Abstract
The SARS‐CoV‐2 virus, responsible for COVID‐19, spread rapidly worldwide and became a pandemic in 2020. In some patients, the virus remains in the respiratory tract, causing pneumonia, respiratory failure, acute respiratory distress syndrome (ARDS), and sepsis, leading to death. Natural flavonoids (aglycone and glycosides) possess broad biological activities encompassing antiinflammatory, antiviral, antitumoral, antiallergic, antiplatelet, and antioxidant effects. While many studies have focused on the effects of natural flavonoids in experimental models, reports based on clinical trials are still insufficient. In this review, we highlight the effects of flavonoids in controlling pulmonary diseases, particularly the acute respiratory distress syndrome, a consequence of COVID‐19, and their potential use in coronavirus‐related diseases. Furthermore, we also focus on establishing a relationship between biological potential and chemical aspects of related flavonoids and discuss several possible mechanisms of action, pointing out some possible effects on COVID‐19.
Keywords: ACE2, acute lung injury, COVID‐19, flavonoids, SARS‐CoV‐2
1. INTRODUCTION
SARS‐CoV‐2 (Severe acute respiratory syndrome‐related coronavirus type 2) infection is the cause of COVID‐19 (coronavirus infectious disease), which spread rapidly worldwide, leading to widespread social fear, economic chaos, and many deaths. The World Health Organization (WHO) declared this disease a pandemic on March 30, 2020, since COVID‐19 reached more than 110 countries. The disease is considered a highly transmissible zoonosis in humans (Guo et al., 2020; Rothan & Byrareddy, 2020). It has an incubation period of 2 to 14 days, and after this period, the patient may remain asymptomatic or develop mild symptoms such as cough, fever, runny nose, and myalgia. In some patients, if the virus remains in the respiratory tract, it can induce pneumonia, respiratory failure, acute respiratory distress syndrome (ARDS), and sepsis (Li et al., 2020; Zhou et al., 2020). The disease has a broad spectrum of symptoms and severity and can lead to death, especially if the patient has comorbidities (Singh, Gupta, & Misra, 2020; South, Diz, & Chappell, 2020). Current studies associate COVID‐19 severity to the “cytokine storm” caused by the virus (Day, Fox, Halsey, Carpenter, & Kottaridis, 2020; Ye, Wang, & Mao, 2020). Once the virus evades the innate respiratory tract defense system, it reaches the lungs and induces an intense inflammatory response. Other studies suggest that the virus triggers a systemic disease by promoting hemophagocytosis through iron dissociation in the form of porphyria, justifying refractory hypoxemia (Wenzhong & Hualan, 2020). The scientific community eagerly seeks to develop a vaccine for prevention or drugs that treat and potentially cure the disease. While these measures are not yet resolved, alternatives to help fight symptoms must be found.
The use of natural products derived from herbs by folk medicine has been described for many years, and their properties are explored for new drug development. Several natural compounds have been described with scientifically proven therapeutic properties: antiviral, antiinflammatory, and antioxidant (Lago et al., 2014; Santana et al., 2016; Slader, Reddel, Jenkins, Armour, & Bosnic‐Anticevich, 2006). In this context, flavonoids are classified as polyphenolic compounds metabolized by plants, fungi, and possess essential biological activities (Nijveldt et al., 2001). Knowing the chemical structure and effects of flavonoids on acute lung inflammation may lead to its application in clinical trials (as monotherapy or associated with known prescribed drugs). This review highlights the potential effects of several flavonoids in reducing acute pulmonary inflammation and ARDS, by inhibiting the enzymatic system associated with SARS‐CoV‐2.
2. CORONAVIRUS, SARS‐COV‐2, COVID‐19, AND ACUTE LUNG INFLAMMATION
The coronaviruses (CoVs) belongs to the Coronaviridae family, and is divided into four genera: α, ß, γ, δ, whereas only α‐ and ß‐CoV are capable of infecting mammals (Guo et al., 2020). There are seven CoVs known to infect humans: HCoV‐NL63, HCoV‐OC43, HCoV‐229E, HCoV‐HKU1, MERS‐CoV, and SARS‐CoV‐1 (Yin & Wunderink, 2018). The virus spike protein (S proteins) resembles a crown, in Latin called a corona, which named this family and is the main feature of CoVs (Schoeman & Fielding, 2019). The main selected proteins are the envelope protein—E, membrane protein—M, spike protein particles—S, and protein‐encapsulated nucleus—N. The S protein is responsible for host cell recognition and membrane fusion. The N protein is responsible for binding the RNA genome, creating the nucleocapsid, and is associated with the replication process. The M protein determines the shape of the viral envelope, and the E protein, in smaller amounts, participates in the assembly and releases by budding (Chan et al., 2020; Schoeman & Fielding, 2019).
The coronavirus that affects humans is endemic and responsible for 15–30% of respiratory tract infections each year. However, in 2002, the SARS‐CoV, a group 2b β‐coronavirus, called the attention since it causes severe respiratory infection, the SARS outbreak in the Guangdong Province of China. Epithelial cells are the primary cells affected by SARS‐CoV in the lung, although some studies showed that it can also affect dendritic cells and macrophages and induces proinflammatory cytokines that may contribute to the severe disease (Law et al., 2005; Peiris et al., 2003; Spiegel, Schneider, Weber, Weidmann, & Hufert, 2006). After 10 years, a novel CoVs appeared in the Middle East in 2012, provoking the Middle East Respiratory Syndrome‐CoV (MERS‐CoV) and induced respiratory infection in Saudi Arabia and other countries in the Middle East (Zaki, van Boheemen, Bestebroer, Osterhaus, & Fouchier, 2012). Unlike SARS‐CoV, MERS‐CoV uses the DPP4 (CD26) receptor to gain entry and effectively replicate in camel cell lines (Raj et al., 2013).
The first case of COVID‐19 was reported in December 2019 in the city of Wuhan, China, and was called the new coronavirus SARS‐CoV‐2 (Rothan & Byrareddy, 2020). Many studies suggest that SARS‐CoV‐2 has arisen in bats, then infected an intermediate host (yet unknown), and suffered several mutations that allowed the virus to infect humans (Chan et al., 2020; Zhou et al., 2020). These authors showed that the new virus has 96% genomic similarity compared to the coronavirus residing in bats (Zhou et al., 2020). Human‐to‐human transmission occurs through coughing, sneezing, fecal‐oral contact, and the virus can trigger respiratory, liver, renal, intestinal, and neurological system complications (Jin et al., 2020).
The SARS‐CoV‐2 has a single‐stranded RNA genome with approximately 30 kb. This genetic material is translated into structural and non‐structural proteins inside the host cell, determining its shape, life cycle, and virulence (Astuti and Ysrafil, 2020). In addition to the structures cited, SARS‐CoV‐2 also has the hemagglutinin esterase (HE) protein in the phospholipid double layer (Chan et al., 2020; Jin et al., 2020). Several studies point that the host cell angiotensin‐converting enzyme 2 (ACE2) is the primary receptor for SARS‐CoV‐2 cell entry (Astuti and Ysrafil, 2020; Chan et al., 2020; Yan et al., 2020), as previously observed in SARS‐CoV (Li et al., 2003). ACE2 is expressed in various organs such as the lungs, heart, brain, kidney, stomach, and liver (Hamming et al., 2004; Imai et al., 2005; Jiang, Gao, Lu, & Zhang, 2013; Olszanecki et al., 2009). Thus, tissue expression and ACE2 distribution are critical for infection and viral tropism. By interacting with ACE2 on cell surfaces, SARS‐CoV‐2 can connect with high affinity (Yan et al., 2020; Zhao et al., 2020), causing a generalized homeostasis imbalance that affects pulmonary, cardiac, circulatory, and renal systems, leading to systemic failure and eventually to patients death (Li, Li, Zhang, & Wang, 2020). When the virus binds to the ACE2 in the respiratory epithelium, the glycoprotein S inserts two subunits: S1, responsible for viral reach and tropism and S2, responsible for the fusion of the viral cell membrane. For this, the virus needs TMPRSS2 (serine transmembrane protease 2) to activate S glycoproteins in the viral envelope, thus associating SARS‐CoV‐2 and ACE2 in the lungs (Hoffmann et al., 2020; Wang, Grunewald, & Perlman, 2020). After membrane fusion, viral RNA replication begins, which proceeds quickly toward cell death, endothelial and epithelial vascular leakage, and pro‐inflammatory cytokines release (Jin et al., 2020). The viral RNA, identified as PAMPs (pathogen‐associated molecular patterns) is detected by Toll‐like (TLR) receptors (Birra et al., 2020) and thus starts a cascade, until activation of the nuclear transcription factor κB (NF‐κB) and consequent release of several systemic inflammatory mediators occurs in the lungs (Alexopoulou, Holt, Medzhitov, & Flavell, 2001; Wu & Chen, 2014). When SARS‐CoV‐2 infection occurs, there is a decrease in ACE2 and an increase in angiotensin II. Reduction of ACE2 is known to be related to alveolar injury and increased vascular permeability (Imai et al., 2005), and it was confirmed in experimental animal models (Imai, Kuba, & Penninger, 2008; Ye & Liu, 2020). In addition, angiotensin I (mild vasoconstrictor) is converted by ACE to angiotensin II, a potent vasoconstrictor. ACE2 converts angiotensin II to angiotensin 1–7, known for its vasodilatory effects (Benigni, Cassis, & Remuzzi, 2010). The ACE2 is also expressed in significant amounts in the pericyte, a mesenchymal cell presented in the endothelium of small vessels, which are essential for endothelial stability. When SARS‐CoV‐2 attacks the vascular system, there is endothelial imbalance and consequent dysfunction in the microcirculation (Chen, Li, Chen, Feng, & Xiong, 2020). The autopsy of fatal COVID‐19 patients reveals the presence of a microthrombi (Dolhnikoff et al., 2020) that can cause the severe form of COVID‐19.
Angiotensin II can bind to angiotensin receptors 1 (AT1) and 2 (AT2) that regulate hemodynamic stability and blood pressure (Arendse et al., 2019; Kreutz et al., 2020). AT1 has a vasoconstrictor effect and accounts for increased vascular permeability inducing inflammation and remodeling (Benigni et al., 2010). AT2 receptors, on the other hand, have a vasodilatory effect and exert anti‐regulatory activity for AT1 (Batenburg, Tom, Schuijt, & Danser, 2005). One hypothesis is that angiotensin II production through AT1 receptors activates the Janus kinase signal transducer and activator of transcription pathways involved in pro‐inflammatory, proliferative, and pro‐fibrotic responses and activates other pathways such as reactive oxygen species production, cell growth, and apoptosis (Seif et al., 2020). The increase in angiotensin II, on the other hand, was related to increased inflammatory activity due to the vital role of AT1 and the recruitment of immune system cells (Forrester et al., 2018).
Lung inflammation is one of the characteristics of several lung diseases such as asthma, chronic obstructive lung diseases, and others (Moldoveanu et al., 2009). However, acute lung inflammation and the ARDS are characterized by an intense inflammation that still kills more than 40% of the patients in the intensive therapy care unit (Bellani et al., 2016).
Acute lung injury (ALI) is characterized by the recruitment of immune cells, neutrophils, macrophages, and lymphocytes, with a high cytokine production such as IL‐6, IL‐1β, and TNF‐α (Bittencourt‐Mernak et al., 2017; Herold, Mayer, & Lohmeyer, 2011). Currently, several experimental animal models mimic inflammatory findings in ALI, such as intratracheal instillation lipopolysaccharide (LPS), viral infection, and sepsis (Bittencourt‐Mernak et al., 2017; Rungsung et al., 2018; Zhang et al., 2017).
It is described that COVID‐19 patient developed a cascade of cytokines and that the immune system often does not respond promptly (Ye et al., 2020). This SARS‐CoV‐2/ACE2 linkage triggers an exaggerated cytokine response, and consequently, an exacerbated inflammatory process, called “cytokine storm” (Jin et al., 2020; Ye et al., 2020). There is still a dysfunction of the renin‐angiotensin system with increased inflammation and vascular permeability, resulting in reduced ACE2 function (Basu, Sarkar, & Maulik, 2020; Li, Li, et al., 2020). Therefore, there is a systemic immune imbalance with significant systemic repercussions.
Respiratory symptoms and pulmonary effects in patients with COVID‐19 are the most discussed features related to severity. Three possibilities have been described regarding manifestations of the disease when it affects the respiratory tract of symptomatic patients: (1) the virus remains in the upper respiratory tract, and due to viral replication, the patient may present symptoms such as sore throat, dry cough, and runny nose; (2) the patient has worsened respiratory symptoms, including dyspnea, hypoxemia, and fever due to the exacerbated immune response; (3) one‐third of patients evolve to respiratory failure such as ARDS and need rapid ventilation support (Rothan & Byrareddy, 2020).
The pathogenesis of COVID‐19 is highly complex and involves suppressing host antiviral and innate immune response, induction of oxidative stress followed by hyper inflammation described as the “cytokine storm,” causing ALI, tissue fibrosis, and pneumonia. Most patients that recovered from severe COVID‐19 showed elevated lung disease severity at days 10–14 after initial symptoms presentations. The lung lesions can be absorbed in 53.0% of patients during the third week after discharge, with no sequelae. However, about 40% of patients had lung ground‐glass opacity (GGO) and fibrous stripe as the main manifestations upon computed tomography images, seen on radiological follow‐ups (Pan et al., 2020).
There is an increase in the cytokines TNF‐α, IL‐1ß, IL‐7, IL‐8, IL‐9, IL‐10, INF‐γ, monocyte chemoattractant protein‐1, and others (Burgos‐Blasco et al., 2020; Ye et al., 2020). Most COVID‐19 patients, especially among elderly patients, had marked lymphopenia and increased neutrophils, but T cell counts in severe COVID‐19 patients surviving the disease were gradually restored (Akbari et al., 2020; Chen et al., 2020). Some critically ill patients showed higher expressions of IL‐1β, IL‐6, TNF‐α, and other cytokines (Akbari et al., 2020). Thus, these inflammatory‐related factors might function as a biomarker to monitor the progression of COVID‐19 disease.
Some new reports showed that COVID‐19 survivors, particularly those developing the severe form, evolved to pulmonary fibrosis (Rogliani et al., 2020; Zhang et al., 2020). Interestingly, Aloufi et al. (2020) reported that lung fibroblasts isolated from idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease patients express higher levels of ACE2. It suggested that the risk of developing pulmonary fibrosis can be associated with increased expression of ACE2, which occurred in the risk group, involving obesity, heart, and aging disorders (Aloufi et al., 2020).
As discussed above, viruses commonly encode proteins that inhibit the immune system, promote viral invasion, and pathogenesis. In this context, flavonoids have been studied for their antiviral effect and inhibition of the virus membrane proteins, preventing cellular invasion (Russo, Moccia, Spagnuolo, Tedesco, & Russo, 2020; Seong, Kim, & Shin, 2018). Although several studies have shown that different flavonoids are beneficial in controlling respiratory diseases, additional studies of the specific effects of flavonoids on molecular mechanisms in lung diseases are needed.
In this review, we focused on flavonoids described in the literature as having potential biological effects against different coronavirus (SARS‐CoV and MERS), respiratory illnesses such as ALI, and ARDS, and those flavonoids showing antioxidant and antiinflammatory properties, focusing on possible relation of the flavonoids' chemical structure and biological function. We also discussed, based on these pieces of evidence, the potential application of flavonoids to SARS‐CoV‐2 and COVID‐19.
3. FLAVONOIDS: CHEMISTRY, OCCURRENCE, ANTIINFLAMMATORY, AND ANTIVIRAL POTENTIAL ACTION
The flavonoids play an essential role among natural products, comprising more than 8,000 different bioactive molecules described in the literature. These metabolites are present in many plant species, including various products and grains (Jucá et al., 2020). Flavonoids are chemically characterized by a general structure of a 15‐carbon skeleton with two phenyls (rings A and B) and one heterocyclic (ring C) unities, also known as C6C3C6 (Figure 1). This class's subgroups include chalcones, flavones, flavonols, flavanones, flavanonols, flavans, anthocyanins, and isoflavonoids (Figure 2), which can contain one or more carbohydrate moieties (glycosylated flavonoids) or is composed exclusively by aglycones.
FIGURE 1.
General and subgroup structures of the flavonoids class
FIGURE 2.

Chemical structure of flavonoids 1–101. (a) Chemical structure of glycosylated flavonoids; (b) Chemical structure of free flavonoids; and (c) Chemical structure of theaflavins and procyanidin derivatives
Several biological properties have been described for these compounds, including antioxidant, antiaging, antiinflammatory, immunomodulatory, cardioprotective, antimicrobial, antifungal, and antiviral activities (Brendler et al., 2020; Jucá et al., 2020; Lago et al., 2014; Santana et al., 2016). As such, these compounds are of interest in the treatment of various illnesses. Several studies with flavonoids have been published, involving pathologies such as viral SARS, ALI and ARDS (Bittencourt‐Mernak et al., 2017; Jo, Kim, Kim, Shin, & Kim, 2019; Zhang et al., 2017); however, to the best of our knowledge, no previous research articles were published until now showing the effects of flavonoids in COVID‐19. However, other authors review the literature to suggest the potential use of flavonoids in COVID‐19 (Russo et al., 2020; Solnier & Fladerer, 2020; Tutunchi, Naeini, Ostadrahimi, & Hosseinzadeh‐Attar, 2020). Many unique features of flavonoids, such as antioxidant and antiinflammatory properties, their ability to inhibit enzymes, to destroy cell membranes, and to prevent virus penetration make them suitable for further tests to demonstrate their beneficial effects against these diseases (Jucá et al., 2020; Nijveldt et al., 2001; Panche, Diwan, & Chandra, 2016).
The leaves of Microcos paniculate, also known as shiral (India, Bengal), has been traditionally used to treat upper airway infections, containing flavonoids such as apigenin C‐glycosides (ACGs), vicenin‐1 (1), vicenin‐2 (2), isoshaftoside (3), shaftoside (4), vitexin (5), isovitexin (6), violanthin (7), and isoviolanthin (8) (Li et al., 2018). Their effects were measured by cytokine level determination and lung inflammation evaluation in situ. ACGs reduced pulmonary edema and microvascular permeability by down‐regulating LPS‐induced TNF‐α, IL‐6, and IL‐1β expression. Metabolic profiling showed that this protective effect was due to suppression of TLR4/TRPC signaling pathway activation. As such, ACGs could be further explored to treat ALI and ARDS.
Obtained from Scutellaria baicalensis, baicalin (9) has shown anti‐apoptosis, antiinflammatory, and antioxidant properties. An in vitro study demonstrated that this compound attenuates oxidative stress and endothelial dysfunction by improving ACE2 activity (Wei et al., 2015). Improved endothelial function impaired Ang II by promoting endothelial‐dependent vasodilation and suppression of human umbilical vein endothelial cells apoptosis. Baicalin decreased the expression of pro‐apoptotic protein Bax and cleaved caspase‐3, involving an increase of Bcl‐2 expression. Baicalin also significantly conversed Ang II to Ang‐(1–7) by ACE2 and Mas receptor mRNA expression and protein expression and up‐regulation of the PI3K/AKT/eNOS pathway (Wei et al., 2015). Astilbin (10), found in Smilax china, shown to diminish LPS‐induced ARDS in vivo and in vitro successfully. This effect was determined by regulating pro‐inflammatory cytokines TNF‐α and IL‐6, MAPK phosphorylation inhibition, suppression of proinflammatory enzyme heparinase, and diminished heparin sulphate degradation (Kong et al., 2016). Glycosylated flavonoids hesperidin (11), naringin (12), and neohesperidin (13), found in different quantities in citrus fruits, were among the compounds subjected to a molecular docking study. The SARS‐CoV S protein has a significant binding affinity to the human ACE2 enzyme, considered to be crucial for virus entrance in host cells (Basu et al., 2020). Therefore, compounds that bind this enzyme could prevent coronavirus infection.
Rutin (14) was investigated for antiinflammatory effects in vivo (Guardia, Rotelli, Juarez, & Pelzer, 2001). At a dosage of 80 mg/kg it was able to decrease paw edema in the acute phase of inflammation (day six) as well as in the chronic phase (days 7 to 30). On days 21st and 30th after induced inflammation, rutin could suppress this effect by 100%. Comparatively, related flavonoids quercetin (15) and hesperidin (11) were also tested and showed smaller significant antiinflammatory properties. A study conducted with licorice flavonoids liquiritigenin (16), liquiritin (17), and liquiritin apioside (18) from Glycyrrhiza uralensis showed that these compounds effectively diminish LPS‐induced pulmonary inflammation by inhibiting inflammatory cell infiltration and inflammatory mediator release, including reduction of TNF‐α and IL‐1β expression. The maximal dosage of those flavonoids (30 mg/kg) produces similar effects as treatment with dexamethasone at 1 mg/kg, the standard drug used for the assays (Xie, Dong, Wu, Yan, & Xie, 2009). These results were consistent with the effects observed in macrophages for daidzein (19), eriodictyol (20), genistein (21), isorhamnetin (22), pelargonidin (23), and naringenin (24) (Hämäläinen, Nieminen, Vuorela, Heinonen, & Moilanen, 2007). The antiinflammatory and antioxidant properties of eriodictyol (20) were also studied in the LPS‐induced ALI model. Eriodictyol demonstrated inhibition of proinflammatory cytokine expression and attenuation of oxidative injury by activating the Nrf2 pathway at a dose of 30 mg/kg (Zhu, Guo, Huang, Wu, & Zhang, 2015). Besides the effects antiinflammatory of narigenin (24), these flavonoids also demonstrated an inhibition of the 3‐chymotrypsin‐like protease (3CLpro), and reduction of ACE receptors activity (Tutunchi et al., 2020).
The in vivo protective effect of synthetic flavonoid LFG‐500 (25) was shown to inhibit cytokines such as TNF‐α, IL‐1β, and IL‐6 in lung tissues after inducing ALI and inflammation by LPS challenge. In vitro effects were investigated as well, where the cytokine inhibition was also observed, by inhibiting NF‐κB activation. In addition, p38 and JNK MAPK pathways were found to be involved in the antiinflammatory properties of compound 25 (Li et al., 2016). According to a review reporting effects of flavonoids in lung diseases, luteolin (26), pinocembrin (27), and oroxylin‐A (28) were described to attenuate LPS‐induced ARDS in vivo and in vitro, affecting proinflammatory cytokine concentrations as well as MAPK ad NF‐κB pathway activation (Kimata et al., 2000). In addition, the therapeutic effect of oroxylin‐A (28) ameliorated the increased of the white blood cells counts, elevated plasma tumor necrosis factor (TNF)‐α, and nitric oxide (NO), increased pulmonary edema, thickened alveolar septa caused by the administration of LPS (Tseng et al., 2012). In vitro pre‐treatment with pinocembrin (27) remarkably regulated the production of TNF‐α, IL‐1β, IL‐6, and IL‐10 via inhibition of IκBα, ERK1/2, JNK, and p38 MAPK phosphorylation. In the mouse model of LPS‐induced ALI, pinocembrin (20 or 50 mg/kg, i.p.) attenuated the development of pulmonary edema, histological severities, as well as neutrophil, lymphocyte, and macrophage infiltration, increased by LPS administration (Soromou et al., 2012).
Other flavonoids were reported to affect the expression of additional pro‐inflammatory genes such as nitric oxide synthase, cyclooxygenase, and lipoxygenase. Luteolin (26), apigenin (29), and chrysin (30) were among those with inhibitory potential against iNOS and NO products. Morin (31) and myricetin (32) were cited for the ability to affect the lipoxygenase enzyme (Havsteen, 2002; O'Leary et al., 2004; Yoon & Baek, 2005). Flavonoids 26, 29–31 also inhibit the enzyme cyclooxygenase. Furthermore, luteolin, apigenin, and fisetin (33) were shown to inhibit the synthesis of cytokines IL‐4 and IL‐13 in vitro (Hirano et al., 2004). Scutellarein (34) and fustin (35) were also among the flavonoids able to inhibit IL‐4. Compounds 26, 29, 31, 32, and 34 were reported to have antiinflammatory effects against asthma models and chronic obstructive pulmonary disease (Coutinho, Muzitano, & Costa, 2009; Kim, Son, Chang, & Kang, 2004; O'Leary et al., 2004). Another study showed that pre‐treatment with luteolin decreased lung edema and protein content in lung tissue and bronchoalveolar lavage fluid (BALF). Furthermore, luteolin pre‐treatment showed a significant reduction in proinflammatory cytokines (IL‐6 and IL‐1β) and attenuation in sepsis‐induced ALI in mice through the suppression in ICAM‐1, NF‐κB, oxidative stress, and partially iNOS pathways (Rungsung et al., 2018). In addition, Kuo et al. (2011) investigated the protective effects and luteolin mechanisms in intratracheal instillation of LPS‐induced ALI in mice. The antioxidant and antiinflammatory effects of luteolin were observed by reducing catalase and superoxide dismutase activities, the levels of oxidative damage and lipid peroxidation, and the secretion of TNF‐α, IL‐8 (KC), and ICAM‐1 in BALF after LPS‐induced ALI. Furthermore, the pre‐treatment with luteolin restored the LPS‐induced decrease in oxygen pressure and increase of carbon dioxide in arterial blood.
Antiinflammatory effects of morin (31) on ALI were studied using LPS‐induced ALI mouse model. Morin showed attenuation in the inflammatory cells and decreased IL‐1β, IL‐18, and IL‐6 cytokines. This flavonoid also decreased lung NLRP3 inflammasome protein levels and improved superoxide dismutase activity (Tianzhu, Shihai, & Juan, 2014).
Fisetin (33), a flavonoid found in several fruits and vegetables, effectively reduced the IL‐6 and TNF‐α release and total protein in BALF, besides improving lung inflammation. In addition, fisetin is related to the inhibition of the Toll‐like receptor 4 (TLR4) and NF‐κB expression (Feng, Jiang, Sun, Fu, & Li, 2016).
Sakuranetin (36), from the Brazilian plant Baccharis retusa, exhibited antiinflammatory activity, significantly reducing the number of neutrophils. In addition, sakuranetin reduced the number of macrophages in bronchoalveolar lavage fluid (BALF) and pro‐inflammatory cytokines like IL‐1β, IL‐8, and TNF‐α in mice exposed to LPS instillation (Bittencourt‐Mernak et al., 2017). These authors showed that sakuranetin could similarly act as an immunomodulatory compound since it was reported that this treatment in mice could modulate the macrophage profile. In addition, sakuranetin has an inhibitory effect in viral RNA synthesis, effective in the inactivation of the influenza B/Lee/40 virus, and could be a good candidate for treating diseases related to the influenza virus (Kwon, Ji, Yim, Kim, & Choi, 2018).
The β‐CoVs, like SARS‐CoV‐2, usually induce the production of polypeptides with ~80 kDa after the genome transcription. This polypeptide is cleaved to generate various proteins through proteolytic processes, mediated by two proteins: papain‐like protease (PLpro) and 3‐chymotrypsin‐like protease (3CLpro). PLpro and 3CLpro are crucial to the virus life cycle and viral coronavirus replication (Lin et al., 2005; Nguyen et al., 2012; Park et al., 2016). As such, they are viable targets for developing drugs against SARS, MERS, and other coronavirus infections (Chen et al., 2006; Lin et al., 2005; Nguyen et al., 2012). The SARS‐CoV PLpro catalytic domain comprises various catalytically active enzymes, transmembrane domains, and domains with unknown function. The associated membrane domain can perform proteolytic cleavage releasing proteins nsp1, nsp2, and nsp3 from the viral polyprotein, which is essential for viral replication. This protease is also a deubiquitinating and deISGylating enzyme, which antagonizes the innate immune response. SARS‐CoV PLpro is a member of the peptidase clan CA (family C16), with a classic catalytic triad in its active site composed of Cys112‐His273‐Asp287 (Báez‐Santos, St. John, & Mesecar, 2015). The 3CLpro generates various essential proteins for viral replication after cleavage of the polyprotein at 11 sites. This protease performs a crucial role in viral replication and is located at the 3′‐end, differently encoding genes of structural/supplemental protein (Anand, Ziebuhr, Wadhwani, Mesters, & Hilgenfeld, 2003; Kumar, Tan, Wang, Lin, & Liang, 2016; Needle, Lountos, & Waugh, 2015; Qamar, Alqahtani, Alamri, & Chen, 2020). Among the glycoside flavonoids with 3CLpro inhibition activity, Chen et al. (2006) described, by molecular docking experiments and enzymatic inhibitions assays, the potential of quercetin‐3‐β‐galactose (37), with IC50 of 42.79 ± 4.97 μM, in a competitive mode. The same study investigated the binding interaction mode between the compound and the virus protease. Eight quercetin‐3‐O‐β‐galactose derivatives were synthesized and after testing the inhibitory potentials, it was observed that the removal of hydroxyl groups (compounds 38–42) decreases the activity. Acetylated glycosylic unity (compound 38) is also unfavorable for activity and the addition of rhamnoside unity on the C‐7 position of quercetin (compound 42) did not affect the functionality against SARS‐CoV. Changing the galactose to fucose (39), arabinose (40) or glucose (41) had no evident effect on inhibitor potency. All IC50 values are represented in Table 1 (Chen et al., 2006).
TABLE 1.
Inhibitory activities of flavonoids 37, 39–41 against SARS‐CoV 3CLpro
| Flavonoid | Activity |
|---|---|
|
Quercetin‐3‐O‐β‐galactoside (37) |
SARS‐CoV 3CLpro Inhibitor IC50 = 42.8 μM |
|
Quercetin‐3‐O‐β‐fucoside (39) |
SARS‐CoV 3CLpro Inhibitor IC50 = 24.1 μM |
|
Quercetin‐3‐O‐β‐arabinoside (40) |
SARS‐CoV 3CLpro Protease inhibitor IC50 = 31.6 μM |
|
Quercetin‐3‐O‐β‐glucoside (41) |
SARS‐CoV 3CLpro Protease inhibitor IC50 = 48.8 μM |
Puerarin (43), isolated from Pichia pastoris, showed low inhibitor activity against 3CLpro, with an IC50 value of 381 μM. Compared with non‐glycosylated derivative (daidzein ‐ 19), the study suggests that the presence or absence of a sugar moiety at ring A does not affect the activity (Nguyen et al., 2012). Glycoside flavonoids rhoifolin (44) and pectolinarin (45) were subjected to a molecular docking study and evaluated by Jo, Kim, Shin, and Kim (2020) against 3CLpro. The series of tested compounds showed high inhibitory values with IC50 of 27.45 and 37.78 μM, respectively. These compounds have an α‐l‐rhamnopyranosyl β‐d‐glucopyranoside and l‐rhamnopyranosyl β‐d‐gluco‐pyranoside moieties. In addition, these sugar groups attached to position C‐7 of the chromen‐4‐one occupy the S1 and S2 sites and S2 and S30 sites, unlike the two flavonols described above. The higher affinity of rhoifolin (44) may be due to orchestrated binding through S1, S2, and S3′ sites (Jo et al., 2020). Studies carried out with roots of Isatidis indigotica, allowed the identification of several compounds, including the flavanone hesperetin (46) and isoflavone daidzein (19), exhibiting IC50 values against SARS‐CoV 3CLpro, through cell‐free cleavage assay, of 60 and 105 μM, respectively (Lin et al., 2005). Fractionation of extract from leaves of Torreya nucifera afforded four flavonoids: amentoflavone (47), bilobetin (48), ginkgetin (49), and sciadopytiscin (50), which demonstrated 3CLpro inhibitory activity. The IC50 values are 8.3, 72.3, 32.0, and 38.4 μM, respectively, and amentoflavone (47) is the most active (8.3 μM) (Ryu et al., 2010).
Nine alkylated chalcones (51–59) isolated from Angelika keiskei displayed in vitro activities against SARS‐CoV proteases, including PLpro. 4‐Hydroxyderricin (51), xanthoangelol (52), xanthoangelol F (53), xanthoangelol D (54), xanthoangelol E (55), xanthoangelol B (56), xanthoangelol G (57), and xanthokeistal A (58) showed IC50 values of 26.0, 11.7, 5.6, 19.3, 1.2, 11.7, 46.4, and 21.1 μM against PLpro, respectively, with xanthoangelol E being the most active. An in silico molecular docking study was performed for compound 55, showing that the hydroperoxyl group forms three hydrogen bonds at different binding sites. In this same study, isobavachalcone (59) displayed an IC50 value of 13.0 μM against SARS‐CoV PLpro. The inhibition mechanisms of flavonoids 52–59 were determined by the effect of the substances on the kinetics of substrate proteolysis and were determined to be non‐competitive. Compound 59 was established as a mixed inhibition type (Park et al., 2016). As observed by the authors, the isoprene unit's length was not relevant for the observed activity. All activities are summarized in Table 2.
TABLE 2.
Inhibitory activities of flavonoids 51–59 against SARS‐CoV 3CLpro and PLpro
| Flavonoid | Activity |
|---|---|
|
4‐Hydroxyderricin (51) |
SARS‐CoV Inhibitor IC50 = 81.4 μM (3CLpro cell‐free) IC50 = 50.8 μM (3CLpro cell‐based) IC50 = 26.0 μM (PLpro) |
|
Xanthoangelol (52) |
SARS‐CoV Protease inhibitor IC50 = 38.4 μM (3CLpro cell‐free) IC50 = 5.8 μM (3CLpro cell‐based) IC50 = 11.7 μM (PLpro) |
|
Xanthoangelol F (53) |
SARS‐CoV Protease inhibitor IC50 = 34.1 μM (3CLpro cell‐free) IC50 = 32.6 μM (3CLpro cell‐based) IC50 = 5.6 μM (PLpro) |
|
Xanthoangelol D (54) |
SARS‐CoV Protease inhibitor IC50 = 26.6 μM (3CLpro cell‐free) IC50 = 9.3 μM (3CLpro cell‐based) IC50 = 19.3 μM (PLpro) |
|
Xanthoangelol E (55) |
SARS‐CoV Protease inhibitor IC50 = 11.4 μM (3CLpro cell‐free) IC50 = 7.1 μM (3CLpro cell‐based) IC50 = 1.2 μM (PLpro) |
|
Xanthoangelol B (56) |
SARS‐CoV Protease inhibitor IC50 = 22.2 μM (3CLpro cell‐free) IC50 = 8.6 μM (3CLpro cell‐based) IC50 = 11.7 μM (PLpro) |
|
Xanthoangelol G (57) |
SARS‐CoV Protease inhibitor IC50 = 129.8 μM (3CLpro cell‐free) IC50 = NT (3CLpro cell‐based) IC50 = 46.4 μM (PLpro) |
|
Xanthokeistal A (58) |
SARS‐CoV Protease inhibitor IC50 = 44.1 μM (3CLpro cell‐free) IC50 = 9.8 μM (3CLpro cell‐based) IC50 = 21.1 μM (PLpro) |
|
Isobavachalcone (59) |
SARS‐CoV Protease inhibitor IC50 = 39.4 μM (3CLpro cell‐free) IC50 = 11.9 μM (3CLpro cell‐based) IC50 = 13.0 μM (PLpro); IC50 = 7.3 μM (PLpro) |
Molecular docking studies developed by Jo et al. (2019) suggested that herbacetin (60) occupies the S1 and S2 sites of MERS‐CoV 3CLpro, and the hydroxyl group at C‐7 position is essential for S1 binding site. Helichrysetin (61) exhibits relevant inhibitory activity and the authors suggest that the presence of a hydroxyl group at C‐4 position is suitable for binding to MERS‐CoV 3CLpro (Jo et al., 2019).
Fractionation of the bioactive extract from Broussonetia papyfera led to the isolation of flavonoids 62–71 (Table 3). When tested in vitro against SARS‐CoV PLpro, broussochalcone B (62), broussochalcone A (63), 4‐hydroxyisolonchocarpin (64), papyriflavonol A (65), 3′‐(3‐methylbut‐2‐enyl)‐3′,4′,7‐trihydroxyflavane (66), kazinol A (67), kazinol B (68), broussoflavan A (69), kazinol F (70), and kazinol J (71) displayed moderate activities, with IC50 values of 11.6, 9.2, 35.4, 3.7, 35.8, 66.2, 31.4, 20.4, 27.8 and 15.2 μM, respectively. The prenylated flavonol derivative 65 showed the highest activity. It was more potent against the catalytic activity of SARS‐CoV PLpro than flavone derivatives such as kaempferol (IC50 = 16.3 μM), quercetin (IC50 = 8.6 μM), and quercetin‐O‐β‐galactose (IC50 = 51.9 μM), probably due to strong hydrophobic interactions with the enzyme. Kinetic studies determined all compounds as non‐competitive inhibitor types. When tested for activity against MERS‐CoV PLpro, none showed high potential, with IC50 values between 39.5 and 171.6 μM (Park et al., 2017).
TABLE 3.
Inhibitory activities of flavonoids 62–71 against SARS‐CoV and MERS‐CoV proteases
| Flavonoid | Activity |
|---|---|
|
Broussochalcone B (62) |
SARS‐CoV Protease inhibitor IC50 = 57.8 μM (3CLpro cell‐free) IC50 = 11.6 μM (PLpro) MERS‐CoV Protease inhibitor IC50 = 27.9 μM (3CLpro) IC50 = 112.9 μM (PLpro) |
|
Broussochalcone A (63) |
SARS‐CoV Protease inhibitor IC50 = 88.1 μM (3CLpro) IC50 = 9.2 μM (PLpro) MERS‐CoV Protease inhibitor IC50 = 36.2 μM (3CLpro) IC50 = 42.1 μM (PLpro) |
|
4‐Hydroxyisolonchocarpin (64) |
SARS‐CoV Protease inhibitor IC50 = 202.7 μM (3CLpro) IC50 = 35.4 μM (PLpro) MERS‐CoV Protease inhibitor IC50 = 193.7 μM (3CLpro) IC50 = 171.6 μM (PLpro) |
|
Papyriflavonol A (65) |
SARS‐CoV Protease inhibitor IC50 = 103.6 μM (3CLpro) IC50 = 3.7 μM (PLpro) MERS‐CoV Protease inhibitor IC50 = 64.5 μM (3CLpro) IC50 = 112.5 μM (PLpro) |
|
3′‐(3‐methylbut‐2‐enyl)‐3′,4′,7‐trihydroxyflavane (66) |
SARS‐CoV Protease inhibitor IC50 = 30.2 μM (3CLpro) IC50 = 35.8 μM (PLpro) MERS‐CoV Protease inhibitor IC50 = 34.7 μM (3CLpro) IC50 = 48.8 μM (PLpro) |
|
Kazinol A (67) |
SARS‐CoV Protease inhibitor IC50 = 84.8 μM (3CLpro) IC50 = 66.2 μM (PLpro) MERS‐CoV Protease inhibitor NA (3CLpro) IC50 = 13.0 μM (PLpro) |
|
Kazinol B (68) |
SARS‐CoV Protease inhibitor IC50 = 233.3 μM (3CLpro) IC50 = 31.4 μM (PLpro) MERS‐CoV Protease inhibitor NA (3CLpro) IC50 = 88.5 μM (PLpro) |
|
Broussoflavan A (69) |
SARS‐CoV Protease inhibitor IC50 = 92.4 μM (3CLpro) IC50 = 30.4 μM (PLpro) MERS‐CoV Protease inhibitor IC50 = 125.7 μM (3CLpro) IC50 = 49.1 μM (PLpro) |
|
Kazinol F (70) |
SARS‐CoV Protease inhibitor IC50 = 43.3 μM (3CLpro) IC50 = 27.8 μM (PLpro) MERS‐CoV Protease inhibitor IC50 = 135.0 μM (3CLpro) IC50 = 39.5 μM (PLpro) |
|
Kazinol J (71) |
SARS‐CoV Protease inhibitor IC50 = 64.2 μM (3CLpro) IC50 = 15.2 μM (PLpro) MERS‐CoV Protease inhibitor IC50 = 109.2 μM (3CLpro) IC50 = 55.0 μM (PLpro) |
Fractionation of bioactive extract from Paulownia tomentosa fruits led to the isolation of flavonoids 72–83. Evaluated against SARS‐CoV PLpro, all compounds showed inhibitory activity. Tomentin A (72), tomentin B (73), tomentin C (74), tomentin D (75), tomentin E (76), 3′‐O‐methyldiplacol (77), 4′‐O‐methoxydiplacol (78), 3′‐O‐methyldiplacone (79), 4′‐O‐methyldiplacone (80), mimulone (81), diplacone (82), and 6‐geranyl‐4′,5,7‐trihydroxy‐3′,5′‐dimethoxyflavanone (83) displayed IC50 values of 6.2, 6.1, 11.6, 12.5, 5.0, 9.5, 9.2, 13.2, 12.7, 14.4, 10.4, and 13.9 μM, respectively. Compounds 72–76, bearing unusual 3,4‐dihydro‐2H‐pyran structures, were more effective in inhibiting the enzyme than the cyclization precursors (Cho et al., 2013). This series of compounds allowed the authors to infer that a 3,4‐dihydro‐2H‐pyran moiety is more effective at inhibiting 3CLpro expression than the open ring precursors. The extract from Psoralea corylifolia seeds was subjected to fractionation to afford six related flavonoids (59 and 84–88). These compounds were then evaluated for potential SARS‐CoV PLpro inhibition in vitro. Bavachinin (84), neobavaisoflavone (85), 4′‐O‐methylbavachalcone (86), and corylinin (87) showed moderate IC50 values of 38.4, 18.3, 10.1, and 32.3 μM, respectively, while isobavachalcone (59) and psoralidin (88) were the most active compounds, with IC50 values of 7.3 and 4.2 μM, respectively. These results suggest that P. corylifolia can be considered a source of potent PLpro inhibitors, and the strongest ones were isobavachalcone and psoralidin. Enzyme kinetic assay determined that all compounds possess a mixed inhibition mode (Kim et al., 2014). Nguyen et al. (2012) compared the activity of ampelopsin (89), epigallocatechin gallate (90), epigallocatechin (91), and gallocatechin gallate (92), from Pichia pastoris (Nguyen et al., 2012). The results suggested that compounds 90 and 92 possess stronger 3CLpro inhibitory activity than 89 and 92, with IC50 values of 73 and 47 μM for 90 and 92, respectively, and 364 mM for 89. Only an inhibitory percentage of 5.4% at 200 μM was observed for 92.
Using molecular docking assays, theaflavin (93) can bind in catalytic pockets of RNA‐dependent RNA‐polymerase (RdRp) of MERS‐CoV, SARS‐CoV, and SARS‐CoV‐2 (Lung et al., 2020). In this way, this compound could be considered a lead compound for inhibitors targeting CoVs RdRp (Lung et al., 2020). A study demonstrated that 3‐theaflavin‐3‐gallate (94) and theaflavin‐3,3′‐digallate (95), both present in black tea, were effective against SARS‐CoV 3CLpro, with IC50 values of 7.0 and 9.5 μM. This suggests that black tea could prevent or alleviate coronavirus infection (Chen et al., 2005). Studies carried out by Zhuang et al. (2009) with extracts from Cinnamomi cortex describe inhibition in wild‐type SARS‐CoV (Zhuang et al., 2009). It was possible to isolate from this extract procyanidin A2 (96), procyanidin B2 (97), and dimer cinnamtannin B1 (98), showing overall moderate inhibitory effects with IC50 values of 120.7, 161.1, and 32.9 μM, respectively (Zhuang et al., 2009).
SARS‐CoV N protein envelopes the genomic RNA, and as such, has a crucial role in the virus particle assembly. It may cause apoptosis of the host cells, upregulate the proinflammatory cytokine production and block innate immune responses. In addition, it has a significant role in replication for this virus and is considered a central target for anti‐SARS drugs. An inhibitor screening of this protein was performed on a biochip platform, with high sensitivity and rapid response. In this work, (−)‐gallocatechin gallate (92) and (−)‐catechin gallate (99) showed high activity. Both compounds, at 0.005 μg/mL, diminished the binding affinity in a concentration‐dependent manner. More than 40% inhibition was displayed at a concentration of 0.05 μg/mL on the biochip platform for 92 and 99. Other flavonoids including kaempferol (100) and quercetin (15), showed no inhibitory activity at the tested concentrations (Roh, 2012).
The flavonoids juglanin (101) was shown to inhibit SARS‐CoV 3‐a‐mediated current, with an IC50 value of 2.5 μM. The activity of this cation‐selective channel can be expressed in the infected cells and virus release (Schwarz et al., 2014).
In summary, flavonoids' favorable effects are related to the antiinflammatory, antioxidant, immunomodulatory, and anti‐viral effects, suggesting that flavonoids can be a promising treatment strategy for conventional drugs against COVID‐19.
4. DISCUSSION AND CONCLUSIONS
A range of studies is, globally, currently trying to find an efficient treatment and/or a vaccine to treat or prevent COVID‐19, a pandemic that has so far caused hundreds of thousands of deaths and has crippled the global economy. In the present review, we reported the biological effects of several flavonoids inhibiting some coronavirus proteins or counteracting lung inflammation and cytokine storm, which is a critical consequence of SARS‐CoV‐2. These polyphenolic compounds, isolated from different plants, have been used to treat numerous viral diseases successfully and can be used, associated with other pharmacological treatments, in the treatment of COVID‐19 as well. Both flavonoids and other compounds derived from plants can also modulate the immune system to improve the organism defense, modulating macrophage profile and natural killer cells, and increasing antiinflammatory mechanisms (Tutunchi et al., 2020).
Based on the different structures of flavonoids 1–101, some structure–activity relationships (SAR) could be established. Initially, it was observed that glycosylated derivatives exhibited improved biological effects than their aglycone derivatives, which could be explained, at least in part, by the possibility of formation of different hydrogen bonds between the flavonoid and catalytic site of other enzymes (Ryu et al., 2010). Considering the structure of flavonoid moiety, the more active derivatives were flavonols, suggesting that an α,β‐unsaturated system (double bond at C‐2/C‐3 and carbonyl group at C‐3) is associated to the presence of an oxygen atom at C‐3, plays an important role in the activity. Furthermore, it was observed that the presence of a catechol unity in the ring B (positions C‐3′ and C‐4′) of different flavonoids is associated with higher potential in relation to related derivatives displaying no substituents at ring B or those presenting only one hydroxyl or methoxyl group at C‐4′ position. Considering the effects of compounds 47–50 and the pronounced activity of flavonoid 47, it is possible to suggest that free hydroxyl groups are crucial since derivatives 48–50, which exhibited methoxyl groups, displayed reduced activity. As reported in the literature (Ryu et al., 2010), the catechol unity is related to the interactions of bioactive compounds and catalytic site by hydrogen bond formation of different enzymes SARS‐CoV or MERS‐CoV protease inhibitors, associated with coronavirus replication. Considering bioflavonoids 47–50, interesting structural‐activity relationships could be established, especially the methylation of hydroxyl groups at C‐7′ and C‐4′, which cause a reduction in the inhibitory activity. However, methoxyl at C‐7 increases the potency, suggesting that methoxyl groups' position in the structures of these related bioflavonoids is associated with their inhibitory potential of SARS‐CoV 3CLpro (Russo et al., 2020; Ryu et al., 2010). Considering chalcone derivatives 51–64, the biological effect (SARS‐CoV protease inhibitor) seems to be associated with the presence of prenyl unities at different position of C6C3C6 moiety – this initial analysis also suggested that the presence of hydroxyl group at C‐4′ is important to the activity – in addition, it is essential to mention that the acyclic prenyl unity at C‐3 seen to be crucial to activity of related compounds since compound 64, a chromene, exhibited reduced potential. A similar profile was observed to prenylated flavanes 66–69 since the effect of compounds, which showed an acyclic unity (66 and 67) were able to inhibit SAR‐CoV and MERS‐CoV more efficiently in comparison to chromene derivatives 68 and 69. In addition, considering the structures of biflavones 47–50, biflavonols 93–98, and their inhibitory effects of SARS‐CoV 3CLpro enzymes, it was observed a positive influence of dimerization (Islam et al., 2020; Ryu et al., 2010).
Studies using molecular docking showed that rutin, which was approved by NMPA (National Medical Products Administration), exhibited the best effect in the binding affinity to inhibit SARS‐CoV‐2 compared to other compounds (Xu et al., 2020). Some authors have also shown that certain flavonoids can interact with the receptor binding of the SARS‐CoV‐2 using in silico analysis (Istifli et al., 2020). In this computational study, the group of flavonoids anthocyanidins, isoflavones, and flavanones showed improved interaction with the target proteins, in special (−)‐epicatechin gallate. Basu et al. (2020) also showed that the structure of ACE2 and spike protein fragment becomes unstable in the presence of hesperidin, suggesting that this compound can have effects on the virus entry. Besides, the effects evaluated by in silico studies showing possible effects of flavonoids in the ACE2 and Spike interaction, we have to consider that flavonoids have an antiinflammatory effect well known, avoiding or reducing lung inflammation and the cytokine storm induced by SARS‐CoV‐2, as shown in Figure 3. In this regard, our group and others showed that flavonoids can inhibit NF‐kB and MAP kinase pathways, and cellular signaling involved in ALI. Moreover, flavonoids can reduce cytokine release in the lung and the neutrophils and macrophage recruitment and activation, respectively, which could be interesting for inhibiting cytokine storm and acute inflammation. We have previously shown that sakuranetin inhibits several features of ALI induced by LPS in mice, improving respiratory function and reducing the weight loss induced by systemic infection (Bittencourt‐Mernak et al., 2017). Although COVID‐19‐induced ALI has a different mechanism from LPS‐induced ALI, the cytokine storm and lung injury have similar features.
FIGURE 3.
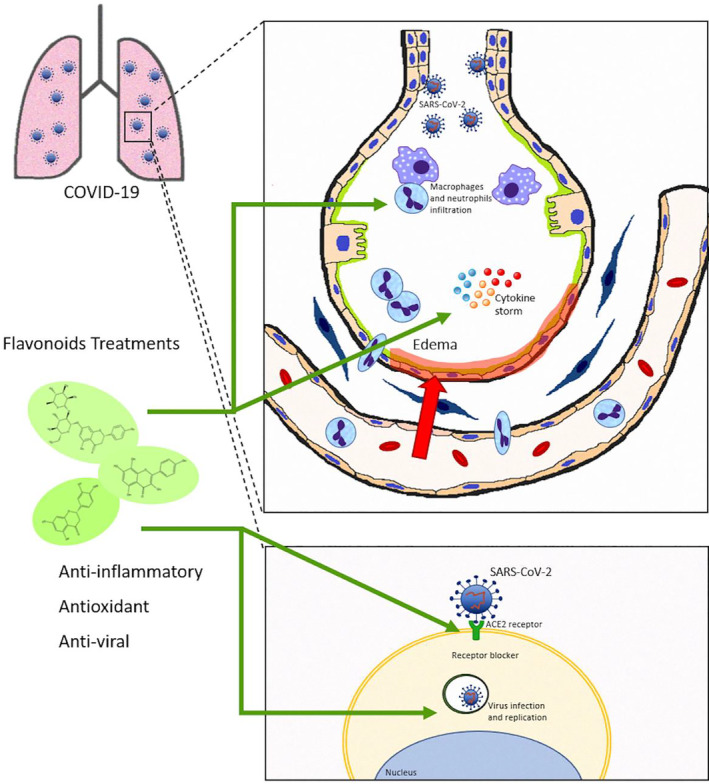
Possible effects of flavonoids on COVID‐19‐induced acute lung injury: COVID‐19 affects several organs, especially the pulmonary system. Severe COVID‐19 is characterized by a cytokine storm and acute lung inflammation that can progress to acute lung injury and systemic inflammation. Acute lung injury is well characterized by endothelium and/or epithelial injury, macrophage activation, neutrophil recruitment, and oxidative stress as well as high cytokine release. The binding of SARS‐CoV‐2 to ACE2 in epithelial cells induces infection and virus replication. The flavonoid has well‐described antiinflammatory, antioxidant, and antiviral effects. The evidence reviewed in the literature shows that flavonoid could be a potential therapeutic target for COVID‐19, since it inhibits the cytokine storm and lung inflammation. In addition, evidence suggests that it can block the entry and replication of the virus, and should be further explored
Therefore, this review emphasized flavonoids' ability to inhibit several features of COVID‐19, which may help researchers working on COVID‐19 drug discovery, an urgent need under the continuing spread of COVID‐19. Of course, in vitro testing of the drug candidates is necessary ‐ however, this review may prove valuable for exploring and developing novel natural anti‐COVID‐19 therapeutic agents in the future, which can be associated with other drugs or not. However, further research and more detailed pharmacological investigations in‐vivo, including PK/PD studies, must be performed to develop new drugs to treat coronavirus diseases, such as COVID‐19.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare related to the data shown on this publication.
ACKNOWLEDGMENTS
The authors would like to thank FAPESP, CNPq, and CAPES for financial support. We would like to express our special thanks to Shahin Shams from the Department of Biomedical Engineering, University of California, Davis, California, USA, who gently revised the manuscript.
Santana FPR, Thevenard F, Gomes KS, et al. New perspectives on natural flavonoids on COVID‐19‐induced lung injuries. Phytotherapy Research. 2021;35:4988–5006. 10.1002/ptr.7131
Funding information Conselho Nacional de Desenvolvimento Científico e Tecnológico, Grant/Award Numbers: 301354/2019‐7, 306278/2015‐4; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Grant/Award Number: financial code 001; Fundação de Amparo à Pesquisa do Estado de São Paulo, Grant/Award Numbers: 2018/06088‐0, 2018/07885‐1, 2020/01221‐4
Contributor Information
Carla Maximo Prado, Email: carla.prado@unifesp.br.
João Henrique Ghilardi Lago, Email: joao.lago@ufabc.edu.br.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study since it is a review paper
REFERENCES
- Akbari, H. , Tabrizi, R. , Lankarani, K. B. , Aria, H. , Vakili, S. , Asadian, F. , … Faramarz, S. (2020). The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID‐19): A systematic review and meta‐analysis. Life Sciences, 258, 118167–118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulou, L. , Holt, A. C. , Medzhitov, R. , & Flavell, R. A. (2001). Recognition of double‐stranded RNA and activation of NF‐κB by Toll‐like receptor 3. Nature, 413, 732–738. [DOI] [PubMed] [Google Scholar]
- Aloufi, N. , Traboulsi, H. , Ding, J. , Fonseca, G. J. , Nair, P. , Huang, S. K. , … Baglole, C. J. (2020). Angiotensin‐converting enzyme 2 (ACE2) expression in COPD and IPF fibroblasts—The forgotten cell in COVID‐19. American Journal of Physiology. Lung Cellular and Molecular Physiology, 320, L152–L157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand, K. , Ziebuhr, J. , Wadhwani, P. , Mesters, J. R. , & Hilgenfeld, R. (2003). Coronavirus main proteinase (3CLpro) structure: Basis for design of anti‐SARS drugs. Science, 300, 1763–1767. [DOI] [PubMed] [Google Scholar]
- Arendse, L. B. , Jan Danser, A. H. , Poglitsch, M. , Touyz, R. M. , Burnett, J. C. , Llorens‐Cortes, C. , … Sturrock, E. D. (2019). Novel therapeutic approaches targeting the renin‐angiotensin system and associated peptides in hypertension and heart failure. Pharmacological Reviews, 71, 539–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuti, Y. I. (2020). Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): An overview of viral structure and host response. Diabetes and Metabolic Syndrome: Clinical Research & Reviews, 14, 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez‐Santos, Y. M. , St. John, S. E. , & Mesecar, A. D. (2015). The SARS‐coronavirus papain‐like protease: Structure, function and inhibition by designed antiviral compounds. Antiviral Research, 115, 21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu, A. , Sarkar, A. , & Maulik, U. (2020). Molecular docking study of potential phytochemicals and their effects on the complex of SARS‐CoV2 spike protein and human ACE2. Scientific Reports, 10, 17699–17713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batenburg, W. W. , Tom, B. , Schuijt, M. P. , & Danser, A. H. J. (2005). Angiotensin II type 2 receptor‐mediated vasodilation. Focus on bradykinin, NO and endothelium‐derived hyperpolarizing factor(s). Vascular Pharmacology, 42, 109–118. [DOI] [PubMed] [Google Scholar]
- Bellani, G. , Laffey, J. G. , Pham, T. , Fan, E. , Brochard, L. , Esteban, A. , … Pesenti, A. (2016). Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. Journal of the American Medical Association, 315, 788–800. [DOI] [PubMed] [Google Scholar]
- Benigni, A. , Cassis, P. , & Remuzzi, G. (2010). Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Molecular Medicine, 2, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birra, D. , Benucci, M. , Landolfi, L. , Merchionda, A. , Loi, G. , Amato, P. , … Moscato, P. (2020). COVID 19: A clue from innate immunity. Immunologic Research, 68, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt‐Mernak, M. I. , Pinheiro, N. M. , Santana, F. P. R. , Guerreiro, M. P. , Saraiva‐Romanholo, B. M. , Grecco, S. S. , … Prado, C. M. (2017). Prophylactic and therapeutic treatment with the flavonone sakuranetin ameliorates LPS‐induced acute lung injury. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 312, L217–L230. [DOI] [PubMed] [Google Scholar]
- Brendler, T. , Al‐Harrasi, A. , Bauer, R. , Gafner, S. , Hardy, M. L. , Heinrich, M. , … Williamson, E. M. (2020). Botanical drugs and supplements affecting the immune response in the time of COVID‐19: Implications for research and clinical practice. Phytotherapy Research, 29, 1–19. [DOI] [PubMed] [Google Scholar]
- Burgos‐Blasco, B. , Güemes‐Villahoz, N. , Santiago, J. L. , Fernandez‐Vigo, J. I. , Espino‐Paisán, L. , Sarriá, B. , … Martinez‐de‐la‐Casa, J. M. (2020). Hypercytokinemia in COVID‐19: Tear cytokine profile in hospitalized COVID‐19 patients. Experimental Eye Research, 200, 108253–108258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, J. F. W. , Kok, K. H. , Zhu, Z. , Chu, H. , To, K. K. W. , Yuan, S. , & Yuen, K. Y. (2020). Genomic characterization of the 2019 novel human‐pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging Microbes & Infections, 9, 221–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.‐N. , Lin, C. P. C. , Huang, K.‐K. , Chen, W.‐C. , Hsieh, H.‐P. , Liang, P.‐H. , & Hsu, J. T.‐A. (2005). Inhibition of SARS‐CoV 3C‐like protease activity by Theaflavin‐3,3′‐digallate (TF3). Evidence‐Based Complementary and Alternative Medicine, 2, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G. , Wu, D. , Guo, W. , Cao, Y. , Huang, D. , Wang, H. , … Ning, Q. (2020). Clinical and immunological features of severe and moderate coronavirus disease 2019. The Journal of Clinical Investigation, 130, 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Li, J. , Luo, C. , Liu, H. , Xu, W. , Chen, G. , … Jiang, H. (2006). Binding interaction of quercetin‐3‐β‐galactoside and its synthetic derivatives with SARS‐CoV 3CLpro: Structure‐activity relationship studies reveal salient pharmacophore features. Bioorganic & Medicinal Chemistry, 14, 8295–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Li, X. , Chen, M. , Feng, Y. , & Xiong, C. (2020). The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS‐CoV‐2. Cardiovascular Research, 116, 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, J. K. , Curtis‐Long, M. J. , Lee, K. H. , Kim, D. W. , Ryu, H. W. , Yuk, H. J. , & Park, K. H. (2013). Geranylated flavonoids displaying SARS‐CoV papain‐like protease inhibition from the fruits of Paulownia tomentosa . Bioorganic & Medicinal Chemistry, 21, 3051–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho, M. A. S. , Muzitano, M. F. , & Costa, S. S. (2009). Flavonoides: Potenciais agentes terapêuticos para o processo inflamatório. Revista Virtual de Química, 1, 241–256. [Google Scholar]
- Day, J. W. , Fox, T. A. , Halsey, R. , Carpenter, B. , & Kottaridis, P. D. (2020). IL‐1 blockade with anakinra in acute leukaemia patients with severe COVID‐19 pneumonia appears safe and may result in clinical improvement. British Journal of Haematology, 190, e57–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolhnikoff, M. , Duarte‐Neto, A. N. , de Almeida Monteiro, R. A. , da Silva, L. F. F. , de Oliveira, E. P. , Saldiva, P. H. N. , … Negri, E. M. (2020). Pathological evidence of pulmonary thrombotic phenomena in severe COVID‐19. Journal of Thrombosis and Haemostasis, 18, 1517–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, G. , Jiang, Z. Y. , Sun, B. , Fu, J. , & Li, T. Z. (2016). Fisetin alleviates lipopolysaccharide‐induced acute lung injury via TLR4‐mediated NF‐κB signaling pathway in rats. Inflammation, 39, 148–157. [DOI] [PubMed] [Google Scholar]
- Forrester, S. J. , Booz, G. W. , Sigmund, C. D. , Coffman, T. M. , Kawai, T. , Rizzo, V. , … Eguchi, S. (2018). Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiological Reviews, 98, 1627–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia, T. , Rotelli, A. E. , Juarez, A. O. , & Pelzer, L. E. (2001). Anti‐inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Il Farmaco, 56, 683–687. [DOI] [PubMed] [Google Scholar]
- Guo, Y. R. , Cao, Q. D. , Hong, Z. S. , Tan, Y. Y. , Chen, S. D. , Jin, H. J. , … Yan, Y. (2020). The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak—An update on the status. Military Medical Research, 7, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen, M. , Nieminen, R. , Vuorela, P. , Heinonen, M. , & Moilanen, E. (2007). Anti‐inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT‐1 and NF‐kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF‐kappaB activation along with their inhibitory effects. Mediators of Inflammation, 2007, 25673–25683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming, I. , Timens, W. , Bulthuis, M. L. C. , Lely, A. T. , Navis, G. J. , & Van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology, 203, 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havsteen, B. H. (2002). The biochemistry and medical significance of the flavonoids. Pharmacology & Therapeutics, 96, 67–202. [DOI] [PubMed] [Google Scholar]
- Herold, S. , Mayer, K. , & Lohmeyer, J. (2011). Acute lung injury: How macrophages orchestrate resolution of inflammation and tissue repair. Frontiers in Immunology, 2, 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano, T. , Higa, S. , Arimitsu, J. , Naka, T. , Shima, Y. , Ohshima, S. , … Tanaka, T. (2004). Flavonoids such as luteolin, fisetin and apigenin are inhibitors of interleukin‐4 and interleukin‐13 production by activated human basophils. International Archives of Allergy and Immunology, 134, 135–140. [DOI] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , … Pöhlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, Y. , Kuba, K. , & Penninger, J. M. (2008). The discovery of angiotensin‐converting enzyme 2 and its role in acute lung injury in mice. Experimental Physiology, 93, 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, Y. , Kuba, K. , Rao, S. , Huan, Y. , Guo, F. , Guan, B. , … Penninger, J. M. (2005). Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature, 436, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, M. T. , Sarkar, C. , El‐Kersh, D. M. , Jamaddar, S. , Uddin, S. J. , Shilpi, J. A. , & Mubarak, M. S. (2020). Natural products and their derivatives against coronavirus: A review of the non‐clinical and pre‐clinical data. Phytotherapy Research, 34, 2471–2492. [DOI] [PubMed] [Google Scholar]
- Istifli, E. S. , Netz, P. A. , Sihoglu Tepe, A. , Husunet, M. T. , Sarikurkcu, C. , & Tepe, B. (2020). In silico analysis of the interactions of certain flavonoids with the receptor‐binding domain of 2019 novel coronavirus and cellular proteases and their pharmacokinetic properties. Journal of Biomolecular Structure & Dynamics, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, T. , Gao, L. , Lu, J. , & Zhang, Y.‐D. (2013). ACE2‐Ang‐(1‐7)‐Mas axis in brain: A potential target for prevention and treatment of ischemic stroke. Current Neuropharmacology, 11, 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, Y. , Yang, H. , Ji, W. , Wu, W. , Chen, S. , Zhang, W. , & Duan, G. (2020). Virology, epidemiology, pathogenesis, and control of covid‐19. Viruses, 12, 372–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, S. , Kim, H. , Kim, S. , Shin, D. H. , & Kim, M. S. (2019). Characteristics of flavonoids as potent MERS‐CoV 3C‐like protease inhibitors. Chemical Biology & Drug Design, 94, 2023–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, S. , Kim, S. , Shin, D. H. , & Kim, M. S. (2020). Inhibition of SARS‐CoV 3CL protease by flavonoids. Journal of Enzyme Inhibition and Medicinal Chemistry, 35, 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucá, M. M. , Cysne Filho, F. M. S. , de Almeida, J. C. , Mesquita, D. d. S. , Barriga, J. R. d. M. , Dias, K. C. F. , … Vasconcelos, S. M. M. (2020). Flavonoids: Biological activities and therapeutic potential. Natural Product Research, 34, 692–705. [DOI] [PubMed] [Google Scholar]
- Kim, D. W. , Seo, K. H. , Curtis‐Long, M. J. , Oh, K. Y. , Oh, J.‐W. , … Park, K. H. (2014). Phenolic phytochemical displaying SARS‐CoV papain‐like protease inhibition from the seeds of Psoralea corylifolia . Journal of Enzyme Inhibition and Medicinal Chemistry, 29, 59–63. [DOI] [PubMed] [Google Scholar]
- Kim, H. P. , Son, K. H. , Chang, H. W. , & Kang, S. S. (2004). Anti‐inflammatory plant flavonoids and cellular action mechanisms. Journal of Pharmacological Sciences, 96, 229–245. [DOI] [PubMed] [Google Scholar]
- Kimata, M. , Shichijo, M. , Miura, T. , Serizawa, I. , Inagaki, N. , & Nagai, H. (2000). Effects of luteolin, quercetin and baicalein on immunoglobulin E‐mediated mediator release from human cultured mast cells. Clinical and Experimental Allergy, 30, 501–508. [DOI] [PubMed] [Google Scholar]
- Kong, G. , Huang, X. , Wang, L. , Li, Y. , Sun, T. , Han, S. , … Wang, X. (2016). Astilbin alleviates LPS‐induced ARDS by suppressing MAPK signaling pathway and protecting pulmonary endothelial glycocalyx. International Immunopharmacology, 36, 51–58. [DOI] [PubMed] [Google Scholar]
- Kreutz, R. , Algharably, E. A. E. H. , Azizi, M. , Dobrowolski, P. , Guzik, T. , Januszewicz, A. , … Burnier, M. (2020). Hypertension, the renin‐angiotensin system, and the risk of lower respiratory tract infections and lung injury: Implications for COVID‐19. Cardiovascular Research, 116, 1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, V. , Tan, K. P. , Wang, Y. M. , Lin, S. W. , & Liang, P. H. (2016). Identification, synthesis and evaluation of SARS‐CoV and MERS‐CoV 3C‐like protease inhibitors. Bioorganic & Medicinal Chemistry, 24, 3035–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, M. Y. , Liao, M. F. , Chen, F. L. , Li, Y. C. , Yang, M. L. , Lin, R. H. , & Kuan, Y. H. (2011). Luteolin attenuates the pulmonary inflammatory response involves abilities of antioxidation and inhibition of MAPK and NFκB pathways in mice with endotoxin‐induced acute lung injury. Food and Chemical Toxicology, 49, 2660–2666. [DOI] [PubMed] [Google Scholar]
- Kwon, D. H. , Ji, J. H. , Yim, S. H. , Kim, B. S. , & Choi, H. J. (2018). Suppression of influenza B virus replication by sakuranetin and mode of its action. Phytotherapy Research, 32, 2475–2479. [DOI] [PubMed] [Google Scholar]
- Lago, J. H. G. , Toledo‐Arruda, A. C. , Mernak, M. , Barrosa, K. H. , Martins, M. A. , Tibério, I. F. L. C. , & Prago, C. M. (2014). Structure‐activity association of flavonoids in lung diseases. Molecules, 19, 3570–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law, H. K. W. , Chung, Y. C. , Hoi, Y. N. , Sin, F. S. , Yuk, O. C. , Luk, W. , … Lau, Y. L. (2005). Chemokine up‐regulation in SARS‐coronavirus‐infected, monocyte‐derived human dendritic cells. Blood, 106, 2366–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. , Yang, D. , Cao, X. , Wang, F. , Jiang, H. , Guo, H. , … Yin, X. (2016). LFG‐500, a newly synthesized flavonoid, attenuates lipopolysaccharide‐induced acute lung injury and inflammation in mice. Biochemical Pharmacology, 113, 57–69. [DOI] [PubMed] [Google Scholar]
- Li, H. , Liu, L. , Zhang, D. , Xu, J. , Dai, H. , Tang, N. , … Cao, B. (2020). SARS‐CoV‐2 and viral sepsis: Observations and hypotheses. Lancet, 395, 1517–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, K. , He, Z. , Wang, X. , Pineda, M. , Chen, R. , Liu, H. , … Guo, J. (2018). Apigenin C‐glycosides of Microcos paniculata protects lipopolysaccharide induced apoptosis and inflammation in acute lung injury through TLR4 signaling pathway. Free Radical Biology & Medicine, 124, 163–175. [DOI] [PubMed] [Google Scholar]
- Li, M. Y. , Li, L. , Zhang, Y. , & Wang, X. S. (2020). Expression of the SARS‐CoV‐2 cell receptor gene ACE2 in a wide variety of human tissues. Infectious Diseases of Poverty, 9, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Moore, M. J. , Vasllieva, N. , Sui, J. , Wong, S. K. , Berne, M. A. , … Farzan, M. (2003). Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature, 426, 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C. W. , Tsai, F. J. , Tsai, C. H. , Lai, C. C. , Wan, L. , Ho, T. Y. , … Chao, P. D. L. (2005). Anti‐SARS coronavirus 3C‐like protease effects of Isatis indigotica root and plant‐derived phenolic compounds. Antiviral Research, 68, 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung, J. , Lin, Y. S. , Yang, Y. H. , Chou, Y. L. , Shu, L. H. , Cheng, Y. C. , … Wu, C. Y. (2020). The potential chemical structure of anti‐SARS‐CoV‐2 RNA‐dependent RNA polymerase. Journal of Medical Virology, 92, 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldoveanu, B. , Otmishi, P. , Jani, P. , Walker, J. , Sarmiento, X. , Guardiola, J. , … Yu, J. (2009). Inflammatory mechanisms in the lung. Journal of Inflammation Research, 2, 1–11. [PMC free article] [PubMed] [Google Scholar]
- Needle, D. , Lountos, G. T. , & Waugh, D. S. (2015). Structures of the Middle East respiratory syndrome coronavirus 3C‐like protease reveal insights into substrate specificity. Acta Crystallographica, Section D: Biological Crystallography, 71, 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T. T. H. , Woo, H. J. , Kang, H. K. , Nguyen, V. D. , Kim, Y. M. , Kim, D. W. , … Kim, D. (2012). Flavonoid‐mediated inhibition of SARS coronavirus 3C‐like protease expressed in Pichia pastoris . Biotechnology Letters, 34, 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijveldt, R. J. , van Nood, E. , van Hoorn, D. E. , Boelens, P. G. , van Norren, K. , & Van Leeuwen, P. A. (2001). Flavonoids: A review of probable mechanisms of action and potential applications. The American Journal of Clinical Nutrition, 74, 418–425. [DOI] [PubMed] [Google Scholar]
- O'Leary, K. A. , De Pascual‐Tereasa, S. , Needs, P. W. , Bao, Y. P. , O'Brien, N. M. , & Williamson, G. (2004). Effect of flavonoids and Vitamin E on cyclooxygenase‐2 (COX‐2) transcription. Mutation Research, Fundamental and Molecular Mechanisms of Mutagenesis, 551, 245–254. [DOI] [PubMed] [Google Scholar]
- Olszanecki, R. , Madej, J. , Suski, M. , Gebska, A. , Bujak‐Gizycka, B. , & Korbut, R. (2009). Angiotensin metabolism in rat stomach wall: Prevalence of angiotensin‐1‐7 formation. Journal of Physiology and Pharmacology, 60, 191–196. [PubMed] [Google Scholar]
- Pan, F. , Ye, T. , Sun, P. , Gui, S. , Liang, B. , Li, L. , … Zheng, C. (2020). Time course of lung changes at chest CT during recovery from Coronavirus disease 2019 (COVID‐19). Radiology, 295, 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panche, A. N. , Diwan, A. D. , & Chandra, S. R. (2016). Flavonoids: An overview. Journal of Nutritional Science, 5, e47–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. Y. , Ko, J. A. , Kim, D. W. , Kim, Y. M. , Kwon, H. J. , Jeong, H. J. , … Ryu, Y. B. (2016). Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS‐CoV. Journal of Enzyme Inhibition and Medicinal Chemistry, 31, 23–30. [DOI] [PubMed] [Google Scholar]
- Park, J.‐Y. , Yuk, H. J. , Ryu, H. W. , Lim, S. H. , Kim, K. S. , Park, K. H. , … Lee, W. S. (2017). Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. Journal of Enzyme Inhibition and Medicinal Chemistry, 32, 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris, J. S. M. , Chu, C. M. , Cheng, V. C. C. , Chan, K. S. , Hung, I. F. N. , Poon, L. L. M. , … Yuen, K. Y. (2003). Clinical progression and viral load in a community outbreak of coronavirus‐associated SARS pneumonia: A prospective study. Lancet, 361, 1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qamar, M. T. u. , Alqahtani, S. M. , Alamri, M. A. , & Chen, L. L. (2020). Structural basis of SARS‐CoV‐2 3CLpro and anti‐COVID‐19 drug discovery from medicinal plants. Journal of Pharmaceutical Analysis, 10, 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj, V. S. , Mou, H. , Smits, S. L. , Dekkers, D. H. W. , Müller, M. A. , Dijkman, R. , … Haagmans, B. L. (2013). Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus‐EMC. Nature, 495, 251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogliani, P. , Calzetta, L. , Coppola, A. , Puxeddu, E. , Sergiacomi, G. , D’Amato, D. , & Orlacchio, A. (2020). Are there pulmonary sequelae in patients recovering from COVID‐19? Respiratory Research, 21, 286–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh, C. (2012). A facile inhibitor screening of SARS coronavirus N protein using nanoparticle‐based RNA oligonucleotide. International Journal of Nanomedicine, 7, 2173–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan, H. A. , & Byrareddy, S. N. (2020). The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. Journal of Autoimmunity, 109, 102433–102436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungsung, S. , Singh, T. U. , Rabha, D. J. , Kumar, T. , Cholenahalli Lingaraju, M. , Parida, S. , … Kumar, D. (2018). Luteolin attenuates acute lung injury in experimental mouse model of sepsis. Cytokine, 110, 333–343. [DOI] [PubMed] [Google Scholar]
- Russo, M. , Moccia, S. , Spagnuolo, C. , Tedesco, I. , & Russo, G. L. (2020). Roles of flavonoids against coronavirus infection. Chemico‐Biological Interactions, 328, 109211–109223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, Y. B. , Jeong, H. J. , Kim, J. H. , Kim, Y. M. , Park, J. Y. , Kim, D. , … Lee, W. S. (2010). Biflavonoids from Torreya nucifera displaying SARS‐CoV 3CLpro inhibition. Bioorganic & Medicinal Chemistry, 18, 7940–7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana, F. P. R. , Pinheiro, N. M. , Mernak, M. I. B. , Righetti, R. F. , Martins, M. A. , Lago, J. H. G. , … Prado, C. M. (2016). Evidences of herbal medicine‐derived natural products effects in inflammatory lung diseases. Mediators of Inflammation, 2016, 2348968–2348981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman, D. , & Fielding, B. C. (2019). Coronavirus envelope protein: Current knowledge. Virology Journal, 16, 69–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, S. , Sauter, D. , Wang, K. , Zhang, R. , Sun, B. , Karioti, A. , … Schwarz, W. (2014). Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Medica, 80, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif, F. , Aazami, H. , Khoshmirsafa, M. , Kamali, M. , Mohsenzadegan, M. , Pornour, M. , & Mansouri, D. (2020). JAK inhibition as a new treatment strategy for patients with COVID‐19. International Archives of Allergy and Immunology, 181, 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong, R. K. , Kim, J. A. , & Shin, O. S. (2018). Wogonin, a flavonoid isolated from Scutellaria baicalensis, has anti‐viral activities against influenza infection via modulation of AMPK pathways. Acta Virologica, 62, 78–85. [DOI] [PubMed] [Google Scholar]
- Singh, A. K. , Gupta, R. , & Misra, A. (2020). Comorbidities in COVID‐19: Outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabetes and Metabolic Syndrome: Clinical Research & Reviews, 14, 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slader, C. A. , Reddel, H. K. , Jenkins, C. R. , Armour, C. L. , & Bosnic‐Anticevich, S. Z. (2006). Complementary and alternative medicine use in asthma: Who is using what? Respirology, 11, 373–387. [DOI] [PubMed] [Google Scholar]
- Solnier, J. , & Fladerer, J. P. (2020). Flavonoids: A complementary approach to conventional therapy of COVID‐19? Phytochemistry Reviews, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soromou, L. W. , Chu, X. , Jiang, L. , Wei, M. , Huo, M. , Chen, N. , … Deng, X. (2012). In vitro and in vivo protection provided by pinocembrin against lipopolysaccharide‐induced inflammatory responses. International Immunopharmacology, 14, 66–74. [DOI] [PubMed] [Google Scholar]
- South, A. M. , Diz, D. I. , & Chappell, M. C. (2020). COVID‐19, ACE2, and the cardiovascular consequences. American Journal of Physiology. Heart and Circulatory Physiology, 318, H1084–H1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel, M. , Schneider, K. , Weber, F. , Weidmann, M. , & Hufert, F. T. (2006). Interaction of severe acute respiratory syndrome‐associated coronavirus with dendritic cells. The Journal of General Virology, 87, 1953–1960. [DOI] [PubMed] [Google Scholar]
- Tianzhu, Z. , Shihai, Y. , & Juan, D. (2014). The effects of morin on lipopolysaccharide‐induced acute lung injury by suppressing the lung NLRP3 inflammasome. Inflammation, 37, 1976–1983. [DOI] [PubMed] [Google Scholar]
- Tseng, T. L. , Chen, M. F. , Tsai, M. J. , Hsu, Y. H. , Chen, C. P. , & Lee, T. J. F. (2012). Oroxylin‐A rescues lps‐induced acute lung injury via regulation of NF‐κB signaling pathway in rodents. PLoS One, 7, e47403–e47413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutunchi, H. , Naeini, F. , Ostadrahimi, A. , & Hosseinzadeh‐Attar, M. J. (2020). Naringenin, a flavanone with antiviral and anti‐inflammatory effects: A promising treatment strategy against COVID‐19. Phytotherapy Research, 34, 3137–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Grunewald, M. , & Perlman, S. (2020). Coronaviruses: An updated overview of their replication and pathogenesis. In Methods in molecular biology (pp. 1–29). Humana Press Inc.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, X. , Zhu, X. , Hu, N. , Zhang, X. , Sun, T. , Xu, J. , & Bian, X. (2015). Baicalin attenuates angiotensin II‐induced endothelial dysfunction. Biochemical and Biophysical Research Communications, 465, 101–107. [DOI] [PubMed] [Google Scholar]
- Wenzhong, L. , & Hualan, L. (2020). COVID‐19: Attacks the 1‐beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. ChemRxiv Preprint. [Google Scholar]
- Wu, J. , & Chen, Z. J. (2014). Innate immune sensing and signaling of cytosolic nucleic acids. Annual Review of Immunology, 32, 461–488. [DOI] [PubMed] [Google Scholar]
- Xie, Y.‐C. , Dong, X.‐W. , Wu, X.‐M. , Yan, X.‐F. , & Xie, Q.‐M. (2009). Inhibitory effects of flavonoids extracted from licorice on lipopolysaccharide‐induced acute pulmonary inflammation in mice. International Immunopharmacology, 9, 194–200. [DOI] [PubMed] [Google Scholar]
- Xu, Z. , Yang, L. , Zhang, X. , Zhang, Q. , Yang, Z. , Liu, Y. , … Liu, W. (2020). Discovery of potential flavonoid inhibitors against COVID‐19 3CL proteinase based on virtual screening strategy. Frontiers in Molecular Biosciences, 7, 556481–556488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, R. , Zhang, Y. , Li, Y. , Xia, L. , Guo, Y. , & Zhou, Q. (2020). Structural basis for the recognition of SARS‐CoV‐2 by full‐length human ACE2. Science, 367, 1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, Q. , Wang, B. , & Mao, J. (2020). The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID‐19. The Journal of Infection, 80, 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, R. , & Liu, Z. (2020). ACE2 exhibits protective effects against LPS‐induced acute lung injury in mice by inhibiting the LPS‐TLR4 pathway. Experimental and Molecular Pathology, 113, 104350–104387. [DOI] [PubMed] [Google Scholar]
- Yin, Y. , & Wunderink, R. G. (2018). MERS, SARS and other coronaviruses as causes of pneumonia. Respirology, 23, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, J.‐H. , & Baek, S. J. (2005). Molecular targets of dietary polyphenols with anti‐inflammatory properties. Yonsei Medical Journal, 46, 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki, A. M. , Van Boheemen, S. , Bestebroer, T. M. , Osterhaus, A. D. M. E. , & Fouchier, R. A. M. (2012). Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. The New England Journal of Medicine, 367, 1814–1820. [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Wu, Z. , Li, J.‐W. , Tan, K. , Yang, W. , Zhao, H. , & Wang, G. Q. (2020). Discharge may not be the end of treatment: Pay attention to pulmonary fibrosis caused by severe COVID‐19. Journal of Medical Virology, 93, 1378–1386. [DOI] [PubMed] [Google Scholar]
- Zhang, R. , Ai, X. , Duan, Y. , Xue, M. , He, W. , Wang, C. , … Liu, H. (2017). Kaempferol ameliorates H9N2 swine influenza virus‐induced acute lung injury by inactivation of TLR4/MyD88‐mediated NF‐κB and MAPK signaling pathways. Biomedicine & Pharmacotherapy, 89, 660–672. [DOI] [PubMed] [Google Scholar]
- Zhao, Y. , Zhao, Z. , Wang, Y. , Zhou, Y. , Ma, Y. , & Zuo, W. (2020). Single‐cell RNA expression profiling of ACE2, the receptor of SARS‐CoV‐2. American Journal of Respiratory and Critical Care Medicine, 202, 756–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X. L. , Wang, X. G. , Hu, B. , Zhang, L. , Zhang, W. , … Shi, Z. L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579, 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, G. F. , Guo, H. J. , Huang, Y. , Wu, C. T. , & Zhang, X. F. (2015). Eriodictyol, a plant flavonoid, attenuates LPS‐induced acute lung injury through its antioxidative and anti‐inflammatory activity. Experimental and Therapeutic Medicine, 10, 2259–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang, M. , Jiang, H. , Suzuki, Y. , Li, X. , Xiao, P. , Tanaka, T. , … Hattori, T. (2009). Procyanidins and butanol extract of Cinnamomi cortex inhibit SARS‐CoV infection. Antiviral Research, 82, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study since it is a review paper


