Abstract
The urban poor in developing countries face challenging living environments, which may interfere with good sleep. Using actigraphy to measure sleep objectively, we find that low-income adults in Chennai, India, sleep only 5.5 hours a night on average despite spending 8 hours in bed. Their sleep is highly interrupted, with sleep efficiency—sleep per time in bed—comparable to those with disorders such as sleep apnea or insomnia. A randomized three-week treatment providing information, encouragement, and improvements to home sleep environments increased sleep duration by 27 minutes a night by inducing more time in bed. Contrary to expert predictions and a large body of sleep research, increased nighttime sleep had no detectable effects on cognition, productivity, decision making, or well being, and led to small decreases in labor supply. In contrast, short afternoon naps at the workplace improved an overall index of outcomes by 0.12 standard deviations, with significant increases in productivity, psychological well-being, and cognition, but a decrease in work time.
I. Introduction
Understanding the lives of the poor is central to modern development economics. Economists have studied many deprivations associated with poverty, such as lack of access to nutrition, water, education, health care, and clean air. This article considers a previously unexamined challenge faced by the urban poor in developing countries: sleep deprivation. People in these settings face many barriers to a good night’s sleep, such as heat, noise, crowding, physical discomfort, and psychological distress. Sleep could be a crucial input to their productivity, well-being, and cognitive function. Yet we know little about how much and how well people in low-income countries sleep, or the returns to policies that seek to increase sleep.
Using state-of-the-art technology to measure sleep objectively, we uncover widespread sleep deprivation in Chennai, India. Our two samples of low-income adults sleep only 5.5 hours a night on average, far below the minimum level recommended by sleep experts (Hirshkowitz et al. 2015; Watson et al. 2015). This is not due to a lack of trying. People spend about eight hours a night in bed, but their sleep is highly disrupted, with 31 awakenings in a typical night. The implied sleep efficiency—time asleep per time in bed—of 70% is much lower than objective measures from general U.S. populations, and similar to those suffering from disorders such as sleep apnea or insomnia in high-income countries (Hedner et al. 2004; Trauer et al. 2015).
An enormous body of research, mostly conducted in sleep labs in rich countries, documents severe negative effects of sleep deprivation on a range of outcomes, from attention and memory to mood and health (Banks and Dinges 2007; Lim and Dinges 2010). While experimental evidence on the effect of increasing sleep in field settings is scarce,1 there is a widely held belief among researchers and the public that reducing sleep deprivation would lead to improvements in economic outcomes (Walker 2017). To document these priors, we surveyed 119 experts from sleep science and economics who predicted sizable economic benefits, including a 7% increase in work output, of increasing sleep by half an hour a night from the low levels observed in our setting.
To measure the economic effects of increasing sleep in the field, we conducted a randomized controlled trial with 452 adults in Chennai. We employed participants for a one-month data entry job with flexible hours, allowing us to precisely measure productivity and labor supply, as well as physical and psychological well-being, cognition, and time, risk, and social preferences. Two night sleep treatments gave participants (i) items to improve their home-sleep environments, (ii) information and verbal encouragement to increase their night sleep (the encouragement treatment), and (iii) for a subset of participants, additional financial incentives to increase night sleep (the incentives treatment). These treatments were cross-randomized with a nap treatment that offered participants the opportunity for a daily half-hour afternoon nap at their workplace.2
The night sleep treatments on average increased nighttime sleep by 27 minutes a night (std. err. = 3 minutes), with larger effects for the incentives (33 minutes) than for the encouragement treatment (20 minutes). The increased sleep duration was entirely driven by additional time in bed—on average 38 minutes a night (std. err. = 4 minutes)—rather than higher sleep efficiency. These results demonstrate that people do have substantial ability to adjust their nighttime sleep through changes in time in bed, but may not be able to increase their sleep efficiency. The low sleep efficiency increases the opportunity cost of sleep: raising sleep duration by 1 minute requires 1.4 more minutes in bed.
Similarly, the nap treatment increased daytime sleep by about 14 minutes a day on average (std. err. = 0.3 minutes), while slightly crowding out nighttime sleep. Although both types of treatments increased 24-hour sleep, the 27-minute increase from the night sleep treatments was significantly higher than the 8-minute increase from the nap treatment (p < .01).
We first examine the effects of each combination of treatments on an overall summary index that aggregates all outcomes as in Anderson (2008). Each of the night sleep treatments alone had no significant effect on this overall index: 0.00 standard deviations (std. err. = 0.07) and −0.05 std. dev. (std. err. = 0.07), respectively, for the encouragement only and incentives only groups. In contrast, naps alone had a positive, marginally significant effect of 0.11 std. dev. (std. err. = 0.07, p = .11). Those who received a night sleep treatment in addition to naps had very similar effects to those with naps only.3 This pattern of results suggests that naps have an overall positive effect on outcomes, whereas increases in night sleep do not. To increase statistical power and streamline the discussion of effects on the individual outcomes, we turn to an analysis that pools the two night sleep treatments and does not allow for an interaction effect of night sleep and nap treatments. The effects of each treatment described below should thus be interpreted as conditional on the distribution of the other treatment (Muralidharan, Romero, and Wüthrich 2019).
In the pooled analysis, we find no significant effect of increased night sleep on the overall index (−0.01 std. dev., std. err. = 0.04), or on summary indices corresponding to four families of outcomes: work, well-being, cognition, and economic preferences. In fact, the pooled night sleep treatment had no significant positive effect on any outcome other than sleep itself. It did not significantly increase productivity at the data entry job, a relatively cognitively demanding task intended to be sensitive to sleep deprivation. Instead, increased sleep came at the cost of lowering labor supply by nine minutes a day, leading to a small (but not statistically significant) decrease in earnings. We reject the median expert prediction of a 7% increase in output (p < .001). Similarly, we find no significant effects on detailed measures of physical and psychological well-being or standard measures of cognition and social, risk, and time preferences.
Why does increased night sleep not have benefits in our setting, contrary to expert predictions and a large body of lab studies? One possibility is that the large effects from lab experiments, which typically dramatically reduce sleep for up to a few nights, do not generalize to marginal, policy-relevant increases in sleep in the field. Another possibility is that the low quality of sleep observed in our setting—as proxied by low efficiency and frequent awakenings—explains the lack of benefits of increased nighttime sleep. Returns to increased sleep could be higher in typical rich-country settings. We cannot adjudicate these reasons, but our results highlight the importance of studying sleep in the field, where outcomes have real stakes and sleep is a choice variable with opportunity costs. They also caution against extrapolating sleep science findings across diverse contexts.
In contrast to night sleep, naps significantly improved a range of outcomes. The nap treatment significantly increased the overall summary index by 0.12 std. dev. (std. err. = 0.04, p = .00), as well as the index variables corresponding to well-being (0.08 std. dev., p = .03), and cognition (0.10 std. dev., p = .08). The effect of naps on work outcomes depends on the comparison. Compared with taking a break, naps increased earnings—the summary variable for the work outcomes—by 0.05 std. dev. (p = .05). However, driven by a decrease in labor supply, naps reduced earnings compared to working during the nap time by 0.10 std. dev. (p = .00), highlighting the importance of taking into account the opportunity costs of sleep.
Considering the individual outcome variables one by one and adjusting for multiple comparisons, naps have significant positive effects on productivity (0.04 std. dev., p = .06), psychological well-being (0.12 std. dev., p = .04), lab measures of cognitive function (0.08 std. dev., p = .06), and attention at work (0.20 std. dev., p = .07).
The nap and night sleep treatments have statistically different effects on the overall index (p = .02). We are less powered to detect differences for the family-level indices. Point estimates are larger for the nap treatment for well-being, cognition, and preferences, but the differences are not statistically significant. At the level of individual outcomes, we find statistically significant differences in effects only for psychological well-being (p = .04). Estimating the per minute effects of the two types of sleep in an instrumental variable analysis, however, we can reject equal per minute effects of naps and nighttime sleep on the summary index (p < .01), on the family indices for cognition (p = .08) and preferences (p = .08), and on some individual outcomes, including labor supply (p = .04) and psychological well-being (p = .05). In every case but labor supply, the effects are more positive for naps.
One possible reason for the different effects of the nap and night sleep treatments is that the timing of sleep may matter. Contrary to hypotheses and some evidence in sleep science (e.g., Nicholson et al. 1985; Mollicone, Van Dongen, and Dinges 2007; Mollicone et al. 2008), naps and night sleep may simply not be close substitutes. An alternative explanation is that sleep quality may play a role, since naps in our study occurred in a more comfortable office environment. We cannot separate these explanations, but hope that future work in similar settings may help answer this question.
Our article makes the following contributions. First, it contributes to a better understanding of the living conditions faced by the poor in developing countries by providing objective measures of sleep. We discover surprisingly low levels of sleep duration and efficiency among the urban poor in Chennai. These findings are consistent with two papers measuring sleep objectively in smaller samples in Sri Lanka and Haiti (Castro et al. 2013; Schokman et al. 2018) and contrast with self-reported measures of sleep, which may fail to capture the low sleep efficiency and its effect on total sleep (Stranges et al. 2012; Gildner et al. 2014; Simonelli et al. 2018).
Second, we build on a recent literature that estimates the causal effect of sleep outside of sleep laboratories. The lack of effects of nighttime sleep we find using a field experiment contrasts with an economics literature that uses natural experiments in rich countries to demonstrate that sleep can have sizable effects on wages (Gibson and Shrader 2018; Giuntella and Mazzonna 2019), hospitalizations (Jin and Ziebarth 2020), accidents (Smith 2016), and civic behaviors (Holbein, Schafer, and Dickinson 2019).4 We speculate that the stark difference in sleep efficiency in our setting compared to rich-country populations contributes to this difference. It could also be that increased night sleep simply does not have high benefits at the margin in the field, as in another recent field experiment (Avery, Giuntella, and Jiao 2019). Additional field studies of sleep, including interventions that improve sleep efficiency, may help reconcile these findings.
Third, we show that afternoon naps in a comfortable office environment have positive effects on a range of outcomes, including productivity, well-being, and cognition. Naps are a common feature of life around the world and are particularly prevalent in tropical countries (Dinges 1992). Naps have been studied in sleep labs, but we have little causal evidence on the effects of naps on worker productivity and other real-world outcomes or consideration of the opportunity cost of naps (Lovato and Lack 2010; Ficca et al. 2010). Our work takes a step toward filling this gap, and shows that naps may be an effective way to combat sleep deprivation. The decline of naps as employment in developing countries shifts toward Western schedules could therefore be costly.
Finally, recent research in behavioral and development economics argues that people in developing countries often underinvest in high-return investments such as preventive health, agricultural inputs, or capital investments (Kremer, Rao, and Schilbach 2019). At first glance, the low levels of sleep we discovered appear to tell the same story, and experts predicted substantial effects of increased sleep. Instead, our evidence suggests that—in this context—people do not underinvest in sleep duration given the environmental constraints that they face. The returns to increasing night sleep in their home environments are low and possibly even negative. To paraphrase Schultz (1964), low-income people in Chennai may be poor but efficiently tired.
II. Measuring Sleep in Chennai
II.A. Measuring Sleep Outside the Lab
The gold standard for objectively measuring sleep in labs is polysomnography (PSG), by recording brain waves, blood oxygen levels, eye movements, and body movements to determine sleep/wake cycles and stages of sleep (Marino et al. 2013). Although highly accurate, this bulky technology is impractical for field studies and may interfere with natural sleep patterns at people’s homes, thus making measuring sleep outside of sleep labs challenging. Self-reported measures are unreliable and correlate only moderately with objective measures because people tend to report time in bed rather than hours asleep, leading to overreporting of sleep duration (Lauderdale et al. 2008; Schokman et al. 2018).
Actigraphs, which resemble wristwatches and infer sleep/wake states from body movement, recently emerged as a viable alternative for field studies. These devices allow researchers to objectively measure sleep in participants’ home environments without interfering with sleep, as these devices are portable, comfortable, and unobtrusive. Validation studies show that actigraphs reliably measure sleep duration. Comparisons between actigraphy and PSG measures show high degrees of accuracy in sleep-wake detection, with 90% minute-by-minute agreement between the two (Sadeh et al. 1995; Marino et al. 2013). Actigraphs have been found to provide valid and clinically useful measures of sleep duration even among people with sleep disorders (Kushida et al. 2001; Smith et al. 2018) and reliably capture treatment effects of various interventions on sleep (Sadeh 2011).
Actigraphs also measure sleep efficiency, defined as time asleep divided by time in bed. This measure is available since—in addition to number of hours asleep—actigraphs also detect when an individual is in bed but not asleep. Sleep efficiency is perhaps the most commonly used proxy for sleep quality in sleep science (Ohayon et al. 2017). Disruptions to sleep, such as brief awakenings during the night, drive down sleep efficiency. In addition, sleep efficiency affects the opportunity cost of sleep, since it indicates the time in bed needed to achieve an hour of actual sleep.
II.B. Sleep Deprivation around the World
While sleep scientists recommend seven to nine hours of sleep a night (Hirshkowitz et al. 2015; Watson et al. 2015), numerous studies show that people in high-income countries sleep less than this (Walker 2017).5 For instance, Lauderdale et al. (2008) measure sleep via actigraphy among a large, diverse population of healthy young adults in Chicago, and report an average sleep duration of 6.1 hours a night, well below the recommended range.
In contrast, there is scant evidence on sleep patterns in developing countries. Sleep deprivation may be widespread and even more severe in the rapidly growing cities of the developing world, where residential structures are often of low quality and people are exposed to excessive heat, noise, crowding, and pollution—all conditions likely to hinder sleep. Even self-reports—which typically overestimate sleep—suggest a substantial share of people in developing countries sleep less than the recommended seven to nine hours. For example, the 4,500 rural, older Indian adults surveyed in Gildner et al. (2014) self-report 7.1 hours of sleep on average, with about 30% of these individuals reporting 6 or fewer hours a night (Selvamani et al. 2018).
Two recent studies in low-income countries measured sleep using actigraphs and identify significant fractions of the population as sleep deprived. In particular, Schokman et al. (2018) finds an average of only six hours a night among 175 adults from urban Sri Lanka. Knutson (2014) finds that 58 adults in Haiti sleep on average seven hours a night in a rural population without electricity.
II.C. Sleep(less) in Chennai
Our first measure of sleep in Chennai comes from the RCT sample of 452 adults recruited for a full-time data entry job for one month. To capture reliable and objective measures of sleep beyond self-reports, all participants wore actigraphs continuously throughout the study.6 Below, we describe sleep during the baseline period (before treatment) in this sample. We then report very similar patterns of sleep in a broader sample in Chennai, which wore actigraphs for three nights.
1. A Typical Night in Chennai
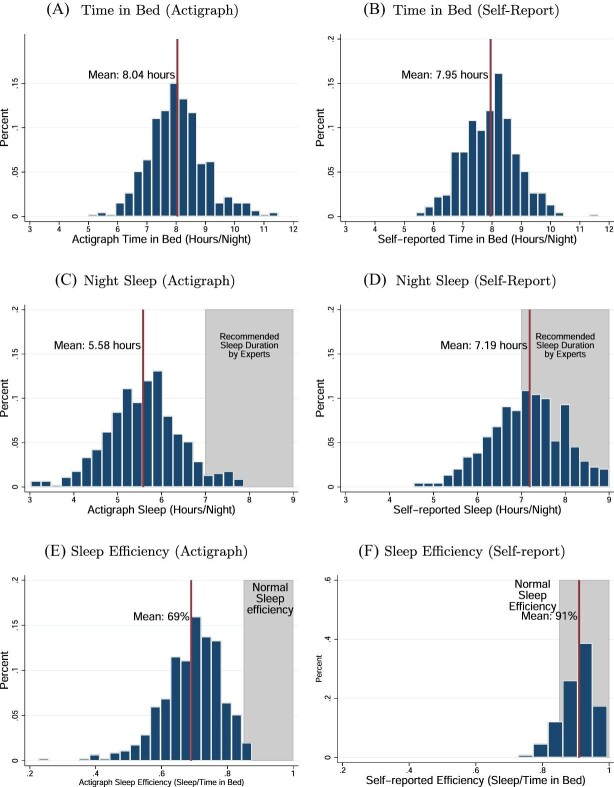
We first provide an example to highlight key features of participants’ sleep patterns. Figure I, Panel A illustrates a typical night for a study participant, using minute-by-minute actigraph measures of sleep (light gray) and wake (dark red) status. This night closely matches the average time in bed, sleep duration, and sleep efficiency in the RCT sample. The participant spends about 8 hours in bed during this night but only achieves 5.6 hours of highly fragmented and interrupted sleep, involving over 30 awakenings. For comparison, we show a less interrupted night with 90% efficiency in Figure I, Panel B. While this night is unusual in Chennai—only 1% of nights in our sample feature such high sleep—it resembles nights of healthy adults in high-income countries who typically enjoy sleep efficiency of 85%–95% (Cole et al. 1992; Carrier et al. 2001; Walker 2017).
Figure I.
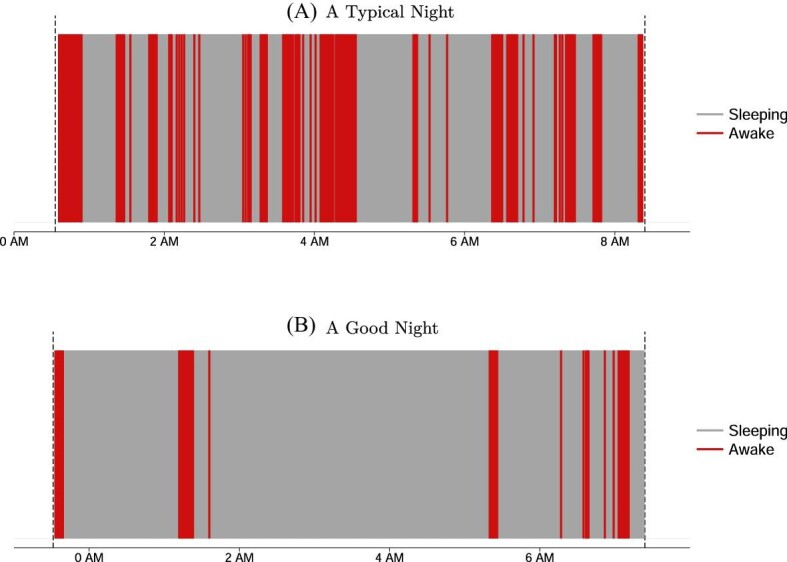
Typical Sleep in Chennai
This figure represents actigraph-measured sleep-wake patterns of two particular nights of two selected study participants. Light gray areas indicate one-minute periods in which the participant was asleep, and dark red areas (color version available online) indicate periods in which the participant was awake according to the actigraph. The gray dashed lines indicate when the participant got into or out of bed. In Panel A we show a typical night in our sample, represented by average levels of time in bed, time asleep, and sleep efficiency. During this particular night, the participant stayed in bed for 7 hours, 45 minutes but slept for only 5 hours, 20 minutes, resulting in a sleep efficiency of 69%, corresponding to the 41st, 40th, and 43rd percentile of the control group, respectively. The participant awoke 31 times during this night, and the longest sleep episode lasted 45 minutes. Panel B depicts a good night of sleep, with sleep patterns similar to those found in the United States and other rich countries: the participant stayed in bed for 7 hours, 53 minutes and slept for 7 hours, 8 minutes, resulting in a sleep efficiency of 90%, corresponding to the 46th, 91st, and 99th percentile of the control group, respectively. In this night, the participant only awoke nine times, and the longest sleep episode lasted 223 minutes.
2. Time in Bed versus Time Asleep
The RCT sample spends roughly eight hours a night in bed before treatment begins, with strong congruence between actigraph measures (Figure II, Panel A) and self-reports (Figure II, Panel B). Time in bed in Chennai is quite similar to that found in U.S. samples.7 Despite this significant time in bed, study participants only enjoy 5.6 hours asleep per night (Table I and Figure II, Panel C), significantly below time in bed and the recommended seven to nine hours. Ninety-five percent of participants slept less than seven hours a night, and 71% slept less than six hours a night on average. In high-income countries, such low average time asleep is typical in populations with disorders such as sleep apnea (Cole et al. 1992; Kushida et al. 2001; Gershon et al. 2012).
Figure II.
Baseline Distributions of Sleep-Related Variables (RCT Sample)
This figure shows the distribution of the sleep-related variables averaged at the participant-level over the baseline period (seven nights) in the RCT sample (N = 452). The left three panels show distributions of actigraph-measured sleep patterns and the right three panels show the corresponding distributions based on self-reports. Panels A and B show hours in bed as measured by actigraphy and by self-reports, respectively. Panels C and D show night sleep duration in hours as measured by actigraphy and by self-reports, respectively. Panels E and F show sleep efficiency (night sleep duration/time in bed) as measured by actigraphy and by self-reports, respectively.
TABLE I.
Sleep Statistics in Two Samples in Chennai
| RCT sample | Broader sample | |
|---|---|---|
| (pretreatment) | ||
| (1) | (2) | |
| Panel A: Night sleep | ||
| Hours in bed | 8.03 | 7.68 |
| (0.97) | (1.23) | |
| Hours asleep | 5.58 | 5.46 |
| (0.87) | (1.15) | |
| Sleep efficiency | 0.70 | 0.71 |
| (0.08) | (0.10) | |
| Number of awakenings | 31.95 | 27.4 |
| (7.95) | (10.14) | |
| Fraction sleeping less than 7 hours | ||
| Participant-level | 0.95 | 0.93 |
| (0.22) | (0.26) | |
| Participant-day-level | 0.89 | 0.87 |
| (0.31) | (0.33) | |
| Fraction sleeping less than 6 hours | ||
| Participant-level | 0.71 | 0.69 |
| (0.46) | (0.46) | |
| Participant-day-level | 0.65 | 0.64 |
| (0.48) | (0.48) | |
| Self-reported hours asleep | 7.20 | 6.42 |
| (0.94) | (1.49) | |
| Panel B: Nap sleep | ||
| Percent napping on a given day | N/A | 0.25 |
| (0.43) | ||
| Duration of naps (conditional on napping) | N/A | 0.85 |
| (0.61) | ||
| Panel C: Total sleep | ||
| Hours asleep | 5.58 | 5.69 |
| (0.87) | (1.15) | |
| Participant-nights | 3,080 | 1,367 |
| Participants | 452 | 439 |
Notes. This table presents average sleep characteristics in two samples in Chennai. Standard deviations are in parentheses. Column (1) presents summary statistics from the RCT sample, only using data from the seven nights in the baseline period (i.e., before any treatments were implemented). Column (2) presents summary statistics from the three nights in our complementary sleep survey across a broader population in Chennai (described in Online Appendix F). All outcomes are objectively measured by actigraphs unless indicated otherwise. All means and standard deviations are at the participant level (i.e., we collapse the data at the participant level by averaging across nights) unless indicated otherwise. The variables shown in the table are: (i) hours in bed (per night, regardless of whether awake or asleep); (ii) hours asleep at night (per night); (iii) sleep efficiency (hours asleep/hours in bed); (iv) number of awakenings per night; (v) proportion of participants with less than seven hours of night sleep; (vi) proportion of participants with less than six hours of night sleep; (vii) self-reported hours asleep at night; (viii) proportion of participants napping on any given day; (ix) duration of naps (in hours) conditional on taking a nap; and (x) total hours asleep per 24 hours (the sum of night sleep and nap sleep).
3. Sleep Efficiency
Average sleep efficiency in our sample is 70% (Figure II, Panel E), far below recommended levels by sleep scientists who found that a minimum of 85% is needed to indicate “high-quality” sleep (Ohayon et al. 2017). Like sleep duration, sleep efficiency is much lower than typically found in high-income countries, and instead resembles U.S.-based patients suffering from sleep disorders such as sleep apnea (Roure et al. 2008) or insomnia (Trauer et al. 2015). Sleep efficiency is low throughout the night, remaining around 70% between 1 to 5 a.m. (when almost everyone is in bed), consistent with interrupted sleep throughout the night (Online Appendix Figure A.I). Participants experience about 32 awakenings on an average night (Table I, column (1)), again comparable to insomniacs in the United States (Lichstein et al. 2006).8
4. Barriers to Sleep
Why is sleep so inefficient? Survey responses highlight the importance of mental and physical distress (e.g., worries, stress, pain, or hunger) as well as environmental factors (Online Appendix, Figure A.IVa). Over 50% of study participants indicate that their sleep is disrupted by heat, noise, and/or light, which the night sleep treatments described below were intended to address.
5. Napping
Naps are relatively common in this population. Seventy-three percent of participants in our study reported taking at least one nap in the week before enrolling in the study. Conditional on napping, the median time reported for a nap is about one hour. The frequency and length of naps in U.S. populations is similar: Dinges (1992) finds that across a broad population of U.S. adults, 61% reported napping at least once a week with an average nap duration of 73 minutes, while in Pilcher, Michalowski, and Carrigan (2001), 74% of healthy adults report napping during a seven-day period.
6. Self-Reported Sleep
Self-reports significantly overestimate time asleep, relative to the objective actigraph measures, consistent with findings in the United States (Lauderdale et al. 2008; Avery, Giuntella, and Jiao 2019).9 Average baseline self-reported sleep duration in our study is 7.2 hours (Figure II, Panel D), quite similar to the average of 7.1 hours found in a representative survey of older adults in rural India described in Gildner et al. (2014). In comparison, average self-reported sleep duration in the United States ranges from 6.8 to 7.9 hours a night (Lauderdale et al. 2008; Watson et al. 2015; Jackson et al. 2018).
7. Broader Population
To investigate the representativeness of our RCT sample, we conducted a supplementary sleep survey with 3,833 individuals across randomly sampled neighborhoods in Chennai.10 A subset (N = 439) completed three nights of actigraph measurements. Despite not using any of the RCT screening criteria for this survey, the nighttime sleep duration and efficiency in this broader sample are similar to that of RCT participants, with an average of 5.5 hours of sleep a night and 71% sleep efficiency (Table I, column (2)). As in the RCT sample, napping is common, with 25% of participants napping on any given day and an average duration of those naps of roughly 50 minutes.
III. Experimental Design
We designed our experiment with three broad goals in mind. First, we aimed to estimate the effects of increased sleep over a few weeks in relatively natural sleep environments (as opposed to depriving people of sleep in lab settings). Second, we wanted to precisely measure labor supply, productivity, and earnings, and thus we employed study participants full-time in a realistic but closely controlled data entry job. Finally, to provide a broad view of the effects of increased sleep, we collected a range of additional outcomes, including cognition, preferences, and well-being.
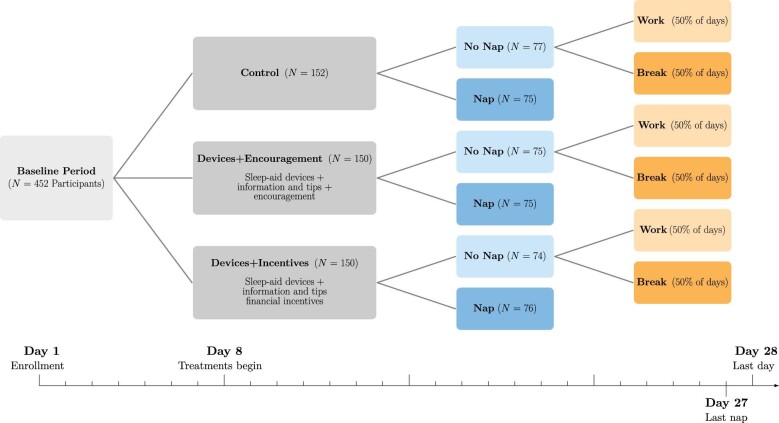
Figure III provides an overview of the experimental design and timeline of the study. Four hundred fifty-two participants worked for 28 days in an office in Chennai, spending most of their workdays doing paid data entry work. Enrollment took place on a rolling basis. The office contained computer work stations for data entry, a break room, booths for surveys and experimental tasks, and nap stations on a separate floor.
Figure III.
RCT Timeline and Experimental Design
This figure presents an overview of the timeline and experimental design of the study. After eight baseline days, the 452 participants were divided into three night sleep treatment groups: control, sleep devices + encouragement, and sleep devices + incentives. Participants in each of these groups were further randomized into a nap group, which was allowed and encouraged to use a nap station in the office in the early afternoon, and a no-nap group. Participants in the no-nap group were further randomized on a daily level (i.e., within individual) either to being allowed to work during the nap period or to take a mandatory pause during that time, with equal probability. The nap treatment ends at day 27, and the participants return the sleep devices on day 28. Endline surveys occur on day 28 or shortly thereafter.
III.A. Interventions to Increase Sleep
For their first eight days in the study, participants remained in a control condition, allowing us to collect rich baseline data. Then, we cross-randomized participants to two night sleep treatments and a nap treatment, stratified by baseline sleep and earnings.
1. Night Sleep Treatments
Each participant was randomly assigned to one of two night sleep treatment groups (encouragement or incentives) or to a control group in equal proportions.
Devices + Encouragement: This treatment involved a bundled intervention to increase night sleep. Individuals were offered (a) information about the benefits of sleep (in particular, generic health benefits) and tips to improve their sleep (such as going to bed at the same time every day, avoiding caffeine after 4 p.m., and avoiding screens before bed), (b) encouragement to increase their sleep as well as daily feedback on their night sleep duration as measured by the actigraph, and (c) loaned devices to improve their sleep environment. The offered devices included eye shades, earplugs, a cot, a mattress, sheets, pillows, and a fan (see Online Appendix Figure A.IIb).11
Devices + Incentives: This group received the same bundled intervention as the Devices + Encouragement group plus financial incentives to increase their actigraph-measured sleep during the treatment period. Each day, participants were paid Rs. 1 per minute of increased sleep for up to two hours of increased sleep (Rs. 120, about $1.70), relative to their baseline period sleep. There was no penalty for sleeping less than in the baseline period.12 To control for any income effects due to the sleep incentive payments, participants in the control and encouragement groups were randomly and anonymously matched to participants in the incentives group and received the same stream of payments, independent of their own sleep.
Control: This group did not receive any of the above treatments. To deal with the concern that loaning items might generate reciprocity effects or affect reported well-being directly, we offered placebo household goods, unrelated to sleep, to a subset of control participants. The total value of these goods was roughly the same as that of the sleep devices and included items such as small kitchen devices, a chair, decorative figurines, and a flashlight. These goods were also returned at the end of the study.13
Given the difficulty of increasing sleep in the field, we took a bundled approach in designing our treatments, working to increase sleep through multiple channels. Participants could respond to the encouragement and incentives by spending more time in bed or by taking steps to increase their sleep efficiency. The tips to improve sleep, such as avoiding caffeine in the evening, turning off the television, or putting away one’s cellphone at night, could plausibly increase sleep efficiency. Finally, the loaned devices could increase sleep efficiency and time in bed, if the devices made it easier to fall asleep or reduced awakenings or if they made time spent in bed more enjoyable.
2. Nap Treatment
Motivated by existing lab evidence that naps can be effective in boosting cognitive function (Lovato and Lack 2010) and can make up for limited nighttime sleep (Mollicone et al. 2008), we cross-randomized the night sleep treatments with a nap intervention. Starting on day 9 of the study, a random subset of individuals were given the opportunity to take a short afternoon nap every day between 1:30 and 2 p.m. Located in a quiet and gender-separated part of the study office, the 25 private nap spaces included a bed, blanket, pillow, table fan, ear plugs, and eye shades (see Online Appendix Figure A.IIc). The actigraphs show that roughly 90% of study participants did sleep during their allotted nap time. Those who did not want to nap were asked to sit quietly or rest in their nap area; they did not have the option to work during this time.
The remaining (no-nap) participants were randomized each day with equal probability to either a work day, on which we allowed them to work through the nap period, or a break day, on which we enforced a half-hour break from data entry during the same period. Break day participants were allowed to engage in any leisure activity they chose, including sitting in a comfortable office break room. By comparing nap and break participants, we isolate the effect of a nap relative to a break of the same length. By instead comparing the nap and work participants, we can estimate the net effect of naps on work output, including the lost work time.
III.B. Outcome Measures
Sleep and work are the two key sets of outcomes of this study. We measured each of them daily using actigraphs and the data entry platform, respectively. Study participants also completed a series of short surveys and experimental tasks throughout the study (see Online Appendix Table A.III). Described in greater detail in Online Appendix C, these measures fall into three broad categories: (i) physical and psychological well-being, (ii) cognition, and (iii) economic preferences.
1. Measures of Sleep
We measure night and nap sleep—sleep duration, time in bed, efficiency, and interruptions—using actigraphs, as described in Section II.A. Ninety-four percent of participants wore their actigraph on any given day, balanced across treatments. We complement these measures with daily self-reports of time in bed, time asleep, and number of awakenings during the night.
2. Work-Related Outcomes
Participants were engaged each day in data entry work. We designed a software interface that presented participants with images containing alphanumeric text and asked them to transcribe the data by typing into text boxes (see Online Appendix Figure A.III). The task was designed to mimic a real-world data entry job.14 Participants were paid for time spent typing as well as the amount of data entered, as described below. This design allows us to precisely measure labor supply, productivity, and earnings.
Labor Supply. Our preregistered measure of labor supply is the active typing time as automatically measured by the data entry software. As in many workplaces, participants were not forced to arrive or leave the office at precise times. On most days, participants could arrive or depart from the office as they chose between 9:30 a.m. and 8 p.m. When in the office, participants could take breaks from work. We can precisely measure even short breaks: if a participant spent two consecutive minutes without typing, the software automatically paused and the break period did not count toward the labor-supply measure. Thus, participants had a fair amount of control over their labor supply, except for time slots set aside for surveys, experimental tasks, and the lunch break.
Earnings. Earnings in the data entry work is our preregistered measure of performance at work and was used as our summary measure of work because it combines labor supply and productivity. It has two components: an “attendance pay” per hour of active typing (one-third of work earnings) and a “performance pay,” a piece rate for each correct character and a penalty per mistake (two-thirds of data entry earnings). Each half hour, piece rates were randomly varied between a low value (Rs. 5 per 1,000 correct characters) and high value (four times as large) with equal probability. The penalty rate remained constant throughout at Rs. 1 per 10 mistakes. The variation in piece rates allows us to benchmark any productivity effects of the sleep treatments against monetary incentives. The participants were paid daily, just before leaving the office for the day.15
Productivity. Our preregistered measure of worker productivity is output divided by active typing hours. Output is the number of correct entries minus (a weighted) number of mistakes. The weight on mistakes was defined as the ratio of the average piece rate and the penalty rate.
3. Well-Being
We collected a wide range of measures of psychological and physical well-being. As preregistered, we examine these variables both as indices and individually. The preregistered measures of mental well-being are happiness, sense of life possibilities (Cantril Scale), life satisfaction, stress, and depression. The measures of physical well-being are performance in a stationary biking task, reported days of illness, self-reported pain, activities of daily living, and blood pressure.16
4. Cognition
Sleep scientists have documented a strong relationship between sleep and cognition in numerous laboratory studies in rich countries (Lim and Dinges 2010; Killgore 2010). We collected (i) laboratory measures of cognitive function borrowed from cognitive psychology and sleep medicine; and (ii) a measure of attention to incentives at work embedded in the data entry task.
Lab Measures of Cognition. Each afternoon, participants completed the Psychomotor Vigilance Task (PVT), a standard measure of alertness and attention used in sleep medicine (Basner and Dinges 2011). Every other day, they completed cognitive tasks measuring memory (Corsi blocks task) and inhibitory control (Hearts and Flowers task), described in detail in Dean, Schilbach, and Schofield (2019) and briefly in Online Appendix C.5. All cognitive tasks were incentivized for performance (e.g., speed, accuracy).
Attention to Work Incentives. To test whether sleep affects the ability to attend to important aspects of one’s work environment, for example, the incentives faced, we randomized the visual salience of piece rates across days starting on day 6 of the baseline period. In the salient condition, the current piece rate was highlighted in different colors for each rate and displayed on the screen at all times. We consider this condition the “full-attention” benchmark, as in Chetty, Looney, and Kroft (2009). In the nonsalient condition, noticing and remembering the piece rate was more challenging. A single muted color was used for both piece rates, and (in the second half of the study) the rate was only visible for the first 15 seconds of a half-hour slot, fading out slowly. Online Appendix Figure A.III provides screenshots of each condition described below. The participant-level attention measure is the difference in average response to piece rate incentives in the full-attention benchmark and in the nonsalient condition.17
5. Preferences
Sleep may affect preferences through its impact on cognition or directly. For instance, sleep has been hypothesized to play a critical role in replenishing self-control (Vohs and Baumeister, 2016) and sleep deprivation has been correlated with cyberloafing at work (Wagner et al. 2012) and cheating (Barnes et al. 2011). Similarly, sleep could alter the weight placed on sure things versus gambles or on others versus the self (McKenna et al. 2007; Anderson and Dickinson 2010; Holbein, Schafer, and Dickinson 2019). To examine such effects we study time preference via financial savings outcomes and choices on a real-effort task, and risk and social preferences via standard experimental economics measures described below.
Savings. We measured savings behavior by providing participants an opportunity to save money in a lockbox at the study office, as in Schilbach (2019). At the end of each work day, after receiving their earnings, individuals had the opportunity to deposit or withdraw money. Participants were randomly assigned to receive daily interest rates between 0% and 2% for any money saved in the box.18 For participants receiving the positive interest rate, at least, the savings account we offered was quite lucrative. The deposits made in this account constitute our main savings outcome.
Effort Discounting. We measured present bias using real-effort choices, following Augenblick and Rabin (2019). Participants made decisions about how many pages to type at the end of the day on a particular date under different piece rates. Using choices elicited both in advance and on the day of the work itself, we structurally estimate an individual-level present bias parameter βi, once each in the baseline and treatment periods. A complete description of the task is in Online Appendix C.6.3.
Social and Risk Preferences. We measured risk and social preferences via standard tasks in the behavioral economics literature. Risk aversion and loss aversion are captured via a multiple price list elicitation similar to those in Holt and Laury (2002), and Charness, Gneezy, and Imas (2013). Social preferences are measured via dictator, ultimatum, and trust games (Camerer 2003).
6. Realism and External Validity
Conducting the study in the context of a month-long data entry job in a controlled environment follows Kaur, Kremer, and Mullainathan (2015) and allows for the provision of afternoon naps and precise measurement of the outcomes described above. However, it also comes with some costs. First, the work environment has some unusual and artificial features, such as regular surveys and laboratory measures of cognition and preferences. Second, any labor supply responses we find might be muted in environments where employers more strictly control schedules. In practice, participants tend to spend about 8 hours at the office each day, with an average arrival time of 10:32 a.m. (std. dev. = 43 minutes) and average departure time of 6:20 p.m. (std. dev. = 57 minutes). This is quite similar to other jobs in our context, with long commutes and unreliable transportation, such that arriving strictly at a given time is difficult.
III.C. Expert Predictions
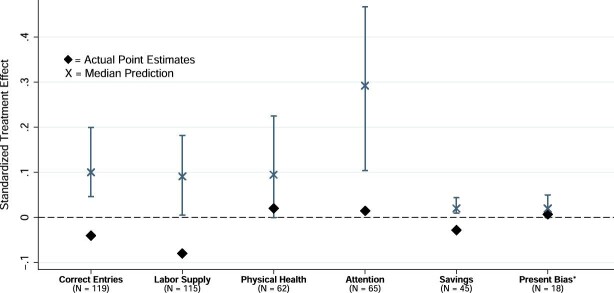
To quantify how our results compare with existing scientific understanding, we conducted surveys of experts in sleep science and economics to elicit their prior beliefs about the treatment effects of the night sleep interventions included in this study (DellaVigna, Pope, and Vivalt 2019). Participation in the survey was solicited via emails to experts in each field. The survey provided information on the design of the study, the magnitude of the increase in night sleep reported in Section IV.B, and the outcome measures described above.19 Three versions of the survey were tailored to different respondents: development and labor economists; behavioral economists; and sleep medicine experts. A total of 28 labor and development economists, 19 behavioral economists, and 77 sleep medicine experts responded to the survey. In an effort to keep the survey short, we did not elicit predictions about the effects of the nap treatment. All experts were provided with relevant benchmarks (e.g., the elasticity of effort with respect to the piece rate) and made predictions on labor supply and work output effects. Both types of economists responded with their beliefs about savings. Only behavioral economists were asked to predict changes in present bias, and only sleep experts were asked to predict changes in sustained attention and physical health. The expert predictions are shown in Figure IV and in Online Appendix Table A.IV and are discussed when presenting results. Further details are provided in Online Appendix C.1.
Figure IV.
Expert Predictions versus RCT Results
This figure summarizes the predictions made by experts in economics and sleep science about the expected treatment effect of our (pooled) night sleep interventions. We normalize each prediction, dividing them by the control group’s standard deviation. For comparison, we also present the actual estimated treatment effect. The bars show the interquartile range (25th to 75th percentiles) of the predictions for a given outcome variable. We also show the median prediction (X) and the actual point estimates (diamond) of the treatment effects. N refers to the number of responses for each outcome variable. This number varies by outcome because different types of experts (e.g., sleep researchers, behavioral economists) were asked about some different outcomes. Correct Entries refers to the number of daily correct characters in the data entry task, that is, a measure of overall output per day. Labor Supply refers to the daily number of hours working in the typing task, that is, time at the office excluding voluntary and scheduled pauses. Physical Health refers to a variable that averages (normalized) systolic and diastolic blood pressure. We flip the sign of this variable so a positive value means an improvement in health (i.e., a reduction in blood pressure). Attention refers to an index pooling inverse response times and minor lapses in the Psychomotor Vigilance Task, a standard lab measure of attention in the sleep literature. Signs are flipped such that higher values refer to increased attention. Savings refers to the daily amount deposited minus the amount withdrawn in the savings box during the experiment. Present Bias refers to the present-bias parameter β. Unlike the other variables, the predictions and point estimate refer to the reduction in present bias (increase in β) rather than a normalized outcome, for ease of interpretation.
III.D. Study Population and Balance Checks
We followed two strategies to recruit our study sample. First, recruiters went to low-income neighborhoods in Chennai and spread information about the study, advertising a one-month data entry job. Second, past participants could refer potential new participants to the study. In both cases, recruiters interviewed individuals to determine their eligibility to participate in the study.
1. Eligibility Criteria and Selection
Interested individuals participated in a two-stage screening process, involving a brief unpaid survey and a home visit to check whether the individual met the study’s eligibility criteria: (i) being 25 to 55 years old; (ii) fluency in the local language (Tamil) and the ability to read and write numbers; (iii) having worked fewer than five days per week and earning an average of Rs. 700 ($10) or less per day worked in the previous month; (iv) living in a dwelling able to accommodate the sleep devices used in night sleep treatments and ownership of three or fewer of the sleep devices being offered in the study; (v) the intention to be in Chennai for the following five weeks; and (vi) no children in the household younger than three years.
Importantly, this recruitment and screening procedure does not seem to select participants on average levels of sleep quantity and efficiency. In Table I, we find very similar patterns of sleep among individuals in Chennai in the broader sleep survey, as described in Online Appendix F.
2. Informed Consent
All participants went through a detailed informed-consent procedure including information about the work task, other experimental measures and surveys, the actigraphs and the randomized treatments. The specific research hypotheses were not shared with participants to avoid demand effects. Instead, the goal of the research was described as work to understand the “difficulties underprivileged people in India face, and how these problems affect their lives.”
3. Sample Characteristics
Online Appendix Table A.I shows sample characteristics. A typical study participant was about 35 years old with one or two children and 10 years of education. Two-thirds of study participants were women. Although only 30% of participants had prior computer experience, participants were eager to learn and improved rapidly in their data entry speed during the baseline period.
4. Balance Checks
We test for baseline imbalances in demographics and baseline measures of outcome variables across the experimental conditions in Online Appendix Tables A.I and A.II. Whether we separately consider each treatment cell (Online Appendix Table A.I) or compare the pooled night sleep treatment groups with the control and the nap and no-nap groups (Online Appendix Table A.II), the treatment groups were well-balanced across key characteristics. For each treatment arm, a joint F-test comparing it to the control group indicated no systematic differences on observable characteristics across groups.
As is expected given the large number of comparisons, a few statistically significant differences across treatment groups did emerge. Most notably among those, participants in the night sleep treatment groups were about a year younger than those in the control group, and baseline productivity and earnings were about 3%–4% lower in the nap group than in the no-nap groups (Online Appendix Table A.II). We control for age and for the participant-level baseline average of the outcome variables, so these imbalances should not affect our results.20
IV. Experimental Results
IV.A. Empirical Framework
Most of our empirical analyses, including all work-related outcomes, estimate treatment effects on outcomes measured at the participant-day level using variants of the following equation:
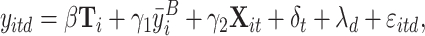 |
(1) |
where yitd is the relevant outcome for participant i on their tth day in the study on calendar date d. Ti is a vector of indicator variables capturing the treatment(s) that participant i was assigned to. β is the vector of coefficients, capturing the effect of each treatment on the outcome of interest.
Following McKenzie (2012), we control for the average baseline value of the outcome variable 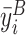 in all specifications, and drop the baseline days from the regression. We also drop days on which participants were absent, since attendance was balanced across groups. Xit includes controls for participants’ age (quartiles) and gender and, where specified, a dummy variable for whether a given no-nap participant i was assigned to work through the nap period or instead to take an enforced break on day t. This allows us to estimate the effect of naps separately compared with working and taking a break.21 Finally, we include day-in-study and calendar date fixed effects, captured by δt and λd, respectively. All standard errors are clustered at the participant level.
in all specifications, and drop the baseline days from the regression. We also drop days on which participants were absent, since attendance was balanced across groups. Xit includes controls for participants’ age (quartiles) and gender and, where specified, a dummy variable for whether a given no-nap participant i was assigned to work through the nap period or instead to take an enforced break on day t. This allows us to estimate the effect of naps separately compared with working and taking a break.21 Finally, we include day-in-study and calendar date fixed effects, captured by δt and λd, respectively. All standard errors are clustered at the participant level.
For some outcomes, such as preferences, we only have one observation in the baseline and one in the treatment period per participant. In those cases, we run participant-level regressions:
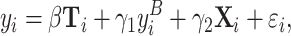 |
(2) |
where again the outcome variable only uses the observations from the treatment period and we control for the baseline observation of the outcome, 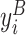 . The vector Xi includes the same gender and age controls. Because these outcome measurements span multiple days (e.g., present bias) or are on a fixed day-in-study (e.g., the endline survey), this specification does not include day-in-study or calendar-date fixed effects or control for whether no-nap participants worked or took a break.
. The vector Xi includes the same gender and age controls. Because these outcome measurements span multiple days (e.g., present bias) or are on a fixed day-in-study (e.g., the endline survey), this specification does not include day-in-study or calendar-date fixed effects or control for whether no-nap participants worked or took a break.
1. Combining Outcomes into Indices
Given the large number of outcomes, we divide them into four major families: work, well-being, cognition, and preferences. We construct a single summary outcome for each family. The work outcomes are naturally summarized by (standardized) earnings in the data entry task, which combines productivity and labor supply into a single quantity. For the other families, we create standardized index variables by residualizing each constituent outcome with respect to day in study and calendar date, standardizing it by the control group’s mean and standard deviation, and then taking a weighted average to form the index. Following Anderson (2008), the weights are the inverse of the covariance matrix of the residualized, standardized outcomes. This ensures that outcomes that are highly correlated receive less weight than outcomes that capture independent information. Signs of outcome variables are flipped when necessary, so positive treatment effects imply an improvement in the outcome.22 We also report treatment effects on an overall index, which combines the four family-level summary outcomes into a single variable. We use the same procedure to create the overall index.
2. Multiple-Hypothesis Testing
We report three approaches to dealing with multiple-hypothesis testing issues caused by observing many outcomes. Our simplest approach is to examine a single overall index variable which combines all outcomes, as described above. Our intermediate approach is to consider outcomes at the level of the four families, using one summary variable for each family, while correcting for the existence of multiple families. Finally, at the level of the individual outcomes, we correct for the existence of multiple outcomes within each family. In each case, we report adjusted p-values that control the family-wise error rate—the probability of at least one false rejection—using a step-down permutation procedure based on Westfall and Young (1993). The adjusted p-values are displayed for the main results in Table IV and Online Appendix Tables A.VII, A.VIII, and A.IX.23
TABLE IV.
Pooled Treatment Effects of Night Sleep and Nap Treatments
| Overall | Work | Well-being | ||||||
|---|---|---|---|---|---|---|---|---|
| Index | Earnings | Productivity | Labor supply | Output | Index | Physical | Psychological | |
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | |
| Night sleep treatments | −0.01 | −0.04 | 0.02 | −0.08 | −0.04 | 0.01 | 0.04 | −0.05 |
| (0.04) | (0.02) | (0.02) | (0.02) | (0.02) | (0.03) | (0.04) | (0.06) | |
| {.79} | {.08} | {.30} | {.00} | {.05} | {.69} | {.35} | {.36} | |
| [.30] | [.32] | [.00] | [.09] | [.97] | [.36] | [.36] | ||
| Nap treatment | 0.12 | −0.02 | 0.04 | −0.09 | −0.01 | 0.08 | 0.05 | 0.12 |
| (0.04) | (0.02) | (0.02) | (0.02) | (0.02) | (0.03) | (0.04) | (0.05) | |
| {.00} | {.23} | {.03} | {.00} | {.77} | {.01} | {.18} | {.02} | |
| [.25] | [.06] | [.00] | [.77] | [.03] | [.19] | [.04] | ||
| Participants | 451 | 451 | 451 | 451 | 451 | 452 | 452 | 452 |
| Unadjusted p-value NS versus nap | {.02} | {.62} | {.49} | {.65} | {.23} | {.16} | {.83} | {.03} |
| FWER-corrected p-value NS versus nap | [.62] | [.66] | [.66] | [.45] | [.41] | [.84] | [.04] | |
| Night sleep treatments | −0.00 | 0.03 | −0.01 | −0.00 | 0.04 | −0.04 | −0.04 | |
| (0.05) | (0.04) | (0.11) | (0.04) | (0.07) | (0.06) | (0.08) | ||
| {.93} | {.51} | {.95} | {.95} | {.58} | {.45} | {.65} | ||
| [.98] | [.76] | [.95] | [.98] | [.64] | [.64] | [.64] | ||
| Nap treatment | 0.10 | 0.08 | 0.20 | 0.07 | 0.13 | 0.03 | 0.07 | |
| (0.05) | (0.04) | (0.11) | (0.04) | (0.06) | (0.05) | (0.08) | ||
| {.03} | {.03} | {.07} | {.05} | {.04} | {.52} | {.33} | ||
| [.08] | [.07] | [.07] | [.15] | [.20] | [.51] | [.51] | ||
| Participants | 452 | 452 | 429 | 452 | 452 | 415 | 415 | |
| Unadjusted p-value NS versus nap | {.12} | {.31} | {.21} | {.22} | {.36} | {.30} | {.32} | |
| FWER-corrected p-value NS versus nap | [.41] | [.31] | [.31] | [.41] | [.36] | [.36] | [.36] | |
Notes. This table shows treatment effects of the pooled night sleep and the nap interventions on the overall index as well as the four families of outcomes: work, well-being, cognition, and preferences. Each row shows coefficients of treatment cells compared to the control group that receives no sleep-related treatments. All work-related regressions are conducted at the participant-day level using equation (1). All other regressions are at the participant level using equation (2). All outcome variables are the same as in Table III. In contrast to Table III, in this table (i) we pool the Devices + Encouragement and the Devices + Incentives treatments to estimate the pooled effect of the two night sleep treatments, and (ii) we do not include a separate indicator for the group that receives both the night sleep and nap treatments. Hence, these estimates should be interpreted as weighted averages of treatment effects in the relevant cells. For instance, the coefficient on the night sleep treatment is the average effect of being assigned to one of the two night sleep treatments (with equal probability), in a population which either receives naps or does not (with equal probability). Below each coefficient, we report (i) the corresponding standard errors in parentheses (·), robust to heteroskedasticity and clustered at the participant-level when applicable, (ii) the unadjusted p-value in curly brackets {·}, and (iii) the Westfall-Young FWER-adjusted p-value in square brackets [·], as described in more detail in Online Appendix E.
3. Pre-Analysis Plan
This study was preregistered on the AEA Registry and ClinicalTrials.gov, including a pre analysis plan (PAP). We deviate from the PAP in some instances. The main deviations (in our view) are the following. First, we prespecified a regression model that included all interactions of treatments. We soon came to realize we were not well-powered for this analysis and that it would lead to a large number of coefficients and comparisons that would be difficult to present and interpret. We still present the prespecified estimates in Tables II and III but complement them with a simplified but higher-powered specification that pools the two night sleep treatments and omits the interactions between nap and night sleep treatments in Table IV. Second, we had not fully specified our approach to multiple-hypothesis testing and made some changes after receiving comments and discussing with experts. We added the overall index variable to parsimoniously aggregate all outcomes. We also redefined the four families of outcomes (work, well-being, cognition, and preferences rather than work and decision making) and created a summary variable for each family. Other deviations are detailed in Online Appendix Section D.
TABLE II.
Treatment Effects on Sleep
| Night sleep | Time in bed | Sleep efficiency | Nap sleep | 24-Hr sleep | |
|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | |
| Devices+Encouragement only | 0.32*** | 0.53*** | −0.54 | 0.00 | 0.33*** |
| (0.08) | (0.09) | (0.66) | (0.00) | (0.08) | |
| Devices+Incentives only | 0.49*** | 0.84*** | −1.14 | 0.00 | 0.49*** |
| (0.08) | (0.09) | (0.70) | (0.00) | (0.08) | |
| Nap only | −0.13* | −0.11 | −0.72 | 0.25*** | 0.09 |
| (0.08) | (0.09) | (0.72) | (0.01) | (0.08) | |
| Devices+Encouragement and nap | 0.21*** | 0.40*** | −1.06 | 0.24*** | 0.42*** |
| (0.07) | (0.08) | (0.66) | (0.01) | (0.08) | |
| Devices+Incentives and nap | 0.48*** | 0.58*** | 0.90 | 0.24*** | 0.70*** |
| (0.08) | (0.09) | (0.68) | (0.01) | (0.08) | |
| Control mean | 5.61 | 8.07 | 69.86 | 0.00 | 5.61 |
| Control std. dev. | 1.20 | 1.37 | 11.28 | 0.00 | 1.20 |
| Participant-nights | 8,454 | 8,454 | 8,454 | 7,191 | 8,035 |
| Participants | 451 | 451 | 451 | 450 | 451 |
Notes. This table reports the treatment effect of the night sleep and nap interventions on sleep patterns. Each column shows the OLS estimates of equation (1) including dummies for each treatment arm and controlling for the average baseline measure of the dependent variable (ANCOVA), age, sex, and day-in-study and date fixed effects. Each row shows coefficients of treatment cells compared to the control group that receives no sleep-related treatments. Night sleep, time in bed, nap sleep, and 24-hour sleep (columns (1), (2), (4), and (5)) are measured in hours. Sleep efficiency (column (3)) is the ratio of night sleep and time in bed (multiplied by 100 for clarity). 24-hour sleep is the sum of nap sleep in the office and night sleep. Standard errors are clustered at the participant level. Stars next to coefficients reflect unadjusted p-values (* significant at 10%; ** at 5%; *** at 1%).
TABLE III.
Fully Disaggregated Treatment Effects of Night Sleep and Nap Treatments
| Overall | Work | Well-being | ||||||
|---|---|---|---|---|---|---|---|---|
| Index | Earnings | Productivity | Labor supply | Output | Index | Physical | Psychological | |
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | |
| Devices+Encouragement only | 0.00 | −0.07** | −0.02 | −0.06* | −0.07** | 0.13*** | 0.16** | 0.02 |
| (0.07) | (0.03) | (0.03) | (0.04) | (0.03) | (0.05) | (0.07) | (0.09) | |
| Devices+Incentives only | −0.05 | −0.07* | −0.02 | −0.08** | −0.08** | 0.05 | 0.08 | −0.02 |
| (0.07) | (0.04) | (0.03) | (0.04) | (0.03) | (0.05) | (0.06) | (0.09) | |
| Nap only | 0.11 | −0.07 | −0.01 | −0.07* | −0.05 | 0.18*** | 0.16** | 0.19** |
| (0.07) | (0.04) | (0.03) | (0.04) | (0.04) | (0.06) | (0.07) | (0.10) | |
| Devices+Encouragement and nap | 0.13* | −0.07* | 0.04 | −0.15*** | −0.04 | 0.13*** | 0.11* | 0.11 |
| (0.07) | (0.04) | (0.03) | (0.04) | (0.03) | (0.05) | (0.06) | (0.09) | |
| Devices+Incentives and nap | 0.08 | −0.07* | 0.04 | −0.17*** | −0.07** | 0.10** | 0.11* | 0.07 |
| (0.06) | (0.04) | (0.03) | (0.04) | (0.03) | (0.05) | (0.07) | (0.09) | |
| Participants | 451 | 451 | 451 | 451 | 451 | 452 | 452 | 452 |
| Devices+Encouragement only | −0.00 | −0.00 | 0.04 | −0.09 | −0.05 | −0.10 | −0.15 | |
| (0.07) | (0.07) | (0.17) | (0.06) | (0.12) | (0.09) | (0.13) | ||
| Devices+Incentives only | −0.03 | 0.04 | −0.12 | −0.04 | 0.11 | −0.16* | −0.06 | |
| (0.08) | (0.07) | (0.19) | (0.07) | (0.12) | (0.08) | (0.13) | ||
| Nap only | 0.09 | 0.07 | 0.15 | −0.01 | 0.12 | −0.09 | −0.01 | |
| (0.07) | (0.07) | (0.17) | (0.07) | (0.12) | (0.09) | (0.14) | ||
| Devices+Encouragement and nap | 0.05 | 0.13* | 0.03 | 0.09 | 0.23* | 0.03 | 0.03 | |
| (0.08) | (0.07) | (0.18) | (0.07) | (0.12) | (0.10) | (0.13) | ||
| Devices+Incentives and nap | 0.14** | 0.09 | 0.32** | 0.01 | 0.11 | −0.10 | 0.01 | |
| (0.07) | (0.07) | (0.16) | (0.07) | (0.12) | (0.09) | (0.14) | ||
| Participants | 452 | 452 | 429 | 452 | 452 | 415 | 415 | |
Notes. This table shows the treatment effects of the five fully disaggregated treatment arms on the overall index as well as the four families of outcomes: work, well-being, cognition, and preferences. Each row shows coefficients of treatment cells compared to the control group that receives no sleep-related treatments. All work-related regressions are conducted at the participant-day level using equation (1). All other regressions are at the participant level using equation (2). The overall index (column (1)) aggregates across the four family-level outcomes. The work outcomes include data entry earnings (the summary variable for the work family, column (2)); productivity (output per hour typing, column (3)); active typing time (column (4)); and output (column (5)). Well-being outcomes include an overall index (column (6)) of the two broad measures of well-being: a physical well-being index (column (7)) and a mental well-being index (column (8)), as described in Section III.B. Cognition measures include an overall index (column (9)) of two measures: lab measurements of attentiveness, memory, and inhibitory control (column (10)); and attention to piece rates in the data entry task (column (11)). Preference measures include an index (column (12)) of three different categories: time preferences (savings, which additionally control for the surveyor on site, and present bias, column (13)), social preferences (column (14)), and risk preferences (column (15)). All indices are a weighted average of their components, in which the weights take into account the covariance structure of the components (Anderson 2008). All dependent variables are normalized with respect to the control group’s mean and standard deviation. When required, outcomes are flipped so that a positive value aligns with what would be considered a “better” outcome. Standard errors in parentheses are robust to heteroscedasticity and clustered at the participant level when applicable. Stars next to coefficients reflect unadjusted p-values (* significant at 10%; ** at 5%; *** at 1%). Online Appendix Table A.VII shows p-values that take into account multiple-hypothesis corrections.
IV.B. Effects on Sleep
1. Overview
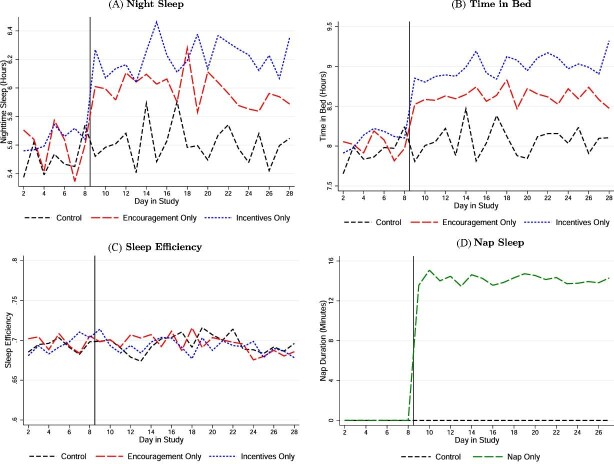
Figure V and Table II show the impacts of our treatments on the different measures of sleep. We find that the two night sleep treatments quickly and substantially increase night sleep by 27 minutes a night on average. Offering short afternoon naps increases daytime sleep by 14 minutes a day. Thus, it is possible to substantially increase sleep in the sleep-deprived population we study through encouragement, incentives, and nap opportunities.
Figure V.
Impacts on Nighttime and Nap Sleep
This figure shows the average of different sleep-related variables for different treatment arms by day in study of the RCT. All outcomes are actigraph measures. We only include workday nights and days in the sample. In Panels A and B, we plot hours of night sleep and hours in bed at night, respectively. Panel C shows sleep efficiency (nighttime sleep/time in bed). In Panel D, we plot the duration of naps at the workplace (in minutes), excluding day 28 because naps were not allowed on that day.
2. Night Sleep Treatments Only
Both night sleep treatments sharply increased sleep, as measured by the actigraphs. On average, individuals who received the Devices + Encouragement and Devices + Incentives treatments only—that is, without also receiving naps—increased their time asleep at night by 19 and 29 minutes a night compared with the control group, respectively (Figure V, Panel A, and Table II, column (1)). Including those who might have additionally received naps, the night sleep treatments increased night sleep by 27 minutes a night on average (Online Appendix Table A.VI). This is a larger gain than typically achieved by sleeping pills and cognitive behavioral therapy for insomnia (Riemann and Perlis, 2009; Trauer et al. 2015).
The increase in sleep was driven entirely by additional time in bed rather than improved sleep efficiency. Both night sleep treatment groups increased their time in bed significantly throughout the treatment period—32 minutes for the encouragement only group and 50 minutes for the incentives only group (Figure V, Panel B, and Table II, column (2)).24 We find no significant changes in sleep efficiency compared to the control group (Figure V, Panel C, and Table II, column (3)), even in the middle of the night when all participants are likely to be in bed (Online Appendix Figure A.Ia).
Increasing time spent in bed is a simple and practical way for our study participants to increase their sleep duration. However, improving sleep efficiency appears to be difficult for participants, even with the aid of the devices and tips surrounding sleep hygiene. Participants faced substantial (implicit) incentives for improvement. For example, a participant in the incentives group who improved their sleep efficiency from 70% to 80% would on average earn an additional Rs. 48—about 20% of average typing earnings—in sleep incentives each night (holding fixed eight hours a night in bed). Yet we saw no changes in sleep efficiency. Improving sleep efficiency may require more substantial interventions, including ways to overcome barriers to sleep—such as mosquitoes, crowding, or psychological distress—that remained unaddressed by our treatments (Online Appendix Figure A.IVa).
3. Nap Treatment Only
The nap intervention was effective at increasing participants’ daytime sleep (Figure V, Panel D, and Table II column (4)). Nearly all participants in the nap treatment (92%) reported falling asleep during their allotted nap time, consistent with actigraph data showing that participants fell asleep in 93% of all nap sessions. The mean actigraph-recorded (unconditional) time asleep during the nap period was 14 minutes, and the median duration was 16 minutes (Online Appendix Figure A.IId).
The “quality” of nap sleep in the office appears to be higher than that of night sleep and naps at home. For instance, sleep efficiency during naps in the office (85%) is higher than efficiency in night sleep (66%) and in naps at home (72%, similar to night sleep), if one excludes in all cases the time taken to first fall asleep.25 The average number of awakenings per minute of sleep achieved is also lower for the office naps. Better sleep quality during naps in the office—compared to both naps and night sleep at home—is consistent with a more comfortable sleep environment in the office.
4. Interactions, Crowd-Out, and Heterogeneity
We find only modest interactions between the sleep treatments in terms of their effects on the various sleep measures. The effect on 24-hour sleep of receiving the encouragement treatment and the nap treatment together is very similar to the sum of the effects of receiving each treatment alone (25 minutes versus 25 minutes, p = .98). The same is largely true for providing incentives and naps together (42 minutes versus 35 minutes, p = .32).
We do find evidence that napping modestly crowds out nighttime sleep: those treated with naps spent seven minutes less in bed at night and slept, on average, eight minutes less per night (Table II, columns (1)–(2)). In contrast, the night sleep treatments do not interfere with naps. Participants randomized to the night sleep treatments did not nap any less when offered a nap (Table II, column (4)). Both treatments increased total time asleep in 24 hours, although naps alone had a substantially smaller and insignificant effect (Table II, column (5)).26
Finally, the effect of the night sleep treatments on sleep quantity and efficiency did not differ significantly by baseline sleep patterns, nor by characteristics such as participants’ gender, age, or baseline earnings (Online Appendix Table A.V). Nor did these factors predict meaningful differences in nap duration for the nap treatment group. The treatments thus seem to have been equally effective at increasing sleep (and leaving efficiency unchanged) for different categories of participants.
IV.C. Overall Effect of Each Treatment on Outcomes
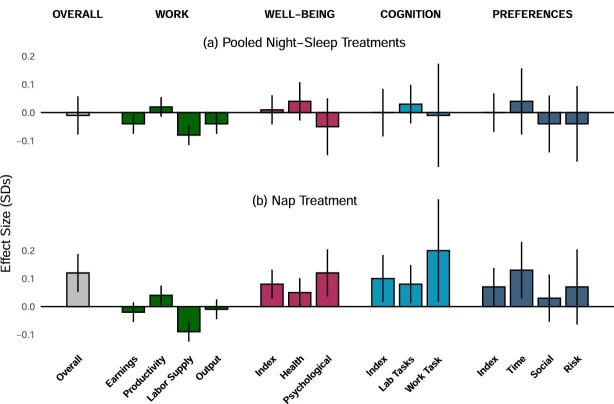
Table III presents the treatment effects for the five combinations of night sleep and nap treatments. Given the large number of outcomes and treatments, we focus on the effects on the overall summary index (column (1)) which parsimoniously and efficiently averages our outcomes.27 Each of the night sleep treatments alone had no effect or a slightly negative (but insignificant) effect on participants: 0.00 std. dev. (std. err. = 0.07) and −0.05 std. dev. (std. err. = 0.07), respectively, for the encouragement only and incentives only groups. In contrast, participants in the nap only treatment experienced positive and marginally significant effects of 0.11 std. dev. (std. err. = 0.07, p = .11). The effect of naps only differs significantly from that of incentives only (p = .02) and suggestively from that of encouragement only (p = .11). Those who received a night sleep treatment in addition to naps had very similar overall effects to those who received naps only: 0.13 and 0.08 std. dev. for the encouragement and nap and incentives and nap groups respectively, compared to 0.11 std. dev. for nap only.
These results provide evidence that naps have an overall positive effect on outcomes, while increases in night sleep do not. However, since each treatment cell has only around 75 participants, this analysis has limited statistical power. The combination of five treatment cells with numerous outcomes also makes discussion of detailed results unwieldy. We therefore turn to a simplified but higher-powered version of this analysis, which pools the two night sleep treatments—which typically have similar and statistically indistinguishable effects —and does not include a separate indicator for the group that receives both the night sleep and nap treatments. The resulting estimates in Table IV should be interpreted as weighted averages of treatment effects in the relevant cells. For instance, in this fully pooled specification, the coefficient on the night sleep treatment is the average effect of being assigned to one of the two night sleep treatments (with equal probability), in a population which either receives naps or does not (with equal probability). In Online Appendix Table A.VIII, we instead pool the two night sleep treatments but include a separate indicator for individuals who received a combination of either night sleep treatment along with the nap treatment.
IV.D. Effect of Night Sleep Treatments
1. Overview
Experts from sleep science and economics predicted that increased night sleep would result in higher work output and labor supply, improved health and attention, increased financial savings, and reduced present bias (Figure IV). In contrast to these predictions and an influential literature in sleep science, we find no effect of the pooled night sleep treatments on any of these outcomes. More generally, we find no positive effects of the night sleep treatments on the four family-level summary variables, or on any of the individual outcomes in our pooled specification (Figure VI and Table IV). Instead, increases in night sleep come at the cost of significantly reduced labor supply and therefore a marginally significant reduction in work output.
Figure VI.
Summary of Treatment Effects
This figure summarizes the treatment effects in our study. We plot the point estimates and 90% confidence intervals for the pooled night sleep interventions in Panel A and the nap intervention in Panel B. All outcomes are standardized, that is, we subtract the mean and divide by the standard deviation of the individuals receiving neither the night sleep nor the nap interventions. The coefficients and confidence intervals are based on the estimates and standard errors in Table IV. The comparison group for the nap treatment is the pooled nap control group, that is, participants not assigned to the nap intervention. The outcome variables, described in more detail in Section III.B, are as follows. Overall index: aggregates across all the outcomes in the table. Work: (i) earnings; (ii) productivity; (iii) active typing time; and (iv) output from the data entry task. Well-being: (i) “Well-being Index,” a composite index of the physical and mental well-being indices; (ii) “Physical,” a physical well-being index, a composite of performance in an endline biking task, self-reported illnesses, self-reported pain, self-reported health, and blood pressure; and (iii) “Psychological,” a mental well-being index, a composite of self-reported depression, happiness, life possibility, life satisfaction, and stress. Cognition: (i) “Cognition Index,” composite index of a lab-based and a work-based measure of cognitive function; (ii) “Lab Tasks,” index of lab measures of attention, memory, and inhibitory control; and (iii) “Work Task,” measure of attention to piece rates in the data entry task. Preferences: (i) “Preferences Index,” composite index of time, social, and risk preference indices; (ii) “Time,” index capturing time preferences, including savings and present bias; (iii) “Social,” index representing social preferences; and (iv) “Risk,” index representing risk preferences.
2. Work Outcomes
The night sleep treatments did not cause significant improvements in productivity, labor supply, output, or earnings (Table IV, columns (2)–(5)). Although the night sleep treatment groups were 1.3% more productive than the control group (column (3)), this difference of 0.02 std. dev. (std. err. = 0.02) is not statistically significant even without multiple-hypothesis testing corrections.
The night sleep treatments reduced labor supply by approximately 10 minutes a day (0.08 std. dev. with std. err. = 0.02, Table IV, column (4)) with no change in days worked (Online Appendix Figure A.VII). This effect on labor supply remains significant with p < .01 when correcting for multiple outcomes in the work family. Given the additional time in bed induced by the night sleep treatments, participants have less time available for work and leisure, which comes at the cost of reduced labor supply. Specifically, participants arrive at work six minutes later in the morning, take two minutes more of breaks at work, and leave for home three minutes earlier on average (Online Appendix Table A.X). While perhaps obvious ex post, the opportunity costs of increasing sleep are typically neglected in the sleep literature. Indeed, the mean expert prediction was an increase in labor supply of 7%, which is strongly rejected by the data (p < .001).
The small increase in productivity was not enough to outweigh the reduction in labor supply, leading to a small and marginally significant decrease in earnings and output, respectively (each 0.04 std. dev., std. err. = 0.02).28 This finding is again in contrast to the mean (median) expert prediction of a 12% (7%) increase in output. The discrepancy can be partly explained by experts overestimating the productivity effects of increased sleep, and partly by their mispredicting that more sleep would increase the time allotted to work. Eighty-three percent of experts made point predictions outside of the 95% confidence interval of our estimate of the effect on output.
Our results also contrast with those from natural experiments studying the economic consequences of sleep. Gibson and Shrader (2018) exploit variation in sunset times in the United States and estimate that 8.5 minutes of additional sleep per night increases earnings by 1.1% in the short run. Giuntella and Mazzonna (2019) use time zone border discontinuities in the United States and find that 19 fewer minutes of sleep are associated with 3% lower earnings. Extrapolating these estimates linearly to our experiment would predict 3.5% and 4.3% increases in earnings, respectively, which we firmly reject (p < .01).
3. Well-Being
Increased night sleep did not significantly improve physical or psychological well-being (Table IV, columns (6)–(8)). We find no positive effect on the index variable that combines the various measures of psychological well-being, in contrast to a largely observational literature that shows associations between self-reported sleep duration or quality and psychological well-being (Kahneman and Krueger 2006; Hamilton et al. 2007; Zhang et al. 2017). In fact, the point estimate is negative (−0.05 std. dev., std. err. = 0.06, Table IV, column (8)). The individual components of this measure also show no significant improvements (Online Appendix Table A.XI).
Similarly, we find no significant effects of increased night sleep on an index of physical well-being (Table IV, column (7)) that combines objective and self-reported measures of health status. We do find positive (not significant) point estimates for some of the underlying components such as performance in a cycling task and self-reported illness, pain, and daily activity (Online Appendix Table A.XI). Of course, three weeks is a short time for effects on physical health and behaviors to emerge. It could be that a longer intervention would generate health improvements in line with the observational literature (e.g., Strine and Chapman 2005; Cappuccio et al. 2008; Giuntella and Mazzonna 2019). It is also worth noting that the disaggregated analysis paints a slightly different picture when it comes to the effects on physical (but not psychological) well-being. Online Appendix Table A.VIII reports that the night sleep only treatment has a positive effect on physical well-being (0.12 std. dev., p = .06)—as does the nap only treatment (0.16 std. dev., p = .02)—when one allows the night sleep and nap cell to have a distinct effect. The interpretation is that the night sleep treatments do increase physical well-being in a population that does not receive any naps, but have no average effect on physical well-being in a population where half the people already take naps. Night sleep and naps thus appear to have a negative interaction effect on physical well-being.
4. Cognition
We find no effects of increased night sleep on cognition (Table IV, columns (9)–(11)). There is no significant effect on an index of laboratory measures of attention, memory, and inhibitory control (Table IV, column (10)), or on any of the individual outcomes, which closely mimic measures used in laboratory studies (Online Appendix Table A.XII). A large body of sleep studies shows that inducing sleep deprivation—typically by keeping participants up all night—substantially worsens performance on these tasks (Lim and Dinges 2010; Killgore 2010). The more modest but sustained and policy-relevant increases in sleep in our setting do not have a corresponding positive effect.
We also find no evidence of effects on attention measured in a more economic domain: how much people react to salient versus nonsalient incentives (Table IV, column (11)). Consistent with limited attention, participants in the control group reacted 16% less to high incentives when piece rates were nonsalient (Online Appendix Table A.XIII). Participants in the night sleep treatments behave quite similarly, reacting 15% less to incentives when they were nonsalient. Increased night sleep did thus not close the gap between responses to salient and nonsalient incentives.
5. Preferences
Consistent with the lack of positive effects of increased night sleep on the above outcomes, we find no evidence of the night sleep treatments affecting time, risk, or social preferences, or on an index combining these outcomes (Table IV, columns (12)–(15)). We detect no significant effect on an index of two measures of time preferences: savings and present bias (Table IV, column (13)). The night sleep treatments did not meaningfully affect savings behavior, leaving deposits and accumulated interest unaffected (Online Appendix Table A.XIV, Panel A). Similarly, we find no effects on present bias estimated from effort choices. We do detect present bias in the control group (β = 0.92, Online Appendix Table A.XIV, Panel B). However, increased sleep does not significantly shift this parameter, in contrast to the view that sleep replenishes self-control (Vohs and Baumeister, 2016).
We also find no evidence of altered risk aversion, loss aversion, or social preferences in standard experimental tasks (Table IV, column (14)–(15)), in contrast to the findings of McKenna et al. (2007), Dickinson and McElroy (2017), Anderson and Dickinson (2010), and Holbein, Schafer, and Dickinson (2019). Although the results are not precise enough to detect small effects, we can rule out changes greater than 0.16 std. dev. at the 95% level for each of these outcomes (Online Appendix Table A.XV).
6. Heterogeneity
We do not find significant evidence of heterogeneity in the effects of the night sleep treatments. Online Appendix Table A.XVI considers effects on the overall index variable and tests for heterogeneity by a number of baseline covariates. Baseline night sleep duration, sleep efficiency, and propensity to nap prior to the study do not interact significantly with the night sleep treatments. Nor do demographics such as gender and age.29
IV.E. Effect of Naps
1. Overview
Naps improved outcomes across a range of domains (Figure VI). Table IV, column (1) reports that naps had an economically meaningful and statistically significant effect on the overall summary index (0.12 std. dev., std. err. = 0.04, p < .01). As shown in Online Appendix Table A.IX, the effect is significant whether naps are compared to taking enforced breaks (0.15 std. dev., p < .01) or to working through the afternoon (0.08 std. dev., p = .03). Given the lack of evidence on the impacts of naps on economically meaningful outcomes in real-world settings (Lovato and Lack 2010; Ficca et al. 2010), this is an important result in itself. In addition, these results serve as a proof of concept that sleep can significantly alter many of the outcomes we study within a short time frame.
2. Work Outcomes
Naps increased productivity. Participants randomized to naps were 2.3% (0.04 std. dev., std. err. = 0.02, p = .06) more productive on average over the day (Table IV, column (3)).30 This effect is sizable, given that productivity is quite inelastic: quadrupling the piece rate increased productivity by only 14%. The productivity effects of naps are similar when compared with the break or the work counterfactuals, suggesting that the effects are due to sleep rather than merely resting (Online Appendix Table A.IX). Online Appendix Figure A.VIII shows that the effects are larger in the afternoon (2.7%) but also evident in the morning (1.9%), suggesting either cumulative effects of regular napping or that participants work harder in the morning in anticipation of the nap.31
By design, nap participants could not work during the 30-minute nap period. They could adjust their labor supply outside of this period, say, by staying at the office longer. We find no evidence of such adjustments (Table IV, column (4)): The nap group spends almost exactly as much time typing as those in the control group on their break days, and 26 minutes (0.20 std. dev.) fewer than the control group on its work days (Online Appendix Table A.IX).
The effects of naps on output and earnings depend on the comparison group (Online Appendix Table A.IX). Compared to taking a break, naps increased total output by 0.05 std. dev. (p = .02). Compared to working, naps instead reduced output by 0.07 std. dev. (p < .01). Earnings closely track output: naps increased overall earnings by Rs. 11 a day (0.05 std. dev., p = .05) compared with taking a break, a sizable increase of 4.1%. In contrast, taking time to nap lowered earnings by Rs. 23 (8.3% or 0.10 std. dev., p < .01) compared to simply working through the break.32
3. Well-Being
Naps significantly improved well-being (Table IV, columns (6)–(8)). The overall effect of 0.08 std. dev. (p = .03) is driven by a 0.12 std. dev. (p = .04) improvement in psychological well-being. The point estimates for all individual components of psychological well-being are positive, with significant effects on happiness, life satisfaction, and sense of life possibility (Online Appendix Table A.XI). The lack of significant effects on physical well-being is perhaps unsurprising, given the limited effects of naps on total sleep. Moreover, physical health benefits of sleep may require more time to emerge, and some of the outcomes, such as the cycling task, were conducted at the end of the study on a day without naps.33
4. Cognition
Naps boost the cognition index—which combines lab measures of cognition with a measure of attention at work—by 0.10 std. dev. (p = .08; Table IV column (9)). The lab measures of cognition increase by 0.08 std. dev. (p = .07; column (10)) on average, driven by an effect on attention (Online Appendix Table A.XII). We find no significant effects on inhibitory control or memory, in contrast to the sleep literature that tends to find broad effects of sleep on many aspects of cognition (Killgore 2010; Lim and Dinges 2010). We also find that naps increased participants’ attention to work incentives by 0.20 std. dev. (p = .07, Online Appendix Table IV, column (11)). The nap group was nearly fully attentive to nonsalient incentives, reacting to them about as much as they reacted to salient incentives (Online Appendix Table A.XIII). This result illustrates the improved attentional resources provided by naps in a real-world work environment.
5. Preferences
Naps have a positive but not significant effect (0.07 std. dev., p = .15) on the index corresponding to the preferences family (Table IV, column (12)). In this family, we find a positive but insignificant effect of naps on an index of patience (0.13 std. dev., p = .20, Table IV, column (13)). This index combines two real-stakes measures of time preferences: savings at the study office and present bias in an effort-discounting task. Naps caused a 14% increase in deposits and a 13% increase in daily net savings (deposits minus withdrawals, Online Appendix Table A.XIV, Panel A). These effects are sizable: randomly providing a 1 percentage point higher daily interest rate increased deposits by 31%. The nap group earned 19% more interest over the course of the study, although this effect is imprecisely estimated.34
Naps also reduce present bias in a real-effort task (Online Appendix Table A.XIV, Panel B). We estimate an average present bias parameter of β = 0.92 in the control group.35 The nap treatment significantly (unadjusted p < .05) reduces present bias to β = 0.98, and time preferences in the nap group are statistically indistinguishable from exponential discounting (i.e., β = 1).
Naps have no significant effects on social or risk preferences (Table IV, columns (14)–(15)). The nap group is not more willing to accept risk or probabilistic losses (Online Appendix Table A.XV). The same table reports that nap participants send 0.16 std. dev. more (std. err. = 0.10) in dictator games, but not in ultimatum or trust games. Nor do naps affect the choices of receivers in ultimatum or trust games. It is worth noting, however, that the measures of social and risk preferences were elicited in the morning, before daily naps, while time preferences were instead measured in the afternoon (Online Appendix Table A.III).
6. Heterogeneity
As with the night sleep treatments, we do not detect statistically significant heterogeneity in the overall effects of naps by baseline sleep or demographic characteristics (Online Appendix Table A.XVI). Notably, those who self-reported napping in the week prior to the study did not see higher effects from the nap treatment. This partly alleviates concerns that the treatment effects capture mainly the effect of reducing naps among the control participants rather than increasing naps in the treatment group.
IV.F. Nap versus Night Sleep Treatments
Table IV reports p-values for differences in the effects of the night sleep and nap treatments on each outcome. We find clear evidence that naps and night sleep treatments have significantly different effects on the overall index variable that combines all outcomes (p = .02). We are relatively underpowered to instead compare the effects on individual outcomes. The only individual outcome on which naps have a significantly different effect than the night sleep treatments is psychological well-being (p = .04). The differences in effects on the indices of well-being, cognition, and preferences are not significant, although the point estimates are larger for naps in each case. For earnings, the point estimates for naps and night sleep treatments are similar and not significantly different.
The nap treatment has larger overall effects than the night sleep treatment, despite causing smaller increases in total (24-hour) sleep. Online Appendix Figure A.IX plots the overall treatment effects for the five disaggregated treatments against the increase in 24-hour sleep generated by the treatment. The figure shows no evidence of a dose response to 24-hour sleep or to night sleep: treatments with larger effects on overall sleep do not have larger effects on the summary of downstream outcomes. In contrast, treatment combinations involving naps have distinctly larger effects than the ones without naps. Naps at the office and nighttime sleep at home are not one-for-one substitutes in our setting, in contrast to some evidence from sleep lab experiments (Mollicone, Van Dongen, and Dinges 2007).
To formally test for differences in the effects of nighttime sleep and nap sleep per minute of sleep, we turn to an instrumental variable (IV) analysis. Specifically, we estimate an IV regression with duration of nighttime sleep and nap sleep as endogenous variables and the five different combinations of treatments as instruments (Online Appendix Table A.XVII).36 As expected given the results above, each additional minute of nap sleep has a substantial effect on the overall index (0.008 std. dev. per minute, or 0.46 std. dev. per hour), significantly different from a minute of nighttime sleep (p < .01). However, the per minute benefits of naps and nighttime sleep also differ significantly for the preferences (p = .08) and cognition (p = .08) indices and suggestively for the well-being index (p = .11).37 The per minute effects are also statistically different—more positive for nap sleep—for psychological well-being (p = .05) and the lab measures of cognition (p = .08). However, naps also lead to larger reductions in labor supply per minute of sleep than nighttime sleep (p = .04). Understandably, a minute of daytime sleep crowds out more work than a minute of nighttime sleep.
V. Discussion and Conclusion
Using state-of-the-art objective measurements, we document a novel fact about sleep in India: low-income adults in Chennai are severely sleep-deprived by usual standards. The strikingly low sleep duration and efficiency in our sample could be a widespread but underappreciated feature of the lives of the urban poor in developing areas. Despite the lack of short-run benefits from sleeping more in our setting, severe sleep deprivation may have serious long-run effects on health and well-being and calls for policy makers’ and researchers’ attention. More systematic research on sleep in developing countries is needed to establish the external validity of our findings and study longer-term effects.
In our setting, substantial increases in sleep duration were achievable through more time in bed, a change that at least in principle lies within people’s choice sets. Increasing sleep efficiency, however, appears to be more difficult. Providing people with tips regarding good sleep hygiene and devices to make their sleep environment more comfortable did not increase efficiency; nor did incentives to achieve more actual sleep (which substantially reward higher sleep efficiency). As a result, increasing sleep duration entailed significant opportunity costs for our study sample.
We find no positive effects of increased night sleep on any of our outcomes, contrasting with predictions made by sleep scientists and economists and evidence from many sleep lab experiments (e.g., Van Dongen et al. 2003; Lim and Dinges 2010; Killgore 2010), and a much smaller body of recent work in economics that uses natural experiments (such as variation in sunset time) to study the effects of sleep (Gibson and Shrader 2018; Giuntella and Mazzonna 2019; Jagnani 2018). It is more consistent with some recent evidence from the field: Avery, Giuntella, and Jiao (2019) find only small gains in academic achievement from inducing college students to increase nighttime sleep.
What explains this unexpected finding? One plausible explanation is the much lower sleep quality—as proxied by sleep efficiency—in our setting compared with those previously studied in rich countries. The low-quality sleep we discovered in Chennai may not offer the same marginal benefits as the sleep typically available in higher-income settings. A more provocative possibility is that the findings from lab studies may not generalize to the field, even in rich countries. The lab experiments used in sleep science induce severe (often total) sleep deprivation (e.g., Lim and Dinges 2010) and typically lack steep incentives to perform well on tasks. We instead study the impacts of a more modest and arguably policy-relevant increase in sleep on highly incentivized outcomes. As one of the first studies of the causal effects of sleep in low-income settings, our experiment is not designed to adjudicate these different possible explanations. It does, however, point to the value of an economic perspective on sleep, which considers sleep as a choice variable, and measures both the benefits and the opportunity costs of sleeping more.
Our results do not imply that more dramatic changes in sleep environments (e.g., improved housing, noise regulations) or in psychological factors hindering sleep (such as stress) could not have large effects. Improving sleep quality could potentially generate more sleep (due to higher sleep efficiency) and greater benefits from each minute of sleep. Identifying interventions to improve sleep efficiency in contexts like ours, and testing whether increased sleep efficiency unlocks the benefits found in sleep research in rich countries, would also be valuable. It could also be that the benefits of increased night sleep manifest over longer time horizons. Consistent with the hypothesis that increased sleep can have meaningful effects, we find the nap treatment has a significant positive effect on an overall index of outcomes, with positive effects on productivity, well-being, and cognition.
The positive effect of naps is an important finding in itself. Naps are a common feature of life around the world, and even more so in tropical settings, where afternoon work may be less productive (Dinges 1992). Yet we know very little about the economic costs and benefits of naps in real-world settings, as concluded by Ficca et al. (2010) in a recent review. As work in developing countries shifts from self-employment to working in firms with set hours, naps may be crowded out. Our findings suggest that this could be costly, because naps provide workers with benefits that night sleep does not. Large firms may be beginning to recognize this, as evidenced by the adoption of nap policies by employers such as Google and Nike (Walker 2017, chapter 15). Given the forgone work time and logistical costs of offering naps, an employer’s decision to provide naps may depend on how much they value their workers’ psychological well-being and attention in the particular work setting.
An obvious question raised by our results is why naps were effective when a larger increase in night sleep had no effect. Naps and night sleep are clearly not substitutes in our setting (Online Appendix Figure A.IX and Table A.XVII). Naps occurred in a pleasant office environment and may therefore have been higher quality than night sleep. Alternatively, naps may simply enter the production function of our outcomes differently than marginal increases in night sleep due to their timing. Naps were timed to coincide with the circadian dip in the mid-afternoon, when individuals are prone to sleepiness and impaired performance. A short burst of sleep during the circadian dip has been shown to be particularly valuable (Takahashi 2003; Lovato and Lack 2010).
Perhaps the broadest question our research raises is to what extent the sizable impacts of sleep in lab studies generalize to field settings. Measuring sleep in the field—made possible by wearables—allows such experiments linking sleep to real-world economic outcomes in a wider range of environments (Handel and Kolstad 2017). We evaluated labor market effects of sleep in the particular context of data entry work. Does sleep matter differently in less cognitively demanding or more physical jobs? In contexts where work is independent or collaborative? For children, as in Jagnani (2018)? Are naps in usual environments equally effective? Systematic measurement and tests across a wider variety of environments would facilitate the estimation of the overall impacts of sleep on the economy.
Supplementary Material
Footnotes
We thank the editors, four anonymous referees, and numerous seminar audiences, colleagues, and interested readers for helpful comments and generous feedback. We are particularly grateful to Isaiah Andrews for patiently answering numerous questions regarding multiple-inference corrections. We thank Alosias A., Vivian Aluoch, Srinivas Balasubramanian, Dillon Bowen, Phoebe Cai, Stephanie Chan-Ahuja, Fiona Chen, Thomas Escande, Juliette Finetti, Isadora Frankenthal, Gabriel Jardanovski, Kannan Kumar, Gunjita Gupta, Erik Hausen, Yihong Huang, Dexin Li, Andrew Locke, Madeline McKelway, Adrien Pawlik, João Pugliese, Jane von Rabeau, Sangeetha Ramanathan, Cory Rand, Maya Roy, Krishna Prasad Srinivasan, Nikkil Sudharsanan, Sifan Xue, Ziqing Yan, and Yanzun Yang for excellent research assistance. We thank Jack Cavanagh for exceptional work reviewing project code and preparing data for publication. We thank all our study participants and staff, all those who took the time to participate in our expert survey, and sleep medicine experts Dr. Dinges, Dr. Basner, Dr. Redline, and Dr. Perlis for their help and guidance on this study. We gratefully acknowledge generous funding and support by the government of Tamil Nadu, the Abdul Latif Jameel Poverty Action Lab, IFMR LEAD, the William F. Milton Fund, the Harvard Global Health Institute, the Program on the Global Development of Aging, the SHASS Research Fund, the Mind, Brain, and Behavior Interfaculty Initiative, the Institute for Translational Medicine and Therapeutics (ITMAT), the Weiss Family Program Fund for Research in Development Economics, and the Pershing Square Venture Fund for Research on the Foundations of Human Behavior. Time devoted to the research reported in this publication was also supported by the National Institute on Aging of the National Institutes of Health under award no. K01AG055691, as well as the Program for Training in Sleep, Circadian and Respiratory Neurobiology in the Division of Sleep Medicine at Harvard Medical School and Brigham and Women’s Hospital, which is supported by a Ruth L. Kirschstein NRSA (T-32 grant) from the National Heart, Lung, and Blood Institute within the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We received IRB approval from Harvard University, protocol no. 14-2294, the Institute for Financial Management and Research in India, and the Indian Council of Medical Research. The experiment was preregistered with a pre-analysis plan on the AEA registry, no. AEARCTR-0002494.
Notable exceptions include Avery, Giuntella, and Jiao (2019), who evaluate commitment contracts to increase sleep among college students in the United States and United Kingdom, and Barnes, Miller, and Bostock (2017), who study the effects of cognitive behavioral therapy for insomnia on job satisfaction and related outcomes.
Altogether, our design results in a control group and five treatment groups (encouragement only, incentives only, naps only, encouragement and naps, incentives and naps). In addition, all those who did not receive naps were randomized each day to either work through the nap period or to take a mandatory break, allowing us to compare naps against work days and break days separately.
The overall effect of naps only differs significantly from that of incentives only (p = .02) and suggestively from that of encouragement only (p = .11). Naps combined with the encouragement treatment had an effect of 0.13 std. dev. (std. err. = 0.07), while naps combined with incentives had an effect of 0.09 std. dev. (std. err. = 0.06), neither of which is significantly different from naps only.
Our findings also contrast with Jagnani (2018), who exploits variation in sunset times in India to show that less time in bed is associated with worse educational outcomes for children. It could be that children are further away from optimal sleep levels, or that sleep quantity may matter more for children and for learning outcomes.
This guideline refers to actual time asleep, not merely time in bed. However, sleep and time in bed are often similar in healthy rich-country populations with high sleep efficiency (Cespedes et al. 2016; Jackson et al. 2018).
Section III provides details on the sample and study design. Participants received a modest daily incentive of Rs. 10 to wear the actigraph, which they forfeited if they removed it. To determine whether the participants wore the devices continuously, a small breakable strap was put through the watch band and checked daily. Compliance rates were high across all groups, with approximately 6% of participants removing the device on any given day.
Cespedes et al. (2016) reports an average of 7.8 hours in bed. Among older participants, Jackson et al. (2018) and Kurina et al. (2015) find 7.2 and 8.4 hours in bed, respectively.
An awakening is defined as a disruption lasting at least 30 seconds. Considering longer awakenings that last for at least five minutes each, we still find an average of 10 such awakenings a night, compared with expert guidelines of 4 or fewer per night (Ohayon et al. 2017).
Despite the overestimation on average, self-reports are moderately correlated with actigraph measures at the individual level (r = 0.48). However, given that self-reported levels of sleep exceed actigraph measures, self-reports also overestimate sleep efficiency relative to actigraph measures (Figure II, Panel F).
For more detail of the survey and population, see Online Appendix F.
Participants were permitted to take more than one of each device, as piloting had suggested that the devices were often shared with family members. They were asked to return—and penalized for not returning—the devices at the end of the study; virtually all complied.
One concern is that participants could game the incentives by strategically reducing their baseline sleep. This is unlikely because participants did not know their treatment status during the baseline period. Also consistent with a lack of gaming, control group participants did not increase their sleep after treatment assignment and, as described in Section II.C, baseline sleep is very similar to levels seen in the broader sleep survey.
The use of placebo item offers to the control group was not randomized, and instead began about halfway through the experiment, after which all control group participants were offered these items. We find no detectable difference in treatment effects based on whether the control group had been offered these placebo goods, and thus we pool all control participants in the analysis.
The data to be digitized were actually artificially generated. By generating the data, we had ready access to the correct answers, allowing us to measure the accuracy of the work easily. We were also able to vary the complexity of the data to be entered across fields. Study participants were unaware of the artificial nature of the data, and we believe they had no reason to not take their work seriously.
Control group participants earned Rs. 283 ( 3.80) a day on average through their typing work (not including additional payments for surveys, experimental tasks, and sleep incentives). For context, GDP per capita in Chennai is approximately
3.80) a day on average through their typing work (not including additional payments for surveys, experimental tasks, and sleep incentives). For context, GDP per capita in Chennai is approximately  9 a day. The piece rate accounted for 57% of typing earnings, while the remaining 43% was compensation for time spent typing.
9 a day. The piece rate accounted for 57% of typing earnings, while the remaining 43% was compensation for time spent typing.
Well-being-related outcomes were preregistered at Clinicaltrials.gov, identifier: NCT03322358.
Formally, it is given by 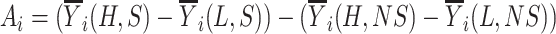 , where
, where 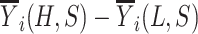 is the average difference of output under high and low piece rates of participant i when incentives are salient, and
is the average difference of output under high and low piece rates of participant i when incentives are salient, and 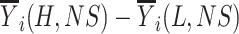 is the same for nonsalient incentives. We residualize output with respect to participant, day in study, and date fixed effects.
is the same for nonsalient incentives. We residualize output with respect to participant, day in study, and date fixed effects.
The interest rates changed twice during the study. Details are provided in Online Appendix D.
The expert surveys were conducted after over half the RCT sample had been acquired, to provide respondents with information on the achieved gains in sleep. However, the paper had not been publicly presented or circulated with results at that time.
Note that we have an imbalance in earnings between the nap and no-nap groups in spite of stratifying on a dummy variable indicating whether the participant’s baseline earnings was above the median. For this dummy variable, we have almost perfect balance, as expected. However, we have a few outliers with very large baseline earnings who all happened to be assigned to the no-nap group. Results are robust to a variety of methods to address this imbalance, including baseline controls for this variable.
For some outcomes, Xit includes additional outcome-specific controls. For work-related outcomes, we control for the fraction of the day worked at high piece rate (which was randomized each day) and the length of the work day (i.e., long or short day, which was also randomized). Table notes specify these additional controls.
This requires taking a normative stance on each variable. Some classifications are relatively uncontroversial: higher productivity and earnings, lower blood pressure and self-reported illness, higher cognitive function and more happiness are all classified as better. We also take the (more arguable) stances that greater patience (lower present bias), higher savings, higher labor supply, and more prosocial behavior in lab experiments all constitute improvements.
More details on our multiple-comparisons corrections procedure are in Online Appendix E. For the main estimates in Table IV, we also provide a range of different corrections, including false-discovery rate corrections in Online Appendix Table A.XVIII.
On average, night sleep treatment participants went to bed 17 minutes earlier at night and got out of bed 25 minutes later in the morning (Online Appendix Table A.X).
To make these measures as comparable as possible, we calculate time in bed for naps (both in the office and at home) as beginning with the minute the participant is first detected to fall asleep. To obtain a comparable number for night sleep, we examine sleep efficiency during the first 15 minutes of night sleep, which is the approximate length of the office naps.
Online Appendix Table A.VI reports estimates from the model that pools the two night sleep treatments and does not separate the cell that receives both a night sleep treatment and the nap treatment. These estimates are more precise, and here the effect of naps on 24-hour sleep is significant. Online Appendix Table A.VI also reports Lee bounds to address imperfect compliance with wearing actigraphs. The conclusions are largely unchanged, consistent with the fact that that noncompliance was relatively low (6%) and balanced across treatment groups.
Online Appendix Table A.VII provides multiple-hypothesis adjusted p-values for the individual outcomes in this specification. The overall index variable aggregates all outcomes and thus does not require multiple-hypothesis testing corrections.
We also do not find any evidence that the effects of the night sleep treatments become more positive as the length of treatment increases (Online Appendix Figure A.VIa).
Note that this does not provide strong evidence that sleep efficiency and baseline sleep duration are irrelevant for the marginal benefits of increased sleep. We have limited statistical power for heterogeneity analysis, and very few study participants have levels of sleep duration or sleep efficiency typically observed in high-income countries.
Online Appendix Table A.VIII reveals that the estimated positive effect of naps on productivity in Table IV is driven by those individuals who also receive the night sleep treatments. Naps alone, instead, have no effect on productivity.
The unadjusted p-values of effects in the afternoon and morning are .01 and .05, respectively. Also evident is a brief dip in productivity in the half hour immediately following the nap. This is consistent with the well-documented phenomenon of temporary sleep inertia after a nap (Lovato and Lack 2010).
The negative effect of naps on earnings (compared to work days) appears to diminish over time. On “long” days, when participants are not restricted to artificially short work hours, the earnings of the nap group converge with the earnings of participants on work days by the end of the study (see Online Appendix Figure A.VI).
Recall, however, that naps only had a positive effect on physical well-being (0.16 std. dev., p = .02) when we allow the night sleep and nap cell to have a separate effect, as reported in Online Appendix Table A.VIII.
We preregistered daily net savings as our main variable of interest. However, this measure suffers from an unanticipated design issue: participants make large one-time withdrawals right before the study ends, which mechanically drives down net savings. We believe deposits more accurately reflect differences in savings behavior, and the accrued interest captures the benefit of savings.
The estimated β is predictive of other behaviors conceptually related to time preference. For example, participants with a lower estimated β arrive at work later and save less (Online Appendix Table A.XIX).
This analysis makes strong assumptions about the linearity of effects (per minute of sleep) and involves exclusion restrictions. Although some of these assumptions might be questionable, we believe Online Appendix Table A.XVII provides valuable information on the differences in effects of the two types of sleep.
The permutation-based Westfall-Young procedure we use to control the family-wise error rate in our other tables cannot be applied to the IV setting, since the permuted first stage leads to a weak-instruments problem. Therefore, we report adjusted p-values calculated using Hochberg’s step-up procedure for controlling the FWER for this table. For the main intent-to-treat results in Table IV, the Hochberg and Westfall-Young adusted p-values are essentially identical, as reported in Online Appendix Table A.XVIII.
Contributor Information
Pedro Bessone, Masschusetts Institute of Technology, United States.
Gautam Rao, Harvard University and National Bureau of Economic Research, United States.
Frank Schilbach, Massaschusetts Institute of Technology and National Bureau of Economic Research, United States.
Heather Schofield, University of Pennsylvania, United States.
Mattie Toma, Harvard University, United States.
Data Availability
Data and code replicating the tables and figures in this article can be found in Bessone et al. (2021) in the Harvard Dataverse https://doi.org/10.7910/DVN/GJ9QPC.
References
- Anderson Clare, Dickinson David L., “Bargaining and Trust: The Effects of 36-h Total Sleep Deprivation on Socially Interactive Decisions,” Journal of Sleep Research, 19 (2010), 54–63. [DOI] [PubMed] [Google Scholar]
- Anderson Michael L., “Multiple Inference and Gender Differences in the Effects of Early Intervention: A Reevaluation of the Abecedarian, Perry Preschool, and Early Training Projects,” Journal of the American Statistical Association, 103 (2008), 1481–1495. [Google Scholar]
- Augenblick Ned, Rabin Matthew, “An Experiment on Time Preference and Misprediction in Unpleasant Tasks,” Review of Economic Studies, 86 (2019), 941–975. [Google Scholar]
- Avery Mallory, Giuntella Osea, Jiao Peiran, “Why Don’t We Sleep Enough? A Field Experiment among College Students,” IZA Discussion Paper no. 12772, 2019. [Google Scholar]
- Banks Siobhan, Dinges David, “Behavioral and Physiological Consequences of Sleep Restriction,” Journal of Clinical Sleep Medicine, 3 (2007), 519–528. [PMC free article] [PubMed] [Google Scholar]
- Barnes Christopher M., Miller Jared A., Bostock Sophie, “Helping Employees Sleep Well: Effects of Cognitive Behavioral Therapy for Insomnia on Work Outcomes,” Journal of Applied Psychology, 102 (2017), 104. [DOI] [PubMed] [Google Scholar]
- Barnes Christopher M., Schaubroeck John, Huth Megan, Ghumman Sonia, “Lack of Sleep and Unethical Conduct,” Organizational Behavior and Human Decision Processes, 115 (2011), 169–180. [Google Scholar]
- Basner Mathias, Dinges David F., “Maximizing Sensitivity of the Psychomotor Vigilance Test (PVT) to Sleep Loss,” Sleep, 34 (2011), 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessone Pedro, Rao Gautam, Schilbach Frank, Schofield Heather, Toma Mattie, “Replication Data for: ‘The Economic Consequences of Increasing Sleep among the Urban Poor’,” (2021), Harvard Dataverse, 10.7910/DVN/GJ9QPC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer Colin F., Behavioral Game Theory: Experiments in Strategic Interaction (Princeton, NJ: Russell Sage Foundation/Princeton University Press, 2003). [Google Scholar]
- Cappuccio Francesco P., Taggart Frances M., Kandala Ngianga-Bakwin, Currie Andrew, Peile Ed, Stranges Saverio, Miller Michelle A., “Meta-Analysis of Short Sleep Duration and Obesity in Children and Adults,” Sleep, 31 (2008), 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier Julie, Land Stephanie, Buysse Daniel J., Kupfer David J., Monk Timothy H., “The Effects of Age and Gender on Sleep EEG Power Spectral Density in the Middle Years of Life (Ages 20–60 Years Old),” Psychophysiology, 38 (2001), 232–242. [PubMed] [Google Scholar]
- Castro L., Poyares D., Leger D., Bittencourt L., Tufik S., “Objective Prevalence of Insomnia in the Sao Paulo, Brazil Epidemiologic Sleep Study,” Annals of Neurology, 74 (2013), 537–546. [DOI] [PubMed] [Google Scholar]
- Cespedes Elizabeth M., Hu Frank B., Redline Susan, Rosner Bernard, Alcantara Carmela, Cai Jianwen, Hall Martica H, Loredo Jose S., Mossavar-Rahmani Yasmin, Ramos Alberto R.et al. , “Comparison of Self-Reported Sleep Duration with Actigraphy: Results from the Hispanic Community Health Study/Study of Latinos Sueño Ancillary Study,” American Journal of Epidemiology, 183 (2016), 561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charness Gary, Gneezy Uri, Imas Alex, “Experimental Methods: Eliciting Risk Preferences,” Journal of Economic Behavior & Organization, 87 (2013), 43–51. [Google Scholar]
- Chetty Raj, Looney Adam, Kroft Kory, “Salience and Taxation: Theory and Evidence,” American Economic Review, 99 (2009), 1145–1177. [Google Scholar]
- Cole Roger J., Kripke Daniel F., Gruen William, Mullaney Daniel J., Christian Gillin J., “Automatic Sleep/Wake Identification from Wrist Activity,” Sleep, 15 (1992), 461–469. [DOI] [PubMed] [Google Scholar]
- Dean Emma, Schilbach Frank, Schofield Heather, “Poverty and Cognitive Function,” in The Economics of Poverty Traps. Barrett Christopher B., Carter Michael R., Chavas Jean-Paul, eds. (Chicago: NBER/University of Chicago Press, 2019), 57–118. [Google Scholar]
- DellaVigna Stefano, Pope Devin, Vivalt Eva, “Predict Science to Improve Science,” Science, 366 (2019), 428–429. [DOI] [PubMed] [Google Scholar]
- Dickinson David L., McElroy Todd, “Sleep Restriction and Circadian Effects on Social Decisions,” European Economic Review, 97 (2017), 57–71. [Google Scholar]
- Dinges David F., “Adult Napping and Its Effects on Ability to Function,” Why We Nap in Stampi Claudio, ed. (Boston: Birkhäuser, 1992, 118–134. [Google Scholar]
- Ficca Gianluca, Axelsson John, Mollicone Daniel, Muto Vincezo, Vitiello Michael, “Naps, Cognition, and Performance,” Sleep Medicine Reviews, 14 (2010), 240–258. [DOI] [PubMed] [Google Scholar]
- Gershon Anda, Thompson Wesley K., Eidelman Polina, McGlinchey Eleanor L., Kaplan Katherine A., Harvey Allison G., “Restless Pillow, Ruffled Mind: Sleep and Affect Coupling in Interepisode Bipolar Disorder,” Journal of Abnormal Psychology, 121 (2012), 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Matthew, Shrader Jeffrey, “Time Use and Labor Productivity: The Returns to Sleep,” Review of Economics and Statistics, 100 (2018), 783–798. [Google Scholar]
- Gildner Theresa, Liebert Melissa, Kowal Paul, Chatterji Somnath, Snodgrass Josh, “Associations between Sleep Duration, Sleep Quality, and Cognitive Test Performance among Older Adults from Six Middle Income Countries: Results from the Study on Global Ageing and Adult Heath (SAGE),” Sleep, 10 (2014), 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuntella Osea, Mazzonna Fabrizio, “Sunset Time and the Economic Effects of Social Jetlag: Evidence from US Time Zone Borders,” Journal of Health Economics, 65 (2019), 210–226. [DOI] [PubMed] [Google Scholar]
- Hamilton Nancy A., Nelson C. A., Stevens N., Kitzman Heather, “Sleep and Psychological Well-Being,” Social Indicators Research, 82 (2007), 147–163. [Google Scholar]
- Handel Benjamin, Kolstad Jonathan, “Wearable Technologies and Health Behaviors: New Data and New Methods to Understand Population Health,” American Economic Review, 107 (2017), 481–485. [DOI] [PubMed] [Google Scholar]
- Hedner Jan, Pillar Giora, Pittman Stephen D., Zou Ding, Grote Ludger, White David P., “A Novel Adaptive Wrist Actigraphy Algorithm for Sleep-Wake Assessment in Sleep Apnea Patients,” Sleep, 27 (2004), 1560–1566. [DOI] [PubMed] [Google Scholar]
- Hirshkowitz Max, Whiton Kaitlyn, Albert Steven M., Alessi Cathy, Bruni Oliviero, DonCarlos Lydia, Hazen Nancy, Herman Johnet al. , “National Sleep Foundation’s Sleep Time Duration Recommendations: Methodology and Results Summary,” Sleep Health, 1 (2015), 40–43. [DOI] [PubMed] [Google Scholar]
- Holbein John B., Schafer Jerome P., Dickinson David L., “Insufficient Sleep Reduces Voting and Other Prosocial Behaviours,” Nature Human Behaviour, 3 (2019), 492. [DOI] [PubMed] [Google Scholar]
- Holt Charles A., Laury Susan K., “Risk Aversion and Incentive Effects,” American Economic Review, 92 (2002), 1644–1655. [Google Scholar]
- Jackson Chandra L., Patel Sanjay R., Braxton Jackson II W., Lutsey Pamela L., Redline Susan, “Agreement between Self-Reported and Objectively Measured Sleep Duration among White, Black, Hispanic, and Chinese Adults in the United States: Multi-Ethnic Study of Atherosclerosis,” Sleep, 41 (2018), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagnani Maulik, “Poor Sleep: Sunset Time and Human Capital Production,” Mimeo, 2018. [Google Scholar]
- Jin Lawrence, Ziebarth Nicolas R., “Sleep, Health, and Human Capital: Evidence from Daylight Saving Time,” Journal of Economic Behavior & Organization, 170 (2020), 174–192. [Google Scholar]
- Kahneman Daniel, Krueger Alan, “Development in the Measurement of Subjective Well-Being,” Journal of Economic Perspectives, 20 (2006), 3–24. [Google Scholar]
- Kaur Supreet, Kremer Michael, Mullainathan Sendhil, “Self-Control at Work,” Journal of Political Economy, 123 (2015), 1227–1277. [Google Scholar]
- Killgore William, “Effects of Sleep Deprivation on Cognition,” Progress in Brain Research, 185 (2010), 105–129. [DOI] [PubMed] [Google Scholar]
- Knutson Kristen L., “Sleep Duration, Quality, and Timing and Their Associations with Age in A Community without Electricity in Haiti,” American Journal of Human Biology, 26 (2014), 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer Michael, Rao Gautam, Schilbach Frank, “Behavioral Development Economics,” in Handbook of Behavioral Economics: Foundation and Applications, vol. 2, B. Douglas Bernheim, Stefano Dellaigna, and David Laibson, eds. (Amsterdam: Elsevier, 2019), 345–458. [Google Scholar]
- Kurina Lianne M., Thisted Ronald A., Chen Jen-Hao, McClintock Martha K., Waite Linda J., Lauderdale Diane S., “Actigraphic Sleep Characteristics among Older Americans,” Sleep Health, 1 (2015), 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushida Clete A., Chang Arthur, Gadkary Chirag, Guilleminault Christian, Carrillo Oscar, Dement William C., “Comparison of Actigraphic, Polysomnographic, and Subjective Assessment of Sleep Parameters in Sleep-Disordered Patients,” Sleep Medicine, 2 (2001), 389–396. [DOI] [PubMed] [Google Scholar]
- Lauderdale Diane S., Knutson Kristen L., Yan Lijing L., Liu Kiang, Rathouz Paul J., “Sleep Duration: How Well Do Self-Reports Reflect Objective Measures? The CARDIA Sleep Study,” Epidemiology, 19 (2008), 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichstein K., Stone K., Donaldson J., Nau S., Soeffing J., Murray D., Lester K., Aguillard N., “Actigraphy Validation with Insomnia,” Sleep, 2 (2006), 232–239. [PubMed] [Google Scholar]
- Lim Julian, Dinges David F., “A Meta-Analysis of the Impact of Short-Term Sleep Deprivation on Cognitive Variables,” Psychological Bulletin, 136 (2010), 375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovato Nicole, Lack Leon, “The Effects of Napping on Cognitive Functioning,” Progress in Brain Research, 185 (2010), 155–166. [DOI] [PubMed] [Google Scholar]
- Marino Miguel, Li Yi, Rueschman Michael N., Winkelman J. W., Ellenbogen J. M., Solet J., Dulin Hilary, Berkman Lisa F., Buxton Orfeu M., “Measuring Sleep: Accuracy, Sensitivity, and Specificity of Wrist Actigraphy Compared to Polysomnography,” Sleep, 36 (2013), 1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna Benjamin S., Dickinson David L., Orff Henry J., Drummond Sean P. A., “The Effects of One Night of Sleep Deprivation on Known-Risk and Ambiguous-Risk Decisions,” Journal of Sleep Research, 16 (2007), 245–252. [DOI] [PubMed] [Google Scholar]
- McKenzie David, “Beyond Baseline and Follow-up: The Case for More T in Experiments,” Journal of Development Economics, 99 (2012), 210–221. [Google Scholar]
- Mollicone D., Van Dongen H., Dinges D., “Optimizing Sleep/Wake Schedules in Space: Sleep during Chronic Nocturnal Sleep Restriction with and without Diurnal Naps,” Acta Astronautica, 60 (2007), 354–361. [Google Scholar]
- Mollicone D., Van Dongen H., Rogers N., Dinges D., “Response Surface Mapping of Neurobehavioral Performance: Testing the Feasibility of Split-Sleep Schedules for Space Operations,” Acta Astronautica, 63 (2008), 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan Karthik, Romero Mauricio, Wüthrich Kaspar, “Factorial Designs, Model Selection, and (Incorrect) Inference in Randomized Experiments,” NBER Working Paper no. w26562, 2019. [Google Scholar]
- Nicholson A., Pascoe P., Roehrs T., Roth T., Spencer M., Stone M., “Sustained Performance with Short Evening and Morning Sleeps,” Aviation, Space, and Environmental Medicine, 56 (1985), 105–114. [PubMed] [Google Scholar]
- Ohayon Maurice, Wickwire Emerson M., Hirshkowitz Max, Albert Steven M., Avidan Alon, Daly Frank J., Dauvilliers Yves, Ferri Raffaele, Fung Constance, Gozal Davidet al. , “National Sleep Foundation’s Sleep Quality Recommendations: First Report,” Sleep Health, 3 (2017), 6–19. [DOI] [PubMed] [Google Scholar]
- Pilcher J., Michalowski K., Carrigan R., “The Prevalence of Daytime Napping and its Relationship to Nighttime Sleep,” Behavioral Medicine, 27 (2001), 71–76. [DOI] [PubMed] [Google Scholar]
- Riemann Dieter, Perlis Michael L., “The Treatments of Chronic Insomnia: A Review of Benzodiazepine Receptor Agonists and Psychological and Behavioral Therapies,” Sleep Medicine Reviews, 13 (2009), 205–214. [DOI] [PubMed] [Google Scholar]
- Roure Nuria, Gomez Silvia, Mediano Olga, Duran Joaquin, de la Peña Monica, Capote Francisco, Teran Joaquin, Masa Juan Fernando, Alonso Maria Luz, Corral Jaimeet al. , “Daytime Sleepiness and Polysomnography in Obstructive Sleep Apnea Patients,” Sleep Medicine, 9 (2008), 727–731. [DOI] [PubMed] [Google Scholar]
- Sadeh Avi, “The Role of Actigraphy in Sleep Medicine: An Update,” Sleep Medicine Review, 15 (2011), 259–267. [DOI] [PubMed] [Google Scholar]
- Sadeh A., Hauri P., Kripke D., Lavie P., “The Role of Actigraphy in the Evaluation of Sleep Disorders,” Sleep, 18 (1995), 288–302. [DOI] [PubMed] [Google Scholar]
- Schilbach Frank, “Alcohol and Self-Control: A Field Experiment in India,” American Economic Review, 109 (2019), 1290–1322. [PubMed] [Google Scholar]
- Schokman Aaron, Bin Yu Sun, Simonelli Guido, Pye Jonathon, Morris Richard, Sumathipala Athula, Siribaddana Sisira H., Hotopf Matthew, Rijsdijk Fruhling, Jayaweera Kaushalyaet al. , “Agreement between Subjective and Objective Measures of Sleep Duration in a Low-Middle Income Country Setting,” Sleep Health, 4 (2018), 543–550. [DOI] [PubMed] [Google Scholar]
- Schultz Theodore William, Transforming Traditional Agriculture (Chicago: University of Chicago Press, 1964). [DOI] [PubMed] [Google Scholar]
- Selvamani Y., Arokiasamy Perianayagam, Chaudhary Mamta, Himanshu, “Association of Sleep Problems and Sleep Duration with Self-Rated Health and Grip Strength among Older Adults in India and China: Results from the Study on Global Aging and Adult Health (SAGE),” Journal of Public Health, 26 (2018), 697–707. [Google Scholar]
- Simonelli Guido, Marshall Nathaniel S., Grillakis Antigone, Miller Christopher B., Hoyos Camilla M., Glozier Nick, “Sleep Health Epidemiology in Low and Middle-Income Countries: A Systematic Review and Meta-Analysis of the Prevalence of Poor Sleep Quality and Sleep Duration,” Sleep Health, 4 (2018), 239–250. [DOI] [PubMed] [Google Scholar]
- Smith Austin C., “Spring Forward at Your Own Risk: Daylight Saving Time and Fatal Vehicle Crashes”, American Economic Journal: Applied Economics, 8 (2016), 65–91. [Google Scholar]
- Smith Michael T., McCrae Christina S., Cheung Joseph, Martin Jennifer L., Harrod Christopher G., Heald Jonathan L, Carden Kelly A., “Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Clinical Practice Guideline,” Journal of Clinical Sleep Medicine, 14 (2018), 1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranges Saverio, Tigbe William, Gomze-Olive Francesc, Thorogood Margaret, Kandala Ngianga-Bakwain, “Sleep Problems: An Emerging Global Epidemic? Findings from the INDEPTH WHO-SAGE Study among More than 40,000 Older Adults from 8 Countries across Africa and Asia,” Sleep, 35 (2012), 1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strine T., Chapman D., “Associations of Frequent Sleep Insufficiency with Health-Related Quality of Life and Health Behaviors,” Sleep Medicine, 6 (2005), 23–27. [DOI] [PubMed] [Google Scholar]
- Takahashi M, “The Role of Prescribed Napping in Sleep Medicine,” Sleep Medicine Reviews, 7 (2003), 227–235. [DOI] [PubMed] [Google Scholar]
- Trauer James M., Qian Mary Y., Doyle Joseph S., Rajaratnam Shantha M. W., Cunnington David, “Cognitive Behavioral Therapy for Chronic Insomnia: A Systematic Review and Meta-Analysis,” Annals of Internal Medicine, 163 (2015), 191–204. [DOI] [PubMed] [Google Scholar]
- Van Dongen Hans P. A., Maislin Greg, Mullington Janet M., Dinges David F., “The Cumulative Cost of Additional Wakefulness: Dose-Response Effects on Neurobehavioral Functions and Sleep Physiology from Chronic Sleep Restriction and Total Sleep Deprivation,” Sleep, 26 (2003), 117–126. [DOI] [PubMed] [Google Scholar]
- Vohs Kathleen D., Baumeister Roy F., Handbook of Self-Regulation: Research, Theory, and Applications (New York: Guilford Press, 2016). [Google Scholar]
- Wagner David T., Barnes Christopher M., Lim Vivien K. G., Lance Ferris D., “Lost Sleep and Cyberloafing: Evidence from the Laboratory and a Daylight Saving Time Quasi-Experiment,” Journal of Applied Psychology, 97 (2012), 1068. [DOI] [PubMed] [Google Scholar]
- Walker Matthew P., Why We Sleep: Unlocking the Power of Sleep and Dreams (New York: Scribner, 2017). [Google Scholar]
- Watson Nathaniel F., Badr Safwan M., Belenky Gregory, Bliwise Donald L., Buxton Orfeu M., Buysse Daniel, Dinges David F., Gangwisch Jameset al. , “Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion,” Sleep, 38 (2015), 1161–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall Peter H., Young S. Stanley, Resampling-Based Multiple Testing: Examples and Methods for p-Value Adjustment (New York: John Wiley & Sons, 1993). [Google Scholar]
- Zhang Jihui, Paksarian Diana, Lamers Femke, Hickie Ian B., He Jianping, Ries Merikangas Kathleen, “Sleep Patterns and Mental Health Correlates in US Adolescents,” Journal of Pediatrics, 182 (2017), 137–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code replicating the tables and figures in this article can be found in Bessone et al. (2021) in the Harvard Dataverse https://doi.org/10.7910/DVN/GJ9QPC.