF-α, IL-6, MIP-1α, and MCP-1 compared to WT mice, and AngII enhanced the MCP-1 expression in SP-AngII mice (Figure 4A). Consistent with this, there was more amount of macrophage infiltration in the heart of SP mice than WT mice. Moreover, AngII induced inflammation response (Figure 4B). Based on the results, we concluded that PGC-1α in CFs might take part in the regulation of inflammation.
Figure 4.
Cardiac fibroblast-specific PGC-1αKO aggravates the inflammatory response in the heart. (A) qPCR analysis of the mRNA expression levels of inflammatory genes (TNF-α, IL-6, MCP-1, MIP-1α). N.S. indicates no significant difference. (B) Representative cross sections of the heart stained for F4/80 and quantitative analysis of the number of positive cells. *p < 0.05, **p < 0.01, ***p < 0.001. Data represent mean ± SEM (n = 5 per group).
PGC-1α KO in CF Promotes the Expression of Fibrosis-Related Genes
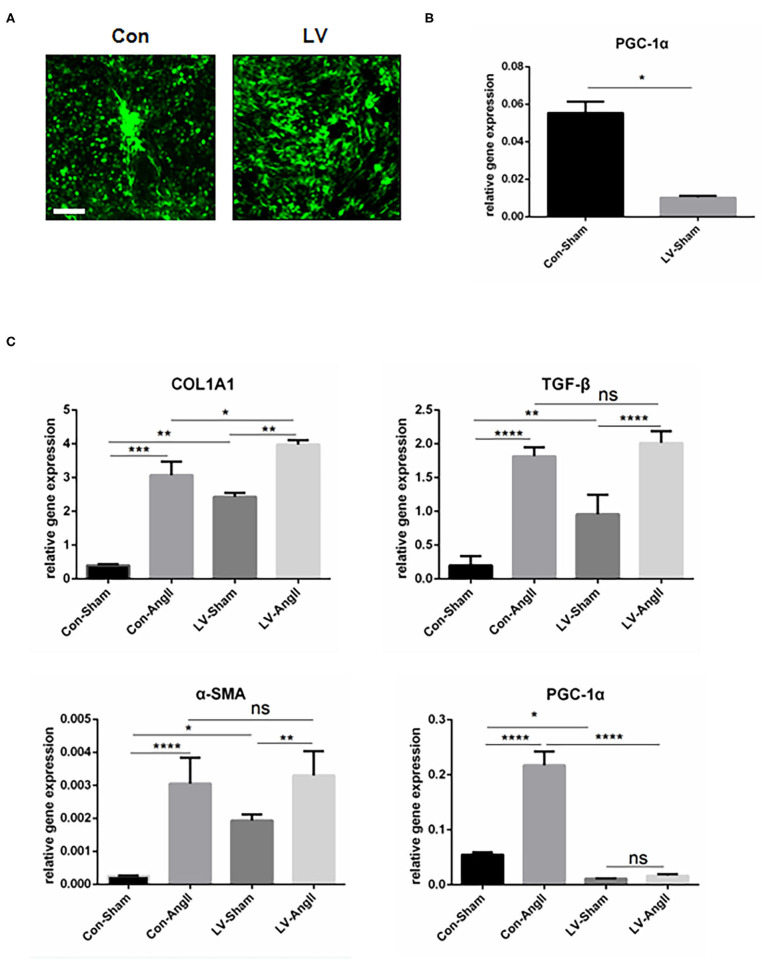
To rule out the effect of cardiomyocytes, CFs were cultured in vitro to find direct evidence that PGC-1α plays a vital role in maintaining the function of CFs. Lentivirus PGC-1α RNA interference (RNAi) was used to knock down PGC-1α expression in CFs (Figures 5A,B). Without the stimulation, cells knocked down of PGC-1α were detected to have higher mRNA levels of Col1a1, TGF-β, and α-SMA at baseline. AngII stimulation resulted in an increased expression of fibrosis markers, including Col1a1, TGF-β, and α-SMA in both experimental group and control group. Also, there is no significant difference in the expression of TGF-β and α-SMA between Con-AngII and LV-AngII cells (Figure 5C). These results indicated that PGC-1α may be involved in mediating the activation of CFs.
Figure 5.
PGC-1α KO in cardiac fibroblast promotes the expression of fibrosis-related genes. (A) Representative CF treated with PGC-1α-knockdown lentivirus (LV) and control virus (Con). (B) qPCR analysis of the mRNA expression levels of PGC-1α in the LV and Con CF groups. (C) qPCR analysis of mRNA expression levels of fibrotic genes (Col1a1, α-SMA, TGF-β). N.S. indicates no significant difference. *p < 0.05, **p < 0.01, ***p < 0.001. Data represent mean ± SEM (n = 3 independent experiments).
Discussion
An increasing number of studies have emphasized the relationship between heart remodeling and metabolism. PGC-1α is an important regulator of mitochondrial biology and energy metabolism, and the repression of PGC-1α accelerates cardiac dysfunction and the clinical signs of heart failure (24). Some mechanistic studies have shown that the protective role of PGC-1α in heart requires the activation of Sirtuin 1 (SIRT1), which is a redox-sensitive enzyme. Furthermore, the SIRT1-PGC-1α axis is vital to the performance of cardiac protection through decreasing inflammation, oxidative stress, fibrosis, and so on (28, 29). However, the underlying mechanism of PGC-1α-mediated cardiac remodeling is still unclear. Herein, we unveil the specific role of PGC-1α in regulating the function of CFs, which accelerates cardiac remodeling including cardiac fibrosis and cardiomyocytes hypertrophy.
Reduced PGC-1α is associated with impaired mitochondrial biology in heart diseases and energy expenditure (30). PGC-1α is also considered as a vital regulator of the scavenging of ROS. There is research reporting that ROS accelerates the deposition of collagen through the activation of p53, which results in severe fibrosis (31). In this study, ROS levels were increased in WT mice after AngII infusion, which has been proved to be associated with inhibition of PGC-1α-activated catalase expression (32). Our data showed a slight increase in PGC-1α expression in the heart of WT mice after treatment with AngII. More importantly, AngII failed to enhance PGC-1α expression in the heart of SP mice, which suggested that AngII-induced the PGC-1α upregulation in whole heart tissue which might be attributed to the impact of CF on other cells. Hence, we proposed that the absence of PGC-1α in CFs exerts impact on other cells in the heart, leading to the aggravation of cardiac remodeling. In addition, PGC-1α is an essential component of inflammatory response. Overexpression of PGC-1α inhibits the production of pro-inflammatory cytokines and promotes the secretion of anti-inflammatory cytokines in the heart, preventing the heart from getting damaged (33–35). In our study, CF-specific PGC-1α knockdown promoted the expression of pro-inflammatory cytokines in the heart, which indicated that PGC-1α in CFs took part in the inflammatory response. Previous report has demonstrated that pro-inflammatory cytokines facilitated the transition of CFs to myofibroblasts (8). According to the research, there are only ~2% activated CFs in the adult heart (36). Our data shows that in CF-specific PGC-1α KO mice, plenty of activated CFs convert into myofibroblasts even without the stimulation of AngII by detecting the high expression of fibrosis markers and cardiac hypertrophy markers. All these data show that PGC-1α in CFs, as a versatile factor, plays a vital role in regulating cardiac remodeling. On the contrary, several researches demonstrate that overexpression of PGC-1α beyond physiological content leads to mitochondrial proliferation and myofibrillar displacement, which finally contributes to cardiac failure (30, 37). Thus, maintaining PGC-1α in a physiological stage is crucial for cardiovascular health. Our results exhibited that both AngII and PGC-1α KO have an influence on cardiac remodeling, which may indicate that PGC-1α and AngII perform through an overlapping pathway. The aforesaid SIRT1 is an essential protein in regulating the fibrosis-related pathway in many organs through regulating gene transcription (38, 39). AngII-induced cardiovascular remodeling is reported to be closely related to the reduction of SIRT1. On the other hand, overexpression of SIRT1 suppresses the ROS-induced p38/mitogen-activated protein kinase (MAPK) pathway, which promotes the activation of CFs (40, 41). Moreover, as mentioned before, PGC-1α plays a protective role through cooperating with the activated SIRT1 (29). AngII and PGC-1α might affect the function of SIRT1, leading to the activation of the p38/MAPK pathway and the transition of CFs. There are some limitations in our study. It remains uncertain whether overexpression of PGC-1α could improve cardiac remodeling. Furthermore, the underlying mechanism that CF-specific knockout of PGC-1α participates in cardiac remodeling remains unclear, which still needs further study.
In conclusion, we have discovered the PGC-1α-mediated pathology of cardiac remodeling, especially cardiac fibrosis. We propose that the absence of PGC-1α in CFs impairs the balance of the synthesis and degeneration of collagen through regulating ROS production and inflammation, leading to deposition of collagens and cardiac remodeling. Our findings reveal that PGC-1α is critical for the cardiac fibrosis by multiple fibrogenic pathways.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by the Committee of Ethics on Animal Experiments at Shanghai Jiao Tong University School of Medicine.
Author Contributions
X-xP and C-cR designed the study. H-jC performed the experiments, analyzed the data, and wrote the manuscript. L-l-qD conducted the in vitro experiments. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by grants from the National Natural Science Foundation of China (Nos. 81922004, 82030006, 81770495, 81870180, and 81700433).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.664626/full#supplementary-material
References
- 1.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. (2007) 117:568–75. 10.1172/JCI31044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drazner MH. The progression of hypertensive heart disease. Circulation. (2011) 123:327–34. 10.1161/CIRCULATIONAHA.108.845792 [DOI] [PubMed] [Google Scholar]
- 3.Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. (1997) 29:1947–58. 10.1006/jmcc.1997.0435 [DOI] [PubMed] [Google Scholar]
- 4.Medugorac I. Characterization of intramuscular collagen in mammalian left ventricle. Basic Res Cardiol. (1982) 77:589–98. 10.1007/BF01908312 [DOI] [PubMed] [Google Scholar]
- 5.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. (2005) 45:657–87. 10.1146/annurev.pharmtox.45.120403.095802 [DOI] [PubMed] [Google Scholar]
- 6.Cleutjens JP, Blankesteijn WM, Daemen MJ, Smits JF. The infarcted myocardium: simply dead tissue, or a lively target for therapeutic interventions. Cardiovasc Res. (1999) 44:232–41. 10.1016/S0008-6363(99)00212-6 [DOI] [PubMed] [Google Scholar]
- 7.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. (2010) 7:30–7. 10.1038/nrcardio.2009.199 [DOI] [PubMed] [Google Scholar]
- 8.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. (2009) 123:255–78. 10.1016/j.pharmthera.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 9.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. (2003) 112:1776–84. 10.1172/JCI200320530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. (2007) 13:952–61. 10.1038/nm1613 [DOI] [PubMed] [Google Scholar]
- 11.Foo IT, Naylor IL, Timmons MJ, Trejdosiewicz LK. Intracellular actin as a marker for myofibroblasts in vitro. Lab Invest. (1992) 67:727–33. [PubMed] [Google Scholar]
- 12.Rönty MJ, Leivonen SK, Hinz B, Rachlin A, Otey CA, Kähäri VM, et al. Isoform-specific regulation of the actin-organizing protein palladin during TGF-beta1-induced myofibroblast differentiation. J Invest Dermatol. (2006) 126:2387–96. 10.1038/sj.jid.5700427 [DOI] [PubMed] [Google Scholar]
- 13.Pho M, Lee W, Watt DR, Laschinger C, Simmons CA, McCulloch CA. Cofilin is a marker of myofibroblast differentiation in cells from porcine aortic cardiac valves. Am J Physiol Heart Circ Physiol. (2008) 294:H1767–78. 10.1152/ajpheart.01305.2007 [DOI] [PubMed] [Google Scholar]
- 14.Kuncio GS, Neilson EG, Haverty T. Mechanisms of tubulointerstitial fibrosis. Kidney Int. (1991) 39:550–6. 10.1038/ki.1991.63 [DOI] [PubMed] [Google Scholar]
- 15.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. (2006) 27:728–35. 10.1210/er.2006-0037 [DOI] [PubMed] [Google Scholar]
- 16.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. (1998) 92:829–39. 10.1016/S0092-8674(00)81410-5 [DOI] [PubMed] [Google Scholar]
- 17.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. (1999) 98:115–24. 10.1016/S0092-8674(00)80611-X [DOI] [PubMed] [Google Scholar]
- 18.Sterbauer H, Oberkofler H, Krempler F, Patsch W. Human peroxisome proliferator activated receptor gamma coactivator 1 (PPARGC1) gene: cDNA sequence, genomic organization, chromosomal localization, and tissue expression. Genomics. (1999) 62:98–102. 10.1006/geno.1999.5977 [DOI] [PubMed] [Google Scholar]
- 19.Knutti D, Kaul A, Kralli A. A tissue-specific coactivator of steroid receptors, identified in a functional genetic screen. Mol Cell Biol. (2000) 20:2411–22. 10.1128/MCB.20.7.2411-2422.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. (2004) 95:568–78. 10.1161/01.RES.0000141774.29937.e3 [DOI] [PubMed] [Google Scholar]
- 21.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest. (2005) 115:547–55. 10.1172/JCI24405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Besse-Patin A, Léveillé M, Oropeza D, Nguyen BN, Prat A, Estall JL. Estrogen signals through peroxisome proliferator-activated receptor-γ coactivator 1α to reduce oxidative damage associated with diet-induced fatty liver disease. Gastroenterology. (2017) 152:243–56. 10.1053/j.gastro.2016.09.017 [DOI] [PubMed] [Google Scholar]
- 23.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. (2005) 3:e101. 10.1371/journal.pbio.0030101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci USA. (2006) 103:10086–91. 10.1073/pnas.0603615103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubey RK, Gillespie DG, Jackson EK. Adenosine inhibits collagen and protein synthesis in cardiac fibroblasts: role of A2B receptors. Hypertension. (1998) 31:943–8. 10.1161/01.HYP.31.4.943 [DOI] [PubMed] [Google Scholar]
- 26.Marmolino D, Manto M, Acquaviva F, Vergara P, Ravella A, Monticelli A, et al. PGC-1alpha down-regulation affects the antioxidant response in Friedreich's ataxia. PLoS ONE. (2010) 5:e10025. 10.1371/journal.pone.0010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. (2004) 18:816–27. 10.1096/fj.03-1273rev [DOI] [PubMed] [Google Scholar]
- 28.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. (2006) 127:1109–22. 10.1016/j.cell.2006.11.013 [DOI] [PubMed] [Google Scholar]
- 29.Waldman M, Cohen K, Yadin D, Nudelman V, Gorfil D, Laniado-Schwartzman M, et al. Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving 'SIRT1 and PGC-1α'. Cardiovasc Diabetol. (2018) 17:111. 10.1186/s12933-018-0757-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. (2000) 106:847–56. 10.1172/JCI10268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Ani B, Alzamil NM, Hewett PW, Al-Hashem F, Bin-Jaliah I, Shatoor AS, et al. Metformin ameliorates ROS-p53-collagen axis of fibrosis and dyslipidemia in type 2 diabetes mellitus-induced left ventricular injury. Arch Physiol Biochem. (2021) 13:1–7. 10.1080/13813455.2020.1869265 [DOI] [PubMed] [Google Scholar]
- 32.Xiong S, Salazar G, San Martin A, Ahmad M, Patrushev N, Hilenski L, et al. PGC-1 alpha serine 570 phosphorylation and GCN5-mediated acetylation by angiotensin II drive catalase down-regulation and vascular hypertrophy. J Biol Chem. (2010) 285:2474–87. 10.1074/jbc.M109.065235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sczelecki S, Besse-Patin A, Abboud A, Kleiner S, Laznik-Bogoslavski D, Wrann CD, et al. Loss of Pgc-1α expression in aging mouse muscle potentiates glucose intolerance and systemic inflammation. Am J Physiol Endocrinol Metab. (2014) 306:E157–67. 10.1152/ajpendo.00578.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisele PS, Furrer R, Beer M, Handschin C. The PGC-1 coactivators promote an anti-inflammatory environment in skeletal muscle in vivo. Biochem Biophys Res Commun. (2015) 464:692–7. 10.1016/j.bbrc.2015.06.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buler M, Aatsinki SM, Skoumal R, Komka Z, Tóth M, Kerkelä R, et al. Energy-sensing factors coactivator peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) and AMP-activated protein kinase control expression of inflammatory mediators in liver: induction of interleukin 1 receptor antagonist. J Biol Chem. (2012) 287:1847–60. 10.1074/jbc.M111.302356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu R, Ma F, Tosevska A, Farrell C, Pellegrini M, Deb A. Cardiac fibroblast proliferation rates and collagen expression mature early and are unaltered with advancing age. JCI Insight. (2020) 5:140628. 10.1172/jci.insight.140628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, et al. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res. (2004) 94:525–33. 10.1161/01.RES.0000117088.36577.EB [DOI] [PubMed] [Google Scholar]
- 38.Li XZ, Cheng LZ, Yan YM, Liu BH, Cheng YX. SIRT1 inhibitory compounds from the roots of Codonopsis pilosula. J Asian Nat Prod Res. (2019) 21:25–32. 10.1080/10286020.2017.1422491 [DOI] [PubMed] [Google Scholar]
- 39.Hwang JS, Kang ES, Han SG, Lim DS, Paek KS, Lee CH, et al. Formononetin inhibits lipopolysaccharide-induced release of high mobility group box 1 by upregulating SIRT1 in a PPARδ-dependent manner. PeerJ. (2018) 6:e4208. 10.7717/peerj.4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burke RM, Dirkx RA, Jr, Quijada P, Lighthouse JK, Mohan A, O'Brien M, et al. Prevention of fibrosis and pathological cardiac remodeling by salinomycin. Circ Res. (2021) 128:1663–78. 10.1161/CIRCRESAHA.120.317791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou L, Zhang S, Bolor-Erdene E, Wang L, Tian D, Mei Y. NAMPT/SIRT1 attenuate Ang II-induced vascular remodeling and vulnerability to hypertension by inhibiting the ROS/MAPK pathway. Oxid Med Cell Longev. (2020) 2020:1974265. 10.1155/2020/1974265 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.